Abstract
Studies linking meditation and brain structure are still relatively sparse, but the hippocampus is consistently implicated as one of the structures altered in meditation practitioners. To explore hippocampal features in the framework of meditation, we analyzed high-resolution structural magnetic resonance imaging data from 30 long-term meditators and 30 controls, closely matched for sex, age, and handedness. Hippocampal formations were manually traced following established protocols. In addition to calculating left and right hippocampal volumes (global measures), regional variations in surface morphology were determined by measuring radial distances from the hippocampal core to spatially matched surface points (local measures). Left and right hippocampal volumes were larger in meditators than in controls, significantly so for the left hippocampus. The presence and direction of this global effect was confirmed locally by mapping the exact spatial locations of the group differences. Altogether, radial distances were larger in meditators compared to controls, with up to 15% difference. These local effects were observed in several hippocampal regions in the left and right hemisphere though achieved significance primarily in the left hippocampal head. Larger hippocampal dimensions in long-term meditators may constitute part of the underlying neurological substrate for cognitive skills, mental capacities, and/or personal traits associated with the practice of meditation. Alternatively, given that meditation positively affects autonomic regulation and immune activity, altered hippocampal dimensions may be one result of meditation-induced stress reduction. However, given the cross-sectional design, the lack of individual stress measures, and the limited resolution of brain data, the exact underlying neuronal mechanisms remain to be established.
Keywords: brain, hippocampus, mapping, meditator, mindfulness, MRI
INTRODUCTION
Research addressing the link between mindfulness practices and structural brain features is still relatively sparse, but the anatomy of the hippocampus has been repeatedly reported to differ between meditators and nonmeditators. For example, a voxel-based morphometry study detected a larger gray matter (GM) concentration in the right hippocampus in meditators compared to controls [Holzel et al., 2008]. In a region-of-interest (ROI) analysis of a different sample, meditators had larger right hippocampal volumes than controls [Luders et al., 2009b]. These findings, based on structural magnetic resonance imaging (MRI), are complemented by outcomes from a diffusion tensor imaging (DTI) study in an overlapping sample [Luders et al., 2011]. This DTI study revealed larger fractional anisotropy (FA) values in meditators in hippocampal white matter (WM) pathways, such as the left and right uncinate fasciculus (connecting the hippocampus with the orbito-frontal cortex) and the ventral part of the right cingulum bundle (connecting the hippocampus with the cingulate gyrus). While these cross-sectional studies cannot determine causal relationships, a recent longitudinal study contrasting structural MRI scans of novice meditators before and after an 8-week mindfulness training program confirmed actual meditation-induced changes in regions of the left hippocampus [Holzel et al., 2011]. Hippocampal differences between meditators and nonmeditators or actual changes of the hippocampus due to meditation as revealed in structural studies are complemented by outcomes from functional imaging studies. Specifically, experiments using positron emission tomography or functional MRI (fMRI) within samples of novice or expert meditators indicated increased brain activation (compared to baseline) during meditation or mindfulness exercises in left and right hippocampal and parahippocampal regions [Engstrom et al., 2010; Holzel et al., 2007; Lazar et al., 2000; Lou et al., 1999, 2005]. Taken together, these findings seem to suggest that the hippocampus is crucially involved in processes related to meditation. To further explore hippocampal features in the framework of meditation, we combined a traditional ROI-based approach with a surface-based anatomical technique to identify regional hippocampal alterations and visualize group differences at high spatial resolution. Specifically, we set out to examine whether long-term meditation practitioners exhibit greater hippocampal dimensions compared with well-matched controls.
MATERIALS AND METHODS Subjects
Subjects
Our study included 30 meditators and 30 control subjects. Brain scans for the control sample were obtained from the ICBM database of normal adults (http://www.loni.ucla.edu/ICBM/Databases/), and criteria for subject screening and eligibility are detailed elsewhere [Mazziotta et al., 2009]. In contrast, all image data as well as subject-specific information for the meditation sample was newly acquired. The recruitment procedure was initiated by distributing study flyers at meditation centers, by postings on center-specific email lists, or by word of mouth through meditators who had already participated in our study. Interested subjects contacted the laboratory and were subsequently prescreened for eligibility via e-mail or phone. Subjects who met study inclusion criteria were scheduled for a single 2-h appointment on the UCLA campus. The final composition of the meditation sample (n = 30) determined the selection of the ICBM control subjects (n = 30). That is, within the image database of controls, we aimed to find the closest fit for each meditator with respect to sex, handedness, and age (the maximum allowed age difference within a sex-matched pair was 2 years). The two resulting samples (meditators/controls) each contained 15 men and 15 women, consisting of 28 right-handers and two left-handers (both left-handers were men). Handedness was determined based on self-reported preference for selected activities (writing, throwing, holding, opening, etc.) using a modified version of the Edinburgh Inventory [Oldfield, 1971]. Age ranged from 24 to 64 years, where 47.3 years was the mean age for meditators as well as for controls (standard deviation: ± 11.7 and 11.8, respectively). Meditators and controls were relatively similar with respect to race, with 22 meditators (73.3%) and 25 controls (83.3%) reporting that they were Caucasian. The remaining eight meditators (MED) and five controls (CTL) indicated to be African American (2 MED/3 CTL), Asian American (5 MED/1 CTL), as well as African American and Asian American (1 MED). One control subject did not provide information on race. As 26 meditators (86.7%) and also 26 controls had, at least, some college experience, both groups were also comparable with respect to their educational background. However, the level of education seemed to be slightly higher in meditators than in controls (i.e., high school degree: 4 MED/7 CTL; bachelor’s degree: 12 MED/16 CTL; master’s degree: 14 MED/7 CTL). Within the meditation sample, years of meditation practice ranged from 5 to 46 years (mean ± SD: 20.2 ± 12.2 years). Information on subject-specific practice amount and meditation style is provided elsewhere [Luders et al., 2012]. All subjects were required to be free of any neurological disorders and gave informed consent according to institutional guidelines (the study was approved by the Institutional Review Board of the University of California, Los Angeles, CA). Subjects constituting the current sample (n = 60) partly overlap with samples of n = 44 (53.33%), n = 54 (78.33%), and n = 60 (100%) whose MRI data were analyzed in prior studies addressing the effects of meditation on voxelwise GM and ROI-specific volumes [Luders et al., 2009b], tract-specific FA [Luders et al., 2011], and anatomical features of the corpus callosum [Luders et al., 2012].
Image Acquisition and Processing
MRI data from all subjects (i.e., controls and meditators) was acquired on the same 1.5T Siemens Sonata scanner (Erlangen, Germany) using an eight-channel head coil and a T1-weighted sequence (magnetization prepared rapid acquisition gradient echo) with the following parameters: repetition time = 1900 ms, echo time = 4.38 ms, flip angle = 15°, 160 contiguous 1 mm sagittal slices, field of view: 256 mm × 256 mm, matrix: 256 × 256, voxel dimensions: 1.0 × 1.0 × 1.0 mm. Automated radio-frequency bias field corrections were applied to correct image volumes for intensity drifts caused by magnetic field in homogeneities [Shattuck et al., 2001]. In addition, all images volumes were placed into a standard space using automated six-parameter rigid-body transformations [Woods et al., 1998]. The hippocampus was labeled manually by one rater (JY.H.) in contiguous coronal brain sections using BrainSuite [Shattuck and Leahy, 2002] and guided by well-established protocols (http://users.loni.ucla.edu/~narr/protocol.php?q=hippotrace). To determine intrarater reliability, the hippocampus was labeled twice in five randomly selected brains revealing intraclass correlations for hippocampus volume of rI = 0.95. In addition, the hippocampus was labeled five times, by the same rater, within one randomly selected brain revealing a volumetric overlap of 85% for all labels. The overlap was defined as the volume of the intersection of the five labels, divided by the mean volume of these labels, multiplied by 100. Finally, to get a sense for the overall reliability, we investigated the association between hippocampal measures resulting from manual and automated procedures. For this purpose, we additionally generated left and right hippocampal labels for all 60 subjects using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Pearson correlations between hippocampal volumes obtained manually (BrainSuite) and automatically (FreeSurfer) revealed significant (P < 0.001) positive correlations for the left (r = 0.78) and right hippocampus (r = 0.59). These outcomes are in close agreement with prior validation studies, where correlations between manual and automated hippocampal segmentations varied from r = 0.80 to r = 0.61 [Cherbuin et al., 2009].
Total Brain Volume Measures
To establish whether meditators and controls differed in total brain volume (TBV), all image volumes were tissueclassified into GM, WM, and cerebrospinal fluid (CSF) using SPM8 (http://www.fil.ion.ucl.ac.uk/spm) and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm.html), as described elsewhere [Luders et al., 2009a]. Tissue volumes were determined based on the respective tissue-classified partitions (i.e., GM, WM, and CSF) in native space. TBV was calculated (in ml) by adding GM, WM, and CSF volumes and statistically compared between meditators and controls. Meditators and controls did not differ significantly with respect to TBV [meditators (mean ± SD): 1446 ± 130 ml; controls (mean ± SD): 1438 ± 130 ml].
Hippocampus Measures
Global measures
After manually labeling the hippocampus (as described above), global left and right hippocampal volumes were established (in mm3) based on the number of voxels constituting the hippocampus labels.
Local measures
To obtain regionally specific information, the manually derived hippocampal labels were first converted into three-dimensional (3D) shape representations of the left and right hippocampus. Then, precisely following the outer contours of the 3D shapes, we automatically generated 3D parametric surface meshes [Thompson et al., 1996; Thompson et al., 2004]. These hippocampal meshes resemble a gridded surface of equally spaced points, where the array of these points is standardized across all subjects establishing a point-by-point correspondence. For each hippocampal mesh, a medial curve was defined along the long axis of the hippocampus. The radial distances (in mm) from this medial axis to each surface point constitute the local descriptors of hippocampal morphology. Global and local hippocampus measures were compared between meditators (n = 30) and controls (n = 30) using independent sample t-tests. As a safeguard against type I error, in global group comparisons, Bonferroni corrections were applied using a threshold of P ≤ 0.025 (to account for the bilateral hippocampus volumes). To explore differential effects across the hippocampal surface, the exact locations of significant group differences were mapped using uncorrected thresholds at P ≤ 0.05.
RESULTS
When comparing global hippocampal measures in meditators versus controls, mean left and right hippocampal volumes were larger in meditators (Table I). The group effect of the left hippocampus (7.2% larger in meditators) remained significant after Bonferroni correction (P = 0.003).
TABLE I.
Hippocampal volumes in mm3 (mean ± SD)
| Hippocampus | Meditators (n = 30) |
Controls (n = 30) |
Mean difference |
|---|---|---|---|
| Right | 3584.63 ± 402.86 | 3465.83 ± 535.20 | 118.8 |
| Left | 3521.47 ± 330.62 | 3269.40 ± 295.67 | 252.1** |
Significant at the 0.01 level.
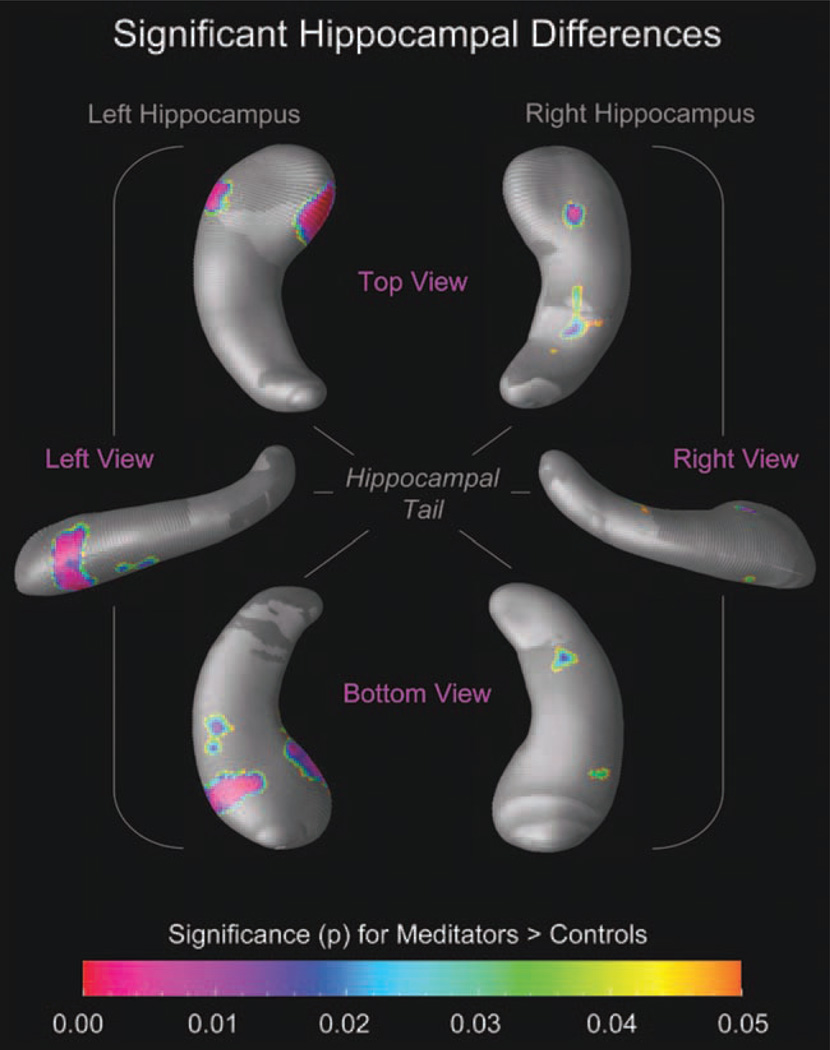
When using the mesh-based approach to characterize effects locally, we detected larger radial distances in meditators compared with controls with up to 15% difference (green–yellow–orange clusters in Fig. 1). Although larger radial distances also occurred in controls compared to meditators, they were only evident in some small and isolated clusters, such as at the very top of the hippocampal head and close to the end of the tail (cyan–blue–purple clusters in Fig. 1). Moreover, while larger radial distances in controls did not reach statistical significance (maps not shown), larger radial distances in meditators were highly significant in several hippocampal subregions (Fig. 2), with most pronounced effects in the head of the left hippocampus.
Figure 1.
Hippocampal differences (in percent). The color bar encodes the magnitude of the group difference (%). Hippocampal maps illustrate larger radial distances in meditators (green–yellow–orange) and in controls (cyan–blue–purple). Group differences of less than 5% are not differentiated by group and shown in gray. Maps on the left display views of the left hippocampus (from the top; left; bottom); maps on the right display views of the right hippocampus (from the top; right; bottom). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
Significant hippocampal differences. The color bar encodes the significance of the group difference (P). Hippocampal maps illustrate significantly larger radial distances in meditators (P ≤ 0.05, uncorrected) compared with controls. Hippocampal regions in gray indicate where no significant group differences were observed (P > 0.05). Maps on the left display views of the left hippocampus (from the top; left; bottom); maps on the right display views of the right hippocampus (from the top; right; bottom). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
This is the first study conducted within a relatively large sample of meditators and well-matched controls, where global measures (hippocampal volumes) were complemented with refined local measures (radial hippocampal distances from surface to central core). Our study revealed robust group differences with larger hippocampal dimensions in meditators compared to controls. Links between meditation and hippocampal anatomy have been previously observed, both in independent [Holzel et al., 2008] and overlapping samples [Luders et al., 2009b] containing fewer subjects1 and using different methodological approaches2. Of note, aside from constituting a more powerful statistical design due to the enlarged sample size, the current study expands our initial study [Luders et al., 2009b] in at least two ways: It not only includes the right as well as the left hippocampus in the volumetric ROI analysis (global measures) but also maps differential effects across the hippocampal surface in 3D (local measures). Global hippocampal effects (i.e., larger hippocampal volumes in meditators) were confirmed locally when inspecting the significance profiles, where the hippocampal boundaries in meditators (especially the boundaries of the left hippocampus) protrude outward more than in controls. In close agreement with these observations, a recent longitudinal study [Holzel et al., 2011] detected significant increases of hippocampal GM in the left hemisphere when comparing brains of novice meditators before and after an 8-week mindfulness training program.
Possible Functional Implications
The larger hippocampal dimensions in meditators, as detected in our study, might be related to increased hippocampal activity during meditation, as observed in functional imaging studies [Engstrom et al., 2010; Holzel et al., 2007; Lazar et al., 2000; Lou et al., 1999, 2005].
The hippocampus is a key structure for memory processes, where increasing evidence suggests hippocampal involvement not only in long-term memory but also in working memory [Bergmann et al., 2012]. Memory consolidation has been previously discussed as a possible underlying mechanism for increased hippocampal activity during meditation [Engstrom et al., 2010]. Similarly, memory consolidation (and/or memory retrieval) might be linked to hippocampal structure, where larger hippocampal dimensions could be advantageous. Our current findings might thus account for previously observed positive effects of meditation practices on memory performance, such as an increased specificity of autobiographical memory or an enhanced capacity of working memory [Chiesa et al., 2011; Heeren et al., 2009; Kozhevnikov et al., 2009; Williams et al., 2000]. With particular relevance to possible links between meditation and working memory, it was also suggested that, during meditation, an attentional shift (from external matters to internal states) occurs “according to a previously established rule kept in working memory” [Baerentsen et al., 2010]. Nevertheless, further research is needed, not only to determine the exact mechanisms of memory consolidation and/or retrieval during meditation but also the specific components of memory affected.
It is also possible that the observed hippocampal alterations in long-term meditators are not at all linked to memory, but to other mental skills associated with the practice of meditation, such as high levels of awareness, attention, and focus. Larger hippocampal dimensions may similarly account for meditators’ habits and abilities to engage in mindfulness behavior, cultivate positive emotions, and retain emotional stability. Davidson et al. [2000], for example, have proposed an active role of the hippocampus in emotional responding. They suggest, “individuals who habitually fail to regulate their affective responses in a context-sensitive fashion may have a functional impairment of the hippocampus.” None of these hypotheses can be tested without actual behavioral measures, so the outcomes of our study revealing increased hippocampal dimensions in active meditators may motivate research combining imaging and behavioral data.
Possible Underlying Mechanisms
Engaging the brain in intense cognitive processes during meditation (i.e., efforts to exercise awareness, attention, concentration, focus, etc.) might directly affect the hippocampus. That is, actively meditating (especially regularly meditating over many years) may induce changes of hippocampal anatomy, particularly in the left hemisphere. Training-induced structural changes of the hippocampus have been repeatedly reported using structural MRI data in subjects unselected for meditation [Boyke et al., 2008; Draganski et al., 2006; Pereira et al., 2007]. Even more importantly, practice-induced changes of hippocampal morphology have been demonstrated in the framework of meditation, as detailed above [Holzel et al., 2011], providing strong support for a causal role of meditation. Macroscopic hippocampal changes become evident due to underlying microscopic events, such as synaptogenesis, angiogenesis, and/or dendritic branching. Another potential mechanism is the actual generation of new hippocampal neurons (neurogenesis). Although neurogenesis was initially thought to occur only during embryonic and early postnatal development, a large body of evidence now confirms that new neurons are generated throughout life from neural progenitor cells within specific brain regions, including the hippocampus [Elder et al., 2006; Eriksson, 2003; Eriksson et al., 1998; Gage, 2002].
Alternatively (or as a complementary mechanism), meditation might shape hippocampal anatomy indirectly via altering (i.e., positively affecting) autonomic regulation and immune activity [Cysarz and Bussing, 2005; Davidson et al., 2003; Kubota et al., 2001]. As summarized elsewhere [Sala et al., 2004], the hippocampus plays a major role in stress regulation, “but is itself highly sensitive to neurotoxic effects of repeated stressful episodes.” For example, loss of hippocampal neurons or disturbances in hippocampal neurogenesis due to stress (and stress-induced cortisol neurotoxicity) has been reported in various psychiatric disorders that are related to stressful events (e.g., anxiety disorder, depressive disorder, or posttraumatic stress disorder). Meditation and mindfulness-based techniques are highly effective in stress reduction, both in terms of subjectively perceived stress as well as objectively measured biomarkers of stress [Holzel et al., 2010; Jensen et al., 2011; Jung et al., 2010, 2011; Mohan et al., 2011]. Thus, the increased hippocampal dimensions in meditation practitioners might constitute an indirect consequence of meditation (i.e., the result of meditation-induced stress-reduction).
On a related note, meditation practices might be accompanied by certain lifestyle choices (e.g., related to diet, smoking, alcohol consumption, physical exercise, recreational activities) which themselves may have secondary consequences (e.g., related to sleep, mental stimulation, physical and mental health). All of those variables (either alone or in combination with each other) may contribute to observable changes in hippocampal structure. While none of these aforementioned variables has been tested in the current study, it is important to acknowledge their potential impact on the observed group differences. Future studies may significantly advance this field of research by capturing possible discriminating features between meditators and controls (aside from the actual practice of meditation) and either statistically controlling for these potential moderator variables or using them as inclusion/exclusion or matching criteria during subject selection.
Finally, a different hippocampal anatomy may constitute an innate brain feature that draws an individual toward meditation and/or helps continuing a regular and longterm practice. As our study is cross-sectional, it is impossible to tease apart the relative contributions of nature and nurture. Any conclusions with respect to the determinants of the observed group differences remain speculative, and further research, preferably incorporating longitudinal designs, is needed.
ACKNOWLEDGMENTS
The authors thank all participants for their dedication and partaking in our study. The authors are also grateful to Trent Thixton who assisted with the acquisition of the image data. For generous support, the authors thank the Brain Mapping Medical Research Organization, the Robson Family and Northstar Fund, and the following Foundations: Brain Mapping Support, Pierson-Lovelace, Ahmanson, Tamkin, William M., and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community, Jennifer Jones-Simon, and Capital Group Companies.
Contract grant sponsor: National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); Contract grant numbers: C06 RR012169, P41 RR013642, R01 AG040060, R01 EB008281, R01 EB007813 and R01 AG020098 (PT).
Footnotes
However, not all subjects from our prior study were included in the current study. Specifically, to minimize the potential impact of age-related brain atrophy, we excluded three meditators aged 69, 69, and 71 years from the current analysis, and we recruited new subjects such that the current sample of 30 meditators included 19 meditators from the original sample.
In our prior study, we conducted a voxel-based gray matter analysis in combination with a hypothesis-driven ROI volumetric analysis. ROIs were defined a priori based on published findings. Since, at that point, the left hippocampus was not implicated in prior studies, it was not defined as an ROI. Thus, only right hippocampal volumes were examined and reported to be significantly larger in meditators.
REFERENCES
- Baerentsen KB, Stodkilde-Jorgensen H, Sommerlund B, Hartmann T, Damsgaard-Madsen J, Fosnaes M, Green AC. An investigation of brain processes supporting meditation. Cogn Process. 2010;11:57–84. doi: 10.1007/s10339-009-0342-3. [DOI] [PubMed] [Google Scholar]
- Bergmann HC, Rijpkema M, Fernandez G, Kessels RP. Distinct neural correlates of associative working memory and long-term memory encoding in the medial temporal lobe. Neuroimage. 2012 Mar 27; doi: 10.1016/j.neuroimage.2012.03.047. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Boyke J, Driemeyer J, Gaser C, Buchel C, May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Anstey KJ, Reglade-Meslin C, Sachdev PS. In vivo hippocampal measurement and memory: A comparison of manual tracing and automated segmentation in a large community-based sample. PLoS ONE. 2009;4:e5265. doi: 10.1371/journal.pone.0005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev. 2011;31:449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Cysarz D, Bussing A. Cardiorespiratory synchronization during Zen meditation. Eur J Appl Physiol. 2005;95:88–95. doi: 10.1007/s00421-005-1379-3. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Urbanowski F, Harrington A, Bonus K, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosom Med. 2003;65:564–570. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, De GR, Gama Sosa MA. Research update: Neurogenesis in adult brain and neuropsychiatric disorders. Mt Sinai J Med. 2006;73:931–940. [PubMed] [Google Scholar]
- Engstrom M, Pihlsgard J, Lundberg P, Soderfeldt B. Functional magnetic resonance imaging of hippocampal activation during silent mantra meditation. J Altern Complement Med. 2010;16:1253–1258. doi: 10.1089/acm.2009.0706. [DOI] [PubMed] [Google Scholar]
- Eriksson PS. Neurogenesis and its implications for regeneration in the adult brain. J Rehabil Med. 2003;(41 Suppl):17–19. doi: 10.1080/16501960310010098. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A, Van BN, Philippot P. The effects of mindfulness on executive processes and autobiographical memory specificity. Behav Res Ther. 2009;47:403–409. doi: 10.1016/j.brat.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Hempel H, Hackl A, Wolf K, Stark R, Vaitl D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci Lett. 2007;421:16–21. doi: 10.1016/j.neulet.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Holzel BK, Ott U, Gard T, Hempel H, Weygandt M, Morgen K, Vaitl D. Investigation of mindfulness meditation practitioners with voxel-based morphometry. SCAN. 2008;3:55–61. doi: 10.1093/scan/nsm038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CG, Vangkilde S, Frokjaer V, Hasselbalch SG. Mindfulness training affects attention-or is it attentional effort? J Exp Psychol Gen. 2011;141:106–23. doi: 10.1037/a0024931. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jung YH, Kang DH, Jang JH, Park HY, Byun MS, Kwon SJ, Jang GE, Lee US, An SC, Kwon JS. The effects of mind-body training on stress reduction, positive affect, and plasma catecholamines. Neurosci Lett. 2010;479:138–142. doi: 10.1016/j.neulet.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Jung YH, Kang DH, Byun MS, Shim G, Kwon SJ, Jang GE, Lee US, An SC, Jang JH, Kwon JS. Influence of brain-derived neurotrophic factor and catechol O-methyl transferase polymorphisms on effects of meditation on plasma catecholamines and stress. Stress. 2011;15:97–104. doi: 10.3109/10253890.2011.592880. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kozhevnikov M, Louchakova O, Josipovic Z, Motes MA. The enhancement of visuospatial processing efficiency through Buddhist Deity meditation. Psychol Sci. 2009;20:645–653. doi: 10.1111/j.1467-9280.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sato W, Toichi M, Murai T, Okada T, Hayashi A, Sengoku A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Brain Res Cogn Brain Res. 2001;11:281–287. doi: 10.1016/s0926-6410(00)00086-0. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Bush G, Gollub RL, Fricchione GL, Khalsa G, Benson H. Functional brain mapping of the relaxation response and meditation. Neuroreport. 2000;11:1581–1585. [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Hum Brain Mapp. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Nowak M, Kjaer TW. The mental self. Prog Brain Res. 2005;150:197–204. doi: 10.1016/S0079-6123(05)50014-1. [DOI] [PubMed] [Google Scholar]
- Luders E, Gaser C, Narr KL, Toga AW. Why sex matters: Brain size independent differences in gray matter distributions between men and women. J Neurosci. 2009a;29:14265–14270. doi: 10.1523/JNEUROSCI.2261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Toga AW, Lepore N, Gaser C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage. 2009b;45:672–678. doi: 10.1016/j.neuroimage.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Clark K, Narr KL, Toga AW. Enhanced brain connectivity in long-term meditation practitioners. Neuroimage. 2011;57:1308–1316. doi: 10.1016/j.neuroimage.2011.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Phillips OR, Clark K, Kurth F, Toga AW, Narr KL. Bridging the hemispheres in meditation: Thicker callosal regions and enhanced fractional anisotropy (FA) in long-term practitioners. Neuroimage. 2012;61:181–187. doi: 10.1016/j.neuroimage.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Woods R, Iacoboni M, Sicotte N, Yaden K, Tran M, Bean C, Kaplan J, Toga AW. The myth of the normal, average human brain—The ICBM experience: (1) subject screening and eligibility. Neuroimage. 2009;44:914–922. doi: 10.1016/j.neuroimage.2008.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Sharma R, Bijlani RL. Effect of meditation on stress-induced changes in cognitive functions. J Altern Complement Med. 2011;17:207–212. doi: 10.1089/acm.2010.0142. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala M, Perez J, Soloff P, Ucelli di NS, Caverzasi E, Soares JC, Brambilla P. Stress and hippocampal abnormalities in psychiatric disorders. Eur Neuropsychopharmacol. 2004;14:393– 405. doi: 10.1016/j.euroneuro.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: An automated cortical surface identification tool. Med Image Anal. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13:856– 876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, de Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Williams JM, Teasdale JD, Segal ZV, Soulsby J. Mindfulness-based cognitive therapy reduces overgeneral autobiographical memory in formerly depressed patients. J Abnorm Psychol. 2000;109:150–155. doi: 10.1037//0021-843x.109.1.150. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Tomogr. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]