Abstract
Adenosine receptors (AR; A1, A2A, A2B, and A3) contract and relax smooth muscle through different signaling mechanisms. Deciphering these complex responses remains difficult because relationships between AR subtypes and various end-effectors (e.g., enzymes and ion channels) remain to be identified. A1AR stimulation is associated with the production of 20-hydroxyeicosatetraenoic acid (20-HETE) and activation of protein kinase C (PKC). 20-HETE and PKC can inhibit large conductance Ca2+/voltage-sensitive K+ (BK) channels that regulate smooth muscle contraction. We tested the hypothesis that activation of A1AR inhibits BK channels via a PKC-dependent mechanism. Patch clamp recordings and Western blots were performed using aortae of wild type (WT) and A1AR knockout (A1KO) mice. There were no differences in whole-cell K+ current or α and β1 subunits expression between WT and A1KO. 20-HETE (100 nM) inhibited BK current similarly in WT and A1KO mice. NECA (5′-N-ethylcarboxamidoadenosine; 10 μM), a non-selective AR agonist, increased BK current in myocytes from both WT and A1KO mice, but the increase was greater in A1KO (52±15 vs. 17±3%; p<0.05). This suggests that A1AR signaling negatively regulates BK channel activity. Accordingly, CCPA (2-chloro-N(6)-cyclopentyladenosine; 100 nM), an A1AR-selective agonist, inhibited BK current in myocytes from WT but not A1KO mice (81±4 vs. 100±7% of control; p<0.05). Gö6976 (100 nM), a PKCα inhibitor, abolished the effect of CCPA to inhibit BK current (99±3% of control). These data lead us to conclude that, in aortic smooth muscle, A1AR inhibits BK channel activity and that this occurs via a mechanism involving PKCα.
Keywords: Large conductance Ca2+/voltage-sensitive K+ channels, 20-hydroxy-eicosatetraenoic acid, 2-chloro-N (6)-cyclopentyladenosine, 5′-N-ethylcarboxamidoadenosine, protein kinase C alpha
INTRODUCTION
Adenosine exerts its effects through four G-protein coupled receptors: the known adenosine receptor (AR) subtypes are A1, A2A, A2B and A3. These AR subtypes play important roles in vascular reactivity, as A1AR and A3AR contract smooth muscle, whereas A2AAR and A2BAR relax smooth muscle (1, 13, 18, 38, 47). It is well accepted that metabolites of arachidonic acid (AA) regulate vascular tone; however, only recently have these pathways been recognized to function downstream of A1AR and A2AAR (10, 14, 30, 36). Epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatetraenoic acid (20-HETE) are produced from arachidonate by epoxygenases and ω-hydroxylases, respectively. EETs are considered to be endothelium-derived hyperpolarizing factors that activate Ca2+-dependent K+ channels and Na+-K+-ATPase (40). 20-HETE in vascular smooth muscle functions as a second messenger to promote depolarization, Ca2+ influx, and contraction of vascular smooth muscle that acts, in part, through protein kinase C (PKC) (28, 49).
Ion channels are important determinants of vascular tone, as they control membrane potential and the intracellular Ca2+ concentration. Large conductance, Ca2+/voltage-sensitive K+ (BK) channels participate in this electromechanical coupling (6, 31). BK channels are activated by membrane depolarization and increases in intracellular Ca2+. 20-HETE has been shown to inhibit BK channels in canine basilar artery (34) and rat renal arterioles (52). BK channels can also be regulated by phosphorylation and are targets of PKC, which reduces open probability (51).
We have shown previously that activation of A1AR couples with the Cyp4a metabolite, 20-HETE and mediates contraction of the aortic smooth muscle through a pathway involving PKCα and/or p-ERK1/2. However, genetic ablation of the A1AR reduced the contractions in response to 20-HETE, in part, by reducing the expression of downstream signaling molecules (PKCα and p-ERK1/2) (22). To further understand the signaling transduction of A1AR and 20-HETE, we performed studies designed to test the hypothesis that activation of A1AR inhibits BK channels via a PKC-dependent mechanism.
MATERIALS AND METHODS
Animals
A1KO mice (originally obtained from Dr. Jurgen Schnermann, NIDDK, NIH) are on C57BL/6 background. A1KO mice were backcrossed 4 generations with C57BL/6 (WT); genotypes were confirmed by polymerase chain reaction. C57BL/6 (WT) mice (originally purchased from The Jackson Laboratory, Bar Harbor, ME) were bred in-house. Equal number of males and females of 14–18 weeks of age were used in our studies, as no gender differences were observed. The Institutional Animal Care and Use Committee of West Virginia University provided regulatory oversight and protocols followed guidelines set forth in The Guide for the Care and Use of Laboratory Animals (National Research Council, 2011). Mice had free access to food and water and were housed on a 12:12 hr light-dark cycle. Mice were killed with an overdose of sodium pentobarbital (150 mg/kg ip) and aortae were quickly harvested into ice-cold physiological saline solution. Adipose and connective tissue were removed under the magnification of a dissecting microscope.
Immunoblot analysis
Aortae from WT and A1KO mice were homogenized with 150 μL radio-immuno precipitation assay buffer containing (mM) 20 Tris-HCl, 150 NaCl, 1 Na2EDTA, 1 EGTA, 2.5 sodium pyrophosphate,1 beta-glycerophosphate, and 1 Na3VO4; plus 1% NP-40, 1% sodium deoxycholate, and 1 μg/ml leupeptin. Samples were vortexed and then centrifuged for 10 min at 13,800 g at 4°C. Protein was measured using the Bradford dye procedure with bovine serum albumin as a standard (Bio-Rad Laboratories; Hercules, CA). The protein extract was divided into aliquots and stored at −80°C. Samples (25 μg of total protein) were loaded on slab gels (10% acrylamide; 1 mm thick), separated by SDS-PAGE, and transferred to nitrocellulose membranes (Hybond-ECL). Protein transfer was confirmed by visualization of prestained molecular weight markers (Bio-Rad). Membranes were blocked with 5% nonfat dry milk and incubated with primary antibody. A 1:5,000 primary antibody dilution used for BK α and β1 subunits (Alomone labortatories, Israel), while 1:10,000 dilutions were used for secondary antibody and β-actin.
Electrophysiology
WT and A1KO mice aortae were digested in a physiological saline solution containing (mg/ml) 2 collagenase type-II, 1 soybean trypsin inhibitor, 1 bovine serum albumin, and 1 elastase for 30 minutes at 37°C. Single cells were liberated by passing the tissue through the tip of a fire-polished Pasteur pipette. The suspension was passed through a 100 μm nylon mesh and spun for 10 minutes at 10,000g. The pellet was resuspended in low Ca2+ physiological saline solution and cells were stored on ice for use within 8 hr. Cells were allowed to attach to glass coverslip, which was then transferred to the recording chamber. Solutions flowed into the recording chamber by gravity at a rate of 2–3 ml/min and the chamber had a volume of 0.2–0.3 ml. BK channel currents were recorded at room temperature from whole-cell patches as described previously (3). Bath solution contained (mM) 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES free acid and 5 Tris base; pH 7.4. Pipette solution contained (mM) 140 KCl, 1 MgCl2, 1 EGTA and 0.281 CaCl2 (pCa 7), 10 HEPES, 1 Mg-ATP, 0.1 Na-GTP, and 5 Tris; pH 7.1. pClamp software and an Axopatch 200B amplifier were used (Molecular Devices; Sunnyvale, CA). Currents were low pass filtered at 1 kHz and digitized at 5 kHz.
Statistics
Data are expressed as mean ± SEM from n number of mice, because the treatment level (i.e., genotype) is on a per mouse basis. For patch clamp experiments, that means results from all cells (≥3) from a single mouse aorta were averaged to represent n = 1. Current-voltage relationships were analyzed by two-way repeated measures analysis of variance (ANOVA). This was followed with Bonferroni post hoc test to determine where differences existed. When only two values were compared (e.g., BK subunit expression) an unpaired t-test was used. P < 0.05 was considered significant in all tests.
RESULTS
Total BK current and BK subunit expression in WT and A1KO mice aortic myocytes
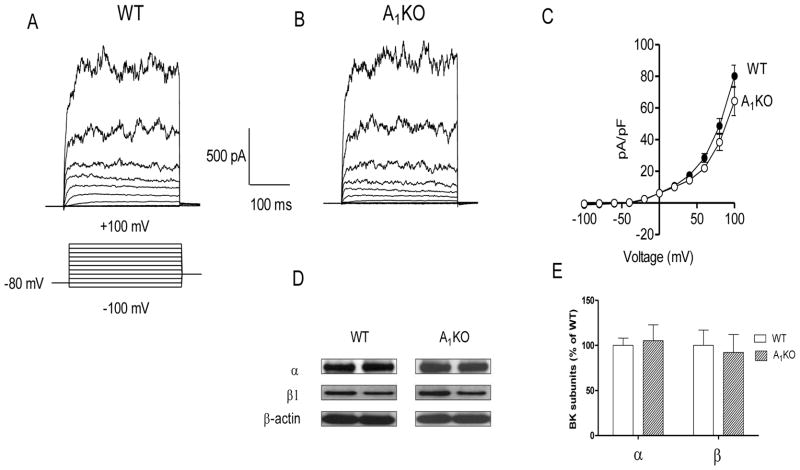
We performed whole-cell patch recordings on aortic smooth muscle cells from WT (Fig. 1A) and A1KO (Fig. 1B) mice; we observed no difference in BK current. That is, whole-cell K+ current in smooth muscle cells was indistinguishable between WT and A1KO mice. Currents were normalized to cell capacitance (i.e., current density). The group data are shown in Fig. 1C. BK α and β1 proteins were expressed in aortae from both WT and A1KO mice. BK α and β1 subunit proteins migrated at 100 and 25 kDa, respectively. There were no differences observed in the two protein levels between genotypes (Figs. 1D and 1E). Thus, the molecular (protein) and functional (current) expression of BK channels was similar in smooth muscle cells from WT and A1KO mice.
Fig. 1. Whole-cell K+ current and BK channel subunit expression is similar in smooth muscle from WT and A1KO mice.
Representative traces of whole-cell K+ current in aortic smooth muscle cells from WT (A) and A1KO mice (B). The voltage template used to elicit the currents in this and subsequent figures is shown below the trace in A; cells were held at −80 mV and stepped from −100 to +100 mV in 20 mV increments. (C) Group data representing whole-cell K+ current in aortic smooth muscle cells from WT (n = 13) and A1KO (n = 20) mice. (D) Representative Western blots from mouse aortae for BK channel subunit expression relative to β-actin (α = 100 kDa; β1 = 25 kDa; β-actin = 42 kDa). (E) Group data for BK α and β1 subunit expression in the aortae of WT (n = 6) and A1KO (n = 6) mice. There were no differences between WT and A1KO mice in whole-cell K+ current or BK protein expression.
Effect of 20-HETE on BK current in WT and A1KO mice aortic myocytes
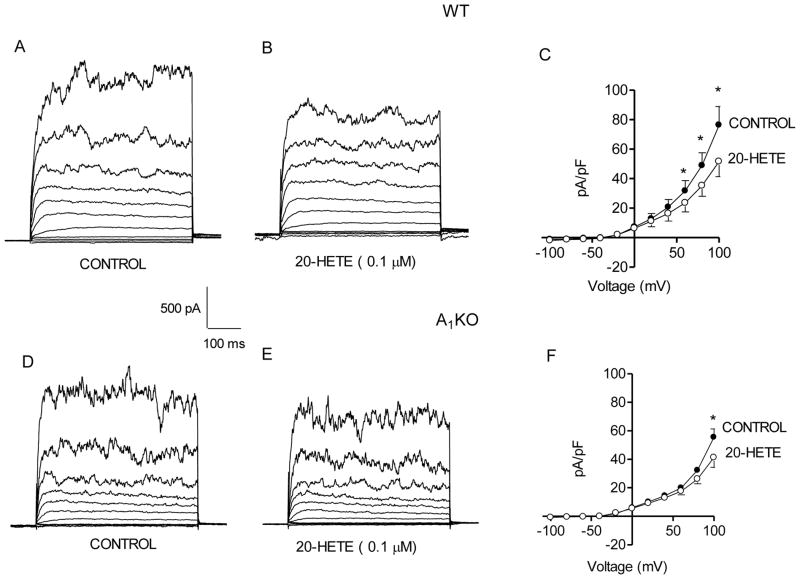
To assess the reported inhibitory effect of 20-HETE on BK channels (23, 52), whole-cell recordings were performed on WT and A1KO myocytes. We observed a decrease in the BK current in both WT (Fig. 2A and B) and A1KO (Fig. 2D and E) smooth muscle cells. Mean current density at +100 mV in WT under control conditions was 76.4 ± 12.5 pA/pF (n=4); this was decreased to 51.6 ± 10.3 pA/pF by 20-HETE (Fig. 2C). In smooth muscle cells from WT mice, 20-HETE decreased current density 33 ± 7%. Similarly in smooth muscle cells from A1KO mice, mean current density was 55.6 ± 10.3 pA/pF (n=4) and this was decreased to 41.4 ± 7 pA/pF by 20-HETE (Fig. 2F). Thus, in myocytes from A1KO mice, 20-HETE decreased current density 24 ± 11%.
Fig. 2. Effect of 20-HETE on BK current in WT and A1KO aortic myocytes.
Representative current traces are shown under control conditions (A) and with 0.1 μM 20-HETE (B) in a smooth muscle cell from a WT mouse. The voltage template was the same as Fig. 1. (C) Group data (n = 5) show the decrease in the BK current by 20-HETE in smooth muscle cells from WT mice. Representative traces are shown under control conditions (D) and with 0.1 μM 20-HETE (E) for a smooth muscle cell from an A1KO mouse. (F) Group data (n = 5) show the decrease in BK current by 0.1 μM 20-HETE in smooth muscle cells from A1KO mice. *p<0.05 compared to the respective control.
Effect of NECA on BK current in WT and A1KO mice aortic myocytes
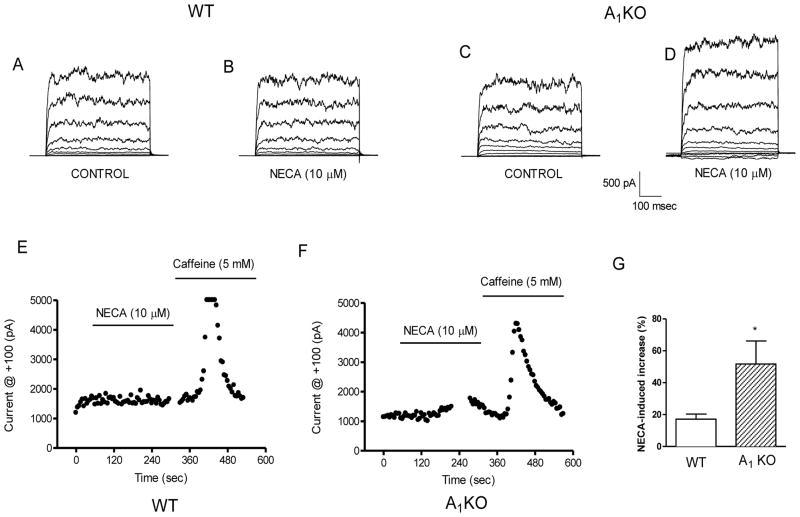
Whole-cell patch recordings were made in WT and A1KO aortic myocytes to determine the effect of NECA on BK current. NECA is a nonselective adenosine receptor agonist and can activate multiple AR subtypes simultaneously. Whole-cell recordings showed prominent BK current in smooth muscle cells from WT and A1KO mice. Caffeine (5 mM) was used as a positive control to release Ca2+ and increase BK current in both WT and A1KO aortic smooth muscle cells (Figs. 3E and 3F). There was very little change in the BK current in the WT aortic myocytes when stimulated with 10 μM NECA (Fig. 3E). In contrast, the BK current in A1KO aortic myocytes was significantly increased by 10 μM NECA (Fig. 3F). The time-dependent increase in BK current with 10 μM NECA in A1KO smooth muscle cells was 52 ± 15% (n=7); this was significantly higher than the response to NECA in smooth muscle cells from WT mice (17 ± 3%; n=9; Fig. 3G). The disparate responses to NECA in smooth muscle cells from WT and A1KO mice suggest that multiple AR subtypes are simultaneously regulating BK channels. Thus, the next experiment was to determine the effect of an A1AR-specific agonist on BK channels in smooth muscle cells from WT and A1KO mice.
Fig. 3. Effect of NECA on BK current in WT and A1KO aortic myocytes.
Representative currents under control conditions (A) and with 10 μM NECA (B) in WT. The voltage template was the same as Fig. 1. Representative currents under control conditions (C) and with 10 μM NECA (D) in smooth muscle cells from A1KO mice. Data showing currents vs. time for 10 μM NECA and 5 mM caffeine in smooth muscle cells from WT (E) and in A1KO (F) mice (blank areas in the time course represent where the protocol was stopped to perform voltage steps) (G) Group data show that NECA increases the BK current more in smooth muscle cells from A1KO mice compared to WT mice. *p < 0.05 for WT vs. A1KO; n=7-9.
Effect of CCPA on BK current in WT and A1KO mice aortic myocytes
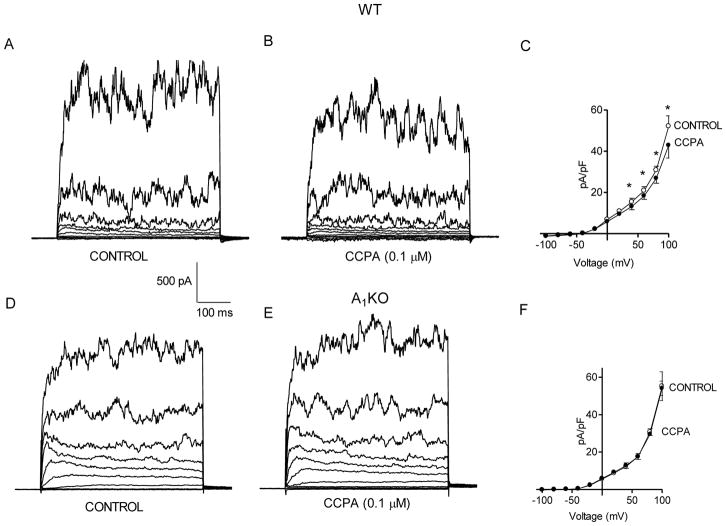
CCPA (100 nM), an A1-selective agonist, decreased BK current in aortic smooth muscle cells from WT mice (Figs. 4A and 4B). The mean current density at +100 mV in WT was 52.1 ± 5.0 pA/pF (n=4) and decreased with the application of CCPA to 42.8 ± 6.2 pA/pF (Fig. 4C). That is, CCPA decreased current density 19 ± 4% in smooth cells from WT mice. In contrast, CCPA had no effect on BK current in smooth muscle cells from A1KO mice (Figs. 4D and 4E). The mean current density at +100 mV in smooth muscle cells from A1KO mice was 55.2 ± 7.7 and 54.1 ± 3.9 pA/pF (n=4) in the absence or presence of 100 nM CCPA, respectively (Fig. 4F). That is, current density in the presence of CCPA was 98 ± 7% of control in smooth muscle cells from A1KO mice.
Fig. 4. Effect of CCPA on BK current in WT and A1KO aortic myocytes.
Representative traces under control conditions (A) and with 0.1 μM CCPA (B) in a smooth muscle cell from a WT mouse. The voltage template was the same as Fig. 1. (C) Group data representing the decrease in the BK current by CCPA in the WT mice (n = 4). Representative traces show current under control conditions (D) and with 0.1 μM CCPA (E) in a smooth muscle cell from an A1KO mouse. (F) Group data illustrate that there is no effect of CCPA on BK current in smooth muscle cells from A1KO mice. *p<0.05 compared to untreated WT (n=4).
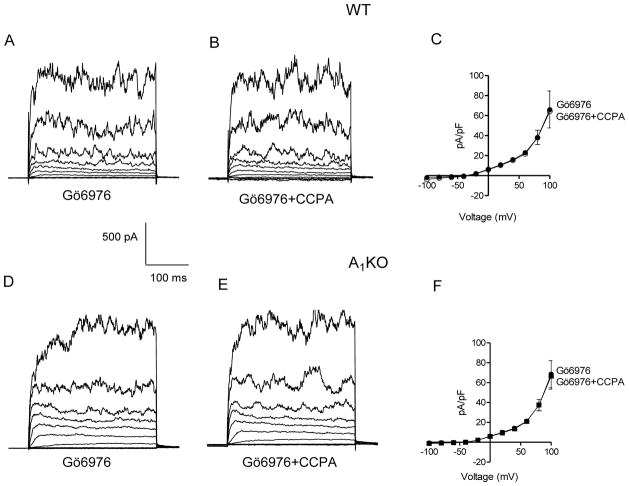
Effect of PKCα inhibition on BK current in WT and A1KO mice aortic myocytes
As shown previously (22, 36), PKCα is downstream of A1AR activation, 20-HETE production, and mediates contraction of smooth muscle. We determined if inhibition of PKCα affected regulation of BK current by A1AR activation in smooth muscle cells from WT and A1KO mice. When PKCα was inhibited with Gö6976 (100 nM) in WT smooth muscle cells, subsequent addition of CCPA (100 nM) was no longer able to inhibit current (Figs. 5A and 5B; compare to Fig. 4A–C). In smooth muscle cells from WT mice, mean current density at +100 mV for Gö6976 was 65.9 ± 18.6 pA/pF vs. 64.2 ± 16.6 pA/pF for CCPA + Gö6976 (Fig. 5C). That is, current density in the presence of CCPA was 99 ± 3% of control in smooth muscle cells from WT mice treated with Gö6976. There was no effect of CCPA on BK current in A1KO smooth muscle cells whether Gö6976 was present or not (Fig 5D–F; note that this is a result similar to that shown in Fig. 4D–F). The mean current density at +100 mV in cells from A1KO mice for Gö6976 was 68.5 ± 13.6 pA/pF vs. 66.6 ± 13.2 pA/pF for CCPA + Gö6976 (Fig. 5F). That is, current density in the presence of CCPA was 97 ± 1% of control in Gö6976-treated smooth muscle cells from A1KO mice.
Fig. 5. Effect of PKCα inhibitor, Gö6976 on BK current in WT and A1KO aortic myocytes.
Representative traces with 0.1 μM Gö6976 (A) and with 0.1 μM Gö6976 + 0.1 μM CCPA (B) in a smooth muscle cell from a WT mouse. The voltage template was the same as Fig. 1. (C) Group data demonstrate the effect of Gö6976 to prevent CCPA-induced inhibition of BK current in smooth muscle cells from WT mice (n =4). Representative traces with 0.1 μM Gö6976 (D) and with 0.1 μM Gö6976 + 0.1 μM CCPA (E) in a smooth muscle cell from an A1KO mouse. (F) Group data representing BK current in Gö6976-treated smooth muscle cells from A1KO mice (n = 4).
DISCUSSION
We tested the hypothesis that activation of A1AR inhibits BK channels in aortic smooth muscle via a PKC-dependent mechanism. This hypothesis was based on previous studies indicating: 1) that A1AR stimulation is associated with 20-HETE production and activation of PKC (22, 36) and 2) 20-HETE and PKC can inhibit BK channels (33, 43, 51, 52). We performed whole-cell patch clamp and Western blot studies using aortic smooth muscle cells and aortae of WT and A1KO mice. No single channel studies were done and this is a limitation of this study. Our major findings included: 1) There were no differences in whole-cell K+ current in aortic smooth muscle cells from WT and A1KO mice, nor were there any differences in the expression of pore-forming α or regulatory β1 subunit proteins. 2) Inhibition of BK current by 20-HETE was similar in aortic smooth muscle cells from WT and A1KO mice. 3) NECA, a non-selective AR agonist increased BK current in aortic smooth muscle cells from both WT and A1KO mice, but the increase was greater in smooth muscle cells from mice lacking the A1AR. 4) CCPA, an A1AR-selective agonist, inhibited BK current in smooth muscle cells from WT, but not A1KO, mice. 5) Inhibition of PKCα with Gö6976 abolished the effect of CCPA to inhibit BK current in smooth muscle cells from WT mice. Together, these data lead us to conclude that, in aortic smooth muscle, A1AR stimulation inhibits BK channel activity and that this occurs via a mechanism involving PKCα.
BK channels are ubiquitously expressed on the sarcolemma of vascular smooth muscles. BK channels are composed of pore-forming α subunits with or without regulatory β subunits. The β1 subunit, however, is commonly found in vascular smooth muscle (3, 24, 32). The α subunit is the voltage- and Ca2+-sensitive pore, while β subunits can modify many characteristics including pharmacology and Ca2+-sensitivity. We observed no difference in the expression of α or β1 BK subunits (Fig. 1), suggesting that the channels are equally expressed in aortic smooth muscle cells from WT and A1KO mice. Further, there were no differences in BK current magnitude between WT and A1KO mice (Fig. 1). We have previously shown that adenosine A1 receptor-mediated smooth muscle contraction is dependent on Cyp4a using wild type and A1AR knockout mice (22, 36). 20-HETE has been shown to inhibit BK channels in rat renal arteriolar smooth muscle cells (45, 52) and we wanted to investigate if A1AR-20-HETE mediated pathway for smooth muscle contraction involved BK channels. We have observed similar results in whole-cell patch recordings (Fig. 2). That is, 20-HETE decreased BK current similarly in smooth muscle cells from both WT and A1KO mice (Fig. 2). In the present study, we used only a single concentration of 20-HETE. This concentration was chosen based on our previous study (22) where we determined the concentration-dependence of aortic contraction to 20-HETE. The concentration of 20-HETE (0.1μM) used in this study is quite modest and it is in the range of 100 μM (45, 52) and 1 μM (52) concentration that have been used by several others as well. 20-HETE is a potent vasoconstrictor as shown previously by significant contractions in the aortae of both WT and A1KO mice (22, 36). 20-HETE activates PKC (22, 23, 33, 36) and PKC may mediate contraction by inhibiting BK channel activity in rat cerebral arteries (4), rabbit portal vein (21), canine basilar artery (34) and rat tail artery (43). This inhibition depends on the sequential phosphorylation of two serines in the C-terminus of the BK α subunit (51). In the present study, when PKCα was antagonized with Gö6976, CCPA could no longer inhibit BK channel current (compare Figs. 4 and 5). This suggests that A1AR signaling through PKCα is negatively coupled to BK channels, perhaps by 20-HETE. The involvement of PKCα in 20-HETE mediated inhibition of BK channels has been shown in canine basilar artery as well (34).
We cannot exclude the possibility that isoforms of PKC other than PKCα regulate BK channels. We relied on experiments with a single inhibitor (Gö6976) at a single concentration (100 nM). However, our previously published data indicate that PKC α (alpha) is the isoform most abundant in mouse smooth muscle (2). Other conventional isoforms were expressed, including beta (β) and gamma (γ), but at lower levels. With regard to conventional PKC isoforms, Gö6976 inhibits PKC α and β, but cannot differentiate between the two, as the IC50 values are close. However, PKC β is not likely to mediate this effect, as we demonstrated previously that activation of adenosine receptors does not increase PKC β in the membrane fraction (2). The amount of PKC in the membrane fraction is an indicator of enzyme activation. In contrast, after adenosine receptor activation, the amount of PKC α in the membrane fraction does increase (2). The IC50 of Gö6976 for PKC γ appears to be at least 10 μM (20). As for novel and atypical isoforms of PKC, Gö6976 does not inhibit PKC δ (delta), ε (epsilon), or ζ (zeta) at the concentration we used (IC50 > 3 μM; (27).
The non-selective adenosine agonist NECA relaxes smooth muscle by acting on A2AR (38, 41, 47), whereas the A1AR-selective agonist CCPA contracts smooth muscle (22, 36). Activation of BK channels by A2AR could lead to membrane potential hyperpolarization and contribute to the relaxation of smooth muscle, whereas inhibition of BK channels by A1AR could cause depolarization and contribute to contraction. We observed that NECA increased BK current significantly in A1KO as compared to the WT (Fig. 3). This suggests that the increase in the BK current could be due to the absence of A1 and the non-selective action of NECA on other adenosine receptors (e.g. A2AR) in the A1KO. Unpublished results from our lab suggest that A2A receptor expression is upregulated in A1KO mice and this might also be a factor in the larger responses to NECA. However, studies investigating the effect of A2A receptor signaling on BK current have not been performed and represent a limitation of this study. There is evidence showing that in the rat pre-glomerular vessels A2AAR, through EETs, activate BK channels mediate vasodilation (9, 39). Our own lab has shown evidence in the mouse aorta that A2AR mediates vasodilation through EETs via sarcolemmal KATP channels (37). In addition, it has been shown in the rats that A3AR restores vascular reactivity after hemorrhagic shock in a ryanodine receptor-mediated and BK channel-dependent pathway (50). There have been no studies showing the interaction of A2BAR and BK channels. Furthermore, by using the A1 selective agonist CCPA we demonstrated a decrease in BK current in smooth muscle cells from WT mice, but no effect in smooth muscle cells from the A1KO mice. This is the first evidence in the literature showing that A1AR activation inhibits BK current. As the A1AR is known to mediate contraction (22, 47, 48), we suggest this may be mediated by inhibition of BK channels. It should be noted, however, that there are reports of A1 activating KATP channels (11) and linking to nitric oxide-dependent smooth muscle relaxation (39). The reasons for such differences are not readily apparent, but may perhaps be attributed to the vascular beds and species.
Reactive oxygen species are known to play a role in modulating BK channel activity. Whether ROS activates or inhibits BK channel is debatable. For instance, studies have shown that hydrogen peroxide (H2O2) has negative effects(12) and positive effects (46) on BK channel activation. However, ROS are generated by the activation of adenosine receptors as shown by us (44) and others (29). Myocardial A2A receptors via H2O2 couple with KATP channels in smooth muscle play a role in reactive hyperemia (44). A1 receptors have been shown to attenuate myocardial stunning by reducing ROS formation by opening KATP channels (29). There have been no studies showing a link between adenosine receptor activation, ROS generation, and BK channel activation. However, we can speculate that A1 receptor activation may involve ROS pathway in modulating BK channel activity, but further studies are needed to test this hypothesis.
There is no clear consensus on mean arterial pressure in WT and A1KO mice. Johansson colleagues (19) generated the first A1KO mouse line in 2001. These authors reported no change in blood pressure or heart rate. Unfortunately, the data were not shown and the paper does not indicate how measurements were made (19). Brown and colleagues (7) reported that MAP was increased in A1KO mice (same mice used by Johansson and colleauges from Fredholm’s laboratory). Specifically, MAP in conscious mice was 85 +/− 1 and 97 +/− 2 mmHg for WT and A1KO mice, respectively (p < 0.05). In addition to changes in salt and water balance and renal hemodynamics, these data support the idea that A1 receptors couple to smooth muscle contraction and increases in total peripheral resistance(7). In contrast, Lee and colleagues reported that telemetered MAP in unstressed A1KO mice was indistinguishable from WT. Specifically, Lee and colleagues (25) report that MAP is 120 +/− 3 vs. 117 +/− 3 mmHg in WT and A1KO mice, respectively. When mice are made hypertensive with Ang II infusions, the blood pressure is less in A1KO mice, reinforcing the idea that A1 receptors couple to smooth muscle contraction and increased total peripheral resistance(25).
Adenosine, via multiple receptor subtypes, contracts and relaxes vascular smooth muscle through several mechanisms, including the regulation of K+ channels (11). While adenosine-mediated increases in KATP channel activity are generally well accepted (44), reports regarding the role of BK channels in adenosine-induced smooth muscle relaxation vary widely. In canine coronary arterioles, vasodilation in response to adenosine is inhibited by iberiotoxin (a very selective BK channel antagonist) (8). Blocking BK channels inhibits vasodilation to 2-chloroadenosine in pig coronary arterioles (5); however, the role of BK channels in this response is abolished in pigs with metabolic syndrome (5). Thus, it could be pathology that explains why BK channels play no role in adenosine-induced vasodilation human coronary arterioles (42), as they are typically collected from patients with heart disease. Conversely, it may be that BK channels play little, if any role, in adenosine-induced vasodilation, as this has been reported in the majority of studies from pig coronary arterioles (15–17). However, it cannot be ignored that BK channels are reported to contribute to adenosine-induced relaxation or vasodilation of rat cerebral arterioles (35), rabbit renal arteries (41), rat aortas (39), and rat preglomerular microvessels (9). Further, adenosine increases a Ca2+-dependent K+ current in smooth muscle cells from the rat mesenteric artery that may be mediated by BK channels (26). At present, there is little consensus regarding the role of BK channels in adenosine-induced smooth muscle relaxation and very little data directly addressing whether adenosine increases BK current in smooth muscle cells isolated from those same arteries or arterioles.
Our lab has previously shown in the coronary artery smooth muscle cells that activation of A1AR leads to signaling via PLC-βIII, PKC-α leading to ERK1/2 phosphorylation. However, this study demonstrates that adenosine receptor signaling converges on BK channels in vascular smooth muscle. The study is novel in showing a relationship between A1AR; Cyp4a product, 20-HETE; PKC-α and BK channel in WT and A1KO aortic myocytes. However this can be extrapolated to understand vasomotor responses in resistance vessels, but this will require direct investigation, outside the scope of the present study. This conduit artery does not contribute to resistance that regulates blood flow, but results from this cell type provide insight into signaling mechanisms that may also be present in smaller arteries and arterioles. From our data, we conclude that A1AR signaling inhibits BK channels via 20-HETE and PKCα.
Acknowledgments
NIH grants HL094447 and HL027339 to S. J. Mustafa and HL-114559 to M. A. Nayeem supported this work. We are thankful to Drew Fancher for his technical help and Dovenia Ponnoth for her help.
LITERATURE CITED
- 1.Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol. 2007;293:H3448–3455. doi: 10.1152/ajpheart.00764.2007. [DOI] [PubMed] [Google Scholar]
- 2.Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H1032–1039. doi: 10.1152/ajpheart.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano S, Tune JD, Dick GM. Bisphenol A activates Maxi-K (K(Ca)1.1) channels in coronary smooth muscle. Br J Pharmacol. 2010;160:160–170. doi: 10.1111/j.1476-5381.2010.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J Gen Physiol. 1996;108:315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Neeb ZP, Bratz IN, Sturek M, Tune JD. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H1629–1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 7.Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- 8.Cabell F, Weiss DS, Price JM. Inhibition of adenosine-induced coronary vasodilation by block of large-conductance Ca(2+)-activated K+ channels. Am J Physiol. 1994;267:H1455–1460. doi: 10.1152/ajpheart.1994.267.4.H1455. [DOI] [PubMed] [Google Scholar]
- 9.Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol. 2006;291:F155–161. doi: 10.1152/ajprenal.00231.2005. [DOI] [PubMed] [Google Scholar]
- 10.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredholm BB, APIJ, Jacobson KA, Klotz KN, Linden J International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 14.Harder DR, Lange AR, Gebremedhin D, Birks EK, Roman RJ. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J Vasc Res. 1997;34:237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- 15.Heaps CL, Bowles DK. Gender-specific K(+)-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol. 2002;92:550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- 16.Hein TW, Kuo L. cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and K(ATP) channels. Circ Res. 1999;85:634–642. doi: 10.1161/01.res.85.7.634. [DOI] [PubMed] [Google Scholar]
- 17.Hein TW, Wang W, Zoghi B, Muthuchamy M, Kuo L. Functional and molecular characterization of receptor subtypes mediating coronary microvascular dilation to adenosine. J Mol Cell Cardiol. 2001;33:271–282. doi: 10.1006/jmcc.2000.1298. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keenan C, Goode N, Pears C. Isoform specificity of activators and inhibitors of protein kinase C gamma and delta. FEBS Lett. 1997;415:101–108. doi: 10.1016/s0014-5793(97)01104-6. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura K, Xiong Z, Teramoto N, Kuriyama H. Roles of inositol trisphosphate and protein kinase C in the spontaneous outward current modulated by calcium release in rabbit portal vein. Pflugers Arch. 1992;421:539–551. doi: 10.1007/BF00375049. [DOI] [PubMed] [Google Scholar]
- 22.Kunduri S, Mustafa S, Ponnoth D, Dick G, Nayeem M. Adenosine A1 receptors link to smooth muscle contraction via CYP4a, PKC-alpha, and ERK1/2. J Cardiovasc Pharmacol. 2013 doi: 10.1097/FJC.0b013e3182919591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 24.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- 25.Lee DL, Bell TD, Bhupatkar J, Solis G, Welch WJ. Adenosine A1-receptor knockout mice have a decreased blood pressure response to low-dose ANG II infusion. Am J Physiol Regul Integr Comp Physiol. 2012;303:R683–688. doi: 10.1152/ajpregu.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Cheung DW. Modulation of Ca(2+)-dependent K(+) currents in mesenteric arterial smooth muscle cells by adenosine. Eur J Pharmacol. 2000;394:35–40. doi: 10.1016/s0014-2999(00)00142-4. [DOI] [PubMed] [Google Scholar]
- 27.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 28.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 29.Narayan P, Mentzer RM, Jr, Lasley RD. Adenosine A1 receptor activation reduces reactive oxygen species and attenuates stunning in ventricular myocytes. J Mol Cell Cardiol. 2001;33:121–129. doi: 10.1006/jmcc.2000.1282. [DOI] [PubMed] [Google Scholar]
- 30.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol. 2008;295:H2068–2078. doi: 10.1152/ajpheart.01333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 32.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 33.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+, K+-ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol. 2002;137:1362–1370. doi: 10.1038/sj.bjp.0704960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paterno R, Faraci FM, Heistad DD. Role of Ca(2+)-dependent K+ channels in cerebral vasodilatation induced by increases in cyclic GMP and cyclic AMP in the rat. Stroke. 1996;27:1603–1607. doi: 10.1161/01.str.27.9.1603. discussion 1607–1608. [DOI] [PubMed] [Google Scholar]
- 36.Ponnoth DS, Nayeem MA, Kunduri SS, Tilley SL, Zeldin DC, Ledent C, Mustafa SJ. Role of omega-hydroxylase in adenosine-mediated aortic response through MAP kinase using A2A-receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R400–408. doi: 10.1152/ajpregu.00481.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponnoth DS, Nayeem MA, Tilley SL, Ledent C, Jamal Mustafa S. CYP-epoxygenases contribute to A2A receptor-mediated aortic relaxation via sarcolemmal KATP channels. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1003–1010. doi: 10.1152/ajpregu.00335.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponnoth DS, Sanjani MS, Ledent C, Roush K, Krahn T, Mustafa SJ. Absence of adenosine-mediated aortic relaxation in A(2A) adenosine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2009;297:H1655–1660. doi: 10.1152/ajpheart.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray CJ, Marshall JM. The cellular mechanisms by which adenosine evokes release of nitric oxide from rat aortic endothelium. J Physiol. 2006;570:85–96. doi: 10.1113/jphysiol.2005.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roman RJ, Maier KG, Sun CW, Harder DR, Alonso-Galicia M. Renal and cardiovascular actions of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol. 2000;27:855–865. doi: 10.1046/j.1440-1681.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 41.Rump LC, Jabbari TJ, von Kugelgen I, Oberhauser V. Adenosine mediates nitric-oxide-independent renal vasodilation by activation of A2A receptors. J Hypertens. 1999;17:1987–1993. doi: 10.1097/00004872-199917121-00032. [DOI] [PubMed] [Google Scholar]
- 42.Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol. 2005;288:H1633–1640. doi: 10.1152/ajpheart.00575.2004. [DOI] [PubMed] [Google Scholar]
- 43.Schubert R, Noack T, Serebryakov VN. Protein kinase C reduces the KCa current of rat tail artery smooth muscle cells. Am J Physiol. 1999;276:C648–658. doi: 10.1152/ajpcell.1999.276.3.C648. [DOI] [PubMed] [Google Scholar]
- 44.Sharifi Sanjani M, Zhou X, Asano S, Tilley SL, Ledent C, Teng B, Dick GM, Mustafa SJ. Interactions between A2A adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol. 2013 doi: 10.1152/ajpheart.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–418. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 46.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001;117:253–274. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288:H1411–1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Yang JN, Arner A, Boels PJ, Fredholm BB. Adenosine A(1) receptors and vascular reactivity. Acta Physiol (Oxf) 2010;199:211–220. doi: 10.1111/j.1748-1716.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- 49.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou R, Chen F, Li Q, Hu DY, Liu LM. Stimulation of the adenosine A3 receptor reverses vascular hyporeactivity after hemorrhagic shock in rats. Acta Pharmacol Sin. 2010;31:413–420. doi: 10.1038/aps.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci U S A. 2009;107:8005–8010. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]