Abstract
Objectives
We evaluated the efficacy of a hepatitis care coordination intervention to improve linkage to hepatitis A virus (HAV) and hepatitis B virus (HBV) vaccination and clinical evaluation of hepatitis C virus (HCV) infection among methadone maintenance patients.
Methods
We conducted a randomized controlled trial of 489 participants from methadone maintenance treatment programs in San Francisco, California, and New York City from February 2008 through June 2011. We randomized participants to a control arm (n = 245) and an intervention arm (n = 244), which included on-site screening, motivational-enhanced education and counseling, on-site vaccination, and case management services.
Results
Compared with the control group, intervention group participants were significantly more likely (odds ratio [OR] = 41.8; 95% confidence interval [CI] = 19.4, 90.0) to receive their first vaccine dose within 30 days and to receive an HCV evaluation within 6 months (OR = 4.10; 95% CI = 2.35, 7.17). A combined intervention adherence outcome that measured adherence to HAV–HBV vaccination, HCV evaluation, or both strongly favored the intervention group (OR = 8.70; 95% CI = 5.56, 13.61).
Conclusions
Hepatitis care coordination was efficacious in increasing adherence to HAV–HBV vaccination and HCV clinical evaluation among methadone patients.
Viral hepatitis is a major public health problem among drug users in the United States. Drug users are at high risk of infection with hepatitis A, B, and C viruses (HAV, HBV, and HCV, respectively) through unsterile injection practices and high-risk sexual activity.1–3 HCV infection can be acquired rapidly by injection drug users, with prevalence rates of 70% or higher among recent-onset injectors.4,5 Cirrhosis, hepatocellular carcinoma, and death are important sequelae of HCV and chronic HBV infection.6,7 Superimposed HBV and HAV infection may exacerbate liver disease among those with chronic HCV infection.8 HIV infection can accelerate disease progression in HCV- and HBV-infected persons.9–11 Given that a significant proportion of this population remains at risk for these infections, HAV–HBV vaccination programs that effectively engage drug users are needed.2,12 Treatment options for HCV are rapidly improving with the introduction of direct-acting antivirals (e.g., telaprevir and boceprevir) and the prospect of interferon-free regimens.13–16
The integration of primary medical care and case management services within drug treatment programs has been associated with increased utilization of outpatient health care services among HIV- and HCV-seropositive drug users. Studies have found increased rates of the use of HIV/AIDS- and HCV-related medical care services in the methadone treatment setting17–19; however, most drug treatment programs do not have the infrastructure to provide on-site HCV treatment.20 Despite advances in HCV treatment, many HCV-positive drug users are not engaged in HCV care,21,22 and many drug users experience missed opportunities for HAV and HBV vaccination.2,23
Drug users experience multiple complex individual, social, and structural barriers to HCV evaluation and treatment. Barriers include lack of knowledge about available effective treatments, low perceived risk of potential long-term adverse health consequences, fear of possible side effects of treatment, high treatment costs, lack of insurance, negative peer norms regarding HCV medications, medical mistrust, and potential provider concerns about treating active drug users.24–31 As has been observed for HIV infection, with HCV infection there is a cascade of care, with decreasing proportions of infected persons knowing their status, having had a clinical evaluation, being engaged in care, being on treatment, completing treatment, and having an optimal virological response.21,30,32
HCV drug efficacy trials focus on optimizing outcomes among those treated, whereas adherence interventions frequently focus on assisting individuals to complete initiated therapy. For drug users with HCV infection, the initial steps in the cascade of care, including screening, identifying those HCV positive, and engaging infected persons in care, remain a substantial gap.21 Care coordination approaches such as case management and patient navigation services have shown promise in engaging and retaining patients in cancer screening and care and have been used in HIV primary care with promising but inconsistent results.33–39 There is a need for rigorously designed research to examine the efficacy of care coordination approaches such as case management and patient navigation as a strategy for improving the efficiency of the HCV cascade of care.
We evaluated the impact of a hepatitis care coordination model integrated in the methadone maintenance treatment (MMT) setting on the following primary outcomes: (1) receipt of the first dose of HAV–HBV vaccine and (2) adherence to an initial appointment with a hepatitis C health care provider. We hypothesized that hepatitis care coordination, including on-site screening, education and counseling, motivational interviewing, on-site vaccination, and case management, would increase rates of adherence to HAV–HBV vaccination and initial appointment with a hepatitis C health care provider more than a control intervention that reflected standard recommendations for the care of drug users.40
METHODS
We conducted an unblinded, 2-armed randomized controlled study in MMT programs in San Francisco, California, and New York City from February 2008 through June 2011. The opiate treatment program in San Francisco provided care for more than 400 opioid-dependent patients per year. During the study period, the participating MMT program offered its patients routine (i.e., nonstudy) hepatitis screening and vaccination through referral arrangements at off-site primary care clinics. The New York City study site consisted of a complex of 5 methadone dosing windows within 1 clinic in Manhattan that provided treatment for approximately 1300 patients per year. During the study period, the MMT program offered its patients routine (i.e., nonstudy) screening for HBV but not for HCV and referred patients offsite for HBV vaccination, if indicated.
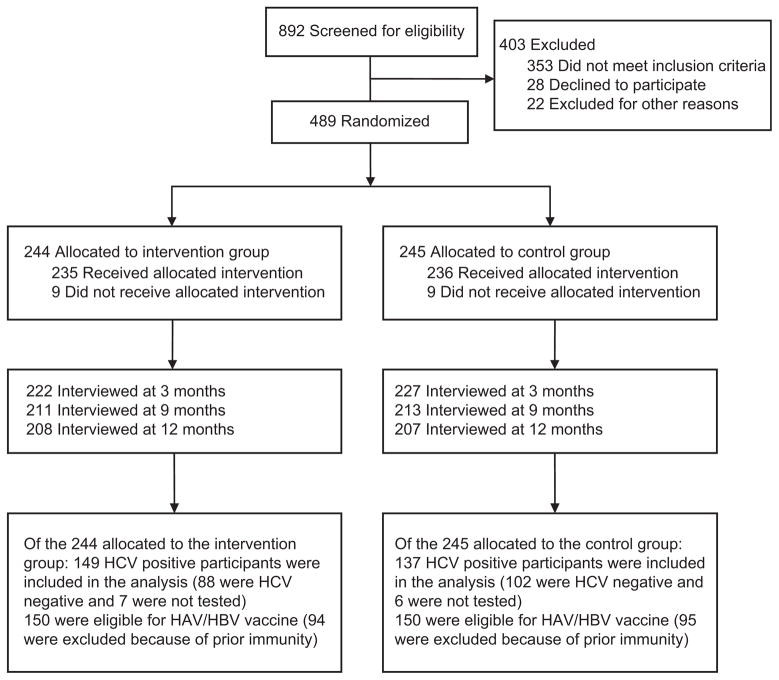
We recruited MMT patients from methadone waiting rooms to participate in eligibility screening. Eligible participants were at least 18 years old; either HCV negative, of unknown HCV status, or, if HCV positive, with no prior medical care or diagnostic evaluation for HCV (i.e., liver biopsy, viral load test, genotype test, liver imaging); and willing to participate in all study-related activities. We excluded potential participants if they were currently enrolled in an HIV- or HCV-related research protocol, could not remain in the study for 12 months, or were unable to provide informed consent because of cognitive impairment or an inability to understand and speak English. Participant disposition from initial contact to trial completion is shown in Figure 1.
FIGURE 1. Allocation of participants in study to promote linkage to hepatitis services: San Francisco, CA, and New York City, February 2008–June 2011.
Note. HAV = hepatitis A virus; HBA = hepatitis B virus; HCV = hepatitis C virus.
Assessments
Participants completed surveys at baseline and at 3, 9, and 12 months after baseline, and we assessed outcomes at 1, 6, and 12 months after baseline and at times of HCV clinical and vaccination visits throughout the study period. At baseline, we used standardized instruments to collect information about participants’ sociodemographics, substance use, health status, and HIV and hepatitis testing and treatment history, by using a combination of interviewer-administered and computer-assisted personal interview. Standardized instruments included the Addiction Severity Index,41 the Beck Depression Inventory-II,42 and the SF-12 Health Survey.43 Follow-up interviews gathered information about whether participants had sought HCV evaluation or treatment both inside and outside of the health care systems where the study was being conducted. In addition, we collected data on use of HCV-related medical services during the 12-month study through a review of the health care systems’ computerized medical records. We excluded from further analysis self-reported health care that was already represented in computerized medical records. We collected blood specimens at baseline only and urine specimens at each assessment.
We collected a blood specimen to test for HAV, HBV, and HCV, and also for HIV if consent was given. We determined HAV status by the total HAV antibody test; a positive test reflects immunity due to either prior natural infection or vaccination. We determined HIV status by HIV enzyme-linked immunosorbent assay and Western blot testing. We determined hepatitis B status by HBV surface antigen, total HBV core antibody, and HBV surface antibody testing. We considered individuals with HBV surface antibody only to be immune because of vaccination. We considered individuals with HBV surface antibody and total HBV core antibody to be immune because of prior disease; these individuals, along with those with isolated total HBV core antibody and those with HBV surface antigen, were considered to have prevalent HBV infection. We considered individuals to need vaccination if they were susceptible to HAV (i.e., were HAV-total-antibody negative), were negative for all HBV markers, or had isolated total HBV core antibody; these individuals were offered vaccination with combined HAV–HBV vaccine. We considered participants to need an HCV clinical evaluation if they were HCV-antibody positive. We tested serum samples by licensed clinical laboratories in California and New York according to manufacturers’ instructions.
We analyzed urine specimens for the presence of cocaine, heroin, amphetamines, barbiturates, benzodiazepines, tetrahydrocannabinol, and methadone by the enzyme-multiplied immunoassay technique.
Enrollment
Participants were individuals who met eligibility criteria, gave written informed consent, completed a baseline assessment, and provided urine and blood specimens. Following baseline assessment, we randomly assigned participants to the hepatitis care coordination group (intervention) or the group receiving HIV and viral hepatitis testing, education, and counseling (control), with both groups stratified by gender. The project statistician employed computer software (SAS version 9.1; SAS Institute, Cary, NC) to generate randomization assignments separately for each of the participating sites, using varying block sizes known only to the statistician.
Outcome Measures
We defined HAV–HBV vaccination adherence as receipt of the first vaccination dose within 30 days of the date of referral (for screened participants negative for total-HAV-antibody or negative for HBV surface antigen and HBV surface antibody, or negative for both HAV and HBV serology). For the HCV evaluation outcome, we defined intervention adherence as attendance at an HCV clinical evaluation within 6 months of the date of referral (for HCV antibody–positive participants). We created a combined intervention adherence index to examine treatment effects over a period of 12 months (for those who required HAV–HBV vaccination, HCV evaluation, or both). We reviewed medical records to verify self-reported vaccinations and attendance at HCV care provider visits in each of the time periods.
Follow-Up
Research interviewers located and assessed participants. Individuals who missed appointments were contacted by telephone and mail. Interviews took approximately 1 hour to complete. Respondents were paid $20 for the baseline assessment and $25, $30, and $35 for the 3-, 9-, and 12-month interviews, respectively. Participants were not paid for attending clinical (including HIV and viral hepatitis counseling and education sessions) or vaccine visits.
Intervention and Control Groups
Participants in both groups received individual 2-session manual-guided HIV and viral hepatitis counseling and education administered by research staff using a laptop-based slide presentation. The education and counseling included HIV and viral hepatitis pretest counseling, voluntary testing, posttest counseling, and the provision of test results at the second session. Basic information about HIV and viral hepatitis transmission, prevention, and treatment as well as the benefits of HAV–HBV immunization was provided by research staff. This educational and counseling session constituted a manual-guided intervention reflecting what is recommended by the Centers for Disease Control and Prevention for all drug users and by various public health agencies for drug treatment programs.40 The posttest counseling session took place 1 to 2 weeks following the pretest counseling session.
Intervention group
The hepatitis care coordination intervention included the individual 2-session HIV and viral hepatitis pretest and posttest counseling and education provided to the control group, with the difference that it was delivered in a motivational interviewing style.44 The intervention group also received serological testing for HIV and hepatitis viruses, on-site vaccination, and, for a period of 6 months, motivational interviewing–enhanced case management assistance with vaccination and off-site HCV evaluations.
Participants who, on the basis of serologic test results, were susceptible to HAV, HBV, or both were offered combination vaccine (Twinrix; GlaxoSmithKline, Rixensart, Belgium) on site at the MMT program. Following the instructions on the package insert, MMT program staff administered the combined HAV–HBV vaccine as closely as possible to the recommended schedule at baseline, 1 month, and 6 months.
The investigative team designed case management sessions to facilitate access to needed hepatitis medical care, including HAV–HBV vaccination and HCV clinical evaluations. Case managers coordinated with primary care and hepatology clinics to schedule HCV evaluation appointments. In San Francisco, participants were referred for clinical visits in a building adjacent to the methadone program; in New York City, participants were referred for the same services 9 blocks away. Case managers scheduled initial and follow-up patient appointments, accompanied patients to these appointments, negotiated new appointments when participants failed to attend medical care visits, and provided follow-up reminder phone calls and letters. Case managers also assisted patients in accessing psychiatric services, alcohol treatment, legal assistance, and social service entitlements. Case management sessions were held weekly, lasted an hour or less, and were not compensated, but transportation tokens or bus or subway cards were provided on a case-by-case basis by interventionists if transportation could affect adherence to the intervention. Case manager caseloads included approximately 15 participants throughout the study. Case managers had bachelor’s degrees and a minimum of 1 year of experience working with substance-abusing populations and were supervised by a psychologist.
A total of 9 participants did not receive the allocated intervention: 4 declined participation, 3 were deemed ineligible by study investigators, 1 elected to discontinue treatment, and 1 was jailed (Figure 1).
Control group
Components of the control group intervention included the 2-session HIV and viral hepatitis pretest and posttest counseling without motivational interviewing, on-site serological testing for HIV and hepatitis viruses, and off-site referral for vaccination and hepatitis evaluation. In San Francisco, participants were referred for vaccination and clinical visits to a building adjacent to the methadone program; in New York City, participants were referred for the same services 9 blocks away. Those randomized to the control group who did not adhere to off-site HAV–HBV vaccination within 30 days following referral for vaccination (at the posttest counseling session) were considered nonadherent to off-site vaccine referral and were then offered on-site vaccination and given reminders.
Nine control participants did not receive the allocated intervention: 5 were jailed, 3 declined participation, and 1 died (Figure 1).
Statistical Analysis
We used an intent-to-treat approach to examine clinical outcomes for all randomized participants who required either HAV–HBV vaccination or HCV clinical evaluation. We used the independent-samples t test (for continuous variables) and the Pearson χ2 test (for categorical variables) to compare the 2 study arms on baseline sociodemographic and clinical characteristics. We calculated time-dependent variables from the date of the second education session, at which participants were given serological results.
We used logistic regression models to compare intervention and control groups on 3 outcome measures: (1) adherence to HAV–HBV vaccination within 30 days, (2) adherence to HCV evaluation within 6 months, and (3) a combined adherence measure consisting of compliance to HAV–HBV vaccination and HCV evaluation, as needed, within 12 months. Covariates in regression models included intervention condition, recruitment site, gender, ethnicity, education, HIV status, and homeless status; we chose these covariates on the basis of hypothesized relationships and relationships identified in earlier literature.21,30,45 We tested separate models for each adherence measure.
We conducted Cox proportional hazards models to compare intervention conditions on the time to receive vaccination or an HCV evaluation, using the same covariates as in the logistic regression models to provide adjusted estimates. We used SAS version 9.2 (SAS Institute, Cary, NC) to test all models.
RESULTS
We randomized a total of 489 individuals (239 in San Francisco, 250 in New York City) to the 2 study groups. Participants of the intervention group (n = 244) and the control group (n = 245) did not differ significantly on any baseline-measured variables, including HIV infection prevalence (Table 1). Overall prevalence rates for immunity to HAV, prevalent HBV, and HCV seropositivity were 55.6%, 42.3%, and 58.5%, respectively, with no differences in prevalence rates between groups (P > .05 for all). There were no differences between the 2 study sites in follow-up rates or in the proportion evaluable for either study endpoint.
TABLE 1.
Demographic and Clinical Characteristics of Participants in Study to Promote Linkage to Hepatitis Services: San Francisco and New York City, February 2008–June 2011
| Characteristic | Intervention Group, (n = 244), No. (%) or Mean ± SD | Control Group, (n = 245), No. (%) or Mean ± SD | Pa |
|---|---|---|---|
| Male gender | 167 (68.4) | 167 (68.2) | .95 |
| Race/ethnicity | .76 | ||
| White | 88 (36.1) | 88 (35.9) | |
| Hispanic | 78 (32.0) | 70 (28.6) | |
| African American | 67 (27.5) | 77 (31.4) | |
| Other | 11 (4.5) | 10 (4.1) | |
| High school education or above | 127 (52.1) | 142 (58.0) | .19 |
| Married | 42 (17.2) | 37 (15.1) | .53 |
| Homeless past 6 mo | 101 (41.4) | 98 (40.0) | .75 |
| Employed | 39 (16.0) | 39 (15.9) | .98 |
| Yearly income < $10 000 | 156 (63.9) | 142 (58.0) | .18 |
| Injection drug use (ever) | 177 (72.5) | 166 (67.8) | .25 |
| Immune to hepatitis A | 136 (55.7) | 136 (55.5) | .78 |
| Hepatitis B status | |||
| Susceptible | 80 (32.8) | 77 (31.4) | .75 |
| Chronic antigen | 3 (1.2) | 3 (1.2) | .99 |
| Immune, vaccination | 39 (16.0) | 44 (18.0) | .56 |
| Immune, disease | 73 (29.9) | 72 (29.4) | .9 |
| Isolated core | 34 (13.9) | 32 (13.1) | .78 |
| Hepatitis C antibody positive | 149 (61.1) | 137 (55.9) | .45 |
| HIV infected | 28 (11.7) | 22 (9.1) | .35 |
| Age, y | 44.7 ± 10.3 | 45.0 ± 9.8 | .48 |
| Heroin use, y | 15.2 ± 10.8 | 14.9 ± 10.5 | .73 |
| Methadone dose, mg | 89.6 ± 39.5 | 89.8 ± 43.3 | .97 |
| No. of d alcohol used in past 30 d | 4.5 ± 8.6 | 6.1 ± 10.2 | .07 |
| Addiction Severity Index | |||
| Alcohol use | 0.10 ± 0.17 | 0.12 ± 0.21 | .19 |
| Drug use | 0.25 ± 0.11 | 0.25 ± 0.11 | .88 |
| Psychiatric | 0.34 ± 0.24 | 0.33 ± 0.24 | .56 |
| Beck Depression Inventory-II (BDI II) depression severity total scores | 18.8 ± 11.50 | 18.70 ± 10.80 | .87 |
| Persons with BDI II depression severity scores ≥ 20 | 116 (47.5) | 113 (46.1) | .75 |
| SF-12 Physical Component Summary | 44.2 ± 11.2 | 43.4 ± 11.7 | .45 |
| SF-12 Mental Component Summary | 38.6 ± 12.3 | 40.6 ± 13.4 | .08 |
Note. Addiction Severity Index composite scores in each domain range from 0 (no problems) to 1 (severe problems). In Beck Depression Inventory II, higher scores indicate more severe depression: minimal = 0–13; mild = 14–19; moderate = 20–28; severe = 29–63. The SF-12 Health Survey (SF-12), Physical Component Summary and Mental Component Summary scores have a range of 0 to 100 and were designed to have a mean score of 50 and a standard deviation of 10 in a representative sample of the US population. Scores greater than 50 represent above-average health status.
Differences in proportions evaluated by Pearson χ2 (2-tailed) and means by t test.
Case Management Services
The average number of case management sessions attended by intervention arm participants was 11.3 (SD = 8.63), with a median of 10 sessions. The average length of time per session was 16.8 minutes (SD = 19.62), with a median of 10 minutes; participants received a mean total of 189.6 minutes (SD = 196.12), with a median of 120 minutes. We used a repeated-measures zero-inflated Poisson regression model to compare rates of illicit drug use and alcohol use (past 30 days) between intervention conditions, controlling for intervention arm, age, gender, race/ethnicity, HIV status, homelessness, and recruitment site over the 12-month study period. There was no significant intervention effect on any of the drug use variables, but the intervention group showed modestly greater reductions in alcohol use (from 45.1% to 37.2%) than the control group (from 44.9% to 41.4%; P = .066).
Adherence to First-Dose HAV–HBV Vaccine
On the basis of serological testing, 300 of the 489 participants required the combined HAV–HBV vaccine (i.e., were found to be susceptible to HAV, HBV, or both). Intervention group participants were more likely than control group participants to receive the first dose of the HAV–HBV vaccine within 30 days of the educational session at which participants were given serological results (76.7% vs 12.0%; odds ratio [OR] = 41.8; 95% confidence interval [CI] = 19.4, 90.0). Participants enrolled at the New York City site were more likely to be vaccinated than participants at the San Francisco site (OR = 3.83; 95% CI = 1.81, 8.11; Table 2). However, in each site examined separately, intervention group participants were more likely than control group participants to receive the first dose of the vaccine within 30 days (for New York City, OR = 28.2; 95% CI = 11.8, 67.7; for San Francisco, OR = 219.6; 95% CI = 19.1, > 999.0). In the survival analysis model, intervention group participants received their first vaccine dose earlier (median days from provision of serological results = 7.00; 95% CI = 5.00, 10.00) than the control group participants (median days = 49.5; 95% CI = 45.0, 62.0). The proportional hazards ratio model indicated that intervention group participants were likely to receive their first dose of the vaccine earlier than the control group participants (hazard ratio [HR] = 3.89; 95% CI = 2.17, 6.99).
TABLE 2.
Effects of Intervention Condition on Adherence to First HAV–HBV Vaccine Dose in Study to Promote Linkage to Hepatitis Services: San Francisco, CA, and New York City, February 2008–June 2011
| Variable | OR (95% CI) |
|---|---|
| Intervention condition | |
| Hepatitis care coordination | 41.8** (19.4, 90.0) |
| Control (Ref) | 1.00 |
| Recruitment location | |
| New York City | 3.83** (1.81, 8.11) |
| San Francisco (Ref) | 1.00 |
| Gender | |
| Female | 1.62 (0.80, 3.30) |
| Male (Ref) | 1.00 |
| Race/ethnicity | |
| White (Ref) | 1.00 |
| African American | 1.03 (0.46, 2.32) |
| Hispanic | 2.51 (1.05, 6.02) |
| Other | 0.41 (0.08, 2.23) |
| Education, y | |
| ≥ 12 | 1.52 (0.77, 3.00) |
| < 12 (Ref) | 1.00 |
| HIV status | |
| Negative | 0.63 (0.19, 2.08) |
| Positive (Ref) | 1.00 |
| Homeless past 6 mo | |
| No | 0.71 (0.36, 1.40) |
| Yes (Ref) | 1.00 |
Note. CI = confidence interval; HAV = hepatitis A virus; HBV = hepatitis B virus; OR = odds ratio.
P < .001.
Adherence to 3-Dose HAV–HBV Vaccine Series
Of the persons in the intervention arm requiring vaccination, 117 of 150 (78.0%) completed the 3-dose vaccine series compared with 14 of 150 (9.3%) of control group participants in their originally assigned arms (OR = 34.44; CI = 17.58, 67.46). Those control group participants who did not initiate vaccination within 30 days were offered on-site vaccine; an additional 90 control group participants then completed the vaccine series so that, overall, more than two thirds of the participants (207 of 300) who required vaccination ultimately completed all 3 doses.
Adherence to HCV Clinical Evaluation
Of the 489 participants, 286 required an HCV evaluation on the basis of serological testing. Intervention group participants were more likely than control group participants to receive an HCV evaluation within the 6-month case management period (65.1% vs 37.2%; OR = 4.10; 95% CI = 2.35, 7.17; Table 3). Participants at the San Francisco site were more likely to receive an HCV evaluation than participants at the New York City site (OR = 3.21; 95% CI = 1.73, 5.95). However, in each site examined separately, intervention group participants were more likely than control group participants to receive an HCV evaluation (data not shown). HIV–HCV coinfected participants were more likely to receive an HCV evaluation than HCV monoinfected participants (OR = 8.02; 95% CI = 2.81, 22.95). In addition, participants who had a stable living arrangement in the past 6 months were more likely than individuals who reported homelessness to receive an HCV evaluation (OR = 2.28; 95% CI = 1.25, 3.33). No other analyzed variables were associated with receipt of an HCV evaluation.
TABLE 3.
Effects of Intervention Condition on Adherence to HCV Clinical Evaluation in Study to Promote Linkage to Hepatitis Services: San Francisco, CA, and New York City, February 2008–June 2011
| Variable | OR (95% CI) |
|---|---|
| Intervention condition | |
| Hepatitis care coordination | 4.10** (2.35, 7.17) |
| Control (Ref) | 1.00 |
| Recruitment location | |
| San Francisco | 3.21** (1.73, 5.95) |
| New York City (Ref) | 1.00 |
| Gender | |
| Female | 0.83 (0.46, 1.49) |
| Male (Ref) | 1.00 |
| Race/ethnicity | |
| White (Ref) | 1.00 |
| African American | 0.93 (0.46, 1.86) |
| Hispanic | 0.79 (0.40, 1.57) |
| Other | 2.33 (0.65, 8.33) |
| Education, y | |
| ≥ 12 | 1.10 (0.64, 1.91) |
| < 12 (Ref) | 1.00 |
| HIV status | |
| Positive | 8.02** (2.81, 22.95) |
| Negative (Ref) | 1.00 |
| Homeless past 6 mo | |
| No | 2.28* (1.25, 3.33) |
| Yes (Ref) | 1.00 |
Note. CI = confidence interval; HCV = hepatitis C virus; OR = odds ratio.
P < .01;
P < .001.
Intervention group participants received an HCV evaluation earlier (median days from provision of serological results = 84.0; 95% CI = 71.0, 113) than did control group participants (median days = 337; 95% CI = 154, undefined). The proportional hazards model showed that intervention group participants in both sites were likely to receive HCV evaluation earlier than the control group participants (in San Francisco, HR = 1.74; 95% CI = 1.10, 2.77; in New York City, HR = 5.00; 95% CI = 2.28, 10.31). Additionally, HIV–HCV coinfected participants were likely to receive an HCV evaluation earlier than HCV monoinfected participants (HR = 2.65; 95% CI = 1.66, 4.22).
Combined Intervention Adherence Outcome
The combined intervention adherence outcome showed strong effects favoring the intervention group. Intervention group participants were more likely to adhere to treatment recommendations (i.e., HAV–HBV vaccination, HCV evaluation, or both) than were control group participants (60.3% vs 16.7%; OR = 8.70; 95% CI = 5.56, 13.61). Additionally, participants who were HIV positive were more likely to have a positive adherence outcome than HIV-negative participants (OR = 3.35; 95% CI = 1.63, 6.86).
DISCUSSION
This is the first randomized controlled trial to examine the efficacy of a hepatitis care coordination model in the methadone treatment setting. We found the hepatitis care coordination intervention to be superior to a control condition in increasing the likelihood of receiving the first dose of the HAV–HBV vaccine and decreasing the time to do so. The vaccination adherence rate of 77% for the hepatitis care coordination intervention is comparable to rates reported in published studies offering vaccination to drug users on-site at syringe exchange sites46,47 and in a drug treatment program.48 On-site vaccination in drug treatment programs has the potential to increase HAV–HBV vaccination among drug users, and this study provides strong evidence that on-site HAV–HBV vaccination can be integrated into methadone treatment programs.
The hepatitis care coordination intervention was also superior to the control condition in increasing adherence to a prescribed HCV clinical evaluation. Intervention participants were more likely to receive an HCV evaluation than those assigned to the control group, and to do so more promptly. Moreover, in an analysis of a combined intervention adherence outcome (i.e., HAV–HBV vaccination, HCV evaluation, or both), the results showed strong effects favoring the hepatitis care coordination intervention group.
Individuals who reported homelessness in the previous 6 months were less likely to participate in an initial HCV evaluation. Homeless individuals may delay or forgo timely and appropriate health care because it may compete with more immediate needs for food and shelter. Previously published findings and the results from our study suggest that additional strategies may be needed to engage HCV-infected homeless persons in HCV care.49,50
This study indicates that on-site HAV–HBV vaccination, HIV and hepatitis education, and motivational interviewing–enhanced patient navigation and case management services in the methadone treatment setting facilitate adherence to HAV–HBV vaccination and engagement in an initial HCV clinical evaluation. Patient navigation and case management are promising components of models of care coordination for cancer and HIV care,38 and the present study demonstrates that these approaches can be effectively applied to the health care needs of HCV-infected drug users in MMT. In fact, this study showed that those who were HIV–HCV coinfected were more likely than HCV-monoinfected persons to have a positive adherence outcome (i.e., receipt of vaccination, HCV evaluation, or both), highlighting the need for the development of enhanced interventions to engage and retain HCV-monoinfected persons in HCV care. As with any multicomponent intervention, the relative contributions of each component (e.g., on-site services vs motivational interviewing vs case management) can be difficult to discern, and further data would be valuable. This study also demonstrated that the intervention could be integrated in 2 different health care organizations. Although site differences emerged regarding the impact of the intervention on both vaccination adherence and HCV clinical evaluation adherence, the intervention was nonetheless efficacious at each site, suggesting potential generalizability. However, further study to identify which individual and structural variables contributed to the site differences is merited; knowledge of these variables might allow refinements to the intervention, which could facilitate the broader dissemination and implementation of the intervention. Additional strategies tailored to address these barriers may be needed.
Limitations of the randomized controlled trial should be noted. We examined the efficacy of the intervention on linkage to an initial HCV clinical evaluation, and further studies are needed to document the impact of the intervention on subsequent steps in the HCV care cascade (including completion of all steps necessary to determine treatment eligibility, treatment acceptance, and adherence to prescribed HCV treatments); nonetheless, the subsequent steps in the HCV care cascade cannot occur without an initial HCV clinical evaluation, which the intervention effectively promoted. Although case management efforts were manualized, procedures allowed case managers to use different reminder and navigation strategies with different participants, and this variability in case management effort may have introduced some variability in the outcomes. Findings may not be fully generalizable to all settings in which drug users or other HCV-infected persons need to be linked to care; our 2 recruitment sites were in drug treatment programs linked to academic medical centers with a full range of resources. However, our sample demographics parallel those of other studies of methadone patients and HCV-infected drug users in other settings,19 and the intervention may have utility in such related settings. Cost constraints prevented testing of HCV-positive patients on-site at the MMT program for HCV viral load to identify chronic HCV infection, and doing so might improve outcomes. Finally, cost and comparative effectiveness data regarding the impact of this intervention are needed.
This study provides new information regarding the efficacy of a model of hepatitis care coordination in methadone treatment designed to increase adherence to HAV–HBV vaccination and HCV clinical evaluation. As the population of untreated chronically HCV-infected persons has aged, the prevalence of HCV-related mortality has risen and now exceeds the mortality rate of HIV.51 Thus, there is a critical need to develop more effective methods to screen, evaluate, and treat HCV infection among drug users and other high-risk populations. A hepatitis care coordination model, which includes on-site vaccination and case management to facilitate linkages to HCV treatment, may help to fill this gap in the continuum of care by providing a more accessible model for MMT programs that are unable to provide on-site HCV clinical care.
Acknowledgments
This study was supported by the National Institutes of Health (grants R01DA020781, R01DA020841, P30 DA 011041, P50DA009253, and U10DA015815).
Footnotes
Reprints can be ordered at http://www.ajph.org by clicking the “Reprints” link.
Contributors
C. L. Masson and D. C. Perlman contributed equally to the study; they originated the study and supervised all aspects of its design, implementation, analysis, interpretation, and writing. K. L. Delucchi performed and oversaw all statistical analyses. C. McKnight, J. Hettema, M. Khalili, A. Min, A. E. Jordan, N. Pepper, J. Hall, N. S. Hengl, C. Young, M. S. Shopshire, J. K. Manuel, L. Coffin, H. Hammer, B. Shapiro, R. M. Seewald, H. C. Bodenheimer Jr, J. L. Sorensen, and D. C. Des Jarlais helped conceptualize ideas, assisted in aspects of implementation, analysis, and interpretation of findings, and reviewed drafts of the article.
Note. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute on Drug Abuse or National Institutes of Health.
Human Participant Protection
The University of California, San Francisco Committee on Human Research and the Beth Israel Medical Center institutional review boards reviewed and approved the study.
Contributor Information
Carmen L. Masson, Department of Psychiatry, University of California, San Francisco.
Kevin L. Delucchi, Department of Psychiatry, University of California, San Francisco.
Courtney McKnight, Beth Israel Medical Center, New York, NY.
Jennifer Hettema, Department of Psychiatry, University of California, San Francisco.
Mandana Khalili, Department of Medicine, University of California, San Francisco.
Albert Min, Beth Israel Medical Center, New York, NY.
Ashly E. Jordan, Beth Israel Medical Center, New York, NY.
Nicole Pepper, Department of Psychiatry, University of California, San Francisco.
Jessica Hall, Department of Psychiatry, University of California, San Francisco.
Nicholas S. Hengl, Department of Psychiatry, University of California, San Francisco.
Christopher Young, Beth Israel Medical Center, New York, NY.
Michael S. Shopshire, Department of Psychiatry, University of California, San Francisco.
Jennifer K. Manuel, Department of Psychiatry, University of California, San Francisco.
Lara Coffin, Beth Israel Medical Center, New York, NY.
Hali Hammer, Department of Family and Community Medicine, University of California, San Francisco.
Bradley Shapiro, Department of Psychiatry, University of California, San Francisco.
Randy M. Seewald, Beth Israel Medical Center, New York, NY.
Henry C. Bodenheimer, Jr, Beth Israel Medical Center, New York, NY.
James L. Sorensen, Department of Psychiatry, University of California, San Francisco.
Don C. Des Jarlais, Beth Israel Medical Center, New York, NY.
David C. Perlman, Beth Israel Medical Center, New York, NY.
References
- 1.Hutin YJ, Sabin KM, Hutwagner LC, et al. Multiple modes of hepatitis A virus transmission among methamphetamine users. Am J Epidemiol. 2000;152(2):186–192. doi: 10.1093/aje/152.2.186. [DOI] [PubMed] [Google Scholar]
- 2.Kuo I, Sherman SG, Thomas DL, Strathdee SA. Hepatitis B virus infection and vaccination among young injection and non-injection drug users: missed opportunities to prevent infection. Drug Alcohol Depend. 2004;73(1):69–78. doi: 10.1016/j.drugalcdep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and C in people who inject drugs: results of systematic reviews. Lancet. 2011;378 (9791):571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garfein RS, Vlahov D, Galai N, et al. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lymphotropic viruses. Am J Public Health. 1996;86(5):655–661. doi: 10.2105/ajph.86.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagan H, Des Jarlais DC, Stern R, et al. HCV synthesis project: preliminary analyses of HCV prevalence in relation to age and duration of injection. Int J Drug Policy. 2007;18(5):341–351. doi: 10.1016/j.drugpo.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses—a model for viral persistence. Immunol Res. 2010;47(1–3):216–227. doi: 10.1007/s12026-009-8152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 8.Vento S, Garofano T, Renzini C, et al. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338(5):286–290. doi: 10.1056/NEJM199801293380503. [DOI] [PubMed] [Google Scholar]
- 9.Thein HH, Qilong Y, Dore GJ, Krahn MD. Natural history of hepatitis C infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22 (15):1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 10.Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19(6):593–601. doi: 10.1097/01.aids.0000163936.99401.fe. [DOI] [PubMed] [Google Scholar]
- 11.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360 (9349):1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 12.Hwang LY, Crimes CZ, Tran TQ, et al. Accelerated hepatitis B vaccine schedule among drug users—a randomized controlled trial. J Infect Dis. 2010;202(10):1500–1509. doi: 10.1086/656776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawlotsky JM. Hepatitis C virus: from discovery to eradication in 40 years? Clin Microbiol Infect. 2011;17(2):105–106. doi: 10.1111/j.1469-0691.2010.03435.x. [DOI] [PubMed] [Google Scholar]
- 14.Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- 15.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- 16.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype I hepatitis C infection: an open-label, randomised, multicentre phase 2 trial [erratum in Lancet. 2010;376(9748):1224] Lancet. 2010;376 (9742):705–716. doi: 10.1016/S0140-6736(10)60934-8. [DOI] [PubMed] [Google Scholar]
- 17.Selwyn PA, Budner NS, Wasserman WC, Arno P. Utilization of on-site primary care services by HIV-seropositive and seronegative drug users in a methadone maintenance program. Public Health Rep. 1993;108(4):492–500. [PMC free article] [PubMed] [Google Scholar]
- 18.Umbricht-Schneiter A, Ginn DH, Pabst KM, Bigelow GE. Providing medical care to methadone clinic patients: referral vs on-site care. Am J Public Health. 1994;84(2):207–210. doi: 10.2105/ajph.84.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez D, Dimova R, Marks KM, et al. Integrated internist–addiction medicine–hepatology model for hepatitis C management for individuals on methadone maintenance. J Viral Hepat. 2012;19(1):47–54. doi: 10.1111/j.1365-2893.2010.01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vassilev ZP, Strauss SM, Astone JM, Friedmann PD, Des Jarlais DC. Provision of on-site medical care to patients with hepatitis C in drug treatment units. J Health Care Poor Underserved. 2004;15(4):663–671. doi: 10.1353/hpu.2004.0075. [DOI] [PubMed] [Google Scholar]
- 21.Grebely J, Raffa JD, Lai C, et al. Low uptake of treatment for hepatitis C virus infection in a large community-based study of inner city residents. J Viral Hepat. 2009;16(5):352–358. doi: 10.1111/j.1365-2893.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 22.Stoové MA, Gifford SM, Dore GJ. The impact of injecting drug use status on hepatitis C-related referral and treatment. Drug Alcohol Depend. 2005;77(1):81–86. doi: 10.1016/j.drugalcdep.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Felsen UR, Fishbein DA, Litwin AH. Low rates of hepatitis A and B vaccination in patients with chronic hepatitis C at an urban methadone maintenance program. J Addict Dis. 2010;29(4):461–465. doi: 10.1080/10550887.2010.509281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stein MD, Maksad J, Clarke J. Hepatitis C disease among injection drug users: knowledge, perceived risk and willingness to receive treatment. Drug Alcohol Depend. 2001;61(3):211–215. doi: 10.1016/s0376-8716(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 25.Heimer R, Clair S, Grau LE, et al. Hepatitis-associated knowledge is low and risks are high among HIV-aware injection drug users in three US cities. Addiction. 2002;97(10):1277–1287. doi: 10.1046/j.1360-0443.2002.t01-1-00211.x. [DOI] [PubMed] [Google Scholar]
- 26.Carey J, Perlman DC, Friedmann P, et al. Knowledge of hepatitis among active drug injectors at a syringe exchange program. J Subst Abuse Treat. 2005;29(1):47–53. doi: 10.1016/j.jsat.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Morrill JA, Shrestha M, Grant RW. Barriers to the treatment of hepatitis C. J Gen Intern Med. 2005;20 (8):754–758. doi: 10.1111/j.1525-1497.2005.0161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watson B, Conigrave KM, Wallace C, Whitfield JB, Wurst F, Haber P. Hazardous alcohol consumption and other barriers to antiviral treatment among hepatitis C positive people receiving opioid maintenance treatment. Drug Alcohol Rev. 2007;26(3):231–239. doi: 10.1080/09595230701247681. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien S, Day C, Black E, Dolan K. Injecting drug users’ understanding of hepatitis C. Addict Behav. 2008;33(12):1602–1605. doi: 10.1016/j.addbeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. doi: 10.1007/s10900-007-9083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munoz-Plaza CE, Strauss S, Astone-Twerell J, et al. Exploring drug users’ attitudes and decisions regarding hepatitis C (HCV) treatment in the US. Int J Drug Policy. 2008;19(1):71–78. doi: 10.1016/j.drugpo.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindenburg CEA, Lambers FAE, Urbanus AT, et al. Hepatitis C testing and treatment among active drug users in Amsterdam: results from the DUTCH-C project. Eur J Gastroenterol Hepatol. 2011;23(1):23–31. doi: 10.1097/MEG.0b013e328340c451. [DOI] [PubMed] [Google Scholar]
- 33.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44(1):26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Christie J, Itzkowitz S, Lihuau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100(3):278–284. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 35.Lebwohl B, Neugut AI, Stavsky E, et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011;45(5):e47–e53. doi: 10.1097/MCG.0b013e3181f595c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen JL, Dilley J, London J, Okin RL, Delucchi KL, Phibbs CS. Case management for substance abusers with HIV/AIDS: a randomized clinical trial. Am J Drug Alcohol Abuse. 2003;29(1):133–150. doi: 10.1081/ada-120018843. [DOI] [PubMed] [Google Scholar]
- 37.Gardner L, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 38.Vargas RB, Cunningham WE. Evolving trends in medical care-coordination for patients with HIV and AIDS. Curr HIV/AIDS Rep. 2006;3(4):149–153. doi: 10.1007/s11904-006-0009-y. [DOI] [PubMed] [Google Scholar]
- 39.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21(suppl 1):S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the US Department of Health and Human Services. MMWR Recomm Rep. 2012;61(RR-5):1–40. [PubMed] [Google Scholar]
- 41.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 43.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Miller R, Rollnick S. Motivational Interviewing: Helping People Change. 3. New York, NY: Guilford Publications; 2013. [Google Scholar]
- 45.Grebely J, Genoway KA, Raffa JD, et al. Barriers associated with the treatment of hepatitis C virus infection among illicit drug users. Drug Alcohol Depend. 2008;93(1–2):141–147. doi: 10.1016/j.drugalcdep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Des Jarlais DC, Fisher DG, Newman JC, et al. Providing hepatitis B vaccination to injection drug users: referral to health clinics vs on-site vaccination at a syringe exchange program. Am J Public Health. 2001;91 (11):1791–1792. doi: 10.2105/ajph.91.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altice FL, Bruce RD, Walton MR, Buitrago MI. Adherence to hepatitis B virus vaccination at syringe exchange sites. J Urban Health. 2005;82(1):151–161. doi: 10.1093/jurban/jti016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mezzelani P, Venturini L, Turrina G, Lugoboni F, Des Jarlais D. High compliance with a hepatitis B vaccination program among intravenous drug users. J Infect Dis. 1991;163(4):923. doi: 10.1093/infdis/163.4.923. [DOI] [PubMed] [Google Scholar]
- 49.Gelberg L, Roberston MJ, Arangua L, et al. Prevalence, distribution, and correlates of hepatitis C infection among homeless adults in Los Angeles. Public Health Rep. 2012;127(4):407–421. doi: 10.1177/003335491212700409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strehlow AJ, Robertson MJ, Zerger S, et al. Hepatitis C among clients of health care for the homeless primary care clinics. J Health Care Poor Underserved. 2012;23 (2):811–833. doi: 10.1353/hpu.2012.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156(4):271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]