Abstract
Background/Aims
Calcium homeostasis requires regulated cellular and interstitial systems interacting to modulate the activity and movement of this ion. Disruption of these systems in the kidney results in nephrocalcinosis and nephrolithiasis, important medical problems whose pathogenesis is incompletely understood.
Methods
We investigated 25 patients from 16 families with unexplained nephrocalcinosis and characteristic dental defects (amelogenesis imperfecta, gingival hyperplasia, impaired tooth eruption). To identify the causative gene, we performed genome-wide linkage analysis, exome capture, next-generation sequencing, and Sanger sequencing.
Results
All patients had biallelic FAM20A mutations segregating with the disease; 20 different mutations were identified.
Conclusions
This autosomal recessive disorder, also known as enamel renal syndrome, of FAM20A causes nephrocalcinosis and amelogenesis imperfecta. We speculate that all individuals with biallelic FAM20A mutations will eventually show nephrocalcinosis.
Keywords: Nephrolithiasis, Urolithiasis, Amelogenesis imperfecta, FAM20B, FAM20C
Introduction
Nephrocalcinosis (NC), diagnosed by radiographs, CT, or increased echogenicity on ultrasound, represents an important renal complication because it can accompany progressive deterioration of glomerular function or nephrolithiasis [1]. In some cases, NC provides a clue to an underlying genetic disorder such as hyperoxaluria or distal renal tubular acidosis with or without deafness [2, 3]. In other cases, NC is a side effect of chronic treatment with various drugs, including loop diuretics and vitamin D. In general, the pathogenesis of NC has not been adequately elucidated, although hypercalciuria appears to be a common finding.
Often, rare genetic diseases reveal previously unrecognized mechanisms of action regarding physiology, cell biology, and metabolism. Here, we present genetic studies into a rare human disease of NC combined with amelogenesis imperfecta (AI), a disorder of abnormal enamel formation and impaired tooth eruption.
Methods
Patients and families were identified in our rare disease renal tubular or dental/craniofacial reference centers and gave informed consent. This study was approved by the institutional review boards and ethics committees of the various centers. All patients had NC confirmed by either ultrasound, X-ray or CT, and all showed characteristic teeth findings with AI and delayed or missing tooth eruption.
In short, multipoint parametric linkage analysis utilizing 2,000 highly polymorphic markers (DeCode, Iceland) across the whole genome in 4 informative families as well as homozygosity mapping for another consanguineous family was used to determine the locus linked to this trait, as published before [4, 5]. Next-generation sequencing using exome capture (Perkin Elmer, USA; Leeds Translational Genomics Unit, UK) was performed on 5 patients from 5 unrelated families; 4 were part of the linkage analysis. Subsequent data analysis was restricted to novel sequence variants within the linked region. The frequency of each variant was examined in >100 ethnically matched alleles available in public databases (1000 Genomes, release 12 – May 2012). Sanger sequencing was performed as described, and mutations were sequenced in family members for segregation analysis.
In detail, genomic DNA was isolated from peripheral blood lymphocytes for all subjects using standard protocols. Genotypes from polymorphic markers for 4 families were generated by DeCode, Iceland. Analyses were carried out as published before with modifications [4]. In short, genotypes were examined using a multipoint parametric linkage analysis and haplotype reconstruction was performed via Allegro and Genehunter for an autosomal recessive model with complete penetrance, disease allele frequency of 0.001 (DeCode map with appropriate allele frequencies). The data were formatted using Mega2 (version 4.0) through Alohomora (version 0.30, Win32); non-informative markers were filtered out. Mendelian inconsistencies were checked using PedCheck (version 1.1); unlikely genotypes were identified and filtered using Merlin (version 1.1 alpha 3). The Allegro haplotype output files were visualized with Haplopainter. In addition and in parallel, whole-genome SNP microarray analysis was performed on genomic DNA for another consanguineous family by AROS Applied Technology; resulting data were analyzed using IBD finder software [5]. Whole-exome sequencing was performed using 3 μg of genomic DNA, which was sheared and ligated to Illumina adapters, according to Agilent’s SureSelect Library Prep protocol. The sample was then size selected (200–300 bp) by agarose gel electrophoresis and enriched for 12 cycles, using PCR prior to hybridization to the SureSelect reagent for 24 h at 65°C. The library was denatured using NaOH and diluted to a concentration of 12 pM, of which 120 μl was hybridized onto a v5 single-read flow cell (Illumina, San Diego, Calif., USA). Samples were prepared for sequencing according to Illumina’s standard amplification, linearization, blocking and primer hybridization protocols. The flow cell was then loaded onto an Illumina GAIIx and sequencing performed for 80 cycles after which the raw data were processed using the Illumina pipeline. The data (qseq) files generated were aligned to the human reference sequence (hg19/GRCh37) using Novoalign short-read alignment software (Novocraft Technologies, Selangor, Malaysia). Duplicate reads and reads mapping to multiple locations were excluded from any analysis. SAMtools and the Genome Analysis Toolkit were used to further process the alignment files for variant calling [6, 7].
For confirmation of variants detected by exome capture/next-generation sequencing and for mutation detection in additional cases, we amplified all coding exons and exon-intron boundaries of FAM20A using standard PCR methodology with intronic (genomic) primers. PCR products were separated on 1% agarose gels with ethidium bromide using electrophoresis and visualized under UV light. Specific bands were cut and DNA was isolated and purified using standard procedures. Bi-directional sequencing of all exons and exon-intron boundaries were performed using a Beckman Coulter CEQ8000 or an Applied Biosystems 3130xl capillary sequencer per the manufacturer’s protocol. Sequencing data was analyzed and compared with the published reference sequence for FAM20A (NG_029809, April 2012, NCBI build 37.3).
Results
We ascertained 25 patients (12 males, 13 females; age 12–64) in 16 families with NC and characteristic dental findings, i.e., the triad of AI, gingival thickening and impairment of tooth eruption (table 1). The diagnosis of NC was made predominantly by nephrologists based upon characteristic imaging findings. Generalized hypoplastic AI was evident from eruption of the deciduous teeth early in childhood with subsequent impaired eruption of the permanent teeth and development of gingival enlargement. Clinical details of some patients have been published [8–11]. None of the parents or offspring of our patients had AI.
Table 1.
FAM20A mutations in patients with NC and AI
| Family | Age, years | Gender | FAM20A mutations |
|---|---|---|---|
| 1 | 21 | male | c.915–918delCTTT; p.F305fsX380 |
|
| |||
| 2 | 27 | female | IVS2 + 1G>A/c.913–914delTT; p.F305fsX378 |
| 31 | male | IVS2 + 1G>A/c.913–914delTT; p.F305fsX378 | |
|
| |||
| 3 | 23 | male | IVS4 + 1G>C/c.1348–1349delTC; p.S450fsX469 |
| 25 | female | IVS4 + 1G>C/c.1348–1349delTC; p.S450fsX469 | |
|
| |||
| 4 | 59 | male | c.1475–1482dupAACCCCAC; p.L495fsX509 |
| 64 | female | c.1475–1482dupAACCCCAC; p.L495fsX509 | |
|
| |||
| 5 | 12 | female | c.406C>T; p.R136X |
|
| |||
| 6 | 20 | male | c.34–35delCT; p.L12fsX78 |
|
| |||
| 7 | 16 | female | c.1513delA; p.I505fsX506 |
| 22 | male | c.1513delA; p.I505fsX506 | |
|
| |||
| 8 | 20 | male | c.1432C>T; p.R478X |
|
| |||
| 9 | 13 | male | c.518T>G; p.L173R |
|
| |||
| 10 | 29 | female | c.727C>T/c.1228–1229delGA; p.R243X/p.D410fsX414 |
|
| |||
| 11 | 19 | female | c.217C>T/c.727C>T; p.R73X/p.R243X |
| 20 | male | c.217C>T/c.727C>T; p.R73X/p.R243X | |
|
| |||
| 12 | 18 | female | c.1369A>T; p.K457X |
|
| |||
| 13 | 14 | female | c.755–757delTCT/c.641–719del79bp; p.F252del/p.I214fsX259 |
| 16 | male | c.755–757delTCT/c.641–719del79bp; p.F252del/p.I214fsX259 | |
|
| |||
| 14 | 21 | female | IVS5 + 2T>G |
|
| |||
| 15 | 24 | male | c.907–908delAG; p.S303fsX378 |
| 31 | male | c.907–908delAG; p.S303fsX378 | |
| 37 | female | c.907–908delAG; p.S303fsX378 | |
|
| |||
| 16 | 17 | female | c.34–35delCT/c.612delC; p.L12fsX78/p.A204fsX215 |
| 18 | female | c.34–35delCT/c.612delC; p.L12fsX78/p.A204fsX215 | |
Mutations are described on the cDNA and predicted protein levels. Listing of one allele indicates homozygosity; two alleles indicate compound heterozygosity. Every patient had biallelic mutations involving insertions, deletions, essential splice sites, missense changes or nonsense changes.
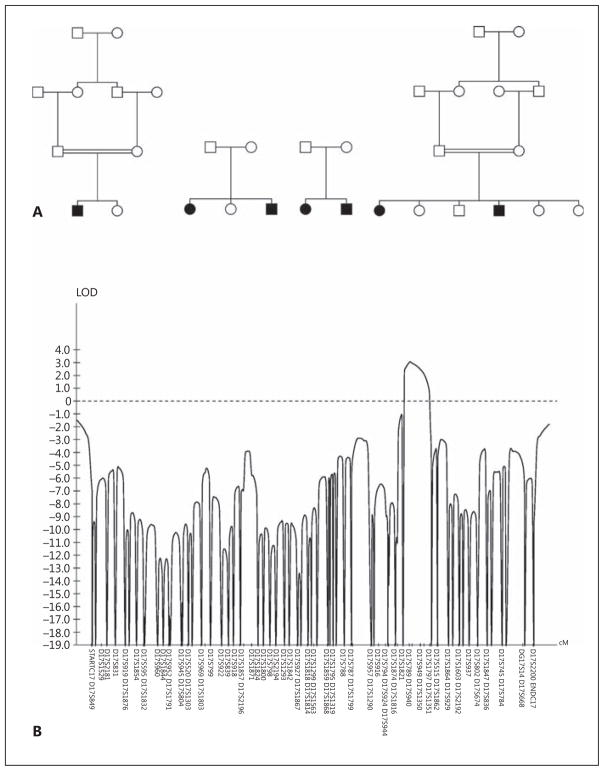
From the 16 families with this disorder, we selected four informative families (fig. 1A) for whole-genome parametric multipoint linkage studies. Using dense (2 cM) polymorphic markers, we identified a single linked locus on chromosome 17q24 with a LOD score of 3.1 (fig. 1B). Haplotype reconstruction placed the locus between flanking markers D17S1821 (97.3 cM) and D17S1797 (106.2 cM), a region of 5.3 million bases containing 41 annotated genes (NCBI build 37.3, October 2011). Independently, homozygosity mapping identified the same locus in another consanguineous family (data not shown).
Fig. 1.
A Pedigrees used for multipoint parametric linkage analysis. Black symbols indicate affected, white unaffected, squares males and circles females. B LOD score analysis for chromosome 17. Note the single significant peak at 17q24.
To identify the disease-causing gene, we performed massive parallel sequencing using exome capture on 5 unrelated patients. Using an autosomal recessive model, limiting our analysis to the linked region, and filtering variants to eliminate common polymorphisms, we found homozygous or compound heterozygous mutations in all 5 patients in only one gene, FAM20A. A total of 20 different mutations (deletions, insertions, and splice site, missense and nonsense mutations) were identified in the homozygous or compound heterozygous state, confirmed by Sanger sequencing, and demonstrated to segregate with the disorder in our 16 families (table 1). FAM20A (cDNA 1,626 bp, protein 541 amino acids, 11 coding exons) is a member of a family of kinase-encoding genes that includes FAM20B and FAM20C.
Discussion
Calcium plays many critical roles in human physiology, serving as an intracellular messenger, an extracellular neuromuscular excitatory ion, and a structural component of bone and teeth. For example, tooth enamel (calcium hydroxyapatite) is the hardest human tissue and can function into old age, despite being the only mineralized tissue with no capacity for cellular repair. Hence, the temporo-spatial concentration of calcium must be exquisitely regulated in different compartments, bound to albumin within the circulation, sequestered by calbindin within cells, including teeth [12], and excreted under tight control by the kidney [13]. Within the vasculature, calcium availability is closely modulated by an intricate interplay between bones and regulatory hormones that involves positive and negative feedback mechanisms (e.g., vitamin D, PTH, calcitonin) [14, 15]. Consequences of dysregulated calcium homeostasis include nephrolithiasis and NC, i.e., precipitation of calcium in the urinary collecting system and renal interstitium, respectively.
The processes that normally maintain calcium balance in renal tissues can be delineated by studying genetic disorders involving NC. A prime example lies in an autosomal recessive disorder of NC combined with AI. Linkage analysis and focused exome sequencing performed on affected families identified the causative gene as FAM20A, previously associated only with a disorder of AI [16, 17].
Previous causes of NC have involved epithelial and paracellular disturbances in calcium transport, predominantly caused by mutations in calcium-specific channels and proteins [18–20]. These systems either reabsorb filtered calcium from the filtrate (urine) across the renal tubular cell or release calcium from the tubular cell into the interstitial compartment. When they malfunction, increased urinary calcium precipitates within the renal tubule (leading to nephrolithiasis and urolithiasis) or within the interstitium (leading to NC); this is invariably accompanied by hypercalciuria.
Our 25 patients exhibited NC and AI, but ascertainment of other patients with biallelic FAM20A mutations will expand the phenotype and define the entire spectrum of this rare disease. Also, two different Fam20a knock-out mouse models exhibited different findings; in one, no kidney findings were reported, whereas the other showed arterial calcification without NC [21, 22]. The patients previously reported with FAM20A mutations and AI may also prove to have NC.
Conclusions
Our findings have implications for diagnosis and treatment. FAM20A is a locally secreted protein with low abundance in saliva [23] and blood [24], suggesting that replacement therapy could provide an option for a treatment or prophylaxis.
Acknowledgments
The authors thank all participating patients and families. We dedicate this work to the late Professor Oliver Wrong, who remained actively involved in these studies from their inception in the 1990s until his passing in February 2012, and whom we miss dearly. Funding for this study was kindly provided by the David and Elaine Potter Charitable Foundation (to R.K.), St Peter’s Trust for Kidney, Bladder and Prostate Research (to D. Bockenhauer, R.J.U., R.K.), Kids Kidney Research (to D. Bockenhauer, R.K.), the Oxalosis & Hyperoxaluria Foundation (to R.K.), the European Union, FP7 EURenOMICS grant agreement No. 305608 (to D. Bockenhauer, R.J.U., R.K.), the Intramural Research Program of the National Human Genome Research Institute (to W.A.G.), the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016 (to H.K.), the Wellcome Trust (to A.M., C.I.), the Sir Jules Thorn Charitable Trust (to C.J., C.I.), the University of Strasbourg (to A.B.-Z.), the French Ministry of Health National Program for Clinical Research (to A.B.-Z.), the Hôpitaux Universitaires de Strasbourg (to A.B.-Z.), the Institut Français pour la Recherche Odontologique (IFRO) (to A.B.-Z.), INSERM (to M.Q., A.B.), the Paris-Descartes University (to M.Q.), the French Ministry of Health and APHP Rothschild Hospital (to M.D.M.), the Paris-Diderot University (to M.D.M., A.A., A.B.), the F.R.S.-FNRS (Fonds de la Recherche Scientifique) (to M.V.), the Minas Gerais State Research Foundation (FAPEMIG) (to H.M.-J.), CAPES Brazil (to A.C.A.), and Deutsche Forschungsgemeinschaft (Ci 107/4-2) (to E.S.).
Footnotes
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial 3.0 License (www.karger.com/OA-license-WT), applicable to the online version of the article only. Distribution for noncommercial purposes only.
References
- 1.Evan AP, Unwin RJ, Williams JC., Jr Renal stone disease: a commentary on the nature and significance of randall’s plaque. Nephron Physiol. 2011;119:p49–p53. doi: 10.1159/000330255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kleta R, Bockenhauer D. Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol. 2006;104:p73–p80. doi: 10.1159/000094001. [DOI] [PubMed] [Google Scholar]
- 3.Vivante A, Lotan D, Pode-Shakked N, Landau D, Svec P, Nampoothiri S, Verma I, Abu-Libdeh A, Bockenhauer D, Dekel B, Anikster Y. Familial autosomal recessive renal tubular acidosis: importance of early diagnosis. Nephron Physiol. 2011;119:p31–p39. doi: 10.1159/000329668. [DOI] [PubMed] [Google Scholar]
- 4.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr IM, Sheridan E, Hayward BE, Markham AF, Bonthron DT. IBDfinder and SNPsetter: tools for pedigree-independent identification of autozygous regions in individuals with recessive inherited disease. Hum Mutat. 2009;30:960–967. doi: 10.1002/humu.20974. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 1000 Genome Project Data Processing Subgroup: The Sequence Alignment/ Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellow EL, Harley KE, Unwin RJ, Wrong O, Winter GB, Parkins BJ. Amelogenesis imperfecta, nephrocalcinosis, and hypocalciuria syndrome in two siblings from a large family with consanguineous parents. Nephrol Dial Transplant. 1998;13:3193–3196. doi: 10.1093/ndt/13.12.3193. [DOI] [PubMed] [Google Scholar]
- 9.Paula LM, Melo NS, Silva Guerra EN, Mestrinho DH, Acevedo AC. Case report of a rare syndrome associating amelogenesis imperfecta and nephrocalcinosis in a consanguineous family. Arch Oral Biol. 2005;50:237–242. doi: 10.1016/j.archoralbio.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Martelli-Junior H, dos Santos Neto PE, de Aquino SN, de Oliveira Santos CC, Borges SP, Oliveira EA, Lopes MA, Coletta RD. Amelogenesis imperfecta and nephrocalcinosis syndrome: a case report and review of the literature. Nephron Physiol. 2011;118:p62–p65. doi: 10.1159/000322828. [DOI] [PubMed] [Google Scholar]
- 11.Hall RK, Phakey P, Palamara J, McCredie DA. Amelogenesis imperfecta and nephrocalcinosis syndrome. Case studies of clinical features and ultrastructure of tooth enamel in two siblings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:583–592. doi: 10.1016/s1079-2104(05)80100-3. [DOI] [PubMed] [Google Scholar]
- 12.Berdal A, Hotton D, Pike JW, Mathieu H, Dupret JM. Cell- and stage-specific expression of vitamin D receptor and calbindin genes in rat incisor: regulation by 1,25-dihydroxyvitamin D3. Dev Biol. 1993;155:172–179. doi: 10.1006/dbio.1993.1016. [DOI] [PubMed] [Google Scholar]
- 13.Bleich M, Shan Q, Himmerkus N. Calcium regulation of tight junction permeability. Ann NY Acad Sci. 2012;1258:93–99. doi: 10.1111/j.1749-6632.2012.06539.x. [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith DJ, Cunningham J. Mineral metabolism and vitamin d in chronic kidney disease – more questions than answers. Nat Rev Nephrol. 2011;7:341–346. doi: 10.1038/nrneph.2011.53. [DOI] [PubMed] [Google Scholar]
- 15.Loupy A, Ramakrishnan SK, Wootla B, Chambrey R, de la Faille R, Bourgeois S, Bruneval P, Mandet C, Christensen EI, Faure H, Cheval L, Laghmani K, Collet C, Eladari D, Dodd RH, Ruat M, Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J Clin Invest. 2012;122:3355–3367. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Sullivan J, Bitu CC, Daly SB, Urquhart JE, Barron MJ, Bhaskar SS, Martelli-Junior H, dos Santos Neto PE, Mansilla MA, Murray JC, Coletta RD, Black GC, Dixon MJ. Whole-exome sequencing identifies FAM20A mutations as a cause of amelogenesis imperfecta and gingival hyperplasia syndrome. Am J Hum Genet. 2011;88:616–620. doi: 10.1016/j.ajhg.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SH, Seymen F, Lee KE, Lee SK, Kweon YS, Kim KJ, Jung SE, Song SJ, Yildirim M, Bayram M, Tuna EB, Gencay K, Kim JW. Novel FAM20A mutations in hypoplastic amelogenesis imperfecta. Hum Mutat. 2012;33:91–94. doi: 10.1002/humu.21621. [DOI] [PubMed] [Google Scholar]
- 18.Shan Q, Himmerkus N, Hou J, Goodenough DA, Bleich M. Insights into driving forces and paracellular permeability from claudin-16 knockdown mouse. Ann NY Acad Sci. 2009;1165:148–151. doi: 10.1111/j.1749-6632.2009.04041.x. [DOI] [PubMed] [Google Scholar]
- 19.Dimke H, Hoenderop JG, Bindels RJ. Molecular basis of epithelial Ca2+ and Mg2+ transport: insights from the TRP channel family. J Physiol. 2011;589:1535–1542. doi: 10.1113/jphysiol.2010.199869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Muller D. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci USA. 2012;109:14241–14246. doi: 10.1073/pnas.1203834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.An C, Ide Y, Nagano-Fujii M, Kitazawa S, Shoji I, Hotta H. A transgenic mouse line with a 58-kb fragment deletion in chromosome 11E1 that encompasses part of the Fam20a gene and its upstream region shows growth disorder. Kobe J Med Sci. 2010;55:E82–E92. [PubMed] [Google Scholar]
- 22.Vogel P, Hansen GM, Read RW, Vance RB, Thiel M, Liu J, Wronski TJ, Smith DD, JeterJones S, Brommage R. Amelogenesis imperfecta and other biomineralization defects in Fam20a and Fam20c null mice. Vet Pathol. 2012;49:998–1017. doi: 10.1177/0300985812453177. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian WJ, Kaleta DT, Petritis BO, Jiang H, Liu T, Zhang X, Mottaz HM, Varnum SM, Camp DG, 2nd, Huang L, Fang X, Zhang WW, Smith RD. Enhanced detection of low abundance human plasma proteins using a tandem IgY12-SuperMix immunoaffinity separation strategy. Mol Cell Proteomics. 2008;7:1963–1973. doi: 10.1074/mcp.M800008-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]