Abstract
Aims
Depression is a recognized risk marker for mortality among acute coronary syndrome (ACS) patients. We hypothesized that ventricular arrhythmia detected by inpatient telemetry monitoring is more frequent among ACS patients with elevated depressive symptoms compared to those without depressive symptoms.
Methods and results
We analyzed data from patients enrolled in a prospective observational study of depression in ACS. Telemetry recordings during the index admission (average recording 21.5 ± 2.8 hours) were analyzed for frequent premature ventricular complexes (PVCs), defined as ≥10 per hour. The self-report Beck Depression Inventory (BDI) was used to assess depressive symptoms. Among 203 ACS patients, frequent PVCs were observed in 28% of patients with moderate depressive symptoms, 28% of those with mild symptoms, and only 11% of those with no/minimal symptoms (p=0.03). Log-transformed PVCs per hour were associated with depressive symptom category (p=0.02). In a multivariable logistic regression model that included age, gender, left ventricular ejection fraction, cardiovascular risk score, heart rate, and QT interval, both mild symptoms (OR 3.13, 95% 1.02–9.57, p=0.046) and moderate to severe symptoms (OR 3.48, 95% CI 1.13–10.68, p=0.029) were independently associated with frequent PVCs.
Conclusion
In this sample of ACS patients, depressive symptoms were independently associated with frequent PVCs during inpatient telemetry monitoring.
Keywords: depression, acute coronary syndrome, arrhythmia
Introduction
Depression is a predictor of early mortality in patients after acute coronary syndrome (ACS), despite adjustment for coronary artery disease (CAD) severity.1, 2 More than one-third of all mortality in patients with CAD is due to sudden cardiac death (SCD), typically from arrhythmia such as sustained ventricular tachycardia or ventricular fibrillation,3 and studies in a variety of populations have suggested that depression is associated with SCD and ventricular arrhythmia.4–7 In a study of patients with implantable cardioverter defibrillators, depressive symptoms were associated with ventricular arrhythmias that resulted in shock for ventricular arrhythmia, and this relationship was stronger among the subgroup of patients with coronary artery disease.8
Cardiac monitoring has been used after ACS to detect arrhythmias, such as ventricular tachycardia or frequent premature ventricular complexes (PVCs). Even in the present era of coronary stenting and effective medical therapies, ventricular arrhythmia continues to be a risk marker for future mortality in ACS.9–12 There have been relatively few prior studies of the relationship between ventricular arrhythmia and depression in CAD patients, with conflicting results.6, 13, 14 One of the initial studies that defined the importance of depression for prognosis after myocardial infarction documented a markedly elevated risk of mortality among patients with both depression and more frequent ventricular arrhythmia in the form of frequent premature ventricular complexes (PVC) (odds ratio for interaction~6).2
In this study we hypothesized that among patients with ACS, depressive symptoms were associated with more frequent ventricular arrhythmia in the form of PVCs measured by inpatient telemetry monitoring. In addition, based on a recent analysis that noted a gender difference in electrocardiographic QT interval changes associated with depressive symptoms,15 we hypothesized that there was a stronger relationship between PVCs and depressive symptoms in women than in men.
Methods
Institutional Review Board approval for this study was obtained at Columbia University. The cohort was drawn from among the first 500 patients enrolled in an ongoing prospective cohort study of depression after ACS, Prescription Use, Lifestyle, and Stress Evaluation (PULSE). Participants for this study were recruited from among patients admitted to Columbia University Medical Center between February 2009 and June 2010.
ACS events were defined according to American Heart Association/American College of Cardiology criteria16 as either acute MI or UA. All patients had symptoms consistent with acute myocardial ischemia and at least one of the following: ischemic electrocardiographic changes (i.e. ST depression and/or T-wave abnormalities), an angiogram indicative of CAD on current admission, and/or documented history of CAD. Patients who presented with an acute rise in serum cardiac enzyme levels were categorized as MI. A study cardiologist confirmed ACS eligibility for all patients. Patients with ST elevation were excluded from the analysis, to prevent possible confounding associated with reperfusion-related arrhythmias in this group.17
The Beck Depression Inventory (BDI),18 a 21-item self-report measure of depressive symptom severity, was administered within one week after the index ACS event. The BDI has repeatedly been associated with long-term mortality after ACS.2, 19–21 Depressive symptoms were categorized according to BDI score: 0 to 4 (none/minimal symptoms), 5 to 9 (mild symptoms), or ≥10 (at least moderate symptoms).
Telemetry monitoring was performed via a 5 electrode continuous telemetry system that was fitted for clinical purposes on patients during their hospital stay (Philips Healthcare). The telemetry system used a template matching algorithm with a sampling rate of 125 to 250 samples per second to differentiate premature ventricular complexes from normal QRS complexes in the ECG signal.22 The sensitivity of the algorithm for PVC detection based on an analysis of 44 non-paced test signals from the 2-channel MIT-Beth Israel Hospital ECG database was 94%, and the false positive rate was 0.3%.22 Summary reports were generated that estimated PVC counts and median heart rate on an hourly basis, and samples of ECG tracings were collected with each summary report (figure 1). These reports were obtained from the medical record and tracings with more than 12 hours of recordings were included in the analysis. A cardiac electrophysiologist (WW) examined each tracing for accuracy blinded to BDI score, and tracings with false positive readings for PVCs were removed. In addition, tracings with ventricular paced rhythms or with atrial fibrillation or atrial flutter were removed from the analysis to minimize false-positive PVC counts. The simple average of PVC counts and heart rate during the recording period were estimated. PVC rates were dichotomized at ≥10 per hour or <10 per hour based on prior studies in patients with acute MI23 and unstable angina 24.
Figure 1.
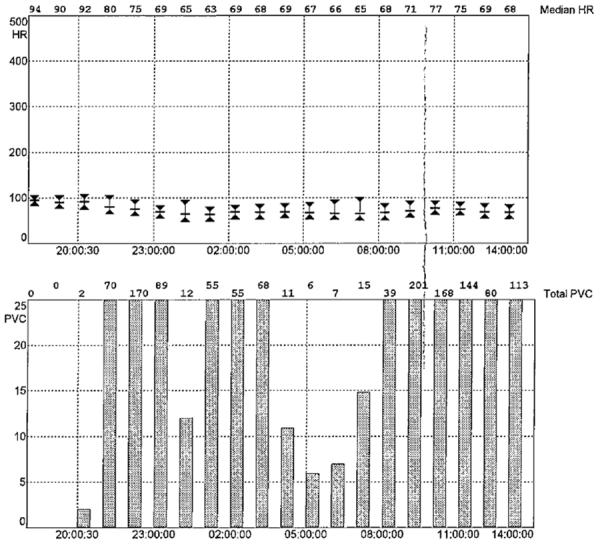
Example of cardiac telemetry report with median heart rate and premature ventricular complex counts per hour.
Twelve-lead ECG tracings from the index admission were obtained and the QT interval was measured from the onset of the QRS complex to the end of the T wave, defined as the point of return of the T wave to the isoelectric line or to the nadir between the T and U waves in cases where a U wave was present.25 QT intervals were corrected for heart rate according to Fridericia's method (QTcF = QT / RR1/3).
Statistical Analyses
Linear-by-linear association χ2 tests for trends and linear regression were used to compare categorical and continuous measurements between groups with no/minimal depressive symptoms, mild symptoms, and at least moderate symptoms. Logistic regression models were used to estimate the relative odds of frequent PVCs (≥10 PVCs per hour) according to BDI category. Other variables included in the analyses were age, gender, non-white race, diabetes, left ejection fraction (LVEF), Global Registry of Acute Coronary Events (GRACE) risk score,26 beta blocker prescription, antidepressant prescription, heart rate, and QTcF. Linear regression was performed with natural log transformation of (PVCs per hour +1) and BDI category as a continuous variable (0 for BDI score 0 to 4, 1 for BDI 5 to 9, or 2 for BDI ≥10). All analyses were performed using SPSS, version 18 (SPSS Inc, Chicago, Illinois).
Results
Among the first 500 patients enrolled in PULSE, 443 patients presented with non ST elevation ACS. Telemetry tracings were retrieved in 237 (53%) patients; the most common reason for lack of tracings was that summary telemetry reports became part of the medical record more than 5 months into the study, after 109 patients had already been enrolled in PULSE. Patients with and without retrieved tracings did not differ on the basis of age (p=0.10), sex (p=0.84), or GRACE risk score (p=0.35). However, patients with retrieved tracings were less likely to have LVEF <0.40 (7.6% versus 14.1%, p=0.03). Among those with retrieved tracings, 13 were removed from the analysis for misspecification of PVCs, 8 for atrial fibrillation as the dominant rhythm, 8 due to predominant ventricular pacing, and 8 due to fewer than 12 hours of recording, resulting in a sample size of 203.
In 200 ACS patients, percutaneous coronary intervention was performed in 152 (76%), and tracings were collected a median of 5 hours after the intervention (interquartile range 3–12 hours). The average length of time of telemetry collection was 21.3 ±3.0 hours per patient. 54 (27%) reported relatively minimal/no depressive symptoms, 68 (33%) had mild symptoms, and 81 (40%) had at least moderate symptoms (Table 1). Women and non-white patients were more likely to report depressive symptoms. The percent of patients who presented with MI and who had reduced LVEF <0.40 did not differ significantly by BDI category. There was a U shaped relationship between beat blocker use and reported depressive symptoms, and as expected, antidepressant use was more common in the groups with higher BDI score. Average heart rate was similar across BDI category, and of note, QTcF was higher in patients with more than minimal/no depressive symptoms.
Table 1.
Demographic and clinical characteristics of ACS patients by depressive symptom status.
| BDI 0–4 (n=53) | BDI 5–9 (n=68) | BDI ≥10 (n=79) | P value | |
|---|---|---|---|---|
| Age | 62.8 (11.8) | 65.4 (10.5) | 64.0 (10.5) | 0.50 |
| Female | 10 (18.9%) | 23 (33.8%) | 40 (50.6%) | <0.01 |
| Non-white | 15 (28.3%) | 28 (41.2%) | 37 (46.8%) | 0.04 |
| Diabetes | 23 (43.4%) | 19 (27.9%) | 34 (43.0%) | 0.84 |
| Non-STEMI | 18 (34.0%) | 24 (35.3%) | 20 (25.3%) | 0.25 |
| LVEF <0.40 | 3 (5.7%) | 2 (2.9%) | 4 (5.1%) | 0.94 |
| Antidepressant | 0 (0%) | 3 (4.4%) | 6 (7.6%) | <0.01 |
| Beta blocker | 39 (73.6%) | 44 (64.7%) | 69 (87.3%) | 0.04 |
| Heart rate (beats per minute) | 67.5 (9.5) | 68.6 (10.8) | 67.6 (9.5) | 0.99 |
| QTcF | 419.6 (22.0) | 432.8 (34.0) | 432.1 (25.3) | 0.019 |
| ≥10 PVCs/hr | 6 (11.1%) | 18 (26.5%) | 23 (29.1%) | 0.02 |
BDI= Beck Depression Inventory; STEMI = ST elevation myocardial infarction; LVEF=left ventricular ejection fraction; QTcF=QT corrected for heart rate using Fridericia's method.
Frequent PVCs were observed in only 11% of those with no/minimal symptoms, 27% of those with mild symptoms, and 29% of patients with moderate depressive symptoms (p for trend 0.02). In a multivariable logistic regression model that included LVEF, GRACE risk score, diabetes, beta blocker use, antidepressant use, heart rate, and QTcF, both mild symptoms (OR 3.13, 95% 1.02–9.57, p=0.046) and moderate to severe symptoms (OR 3.48, 95% CI 1.13–10.68, p=0.029) were independently associated with frequent PVCs. Log-transformed PVCs per hour were also associated with BDI category (1.16 ±1.25 for no/minimal symptoms, 1.61±1.47 for mild symptoms, 1.78±1.53 for moderate symptoms, p for trend 0.018, Figure 3). In a multivariable linear regression that used the log-transform as the dependent variable, BDI category remained associated with more frequent PVCs (Beta coefficient 0.30, 95% CI 0.04–0.56, p=0.022).
Figure 3.
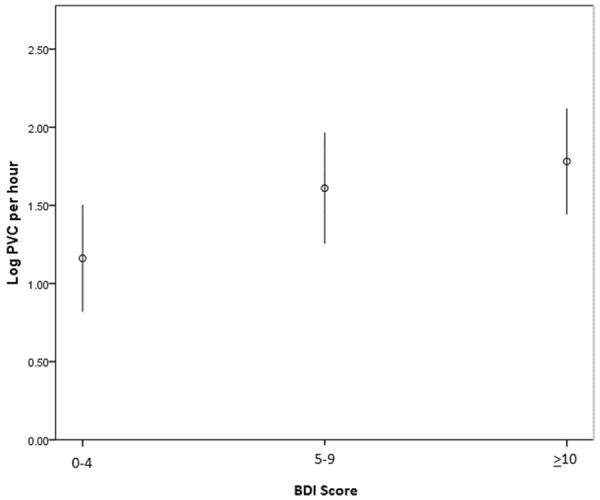
Mean natural log-transformed PVCs per hour in 203 ACS patients, by depressive symptom score (error bars indicate 95% confidence intervals).
We did not detect a difference in the relationship between PVCs and BDI category by gender. Although our numbers were relatively small, the multivariable odds ratios for frequent PVCs by BDI category were similar in men (odds ratio 3.03 for mild symptoms, 4.52 for moderate or more) and women (odds ratio 3.85 for mild symptoms, 3.74 for moderate or more). Interaction terms for BDI category and gender were not statistically significant in multivariable logistic regression models (p=0.66).
Discussion
In this analysis of patients with non-ST elevation ACS, we found that depressive symptoms were associated with more frequent PVCs captured by inpatient cardiac telemetry monitoring. Even mild depressive symptoms, represented by BDI score ≥5, were associated with frequent PVCs compared with no/minimal depressive symptoms. This relationship held despite adjustment for multiple potential confounders, including gender, left ventricular ejection fraction, cardiovascular risk score, and QT interval. Thus, depressive symptoms may be associated with PVCs through mechanisms other than through QT interval prolongation.
There has been conflicting evidence as to whether ambient ventricular arrhythmia is more common in ACS patients with depression compared with those without depression. In a study of 103 patients with coronary artery disease, Carney documented more frequent ventricular arrhythmia by 24-hour ECG monitoring in 21 depressed patients (23.8 percent) compared with 82 non-depressed patients (3.7 percent), although patients with depression were also much less likely to be treated with beta blocker.13 By contrast, an analysis of 353 post-MI patients from the Cardiac Arrhythmia Pilot Study indicated a lack of relationship between depressive symptoms and PVC rate by 24-hour ECG monitoring.14 However, an inclusion criterion for this study was at least 10 PVCs per hour by baseline ambulatory ECG monitoring, making this a highly selected sample. In the Very Anxious Group Under Scrutiny (VAGUS) study, among 940 patients referred for cardiac catheterization, during median follow-up of 3 years both depressive symptoms by BDI score and phobic anxiety were associated with ventricular arrhythmia episodes captured from medical record review.27 To our knowledge ours is the first study to show that even mild depressive symptoms are associated with more frequent ventricular arrhythmia, and adds to the current evidence base by reporting on an inpatient sample with ACS, and by adjusting for more potential confounders than prior analyses.
There are several possible interpretations of our findings. Depressive symptoms may be a marker for more severe cardiac disease that is not detected by other measures such as cardiac risk score or LVEF. However, it is also plausible that depression itself has a causative role in more frequent ventricular ectopy. Some,28–30 but not all,31, 32 studies in coronary heart disease patients have suggested that depression is associated with reduced parasympathetic modulation of heart rate as indicated by measures of heart rate variability. In a case control study involving 40 patients with recent acute MI, Carney and colleagues showed that QT interval variability by 24-hour Holter monitoring, a predictor of arrhythmia risk, was significantly greater in those with major depression.33 In addition, coronary sinus sampling has indicated that certain subtypes of depression are associated with elevated cardiac norepinephrine spillover.34 Given the recognized relationships between autonomic nervous system activity and risk of cardiac arrhythmia and sudden cardiac death,35–37 depressive symptoms may trigger more frequent ventricular arrhythmia through mechanisms involving autonomic dysfunction.
Our analysis has several important limitations. The cardiac telemetry monitoring used for PVC detection was collected primarily for clinical purposes and despite close review of each ECG signal that was included with the summary reports, there may be misspecification of PVC counts. We would not however, expect the error rate to differ by BDI score and confound our findings on this basis. We also do not yet have data on cardiac event rates for this analysis and cannot evaluate how PVC counts interacted with the relationship between depressive symptoms in predicting cardiac mortality. Other physiologic measures such as heart rate variability were also not available to us as we did not have full disclosure ECG data. Despite this, PVC rates are an easily obtainable clinical measure and may still serve as a useful indicator of cardiac risk in ACS patients with depressive symptoms.
In summary, we found in this ACS sample that frequent PVCs detected by inpatient cardiac telemetry were associated with mild depressive symptoms. Further studies are warranted to assess the prognostic importance of ventricular arrhythmia in depressed patients and to define the mechanism of this relationship.
Figure 2.
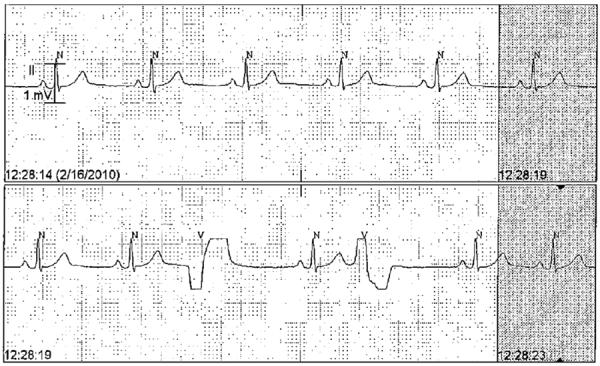
Sample of ECG tracing from cardiac telemetry report. `N' markers denote normal QRS complexes detected by the automatic algorithm, and `V' markers denote PVCs.
Table 2.
Multivariable logistic regression model of frequent PVCs.
| Odds ratio (95% CI) | P value | |
|---|---|---|
| BDI 5–9 | 3.13 (1.02–9.57) | 0.046 |
| BDI ≥10 | 3.48 (1.13–10.68) | 0.029 |
| Age | 1.00 (0.94–1.06) | 0.92 |
| Female | 0.85 (0.38–1.90) | 0.69 |
| Non-white | 1.33 (0.63–2.81) | 0.46 |
| Diabetes | 0.97 (0.44–2.14) | 0.94 |
| GRACE score | 1.03 (1.01–1.05) | <0.01 |
| LVEF (per 0.15 decrease) | 1.97 (1.10–3.52) | 0.023 |
| Beta blocker | 1.00 (0.42–2.40) | 1.00 |
| Antidepressant | 1.13 (0.24–5.31) | 0.88 |
| Heart rate | 1.00 (0.97–1.04) | 0.85 |
| QTcF | 1.00 (0.99–1.02) | 0.81 |
Numbers in parentheses represent standard deviation for continuous measures.
BDI=Beck Depression Inventory; GRACE=Global Registry of Acute Coronary Events; LVEF=left ventricular ejection fraction; QTcF=QT corrected for heart rate using Fridericia's method.
ACKNOWLEDGEMENTS
This work was supported by grants HL-088117, HC-25197, HL-076857, and HL-084034 from the National Institutes of Health, and by a Scientist Development Grant to Dr. Whang from the American Heart Association Founders Affiliate.
References
- 1.van Melle JP, de Jonge P, Spijkerman TA, Tijssen JG, Ormel J, van Veldhuisen DJ, van den Brink RH, van den Berg MP. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–22. doi: 10.1097/01.psy.0000146294.82810.9c. [DOI] [PubMed] [Google Scholar]
- 2.Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91(4):999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 4.Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, Simon G, Siscovick DS. Clinical depression and risk of out-of-hospital cardiac arrest. Archives of Internal Medicine. 2006;166(2):195–200. doi: 10.1001/archinte.166.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Brackett CD, Powell LH. Psychosocial and physiological predictors of sudden cardiac death after healing of acute myocardial infarction. American Journal of Cardiology. 1988;61(13):979–83. doi: 10.1016/0002-9149(88)90110-5. [DOI] [PubMed] [Google Scholar]
- 6.Follick MJ, Gorkin L, Capone RJ, Smith TW, Ahern DK, Stablein D, Niaura R, Visco J. Psychological distress as a predictor of ventricular arrhythmias in a post-myocardial infarction population. Am Heart J. 1988;116(1 Pt 1):32–6. doi: 10.1016/0002-8703(88)90246-3. [DOI] [PubMed] [Google Scholar]
- 7.Lown B. Sudden cardiac death: biobehavioral perspective. Circulation. 1987;76(1 Pt 2):I186–96. [PubMed] [Google Scholar]
- 8.Whang W, Albert CM, Sears SF, Jr, Lampert R, Conti JB, Wang PJ, Singh JP, Ruskin JN, Muller JE, Mittleman MA, Investigators TS. Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: results from the Triggers of Ventricular Arrhythmias (TOVA) study. J Am Coll Cardiol. 2005;45(7):1090–5. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 9.Makikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G, Huikuri HV. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26(8):762–9. doi: 10.1093/eurheartj/ehi188. [DOI] [PubMed] [Google Scholar]
- 10.Maggioni AP, Zuanetti G, Franzosi MG, Rovelli F, Santoro E, Staszewsky L, Tavazzi L, Tognoni G. Prevalence and prognostic significance of ventricular arrhythmias after acute myocardial infarction in the fibrinolytic era. GISSI-2 results. Circulation. 1993;87(2):312–22. doi: 10.1161/01.cir.87.2.312. [DOI] [PubMed] [Google Scholar]
- 11.Hohnloser SH, Klingenheben T, Zabel M, Schopperl M, Mauss O. Prevalence, characteristics and prognostic value during long-term follow-up of nonsustained ventricular tachycardia after myocardial infarction in the thrombolytic era. J Am Coll Cardiol. 1999;33(7):1895–902. doi: 10.1016/s0735-1097(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 12.Drogemuller A, Seidl K, Schiele R, Schneider S, Gitt A, Gottwik M, von Leitner ER, Poppe C, Rettig-Sturmer G, Senges J, Group Ms. Prognostic value of non-sustained ventricular tachycardias after acute myocardial infarction in the thrombolytic era: importance of combination with frequent ventricular premature beats. Z Kardiol. 2003;92(2):164–72. doi: 10.1007/s00392-003-0890-y. [DOI] [PubMed] [Google Scholar]
- 13.Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. Am J Med. 1993;95(1):23–8. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- 14.Follick MJ, Ahern DK, Gorkin L, Niaura RS, Herd JA, Ewart C, Schron EB, Kornfeld DS, Capone RJ. Relation of psychosocial and stress reactivity variables to ventricular arrhythmias in the Cardiac Arrhythmia Pilot Study (CAPS) Am J Cardiol. 1990;66(1):63–7. doi: 10.1016/0002-9149(90)90737-l. [DOI] [PubMed] [Google Scholar]
- 15.Whang W, Julien HM, Higginbotham L, Soto AV, Broodie N, Bigger JT, Garan H, Burg MM, Davidson KW. Women, but not men, have prolonged QT interval if depressed after an acute coronary syndrome. Europace. 2012;14(2):267–71. doi: 10.1093/europace/eur246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon CP, Battler A, Brindis RG, Cox JL, Ellis SG, Every NR, Flaherty JT, Harrington RA, Krumholz HM, Simoons ML, Van De Werf FJ, Weintraub WS, Mitchell KR, Morrisson SL, Anderson HV, Cannom DS, Chitwood WR, Cigarroa JE, Collins-Nakai RL, Gibbons RJ, Grover FL, Heidenreich PA, Khandheria BK, Knoebel SB, Krumholz HL, Malenka DJ, Mark DB, McKay CR, Passamani ER, Radford MJ, Riner RN, Schwartz JB, Shaw RE, Shemin RJ, Van Fossen DB, Verrier ED, Watkins MW, Phoubandith DR, Furnelli T. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee) J Am Coll Cardiol. 2001;38(7):2114–30. doi: 10.1016/s0735-1097(01)01702-8. [DOI] [PubMed] [Google Scholar]
- 17.Majidi M, Kosinski AS, Al-Khatib SM, Lemmert ME, Smolders L, van Weert A, Reiber JHC, Tzivoni D, Bär FWHM, Wellens HJJ, Gorgels APM, Krucoff MW. Reperfusion ventricular arrhythmia `bursts' in TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction: a more precise definition of reperfusion arrhythmias. Europace. 2008;10(8):988–997. doi: 10.1093/europace/eun123. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Lesperance F, Frasure-Smith N, Juneau M, Theroux P. Depression and 1-year prognosis in unstable angina. Arch Intern Med. 2000;160(9):1354–60. doi: 10.1001/archinte.160.9.1354. [DOI] [PubMed] [Google Scholar]
- 20.Whang W, Shimbo D, Kronish IM, Duvall WL, Julien H, Iyer P, Burg MM, Davidson KW. Depressive symptoms and all-cause mortality in unstable angina pectoris (from the Coronary Psychosocial Evaluation Studies [COPES]) Am J Cardiol. 2010;106(8):1104–7. doi: 10.1016/j.amjcard.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, Mankad S, Rogers WJ, Sopko G, Cornell CE, Sharaf B, Merz CN. Depression is associated with cardiac symptoms, mortality risk, and hospitalization among women with suspected coronary disease: the NHLBI-sponsored WISE study. Psychosom Med. 2006;68(2):217–23. doi: 10.1097/01.psy.0000195751.94998.e3. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Yeo CL, Aguirre A. Computers in Cardiology, 1999. 1999. The design and evaluation of a new multi-lead arrhythmia monitoring algorithm; pp. 675–678. [Google Scholar]
- 23.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–62. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 24.Lanza GA, Cianflone D, Rebuzzi AG, Angeloni G, Sestito A, Ciriello G, La Torre G, Crea F, Maseri A. Prognostic value of ventricular arrhythmias and heart rate variability in patients with unstable angina. Heart. 2006;92(8):1055–63. doi: 10.1136/hrt.2005.070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lepeschkin E, Surawicz B. The measurement of the Q-T interval of the electrocardiogram. Circulation. 1952;6(3):378–88. doi: 10.1161/01.cir.6.3.378. [DOI] [PubMed] [Google Scholar]
- 26.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 27.Watkins LL, Blumenthal JA, Davidson JR, Babyak MA, McCants CB, Jr, Sketch MH., Jr Phobic anxiety, depression, and risk of ventricular arrhythmias in patients with coronary heart disease. Psychosom Med. 2006;68(5):651–6. doi: 10.1097/01.psy.0000228342.53606.b3. [DOI] [PubMed] [Google Scholar]
- 28.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O'Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104(17):2024–8. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 29.Stein PK, Carney RM, Freedland KE, Skala JA, Jaffe AS, Kleiger RE, Rottman JN. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res. 2000;48(4–5):493–500. doi: 10.1016/s0022-3999(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 30.Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Archives of Internal Medicine. 2005;165(13):1486–91. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 31.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2005;62(6):661–6. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frasure-Smith N, Lesperance F, Irwin MR, Talajic M, Pollock BG. Brain Behav Immun. 2009. The relationships among heart rate variability, inflammatory markers and depression in coronary heart disease patients. [DOI] [PubMed] [Google Scholar]
- 33.Carney RM, Freedland KE, Stein PK, Watkins LL, Catellier D, Jaffe AS, Yeragani VK. Effects of depression on QT interval variability after myocardial infarction. Psychosom Med. 2003;65(2):177–80. doi: 10.1097/01.psy.0000033129.21715.4b. [DOI] [PubMed] [Google Scholar]
- 34.Barton DA, Dawood T, Lambert EA, Esler MD, Haikerwal D, Brenchley C, Socratous F, Kaye DM, Schlaich MP, Hickie I, Lambert GW. Sympathetic activity in major depressive disorder: identifying those at increased cardiac risk? J Hypertens. 2007;25(10):2117–24. doi: 10.1097/HJH.0b013e32829baae7. [DOI] [PubMed] [Google Scholar]
- 35.Zipes DP, Levy MN, Cobb LA, Julius S, Kaufman PG, Miller NE, Verrier RL. Sudden cardiac death. Neural-cardiac interactions. Circulation. 1987;76(1 Pt 2):I202–7. [PubMed] [Google Scholar]
- 36.Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85(1 Suppl):I77–91. [PubMed] [Google Scholar]
- 37.Chen LS, Zhou S, Fishbein MC, Chen PS. New perspectives on the role of autonomic nervous system in the genesis of arrhythmias. J Cardiovasc Electrophysiol. 2007;18(1):123–7. doi: 10.1111/j.1540-8167.2006.00590.x. [DOI] [PubMed] [Google Scholar]


