Abstract
Background
African-Americans are consistently underrepresented in cancer clinical trials. Minority under-enrollment may be, in part, due to differences in the way clinical trials are discussed in oncology visits with African-American versus White patients.
Objective
To investigate differences in oncologist-patient communication during offers to participate in clinical trials in oncology visits with African-American and White patients.
Methods
From an archive of video recorded oncology visits, we selected all visits with African-American patients that included a trial offer (n=11) and a matched sample of visits with demographically/medically comparable White patients (n=11). Using mixed qualitativequantitative methods, we assessed differences by patient race in (1) word count of entire visits and (2) frequency of mentions and word count of discussions of clinical trials and key elements of consent.
Results
Visits with African-American patients, compared to visits with White patients, were shorter overall and included fewer mentions of and less discussion of clinical trials. Also, visits with African-Americans included less discussion of the purpose and risks of trials offered, but more discussion of voluntary participation.
Discussion and Conclusions
African-American patients may make decisions about clinical trial participation based on less discussion with oncologists than do White patients, as shown by a discourse analysis of two interactions. Possible explanations include a less active communication style of African-Americans in medical visits, oncologists’ concerns about patient mistrust, and/or oncologist racial bias. Findings suggest oncologists should pay more conscious attention to developing the topic of clinical trials with African-American patients, particularly purpose and risks.
Keywords: patient-physician communication, cancer, health care disparities, minorities, discourse analysis
Introduction
Health disparities are differences in health or health risks in which disadvantaged social groups, such as racial/ethnic minorities, women, or the poor, systematically experience worse health or greater health risks than more advantaged groups for reasons that could be addressed by social policies.1 In the United States, racial disparities exist in cancer outcomes: African-Americans with cancer have lower five-year survival rates and higher mortality rates compared to White patients.2 One very likely cause of these differences is racial disparities in health care.3, 4 In cancer research, African-Americans are consistently underrepresented in clinical trial recruitment and enrollment. Underrepresentation may contribute to health disparities in two ways: first, clinical trials are considered state-of-the-art cancer management for all patients, and thus all patients should have access to clinical trials; second, findings from research conducted without adequate minority representation may not be generalizable to minority populations.5-8
Studies have shown that the communication between oncologists and patients influences decision-making about participation in clinical trials.9-11 Prior studies using patient self-reported perceptions of clinical interactions and observations of audio and/or video recorded interactions have also demonstrated consistently that the quality of communication between physicians and African-American patients, as compared to White patients, is of lower quality.4, 12-18 For example, systematic observational analyses of video and/or audio recorded clinical interactions have shown that physicians use more patient-centered communication with and provide more information to White patients than African-American patients, and that African American patients participate less actively in clinical interactions, such as by asking questions or stating concerns.14, 15, 19-21 However, we found no studies using real-time interactional data from actual oncology visits to investigate whether there are differences by patient race in physician-patient communication about clinical trials. These communication differences, if they exist, would suggest that African-Americans may make less informed decisions about clinical trial participation or may be less likely to agree to participate. These differences would, therefore, contribute to underrepresentation in clinical trials and to racial health disparities in cancer care.
Thus the purpose of this study was to compare physician-patient communication during offers to participate in clinical trials in oncology visits with African-American and with White patients. Using word count as an objective measure of the amount of actual face-to-face discussion between oncologists and patients, we first compared the length of the entire visits in which clinical trials were offered for African-American vs. White patients. Second, we analyzed differences in offers to participate in clinical trials as a topic of discussion during the visits. Finally, we analyzed differences in an important type of information within the topic of clinical trials, the five key elements of consent, as subtopics – the purpose of the study, its potential risks and benefits, alternatives to participation, and the voluntary nature of participation. These elements of consent are identified by federal regulations as necessary to obtaining informed consent.22
Patients and Methods
Data for this secondary analysis were taken from an archive of transcripts of oncology visits video-recorded between April 2002 and March 2006 in multidisciplinary outpatient clinics at two comprehensive cancer centers.10, 11 All patients and physicians provided informed consent as required by the Institutional Review Boards at both institutions. Patients were recruited for the study on their first visit to a participating oncologist if clinic staff indicated they were potentially eligible for any clinical trial. The parent study included 235 video recorded visits with patients who were potentially eligible for clinical trials, but only 47 of these visits included explicit offers of clinical trials to patients. Of these, 11 patients were African-American.
Sample
From the parent study, transcripts of all visits that included the explicit offer of a clinical trial to an African American patient (n=11) were selected. Rather than analyzing transcripts of all visits with White patients for comparison, we selected a sample of White patients (n=11), matched to the African-American patients to the extent possible by factors in the following order: SEER23 diagnostic codes for type of cancer, education, income, gender, and age (Table 1). Eleven different oncologists saw the patients in the 22 visits. For African-American patients, ten of the 11 visits (91%) were with White oncologists; the remaining visit was with an African-American physician. For White patients, ten of the 11 visits (91%) were with White oncologists; the remaining visit was with an Asian physician.
Table 1.
Patient Characteristics
| African-American | White | ||
|---|---|---|---|
| Total | |||
| Race | 11 (50%) | 11 (50%) | 22 (100%) |
| Type of Cancer (SEER4 diagnostic codes) | |||
| Digestive | 3 (27%) | 3 (27%) | 6 (27%) |
| Genital | 3 (27%) | 2 (18%) | 5 (23%) |
| Breast | 3 (27%) | 1 (9%) | 4 (18%) |
| Oral | 1 (4%) | 1 (4%) | 2 (9%) |
| Myeloma | 1 (4%) | 1 (4%) | 2 (9%) |
| Respiratory | 0 (0%) | 3 (27%) | 3 (14%) |
| Education | |||
| High school, trade school or less | 5 (45%) | 5 (45%) | 10 (45%) |
| Some college or greater | 6 (55%) | 6 (55%) | 12 (55%) |
| Income | |||
| ≤ $39,999 | 6 (55%) | 3 (27%) | 9 (41%) |
| ≥ $40,000 | 3 (27%) | 6 (55%) | 9 (41%) |
| Mean/SD | 37,700/19.4 | 57,200/34.6 | 47,700/28.7 |
| Independent t-test | p=.1702 | ||
| Gender | |||
| Male | 7 (64%) | 6 (55%) | 13 (59%) |
| Female | 4 (36%) | 5 (45%) | 9 (41%) |
| Age | |||
| ≤ 59 | 6 (55%) | 1 (4%) | 7 (32%) |
| ≥ 60 | 5 (45%) | 10 (96%) | 15 (68%) |
| Mean/SD | 60.5/12.8 | 65.8/6.9 | 63.2/10.4 |
| Independent t-test | p=.2444 | ||
Procedures
We used mixed qualitative-quantitative methods for coding and analysis in this study. To extract the data we used discourse analysis, a qualitative method for analyzing transcripts of talk by topics and subtopics. We chose to use discourse analysis because it takes an interactional perspective, which allows us to analyze how oncologists organize offers to participate in clinical trials and how patients respond as part of the ongoing interaction.24 We adopted definitions of topic and subtopic following Chafe.25 Specifically, we defined a topic as a coherent set of utterances about a main idea; a topic can be as short as an utterance or two or as long as a lengthy discussion. We similarly defined a subtopic as a set of utterances about a subsidiary idea within the main idea of the topic. Topics and subtopics are identifiable by multiple linguistic criteria: pauses; shifts in content; discourse topic markers such as so, now, and OK; and items in lists (for subtopics). To create the database for this study, one author (EB) extracted all mentions of clinical trials in the transcripts of the 22 visits, by oncologists, patients, and patients’ companions. Mentions are defined as the sets of utterances related to a topic or subtopic. Within the extracted mentions of the topic of clinical trials, two authors (EB and AW) then independently extracted mentions of any of the five key elements of consent noted earlier – purpose, risks, benefits, alternatives, and voluntary participation—as subtopics. Elements of consent were defined following the 45 CFR 46 (2005/1999) federal regulations and guidance.22 Overall percent agreement between authors on identification of subtopics was 87.4%; discrepancies were resolved through discussion. Table 2 provides definitions and examples of elements of consent.
Table 2.
Coding for Elements of Consent
| Element of Consent | n= | % agree |
Definition | Example |
|---|---|---|---|---|
| Purpose | 54 | 92.6 | A statement of the scientific question of the research (NIH, 2008). |
And I’ll go through one of the research studies we’re doing here, trying to figure out some new ways to mix and match. |
| Risks | 56 | 80.4 | A statement of the probability and magnitude of harm or discomfort that may arise from participating in research (45 CFR 46, 2005/1999), including both side effects and negative outcomes (NIH, 2008). |
The consent form has all the information that is about the trial, what the drug is, why we are using it, and what its potential side effects are. Or if it’s [the tumor] against the blood vessel and the blood vessel can rupture, so that’s always a small risk. It’s a small risk with Gleevec, it’s been a small risk with this [experimental drug]. |
| Benefits | 59 | 88.1 | A statement of the direct benefits to the patient, including access to the treatment, care, and education patients receive on the trial, as well as their feelings of autonomy and altruism derived from participating in the research; a statement of indirect benefits to others or society, including the likely importance of the scientific knowledge resulting from the clinical trial (NIH, 2008). |
And, um, it’s one pill a day and … the … early information that we have is that the combination-- th-this combination might be one and a half or two times more effective than the older one that I was telling you about. OK. PH: But the information that’s derived will go on to further help medical research -- PT: And other people. PH: -- may be able to help others in the future know if there’s a difference or not. |
| Alternatives | 43 | 83.7 | Disclosure of appropriate alternative treatments, if any, that might be advantageous to the patient (45 CFR 46, 2005/1999). |
So among the drugs that we can give to you there are three options. Either the standard chemotherapy drugs, which have a ten to fifteen percent chance of working. And they are—they could have some potential side effects. The second option is to try one of the newer, more selective drugs … which has the same probability of working, same chance it works but [is] easier to take. |
| Vol. Part. | 42 | 92.8 | A statement that participation is voluntary (45 CFR 46, 2005/1999). |
This is also voluntary. And if you decide either now or later that you don’t wanna participate we’ll still take care of you, we’re not gonna chase you away for it. |
| Total | 254 | 87.4 |
In the quantitative analysis, we calculated the amount of time spent discussing these aspects of clinical trials. To do this, we calculated the word count during each topic and subtopic mention using Microsoft Word Count. The study sample (22 visits) was quite small, making tests of the statistical significance of differences between average word counts inadvisable. Therefore, we assessed the size of the effect of patient race on the discussion of clinical trials using Cohen’s d,26 which is the difference between two means divided by the pooled standard deviation for the two samples. It is, thus, the size of a difference expressed in units of standard deviations. Cohen’s d is not dependent on sample size and is often used to describe the magnitude of the difference between two means. Cohen proposed the following guidelines for the interpretation of effect sizes: small (≥ .2), medium (≥ .5), and large (≥ .8).
Results
Table 3 presents the mean word count of the entire visits, the frequency (i.e., number of times a topic or subtopic was mentioned during a visit) and mean word count of mentions of clinical trials as a topic, and the frequency and mean word count of mentions of elements of consent as subtopics in offers to participate in clinical trials with African-American and with White patients.
Table 3.
Word Count of Entire Visits, Frequency and Word Count of Mentions of Clinical Trials as a Topic, Frequency and Word Count of Mentions of Elements of Consent as Subtopics
| African-American | White | ||||
|---|---|---|---|---|---|
|
| |||||
| Mean | SD | Mean | SD | d= | |
| Entire Visit | |||||
| Mean word count | 4877.73 | 2519.06 | 7247.18 | 2890.36 | d=.8740 |
| Topic of Clinical Trials | |||||
| Mean times trial mentioned | 2.73 | 1.01 | 4.27 | 1.49 | d=1.2099 |
| Mean word count | 1089.64 | 330.62 | 1867.09 | 980.48 | d=1.0618 |
| Subtopics of Elements of Consent | |||||
| Purpose | |||||
| Mean times Purpose ment. | 2.36 | 1.29 | 2.55 | 1.81 | d=.1209 |
| Mean word count | 90.91 | 58.36 | 181.22 | 124.77 | d=.9272 |
| Benefits | |||||
| Mean times Benefits ment. | 2.64 | 1.80 | 2.73 | 1.76 | d=.0505 |
| Mean word count | 181.27 | 144.41 | 200.10 | 161.23 | d=.1230 |
| Risks | |||||
| Mean times Risks ment. | 1.91 | 1.87 | 3.18 | 2.48 | d=.5782 |
| Mean word count | 211.90 | 192.73 | 390.27 | 338.46 | d=.6477 |
| Alternatives | |||||
| Mean times Alt. ment. | 2.00 | 1.61 | 1.91 | 1.58 | d=.0564 |
| Mean word count | 136.20 | 165.48 | 172.33 | 180.99 | d=.2084 |
| Voluntary Participation | |||||
| Mean times Vol. Part. ment. | 2.18 | 1.60 | 1.55 | 1.44 | d=.4139 |
| Mean word count | 123.00 | 59.70 | 107.25 | 105.97 | d=.1831 |
Mean word count of entire visits
Mean word count of the entire visit was less for African-American than White patients (4877.73African-Americans vs. 7247.18Whites, d=.8740).
Frequency and mean word count of mentions of clinical trials as a topic
The topic of clinical trials was mentioned less frequently during visits with African-American than White patients (M=2.73African-Americans vs. 4.27Whites, d=1.2099). When the topic of clinical trials was mentioned, mean word count during mentions was also less for African-American patients (M=1089.64African-Americans vs. 1867.09Whites, d=1.0618).
Frequency and mean word count of mentions of elements of consent as subtopics
The patterns of effect size were mixed for the subtopics of elements of consent. For purpose, the effect of race on the frequency of mentions was minimal (M=2.36African-Americans vs. 2.55Whites, d=.1209); however, when purpose was mentioned, the mean word count during mentions was substantially less for African-American patients (M=90.91 African-Americans vs. 181.22 Whites, d=.9272). For benefits, the effect of race was minimal for both frequency of mentions (M=2.64African-Americans vs. 2.73Whites, d=.0505) and mean word count (M = 181.27African-Americans vs. 200.10Whites, d=.1230). Risks, however, were mentioned less frequently for African-American patients (M=1.91African-Americans vs. 3.18Whites, d=.5782) and the mean word count when risks were mentioned was also less for African-American patients (M=211.900African-Americans vs. 390.27Whites, d=.6477). For alternatives, the effect of race was minimal for frequency of mentions (M=2.00African-Americans vs. 1.91Whites, d=.0564) and small for mean word count (M=136.20African-Americans vs 172.33Whites, d=.2084). There were more frequent mentions of voluntary participation for African-American patients (M=2.18African-Americans vs. 1.55Whites, d=.4139) and the mean word count for voluntary participation with African-American patients was also marginally greater (M=123.00African-Americans vs. 107.25Whites, d=.1831).
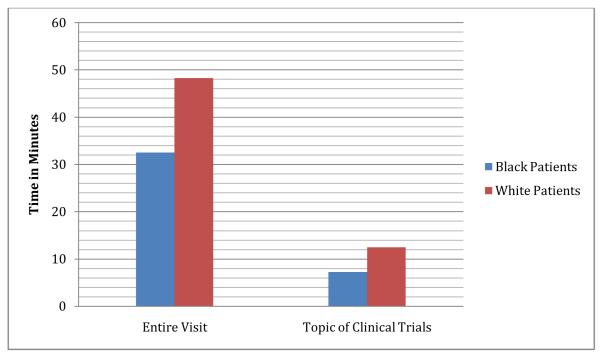
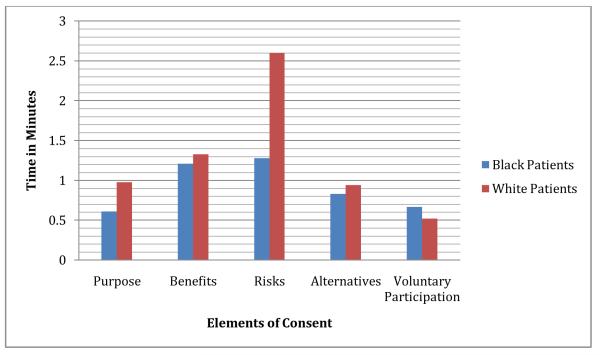
In a different way to summarize the findings of this study, we converted mean word count to mean time of discussion, using an estimate of 150 words per minute of talk adapted from Yuan et al (2006).27 This estimate takes into account individual variation in rate of speech. Figures 1 and 2 illustrate the same findings in a different way: visits with African-American patients include less time spent overall (32 minutes, 30 secondsAfrican-Americans vs. 48 minutes 19 secondsWhites,), less time spent discussing clinical trials (7 minutes, 16 secondsAfrican-Americans vs. 12 minutes, 27secondsWhites), and half as much time spent discussing risks (85 secondsAfrican-Americans vs. 155 secondsWhites), but visits with African-American and White patients include almost the same amount of time discussing benefits (73 secondsAfrican-Americans vs. 80 secondsWhites).
Figure 1.
Time of Entire Visit and Time on Topic of Clinical Trials
Figure 2.
Time on Subtopics of Elements of Consent
Discussion and Conclusions
This is the first study to use a linguistic analysis to compare offers to participate in cancer clinical trials by patient race, and the findings indicate a disparity of words. Oncology visits in which clinical trials are offered to African-American patients, as compared to White patients, included less discussion overall, fewer mentions of and discussion of clinical trials, and less discussion of the purpose of a clinical trial and the risks of participation. The only aspect of clinical trials and elements of consent that received more discussion during visits with African-American patients was voluntary participation.
Findings raise concerns that African-American patients may make decisions about clinical trial participation based on less discussion with oncologists than White patients. More specifically, there is a worrisome disparity in the pattern of information about the risks and benefits of trial participation in discussions with African-American and with White patients: African-American patients engage in the same amount of discussion about the benefits of trial participation as White patients, but notably less discussion about the risks. Providing information about the benefits of trial participation is a powerfully persuasive strategy for oncologists to use with patients in discussing a trial,9, 28 and our study shows that oncologists appear to use this strategy similarly with African-American and with White patients. But the ethics of clinical trial recruitment require that patients also receive adequate information about the risks of the trial, and here African-American patients experience notably less discussion.
The following examples from the same oncologist in visits with an African-American patient and with a White patient illustrate differences in the discussion of risks by race: Example One [African-American patient]:
Oncologist: [A]t any time if you say ‘It’s making me too sick, I’ve had this side effect, it’s too bad’ [Patient: Right.] we’ll pull you off and try one of the other possible regimens. [Patient: Right.]
Example Two [White patient]:
Patient: So on this, would you say fatigue will [stop me] from doing anything? Oncologist: It varies. There’s some people that the fatigue certainly does. There are other people that say they notice it and that they feel better the weeks off. That it doesn’t stop—
Patient: Will I be able to [golf] without a problem?
Family: You don’t [golf] anyway.
Oncologist: I can’t predict how it would be for you but … I mean if the fatigue is such for you that you can’t get out of bed, we can certainly adjust the dose, if—And you can always withdraw from the trial, too. If you say at some point, hey this isn’t worth it, I’m just lying here because I’m so tired. I don’t expect that, that’ll happen and we certainly haven’t seen that much of it. Some people do complain of the fatigue.
The contrasts here are not solely in amount of discussion, but also in interactional form, elaboration, and reassurance. Both examples include references to side effects and withdrawal from the trial. In Example Two, however, the discussion of fatigue as a specific side effect takes place within a substantive dialogue between the patient and the oncologist, not as part of a short monologue mentioning side effects in general, as in Example One. The discussion of fatigue is also elaborated, personalized by the patient and family member with its joking talk of golf and reassurance about managing side effects. In Example One, the burden of discussing side effects and any subsequent decision to withdraw seems to be solely the patient’s. In Example Two, the burden seems more shared between the patient and the oncologist as part of a therapeutic alliance related not only to treatment on the trial but also to quality of life during treatment.
Why is there a disparity of words in discussing clinical trials with African-American patients? One possible explanation has been suggested by prior researchers, and is illustrated in the examples above--that African-American patients may have a less active interactive style in oncology visits, asking fewer questions, providing low uptake responses to physician contributions that do not generate more topic development (e.g., “right”), and providing fewer high uptake responses that keep topics developing (e.g., personalizing information).14, 15, 19 This study suggests that something similar may occur for discussions of clinical trials with African-American patients, particularly with respect to risks.
Another possible explanation is that oncologists may be less willing to fully discuss clinical trial participation with African-American patients due to concerns about African-Americans’ mistrust. Mistrust in physicians and medical institutions has been shown to be greater among African Americans than among whites, in great part due to the legacy of racism and poorer health care for minorities in the U.S.3, 29-33 In this study, voluntary participation was the only element of consent discussed more with African-American patients than with White patients, suggesting that oncologists are sensitive to the issue of mistrust when discussing clinical trials with African-American patients. It may be that oncologists do not persist in offering clinical trials to African-American patients – not mentioning the topic multiple times and/or not developing the topic if African-American patients seem less responsive -- out of concerns about harming the clinical relationship.
Yet another explanation is that oncologists’ racial attitudes and beliefs lead to differences in communication. Recent research in cognitive and social psychology suggests certain stereotypes about African-American patients (e.g., that African-Americans are non-compliant), even among physicians who genuinely and explicitly claim egalitarian attitudes, can make clinical communication less patient-centered.34-37
The literature on minority recruitment in clinical trials has repeatedly identified patient, provider, and system barriers to minority enrollment in clinical trials, two decades after the NIH Revitalization Act of 1993.38-41 In their review of provider barriers to enrollment in clinical trials, Howerton and colleagues42 identified communication practices as a barrier, along with provider attitudes about adherence and minority patient mistrust of medical research; however, these studies were based on retrospective survey and focus group methodologies, not prospective methodologies investigating face-to-face interactions between oncologists and patients. By measuring the amount of actual discussion about clinical trials, the present study identified specific disparities in communication: perhaps because oncologists are sensitive to the issues of racism and the importance of emphasizing that participation in clinical trials is voluntary for African-American patients, mentions of clinical trials occur less often and with less discussion in visits with African-American patients particularly about the risks of participation.
This study had several limitations that suggest directions for future research. First, the sample size was small; however, as far as we know, this is the first study of clinical trial recruitment to directly compare offers to African-American versus White patients using real-time interactional data, and the results generally showed substantial effect sizes. Future comparative studies should include larger numbers of oncology visits with African-American patients. Second, visits with African-American patients were almost all race-discordant in this study, reflecting not only the race of the participating oncologists, but also the demographics of oncology as a sub-specialty in medicine.43 Third, this study looked at just one type of information within offers to participate in clinical trials, the discussion of key elements in informed consent. Future studies should look at other informational dimensions of offers to participate in clinical trials, such as descriptions of the procedures of the trial and discussions of the trial regimen in comparison to standard treatment. Similarly, some information was not available from the parent study, such as patients’ cancer stage and prognosis, the type of trial discussed (e.g., whether it included a randomized controlled trial), and what patients understood about trials offered. Future studies should assess relationships between the quality of communication and these variables. Fourth, the small sample size did not allow a meaningful analysis of outcomes, such as patient decisions about participation. Finally, this study looked at just one minority population, African-American Americans in the United States. The NIH Revitalization Act41 identified multiple populations of underrepresented patients, including Latinos/Hispanics, women, older adults, rural residents, and economically disadvantaged groups. Ideally, research using real-time interactional data from oncology visits should be conducted on other underrepresented populations to discover whether there are other disparities of communication in offers to participate in cancer clinical trials.
This research suggests that deliberative discussions about pros and cons of decisions may help overcome the effects of implicit attitudes.44 In communication about medical research, already fraught with racial issues in the United States, oncologists may need to pay more conscious attention to achieving the goals of patient-centered communication and shared decision-making in offers to participate in clinical trials in general and in discussions of the risks of trial participation in particular. Thus, to improve the quality of shared decision-making about trials, oncologists could be more conscious of mentioning and developing the topic of clinical trials in oncology visits with African-American patients, and provide more information about the risks of trial participation. More mentions and development of the topic of clinical trials may lead to greater enrollment, improving the representation of African-American Americans in clinical trials; more discussion of purpose and risks may help to achieve shared decision-making.
Clinical trials remain the gold standard of progress in cancer research and patient care, but low enrollment, particularly among minorities, threatens that progress and may contribute to disparities in cancer care and outcomes. Studies of real-time recruitment such as this one can identify racial disparities in communication that may lead to minority underrepresentation in clinical trials and suggest communication strategies that may lead to greater minority enrollment in clinical trials.
Acknowledgements
We thank Tanina Foster, M.Ed, who provided assistance with data collection and the Wayne State University Karmanos Cancer Institute Behavioral and Field Research Core, which provided assistance with data collection and management.
Funding: The authors disclosed receipt of the following financial support for the research: National Cancer Institute RO1CA75003 and P30CA 22453
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Braveman P. Health disparities and health equity: concepts and measurement. Annu Rev Public Health. 2006;27:167–94. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: a cancer journal for clinicians. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Smedley BD, Stith AY, Nelson AR. Unequal treatment: Confronting racial and ethnic disparities in health care. National Academies Press; Washington DC: 2003. [PubMed] [Google Scholar]
- 4.Penner LA, Eggly S, Griggs JJ, Orom H, Underwood W, Albrecht TL. Life-Threatening Disparities: The Treatment of Black and White Cancer Patients. J Soc Issues. 2012;68:328–257. doi: 10.1111/j.1540-4560.2012.01751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouad MN. Enrollment of minorities in clinical trials: did we overcome the barriers? Contemporary clinical trials. 2009;30:103–4. doi: 10.1016/j.cct.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Bolen S, Tilburt J, Baffi C, Gary TL, Powe N, Howerton M, et al. Defining “success” in recruitment of underrepresented populations to cancer clinical trials: moving toward a more consistent approach. Cancer. 2006;106:1197–204. doi: 10.1002/cncr.21745. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt ME, Simone JV, National Cancer Policy Board (U.S.) Ensuring quality cancer care. National Academy Press; Washington, D.C.: 1999. [Google Scholar]
- 8.Nass SJ, Moses HL, Mendelsohn J. A National Cancer Clinical Trials System for the 21st Century: Reinvigorating the NCI Cooperative Group Program Institute of Medicine. Committee on Cancer Clinical Trials and the NCI Cooperative Group Program; 2010. [PubMed] [Google Scholar]
- 9.Albrecht TL, Blanchard C, Ruckdeschel JC, Coovert M, Strongbow R. Strategic physician communication and oncology clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:3324–32. doi: 10.1200/JCO.1999.17.10.3324. [DOI] [PubMed] [Google Scholar]
- 10.Albrecht TL, Eggly SS, Gleason ME, Harper FW, Foster TS, Peterson AM, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:2666–73. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggly S, Albrecht TL, Harper FW, Foster T, Franks MM, Ruckdeschel JC. Oncologists’ recommendations of clinical trial participation to patients. Patient Educ Couns. 2008;70:143–8. doi: 10.1016/j.pec.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139:907–15. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 13.Gordon HS, Street RL, Jr., Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients’ perceptions of physician communication. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:904–9. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 14.Siminoff LA, Graham GC, Gordon NH. Cancer communication patterns and the influence of patient characteristics: disparities in information-giving and affective behaviors. Patient Educ Couns. 2006;62:355–60. doi: 10.1016/j.pec.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Eggly S, Harper FW, Penner LA, Gleason MJ, Foster T, Albrecht TL. Variation in question asking during cancer clinical interactions: A potential source of disparities in access to information. Patient Educ Couns. 2011;82:63–8. doi: 10.1016/j.pec.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. American journal of public health. 2004;94:2084–90. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jean-Pierre P, Fiscella K, Griggs J, Joseph JV, Morrow G, Carroll J, et al. Race/ethnicity-based concerns over understanding cancer diagnosis and treatment plan. Journal of the National Medical Association. 2010;102:184–9. doi: 10.1016/s0027-9684(15)30524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song L, Hamilton JB, Moore AD. Patient-healthcare provider communication: Perspectives of African American cancer patients. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2011 doi: 10.1037/a0025334. [DOI] [PubMed] [Google Scholar]
- 19.Gordon HS, Street RL, Jr., Sharf BF, Souchek J. Racial differences in doctors’ information-giving and patients’ participation. Cancer. 2006;107:1313–20. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 20.Cegala DJ, Chisolm DJ, Nwomeh BC. Further examination of the impact of patient participation on physicians’ communication style. Patient Education and Counseling. 2012 doi: 10.1016/j.pec.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Street RL, Jr., Gordon H, Haidet P. Physicians’ communication and perceptions of patients: is it how they look, how they talk, or is it just the doctor? Social science & medicine. 2007;65:586–98. doi: 10.1016/j.socscimed.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Services UDoHaH, editor. CFR. 2009;45:46. Code of federal regulations title 45 public welfare part 46 protection of human subjects, 1/09 update. 2009. [Google Scholar]
- 23.Surveillance Epidemiology and End Results. 2012.
- 24.Roberts C, Sarangi S. Theme-oriented discourse analysis of medical encounters. Medical Education. 2005;39:632–40. doi: 10.1111/j.1365-2929.2005.02171.x. [DOI] [PubMed] [Google Scholar]
- 25.Chafe W. The analysis of discourse flow. In: Schiffrin D, Tannen D, Hamilton HE, editors. The Handbook of Discourse Analysis. Blackwell; Oxford: 2003. [Google Scholar]
- 26.Cohen J. Statistical power analysis for the behavioral sciences. Rev. ed Academic Press; New York: 1977. [Google Scholar]
- 27.Yuan J, Liberman M, Cieri C. Towards an integrated understanding of speaking rate in conversation. Interspeech. 2006:541–4. [Google Scholar]
- 28.Barton E, Eggly S. Ethical or Unethical Persuasion? The Rhetoric of Offers to Participate in Clinical Trials. Written Communication. 2009;26:295–319. [Google Scholar]
- 29.Skloot R. The immortal life of Henrietta Lacks. Crown Publishers; New York: 2010. [Google Scholar]
- 30.Katz RV, Kegeles SS, Kressin NR, Green BL, James SA, Wang MQ, et al. Awareness of the Tuskegee Syphilis Study and the US presidential apology and their influence on minority participation in biomedical research. American journal of public health. 2008;98:1137–42. doi: 10.2105/AJPH.2006.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halbert CH, Weathers B, Delmoor E, Mahler B, Coyne J, Thompson HS, et al. Racial differences in medical mistrust among men diagnosed with prostate cancer. Cancer. 2009;115:2553–61. doi: 10.1002/cncr.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21:879–97. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: the implications of psychological processes for understanding racial disparities in health and health care. Social science & medicine. 2008;67:478–86. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Penner LA, Dovidio JF, West T, Albrecht TL, Gaertner SL, Dailey RK, et al. Aversive Racism and Medical Interactions with Black Patients: A Field Study. Annals of Behavioral Medicine. 2010;39:24. doi: 10.1016/j.jesp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Medical care. 2008;46:678–85. doi: 10.1097/MLR.0b013e3181653d58. [DOI] [PubMed] [Google Scholar]
- 36.Cooper LA, Roter DL, Carson KA, Beach MC, Sabin JA, Greenwald AG, et al. The Associations of Clinicians’ Implicit Attitudes About Race With Medical Visit Communication and Patient Ratings of Interpersonal Care. American journal of public health. 2012;102:979–87. doi: 10.2105/AJPH.2011.300558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair IV, Steiner JF, Fairclough DL, Hanratty R, Price DW, Hirsh HK, et al. Clinicians’ implicit ethnic/racial bias and perceptions of care among black and latino patients. Ann Fam Med. 2013;11:43–52. doi: 10.1370/afm.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colon-Otero G, Smallridge RC, Solberg LA, Jr., Keith TD, Woodward TA, Willis FB, et al. Disparities in participation in cancer clinical trials in the United States : a symptom of a healthcare system in crisis. Cancer. 2008;112:447–54. doi: 10.1002/cncr.23201. [DOI] [PubMed] [Google Scholar]
- 39.Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112:228–42. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 40.Adams-Campbell LL, Ahaghotu C, Gaskins M, Dawkins FW, Smoot D, Polk OD, et al. Enrollment of African Americans onto clinical treatment trials: study design barriers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:730–4. doi: 10.1200/JCO.2004.03.160. [DOI] [PubMed] [Google Scholar]
- 41.Freedman LS, Simon R, Foulkes MA, Friedman L, Geller NL, Gordon DJ, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993--the perspective of NIH clinical trialists. Controlled clinical trials. 1995;16:277–85. doi: 10.1016/0197-2456(95)00048-8. discussion 86-9, 93-309. [DOI] [PubMed] [Google Scholar]
- 42.Howerton MW, Gibbons MC, Baffi CR, Gary TL, Lai GY, Bolen S, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007;109:465–76. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 43.Newman LA, Pollock RE, Johnson-Thompson MC. Increasing the pool of academically oriented African-American medical and surgical oncologists. Cancer. 2003;97:329–34. doi: 10.1002/cncr.11027. [DOI] [PubMed] [Google Scholar]
- 44.van Ryn M, Saha S. Exploring unconscious bias in disparities research and medical education. JAMA : the journal of the American Medical Association. 2011;306:995–6. doi: 10.1001/jama.2011.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]