Abstract
Background
While atrial fibrillation (AF) triggers are known, the underlying AF substrate is less well understood. The goal of our study was to explore correlations between electrophysiological and structural characteristics of atria in patients with paroxysmal AF and individuals at AF risk.
Methods
Patients in sinus rhythm (N=90, age 57±10y; 55 men [63.2%]) with structural heart disease and paroxysmal AF (n=12 [13%]), or with AF risk factors and LVEF > 35% (n=78), underwent SAECG and cardiac magnetic resonance study. Interatrial and epicardial fat was analyzed with a Dark-blood DIR-prepared Fat-Water-separated sequence in the horizontal longitudinal axis. All local P-wave extrema were identified on SAECG leads during sinus rhythm. A P-wave fragmentation (Pf ) was defined as an absolute difference between adjacent extrema which was above 3 standard deviations of noise, and was normalized by the duration of the P-wave in the corresponding lead.
Results
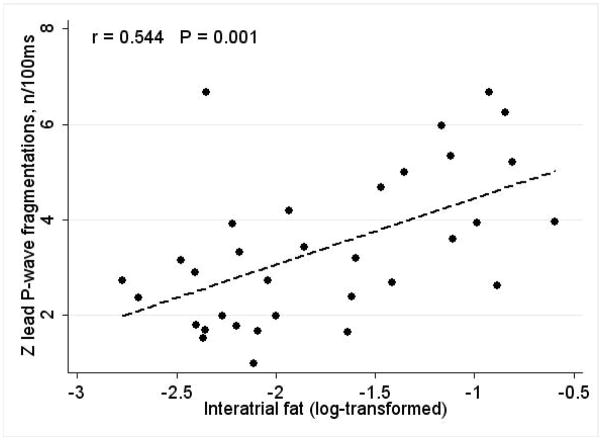
The Pf was greater on the filtered than on the unfiltered P-SAECG signal (13.1±3.8 vs. 3.4±1.2; P < 0.0001). Pf was the greatest on the Y lead (13.0±3.5 on Y lead vs. 12.1±3.4 on Z lead; P=0.003. Pf on Z lead correlated with interatrial fat index (r=0.544; P=0.001). Epicardial fat significantly correlated with body mass index [BMI] (r=0.302; P=0.015). After adjustment for BMI, left atrium (LA) size, epicardial fat and interatrial septum width, interatrial fat independently associated with the Pf on Z lead [β-coefficient 0.009 (95%CI 0.0003–0.019); P=0.043].
Conclusions
Infiltrated atrial fat correlates with discontinuous conduction on posterior LA wall and represents AF early substrate.
Keywords: atrial fibrillation, P-SAECG, cardiac magnetic resonance, infiltrated atrial fat
An estimated 2.23 million people in the United States have diagnosed atrial fibrillation (AF)1. AF increases the risk of stroke and death 2;3 and leads to important socioeconomic consequences such as chronic disease management, disability, and increased hospital admissions. Moreover, the AF burden is increasing due to the aging US population since AF tends to be more prevalent in the elderly 4;5. While our understanding of AF pathophysiology has significantly advanced over the last two decades, the treatment of AF is rarely curative, and in many cases AF progresses from paroxysmal AF to persistent AF 6. The development of a strategy for the primary prevention of AF is urgently needed. However, the substrate and the mechanisms of early AF genesis are not fully understood.
Underlying structural heart diseases, such as coronary heart disease (CHD), hypertensive heart disease, valvular heart disease, cardiomyopathy, and heart failure (HF), predispose patients to AF7. Atrial fibrosis8 is associated with clinically manifest AF. Epicardial fat was shown associated with AF9,10. In the Framingham Heart Study (FHS) epicardial fat was associated with prevalent AF11. Experiments showed paracrine properties of epicardial fat and release of pro-inflammatory and pro-fibrotic substances12. At the same time, autopsy studies demonstrated that fat can infiltrate myocardium13. However, infiltrated atrial fat has not been previously studied in-vivo in patients with paroxysmal AF and in individuals at risk of AF. Only recently technology to quantify in-vivo infiltrated myocardial fat became available14.
Electrophysiologic studies have shown that an abnormally prolonged and fractionated atrial electrogram, which is a sign of delayed and non-uniform conduction through the atria, is characteristic of patients with AF 15;16. Modeling studies have shown that atrial electrogram fractionation is a measure of fibrosis and discontinuous conduction17. Abnormally prolonged P-waves on signal-averaged electrocardiograms (P-SAECGs) predicted AF paroxysms in HF patients 18 and patients after cardiac surgery 19. Still, very few studies have explored atrial electrophysiological properties and atrial structural characteristics in patients at risk of AF, without clinically manifest AF. We hypothesized that number of P-wave fragmentations on P-SAECG correlates with infiltrated atrial fat and represents early substrate of AF.
Methods
Study Population
We present the results of analysis of a baseline visit of an ongoing prospective observational cohort study of patients with structural heart disease without overt systolic heart failure (NCT01353131). Study protocol was approved by the Johns Hopkins Institutional Review Board, and all study participants gave written, informed consent upon entering the study. Study design was reported previously 20;21. Study included patients of the Johns Hopkins Hospital with structural heart disease, who have had a 12-lead ECG recorded between October 1, 2009 and March 31, 2010. Patients younger than 21 and older than 70 years, with left ventricular (LV) systolic dysfunction [LV ejection fraction (EF) ≤ 35%], having chronic renal insufficiency with estimated glomerular filtration rate (eGFR) ≤ 30mL/min, and contraindication to contrast-enhanced CMR were excluded.
P –wave ECG analysis
A standard 12-lead ECG and SAECG (MAC 5000, GE Medical Systems, Milwaukee, WI) was recorded at rest. Cardioactive drugs were not withdrawn before the ECG recording. Orthogonal XYZ leads were continuously recorded with 1000Hz sampling rate and 1μV amplitude resolution. Ectopic atrial and ventricular beats were eliminated and 350 clean sinus beats were collected, aligned, and averaged. Averaged signals for the X, Y, and Z leads were also combined into a vector sum, which was calculated as the Euclidean norm of the X, Y, and Z leads. An ECG Research Workstation, Hires Module (GE Marquette Medical Systems, Milwaukee, WI) was used to translate the proprietary file format and extract unfiltered, averaged X, Y, and Z leads, and the vector sum of the X, Y, and Z leads.
Custom-made software running on MATLAB R 2012a for Mac OS X (The Mathworks, Inc., Natick, MA) was developed. The ECG signal was filtered using a bandpass 40–300 Hz filter. The averaged P-wave on the unfiltered X, Y, and Z leads, filtered X,Y, and Z leads, the unfiltered vector sum, and filtered vector sum were analyzed by 2 investigators (SM, LT), blinded to the CMR results. Unfiltered and filtered P-wave duration on each lead was measured (Figure 1).
Figure 1. P wave fragmentations measurement.
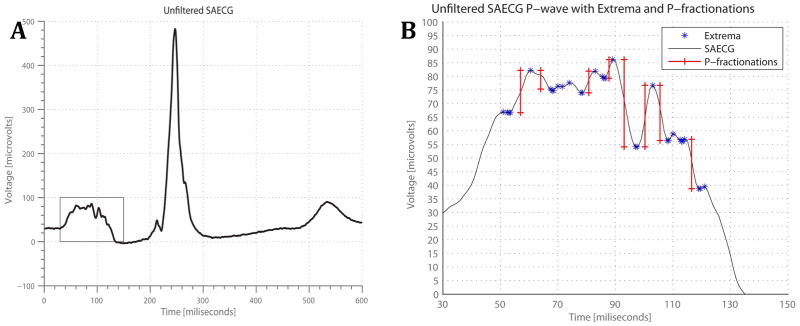
(A) Representative example of SAECG, with the p-wave outlined in the rectangle; (B) P wave further zoomed into the rectangular section. All of the local extrema are shown by stars, and the thresholded P-fragmentations, are shown by vertical lines.
P- fragmentations
P-fragmentations (Pf ) were measured on unfiltered X, Y, and Z leads, filtered X, Y, and Z leads (bandpass 40–300 Hz filter, using zero phase filtering), and the vector sum (Euclidean norm) of the filtered and unfiltered X, Y, and Z leads. First, the baseline noise level was measured on the segment from the end of the T-wave to the beginning of the P-wave. The noise threshold (3SD) was individually determined for each set of data. Local extrema were defined to be those points whose voltages were either strictly greater or less than both the previous and consequent data points. The voltage differences between adjacent local extrema were calculated. If such a difference exceeded pre-defined 3SDs noise threshold, a P-fragmentation was detected. The number of P-fragmentations, average width, and height of the P-fragmentations were calculated. The P-fragmentations were then normalized by the P-wave duration of corresponding lead, per 100ms of the duration of the P-wave. The ECG shown in Figure 1 represents a patient’s SAECG signal, as well as the detection of the differences in adjacent extrema (stars) and the detection of the P-fragmentations (vertical lines).
Atrial CMR Analysis
Patients underwent CMR with a 1.5-T scanner (Siemens Healthcare, Avanto, Erlangen, Germany) on the same day as the ECG recordings. All CMR analyses were performed on dedicated workstations with ARGUS (Siemens Healthcare, PA). All analyses were performed by three experienced observers who were blind to the results of the ECG analysis (NM, PR, CL). The endocardial and epicardial borders of the LV were outlined at end-diastole and end-systole on short-axis cine images. The papillary muscles were included in the left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV), and were excluded from the LV mass. LVEF, LVEDV and LVESV were calculated using Simpson’s rule. LV mass was determined by the sum of the myocardial areas in the end-diastolic phase. LVEDV, LVESV, and LV mass were normalized by body surface area (BSA), and respective indices calculated. BSA was calculated using the Mosteller equation. It was previously shown that BSA impacts LA size parameters22. Total area of the LA and right atrium (RA) were measured and normalized by BSA as well.
A representative example of a patient’s image from our CMR analysis is presented in Figure 2. The presence of interatrial fat was assessed with a Dark-blood DIR-prepared Fat-Water-separated sequence14;23 in the horizontal longitudinal axis (4 chamber view). An OsiriX DICOM viewer (Geneva, Switzerland) was used to quantify the area of the structure of interest. The width of the cephaled portion of the interatrial septum was measured in this study at the level of the fossa ovalis, as described by Shirani et al24. Interatrial fat area was measured, and normalized by BSA. Epicardial fat area was measured in the horizontal longitudinal axis only, and was normalized by BSA as well.
Figure 2. Cardiac magnetic resonance fat image.
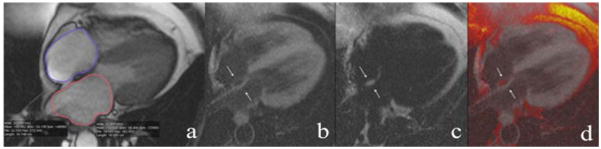
Representative example of Fat-Water DB IR GRE sequence. For the same patient as above, (a) HLA cine MRI during atrial diastole showing left and right atria areas measurement. The bottom row shows the Dark-blood DIR-prepared-Fat-Water-separated sequences. (b) “Water image”-fat signal is suppressed (c) “Fat image” the water signal is suppressed. (d) Fused image (fat and water images added). A cushion of fat deposit in the atrial septum is seen in black in the water image, white in the fat image and black in the fused image (white arrows).
Statistical Analysis
Statistical analysis was computed using STATA 12 (StataCorp LP, College Station, TX). We first explored the distribution of each of our variables. Distribution of interatrial fat and interatrial fat index variables were skewed, and therefore, these variables were log-transformed before entering further analyses. Paired t-test was used to compare atrial electrophysiological parameters measured on different ECG leads (XYZ), and to compare parameters measured on filtered and unfiltered ECG signal. The results are presented as mean ± SD. Categorical variables were compared by Pearson’s chi-square test. Pairwise correlations were studied between normally-distributed variables. Multiple linear regression analysis was performed to determine the predictors of electrophysiological atria characteristic of slow discontinuous conduction Pf. Structural atrial parameters (LA area index, interatrial septum width, epicardial fat index and log-transformed interatrial fat) were adjusted by the body mass index (BMI) in this multiple linear regression model.
Results
Study population
The study population consisted of 90 patients. The mean age was 57±10 years. Slightly more than half of the study population was comprised of men [n=55 (61%)], and whites [n=53 (59%)]. At the same time, the mean LVEF was normal (60.5±9.0), and most of the participants had NYHA class I HF [n=67 (74%)]. Clinical characteristics of the study participants are presented in Table 1. The routine 12 lead ECG parameters and the heart chambers size parameters were within normal range (Table 2).
Table 1.
Clinical characteristics of study participants
| Characteristic | N = 90 |
|---|---|
| Age (SD), y | 59.1(9.3) |
| Females, n(%) | 32(35.5) |
| Whites, n(%) | 34(37.9) |
| Hypertension, n(%) | 63(70) |
| Hypertension Hx (SD), y | 9.9(7.3) |
| Diabetes mellitus, n(%) | (22.2) |
| Diabetes mellitus Hx (SD), y | 8.3(6.2) |
| Body mass index (SD), kg/m2 | 31.0(15.1) |
| History of MI, n(%) | 17(18.9) |
| History of PCI / CABG, n(%) | 19(21.1) |
| NYHA class ≥ II, n(%) | 23(25.6) |
| Systolic blood pressure (SD), mmHg | 147.7(22.2) |
| Diastolic blood pressure (SD), mmHg | 87.7(12.7) |
| eGFR, ml/min | 64.6(2.3) |
| Glucose (SD), mg/dL | 119.8(49.6) |
| Beta-blockers, n(%) | 12(13.3) |
| ACE-I/ARBs, n(%) | 35(38.9) |
| TZD, n(%) | 19(21.1) |
| Ca antagonists, n(%) | 30(30.0) |
| Statins, n(%) | 52(57.8) |
| Current or former smoker, n(%) | 45(50.0) |
Table 2.
Electrophysiological and structural parameters of the heart atria and ventricles.
| Characteristic | N=90 |
|---|---|
| P duration (SD), ms | 112.0(13.1) |
| PQ interval (SD), ms | 176.9(32.6) |
| Heart rate (SD), bpm | 65.7(10.4) |
| QRS duration (SD), ms | 101.6(21.6) |
| P-axis (SD), deg | 48.3(32.1) |
| QTc duration (SD), ms | 429.0(24.5) |
| X lead Pf, n/100ms | 3.2(1.2) |
| Y lead Pf, n/100ms | 4.4(1.9) |
| Z lead Pf, n/100ms | 3.1(1.4) |
| X40–300 Hz Pf, n/100ms | 11.0(3.3) |
| Y40–300 Hz Pf, n/100ms | 13.0(3.5) |
| Z40–300 Hz Pf, n/100ms | 12.1(3.4) |
| SAECG40–300 Hz Pf, n/100ms | 13.2(1.2) |
| LVEF (SD), % | 60.5(9.0) |
| LV mass index (SD), g/m2 | 66.9(17.7) |
| LVESVI (SD), ml/m2 | 27.8(11.4) |
| LVEDVI (SD), ml/m2 | 67.7(16.6) |
| Right atrium area index (SD), cm2/m2 | 9.1(2.8) |
| Left atrium area index (SD), cm2/m2 | 10.5(2.4) |
| Epicardial fat index (SD), cm2/m2 | 7.5(2.4) |
| Epicardial fat(SD), cm2 | 14.6(5.7) |
| Interatrial fat index (SD), cm2/m2 | 0.23(0.26) |
| Interatrial fat (SD), cm2 | 0.46(0.53) |
| Atrial septum width (SD), cm | 0.52(0.25) |
Comparison of electrophysiological characteristics
The Pf was greater on the filtered than on the unfiltered P-SAECG signal (13.1±3.8 vs. 3.4±1.2; P < 0.0001). All of the parameters that were observed were more pronounced on the Y lead (Table 2). Pf was the greatest on the Y lead (13.0±3.5 on Y lead vs. 12.1±3.4 on Z lead; P=0.003), and Pf was the smallest on the X lead (11.0±3.3 on X lead vs. 13.0±3.5on Y lead; P=0.0003). Differences in the number of unfiltered Pf mirrored the differences in the number of filtered Pf. The width of filtered Pf was similar in all leads (6.3±1.3 ms on X lead vs. 6.3±1.4 ms on Y lead, vs. 6.4±1.3 ms on Z lead; NS). At the same time, the width of unfiltered Pf was different across the ECG leads. The widest unfiltered Pf were seen on X lead (25.9±10.2 ms on X lead vs. 19.8±9.0 ms on Y lead; P<0.0001), and the narrowest unfiltered Pf were noticed on the Y lead (19.8±9.0 ms on Y lead vs. 23.3±8.8 ms on Z lead; P=0.0006). Amplitude of both filtered and unfiltered Pf similarly differed across the X, Y, and Z leads. The largest Pf amplitude was observed on Y lead (46.5±22.3 μV on Y lead vs. 40.4±19.1 μV on Z lead; P=0.036) and the smallest Pf amplitude was measured on X lead (35.6±19.0 μV on X lead vs. 46.5±22.3 μV on Y lead; P=0.0006).
Correlations between electrophysiological characteristics and atrial structures
Pf moderately strongly positively correlated with interatrial fat index (Figure 3): the larger interatrial fat area, the higher the Pf on the Z lead was. Interatrial fat area did not correlate with BMI, whereas epicardial fat significantly correlated with BMI (r=0.302; P=0.015).
Figure 3. Correlations between the number of P-fragmentations and interatrial fat.
Scatterplot of the number of P-fragmentations, normalized by P wave width (n/100 ms) on Z SAECG lead (Y) against log-transformed interatrial fat (X).
In order to determine which heart atrial structures affected the characteristics (width, amplitude) of the P-fractionations, we ran multiple regression analyses, separately for Pf on X, Y and Z leads. Size of LA, width of interatrial septum, interatrial and epicardial fat, and BMI, were included in each multiple linear regression model. Only interatrial fat (log-transformed) independently positively associated with the number of Pf on Z lead (Table 3), but not on other ECG leads.
Table 3.
Multiple linear regression model predicting the number of Pf on Z lead.
| Predictor | β coefficient (95% CI) | P value |
|---|---|---|
| Epicardial fat index, cm2/m2 | −0.0021(−0.0049 to 0.00073) | 0.141 |
| Log-transformed Interatrial fat | 0.009(0.0003 to 0.019) | 0.043 |
| Atrial septum width, cm2/m2 | −0.005(−0.031 to 0.020) | 0.671 |
| Left atrium area index, cm2/m2 | −0.001(−0.0007 to 0.0010) | 0.296 |
| Body mass index, kg/m2 | 0.0001(−0.0008 to 0.0010) | 0.770 |
Discussion
In this study, we showed that infiltrating interatrial fat was independently associated with the number of Pf on Z SAECG lead in patients with paroxysmal AF and individuals at risk of AF. While Pf on Y SAECG lead were larger and more numerous, as compared to Pf on other ECG leads, only Z lead Pf significantly correlated with interatrial fat, which underscores importance of the posterior atrial wall in the development of electrophysiological AF substrate at an early stage, and suggests that Pf on Z lead reflects discontinuous conduction on the posterior LA wall. Importantly, interatrial fat was a significant predictor of Pf on the Z SAECG lead after adjustment for LA area index, atrial septum width, BMI and epicardial fat.
Infiltrated interatrial and epicardial fat: role in AF
The role of epicardial fat in the AF development has been previously shown. The epicardial adipose tissue was shown to secrete proinflammatory cytokines (tumor necrosis factor-alpha, interleukin-1, interleukin-6) 25, as well as anti-inflammatory substances 26. Kourliouros et al. 27 showed that an increase in the adiponectin release from epicardial fat was associated with freedom from AF after cardiac surgery.
Recent advancement in imaging modalities established that the epicardial fat volume of the entire heart is independently associated with AF 11;28. Large clusters of epicardial adipose tissue, adjacent to the posterior LA wall 29, anterior roof, LA appendage, and lateral mitral isthmus 10 were described in AF patients. Epicardial fat volume and the location of the fat were both associated with the sites of high dominant frequency or complicated fractionated atrial electrograms during AF 30. Shin et al 31 showed that AF patients had significantly larger periatrial epicardial adipose tissue thickness and larger width of interatrial septum as measured by multislice computer tomography.
However, little attention was paid to another important feature of a fatty heart-- the fatty deposits in the atrial septum and infiltration of endocardium and mid-myocardium by adipocytes 24;28. Pathology studies 24;28 showed that fatty deposits in the atrial septum are proportional to the volume of the epicardial heart and correlate with history of atrial arrhythmia, including AF and conduction abnormalities (sinoatrial and atrioventricular block). Massive fatty deposits in the atrial septum (≥ 2cm) served as a marker of adipose tissue infiltrates in the RV myocardium, and the endocardium of RA, LA, RV and LV. Even after excision of epicardial fat in such hearts, adipose tissue comprised 30–50% of the cardiac weight at necropsy 28. Kellman et. al. recently developed methodology of in-vivo imaging of the infiltrated interatrial fat14,23, which was used in this study. Our study underscored the importance of infiltrated interatrial fat imaging and quantification, and for the first time demonstrated association between infiltrated interatrial fat and discontinuous LA conduction. We speculate that infiltrated atrial fat precedes development of atrial fibrosis and characterizes early pre-clinical AF substrate. Further prospective studies are needed to test this hypothesis.
Atrial electrophysiology, characterized by P-SAECG
The signal averaging technique in ECG analysis was introduced nearly 40 years ago 32;33. Multiple studies have shown that prolonged P-SAECG duration is associated with AF 34;35. Various technical approaches were considered for characterization of P-wave SAECG duration 19 and atrial late potentials 36. For example, a lower root mean square voltage of the last 20 ms of the P wave (RMS 20) was shown linked with AF37. At the same time, invasive electrophysiological studies revealed that a fractionated atrial electrogram (A-EGM) during sinus rhythm is typical for paroxysmal AF 16. Tanigawa et al 16 defined quantitative criteria for abnormal A-EGM as duration of A-EGM ≥ 100 ms and/or ≥ 8 fragmented deflections. However, the quantification of P-fragmentations was not previously implemented on P-SAECG. In this study, we normalized the number of P-fragmentations by the duration of P-wave on the corresponding SAECG lead to refine the SAECG metric, analogous to the fractionated intracardiac A-EGM. In our study, Pf characteristics on the Z SAECG lead provided the most meaningful results. Such local expression of Pf is explained by the relationship between the axis of SAECG lead and the sum LA axis, and is consistent with what has been previously reported in literature 38;39. Since P-wave duration on SAECG may change dramatically under different states of the autonomic nervous system 40, quantification of P-fragmentations on Z lead might become a valuable marker to complement P-SAECG duration for risk stratification of AF, and as a surrogate for non-invasive assessment of fractionated A-EGM. Future studies are needed to test this hypothesis.
We speculate that Pf on Z SAECG lead characterizes discontinuous conduction on the posterior wall of the LA. However, this hypothesis should be further tested. Moreover, it is important to emphasize that results of our study should be interpreted carefully. Observed association between P-fragmentations and interatrial fat has moderate strength. Therefore, other, unmeasured in our study factors (e.g. LA fibrosis, ganglionated plexuses activity) could be responsible for Pf appearance on P-SAECG.
Limitations
Several limitations of this study should be considered. Small study population did not allow us to fully characterize non-linear relationships between P-fragmentations and LA structures. Our study is cross-sectional and is not a prospective observational study, which would be potentially needed to establish and confirm a cause-effect relationship. Furthermore, we did not measure the fat volumetrically. There is no doubt that the absence of volumetric measurement of epicardial fat in this study imposed significant limitation for quantification of epicardial fat, which, however, was not the goal of the study. At the same time, the approach of quantification of infiltrated interatrial fat on a longitudinal 4-chamber view provided a certain advantage. Results of our study suggest that quantification of interatrial fat in a single view might be sufficient for detection and quantification of infiltrated adipose tissue in the atria. In such case area of interatrial fat in the septum serves as a marker of the degree of the general intra-atrial fat infiltration. This opens a new avenue for potentially wide implementation of the proposed approach in routine CMR procedure. However, additional studies have to be conducted first to test this hypothesis.
Conclusion
Infiltrating interatrial fat is independently associated with the number of P-wave fragmentations on Z SAECG lead, likely reflecting discontinuous conduction on the posterior wall of the LA. Further study of normalized by P-SAECG width P-fragmentations is warranted. Infiltrating interatrial fat and P-fragmentations characterize AF substrate in patients with paroxysmal AF and individuals with structural heart disease at risk of AF early in the structural heart disease continuum.
Acknowledgments
Financial support & relationships with industry: Study was partially supported by Cardiac Translational Research Implementation Program (C-TRIP) grant from NIH/National Heart, Lung and Blood Institute #P20HL101397 and the Leducq Foundation.
Footnotes
Clinical Trial Registration: NCT01353131
Reference List
- 1.Feinberg WM, Blackshear JL, Kronmal R, Hart RG. Prevalence, Age Distribution, and Gender of Patients With Atrial Fibrillation. Arch Intern Med. 1995;155:469–73. [PubMed] [Google Scholar]
- 2.Wolf P, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991 Aug;22(8):983–8. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Wolf PA, Agostino RBD, Silbershatz H, Kannel WB, Levy D. Clinical Investigation and Reports Impact of Atrial Fibrillation on the Risk of Death The Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 4.Furberg CD, Psaty BM, Manolio TA, Gardin JM, Smith VE, Rautaharju PM. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994 Aug 1;74(3):236–41. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998 Sep 8;98(10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011 Nov 15;124(20):2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 7.Krahn AD, Manfreda J, Tate RB, Mathewson FAL, Cuddy TE. The Natural History of Atrial Fibrillation: Incidence, Risk Factors, and Prognosis in the Manitoba Follow-Up Study. The American Journal of Medicine. 1995;98:476–84. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 8.Teh AW, Kistler PM, Lee G, Medi C, Heck PM, Spence SJ, Morton JB, Sanders P, Kalman JM. Long-term effects of catheter ablation for lone atrial fibrillation: Progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012 Apr;9(4):473–80. doi: 10.1016/j.hrthm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, Santucci P, Wilber DJ, Akar JG. Pericardial Fat Is Independently Associated With Human Atrial Fibrillation. J Am Coll Cardiol. 2010 Aug 31;56(10):784–8. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 10.Tsao HM, Hu WC, Wu MH, Tai CT, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Wu TJ, Sheu MH, Chang CY, Chen SA. Quantitative Analysis of Quantity and Distribution of Epicardial Adipose Tissue Surrounding the Left Atrium in Patients With Atrial Fibrillation and Effect of Recurrence After Ablation. The American Journal of Cardiology. 2011 May 15;107(10):1498–503. doi: 10.1016/j.amjcard.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Thanassoulis G, Massaro JM, O’Donnell CJ, Hoffmann U, Levy D, Ellinor PT, Wang TJ, Schnabel RB, Vasan RS, Fox CS, Benjamin EJ. Pericardial Fat Is Associated With Prevalent Atrial Fibrillation / Clinical Perspective. Circulation: Arrhythmia and Electrophysiology. 2010 Aug 1;3(4):345–50. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard Al, Atassi F, Amour J, Leprince P, Dutour A, Clément K, Hatem SpN. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. European Heart Journal. 2013 Mar 22; doi: 10.1093/eurheartj/eht099. [DOI] [PubMed] [Google Scholar]
- 13.Tansey DK, Aly Z, Sheppard MN. Fat in the right ventricle of the normal heart. Histopathology. 2005 Jan;46(1):98–104. doi: 10.1111/j.1365-2559.2005.02054.x. [DOI] [PubMed] [Google Scholar]
- 14.Kellman P, Hernando D, Shah S, Zuehlsdorff S, Jerecic R, Mancini C, Liang ZP, Arai AE. Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium. Magn Reson Med. 2009 Jan;61(1):215–21. doi: 10.1002/mrm.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosio FG, Palacios J, Vidal JM, Cocina EG, Gomez-Sanchez MA, Tamargo L. Electrophysiologic studies in atrial fibrillation. Slow conduction of premature impulses: a possible manifestation of the background for reentry. Am J Cardiol. 1983 Jan 1;51(1):122–30. doi: 10.1016/s0002-9149(83)80022-8. [DOI] [PubMed] [Google Scholar]
- 16.Tanigawa M, Fukatani M, Konoe A, Isomoto S, Kadena M, Hashiba K. Prolonged and fractionated right atrial electrograms during sinus rhythm in patients with paroxysmal atrial fibrillation and sick sinus node syndrome. J Am Coll Cardiol. 1991 Feb;17(2):403–8. doi: 10.1016/s0735-1097(10)80106-8. [DOI] [PubMed] [Google Scholar]
- 17.Jacquemet V, Henriquez CS. Genesis of complex fractionated atrial electrograms in zones of slow conduction: a computer model of microfibrosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2009 Jun;6(6):803–10. doi: 10.1016/j.hrthm.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada T, Fukunami M, Shimonagata T, Kumagai K, Ogita H, Asano Y, Hirata A, Masatsugu H, Hoki N. Prediction of paroxysmal atrial fibrillation in patients with congestive heart failure: a prospective study. J Am Coll Cardiol. 2000 Feb;35(2):405–13. doi: 10.1016/s0735-1097(99)00563-x. [DOI] [PubMed] [Google Scholar]
- 19.Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Edith M. Value of the P-Wave Signal-Averaged ECG for Predicting Atrial Fibrillation After Cardiac Surgery. Circulation. 1993;88:2618–22. doi: 10.1161/01.cir.88.6.2618. [DOI] [PubMed] [Google Scholar]
- 20.Mewton N, Rizzi P, Strauss D, Verrier RL, Nearing B, Tereshchenko L, Moxley J, Marchlinsky FE, Wu KC, Killian T, Liu CY, Cox C, Spooner PM, Lima JAC. Screening for Arrhythmogenic Myocardial Substrate by 12-Lead Ecg Qrs Score, Qrs-T Angle, Late Potentials and T-Wave Alternans. J Am Coll Cardiol. 2012;59(13):E737. [Google Scholar]
- 21.Strauss DG, Mewton N, Verrier RL, Nearing B, Killian T, Marchlinsky FE, Tereshchenko LG, Wu KC, Cox C, Spooner PM, Lima JA. Screening Entire Health System ECG Databases to Identify Patients with Arrhythmogenic Myocardial Substrate at Increased Risk of Death. Circulation. 2011;124(21) [Google Scholar]
- 22.Maceira AM, Cosin-Sales J, Roughton M, Prasad SK, Pennell DJ. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kellman P, Hernando D, Arai AE. Myocardial Fat Imaging. Curr Cardiovasc Imaging Rep. 2010 Apr;3(2):83–91. doi: 10.1007/s12410-010-9012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirani J, Roberts WC. Clinical, Electrocardiographic and Morphologic Features of Massive Fatty Deposits (“Lipomatous Hypertrophy”) in the Atrial Septum. J Am Coll Cardiol. 1993;22(1):226–38. doi: 10.1016/0735-1097(93)90839-s. [DOI] [PubMed] [Google Scholar]
- 25.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O’Brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003 Nov 18;108(20):2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 26.Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res. 2008 Jul;40(7):442–5. doi: 10.1055/s-2008-1062724. [DOI] [PubMed] [Google Scholar]
- 27.Kourliouros A, Karastergiou K, Nowell J, Gukop P, Tavakkoli Hosseini M, Valencia O, Mohamed Ali V, Jahangiri M. Protective effect of epicardial adiponectin on atrial fibrillation following cardiac surgery. European Journal of Cardio-Thoracic Surgery. 2011 Feb 1;39(2):228–32. doi: 10.1016/j.ejcts.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. The American Journal of Cardiology. 1995 Aug 15;76(5):414–8. doi: 10.1016/s0002-9149(99)80116-7. [DOI] [PubMed] [Google Scholar]
- 29.Batal O, Schoenhagen P, Shao M, Ayyad AE, Van Wagoner DR, Halliburton SS, Tchou PJ, Chung MK. Left atrial epicardial adiposity and atrial fibrillation. Circulation Arrhythmia and electrophysiology. 2010 Jun;3(3):230–6. doi: 10.1161/CIRCEP.110.957241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagashima K, Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune M, Mano H, Sonoda K, Hiro T, Nikaido M, Hirayama A. Does location of epicardial adipose tissue correspond to endocardial high dominant frequency or complex fractionated atrial electrogram sites during atrial fibrillation? Circ Arrhythm Electrophysiol. 2012 Aug 1;5(4):676–83. doi: 10.1161/CIRCEP.112.971200. [DOI] [PubMed] [Google Scholar]
- 31.Shin S, Yong H, Lim H, Na J, Choi C, Choi J, I, Kim S, Kim J, Kim E, Park S, Rha SW, Park C, Seo H, Oh D, Kim YH. Total and Interatrial Epicardial Adipose Tissues Are Independently Associated With Left Atrial Remodeling in Patients With Atrial Fibrillation. J CARDIOVASC ELECTROPHYSIOL. 2011 Jun;22(6):647–55. doi: 10.1111/j.1540-8167.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 32.Berbari EJ, Lazzara R, El-Sherif N, Scherlag BJ. Extracardiac recordings of His-Purkinje activity during conduction disorders and junctional rhythms. Circulation. 1975 May;51(5):802–10. doi: 10.1161/01.cir.51.5.802. [DOI] [PubMed] [Google Scholar]
- 33.Sadanandan S, Steinberg JS. Signal-Averaged P Wave: Technique and Clinical Applications. Annals of Noninvasive Electrocardiology. 1999 Oct 1;4(4):401–7. [Google Scholar]
- 34.Darbar D, Hardy A, Haines JL, Roden DM. Prolonged signal-averaged P-wave duration as an intermediate phenotype for familial atrial fibrillation. J Am Coll Cardiol. 2008 Mar 18;51(11):1083–9. doi: 10.1016/j.jacc.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guidera SA, Steinberg JS. The signal-averaged P wave duration: a rapid and noninvasive marker of risk of atrial fibrillation. J Am Coll Cardiol. 1993 Jun;21(7):1645–51. doi: 10.1016/0735-1097(93)90381-a. [DOI] [PubMed] [Google Scholar]
- 36.Darbar D, Jahangir A, Hammill SC, Gersh BJ. P wave signal-averaged electrocardiography to identify risk for atrial fibrillation. Pacing Clin Electrophysiol. 2002 Oct;25(10):1447–53. doi: 10.1046/j.1460-9592.2002.01447.x. [DOI] [PubMed] [Google Scholar]
- 37.Budeus M, Hennersdorf M, Felix O, Reimert K, Perings C, Wieneke H, Erbel R, Sack S. Prediction of atrial fibrillation in patients with cardiac dysfunctions: P wave signal-averaged ECG and chemoreflexsensitivity in atrial fibrillation. Europace. 2007 Aug;9(8):601–7. doi: 10.1093/europace/eum054. [DOI] [PubMed] [Google Scholar]
- 38.Holmqvist F, Carlson J, Waktare JE, Platonov PG. Noninvasive evidence of shortened atrial refractoriness during sinus rhythm in patients with paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2009 Mar;32(3):302–7. doi: 10.1111/j.1540-8159.2008.02236.x. [DOI] [PubMed] [Google Scholar]
- 39.Holmqvist F, Platonov PG, McNitt S, Polonsky S, Carlson J, Zareba W, Moss AJ. Abnormal P-wave morphology is a predictor of atrial fibrillation development and cardiac death in MADIT II patients. Ann Noninvasive Electrocardiol. 2010 Jan;15(1):63–72. doi: 10.1111/j.1542-474X.2009.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheema AN, Ahmed MW, Kadish AH, Goldberger JJ. Effects of autonomic stimulation and blockade on signal-averaged P wave duration. J Am Coll Cardiol. 1995 Aug;26(2):497–502. doi: 10.1016/0735-1097(95)80028-f. [DOI] [PubMed] [Google Scholar]