Abstract
While protein-based therapeutics is well-established in the market, development of nucleic acid therapeutics has lagged. Short interfering RNAs (siRNAs) represent an exciting new direction for the pharmaceutical industry. These small, chemically synthesized RNAs can knock down the expression of target genes through the use of a native eukaryotic pathway called RNA interference (RNAi). Though siRNAs are routinely used in research studies of eukaryotic biological processes, transitioning the technology to the clinic has proven challenging. Early efforts to design an siRNA therapeutic have demonstrated the difficulties in generating a highly-active siRNA with good specificity and a delivery vehicle that can protect the siRNA as it is transported to a specific tissue. In this review article, we discuss design considerations for siRNA therapeutics, identifying criteria for choosing therapeutic targets, producing highly-active siRNA sequences, and designing an optimized delivery vehicle. Taken together, these design considerations provide logical guidelines for generating novel siRNA therapeutics.
Keywords: siRNA therapeutic, RNAi, liver cancer, siRNA design, delivery vehicle design
1. Introduction
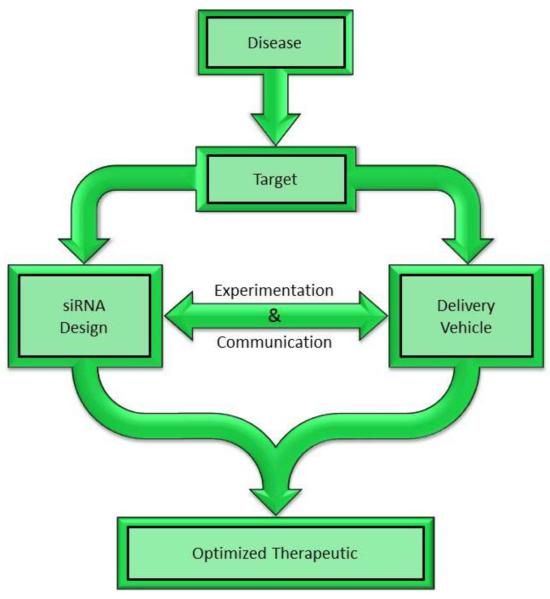
The “big data” that is available and being generated on biological systems has greatly expanded the number of potential targets for treating disease. This is especially true for sequencing data yielding targets for proposed genetic therapies. As one such proposed therapy, siRNAs offer unique advantages that make them an ideal platform for therapeutic development. Perhaps the strongest argument in support of RNAi-based therapeutics is the ability to design the active agent, the siRNA, and its delivery vehicle simultaneously and mostly independent of each other for the knockdown of specific targets in specific tissues (Figure 1). Additionally, unlike protein-based biologics, little process development would be required for scale-up of siRNA-based therapeutics, as the synthesis and purification protocols for nucleic acids are well established.
Figure 1.
siRNA design overview. siRNA therapeutic design should, in the short term, begin by addressing diseases with unmet therapeutic need that are treatable by siRNA-based therapeutics. While initial siRNA and delivery vehicle design begins with some design heuristics, as discussed in the following sections, a completely optimized therapeutic will require concomitant, parallel development of each of the constituents followed by experimental validation of the optimized therapeutic.
Effective silencing and the desired therapeutic effect depend upon the targeted delivery of an siRNA that is both specific and active against the intended target. Furthermore, the choice of an appropriate target largely determines the efficacy of an siRNA-based therapeutic. We therefore discuss the design of siRNA therapeutics in three broad categories: i.) selection of a disease and target mRNA, ii.) design of a highly-active siRNA, and iii.) design of the siRNA delivery vehicle. This review will discuss these aspects of siRNA therapeutic design and where relevant, use liver cancer to demonstrate key/fundamental design characteristics.
2. Choosing a Target for siRNA Mediated Silencing
Selecting a disease to target with any new therapeutic modality begins by choosing a disease for which current therapeutics do not work satisfactorily, thereby avoiding direct competition of the new therapeutic with existing, successful drugs. As the majority of drugs accumulate in the liver, diseases of the liver provide relatively easier targets for early-stage development of any therapeutic. With that in mind, we have chosen advanced liver cancer, specifically hepatocellular carcinoma (HCC), as an example for development of our hypothetical siRNA-based therapeutic. Liver cancer is the 6th most common cancer and the third leading cause of cancer related deaths, with ~750,000 new cases diagnosed annually worldwide [1]. Moreover, it has one of the highest rates of mortality, with only a 14% 5-year survival rate in the United States [2]. The high mortality rate highlights two needs, for earlier diagnosis of the disease and for improved treatments of advanced-stage liver cancer, the most common of which is hepatocellular carcinoma (HCC) [2]. The only approved treatment for advanced stage HCC is Sorafenib, a tyrosine kinase inhibitor having anti-angiogenic activity. Unfortunately, use of Sorafenib only increases median survival rate from 7.9 to 10.7 months over the placebo group [3]. While a number of clinical trials are currently underway using tyrosine kinase inhibitors, monoclonal antibodies, and one siRNA based treatment [4–7], continued development of therapeutics of all kinds for HCC is warranted.
For any disease, multiple proteins are potentially feasible drug targets. In order to select a particular protein target to knock down, it is useful to evaluate, if available, kinetic information about the transcriptional and translational regulation of the target under normal and diseased conditions. Typical siRNA-mediated knockdown only reduces protein levels to ~20% of baseline, and this effect is transient, lasting from a few hours to a couple of weeks before protein levels recover [8,9]. Thus, an siRNA therapeutic should only target mRNAs where this incomplete, transient knockdown is sufficient to achieve a therapeutic effect. To achieve a maximal therapeutic effect, it is also important to select a target where: (i) the protein half-life is shorter than that of the siRNA [8,10], (ii) the mRNA transcription rate is slower than the rate of siRNA-complex turnover [11], (iii) the concentration of mRNA is sufficiently low to permit silencing at minimal siRNA concentrations [12–14], and (iv) no feedback mechanism will upregulate transcription in response to the knockdown. Satisfying these conditions is possible through both, selecting appropriate target genes and engineering of siRNAs for maximal activity and stability. Engineering of the siRNA will be discussed in the next section.
In the development of an actual therapeutic, we would determine all of the necessary kinetic parameters experimentally. We would then select the target with the shortest protein half-life and confirm that its mRNA half-life is not extraordinarily short. Additional experiments that might be required would be to determine the basal levels of the mRNA targets (molecules/cell), as low expression level targets are more amenable to siRNA-mediated silencing. Using HCC as our example system, we examined five targets that have been previously identified and verified as HCC drug targets (Table 1). We were able to locate published values for protein and some mRNA half-lives [10,15–20]. VEGFR was found to have the shortest protein half-life, 70 min, and the known transcript half-lives were all between ~2–9 h [20], a small variability relative to the protein half-lives. Based on protein half-life alone, we concluded that VEGFR would be the most amenable HCC target for the development of an siRNA therapeutic.
Table 1.
Overview of some HCC targets.
| Target | Gene Function | Current Clinical Drugs Targeting | Protein Half-life | References |
|---|---|---|---|---|
| VEGFR/VEGFR2 | Angiogenesis | Sorafenib, Brivanib, Sunitinib, Cediranib, BIBF1120, Pazopanib, Regorafenib, E7080, TSU-68, Vandetanib, Ramucirumab, IMC-1121B | 70 min | [5,15,21] |
| EGFR | Signal Transduction | Erlotinib, Cetuximab, Gefitinib, Lapatinib, Vandetanib | 10 h | [5,16,21] |
| Bcr-ABL | Cell Proliferation | Destanib | 40 h | [5,10,17,21] |
| MEK | Signal Transduction | AZD6244 | 6 h | [5,18,21] |
| PDGFR | Angiogenesis/Signal Transduction | Sorafenib, Brivanib, Linifanib, BIBF1120, Pazopanib, TSU-68 | 3 h | [5,19,21] |
3. siRNA Design Considerations
siRNA mediated silencing possesses three characteristics that are desirable for a therapeutic modality: (i) it operates post-transcriptionally, (ii) the specificity of knockdown is high, and (iii) the same target mRNA can be inhibited by many different siRNA sequences. As siRNA sequences can have widely varying activities, significant effort has been spent identifying factors that enhance siRNA activity and reduce off-targeting and non-specific effects [22,23]. These features can be split coarsely into two categories, those related to siRNA activity and those related to siRNA specificity. siRNA activity is influenced by, among other factors, strand selection, the structure of the mRNA target region, base preferences, and overall siRNA G/C content. In comparison, siRNA specificity depends on strand selection, immunogenicity, and uniqueness of the target sequence. Designing an effective siRNA depends upon proper weighting of each of these factors; siRNA selection algorithms typically weight these factors based upon analyses of siRNA activity data [24]. As it stands, the rules for selecting active siRNAs continue to evolve, and it behooves the researcher to design siRNAs with features that are most strongly predictive of high activity. This section will discuss siRNA design considerations that are substantiated by biochemical evidence.
3.1. Details of the RNAi Mechanism
A canonical siRNA is a 21 nt duplex with 19 internal base pairs, 5' phosphates, and dinucleotide 3' overhangs [25–30]. When exogenous siRNAs enter the cytoplasm, they are first recognized by a protein complex called the RISC Loading complex (RLC), which is responsible for properly orienting the siRNA duplex as it hands off the guide strand to the active RNA Induced Silencing Complex (RISC) [30–35]. The RLC is a ribonucleoprotein complex minimally made up of Argonaute 2 (Ago2), Dicer, and TAR RNA Binding Protein (TRBP) [30,33,36–38]. The RLC orients the siRNA in the complex, and, in doing so, selects, sometimes incorrectly, one strand to serve as the guide strand that will target RISC to the mRNA [33,39–42]. The passenger strand (i.e., the strand not selected by the RLC) is concomitantly cleaved and removed [33,43–46]. The active RISC then binds to its target mRNA by Watson-Crick base pairing. In a similar fashion to how the passenger strand is removed, RISC, specifically the Ago2 RNaseIII, cleaves the target at the center of the region complementary to the siRNA, resulting in degradation of the mRNA [28,42,47]. RISC is a multiple turnover enzyme that can then target other complementary mRNAs [42,46–49]. Additional details of the RNAi mechanism and siRNA-mediated silencing can be found in other sources [50,51].
3.2. Differential Terminal Hybridization Stability
For siRNAs to silence the intended target, they must be oriented by the RLC to ensure incorporation of the intended guide strand into the active RISC [30,33,37,38,40,46]. Incorporation of the unintended strand (i.e., the intended passenger strand) leads to the formation of a RISC that cannot cleave the intended target (reducing the activity of the siRNA therapeutic) and that can cause off-target effects (reducing the specificity of the siRNA) [39,40,52]. The difference in the activity of one strand relative to the other is termed functional asymmetry [40]. Functional asymmetry is a function of multiple factors, including RISC stability, RISC turnover rate, and, most directly, biased incorporation of one siRNA strand into RISC [33,48,53].
Biased strand incorporation occurs due to the recognition of differences in the termini of the siRNAs by the RLC proteins [33,37–40,54,55]. Based on early studies of functional asymmetry and biased strand loading [40], it was concluded that differential terminal hybridization stability, the difference in hybridization free energy between the two ends of the siRNA, was predictive of functional asymmetry due to biased strand loading [33,40]. These studies determined that the strand whose 5' end is less stably hybridized (higher hybridization free energy) to the complementary strand is preferentially loaded into RISC [33,37,38]. Initially, differential hybridization stabilities were calculated using the four terminal base pairs at each end of the siRNA [40,56]. More recent work suggests that using only one nearest neighbor provides a more predictive calculation [54,57]. Nonetheless, when selecting candidate siRNAs, it is recommended to reject siRNAs with an unfavorable differential stability. The remaining candidates can then be pared down further using the sequence characteristics and other design criteria described below.
3.3. 5' Nucleotide Preference
A number of positional base preferences, within the siRNA, are shown to be correlated to siRNA activity [22,58–68]. Most common among these are preferences affecting nucleotides at or near the 5'-termini of the siRNA strands [22,54,58,60,62,69]. To date, there is incomplete consensus among datasets as to what the most predictive base preferences are, nor is there strong biochemical evidence explaining the reason for specific base preferences. The exception is the two 5' terminal nucleotides [22,37,54,58,59,61,62,65,66], wherein Ago2 was identified as having a nucleotide specificity loop that shows significantly higher affinity interactions with U and A bases and lower affinity interactions with C and G bases [53]. By classifying the pool of siRNA strands according to their 5'-terminal nucleotides, the pool of candidates can be further limited to those sequences with the nucleotides that give the greatest likelihood of high activity [37,54].
3.4. mRNA Target Region
mRNA secondary structure precludes siRNA binding through steric hindrance of RISC binding and cleavage [70,71]. Conveniently, mRNA secondary structure can be easily, and relatively accurately, predicted in silico, using the nearest neighbor approach to predict the most thermodynamically stable structures [57,61,72–75]. If more certainty about the structure is desired/required, experimental structural analyses can be used to refine the predicted structures [71,76–81]. That said, the actual mRNA structure in a living cell is dynamic and any approach will provide only incomplete information that can guide choices of potential target regions. Using the available structural information, it is recommended, given a choice, to target regions of greater accessibility, especially at the 5' and 3' ends of the target region [75].
3.5. Immunogenicity
siRNAs can cause a number of immunogenic and cytotoxic responses, some of which arise due to their dsRNA structure and others that are sequence specific [82–85]. Immune receptors for siRNAs reside on the surface of the cell, within endocytotic vesicles, and within the cytosol [83]. While it is possible to prevent interaction of the siRNA with cell surface and endosomal receptors by protecting/inhibiting accessibility to the siRNA with a delivery vehicle (see section 4.1 below for additional details), it is still worthwhile to design siRNAs that avoid the use of immunostimulatory sequence motifs recognized by these receptors.
In general, cytosolic receptors for RNA are uniform in their expression across all cell types and recognize mainly structural features as opposed to sequence motifs [83]. Among these receptors are OAS1, PKR, and RIG-I [83,86–91]. The canonical siRNA structure does not activate PKR or RIG-1; these receptors are more sensitive to dsRNA longer than 30 bp or having 5'-triphosphates [87,89,92]. Unlike PKR and RIG-1, OAS1 is also activated by a sequence motif, NNWW(N9)WGN, in dsRNA as short as 19bp [86].
Cell surface and endosomal receptors for RNA, mainly the toll-like receptors (TLRs) [83,93], recognize specific sequence motifs, and their expression varies by cell type [83,94]. TLR3 (cell surface and endosomal) is a receptor for dsRNA [95,96]. TLRs 7 and 8 (endosomal) are receptors for ssRNA and are primarily responsible for recognition of specific RNA sequences, termed pathogen-associated molecular patterns (PAMPs) [94,96–105]. The recognition of siRNAs by TLR3 remains an area of investigation, as its activation was shown in clinical trials using naked siRNAs [103]. However, TLR3 in vitro was not activated by the canonical naked siRNA [94]. The recognition of siRNAs by TLRs 7 and 8 requires endocytosis of the siRNA followed by duplex melting within the acidic vesicle exposing the ssRNA bases to the TLR receptors, initiating an immune response [102,106]. When designing an siRNA, the immunostimulatory motifs to avoid include: GUCCUUCAA [107], UGUGU [98], UGU [98], UCA [108], GU-rich sequences (Heil 2004), AU-rich sequences [105], and U-rich sequences [102]. In addition to the immunostimulatory sequences, the UGGC motif has been shown to be cytotoxic via non-immune mechanisms [109].
3.6. Non-Specific Effects
Specificity of an siRNA begins with the selection of a sequence that is unique in the transcriptome of the target cells. However, sequences should also be chosen with consideration given to the possibility of off-target effects resulting from partial complementarity and miRNA-like targeting [84]. Repetitive sequences should also be avoided, in particular, the GGGG motif because it forms a G-quartet secondary structure [110].
miRNA-like targeting occurs when siRNAs have seed region complementarity with the 3' UTR of an mRNA, resulting in translational repression of the untargeted transcript [111–117]. Moreover, mRNAs that are regulated by miRNAs are also more susceptible to miRNA-like off-targeting due to extended 3' UTRs and the preexistence of miRNA target sites [118]. Avoiding miRNA-like targeting effects is complicated by an inability to accurately predict seed sequences [116,118,119]. miRNA-like targeting is influenced by the surrounding sequence of the target, the position of the target in the mRNA, and the repetitiveness of the target sequence [114,116,120,121]. As such the best way to account for miRNA-like targeting is to avoid seed sequences that have already been identified (http://www.mirbase.org) [122], so as not to unnecessarily limit the sequence space by avoiding false positives. A number of other tools exist that can predict off-targeting and may prove useful in determining the overall likelihood an siRNA will have strong off-target effects [123,124].
Methods to manipulate the siRNA to avoid off-target effects are not well established. However, there are a number of algorithms that do take off-targeting into account beyond a simple BLAST search [64,123,125]. Nonetheless, off-target effects are particularly challenging to avoid for a number of reasons. First, mismatches are tolerated within the duplex, however where mismatches are tolerated is only partially known (Figure 2) [126–128]. Second, predicting miRNA-like targeting is inaccurate and prediction of seed region interactions often leads to overestimation of off-targeting [118]. Lastly, siRNAs can cause translational repression, post-transcriptional silencing, or P-body based degradation and/or repression [24,129–135], making the cause of the off-target effects difficult to interpret. Still, as with immunogenicity, the only way to fully ensure that off-target effects do not occur is by post-hoc analysis using techniques such as RNA microarrays or parallel sequencing to quantify the levels of all untargeted transcripts [136–138] and antibody microarrays and mass spectrometry to check for off-target translational repression [139,140].
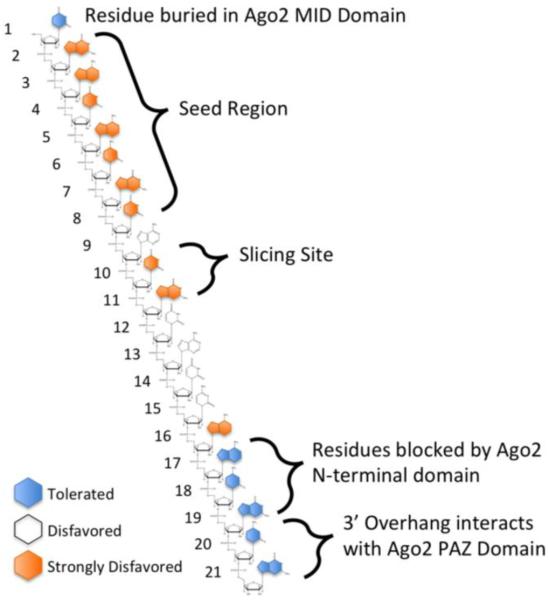
Figure 2.
Tolerance for mismatches between an siRNA and its target. siRNA seed region (bases 2–8) [112,143] and splice site (bases 10–11) [127,132,144] are the least tolerant of mismatches because of their active role in silencing. The 1st nucleotide [53,145], the nucleotides of the 3'overhang (bases 20–21) [146,147], and bases 17–19 [144,148] are the most tolerant of mismatches, because their ability to base pair is at least partially blocked by Ago2. Position and base specific mismatches are tolerated at positions 8–16 [141].
Genetic variation should also be considered, particularly single nucleotide polymorphisms (SNPs). Target regions with as little as one nucleotide difference can change target knockdown efficiency significantly [128,141]. Thus, in the short term, it makes sense to avoid targeting regions where SNPs occur to ensure the broad utility of any therapeutic. Going forward, the existence of inexpensive sequencing and synthesis technologies may allow the design of patient-specific siRNA therapeutics, accounting for each patient's unique genotype.
From our perspective, the problem of avoiding non-specific effects reverts, largely, to the problem of designing an siRNA with the greatest specific activity. The most active siRNAs can be used at the lowest possible concentrations, decreasing off-target effects while still achieving a therapeutic effect [14]. Pooling siRNAs against a single target has been suggested as a means to improve specificity of silencing [142]. This effect is principally attributed to the reduced concentration of any given siRNA in the pool. However, we expect that design of the most active and specific RNA using guidelines such as those presented here will maximize the therapeutic window beyond what is possible by pooling. Thus, we would consider accounting for the factors described here as being of lesser importance relative to those that are predictive/indicative of the highest specific activity of a sequence.
3.7. Other siRNA Design Criteria
Other factors shown to influence siRNA activity include the G/C content (i.e., the overall duplex stability) of the siRNA [22,39,149], the secondary structure of the guide strand [150,151], internal repeats [22], palindromic sequences [152], and positional base preferences along the siRNA [22,58–68,153]. More recently, additional structural criteria, even to the level of tertiary structure [154], have been identified as valuable in predicting siRNA activity. While each of these factors may be important in siRNA design, either their overall influence is thought to be minimal compared to the other selection criteria or there is a lack of consensus on how to implement the feature as a selection criterion. As such, selection of siRNAs using the mentioned criteria should be done as a second order set of rules to distinguish only the most active and specific siRNAs.
3.8. Non-Canonical siRNA Structural Designs
Using non-canonical siRNA structures in large part changes the point of entry into the RNAi pathway or changes the way in which the RNAi proteins interact with the non-canonical siRNA [155]. From the bottom up, ssRNAs are capable of being loaded by Ago2, albeit inefficiently, to form an active RISC in vitro [47,144,156,157] and in vivo when chemically modified [158,159]. By only providing one siRNA strand to enter RISC, proper strand selection is no longer a design characteristic. Other structures are designed to bias strand incorporation by altering the length of the passenger strand (asymmetric interfering RNAs (aiRNAs) and asymmetric short-duplex siRNAs (asiRNAs) [160–162]), the length of the 3' overhang (fork-siRNAs (fsiRNAs) [163]), or by assembling a duplex with a segmented passenger strand (small internally segmented interfering RNAs (sisiRNAs) [164]). A more recently tested structure utilizes a “bulge” at the second position of the guide strand, creating a perturbation at the first base involved in the seed region [165]; the use of this modification to the canonical siRNA structure was shown to decrease off-targeting by miRNA-like activity. Other non-canonical structures exploit the ability for longer RNAs to enter RISC more efficiently; these structures are called Dicer substrate RNAs (DsiRNAs) [166–169].
3.9. Incorporation of Chemical Modifications
Chemical modifications in siRNAs can increase their stability (specifically with regards to nuclease degradation), minimize immunogenicity, and, to the extent possible, improve the activity of the siRNA [85,102,107,170–175]. Chemical modifications of the siRNA focus on changing the phosphodiester backbone, ribose sugar, nucleotide base, and 2'-OH ribose group. Effective use of chemical modifications requires the substitution of the new chemical moiety at a position within the siRNA where the additional group or structural alteration does not inhibit normal siRNA function. In general, the rationale behind chemical modifications is to incorporate small perturbations in the siRNA structure to prevent recognition and/or binding of the siRNA by nucleases and the immune receptors for RNA. The most common modifications include altering the 2'-OH group to a 2'-O-methyl or 2'-F to prevent recognition of the RNA by nucleases and TLR7 and TLR8 [85,173,176–181]. Unfortunately, no rules exist defining which chemical modifications are most useful and how they are best applied.
The 3' overhangs are also a common location for chemical modifications for two reasons: (i) they may provide a site of attack for endoribonucleases and (ii) chemical modifications, even bulky ones, are typically well tolerated at these positions [182]. Phosphorothioate and phosphorodithioate modifications to the backbone of the siRNA generate siRNAs with nuclease resistance, but the number and positions of modifications are important in retaining siRNA activity [176,183,184]. A more comprehensive understanding of the RNAi mechanism as well as the effects of various chemical modifications will aid in rational design of siRNAs with an even greater therapeutic index. A more extensive review discussing chemical modifications of siRNAs can be found elsewhere [185].
4. Delivery
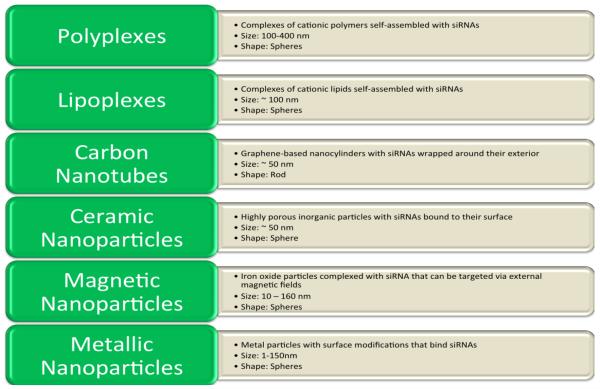
siRNAs are hydrophilic, due to their anionic backbone, and do not readily diffuse across cellular membranes. Moreover, naked siRNAs are rapidly filtered from circulation, degraded, and can initiate immune/inflammatory responses (as stated above) [7,83]. Thus, delivery vehicles must be used to protect/conceal the siRNA while facilitating its transport to the cytoplasm of the targeted cells. By varying characteristics such as size, charge, shape, chemistry, and the means of directing the vehicle-siRNA complexes to the target cells, well-designed delivery vehicles can markedly increase the therapeutic efficacy of a given siRNA [186]. There are many different types of delivery vehicles that have been developed for siRNA-based therapeutics, with lipoplexes and polyplexes currently being the most commonly applied, each possessing different physical and chemical characteristics, as well as offering distinct advantages and disadvantages (Figure 3). The following sections describe a design approach in which the desired properties of the delivery vehicle are selected based on the unique characteristics of the targeted disease. As an illustrative example, these guidelines are applied to the development of a hypothetical HCC therapeutic. The following sections describe these various vehicle types and outline approaches for the design of an siRNA delivery vehicle.
Figure 3.
Types of non-viral delivery vehicles being utilized in developing siRNA therapeutics. For a more comprehensive review of each particle the following are suggested: Polyplexes [187–189], Lipoplexes [190–192], Carbon Nanotubes [184,193–195], Ceramic Nanoparticles [196–198], Magnetic Nanoparticles [199,200] and Metallic Nanoparticles [201–203]. Additional sources discussing the breadth of delivery vehicles that have been tested to date: [6,7,204–206].
4.1. Accessing the Cell Cytoplasm
The purpose of the delivery vehicle is to protect the siRNA and deliver it to the cytoplasm of the target cells where it can be recognized by the proteins of the RNAi pathway. To reach the cytoplasm, vehicle-siRNA complexes can, as is the case with many lipoplexes, cross the membrane directly [207] or be internalized by endocytosis followed by escape from the vesicle, often by vesicle rupture [208–210]. Vehicle-siRNA complexes are formed in two ways: self-assembly by electrostatics (typical) and covalent attachment (rare). For self-assembled complexes, siRNAs attach on the surface or are fully encapsulated depending on the structure and flexibility of the vehicles. The fully formed complex reduces siRNA degradation by serum nucleases, recognition by toll-like receptors (TLRs), and other molecules of the innate immune system [83]. siRNAs that are not fully encapsulated are more likely to be recognized by serum nucleases and TLRs. While the affinity of the vehicle for the siRNA is critical for complex formation, the complex must dissociate upon cell entry to allow the siRNA to be bound by the pathway proteins [211].
The various endocytotic mechanisms take up species of particular sizes as follows: lipid rafts (40–50 nm), caveolae-mediated endocytosis (50–60 nm), clathrin-mediated endocytosis (<200 nm), macropinocytosis (500–10,000 nm, and phagocytosis (<10,000 nm) [212,213]. Current data suggests that delivery leading to siRNA silencing can occur through multiple uptake mechanisms depending on the vehicle type [186,214,215]. Additionally, all endocytotic pathways efficiently incorporate complexes with a net positive charge [216].
When taking these factors into account, the endocytotic pathways used most frequently by the diseased cells will dictate both the characteristics of the chosen delivery vehicle. For our hypothetical case of liver cancer, HCCs primarily use pinocytosis for the uptake of extracellular components. As such, our vehicle-siRNA complexes should have a maximum diameter of 10,000 nm and a net positive charge [217]. These design requirements do not eliminate any candidate vehicle types (Figure 3) from potentially being used in a therapeutic for the treatment of HCCs.
4.2. Routes of Systemic Delivery
Each tissue is connected to multiple transport systems (e.g., circulatory, lymphatic, etc.). Delivery to the intended target can be enhanced by pairing the characteristics of a therapeutic complex to the characteristics of the transport system that will deliver the complex to the target cells. [218,219]. The goal then becomes to maximize the residence time of the therapeutic complex in a given transport system before it is degraded or sequestered. For diseases in tissues that are difficult to access by transport systems, e.g., prostate, eye, neck, and brain, localized delivery is often more appropriate [205].
The circulatory system is the most common route for therapeutic delivery. In the circulatory system, particles are actively filtered based on size. Particles less than 10 nm in size are filtered by the kidneys within hours of injection, and particles larger than 200 nm are retained within the spleen [220,221]. Thus, to improve the bioavailability of the complexes, it is optimal to design complexes to between 50–200 nm [222].
Bioavailability can be further improved by avoiding recognition and clearance of the complexes by phagocytes. While particle size can be manipulated to avoid phagocytosis, the size range and how much size changes affect clearance differ among particle types [223]. Shape has been shown to have a more consistent effect, with shapes containing large tangential planes (e.g., rods) being phagocytosed more efficiently than particles of uniform diameter (e.g., spheres) [224–226]. Complexes of high zeta potential (>25 mV) are also phagocytosed more efficiently than complexes with potential below 15 mV [227]. PEGylation and the use of hydrophilic polymer coatings have also been shown to inhibit phagocytosis [228–230].
Based on the intent to reach HCCs in the liver as well as metastatic cells, the cardiovascular system provides the best system for delivery. Bioavailability within the system is thus optimized using spherically shaped complexes, 50–200 nm in diameter, with a 0 to 15 mV zeta potential, with PEGylation as needed to achieve the desired circulating half-life. These complex characteristics would reduce clearance from circulation, increasing their access to HCCs.
4.3. Delivery Specifically to Target Cells
Having designed the complex for maximal bioavailability, consideration must now be given to trafficking out of the transport system to the target cells. For instance, tumors, including HCC, are susceptible to enhanced permeability and retention (EPR) which results in an increased uptake and sequestration of particles 100–500 nm in size from the circulation (“passive” targeting) [231–233]. Designing the size of complexes to be 100–200 nm will result in enhanced accumulation in the vicinity of an HCC tumor, without compromising other design characteristics.
Delivery vehicles can also be modified with surface ligands (e.g., peptides, antibodies, or aptamers) that guide and aid uptake by the target cells (“active targeting”) [234]. While easily applied for complexes with encapsulated siRNA, complexes with surface attached siRNA will have reduced surface area for siRNA attachment with the addition of each ligand. Active targeting has proven effective in HCC models [235–237], but the strategies for functionalizing delivery vehicles with ligands are not well defined. In situations where targeting cannot be improved through molecular approaches, magnetic nanoparticles can be directed to a specific target region via external magnetic fields, though this can be logistically difficult [200].
4.4. Methods of Administration
Multiple approaches can be applied for local or systemic administration to the body. Current therapeutics favor localized delivery (intranasal, intraocular, intratumoral, etc.) for their specificity in reaching a target tissue [238], though this is difficult for relatively remote tissues where the disease may be systemic, as for metastatic HCC. In such cases, the circulatory system can be used to access cells at nearly any location in the body, with the possible exception of the brain due to the blood-brain barrier. For access to the circulatory system, transdermal and gastrointestinal absorption can be used, but intravenous (IV) injection is optimal for the immediate delivery of known concentrations of a therapeutic [239]. For IV therapeutics, complexes should be designed to be stable for long-term storage in a saline or intravascular compatible solution. While systemic delivery always has the potential to allow non-specific effects outside the target tissue, these effects can be mitigated through the inclusion of cell-specific targeting moieties that limit uptake by non-targeted cells while not preventing access to targeted cells at remote locations.
4.5. Selection of a Specific Delivery Vehicle for Targeting HCCs
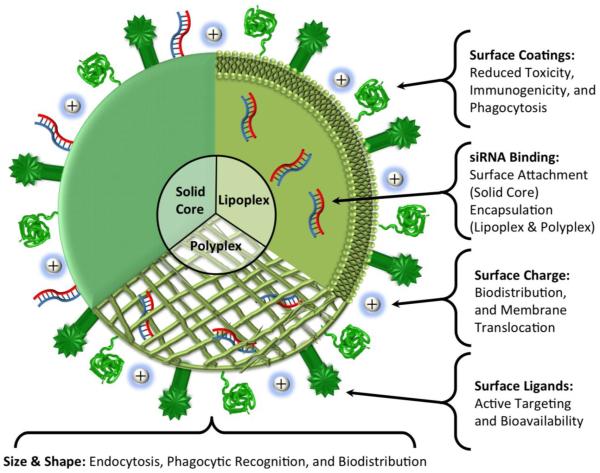
Each of the variety of delivery vehicle types has unique characteristics that are advantageous for particular applications. However, some vehicle types may not perform as well as others in our case of targeting HCCs. Carbon nanotubes are readily phagocytosed [195]. Ceramic, magnetic, and metallic delivery vehicles have issues with long term accumulation and cytotoxicity [198,201]. Thus, the delivery vehicles most likely to be successful for this application are lipoplexes or polyplexes (Figure 4) [207,235,239,240].
Figure 4.
Design characteristics of vehicle-siRNA complexes. A variety of characteristics can be manipulated to improve the bioavailability and biodistribution of vehicle-siRNA complexes while reducing their cytotoxicity and immunogenicity.
Further comparison of these two platforms can be based on available experimental data, in particular published results from clinical trials. One type of lipoplex, stable nucleic acid lipid particles (SNALPs), is currently being used in 6 of 10 clinical trials for systemic delivery of siRNAs and has achieved therapeutic efficacy at low doses in vivo (ED50 = 0.3 mg/kg) [6,7,190,191,241]. Cytotoxicity and pulmonary inflammation related to SNALPs raise concerns for their therapeutic application and has been the result of clinical trials being terminated [242,243]. Polyplexes are currently being applied in one clinical trial for the systemic delivery of siRNA with therapeutic effects at ED50 = 2 mg/kg in vivo and TD50 = 27 mg/kg in vivo [244–246]. Recent studies have shown that biodegradable bonds within the polymer have further reduced toxicity and improved polyplex residence time due to decreased recognition by phagocytes [187,247–249], even though, no toxicity was observed in clinical trials [244]. The choice between a lipoplex or polyplex platform reduces to the preference of increased delivery efficiency (lipoplexes) or reduced toxicity (polyplexes).
5. Conclusions
Current data permits mechanism-driven design of both siRNAs and their delivery vehicles, at least to some extent. Perhaps one of most exciting aspects of siRNA design is the ability to target so many different disease states once an effective delivery vehicle exists for a given tissue type, as exemplified by the SNALP and polyplex platforms currently in clinical trials. In the future, the “plug and play” nature of these combinations of siRNA and vehicle could potentially support the development of personalized therapeutics on a patient by patient basis. Still more comprehensive methods for siRNA sequence selection and modification will be driven by a more complete understanding of the RNAi mechanism. Continuing advances in vehicle chemistry and careful analysis of vehicle-siRNA complex structures will improve the specificity and efficiency of siRNA therapeutics.
Acknowledgments
Financial support for this work was provided in part by Michigan State University (MSU Foundation, Center for Systems Biology, and MSU Graduate School), the National Institutes of Health (GM079688, GM089866, RR024439, DK081768, DK088251), and the National Science Foundation (CBET 0941055).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. [(accessed on 26 November 2012)];Globocan 2008 v2.0, cancer incidence and mortality worldwide: Iarc cancerbase no. 10. Available online: http://globocan.iarc.fr/
- 2.American cancer society [(accessd on 26 November 2012)];Cancer facts & figures. 2012 Available online: http://www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-figures-2012/
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 4.Zhu AX, Duda DG, Sahani DV, Jain RK. Hcc and angiogenesis: Possible targets and future directions. Nat. Rev. Clin. Oncol. 2011;8:292–301. doi: 10.1038/nrclinonc.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burnett JC, Rossi JJ, Tiemann K. Current progress of sirna/shrna therapeutics in clinical trials. Biotechnol. J. 2011;6:1130–1146. doi: 10.1002/biot.201100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haussecker D. The business of rnai therapeutics in 2012. Mol. Ther. Nucleic Acids. 2012;1:e8. doi: 10.1038/mtna.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei J, Jones J, Kang J, Card A, Krimm M, Hancock P, Pei Y, Ason B, Payson E, Dubinina N, et al. Rna-induced silencing complex-bound small interfering rna is a determinant of rna interference-mediated gene silencing in mice. Mol. Pharmacol. 2011;79:953–963. doi: 10.1124/mol.110.070409. [DOI] [PubMed] [Google Scholar]
- 9.Bartlett DW. Insights into the kinetics of sirna-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34:322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spiller DG, Giles RV, Broughton CM, Grzybowski J, Ruddell CJ, Tidd DM, Clark RE. The influence of target protein half-life on the effectiveness of antisense oligonucleotide analog-mediated biologic responses. Antisense Nucleic Acid Drug Dev. 1998;8:281–293. doi: 10.1089/oli.1.1998.8.281. [DOI] [PubMed] [Google Scholar]
- 11.Larsson E, Sander C, Marks D. Mrna turnover rate limits sirna and microrna efficacy. Mol. Syst. Biol. 2010;6:1–9. doi: 10.1038/msb.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy S, Wang D, Ruvkun G. A conserved sirna-degrading rnase negatively regulates rna interference in c. Elegans. Nature. 2004;427:645–649. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- 13.Bian Y, Zhou W, Zhao Y, Li X, Geng W, Hao R, Yang Q, Huang W. High-dose sirnas upregulate mouse eri-1 at both transcription and posttranscription levels. PLoS ONE. 2011;6:e26466. doi: 10.1371/journal.pone.0026466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caffrey DR, Zhao J, Song Z, Schaffer ME, Haney SA, Subramanian RR, Seymour AB, Hughes JD. Sirna off-target effects can be reduced at concentrations that match their individual potency. PLoS ONE. 2011;6:e21503. doi: 10.1371/journal.pone.0021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calera MR, Venkatakrishnan A, Kazlauskas A. Ve-cadherin increases the half-life of vegf receptor 2. Exp. Cell Res. 2004;300:248–256. doi: 10.1016/j.yexcr.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Schlessinger J. The epidermal growth factor receptor as a multifunctional allosteric protein. Biochemistry. 1988;27:3119–3123. doi: 10.1021/bi00409a002. [DOI] [PubMed] [Google Scholar]
- 17.Dhut S, Chaplin T, Young BD. Bcr-abl and bcr proteins: Biochemical characterization and localization. Leukemia. 1990;4:745–750. [PubMed] [Google Scholar]
- 18.Wang PY, Rao JN, Zou T, Liu L, Xiao L, Yu TX, Turner DJ, Gorospe M, Wang JY. Post-transcriptional regulation of mek-1 by polyamines through the rna-binding protein hur modulating intestinal epithelial apoptosis. Biochem. J. 2010;426:293–306. doi: 10.1042/BJ20091459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keating MT, Williams LT. Processing of the platelet-derived growth factor receptor. Biosynthetic and degradation studies using anti-receptor antibodies. J. Biol. Chem. 1987;262:7932–7937. [PubMed] [Google Scholar]
- 20.Yang E, van Nimwegen E, Zavolan M, Rajewsky N, Schroeder M, Magnasco M, Darnell JE. Decay rates of human mrnas: Correlation with functional characteristics and sequence attributes. Genome Res. 2003;13:1863–1872. doi: 10.1101/gr.1272403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational sirna design for rna interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by rnai. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 24.Naito Y, Ui-Tei K. Sirna design software for a target gene-specific rna interference. Front. Genet. 2012;3:102. doi: 10.3389/fgene.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of rna interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 26.Zamore PD, Tuschl T, Sharp PA, Bartel DP. Rnai: Double-stranded rna directs the atp-dependent cleavage of mrna at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 27.Nykänen A, Haley B, Zamore PD. Atp requirements and small interfering rna structure in the rna interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 28.Elbashir SM, Martinez J, Patkaniowska A, Lendeckel W, Tuschl T. Functional anatomy of sirnas for mediating efficient rnai in drosophila melanogaster embryo lysate. EMBO J. 2001;20:6877–6888. doi: 10.1093/emboj/20.23.6877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima WF, Murray H, Nichols JG, Wu H, Sun H, Prakash TP, Berdeja AR, Gaus HJ, Crooke ST. Human dicer binds short single-strand and double-strand rna with high affinity and interacts with different regions of the nucleic acids. J. Biol. Chem. 2009;284:2535–2548. doi: 10.1074/jbc.M803748200. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai K, Amarzguioui M, Kim D, Alluin J, Heale B, Song M, Gatignol A, Behlke MA, Rossi JJ. A role for human dicer in pre-risc loading of sirnas. Nucleic Acids Res. 2011;39:1510–1525. doi: 10.1093/nar/gkq846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu QH, Rand TA, Kalidas S, Du FH, Kim HE, Smith DP, Wang XD. R2d2, a bridge between the initiation and effector steps of the drosophila rnai pathway. Science. 2003;301:1921–1925. doi: 10.1126/science.1088710. [DOI] [PubMed] [Google Scholar]
- 32.Tomari Y, Du T, Haley B, Schwarz D, Bennett R, Cook H, Koppetsch B, Theurkauf W, Zamore P. Risc assembly defects in the drosophila rnai mutant armitage. Cell. 2004;116:831–841. doi: 10.1016/s0092-8674(04)00218-1. [DOI] [PubMed] [Google Scholar]
- 33.Tomari Y, Matranga C, Haley B, Martinez N, Zamore PD. A protein sensor for sirna asymmetry. Science. 2004;306:1377–1380. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 34.Chendrimada T, Gregory R, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. Trbp recruits the dicer complex to ago2 for microrna processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammond SM, Bernstein E, Beach D, Hannon GJ. An rna-directed nuclease mediates post-transcriptional gene silencing in drosophila cells. Nature. 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 36.MacRae IJ, Ma E, Zhou M, Robinson CV, Doudna JA. In vitro reconstitution of the human risc-loading complex. Proc. Natl. Acad. Sci. USA. 2008;105:512–517. doi: 10.1073/pnas.0710869105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gredell JA, Dittmer MJ, Wu M, Chan C, Walton SP. Recognition of sirna asymmetry by tar rna binding protein. Biochemistry. 2010;49:3148–3155. doi: 10.1021/bi902189s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noland CL, Ma E, Doudna JA. Sirna repositioning for guide strand selection by human dicer complexes. Mol. Cell. 2011;43:110–121. doi: 10.1016/j.molcel.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khvorova A, Reynolds A, Jayasena SD. Functional sirnas and mirnas exhibit strand bias. Cell. 2003;115:209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz D, Hutvagner G, Du T, Xu Z, Aronin N, Zamore P. Asymmetry in the assembly of the rnai enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton A, Baulcombe D. A species of small antisense rna in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 42.Elbashir SM, Lendeckel W, Tuschl T. Rna interference is mediated by 21-and 22-nucleotide rnas. Genes Dev. 2001;15:188–200. doi: 10.1101/gad.862301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rand TA, Petersen S, Du F, Wang X. Argonaute2 cleaves the anti-guide strand of sirna during risc activation. Cell. 2005;123:621–629. doi: 10.1016/j.cell.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Leuschner PJF, Ameres SL, Kueng S, Martinez J. Cleavage of the sirna passenger strand during risc assembly in human cells. EMBO Rep. 2006;7:314–320. doi: 10.1038/sj.embor.7400637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matranga C, Tomari Y, Shin C, Bartel D, Zamore P. Passenger-strand cleavage facilitates assembly of sirna into ago2-containing rnai enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 46.Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, Tomari Y. Atp-dependent human risc assembly pathways. Nat. Struct. Mol. Biol. 2010;17:17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rivas FV, Tolia NH, Song JJ, Aragon JP, Liu JD, Hannon GJ, Joshua-Tor L. Purified argonaute2 and an sirna form recombinant human risc. Nat. Struct. Mol. Biol. 2005;12:340–349. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 48.Haley B, Zamore PD. Kinetic analysis of the rnai enzyme complex. Nat. Struct. Mol. Biol. 2004;11:599–606. doi: 10.1038/nsmb780. [DOI] [PubMed] [Google Scholar]
- 49.Liu JD, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian rnai. Sci. Signal. 2004;305:1437–1437. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 50.Snead NM, Rossi JJ. Biogenesis and function of endogenous and exogenous sirnas. Wiley Interdiscip. Rev. RNA. 2010;1:117–131. doi: 10.1002/wrna.14. [DOI] [PubMed] [Google Scholar]
- 51.Carthew RW, Sontheimer EJ. Origins and mechanisms of mirnas and sirnas. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microrna/short hairpin rna pathways. Nature. 2006;441:537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 53.Frank F, Sonenberg N, Nagar B. Structural basis for 5'-nucleotide base-specific recognition of guide rna by human ago2. Nature. 2010;465:818–822. doi: 10.1038/nature09039. [DOI] [PubMed] [Google Scholar]
- 54.Walton SP, Wu M, Gredell JA, Chan C. Designing highly active sirnas for therapeutic applications. FEBS J. 2010;277:4806–4813. doi: 10.1111/j.1742-4658.2010.07903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Betancur JG, Tomari Y. Dicer is dispensable for asymmetric risc loading in mammals. RNA. 2012;18:24–30. doi: 10.1261/rna.029785.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutvagner G. Small rna asymmetry in rnai: Function in risc assembly and gene regulation. FEBS Lett. 2005;579:5850–5857. doi: 10.1016/j.febslet.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 57.Lu ZJ, Mathews DH. Efficient sirna selection using hybridization thermodynamics. Nucleic Acids Res. 2008;36:640–647. doi: 10.1093/nar/gkm920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ui-Tei K, Naito Y, Takahashi F, Haraguchi T, Ohki-Hamazaki H, Juni A, Ueda R, Saigo K. Guidelines for the selection of highly effective sirna sequences for mammalian and chick rna interference. Nucleic Acids Res. 2004;32:936–948. doi: 10.1093/nar/gkh247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jagla B, Aulner N, Kelly PD, Song D, Volchuk A, Zatorski A, Shum D, Mayer T, de Angelis DA, Ouerfelli O, et al. Sequence characteristics of functional sirnas. RNA. 2005;11:864–872. doi: 10.1261/rna.7275905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huesken D, Lange J, Mickanin C, Weiler J, Asselbergs F, Warner J, Meloon B, Engel S, Rosenberg A, Cohen D, et al. Design of a genome-wide sirna library using an artificial neural network. Nat. Biotechnol. 2005;23:995–1001. doi: 10.1038/nbt1118. [DOI] [PubMed] [Google Scholar]
- 61.Ladunga I. More complete gene silencing by fewer sirnas: Transparent optimized design and biophysical signature. Nucleic Acids Res. 2006;35:433–440. doi: 10.1093/nar/gkl1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shabalina SA, Spiridonov AN, Ogurtsov AY. Computational models with thermodynamic and composition features improve sirna design. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amarzguioui M, Prydz H. An algorithm for selection of functional sirna sequences. Biochem. Biophys. Res. Commun. 2004;316:1050–1058. doi: 10.1016/j.bbrc.2004.02.157. [DOI] [PubMed] [Google Scholar]
- 64.Gong W, Ren Y, Xu Q, Wang Y, Lin D, Zhou H, Li T. Integrated sirna design based on surveying of features associated with high rnai effectiveness. BMC Bioinformatics. 2006;7:516. doi: 10.1186/1471-2105-7-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takasaki S, Kotani S, Konagaya A. An effective method for selecting sirna target sequences in mammalian cells. Cell Cycle. 2004;3:788–793. [PubMed] [Google Scholar]
- 66.Holen T. Efficient prediction of sirnas with sirnarules 1.0: An open-source java approach to sirna algorithms. RNA. 2006;12:1620–1625. doi: 10.1261/rna.81006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takasaki S. Selecting effective sirna target sequences by using bayes' theorem. Comput. Biol. Chem. 2009;33:368–372. doi: 10.1016/j.compbiolchem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Katoh T, Suzuki T. Specific residues at every third position of sirna shape its efficient rnai activity. Nucleic Acids Res. 2007;35:e27. doi: 10.1093/nar/gkl1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seitz H, Tushir JS, Zamore PD. A 5'-uridine amplifies mirna/mirna* asymmetry in drosophila by promoting rna-induced silencing complex formation. Silence. 2011;2:4. doi: 10.1186/1758-907X-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown KM, Chu CY, Rana TM. Target accessibility dictates the potency of human risc. Nat. Struct. Mol. Biol. 2005;12:469–470. doi: 10.1038/nsmb931. [DOI] [PubMed] [Google Scholar]
- 71.Ameres SL, Martinez J, Schroeder R. Molecular basis for target rna recognition and cleavage by human risc. Cell. 2007;130:101–112. doi: 10.1016/j.cell.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 72.Mathews D, Sabina J, Zuker M, Turner D. Expanded sequence dependence of thermodynamic parameters improves prediction of rna secondary structure. J. Mol. Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- 73.Ding Y, Chan CY, Lawrence CE. Sfold web server for statistical folding and rational design of nucleic acids. Nucleic Acids Res. 2004;32:W135–W141. doi: 10.1093/nar/gkh449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tafer H, Ameres SL, Obernosterer G, Gebeshuber CA, Schroeder R, Martinez J, Hofacker IL. The impact of target site accessibility on the design of effective sirnas. Nat. Biotechnol. 2008;26:578–583. doi: 10.1038/nbt1404. [DOI] [PubMed] [Google Scholar]
- 75.Gredell J, Berger A, Walton S. Impact of target mrna structure on sirna silencing efficiency: A large-scale study. Biotechnol. Bioeng. 2008;100:744–755. doi: 10.1002/bit.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vickers TA, Koo S, Bennett CF, Crooke ST, Dean NM, Baker BF. Efficient reduction of target rnas by small interfering rna and rnase h-dependent antisense agents. A comparative analysis. J. Biol. Chem. 2003;278:7108–7118. doi: 10.1074/jbc.M210326200. [DOI] [PubMed] [Google Scholar]
- 77.Bohula EA, Salisbury AJ, Sohail M, Playford MP, Riedemann J, Southern EM, Macaulay VM. The efficacy of small interfering rnas targeted to the type 1 insulin-like growth factor receptor (igf1r) is influenced by secondary structure in the igf1r transcript. J. Biol. Chem. 2003;278:15991–15997. doi: 10.1074/jbc.M300714200. [DOI] [PubMed] [Google Scholar]
- 78.Overhoff M, Alken M, Far RK-K, Lemaitre M, Lebleu B, Sczakiel G, Robbins I. Local rna target structure influences sirna efficacy: A systematic global analysis. J. Mol. Biol. 2005;348:871–881. doi: 10.1016/j.jmb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 79.Schubert S, Grünweller A, Erdmann VA, Kurreck J. Local rna target structure influences sirna efficacy: Systematic analysis of intentionally designed binding regions. J. Mol. Biol. 2005;348:883–893. doi: 10.1016/j.jmb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 80.Shao Y, Chan CY, Maliyekkel A, Lawrence CE, Roninson IB, Ding Y. Effect of target secondary structure on rnai efficiency. RNA. 2007;13:1631–1640. doi: 10.1261/rna.546207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoshinari K, Miyagishi M, Taira K. Effects on rnai of the tight structure, sequence and position of the targeted region. Nucleic Acids Res. 2004;32:691–699. doi: 10.1093/nar/gkh221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sledz CA, Holko M, de Veer MJ, Silverman RH, Williams BRG. Activation of the interferon system by short-interfering rnas. Nat. Cell Biol. 2003;5:834–839. doi: 10.1038/ncb1038. [DOI] [PubMed] [Google Scholar]
- 83.Samuel-Abraham S, Leonard JN. Staying on message: Design principles for controlling nonspecific responses to sirna. FEBS J. 2010;277:4828–4836. doi: 10.1111/j.1742-4658.2010.07905.x. [DOI] [PubMed] [Google Scholar]
- 84.Jackson A. Recognizing and avoiding sirna off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010;9:57–67. doi: 10.1038/nrd3010. [DOI] [PubMed] [Google Scholar]
- 85.Robbins M, Judge A, Liang L. 2'-o-methyl-modified rnas act as tlr7 antagonists. Mol. Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- 86.Kodym R, Kodym E, Story MD. 2'-5'-oligoadenylate synthetase is activated by a specific rna sequence motif. Biochem. Biophys. Res. Commun. 2009;388:317–322. doi: 10.1016/j.bbrc.2009.07.167. [DOI] [PubMed] [Google Scholar]
- 87.Manche L, Green SR, Schmedt C, Mathews MB. Interactions between double-stranded rna regulators and the protein kinase dai. Mol. Cell. Biol. 1992;12:5238–5248. doi: 10.1128/mcb.12.11.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bevilacqua PC, Cech TR. Minor-groove recognition of double-stranded rna by the double-stranded rna-binding domain from the rna-activated protein kinase pkr. Biochemistry. 1996;35:9983–9994. doi: 10.1021/bi9607259. [DOI] [PubMed] [Google Scholar]
- 89.Marques JT, Devosse T, Wang D, Zamanian-Daryoush M, Serbinowski P, Hartmann R, Fujita T, Behlke MA, Williams BR. A structural basis for discriminating between self and nonself double-stranded rnas in mammalian cells. Nat. Biotechnol. 2006;24:559–565. doi: 10.1038/nbt1205. [DOI] [PubMed] [Google Scholar]
- 90.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-i and melanoma differentiation-associated gene 5. J. Exp. Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gantier MP, Williams BRG. The response of mammalian cells to double-stranded rna. Cytokine Growth Factor Rev. 2007;18:363–371. doi: 10.1016/j.cytogfr.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nallagatla SR, Hwang J, Toroney R, Zheng X, Cameron CE, Bevilacqua PC. 5'-triphosphate-dependent activation of pkr by rnas with short stem-loops. Science. 2007;318:1455–1458. doi: 10.1126/science.1147347. [DOI] [PubMed] [Google Scholar]
- 93.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 94.Weber C, Müller C, Podszuweit A, Montino C, Vollmer J, Forsbach A. Toll-like receptor (tlr) 3 immune modulation by unformulated small interfering rna or DNA and the role of cd14 (in tlr-mediated effects) Immunology. 2012;136:64–77. doi: 10.1111/j.1365-2567.2012.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded rna and activation of nf-kappab by toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 96.Kariko K, Bhuyan P, Capodici J, Weissman D. Small interfering rnas mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J. Immunol. 2004;172:6545–6549. doi: 10.4049/jimmunol.172.11.6545. [DOI] [PubMed] [Google Scholar]
- 97.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded rna via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 98.Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic sirna. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 99.Jackson AL, Burchard J, Leake D, Reynolds A, Schelter J, Guo J, Johnson JM, Lim L, Karpilow J, Nichols K, et al. Position-specific chemical modification of sirnas reduces “off-target” transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of tlr7-mediated recognition of single-stranded rna. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 101.Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C. Nucleic acid agonists for toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur. J. Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- 102.Goodchild A, Nopper N, King A, Doan T, Tanudji M, Arndt GM, Poidinger M, Rivory LP, Passioura T. Sequence determinants of innate immune activation by short interfering rnas. BMC Immunol. 2009;10 doi: 10.1186/1471-2172-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJC, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Karikó K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by sirna via tlr3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reynolds A, Anderson EM, Vermeulen A, Fedorov Y, Robinson K, Leake D, Karpilow J, Marshall WS, Khvorova A. Induction of the interferon response by sirna is cell type- and duplex length-dependent. RNA. 2006;12:988–993. doi: 10.1261/rna.2340906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forsbach A, Nemorin J-G, Montino C, Müller C, Samulowitz U, Vicari AP, Jurk M, Mutwiri GK, Krieg AM, Lipford GB, Vollmer J. Identification of rna sequence motifs stimulating sequence-specific tlr8-dependent immune responses. J. Immunol. 2008;180:3729–3738. doi: 10.4049/jimmunol.180.6.3729. [DOI] [PubMed] [Google Scholar]
- 106.Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded sirnas is sequence-dependent and requires endosomal localization. J. Mol. Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 107.Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of ifn-α by short interfering rna in plasmacytoid dendritic cells through tlr7. Nat. Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 108.Jurk M, Chikh G, Schulte B, Kritzler A, Richardt-Pargmann D, Lampron C, Luu R, Krieg AM, Vicari AP, Vollmer J. Immunostimulatory potential of silencing rnas can be mediated by a non-uridine-rich toll-like receptor 7 motif. Nucleic Acid Ther. 2011;21:201–214. doi: 10.1089/nat.2011.0298. [DOI] [PubMed] [Google Scholar]
- 109.Fedorov Y, Anderson EM, Birmingham A, Reynolds A, Karpilow J, Robinson K, Leake D, Marshall WS, Khvorova A. Off-target effects by sirna can induce toxic phenotype. RNA. 2006;12:1188–1196. doi: 10.1261/rna.28106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shafer RH, Smirnov I. Biological aspects of DNA/rna quadruplexes. Biopolymers. 2000;56:209–227. doi: 10.1002/1097-0282(2000/2001)56:3<209::AID-BIP10018>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 111.Doench JG, Petersen CP, Sharp PA. Sirnas can function as mirnas. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lambert NJ, Gu SG, Zahler AM. The conformation of microrna seed regions in native micrornps is prearranged for presentation to mrna targets. Nucleic Acids Res. 2011;39:4827–4835. doi: 10.1093/nar/gkr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gu S, Jin L, Zhang F, Huang Y, Grimm D, Rossi JJ, Kay MA. Thermodynamic stability of small hairpin rnas highly influences the loading process of different mammalian argonautes. Proc. Natl. Acad. Sci. U. S. A. 2011;108:9208–9213. doi: 10.1073/pnas.1018023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bartel D. Micrornas: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microrna targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 116.Lin X, Ruan X, Anderson MG, Mcdowell JA, Kroeger PE, Fesik SW, Shen Y. Sirna-mediated off-target gene silencing triggered by a 7 nt complementation. Nucleic Acids Res. 2005;33:4527. doi: 10.1093/nar/gki762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai EC. Micro rnas are complementary to 3 ' utr sequence motifs that mediate negative post-transcriptional regulation. Nat. Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 118.Schultz N, Marenstein DR, De Angelis DA, Wang W-Q, Nelander S, Jacobsen A, Marks DS, Massagué J, Sander C. Off-target effects dominate a large-scale rnai screen for modulators of the tgf-β pathway and reveal microrna regulation of tgfbr2. Silence. 2011;2:3. doi: 10.1186/1758-907X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Didiano D, Hobert O. Perfect seed pairing is not a generally reliable predictor for mirna-target interactions. Nat. Struct. Mol. Biol. 2006;13:849–851. doi: 10.1038/nsmb1138. [DOI] [PubMed] [Google Scholar]
- 120.Doench JG, Sharp PA. Specificity of microrna target selection in translational repression. Genes Dev. 2004;18:504–511. doi: 10.1101/gad.1184404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Broderick JA, Salomon WE, Ryder SP, Aronin N, Zamore PD. Argonaute protein identity and pairing geometry determine cooperativity in mammalian rna silencing. RNA. 2011;17:1858–1869. doi: 10.1261/rna.2778911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kozomara A, Griffiths-Jones S. Mirbase: Integrating microrna annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sigoillot FD, Lyman S, Huckins JF, Adamson B, Chung E, Quattrochi B, King RW. A bioinformatics method identifies prominent off-targeted transcripts in rnai screens. Nat. Methods. 2012;9:363–366. doi: 10.1038/nmeth.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sudbery I, Enright AJ, Fraser AG, Dunham I. Systematic analysis of off-target effects in an rnai screen reveals micrornas affecting sensitivity to trail-induced apoptosis. BMC Genomics. 2010;11:175. doi: 10.1186/1471-2164-11-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anderson EM, Birmingham A, Baskerville S, Reynolds A, Maksimova E, Leake D, Fedorov Y, Karpilow J, Khvorova A. Experimental validation of the importance of seed complement frequency to sirna specificity. RNA. 2008;14:853–861. doi: 10.1261/rna.704708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Snove O, Holen T. Many commonly used sirnas risk off-target activity. Biochem. Biophys. Res. Commun. 2004;319:256–263. doi: 10.1016/j.bbrc.2004.04.175. [DOI] [PubMed] [Google Scholar]
- 127.Holen T, Moe SE, Sorbo JG, Meza TJ, Ottersen OP, Klungland A. Tolerated wobble mutations in sirnas decrease specificity, but can enhance activity in vivo. Nucleic Acids Res. 2005;33:4704–4710. doi: 10.1093/nar/gki785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwarz DS, Ding H, Kennington L, Moore JT, Schelter J, Burchard J, Linsley PS, Aronin N, Xu Z, Zamore PD. Designing sirna that distinguish between genes that differ by a single nucleotide. PLoS Genet. 2006;2:1307–1318. doi: 10.1371/journal.pgen.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jackson AL, Burchard J, Schelter J, Chau BN, Cleary M, Lim L, Linsley PS. Widespread sirna “off-target” transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saxena S, Jónsson ZO, Dutta A. Small rnas with imperfect match to endogenous mrna repress translation. Implications for off-target activity of small inhibitory rna in mammalian cells. J. Biol. Chem. 2003;278:44312–44319. doi: 10.1074/jbc.M307089200. [DOI] [PubMed] [Google Scholar]
- 131.Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, Wolfsberg TG, Umayam L, Lee JC, Hughes CM, Shanmugam KS, Bhattacharjee A, Meyerson M. Short interfering rnas can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc. Natl. Acad. Sci. USA. 2004;101:1892–1897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aleman LM, Doench J, Sharp PA. Comparison of sirna-induced off-target rna and protein effects. RNA. 2007;13:385–395. doi: 10.1261/rna.352507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. Microrna-dependent localization of targeted mrnas to mammalian p-bodies. Nat. Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teixeira D, Sheth U, Valencia-Sanchez MA, Brengues M, Parker R. Processing bodies require rna for assembly and contain nontranslating mrnas. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Behm-Ansmant I, Rehwinkel J, Izaurralde E. Micrornas silence gene expression by repressing protein expression and/or by promoting mrna decay. Cold Spring Harb. Symp. Quant. Biol. 2006;71:523–530. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 136.Semizarov D, Frost L, Sarthy A, Kroeger P, Halbert DN, Fesik SW. Specificity of short interfering rna determined through gene expression signatures. Proc. Natl. Acad. Sci. USA. 2003;100:6347–6352. doi: 10.1073/pnas.1131959100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Persengiev SP, Zhu X, Green MR. Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering rnas (sirnas) RNA. 2004;10:12–18. doi: 10.1261/rna5160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wilson PA, Plucinski M. A simple bayesian estimate of direct rnai gene regulation events from differential gene expression profiles. BMC Genomics. 2011;12:250. doi: 10.1186/1471-2164-12-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sydor JR, Nock S. Protein expression profiling arrays: Tools for the multiplexed high-throughput analysis of proteins. Proteome Sci. 2003;1:3. doi: 10.1186/1477-5956-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pan S, Aebersold R, Chen R, Rush J, Goodlett DR, McIntosh MW, Zhang J, Brentnall TA. Mass spectrometry based targeted protein quantification: Methods and applications. J. Proteome Res. 2009;8:787–797. doi: 10.1021/pr800538n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Huang H, Qiao R, Zhao D, Zhang T, Li Y, Yi F, Lai F, Hong J, Ding X, Yang Z, et al. Profiling of mismatch discrimination in rnai enabled rational design of allele-specific sirnas. Nucleic Acids Res. 2009;37:7560–7569. doi: 10.1093/nar/gkp835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kittler R, Surendranath V, Heninger A-K, Slabicki M, Theis M, Putz G, Franke K, Caldarelli A, Grabner H, Kozak K, et al. Genome-wide resources of endoribonuclease-prepared short interfering rnas for specific loss-of-function studies. Nat. Methods. 2007;4:337–344. doi: 10.1038/nmeth1025. [DOI] [PubMed] [Google Scholar]
- 143.Parker JS, Parizotto EA, Wang M, Roe SM, Barford D. Enhancement of the seed-target recognition step in rna silencing by a piwi/mid domain protein. Mol. Cell. 2009;33:204–214. doi: 10.1016/j.molcel.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Birmingham A, Anderson EM, Reynolds A, Ilsley-Tyree D, Leake D, Fedorov Y, Baskerville S, Maksimova E, Robinson K, Karpilow J, Marshall WS, Khvorova A. 3' UTR seed matches, but not overall identity, are associated with rnai off-targets. Nat. Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- 145.Boland A, Tritschler F, Heimst Auml Dt S, Izaurralde E, Weichenrieder O. Crystal structure and ligand binding of the mid domain of a eukaryotic argonaute protein. EMBO Rep. 2010;11:522–527. doi: 10.1038/embor.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ma J-B, Ye K, Patel DJ. Structural basis for overhang-specific small interfering rna recognition by the paz domain. Nature. 2004;429:318–322. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lingel A, Simon B, Izaurralde E, Sattler M. Nucleic acid 3'-end recognition by the argonaute2 paz domain. Nat. Struct. Mol. Biol. 2004;11:576–577. doi: 10.1038/nsmb777. [DOI] [PubMed] [Google Scholar]
- 148.Sashital DG, Doudna JA. Structural insights into rna interference. Curr. Opin. Struct. Biol. 2010;20:90–97. doi: 10.1016/j.sbi.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vert J-P, Foveau N, Lajaunie C, Vandenbrouck Y. An accurate and interpretable model for sirna efficacy prediction. BMC Bioinformatics. 2006;7:520. doi: 10.1186/1471-2105-7-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Patzel V, Rutz S, Dietrich I, Köberle C, Scheffold A, Kaufmann SHE. Design of sirnas producing unstructured guide-rnas results in improved rna interference efficiency. Nat. Biotechnol. 2005;23:1440–1444. doi: 10.1038/nbt1151. [DOI] [PubMed] [Google Scholar]
- 151.Köberle C, Kaufmann SHE, Patzel V. Selecting effective sirnas based on guide rna structure. Nat. Protoc. 2006;1:1832–1839. doi: 10.1038/nprot.2006.206. [DOI] [PubMed] [Google Scholar]
- 152.Hossbach M, Gruber J, Osborn M, Weber K, Tuschl T. Gene silencing with sirna duplexes composed of target-mrna-complementary and partially palindromic or partially complementary single-stranded sirnas. RNA Biol. 2006;3:82–89. doi: 10.4161/rna.3.2.3110. [DOI] [PubMed] [Google Scholar]
- 153.Vermeulen A, Behlen L, Reynolds A, Wolfson A, Marshall WS, Karpilow J, Khvorova A. The contributions of dsrna structure to dicer specificity and efficiency. RNA. 2005;11:674–682. doi: 10.1261/rna.7272305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sciabola S, Cao Q, Orozco M, Faustino I, Stanton RV. Improved nucleic acid descriptors for sirna efficacy prediction. Nucleic Acids Res. 2012 doi: 10.1093/nar/gks1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Snead NM, Rossi JJ. Rna interference trigger variants: Getting the most out of rna for rna interference-based therapeutics. Nucleic Acid Ther. 2012;22:139–146. doi: 10.1089/nat.2012.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T. Single-stranded antisense sirnas guide target rna cleavage in rnai. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 157.Holen T, Amarzguioui M, Babaie E, Prydz H. Similar behaviour of single-strand and double-strand sirnas suggests they act through a common rnai pathway. Nucleic Acids Res. 2003;31:2401–2407. doi: 10.1093/nar/gkg338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lima WF, Prakash TP, Murray HM, Kinberger GA, Li W, Chappell AE, Li CS, Murray SF, Gaus H, Seth PP, et al. Single-stranded sirnas activate rnai in animals. Cell. 2012;150:883–894. doi: 10.1016/j.cell.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 159.Haringsma HJ, Li JJ, Soriano F, Kenski DM, Flanagan WM, Willingham AT. Mrna knockdown by single strand rna is improved by chemical modifications. Nucleic Acids Res. 2012;40:4125–4136. doi: 10.1093/nar/gkr1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chu C-Y, Rana TM. Potent rnai by short rna triggers. RNA. 2008;14:1714–1719. doi: 10.1261/rna.1161908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun X, Rogoff HA, Li CJ. Asymmetric rna duplexes mediate rna interference in mammalian cells. Nat. Biotechnol. 2008;26:1379–1382. doi: 10.1038/nbt.1512. [DOI] [PubMed] [Google Scholar]
- 162.Chang CI, Yoo JW, Hong SW, Lee SE, Kang HS, Sun X, Rogoff HA, Ban C, Kim S, Li CJ, et al. Asymmetric shorter-duplex sirna structures trigger efficient gene silencing with reduced nonspecific effects. Mol. Ther. 2009;17:725–732. doi: 10.1038/mt.2008.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hohjoh H. Enhancement of rnai activity by improved sirna duplexes. FEBS Lett. 2004;557:193–198. doi: 10.1016/s0014-5793(03)01492-3. [DOI] [PubMed] [Google Scholar]
- 164.Bramsen JB, Laursen MB, Damgaard CK, Lena SW, Babu BR, Wengel J, Kjems J. Improved silencing properties using small internally segmented interfering rnas. Nucleic Acids Res. 2007;35:5886–5897. doi: 10.1093/nar/gkm548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dua P, Yoo JW, Kim S, Lee D-K. Modified sirna structure with a single nucleotide bulge overcomes conventional sirna-mediated off-target silencing. Mol. Ther. 2011;19:1676–1687. doi: 10.1038/mt.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Amarzguioui M, Lundberg P, Cantin E, Hagstrom J, Behlke MA, Rossi JJ. Rational design and in vitro and in vivo delivery of dicer substrate sirna. Nat. Protoc. 2006;1:508–517. doi: 10.1038/nprot.2006.72. [DOI] [PubMed] [Google Scholar]
- 167.Collingwood MA, Rose SD, Huang L, Hillier C, Amarzguioui M, Wiiger MT, Soifer HS, Rossi JJ, Behlke MA. Chemical modification patterns compatible with high potency dicer-substrate small interfering rnas. Oligonucleotides. 2008;18:187–200. doi: 10.1089/oli.2008.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tanudji M, Machalek D, Arndt GM, Rivory L. Competition between sirna duplexes: Impact of rna-induced silencing complex loading efficiency and comparison between conventional-21 bp and dicer-substrate sirnas. Oligonucleotides. 2010;20:27–32. doi: 10.1089/oli.2009.0195. [DOI] [PubMed] [Google Scholar]
- 169.Foster DJ, Barros S, Duncan R, Shaikh S, Cantley W, Dell A, Bulgakova E, O'Shea J, Taneja N, Kuchimanchi S, et al. Comprehensive evaluation of canonical versus dicer-substrate sirna in vitro and in vivo. RNA. 2012;18:557–568. doi: 10.1261/rna.031120.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Turner JJ, Jones SW, Moschos SA, Lindsay MA, Gait MJ. Maldi-tof mass spectral analysis of sirna degradation in serum confirms an rnase a-like activity. Mol. Biosyst. 2006;3:43–50. doi: 10.1039/b611612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Volkov AA, Kruglova N.y.S., Meschaninova MI, Venyaminova AG, Zenkova MA, Vlassov VV, Chernolovskaya EL. Selective protection of nuclease-sensitive sites in sirna prolongs silencing effect. Oligonucleotides. 2009;19:191–202. doi: 10.1089/oli.2008.0162. [DOI] [PubMed] [Google Scholar]
- 172.Hong J, Huang Y, Li J, Yi F, Zheng J, Huang H, Wei N, Shan Y, An M, Zhang H, et al. Comprehensive analysis of sequence-specific stability of sirna. FASEB J. 2010;24:4844–4855. doi: 10.1096/fj.09-142398. [DOI] [PubMed] [Google Scholar]
- 173.Allerson CR, Sioufi N, Jarres R, Prakash TP, Naik N, Berdeja A, Wanders L, Griffey RH, Swayze EE, Bhat B. Fully 2'-modified oligonucleotide duplexes with improved in vitro potency and stability compared to unmodified small interfering rna. J. Med. Chem. 2005;48:901–904. doi: 10.1021/jm049167j. [DOI] [PubMed] [Google Scholar]
- 174.Bramsen JB, Laursen MB, Nielsen AF, Hansen TB, Bus C, Langkjaer N, Babu BR, Hojland T, Abramov M, Van Aerschot A, Odadzic D, et al. A large-scale chemical modification screen identifies design rules to generate sirnas with high activity, high stability and low toxicity. Nucleic Acids Res. 2009;37:2867–2881. doi: 10.1093/nar/gkp106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kenski DM, Butora G, Willingham AT, Cooper AJ, Fu W, Qi N, Soriano F, Davies IW, Flanagan WM. Sirna-optimized modifications for enhanced in vivo activity. Mol. Ther. Nucleic Acids. 2012;1:e5. doi: 10.1038/mtna.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Braasch DA, Jensen S, Liu Y, Kaur K, Arar K, White MA, Corey DR. Rna interference in mammalian cells by chemically-modified rna. Biochemistry. 2003;42:7967–7975. doi: 10.1021/bi0343774. [DOI] [PubMed] [Google Scholar]
- 177.Chiu Y-L, Rana TM. Sirna function in rnai: A chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Manoharan M, Akinc A, Pandey RK, Qin J, Hadwiger P, John M, Mills K, Charisse K, Maier MA, Nechev L, et al. Unique gene-silencing and structural properties of 2'-fluoro-modified sirnas. Angew. Chem. Int. Ed. Engl. 2011;50:2284–2288. doi: 10.1002/anie.201006519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cekaite L, Furset G, Hovig E, Sioud M. Gene expression analysis in blood cells in response to unmodified and 2-modified sirnas reveals tlr-dependent and independent effects. J. Mol. Biol. 2007;365:90–108. doi: 10.1016/j.jmb.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 180.Tluk S, Jurk M, Forsbach A, Weeratna R, Samulowitz U, Krieg AM, Bauer S, Vollmer J. Sequences derived from self-rna containing certain natural modifications act as suppressors of rna-mediated inflammatory immune responses. Int. Immunol. 2009;21:607–619. doi: 10.1093/intimm/dxp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fucini RV, Haringsma HJ, Deng P, Flanagan WM, Willingham AT. Adenosine modification may be preferred for reducing sirna immune stimulation. Nucleic Acid Ther. 2012;22:205–210. doi: 10.1089/nat.2011.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Haupenthal J, Baehr C, Kiermayer S, Zeuzem S, Piiper A. Inhibition of rnase a family enzymes prevents degradation and loss of silencing activity of sirnas in serum. Biochem. Pharmacol. 2006;71:702–710. doi: 10.1016/j.bcp.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 183.Amarzguioui M. Tolerance for mutations and chemical modifications in a sirna. Nucleic Acids Res. 2003;31:589–595. doi: 10.1093/nar/gkg147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang X, Sierant M, Janicka M, Peczek L, Martinez C, Hassell T, Li N, Li X, Wang T, Nawrot B. Gene silencing activity of sirna molecules containing phosphorodithioate substitutions. ACS Chem. Biol. 2012;7:1214–1220. doi: 10.1021/cb300078e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bramsen JB, Kjems J. Development of therapeutic-grade small interfering rnas by chemical engineering. Front. Genet. 2012;3:154–154. doi: 10.3389/fgene.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang J, Byrne JD, Napier ME, DeSimone JM. More effective nanomedicines through particle design. Small. 2011;7:1919–1931. doi: 10.1002/smll.201100442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Elsabahy M, Wooley KL. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Siegwart DJ, Whitehead KA, Nuhn L, Sahay G, Cheng H, Jiang S, Ma M, Lytton-Jean A, Vegas A, Fenton P, et al. Combinatorial synthesis of chemically diverse core-shell nanoparticles for intracellular delivery. Proc. Natl. Acad. Sci. USA. 2011;108:12996–13001. doi: 10.1073/pnas.1106379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ulery BD, Nair LS, Laurencin CT. Biomedical applications of biodegradable polymers. J. Polym. Sci. B Polym. Phys. 2011;49:832–864. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Whitehead KA, Sahay G, Li GZ, Love KT, Alabi CA, Ma M, Zurenko C, Querbes W, Langer RS, Anderson DG. Synergistic silencing: Combinations of lipid-like materials for efficacious sirna delivery. Mol. Ther. 2011;19:1688–1694. doi: 10.1038/mt.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DWY, Stebbing D, Crosley EJ, Yaworski E, et al. Rational design of cationic lipids for sirna delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 192.Malam Y, Loizidou M, Seifalian AM. Liposomes and nanoparticles: Nanosized vehicles for drug delivery in cancer. Trends Pharmacol. Sci. 2009;30:592–599. doi: 10.1016/j.tips.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 193.Mutlu GM, Budinger GRS, Green AA, Urich D, Soberanes S, Chiarella SE, Alheid GF, McCrimmon DR, Szleifer I, Hersam MC. Biocompatible nanoscale dispersion of single-walled carbon nanotubes minimizes in vivo pulmonary toxicity. Nano Lett. 2010;10:1664–1670. doi: 10.1021/nl9042483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cheung W, Pontoriero F, Taratula O, Chen AM, He HX. DNA and carbon nanotubes as medicine. Adv. Drug Deliv. Rev. 2010;62:633–649. doi: 10.1016/j.addr.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 195.Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008;5:487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li Y, Sun L, Jin M, Du Z, Liu X, Guo C, Li Y, Huang P, Sun Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma hepg2 cells. Toxicol. In Vitro. 2011;25:1343–1352. doi: 10.1016/j.tiv.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 197.Slowing II, Trewyn BG, Giri S, Lin VSY. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007;17:1225–1236. [Google Scholar]
- 198.Asefa T, Tao Z. Biocompatibility of mesoporous silica nanoparticles. Chem. Res. Toxicol. 2012;25:2265–2284. doi: 10.1021/tx300166u. [DOI] [PubMed] [Google Scholar]
- 199.Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, Bohm IB, Heverhagen JT, Prosperi D, Parak WJ. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012;41:4306–4334. doi: 10.1039/c2cs15337h. [DOI] [PubMed] [Google Scholar]
- 200.Prijic S, Sersa G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol. Oncol. 2011;45:1–16. doi: 10.2478/v10019-011-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40:1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 202.Cho EC, Zhang Q, Xia Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011;6:385–391. doi: 10.1038/nnano.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kong WH, Bae KH, Jo SD, Kim JS, Park TG. Cationic lipid-coated gold nanoparticles as efficient and non-cytotoxic intracellular sirna delivery vehicles. Pharm. Res. 2012;29:362–374. doi: 10.1007/s11095-011-0554-y. [DOI] [PubMed] [Google Scholar]
- 204.Daka A, Peer D. Rnai-based nanomedicines for targeted personalized therapy. Adv. Drug Deliv. Rev. 2012;64:1508–1521. doi: 10.1016/j.addr.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 205.Parveen S, Misra R, Sahoo SK. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 206.Burnett JC, Rossi JJ. Rna-based therapeutics: Current progress and future prospects. Chem. Biol. 2012;19:60–71. doi: 10.1016/j.chembiol.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.De Ilarduya CT, Sun Y, Duezguenes N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 208.De Planque MRR, Aghdaei S, Roose T, Morgan H. Electrophysiological characterization of membrane disruption by nanoparticles. ACS Nano. 2011;5:3599–3606. doi: 10.1021/nn103320j. [DOI] [PubMed] [Google Scholar]
- 209.Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- 210.Ding HM, Tian WD, Ma YQ. Designing nanoparticle translocation through membranes by computer simulations. ACS Nano. 2012;6:1230–1238. doi: 10.1021/nn2038862. [DOI] [PubMed] [Google Scholar]
- 211.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 213.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 214.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 215.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 216.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine. 2012;7:5577–5591. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Goncalves C, Mennesson E, Fuchs R, Gorvel JP, Midoux P, Pichon C. Macropinocytosis of polyplexes and recycling of plasmid via the clathrin-dependent pathway impair the transfection efficiency of human hepatocarcinoma cells. Mol. Ther. 2004;10:373–385. doi: 10.1016/j.ymthe.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 218.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 219.Moghimi SM, Hunter AC, Andresen TL. Factors controlling nanoparticle pharmacokinetics: An integrated analysis and perspective. Annu. Rev. Pharmacol. Toxicol. 2012;52:481–503. doi: 10.1146/annurev-pharmtox-010611-134623. [DOI] [PubMed] [Google Scholar]
- 220.Venturoli D, Rippe B. Ficoll and dextran vs. Globular proteins as probes for testing glomerular permselectivity: Effects of molecular size, shape, charge, and deformability. Am. J. Physiol. Renal Physiol. 2005;288:F605–F613. doi: 10.1152/ajprenal.00171.2004. [DOI] [PubMed] [Google Scholar]
- 221.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 222.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 223.Vonarbourg A, Passirani C, Saulnier P, Benoit JP. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–4373. doi: 10.1016/j.biomaterials.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 224.Doshi N, Mitragotri S. Macrophages recognize size and shape of their targets. PLoS ONE. 2010;5:e10051. doi: 10.1371/journal.pone.0010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm. Res. 2009;26:235–243. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 226.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 228.Galvin P, Thompson D, Ryan KB, McCarthy A, Moore AC, Burke CS, Dyson M, MacCraith BD, Gun'ko YK, Byrne MT, et al. Nanoparticle-based drug delivery: Case studies for cancer and cardiovascular applications. Cell. Mol. Life Sci. 2012;69:389–404. doi: 10.1007/s00018-011-0856-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schipper ML, Iyer G, Koh AL, Cheng Z, Ebenstein Y, Aharoni A, Keren S, Bentolila LA, Li JQ, Rao JH, et al. Particle size, surface coating, and pegylation influence the biodistribution of quantum dots in living mice. Small. 2009;5:126–134. doi: 10.1002/smll.200800003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lemarchand C, Gref R, Couvreur P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004;58:327–341. doi: 10.1016/j.ejpb.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 231.Dufort S, Sancey L, Coll JL. Physico-chemical parameters that govern nanoparticles fate also dictate rules for their molecular evolution. Adv. Drug Deliv. Rev. 2012;64:179–189. doi: 10.1016/j.addr.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 232.Noguchi Y, Wu J, Duncan R, Strohalm J, Ulbrich K, Akaike T, Maeda H. Early phase tumor accumulation of macromolecules: A great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the epr effect in macromolecular therapeutics: A review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 234.Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008;60:1615–1626. doi: 10.1016/j.addr.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 235.Singh S, Sharma A, Robertson GP. Realizing the clinical potential of cancer nanotechnology by minimizing toxicologic and targeted delivery concerns. Cancer Res. 2012;72:5663–5668. doi: 10.1158/0008-5472.CAN-12-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lo A, Lin C-T, Wu H-C. Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol. Cancer Ther. 2008;7:579–589. doi: 10.1158/1535-7163.MCT-07-2359. [DOI] [PubMed] [Google Scholar]
- 237.Yan HB, Tram K. Glycotargeting to improve cellular delivery efficiency of nucleic acids. Glycoconj. J. 2007;24:107–123. doi: 10.1007/s10719-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 238.Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, de Fougerolles T, Maraganore J. A status report on rnai therapeutics. Silence. 2010;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.De Fougerolles AR. Delivery vehicles for small interfering rna in vivo. Hum. Gene Ther. 2008;19:125–132. doi: 10.1089/hum.2008.928. [DOI] [PubMed] [Google Scholar]
- 240.Wu Y, Ho YP, Mao YC, Wang XM, Yu B, Leong KW, Lee LJ. Uptake and intracellular fate of multifunctional nanoparticles: A comparison between lipoplexes and polyplexes via quantum dot mediated forster resonance energy transfer. Mol. Pharm. 2011;8:1662–1668. doi: 10.1021/mp100466m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Strumberg D, Schultheis B, Traugott U, Vank C, Santel A, Keil O, Giese K, Kaufmann J, Drevs J. Phase i clinical development of atu027, a sirna formulation targeting pkn3 in patients with advanced solid tumors. Int. J. Clin. Pharmacol. Ther. 2012;50:76–78. doi: 10.5414/cpp50076. [DOI] [PubMed] [Google Scholar]
- 242.Lonez C, Vandenbranden M, Ruysschaert JM. Cationic liposomal lipids: From gene carriers to cell signaling. Prog. Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 243.Barros SA, Gollob JA. Safety profile of rnai nanomedicines. Adv. Drug Deliv. Rev. 2012;64:1730–1737. doi: 10.1016/j.addr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 244.Davis ME. The first targeted delivery of sirna in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol. Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 245.Heidel JD, Davis ME. Clinical developments in nanotechnology for cancer therapy. Pharm. Res. 2011;28:187–199. doi: 10.1007/s11095-010-0178-7. [DOI] [PubMed] [Google Scholar]
- 246.Rozema DB, Lewis DL, Wakefield DH, Wong SC, Klein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, et al. Dynamic polyconjugates for targeted in vivo delivery of sirna to hepatocytes. Proc. Natl. Acad. Sci. USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 248.Reilly MJ, Larsen JD, Sullivan MO. Histone h3 tail peptides and poly(ethylenimine) have synergistic effects for gene delivery. Mol. Pharm. 2012;9:1031–1040. doi: 10.1021/mp200372s. [DOI] [PubMed] [Google Scholar]
- 249.Zhou J, Liu J, Cheng CJ, Patel TR, Weller CE, Piepmeier JM, Jiang Z, Saltzman WM. Biodegradable poly(amine-co-ester) terpolymers for targeted gene delivery. Nat. Mater. 2012;11:82–90. doi: 10.1038/nmat3187. [DOI] [PMC free article] [PubMed] [Google Scholar]