Abstract
Background
Methods that predict prognosis and response to therapy in pulmonary hypertension (PH) are lacking. We tested whether the noninvasive estimation of hemodynamic parameters during 6MWT in PH patients provides information that can improve the value of the test.
Methods
We estimated hemodynamic parameters during the 6MWT using a portable, signal-morphology based, impedance cardiograph (PhysioFlow Enduro, Paris, France) with real time wireless monitoring via a bluetooth USB adapter.
Results
We recruited 48 subjects in the study (30 with PH and 18 healthy controls). PH patients had significantly lower maximum SV and CI and slower CO acceleration and decelerations slopes during the test when compared with healthy controls. In PH patients, CI change was associated with total distance walked (R=0.62, p<0.001) and percentage of predicted (R=0.4, p=0.03), HR recovery at 1 min (0.57, p<0.001), 2 min (0.65, p<0.001) and 3 min (0.66, p<0.001). Interestingly, in PH patients CO change during the test was predominantly related to an increase in SV instead of HR.
Conclusions
Estimation of hemodynamic parameters such as cardiac index during six-minute walk test is feasible and may provide useful information in patients with pulmonary hypertension.
Keywords: Cardiac output, Six-minute walk test, Pulmonary Hypertension
Introduction
Pulmonary hypertension (PH) is a condition characterized by elevated pulmonary pressure that can lead to right heart failure and death 1. There is a pressing need to identify affordable noninvasive methodologies that can provide accurate information on severity of disease, prognosis, and response to therapy. Traditionally, six-minute walk test (6MWT) has been the examination of choice to assess response to treatments in PH and therefore the most common primary endpoint of studies that evaluated therapies in PH 2–10. Recent investigations have questioned its value, since change in 6MWT distance after treatment was not a predictor of hospitalization for PH, need for PH rescue therapy, lung transplantation, or mortality 11,12. However, supporters of the 6MWT express that it is a measure of exercise capacity and argue that this tool was never intended to be a surrogate for pulmonary hemodynamics or severity of pulmonary vascular disease 13. In view of these limitations, investigators have made efforts to increase the ability of the 6MWT to predict clinical outcomes with moderate success14,15.
In order to enhance the information, obtained from this simple and commonly used examination, we performed non-invasive estimations of a variety of hemodynamic parameters using a signal morphology-based impedance cardiograph. Impedance cardiography 16 is a well studied methodology that is based on the Ohm’s law that states that when a constant current travels through a conductor, changes in voltage are directly proportional to variations in impedance 17. Bio-impedance decreases across the chest during systole as a result of an increase in aortic blood volume and flow velocity.
We have previously demonstrated the value of signal morphology based impedance cardiography in patients with PH using the PhysioFlow Lab1 (Manatec Biomedical, Paris, France), a device that calculates stroke volume (SV) independently of baseline impedance 16, a source of inaccuracies in the measurements. We found that the cardiac output (CO) estimated by this device had a high consistency, acceptable accuracy, and precision when compared with thermodilution 16. In support of our findings, other investigators have tested a similar technology called bioreactance in patients with PH and found it to be precise and reliable in measuring CO 18.
In the present study, we obtained real time noninvasive estimations of hemodynamic parameters during 6MWT using a portable and miniaturized version of the PhysioFlow lab1, called Physioflow enduro (Manatec Biomedical, Paris, France). We compared measurements obtained in patients with PH and age- and gender- matched healthy controls. In both groups we determined the extent of increase in CO during the walk, and evaluated the unique contribution of stroke volume (SV) and heart rate (HR) to this increase. In addition, we tested whether the hemodynamic estimations by impedance cardiography are associated with severity of PH disease. Preliminary results of this study have been previously reported in the form of abstracts 19,20.
Methods
This prospective study was approved by the Cleveland Clinic Institutional Review Board (study number 11–226). Written informed consent was obtained from all subjects before enrollment. Patients were asked to participate before the 6MWT. The study was performed between August 2011 and November 2012. The 6MWT was ordered by the patient’s pulmonary physicians as an initial or follow-up assessment of their PH. We included patients with PH confirmed by right heart catheterization (RHC) (n=30) and healthy controls (n=18) matched for age and gender. Patients on PH-specific therapies and treatment naïve patients were included. Healthy controls were recruited by placing recruitment flyers in our outpatient clinic. A different group of seven healthy individuals had two 6MWTs, thirty minutes apart, to determine test-retest reliability.
We used a portable (dimensions 11.5 x 8.5 x 1.8 cm, weight of < 200 g), new generation, signal morphology-based impedance cardiograph with real time wireless monitoring via a bluetooth USB adapter (PhysioFlow Enduro, Paris, France) (Figure 1). The basic principle of this methodology is that variations in the impedance (Z) to an alternating high-frequency (75 KHz) and low magnitude (1.8 mA) current, across the thorax during cardiac ejection, result in a specific waveform that can be used to calculate stroke volume (SV)21.
Figure 1. Portable impedance cardiograph.
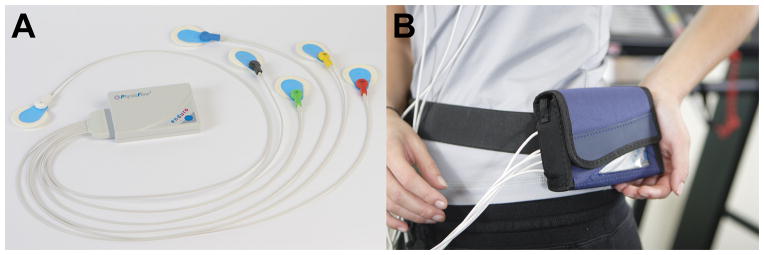
Panel A shows the device with the cable electrodes and panel B depicts the device in the custom fit pouch that helps maintains its stability during the six-minute walk test.
We prepped the skin with a mildly abrasive gel (NuPrep) and 70% isopropyl alcohol. Once the skin was dry we placed electrodes (pregelled Ag/AgCl, Skintact model FS-TB wet-gel; Leonhard Lang Gmbh, Austria) at the left base of the neck above the supraclavicular fossa (n=2), left paraspinal muscles (close to the spine) at the level of the xyfoid process (n=2), right upper (n=1) and left lower chest (n=1). All electrodes were connected to the portable impedance cardiograph via an electrode cable. We affixed the electrodes in place with paper tape (3M micropore paper tape). The impedance cardiograph was then placed in a belt pouch to maintain stability during the walk.
We connected the device to a portable computer that had a type 1 Bluetooth-USB adapter with external antenna that supported 300 m of wireless transmission (SENA UD100 Bluetooth USB Adapter, Sena Technologies, Inc, Korea). Once pertinent data such as age, weight, height and blood pressure were entered, the device then autocalibrated for 30 consecutive beats, a necessary process to detect the rate of variation of the impedance signal used for the calculation of the initial stroke volume index. A detailed explanation of the methodology used has been previously published 16,22,23.
The 6MWT was performed according to ATS standards 24. We obtained the reference standards for the distance walk during the 6MWT from Enright et al 25. Patients remained seated for approximately 10 minutes before the test (during consenting, electrode placement and connection of the device). We then recorded impedance measurements before (2 min), during (6 min) and after the walk (3 min). We obtained real time determinations of HR, SV, CI, EF and EDV every 15 seconds.
Absolute cardiac index change was calculated as the difference between the maximal CI during the walk and the CI at baseline. Similarly, SV change was obtained by subtracting maximal SV during the walk and SV at baseline. Heart rate and CI recovery were obtained by subtracting either the heart rate or CI at the sixth minute of the 6MWT from the values recorded at the first minute of recovery. The cardiac output slopes were obtained from measurements obtained immediately before the walk and the first minute into the six-minute walk. The cardiac output deceleration slope was obtained just before the finalization of the walk and the first minute of recovery. The method of measuring the acceleration and deceleration slopes was established before the initiation of the study. We aimed to capture the acceleration and deceleration slopes during the first minute of the walk and recovery, respectively; since these are the periods during 6MWT when the more pronounced hemodynamic changes occur. For the calculation of the slopes we used all the measurements obtained during each of the two-minute intervals (n=8).
To test reproducibility seven healthy control subjects participated in two six-minute walk sessions separated by 30 minutes of rest. Patients were disconnected after the end of the first session and reconnected before the second test. Tests were performed under the same conditions.
Statistical analysis
Continuous variables were summarized using mean and standard deviation. The single measures intraclass correlation coefficient (ICC) values were computed to assess test-retest reliability of the measurements. An intraclass correlation coefficients value of 1 is considered as highest reproducibility. We calculated semipartial correlations using linear regression with cardiac index change as dependent variable and HR change or SV change as covariates. We used T-test and Fischer’s exact test to compare numerical and categorical variables, respectively. Analysis of covariance (ANCOVA) was applied to obtain means adjusted for covariates and Pearson correlation to identify the strength of association between variables. We utilized binary logistic regression to compare the hemodynamic response during 6MWT between patients with PH and matched healthy controls. All the P values were reported as two tailed. A P value of <0.05 was prespecified as indicative of statistical significance. The statistical analyses were performed using the statistical package SPSS, Version 20 (IBM; Armonk, N.Y., USA).
Results
Patient’s characteristics
Causes of PH (n=30) included pulmonary arterial hypertension (PAH) (n=23, 76 %) and others (n=7, 24%). Pulmonary arterial hypertension was mainly attributed to idiopathic or heritable (n=14) PAH (Table 1). The New York Heart Association (NYHA) functional class in PH patients was 2.3 (±0.7). All but four (13 %) of the patients in the PH group were receiving PH-specific therapies. Treatments included phosphodiestearase 5-inhibitors in 19 (63 %), endothelin receptor antagonists in 17 (57 %) and prostacyclin analogs in 12 (40 %). Monotherapy, dual therapy and triple therapy were administered to 8 (27 %), 14 (47 %) and 4 (13 %), respectively. Brain natriuretic peptide within 3 months of the test was 161.7 (212) pg/mL. Echocardiography within 3 months of the test revealed a mean (SD) right ventricular systolic pressure of 73 (22) mm Hg. RV function was normal, mildly, moderately or severely dysfunctional in 18 %, 30 %, 30 % and 22 % of the patients, respectively. On RHC, performed a median (interquartile range) of 10.5 (4–25) months before the 6MWT, patients had a right atrial pressure of 9 ± 5 mm Hg, mean pulmonary artery pressure of 49 ± 17 mm Hg, CO of 5 ± 1.8 L/min, CI of 2.7 ± 1 L/min/m2 and pulmonary vascular resistance of 9.3 ± 7 Wood Units.
Table 1.
Causes of PH by group.
| WHO group 1 | n (%) |
|---|---|
| I (PAH) | 23 (76 %) |
| -idiopathic/heritable | 14 |
| -congenital heart disease | 4 |
| -connective tissue disease | 3 |
| -portopulmonary hypertension | 1 |
| -hemolytic disorder | 1 |
| II (Heart disease) | 2 (7 %) |
| -left ventricular diastolic dysfunction | 2 |
| III (Lung disease) | 2 (7%) |
| -COPD | 2 |
| IV (CTEPH) | 1 (3 %) |
| V (miscellaneous) | 2 (7%) |
| -sarcoidosis | 2 |
Abbreviations: CTEPH: chronic thromboembolic pulmonary hypertension, PAH: pulmonary arterial hypertension.
Age was 51.8 ± 15 and 49 ± 11 years for patients with PH and healthy controls, respectively (p=0.5). Women were 70% and 56% (p=0.3) of the patients for PH and healthy controls, respectively.
Six-minute walk test
-Traditional determinations
Results of the traditional 6MWT measurements are shown in table 2. Compared with healthy controls, PH patients walked less with similar heart rate at rest and during the test and had reduced heart rate recovery at 1, 2 or 3 minutes. The test-retest reliability of distance walk in six-minute (n=7) had an intraclass correlation coefficient (95% CI) of 0.83 (0.31–0.97). Meanwhile, maximal heart rate during the test had an intraclass correlation coefficient (95% CI) of 0.95 (0.73–0.99).
Table 2.
Traditional parameters measured during six-minute walk test
| Healthy controls Mean (SD) or n (%) |
PH Mean (SD)or n (%) |
p | |
|---|---|---|---|
| n | 18 | 30 | |
| Distance walked (m) | 560 (65) | 380 (117) | <0.001 |
| Distance walked (% of predicted) | 99 (14) | 67 (16) | <0.001 |
| Need of O2 at baseline (%) | 0 (0) | 4 (13) | 0.28 |
| Need of O2 during walking (%) | 0 (0) | 14 (47) | 0.001 |
| Borg dyspnea scale | 1.3 (1) | 3.3 (3) | 0.02 |
| Borg fatigue scale | 1.2 (1) | 2.6 (2) | 0.001 |
| HR at baseline (bpm) | 76 (14) | 83 (12) | 0.09 |
| HR at six minutes (bpm) | 127 (18) | 117 (20) | 0.09 |
| HR change (bpm) | 52 (23) | 44 (23) | 0.24 |
| HR recovery at 1 min (bpm) | 31 (16) | 20 (12) | 0.01 |
| HR recovery at 2 min (bpm) | 40 (16) | 28 (14) | 0.01 |
| HR recovery at 3 min (bpm) | 45 (16) | 33 (16) | 0.01 |
Abbreviations: bpm: beats per minute, HR: heart rate.
-Hemodynamic determinations
PH patients had significantly lower maximum SV and CI and slower CO acceleration and decelerations slopes during the test when compared with healthy controls (table 3). When PH patients were compared with controls using binary logistic regression, SV (OR (95% CI): 0.94 (0.89–0.99), CI change (OR (95% CI): 0.58 (0.35–0.94)), EDV max (OR (95%CI): 0.98 (0.97–0.99)), EDV change (OR (95% CI): 0.97 (0.94–0.99), acceleration (OR (95% CI): 0.73 (0.59–0.90)) and deceleration slopes (OR (95% CI): 1.8 (1.2–2.7)) per 0.1 units were able to discriminate between the groups. When adjusted by distance walked EDV max (OR (95% CI): 0.98 (0.97–0.99)) and EDV change (OR (95% CI): 0.98 (0.96–0.99)) were the only variables that continued to be significant predictors of the presence of PH. The estimated means (SD) of the change in CI adjusted by total distance walked were 2.9 (1.4) and 3 (1.3) L/min/m2 for the healthy control and PH groups, respectively (p=0.77). The estimated means (SD) of the EDV change adjusted by total distance walk were 61.8 (34) and 34.9 (31) mL for the healthy controls and PH groups, respectively (p=0.02).
Table 3.
Hemodynamic parameters measured during six-minute walk test
| Healthy controls Mean (SD) |
PH Mean (SD) |
p | |
|---|---|---|---|
| n | 18 | 30 | |
| SV at baseline (mL) | 62 (19) | 51 (17) | 0.046 |
| SV max (mL) | 95 (26) | 75 (22)* | 0.006 |
| SV change (mL) | 34 (12) | 24 (14) | 0.02 |
| CI at baseline (L/min/m2) | 2.3 (0.9) | 2.3 (0.7) | 0.81 |
| CI max (L/min/m2) | 5.9 (1.7) | 4.9 (1.5)* | 0.04 |
| CI change (L/min/m2) | 3.6 (1.4) | 2.6 (1.2)* | 0.02 |
| CI recovery at 1 min (L/min/m2) | 1.7 (1) | 0.6 (0.6)* | <0.001 |
| CO acceleration slope (L/min/s) | 0.08 (0.04) | 0.04 (0.03)* | 0.001 |
| CO deceleration slope (L/min/s) | −0.06 (0.03) | −0.02 (0.03)* | <0.001 |
| EF at baseline (%) | 48 (17) | 44 (13) | 0.32 |
| EF max (%) | 60 (16) | 55 (16) | 0.27 |
| EF change (%) | 12 (4) | 11 (8) | 0.59 |
| EDV at baseline | 135 (31) | 126 (43) | 0.42 |
| EDV max | 195 (46) | 161 (46) | 0.02 |
| EDV change | 59 (30) | 36 (27)* | 0.007 |
Abbreviations: CI: cardiac index, CO: cardiac output, EDV: end-diastolic volume, EF: ejection fraction, LVET: left ventricular ejection time, SV: stroke volume.
In PH individuals, CO variation was predominantly associated with changes in stroke volume rather than heart rate as suggested by a higher standardized Beta coefficient and semi-partial correlations. SV change and HR change uniquely explained 66 % and 29 % of the CO change variance accounted. The rest of the variance was explained by overlap of the variables stroke volume and heart rate change. To the contrary, in healthy volunteers the contribution of HR and SV was more balanced with a greater degree of overlap (Table 4).
Table 4.
Linear regression using cardiac index change as dependent variable and HR change or SV change as covariates.
| Healthy controls | PH | |
|---|---|---|
| HR change standardized Beta coefficient | 0.5 | 0.54 |
| SV change standardized Beta coefficient | 0.59 | 0.82 |
| HR change semipartial correlation | 0.43 | 0.54 |
| SV change semipartial correlation | 0.51 | 0.81 |
| HR change unique contribution to CO change (%) | 18 | 29 |
| SV change unique contribution to CO change (%) | 26 | 66 |
Abbreviations: CO: cardiac output, HR: heart rare, SV: stroke volume.
The baseline CO intraclass correlation coefficient (95% CI) for single measures was 0.91 (0.56–0.98) with a coefficient of variation of 5.7 %. Maximal CO and SV during exercise as well as acceleration slope had an intraclass correlation coefficients (95% CI) of 0.92 (0.62–0.98), 0.97 (0.83–0.99) and 0.88 (0.46–0.98), respectively.
Comparison of traditional versus cardiac index change during six-minute walk test
In patients with PH, CI change was associated with distance walked either in absolute value (R=0.62, p<0.001) or percent of predicted (R=0.4, p=0.03) (Table 5). CI change was not associated with HR at baseline (R=-0.01, p=0.98) but with maximal HR (R=0.48, p=0.007) and HR recovery at 1 min (0.57, p<0.001), 2 min (0.65, p<0.001) and 3 min (0.66, p<0.001).
Table 5.
Correlation matrix between CI change and traditional six-minute walk parameters
| Healthy controls R (p) |
PH R (p) |
|
|---|---|---|
| n | 18 | 30 |
| Distance walked (m) | 0.17 (0.5) | 0.62 (<0.001) |
| Distance walked (% of predicted) | 0.5 (0.04) | 0.40 (0.03) |
| HR at baseline | −0.38 (0.13) | −0.01 (0.98) |
| HR max | 0.59 (0.01) | 0.48 (0.007) |
| HR recovery at 1 min | 0.77 (<0.001) | 0.57 (0.001) |
| HR recovery at 2 min | 0.69 (0.002) | 0.65 (<0.001) |
| HR recovery at 3 min | 0.64 (0.004) | 0.66 (<0.001) |
Abbreviations: HR: heart rate.
Distance walked in six-minute and impedance cardiography determinations
Total distance walked and percentage of predicted were associated with HR at six minutes (R-0.52, p=0.007 and R=0.52, p=0.001, respectively), HR change (R=0.43, p=0.02) and HR recovery at 1 minute (R=0.64, p<0.001 and R=0.39, p=0.032, respectively), HR recovery at 2 minute and 3 minute (R=0.66, p<0.001, R=0.69, p<0.001, respectively). Total distance walked was also associated with the CO acceleration and decelerations slopes (R=0.51, p=0.005; R= −0.4, p=0.03), maximal CI (R= 0.44, p=0.014) and CI change (R=0.62, p<0.001). When HR recovery in 1 minute and CI change were included in a liner regression model both variables remained significant predictors of the distance walked at six minutes (data not shown).
Comparison of impedance cardiography determinations with indicators of PH disease severity
NYHA functional class was inversely associated with maximal CI (R=−0.44, p=0.02), acceleration slope (R=−0.39, p=0.04), CI recovery in 1 minute (R=−0.48, p=0.01), CI change (R=−0.48, p=0.01). NYHA class was directly associated with deceleration slope (R= 0.46, p=0.01). No significant associations were identified between BNP and estimated hemodynamic parameters; however there was a suggestion of an indirect association with acceleration slope (R-0.34, p=0.1) and CI change (R:−0.32, p=0.1). Similarly, there was a suggestion that CI recovery at 1 minute (3.3 versus 4.2 L/min/m2, p=0.06) was lower in patients with moderate or severe RV dysfunction than those with normal RV function or mild dysfunction. Right ventricular systolic pressure estimated by echocardiography was not associated with impedance cardiography determinations during 6MWT. Even though the RHC was performed a median of 10.5 months before the 6MWT, we found that the thermodilution CO during RHC directly correlated with 6MWT maximal CO (R=0.42, p=0.03), acceleration slope (R=0.59, p=0.002), CO change (R=0.54, p=0.004) and CO recovery in 1 minute (R=0.43, p=0.035). Right atrial pressure, mean pulmonary artery pressure and pulmonary vascular resistance were not associated with parameters estimated by impedance cardiography during 6MWT. PH patients were followed a median (interquartile range) of 19 (8–21) months and all but one patient were alive up to June 2013. The PH patient that died during follow up had low maximal CI (3.5 L/min/m2), CI change (1.2 L/min/m2) and CI recovery in 1 minute (0.5 L/min/m2) during 6MWT.
Discussion
Although the 6MWT is an easy to perform, inexpensive, reproducible, and safe method for assessing functional exercise capacity in patients with different lung diseases 26, it has limitations both in clinical practice and research 27,28. There is a need to discover new methods that can accurately predict prognosis and treatment response in PH. Recent advances in technology permitted the development of a portable, signal morphology-based impedance cardiograph that can continuously measure a variety of hemodynamic parameters such as CI. In this pilot study we showed that is possible to incorporate this novel and noninvasive technology as part of the traditional 6MWT. Impedance cardiography provided real time estimations of several hemodynamic parameters during 6MWT. In addition, this study showed that in PH patients variations in SV are of critical importance in explaining the CO increases during the test. Our study has also confirmed the reliability of PhysioFlow measurements during 6MWT in addition to the previously demonstrated validity compared to hemodynamic measurements obtained during a right heart catheterization at rest 22,29,30.
Cardiac output is commonly impaired during activities in patients with PH, despite relatively normal values at rest 31. During six minute walk test, PH patients walked less distance, had lower HR recovery values, maximum SV and CI change as well as slower acceleration and decelerations CO slopes compared with healthy controls. In support of our findings, a study on exercise showed slower increases and decreases in oxygen consumption in PH patients than in matched controls 32. The attenuated rise and decline in CO can be due to autonomic imbalance and/or pulmonary vascular alteration that affect the SV 33,34. Interestingly, a study in PH patients using impedance cardiography (PhysioFlow lab1) during incremental cardiopulmonary exercise test on a cycle ergometer showed that lower absolute changes in SV and CI were related with death and atrial septostomy 21.
Distance walked at six-minute was associated with chronotropic response (HR at six minute and HR change) and CI changes, as observed by other investigators 35,36. Interestingly, the differences in hemodynamic parameters between patients with PH and healthy controls disappeared when adjusted by distance walked, except for EDV that remained significantly lower in PH patients than controls. Lower EDV may reflect a worse hemodynamic state characterized by a decrease in the left ventricular size due to negative left-to-right transseptal pressure gradient 37. This left ventricular underfilling may affect cardiac performance by decreasing the CI during exercise 38.
Heart rate recovery, a determination that has proven to be of value in PH 14,39, had a good direct association with CO change during the test. Lower heart rate recovery likely represents an autonomic imbalance with increase sympathetic drive and impaired parasympathetic activity 40,41, in part explained by a decrease in cardiac output among other factors 39.
The standard 6MWT does not provide any insight into the mechanisms of exercise limitation. The factors that limit exercise capacity include an imbalance between the oxygen requirements and supply. In patients with PH the peak SV during exercise is limited by the increase in pulmonary artery pressure 42–44. We observed a predominant unique contribution of SV to CO change in patients with PH compared to controls. A study comparing the results of 6MWT with cardiopulmonary exercise in PH patients showed that the O2 pulse, a surrogate of stroke volume, was lower in patients with PH than in controls and it directly correlated with distance walked, thus limitations in the latter may be a reflection of insufficient systemic O2 delivery during the activity 26 and/or a reflection of impaired oxygen utilization due to reduced mitochondrial function 45.
We tested whether the hemodynamic estimations provided by impedance cardiography during 6MWT were associated with traditional variables that predict severity of disease in PH. In addition to 6MW distance, we found that NYHA functional class had a good association with impedance cardiography determinations. Interestingly, CO measured during RHC was associated with CO change, acceleration slope and recovery at 1 minute. Even though this was not the main aim of our study, we found a suggestion that hemodynamic estimations by impedance cardiography during 6MWT may track with disease severity, supporting that this test is worthy of further investigations to better clarify its clinical role in PH.
Our study has limitations a) PH was considered not present in patients with pulmonary disease based on echocardiography, a methodology that has its limitations and could lead to misclassifications, b) some of the comparisons may be underpowered to detect a difference; however the main purpose of this pilot study was to test the feasibility of incorporating this noninvasive methodology to an established examination and determine the relative contribution of HR and SV to CO change during six minute walk test, c) the study was not designed to test whether portable impedance cardiography can predict the severity of disease or response to treatment, and d) most of the PH patients were receiving PH-specific treatment thus the results observed in this group of patients are not applicable to treatment naïve individuals.
Conclusions
Estimation of hemodynamic parameters such us cardiac index during 6MWT is feasible and reliable. In patients with PH, stroke volume plays a more predominant role in the CO increase during six-minute walk test than heart rate. Further studies are necessary to test whether absolute changes in SV and CI during six-minute walk test can provide useful information regarding prognosis and response to therapy in PH patients.
Acknowledgments
Funding sources: This publication was made possible by CTSA KL2 [Grant # RR024990] (A.R.T.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Manatec Biomedical, the makers of PhysioFlow Enduro had no role in the design of the study, data collection or analysis, or in the manuscript preparation. The company provided the equipment for testing and technical support.
We appreciate the invaluable help provided by the pulmonary function test laboratory personnel at Cleveland Clinic that made this study possible. We are thankful with Manatec Biomedical for providing us with PhysioFlow Enduro and technical assistance.
Abbreviations
- CI
cardiac index
- CO
cardiac output
- EDV
end-diastolic volume
- EF
ejection fraction
- LVET
left ventricular ejection time
- RHC
right heart catheterization
- SV
stroke volume
- TFI
thoracic fluid index
Footnotes
Conflict of interest statements:
Adriano R. Tonelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Laith Alkukhun MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Vineesha Arelli MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Jose Ramos RT: Supervisor of Pulmonary Function, The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Jennie Newman LPN: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Kevin McCarthy: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Bohdan Pichurko MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Omar A. Minai MD: Member Scientific Advisory Board: Actelion, Gilead, United Therapeutics, Pfizer and Bayer. Member Speakers Bureau: Actelion, Gilead and United Therapeutics.
Raed A. Dweik MD: The author has no significant conflicts of interest with any companies or organization whose products or services may be discussed in this article.
Abstract presentations: Preliminary results of this study have been previously reported in the form of abstracts at the American Thoracic Association and the Clinical and Translational Science meetings in 2012.
Contributions of authors:
Adriano R. Tonelli MD: Participated in the design of the study, data collection, statistical analysis, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted. Dr Tonelli is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Laith Alkukhun MD: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Vineesha Arelli MD: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Jose Ramos RT: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Jennie Newman LPN: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Kevin McCarthy: Participated in the data collection, interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Bohdan Pichurko MD: Participated in the interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Omar A. Minai MD: Participated in the interpretation of the results and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
Raed A. Dweik MD: Participated in the design of the study, interpretation of the results, writing and critical revision of the manuscript for important intellectual content and final approval of the manuscript submitted.
References
- 1.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009 Jun 30;54(1 Suppl):S43–54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008 Jun 10;117(23):3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2002 Mar 15;165(6):800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 4.Simonneau G, Rubin LJ, Galie N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med. 2008 Oct 21;149(8):521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 5.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002 Mar 21;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med. 2005 Nov 17;353(20):2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 7.Galie N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009 Jun 9;119(22):2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 8.Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann Intern Med. 2000 Mar 21;132(6):425–434. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 9.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002 Aug 1;347(5):322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010 May 4;55(18):1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Savarese G, Paolillo S, Costanzo P, et al. Do changes of 6-minute walk distance predict clinical events in patients with pulmonary arterial hypertension?: a meta-analysis of 22 randomized trials. J Am Coll Cardiol. 2012 Sep 25;60(13):1192–1201. doi: 10.1016/j.jacc.2012.01.083. [DOI] [PubMed] [Google Scholar]
- 12.Macchia A, Marchioli R, Marfisi R, et al. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007 Jun;153(6):1037–1047. doi: 10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 13.Rich S. The 6-minute walk test as a primary endpoint in clinical trials for pulmonary hypertension. J Am Coll Cardiol. 2012 Sep 25;60(13):1202–1203. doi: 10.1016/j.jacc.2012.03.080. [DOI] [PubMed] [Google Scholar]
- 14.Minai OA, Gudavalli R, Mummadi S, Liu X, McCarthy K, Dweik RA. Heart rate recovery predicts clinical worsening in patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012 Feb 15;185(4):400–408. doi: 10.1164/rccm.201105-0848OC. [DOI] [PubMed] [Google Scholar]
- 15.Batal O, Khatib OF, Dweik RA, Hammel JP, McCarthy K, Minai OA. Comparison of baseline predictors of prognosis in pulmonary arterial hypertension in patients surviving </=2 years and those surviving >/=5 years after baseline right-sided cardiac catheterization. Am J Cardiol. 2012 May 15;109(10):1514–1520. doi: 10.1016/j.amjcard.2012.01.366. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli AR, Alnuaimat H, Li N, Carrie R, Mubarak KK. Value of impedance cardiography in patients studied for pulmonary hypertension. Lung. 2011 Oct;189(5):369–375. doi: 10.1007/s00408-011-9299-y. [DOI] [PubMed] [Google Scholar]
- 17.Sodolski T, Kutarski A. Impedance cardiography: A valuable method of evaluating haemodynamic parameters. Cardiol J. 2007;14(2):115–126. [PubMed] [Google Scholar]
- 18.Rich JD, Archer SL, Rich S. Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur Respir J. 2012 Oct 25; doi: 10.1183/09031936.00102212. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli A, Arelli V, Ramos J, McCarthy K, Pichurko B, Dweik R. Determination of hemodynamic parameters during 6-minute walk test in pulmonary hypertension. Clinical and Translational Science. 2012;5(2):141–210. doi: 10.1111/cts.12090. P168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arelli V, Ramos J, McCarthy K, Pichurko B, Dweik R. Determination of hemodynamic parameters during 6-minute walk test in pulmonary hypertension. Am J Respir Crit Care Med. 2012;185:A4093. [Google Scholar]
- 21.Ferreira EM, Ota-Arakaki JS, Barbosa PB, et al. Signal-morphology impedance cardiography during incremental cardiopulmonary exercise testing in pulmonary arterial hypertension. Clin Physiol Funct Imaging. 2012 Sep;32(5):343–352. doi: 10.1111/j.1475-097X.2012.01135.x. [DOI] [PubMed] [Google Scholar]
- 22.Charloux A, Lonsdorfer-Wolf E, Richard R, et al. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: comparison with the “direct” Fick method. Eur J Appl Physiol. 2000 Jul;82(4):313–320. doi: 10.1007/s004210000226. [DOI] [PubMed] [Google Scholar]
- 23.Hsu AR, Barnholt KE, Grundmann NK, Lin JH, McCallum SW, Friedlander AL. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J Appl Physiol. 2006 Jun;100(6):2031–2040. doi: 10.1152/japplphysiol.00806.2005. [DOI] [PubMed] [Google Scholar]
- 24.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul 1;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 25.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998 Nov;158(5 Pt 1):1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 26.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 27.Heresi GA, Dweik RA. Strengths and limitations of the six-minute-walk test: a model biomarker study in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011 May 1;183(9):1122–1124. doi: 10.1164/rccm.201012-2079ED. [DOI] [PubMed] [Google Scholar]
- 28.Gabler NB, French B, Strom BL, et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012 Jul 17;126(3):349–356. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richard R, Lonsdorfer-Wolf E, Charloux A, et al. Non-invasive cardiac output evaluation during a maximal progressive exercise test, using a new impedance cardiograph device. Eur J Appl Physiol. 2001 Aug;85(3–4):202–207. doi: 10.1007/s004210100458. [DOI] [PubMed] [Google Scholar]
- 30.Bougault V, Lonsdorfer-Wolf E, Charloux A, Richard R, Geny B, Oswald-Mammosser M. Does thoracic bioimpedance accurately determine cardiac output in COPD patients during maximal or intermittent exercise? Chest. 2005 Apr;127(4):1122–1131. doi: 10.1378/chest.127.4.1122. [DOI] [PubMed] [Google Scholar]
- 31.Argiento P, Chesler N, Mule M, et al. Exercise stress echocardiography for the study of the pulmonary circulation. Eur Respir J. 2010 Jun;35(6):1273–1278. doi: 10.1183/09031936.00076009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riley MS, Porszasz J, Engelen MP, Shapiro SM, Brundage BH, Wasserman K. Responses to constant work rate bicycle ergometry exercise in primary pulmonary hypertension: the effect of inhaled nitric oxide. J Am Coll Cardiol. 2000 Aug;36(2):547–556. doi: 10.1016/s0735-1097(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 33.Vasilopoulou MK, Vogiatzis I, Nasis I, et al. On- and off-exercise kinetics of cardiac output in response to cycling and walking in COPD patients with GOLD Stages I-IV. Respir Physiol Neurobiol. 2012 May 31;181(3):351–358. doi: 10.1016/j.resp.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 34.Chiappa GR, Borghi-Silva A, Ferreira LF, et al. Kinetics of muscle deoxygenation are accelerated at the onset of heavy-intensity exercise in patients with COPD: relationship to central cardiovascular dynamics. J Appl Physiol. 2008 May;104(5):1341–1350. doi: 10.1152/japplphysiol.01364.2007. [DOI] [PubMed] [Google Scholar]
- 35.Provencher S, Chemla D, Herve P, Sitbon O, Humbert M, Simonneau G. Heart rate responses during the 6-minute walk test in pulmonary arterial hypertension. Eur Respir J. 2006 Jan;27(1):114–120. doi: 10.1183/09031936.06.00042705. [DOI] [PubMed] [Google Scholar]
- 36.Provencher S, Herve P, Sitbon O, Humbert M, Simonneau G, Chemla D. Changes in exercise haemodynamics during treatment in pulmonary arterial hypertension. Eur Respir J. 2008 Aug;32(2):393–398. doi: 10.1183/09031936.00009008. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Tei C, Nakao S, et al. Diastolic bulging of the interventricular septum toward the left ventricle. An echocardiographic manifestation of negative interventricular pressure gradient between left and right ventricles during diastole. Circulation. 1980 Sep;62(3):558–563. doi: 10.1161/01.cir.62.3.558. [DOI] [PubMed] [Google Scholar]
- 38.Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence-based review. J Heart Lung Transplant. 2010 Feb;29(2):159–173. doi: 10.1016/j.healun.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Ramos RP, Arakaki JS, Barbosa P, et al. Heart rate recovery in pulmonary arterial hypertension: relationship with exercise capacity and prognosis. Am Heart J. 2012 Apr;163(4):580–588. doi: 10.1016/j.ahj.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 40.Wensel R, Jilek C, Dorr M, et al. Impaired cardiac autonomic control relates to disease severity in pulmonary hypertension. Eur Respir J. 2009 Oct;34(4):895–901. doi: 10.1183/09031936.00145708. [DOI] [PubMed] [Google Scholar]
- 41.Ciarka A, Doan V, Velez-Roa S, Naeije R, van de Borne P. Prognostic significance of sympathetic nervous system activation in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010 Jun 1;181(11):1269–1275. doi: 10.1164/rccm.200912-1856OC. [DOI] [PubMed] [Google Scholar]
- 42.Groepenhoff H, Westerhof N, Jacobs W, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Exercise stroke volume and heart rate response differ in right and left heart failure. Eur J Heart Fail. 2010 Jul;12(7):716–720. doi: 10.1093/eurjhf/hfq062. [DOI] [PubMed] [Google Scholar]
- 43.Deboeck G, Niset G, Lamotte M, Vachiery JL, Naeije R. Exercise testing in pulmonary arterial hypertension and in chronic heart failure. Eur Respir J. 2004 May;23(5):747–751. doi: 10.1183/09031936.04.00111904. [DOI] [PubMed] [Google Scholar]
- 44.Holverda S, Gan CT, Marcus JT, Postmus PE, Boonstra A, Vonk-Noordegraaf A. Impaired stroke volume response to exercise in pulmonary arterial hypertension. J Am Coll Cardiol. 2006 Apr 18;47(8):1732–1733. doi: 10.1016/j.jacc.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 45.Tuder RM, Davis LA, Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med. 2012 Feb 1;185(3):260–266. doi: 10.1164/rccm.201108-1536PP. [DOI] [PMC free article] [PubMed] [Google Scholar]


