Abstract
BACKGROUND AND PURPOSE
Various CTP parameters have been used to identify ischemic penumbra. The purpose of this study was to determine the optimal CTP parameter and threshold to distinguish true “at-risk” penumbra from benign oligemia in acute stroke patients without reperfusion.
MATERIALS AND METHODS
Consecutive stroke patients were screened and 23 met the following criteria: 1) admission scanning within 9 hours of onset, 2) CTA confirmation of large vessel occlusion, 3) no late clinical or radiographic evidence of reperfusion, 4) no thrombolytic therapy, 5) DWI imaging within 3 hours of CTP, and 6) either CT or MR follow-up imaging. CTP was postprocessed with commercial software packages, using standard and delay-corrected deconvolution algorithms. Relative cerebral blood flow, volume, and mean transit time (rCBF, rCBV and rMTT) values were obtained by normalization to the uninvolved hemisphere. The admission DWI and final infarct were transposed onto the CTP maps and receiver operating characteristic curve analysis was performed to determine optimal thresholds for each perfusion parameter in defining penumbra destined to infarct.
RESULTS
Relative and absolute MTT identified penumbra destined to infarct more accurately than CBF or CBV*CBF (P < .01). Absolute and relative MTT thresholds for defining penumbra were 12s and 249% for the standard and 13.5s and 150% for the delay-corrected algorithms, respectively.
CONCLUSIONS
Appropriately thresholded absolute and relative MTT-CTP maps optimally distinguish “at-risk” penumbra from benign oligemia in acute stroke patients with large-vessel occlusion and no reperfusion. The precise threshold values may vary, however, depending on the postprocessing technique used for CTP map construction.
Introduction
CTP imaging has been used in the admission evaluation of acute ischemic stroke patients to define both the infarct core and the potentially salvageable ischemic penumbra (1,2). For strokes caused by large vessel occlusion, the admission core can expand without early, robust reperfusion (3–5). Salvage of “at-risk” penumbra is the goal of intravenous and intra-arterial acute stroke therapies in such cases, and hence a rapid and accurate assessment of ischemic penumbra could be desirable (4–7). Although infarct core assessment by using DWI has been validated as highly accurate,(8) selection of perfusion parameters and thresholds to identify penumbra remains a challenge, especially when MR imaging is unavailable and the perfusion data have been obtained with advanced CT imaging (6).
Indeed, in much of the literature, true “at-risk” penumbra has been overestimated by including hypoperfused regions of benign oligemia tissue with delay in contrast arrival time but without clinically relevant ischemia (9–11). Studies using various perfusion parameters to estimate penumbra, including CBF and MTT, have reached different conclusions depending, in part, on variables such as scan acquisition time, patient cohort, and type of postprocessing software. For example, although one important CTP study of stroke patients with and without recanalization identified rMTT as the optimal parameter for defining “at-risk” penumbra, those results—and corresponding thresholds—might not be a priori generalizable to patients with either “current generation” longer CTP acquisition times or to CTP maps processed by using newer “delay-corrected” software algorithms (1,2,12–14). Current delay-corrected postprocessing algorithms might be especially critical when major intra- or extracranial circulatory derangements are present (12–16). It has recently been reported that commercial software packages using delay-sensitive deconvolution algorithms can overestimate penumbra—and, hence, final infarct volume—whereas penumbra estimated with delay-insensitive software may better correlate with final infarct volume (12,14).
The purpose of our study, therefore, was to systematically evaluate multiple CTP parameter maps (CBF, CBV, MTT, and the product of CBF*CBV), processed by using both standard and delay-corrected deconvolution algorithms, to determine which parameter—and threshold—optimally identifies critically ischemic “at-risk” penumbral tissue destined to infarct in acute stroke patients with large artery occlusion without reperfusion.
Materials and Methods
Patient Selection
We reviewed the records of all consecutive patients admitted with the diagnosis of acute ischemic stroke from December 2006 to April 2008. Patients who met the following criteria were included in the analysis: 1) admission scanning within 9 hours of onset, 2) CTA confirmation of large vessel occlusion, 3) no late clinical or radiographic evidence of reperfusion, 4) no thrombolytic therapy, 5) DWI imaging within 3 hours of CTP, and 6) either CT or MR follow-up imaging performed beyond 48 hours, but not between 3–7 days to avoid fogging effects. Approval for the study, with waiver of patient consent, was granted by the institutional review board, and the study was conducted in compliance with HIPAA.
Imaging Acquisition
CTP was performed on a multidetector helical scanner (64 section LightSpeed; GE Healthcare, Milwaukee, Wisconsin) as a 66-second biphasic cine series, beginning 5 seconds after power injection of 40 mL of contrast at 7 mL/s (Isovue Multipack–370; Bracco Diagnostics, Princeton, New Jersey). Image acquisition was every half second for the first 40 seconds, which was followed by a 2-second pause and 8 more acquisitions every 3 seconds. Imaging parameters were 80 kVp, 200 mAs, 1-second rotation time. Coverage consisted of 2 slabs positioned parallel and superior to the orbital roof. Each slab consisted of 8 sections of 5-mm thickness.
DWI was obtained on a 1.5T Signa scanner (GE Healthcare) by using single-shot, spin-echo echo-planar imaging. High-b-value images (b = 1000 seconds/mm2) were acquired in 6 different gradient directions, in addition to a single low-b-value (b = 0 seconds/mm2) image. Other parameters were repetition time of 5000 ms, time to echo of 90–100 ms, field of view of 22 × 22 cm, image matrix of 128 × 128, section thickness of 5 mm with a 1 mm gap, and 5 signal intensity averages.
Image Analysis
CTP maps were postprocessed by using delay-corrected software (research version, CTP5 “DC”; GE Healthcare) and standard deconvolution software packages (CTP3 “Std,” GE Healthcare). Admission DWI images and follow-up images (CT or MR images) were coregistered to admission CT perfusion data by using a fully automated rigid method (CTI Molecular Imaging-Reveal-MVS 6.2; Mirada Solution, Oxford, United Kingdom). The images were manually adjusted in cases of unsatisfactory coregistration. After coregistration, on the section with the largest tissue evolved to infarction, the visually detected infarct core was semiautomated outlined based on admission DWI lesion. The “final infarct lesion,” based on follow-up noncontrast CT or MR imaging, was also semiautomatically outlined. Temporally averaged cine CTP images were served for segmentation of gray matter, white matter, and basal ganglia, and contralateral normal side. The normal contralateral hemisphere was used to calculate relative (normalized) cerebral blood flow, volume, and mean transit time (rCBF, rCBV, and rMTT, respectively). All ROIs were transposed onto the CBF, CBV, and MTT maps created by each software package, and on the product of the CBF*CBV map, which was calculated by multiplying CBF by CBV maps with the commercial Analyze program (Analyze 8.1; Analyze-Direct, Mayo Clinic, Rochester, Minnesota). The voxel values were also recorded by using a commercial analysis program. All analyses were performed for both absolute and relative (ie, normalized) voxel values. Normalized perfusion parameter values were calculated by dividing each voxel value by the mean of the contralateral reference region of interest for the whole lesion and also separately for GM, WM, and BG, by dividing each voxel value in GM, WM, and BG by the mean of the corresponding contralateral normal GM, WM, and BG values.
Statistical Analysis
A paired t test was used to calculate the difference between the infarct core and final infarct size for each patient. ROC curve analysis was used to calculate the accuracy, optimal thresholds, sensitivities, and specificities for each perfusion parameter (CBF, rCBF, CBV, rCBV, MTT, rMTT) and the product of CBF*CBV and rCBF*rCBV for each of the 2 platforms.
For each CTP parameter map, both relative and absolute, ROC curves depicting the sensitivity/specificity for distinguishing final infarct voxels from noninfarct voxels were generated from the pooled voxel values for all patients. Thresholds were then calculated as the optimum ROC operating point, with equally attributed weights to specificity and sensitivity; overall accuracy was estimated as the AUC. All P values are reported to the .01 level of significance. STATA version 10 (Stata Corp, College Station, Texas) was used for all analyses.
Results
A review of 98 records identified 23 patients eligible for analysis; 15 (65%) were women and the mean age was 79 years (range 59–92 years, SEM 2). Other demographics included (median [interquartile range]) admission NIHSS score 13 (6–18), symptom onset to CTP time 4.2 hours (2.7–5.5), and CTP to DWI interval 32 minutes (25–42). After a delay ranging from 2–200 days, 2 days median (2–33 days interquartile range), 19 patients underwent a follow-up noncontrast CT examination and 4 patients underwent a follow-up MR imaging examination. Admission CTA demonstrated arterial occlusion as follows: ICA terminus (3 patients), M1 (8 patients), M2 (10 patients), A1 and M1 (2 patients). All patients had larger final infarct volumes compared with the admission DWI infarct core (P < .0001), as shown in Fig 1. More than 1 million voxels were analyzed.
Figure 1.
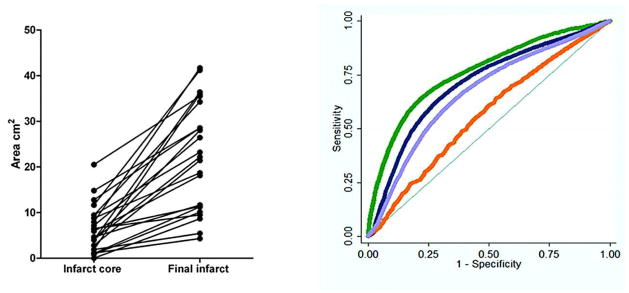
Expansion of core infarct size (area of selected axial section) between the admission DWI scan and the coregistered follow-up CT or MR imaging was an inclusion criterion, and was present in all patients (left). Sample ROC curves (right) showing the sensitivity/specificity of different CTP parameter thresholds used to define “at-risk” penumbra destined to infarct, comparing maps processed by using standard software. Green curves represent rMTT; blue curves, rCBF; orange, rCBV; and purple, the rCBF*rCBV voxel product value maps.
Fig 1 shows sample ROC curves for the CTP parameters postprocessed with the Std software. ROC analysis found significantly higher AUC for absolute and relative MTT than for the other CTP parameters for identification of brain tissue destined to infarct (all Ps <0.01) when using both the Std and DC software.
The corresponding AUCs for CTP parameters obtained with the Std software were MTT 0.76, rMTT 0.78, CBF 0.73, rCBF 0.74, CBV 0.56, rCBV 0.59, CBF*CBV 0.67, and rCBF*rCBV 0.70. AUCs for the DC software were 0.72, 0.71, 0.68, 0.68, 0.52, 0.52, 0.63, and 0.63, respectively. The detailed AUCs, thresholds, sensitivity, and specificity values for MTT are provided in the Table. The next accurate parameter was CBF.
Table.
Optimal absolute and rMTT thresholds for identification of penumbra destined to infarct
| aMTT | AUC | aT (s) | Sens | Spec | rMTT | AUC | rT (%) | Sens | Spec | |
|---|---|---|---|---|---|---|---|---|---|---|
| Std software | All | 0.76 | 12.0 | 0.650 | 0.761 | All | 0.78 | 249 | 0.646 | 0.796 |
| GM | 0.76 | 12.0 | 0.662 | 0.750 | GM | 0.78 | 250 | 0.657 | 0.785 | |
| WM | 0.77 | 13.1 | 0.648 | 0.778 | WM | 0.78 | 250 | 0.676 | 0.778 | |
| BG | 0.61 | 13.3 | 0.289 | 0.938 | BG | 0.63 | 198 | 0.326 | 0.922 | |
|
| ||||||||||
| DC software | All | 0.72 | 13.5 | 0.639 | 0.704 | All | 0.71 | 150 | 0.649 | 0.692 |
| GM | 0.73 | 12.0 | 0.732 | 0.636 | GM | 0.73 | 142 | 0.713 | 0.663 | |
| WM | 0.71 | 14.4 | 0.615 | 0.718 | WM | 0.70 | 167 | 0.599 | 0.725 | |
| BG | 0.63 | 13.8 | 0.377 | 0.796 | BG | 0.63 | 114 | 0.542 | 0.641 | |
Note: All whole-brain optimal thresholds. GM indicates gray matter; BG, basal ganglia region-specific thresholds; aT, absolute thresholds (sec); rT, relative thresholds; sens, sensitivity; spec, specificity; std, standard algorithm; DC, delay-corrected algorithm; aMTT, absolute MTT.
The optimal MTT threshold (operating point) predicting penumbra destined to infarct was 12 seconds (sensitivity = 65%; specificity = 76%), or 249% rMTT of the contralateral normal side (sensitivity = 65%; specificity = 80%) with Std software, whereas with DC software, the optimal MTT threshold was 13.5 seconds (sensitivity = 64%; specificity = 70%), or 150% rMTT (sensitivity = 65%; specificity = 70%). Fig 2 illustrates the study technique on a patient as an example of the optimized MTT thresholds reported here.
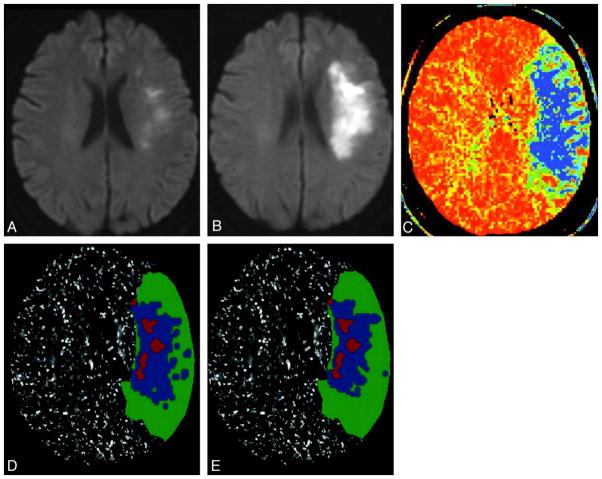
Figure 2.
Example of thresholded MTT map prediction of penumbra destined to infarct in a 70-year-old woman presenting with left hemispheric stroke symptoms. Ictus-to-CTP imaging time was 5 hours 33 minutes, admission NIHSS score was 6, and follow-up MR imaging was performed 44 hours after admission CTP scanning; NIHSS score was 12. Infarct core is segmented on the admission DWI scan (A, and red overlays on D, E), and final infarct volume is segmented on follow-up DWI scan (B). CT-MTT map shows blue/green regions with increased mean transit time (C). D and E, respectively, show the optimally thresholded absolute-MTT (12 seconds threshold) and relative-MTT (249% threshold) maps, both postprocessed by using standard algorithm, which distinguish benign oligemia (green overlays) from true “at-risk” ischemic penumbra (blue overlays).
Discussion
We have shown that appropriately thresholded MTT maps, processed with both delay-sensitive and delay-insensitive deconvolution algorithms, can optimally identify “at-risk” penumbra destined to infarct, but that the specific thresholds vary with postprocessing technique.
Our results expand on and overcome several potential limitations of prior studies, including distinguishing clinically relevant ischemia from benign oligemia, use of a longer “current generation” CTP acquisition time (older 40–50 second protocols could result in time-attenuation curve truncation in patients with large vessel occlusion or atrial fibrillation, causing overestimation of the CBV and MTT lesions), and comparison between standard and delay-corrected postprocessing software (1,2,12–15). In addition, although it has been reported in some studies that the CBF*CBV interaction parameter may provide more accurate prediction of penumbra destined to infarct than CBF alone in patients without recanalization, the comparison between CBF*CBV and MTT has not previously been emphasized (17,18). Moreover, the CBF thresholds used in those studies were predefined rather than optimized (17,18). With regard to the variability in thresholds between different postprocessing algorithms, it is noteworthy that different digital signal intensity processing approaches—even when the same deconvolution algorithm is used—can result in marked variations in CTP maps. Specifically, fixed-point processing has the advantage of speed, whereas floating point processing has higher dynamic range, better precision, and higher signal intensity to noise (19).
Another important feature of our study was the heterogeneity of our stroke cohort, which included patients with both ACA and M2 segmental MCA occlusions, in addition to the ICA terminus and MCA stem occlusions of earlier studies (1,20). MCA occlusive strokes can be classified into different clinical and hemodynamic subtypes depending on the level of the occlusion, which may improve the generalizability of our results (21). For example, stroke patients with proximal MCA occlusion are more likely to present with core/penumbra mismatch and have final infarct growth into penumbra, compared with those with distal MCA occlusion, in whom the admission core lesion is a stronger predictor of final infarct size.
One prior study of CTP ischemic thresholds, by using a shorter, 60-second acquisition protocol, found that tissue with <50% reduction in CBF is likely to survive, whereas a >66% reduction is likely to infarct (22). Although another important study in 2006 found that rMTT provided the most accurate estimate of penumbra, those results—and thresholds—might not be generalizable to different cohorts with different distributions of vascular lesions and hemodynamics (ie, misery perfusion), or to newer, longer CTP acquisition protocols with different postprocessing platforms (1,23). Indeed, a recent (2011) study not only concluded that CBF maps acquired by using newer, longer scan protocols provide more accurate estimates of infarct core than do CBV maps but also that the marked variability across different postprocessing softwares limits the generalizability of parameter map thresholds between platforms (23) Our current study extends and complements these results for “core” by distinguishing noncritical hypoperfusion (“benign oligemia”) from true critical ischemia (“at-risk” penumbra).
Potential limitations of our study include the relatively small number of patients identified with large vessel occlusion but without clinical evidence or imaging findings of reperfusion. Additionally “2-slab”—as opposed to “whole brain”—CTP coverage may have decreased our power to detect more subtle differences between the parameter maps and related threshold values, though because our analysis included only coregistered pixels that were present in the admission and follow-up images, this consideration does not negate our results. Moreover, a limitation inherent to all stroke imaging studies is that they represent a “snapshot” in time; hence, our study lacked sufficient patients to stratify by time postictus. In addition, the relatively small number of patients in our final study group (of 98 potential subjects, we were only able to identify 23 who met all inclusion criteria) precluded additional stratification according to time-to-follow-up or follow-up technique (CT versus MR imaging). Finally, although software availability precluded us from directly comparing MTT to other transit-time measures such as Tmax, a number of recent studies, many of which included xenon CT and PET reference standards, have suggested that MTT and Tmax maps—when appropriately thresholded—are largely equivalent in their assessment of penumbra, and, indeed, in some instances MTT may exceed Tmax in accuracy (10,11,24).
Conclusions
Our study has shown that appropriately thresholded CTP-MTT maps can optimally identify “at-risk” penumbral tissue destined to infarct in acute stroke patients with large vessel occlusion who do not reperfuse, but that the specific thresholds vary with postprocessing technique. Our results expand on those of previous studies, some of which did not distinguish true ischemic hypoperfusion from benign oligemia, and many of which used either “first generation” short CTP acquisition times (45 seconds or less, which could result in truncation of time-attenuation curves) or delay-sensitive postprocessing algorithms. These results may be of value in future clinical trials of stroke treatments, by more accurately determining the volume and location of critically ischemic but potentially viable brain parenchyma as a target for therapy.
Acknowledgments
This work was supported by the Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network grant funded by the NIH (1 P50 NS051343-01A2 and R01NS050041), Agency for Healthcare Research and Quality grant (AHRQ) R01 HS11392-01A1, and Massachusetts General Hospital Clinical Research Center (No. 1 UL1 RR025758-01), Harvard Clinical and Translational Science Center, from the National Center for Research Resources.
ABBREVIATIONS
- a
absolute measures
- ACA
anterior cerebral artery
- AUC
area under the curve
- BG
basal ganglia
- GM
gray matter
- r
relative (normalized) measures
- ROC
receiver operating characteristic
Footnotes
Abstract previously presented at Annual Meeting of the American Society of Neuroradiology, May 15–20, 2010; Boston, Massachusetts.
Disclosures
Shahmir Kamalian—RELATED: Grant: SPOTRIAS, Comments: My salary was from Specialized Programs of Translational Research in Acute Stroke (SPOTRIAS) Network grant funded by the National Institutes of Health (NIH/National Institute of Neurological Disorders and Stroke; one P50 NS051343–01A2). Stuart Pomerantz—discloses research support from GE Healthcare not related to CT perfusion. Ramon González—RELATED: Grant: NIH,* Penumbra;* UNRELATED: Grants/Grants Pending: NIH,* Penumbra.* Michael Lev—RELATED: Grant: NIH,* GE Healthcare,* Comments: Research funding from GE Healthcare for relevant CT scanning hardware, software, and research assistant support; NIH SPOTRIAS Network grant (P50 NS051343); Agency for Healthcare Research and Quality grant (R01 HS11392); MGH Clinical Research Center (No. one UL1 RR025758–01) Harvard Clinical and Translational Science Center (National Center for Research Resources); UNRELATED: Consultancy: GE Healthcare, CoAxia, Millennium Pharmaceuticals; Grants/Grants Pending: DOD, NBIB; Other: Common stock in GE Healthcare < $10K; Honoraria for various academic Grand Rounds, National Medical/Imaging meetings, ≪ $10K.
References
- 1.Wintermark M, Flanders AE, Velthuis B, et al. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–85. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Albers GW, Alexandrov AV, et al. Acute stroke imaging research roadmap. Stroke. 2008;39:1621–28. doi: 10.1161/STROKEAHA.107.512319. [DOI] [PubMed] [Google Scholar]
- 3.Baird AE, Benfield A, Schlaug G, et al. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–89. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 4.Nagakane Y, Christensen S, Brekenfeld C, et al. EPITHET: positive result after reanalysis using baseline diffusion-weighted imaging/perfusion-weighted imaging co-registration. Stroke. 2011;42:59–64. doi: 10.1161/STROKEAHA.110.580464. [DOI] [PubMed] [Google Scholar]
- 5.Marks MP, Olivot JM, Kemp S, et al. Patients with acute stroke treated with intravenous tPA 3–6 hours after stroke onset: correlations between MR angiography findings and perfusion- and diffusion-weighted imaging in the DEFUSE study. Radiology. 2008;249:614–23. doi: 10.1148/radiol.2492071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obach V, Oleaga L, Urra X, et al. Multimodal CT-assisted thrombolysis in patients with acute stroke: a cohort study. Stroke. 2011;42:1129–31. doi: 10.1161/STROKEAHA.110.605766. [DOI] [PubMed] [Google Scholar]
- 7.Parsons MW, Christensen S, McElduff P, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010;30:1214–25. doi: 10.1038/jcbfm.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177–85. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kucinski T, Naumann D, Knab R, et al. Tissue at risk is overestimated in perfusion- weighted imaging: MRimaging in acute stroke patients without vessel recanalization. AJNR Am J Neuroradiol. 2005;26:815–19. [PMC free article] [PubMed] [Google Scholar]
- 10.Kane I, Carpenter T, Chappell F, et al. Comparison of 10 different magnetic resonance perfusion imaging processing methods in acute ischemic stroke: effect on lesion size, proportion of patients with diffusion/perfusion mismatch, clinical scores, and radiologic outcomes. Stroke. 2007;38:3158–64. doi: 10.1161/STROKEAHA.107.483842. [DOI] [PubMed] [Google Scholar]
- 11.Takasawa M, Jones PS, Guadagno JV, et al. How reliable is perfusion MR in acute stroke? Validation and determination of the penumbra threshold against quantitative. PET Stroke. 2008;39:870–77. doi: 10.1161/STROKEAHA.107.500090. [DOI] [PubMed] [Google Scholar]
- 12.Konstas AA, Lev MH. CT perfusion imaging of acute stroke: the need for arrival time, delay insensitive, and standardized postprocessing algorithms? Radiology. 2010;254:22–25. doi: 10.1148/radiol.09091610. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer PW, Mui K, Kamalian S, et al. Avoiding “pseudo-reversibility” of CT-CBV infarct core lesions in acute stroke patients after thrombolytic therapy: the need for algorithmically “delay-corrected” CT perfusion map postprocessing software. Stroke. 2009;40:2875–78. doi: 10.1161/STROKEAHA.109.547679. [DOI] [PubMed] [Google Scholar]
- 14.Kudo K, Sasaki M, Yamada K, et al. Differences in CT perfusion maps generated by different commercial software: quantitative analysis by using identical source data of acute stroke patients. Radiology. 2010;254:200–09. doi: 10.1148/radiol.254082000. [DOI] [PubMed] [Google Scholar]
- 15.Calamante F, Gadian DG, Connelly A. Delay and dispersion effects in dynamic susceptibility contrast MRI: simulations using singular value decomposition. Magn Reson Med. 2000;44:466–73. doi: 10.1002/1522-2594(200009)44:3<466::aid-mrm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Wu O, Ostergaard L, Weisskoff RM, et al. Tracer arrival timing-insensitive technique for estimating flow in MR perfusion-weighted imaging using singular value decomposition with a block-circulant deconvolution matrix. Magn Reson Med. 2003;50:164–74. doi: 10.1002/mrm.10522. [DOI] [PubMed] [Google Scholar]
- 17.Murphy BD, Fox AJ, Lee DH, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–77. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 18.Murphy BD, Fox AJ, Lee DH, et al. White matter thresholds for ischemic penumbra and infarct core in patients with acute stroke: CT perfusion study. Radiology. 2008;247:818–25. doi: 10.1148/radiol.2473070551. [DOI] [PubMed] [Google Scholar]
- 19.Smith SW. WSS, editor. The Scientist and Engineer’s Guide to Digital Signal Processing. Sacramento, California: California Technical Pub; 1997. Digital signal processors; pp. 503–34. [Google Scholar]
- 20.Wintermark M, Reichhart M, Thiran JP, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002;51:417–32. doi: 10.1002/ana.10136. [DOI] [PubMed] [Google Scholar]
- 21.Rordorf G, Koroshetz WJ, Copen WA, et al. Regional ischemia and ischemic injury in patients with acute middle cerebral artery stroke as defined by early diffusion-weighted and perfusion-weighted MRI. Stroke. 1998;29:939–43. doi: 10.1161/01.str.29.5.939. [DOI] [PubMed] [Google Scholar]
- 22.Cenic A, Nabavi DG, Craen RA, et al. Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol. 1999;20:63–73. [PubMed] [Google Scholar]
- 23.Kamalian S, Kamalian S, Maas MB, et al. CT cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke. 2011;42:1923–28. doi: 10.1161/STROKEAHA.110.610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen S, Mouridsen K, Wu O, et al. Comparison of 10 perfusion MRI parameters in 97 sub-6-hour stroke patients using voxel-based receiver operating characteristics analysis. Stroke. 2009;40:2055–61. doi: 10.1161/STROKEAHA.108.546069. [DOI] [PubMed] [Google Scholar]