Abstract
Past work has suggested that the medial superior temporal (MST) area is involved in the initiation of three kinds of eye movements at short-latency by large-field visual stimuli. These eye movements consist of: 1) version elicited by linear motion (the ocular following response, OFR), 2) vergence elicited by binocular parallax (the disparity vergence response, DVR), 3) vergence elicited by global motion towards or away from the fovea (the radial-flow vergence response, RFVR). We investigated this hypothesis by recording the effects of ibotenic acid injections in the superior temporal sulcus (STS) of both hemispheres in five monkeys. After the injections, all three kinds of eye movements were significantly impaired, the magnitude of the impairments often showing a strong correlation with the extent of the morphological damage in the three subregions of the STS: MSTd on the anterior bank, MSTl and MT on the posterior bank. However, the extent of the lesions in the three subregions often co-varied, rendering it difficult to assess their relative contributions to the various deficits. The effects of the lesions on other aspects of oculomotor behavior that are known to be important for the normal functioning of the three tracking mechanisms (e.g., ocular stability, fixation disparity) were judged to be generally minor and to contribute little to the impairments. We conclude that, insofar as MST sustained significant damage in all injected hemispheres, our findings are consistent with the hypothesis that MST is a primary site for initiating all three visual tracking eye movements at ultra-short latencies.
Keywords: Ocular following response, disparity vergence, radial-flow vergence
Introduction
Recent studies in primates have revealed three visually driven ocular tracking mechanisms that are activated in machine-like fashion at ultra-short latencies and are thought to help to stabilize the gaze of the moving observer in 3-D (Miles, 1998). In this scheme, two of these mechanisms generate vergence eye movements to help maintain binocular alignment on objects that lie ahead: the disparity vergence response (DVR), which is driven by the changes in binocular parallax (Busettini et al., 1996; Masson et al., 1997), and the radial-flow-vergence response (RFVR), which is driven by the radial patterns of optic flow (Busettini et al., 1997; Inoue et al., 1998a). The third mechanism, the ocular following response (OFR), helps to stabilize gaze on objects that move within the plane of fixation and generates version eye movements in response to the planar motion (Kawano and Miles, 1986; Miles and Kawano, 1986; Miles et al., 1986).
This paper is concerned with the neural mediation of these responses and focuses on the role of the medial superior temporal area (MST) within the superior temporal sulcus (STS) of the monkey’s cortex. This area is known to contain many neurons that respond vigorously to visual motion with directional selectivity (Van Essen et al., 1981; Desimone and Ungerleider, 1986), and others that are sensitive to binocular disparity or to the patterns of optic flow experienced by the moving observer (Maunsell and Van Essen, 1983a; Tanaka et al., 1986; Tanaka and Saito, 1989; Roy and Wurtz, 1990; Duffy and Wurtz, 1991; Roy et al., 1992; Eifuku and Wurtz, 1999). We have recorded neurons in MST that discharge in relation to the large-field visual stimuli used to elicit the OFR (Kawano et al., 1994; Takemura et al., 2000), the DVR (Takemura et al., 2001; Takemura et al., 2002) and the RFVR (unpublished observations). Most of the changes in neural activity preceded the associated eye movements, and some occurred early enough for them to have a causal role in producing even the earliest ocular responses. These electrophysiological experiments suggested that, despite their ultra-short latency, all three visual tracking eye movements could be mediated at least in part by MST neurons.
The present study was undertaken to test this hypothesis by documenting the effects of bilateral chemical lesions in the STS, which included varying amounts of MSTd, MSTl and MT, on the three short-latency visual tracking eye movements in five monkeys. We report that ibotenic acid injections into the STS could abolish the initial OFR and the initial RFVR and significantly attenuate the initial DVR. These findings are consistent with our hypothesis that the MST has a major role in the production of all three of these ocular tracking responses.
Preliminary results have been reported previously (Takemura and Kawano, 2004).
Materials and Methods
Animal preparation
Data were collected from five adolescent Japanese monkeys (Macaca Fuscata), weighing 5–9 kg. Many of the methods and procedures were very similar to those used in our previous studies (Kawano et al., 1994; Inoue et al., 1998a; Takemura et al., 2001) and will be given only in brief here. All animals had been previously trained to fixate a small target spot on a tangent screen for a liquid reward (Wurtz, 1969). Under aseptic conditions and sodium pentobarbital anesthesia, a cylinder for chronic recording of single neuron activity was implanted vertically over a trephine hole positioned above the posterior parietal cortex. This cylinder was subsequently used for the ibotenic acid injections. A bracket attached to the skull allowed the head to be secured in the standard stereotaxic position during the experiments. Eye movements were recorded using scleral search coils implanted around both eyes (Fuchs and Robinson, 1966; Judge et al., 1980). One animal (monkey P) had been previously used for single unit recordings in the left nucleus of the optic tract (NOT) and in the left dorsolateral pontine nucleus (DLPN) but its oculomotor behavior was within the normal limits after those recordings. All experimental protocols were approved by the AIST Animal Care and Use Committee.
Behavioral paradigms and visual stimuli
The presentation of stimuli and the collection, storage, and display of data were controlled by two personal computers (NEC PC98). The behavioral paradigms and visual stimuli used in this study were identical to those of Shidara and Kawano (1993) for the OFR, Takemura et al. (2001) for the DVR, and Inoue et al. (1998a) for the RFVR. In brief, during the recording sessions, the monkey sat in a primate chair with its head secured in place, and facing a translucent tangent screen (114 × 114 cm) at a distance of 500 mm onto which random-dot patterns (subtense, 90°×90°) could be back-projected. Mirror galvanometers in the projection path in X/Y configuration allowed the patterns to be moved under computer control. The density of dots was ~50% and the smallest dots in the patterns subtended ~1.5° of arc. Five paradigms were used, which in brief were as follows:
In the OFR paradigm, the random-dot pattern filled the screen and 50 ms after the end of a 10° leftward centering saccade started to move at constant velocity (range, 10–160°/s) in one of the four cardinal directions; the motion lasted 150 ms and then the screen was blanked. In the DVR paradigm, two projectors were used with crossed polarizers to achieve dichoptic viewing such that the two eyes saw identical random-dot patterns that filled the screen and initially overlapped exactly (zero binocular disparity); horizontal disparity steps (crossed and uncrossed, ranging in amplitude from 0.5 to 6°) were applied 50 ms after 10° leftward centering saccades, by displacing the two images equally in opposite directions for 230 ms. In the RFVR paradigm, the visual stimulus was a two-frame movie, the initial random-dot pattern being replaced by a second one (simulating the same image viewed from a slightly different distance) 50 ms after a leftward centering saccade; the focus of expansion/contraction was located at the center of the screen and was therefore imaged in—or close to—the fovea; this looming step involved a 5% change in the eccentricity of the dots with respect to the screen center without any change in their size: the “pure radial-flow stimulus” of Busettini et al (1997); note that previous studies indicated that the RFVR is mostly a transient response to stimulus onset and multiple steps (at up to 100Hz, which approximates smooth motion) elicit essentially the same result, i.e., adding further apparent-motion steps adds little to the response (Miles et al., 2004). In all three paradigms, if no saccades were detected during the stimulus presentation period, then the data were stored on a hard disk and the animal was given a drop of fruit juice; otherwise, the trial was aborted and fluid was withheld; at this point, the random-dot patterns were blanked for 0.5 s by a mechanical shutter and then reappeared once more for the start of the next trial. Note that all experiments included saccade-only controls in which the initial patterns remained present and unchanging throughout the trial. The luminance of the patterns ranged from 2.7 cd/m2 (white dots) to 0.3 cd/m2 (black background area) for the RFVR paradigm (average, 1.5 cd/m2), and from 1.0 cd/m2 to 0.05 cd/m2 for the DVR paradigm (average, 0.5 cd/m2). The stimulus patterns used for the DVR paradigm were somewhat darker than those used for RFVR paradigm because of the polarizing filters. The brighter stimulus pattern was also used for the OFR paradigm, except with monkeys N and R for which the polarizers remained in place (but the dichoptic images always exactly overlapped).
In two monkeys we also examined the effects of the MST injections on smooth pursuit and optokinetic eye movements to permit comparison with previous lesion studies (Dürsteler et al., 1987; Dürsteler and Wurtz, 1988). In the smooth-pursuit paradigm, the target was a projected LED spot whose horizontal position was controlled by a mirror galvanometer in the projector light path. At the start of each trial, a stationary target spot appeared at the center of the screen and the monkey was required to fixate it for 700–900 ms. On “pursuit” trials the target moved to the right or left at one of three selected speeds (10, 20, 30 °/s) and, after a grace period of 100 ms to allow the monkey to catch up with the target, the monkey was required to track it with smooth eye movements for the remaining time that it was visible (600 ms). On “fixation” trials, the target remained stationary and the monkey had to fixate it. The trial type (“pursuit” or “fixation”), as well as the direction and speed of target motion on the “pursuit” trials, were randomized from trial to trial. In the optokinetic paradigm two random dot patterns moving to the right or left at one of three selected speeds (20, 40, 60°/s) were back-projected on the screen one after the other to achieve continuous smooth motion. To replicate the traditional optokinetic stimulation used in previous lesion studies, the screen was initially dark and then the moving pattern suddenly came into view (by operating a shutter). This optokinetic stimulus remained on for 15 s, after which time the screen was blanked and recording continued for a further 15 s: optokinetic afternystagmus (OKAN). The monkey was rewarded with drops of fluid for staying alert (apparent from the brisk quick phases).
Lesion procedures
Prior microelectrode recordings, involving 10–64 penetrations per hemisphere, allowed us to determine the likely location of the MST within the STS of both hemispheres in all five monkeys. In 7/10 hemispheres, the penetrations were made with glass-coated tungsten microelectrodes that entered the parietal cortex, advanced through the anterior bank of the STS, and then ended in the posterior bank or floor of the STS. After reconstructing these penetrations and using the selectivity for speed and direction of motion as well as the size of the receptive fields to identify putative MT and MST neurons in both hemispheres, we implanted 4 or 5 guide tubes whose tips were positioned 3–5 mm above the regions thought to encompass the MST in both hemispheres. These guide tubes were to be used later for injecting ibotenic acid, but we first used them to introduce tungsten microelectrodes to confirm that they were located above a region containing abundant directionally selective neuronal activity characteristic of MT and MST. In 3/10 hemispheres, the neuronal recordings were made only through the guide tubes and accurate reconstruction of these electrode tracks was not possible.
The pre-injection eye-movement data were obtained over a period of several days after the guide tubes had been positioned in both hemispheres. Thus, any effects due to the prior microelectrode penetrations and the implantation of the guide tubes would be manifest in the eye-movement data obtained before any injections were made. After completing the collection of the pre-injection data, these guide tubes were used to make multiple pressure injections of ibotenic acid (15 mg/ml in a basic saline solution) over a two-day period in monkeys Q, P and S, a three-day period in monkey N and a seven-day period in monkey R. We used a Hamilton microsyringe with an injection tube whose outer diameter was 0.7 mm (Crist Instruments, Hagerstown, MD), to inject at a rate of 0.2 μl/min, which subsequent histology indicated was sufficiently slow to prevent backflow of ibotenic acid around the microsyringe. To avoid leakage of ibotenic acid during withdrawal, the microsyringe was not raised for at least 15 min after completion of the injection(s) through a given guide tube. The number of injection sites and total amount of ibotenic acid injected are summarized in Table 1. For each monkey, the post-injection eye-movement data were obtained over a period of 2–3 days commencing one day after completing the bilateral injections.
Table 1.
Number and volume of the ibotenic acid injections.
| L-STS | R-STS | Total Volume (μl) | |||||
|---|---|---|---|---|---|---|---|
| No. of guide tubes | No. of injection sites (Volume, μl) | No. of guide tubes | No. of injection sites (Volume, μl) | ||||
| anterior bank | posterior bank | anterior bank | posterior bank | ||||
| Monkey Q | 4 | 4 (8.0) | 4 (8.0) | 4 | 4 (8.0) | 4 (8.0) | 32.0 |
| Monkey N | 5 | 6 (12.3) | 5 (10.1) | 4 | 4 (8.0) | 3 (6.0) | 36.4 |
| Monkey P | 4 | 5 (9.4) | 4 (8.0) | 5 | 5 (10.0) | 5 (10.0) | 37.4 |
| Monkey R | 5 | 6 (24.0) | 5 (22.7) | 5 | 5 (10.0) | 5 (10.0) | 66.7 |
| Monkey S | 4 | 5 (10.0) | 4 (8.1) | 4 | 5 (10.0) | 4 (8.0) | 36.1 |
Data collection and assessment of the eye-movement deficits
In the OFR paradigm, we recorded only the horizontal and vertical positions of the right eye. In the DVR and RFVR paradigms we recorded only the horizontal positions of both eyes. The eye-position measures were hardware differentiated to obtain eye velocity measures. These position and velocity voltage signals were smoothed with a 6-pole Bessel filter (3 dB at 100 Hz) and then digitized at 1000 Hz with a resolution of 12 bits. Voltage signals encoding the positions of the mirror galvanometers controlling the positions of the patterns and target spots on the screen were digitized at 500 Hz with a resolution of 12 bits. All data were stored and transferred to a workstation (SunSparc) for analysis. Trials with saccadic intrusions during the experiment were deleted. The mean eye velocity data obtained in the saccade-only control trials were subtracted from the eye velocity data obtained in all other trials and all quantitative analyses were performed on these “adjusted” data profiles. The horizontal eye position and velocity data obtained in the DVR and RFVR paradigms were used to compute the horizontal vergence position angle and the horizontal vergence velocity (left eye minus right eye), respectively. We used the convention that rightward (and upward) deflections of the stimuli or eyes were positive, hence convergence had a positive sign. Mean response profiles to a particular stimulus over time, synchronized to stimulus onset (time zero), were computed for eye position, eye velocity, horizontal vergence position and vergence velocity for the complete data sets obtained before and after the injections. Mean eye acceleration profiles were obtained by digital differentiation of the mean eye velocity data and were used to estimate the latency of onset of the OFR (criterion for onset, 100°/s2). The initial open-loop OFRs were quantified by measuring the change in the position of the right eye over the time period 50–100 ms measured from stimulus onset. The initial open-loop DVR and RFVRs were quantified by measuring the change in vergence position over the same time period. To assess the magnitude of the deficits in the ocular responses after the ibotenic acid injections, we calculated a percentage reduction, defined as [(Rpre − Rpost)/Rpre] × 100%, where Rpre and Rpost are the response measures for the initial open-loop OFR, DVR or RFVR before and after the injections, respectively.
Morphological reconstruction of the locations and extents of the lesions
At the conclusion of the post-injection recordings from a given monkey, that animal was deeply anesthetized with sodium pentobarbital and perfused through the heart with normal saline followed by 10% Formalin. The animal’s brain was removed, and 50-μm frozen sections were cut in the sagittal plane. After mounting on microscope slides, sections were stained with cresyl violet for cell bodies and with a modified silver stain for myelinated fibers (Gallyas, 1979). Maps of the STS region of cortex were created, based on the method of Van Essen and Maunsell (1980), and the extent of the lesions was drawn on these maps (Fig. 1). The densely myelinated zones in the posterior bank (MT) and in the anterior bank (DMZ) were identified and transferred onto the unfolded maps (Newsome et al., 1985; Ungerleider and Desimone, 1986). In locating MST, we followed the precedent of Komatsu and Wurtz (1988), who defined it as that region of the STS outside MT that contains a preponderance of directionally selective neurons. These workers showed that neurons in MST could be distinguished from neurons in MT by their larger field size for a given retinal eccentricity, and that the rostro-caudal extent of MST was definable morphologically—encompassing the DMZ anteriorly and extending up to the boundary of MT posteriorly—but the medio-lateral boundaries had no exact morphological correlates. In estimating the extent of the involvement of MST in our lesions we have assumed that the MST encompassed the entire region of the STS extending between the two myelinated zones, with MSTl occupying the posterior bank and MSTd the anterior bank (Komatsu and Wurtz, 1988). The areas affected by the ibotenic acid injections were identified using the morphological criteria of Newsome et al., (1985). Surface area measurements of the lesions and the various subdivisions of the STS were made from the maps with the use of a scanner and a program designed for measuring area.
Figure 1.
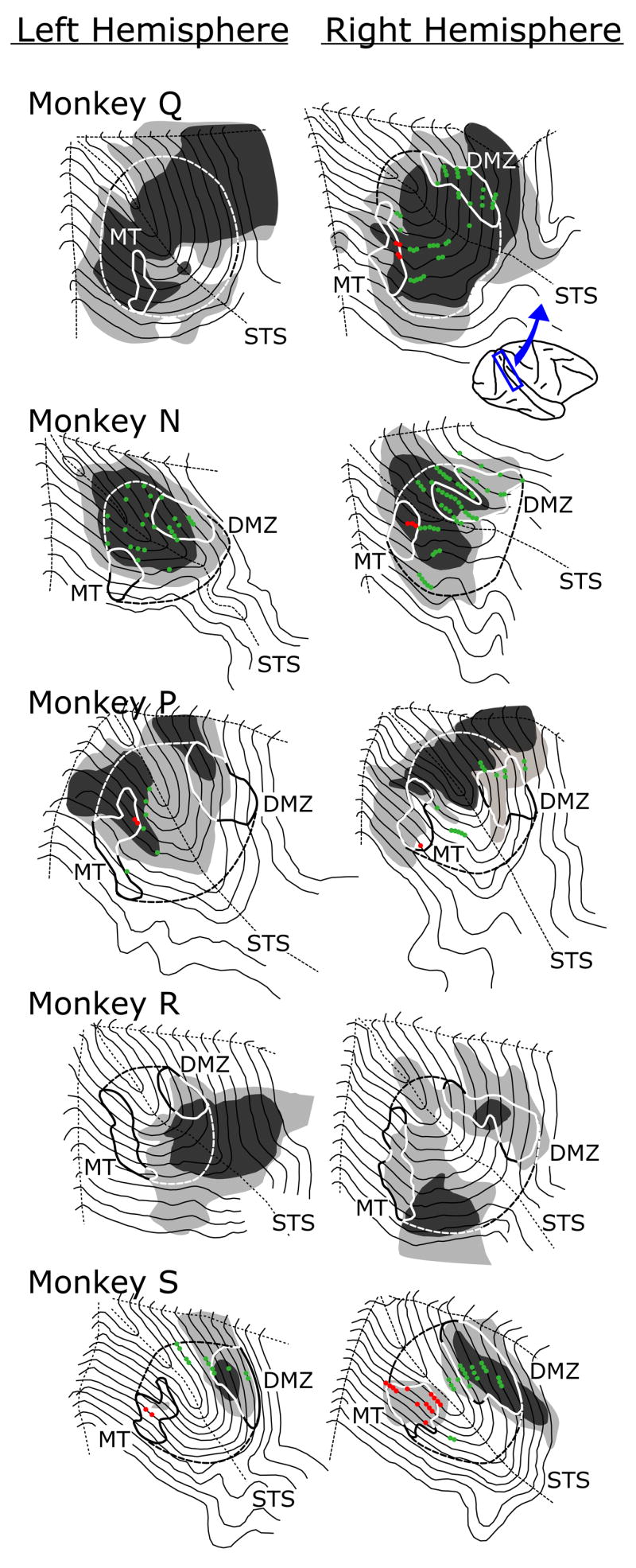
Two-dimensional maps of the STS in 10 hemispheres of five monkeys reconstructed from parasagittal sections using the method of Van Essen and Maunsell (1980). The maps of the left hemisphere are left-right reversed to facilitate easy comparison with the maps of the right hemisphere. Inset shows a view of the right hemisphere with the general area of the STS indicated. Contour lines depict the unfolded layer IV. Solid boundary lines delineate the densely myelinated zones in the anterior bank (DMZ) and the posterior bank (MT) of the STS. Dashed lines depict the approximate medial and lateral boundaries of the MST, which is subdivided into MSTd anteriorly and MSTl posteriorly by the dotted lines depicting the floor of the STS. Black shading indicates areas with morphological damage extending through all layers of the cortex (“complete” lesion) and grey shading indicates areas with damage to some but not all cortical layers (“partial” lesions). Circles indicate the approximate recording sites of neurons that responded to visual motion and showed directional selectivity (red, MT neurons; green, MST neurons).
Results
Reconstruction of the STS lesions
Maps of the STS region of cortex, reconstructed from sagittal sections, are shown in Fig. 1 for all 10 hemispheres that were injected in 5 monkeys. To facilitate easy comparisons of the lesions on the two sides of the brain, maps from the left hemisphere are left-right reversed. The densely myelinated zones in the posterior bank (MT) and in the anterior bank (DMZ) were identified together with the locations of 23 neurons recorded in 8 penetrations that were deemed to be MT neurons (filled red circles in Fig. 1) and a further 127 neurons that were recorded in 21 penetrations and deemed to be MST neurons (filled green circles in Fig. 1). These recordings were made with rigid glass-coated microelectrodes advanced through the depth of the cortex, permitting reconstruction of the entire penetration from the surface of the cortex to the floor of the STS.1 While these electrophysiological recordings provided good confirmation of the locations of some parts of MST, and proved useful for positioning the guide tubes over the putative MST region, it is evident from Fig. 1 that they were generally not sufficient to define the exact medio-lateral extent of the MST. In specifying its medio-lateral boundaries in Fig. 1 we assumed that MST encompassed the entire region of the STS between the MT and the DMZ with a slight medial extension: see the dashed lines in Fig. 1. We will use the floor of the sulcus of the STS to subdivide the MST into an anterior region that approximates MSTd and a posterior region that approximates MSTl. Table 1 summarizes the number of guide tubes and injection sites together with the volume of ibotenic acid injected in each monkey. The initial injections in monkey R were ineffective (probably because the ibotenic acid was not fully dissolved) and a second series was required, hence the unusually large volume in that monkey. The regions affected by the ibotenic acid injections were identified using the morphological criteria of Newsome et al (1985), and were subdivided into areas in which all 6 layers were affected—designated as “complete” lesions (black in Fig. 1)—and areas in which only some layers showed morphological changes—designated as “partial” lesions (grey in Fig. 1). The background silver stain in the lesioned areas sometimes obscured the dense myelination, leading us to underestimate the full extent of MT and the DMZ in those cases. This was especially true on the left side in monkey Q, where we were unable to discern the DMZ (and so assumed that it had been completely lesioned); in this case, the boundary of MST was drawn to approximate that on the opposite side.
Table 2 has three sections that each summarizes our quantitative estimates of the extent of the partial and/or complete lesions in one of the three subregions of the STS—MSTd, MSTl, MT—in both hemispheres for each of the five monkeys. The entries in each section indicate the area in mm2 of the morphological changes within the given subregion; in addition, these values are each expressed as a percentage of the area of their respective subregion (entries in parentheses), and we will refer to these as the “percentage extent” of the lesions. Note that the “total” area of the lesions refers to their overall extent and is given by the sum, “partial + complete”. The extent of the lesions varied considerably among the five monkeys and the order in which their data are shown in Fig. 1 and listed in Table 2—Q, N, P, R, S—generally accords with their percentage total lesions, which were largest in monkey Q and smallest in monkey S. In monkey Q, these total lesions included much of MST and MT on both sides. However, in monkeys N and P significant portions of MST on both sides (especially laterally) as well as the lateral (foveal) regions of MT on the left side were completely spared, and in monkeys R and S even more of MST (especially the floor of the sulcus and the posterior bank in monkey S bilaterally) and almost all of MT on the left side were intact. Bilateral asymmetries, based on the differences in the percentage total lesions in the two hemispheres of a given monkey, were generally minor for MST, averaging only 9% for MSTd (range, 0–15%) and 15% for MSTl (range, 1–35%), but could be appreciable for MT, averaging 41% (range, 0–90%). The proportions of the three subdivisions of the STS that sustained damage to all layers (“complete” lesion) ranged from 0–100%, and were <50% in two thirds (20/30) of the cases listed in Table 2. It is perhaps not surprising that the percentage extents of the lesions in a given subdivision of a given monkey tended to be positively correlated, and the r2 values for the “complete” lesion data were very high: 0.97 for MSTl vs. MSTd, 0.93 for MST vs, MT, 0.85 for MSTl vs. MT, and 0.95 for MSTd vs. MT. The equivalent r2 values for the “total” lesion data were much lower: 0.71, 0.49, 0.28, and 0.77. Note that there was clear gliosis along the path of the guide tubes in the overlying area 7a but there was no evidence of any additional damage such as might be expected from ibotenic acid due to backflow around the microsyringe or leakage during withdrawal of the microsyringe.
Table 2.
Estimated area of the lesions in the three subregions of the STS.
| Left MSTd | Right MSTd | |||||
|---|---|---|---|---|---|---|
| partial | complete | total | partial | complete | total | |
| monkey Q | 55.2 (17) | 208 (65) | 263.2 (83) | 37 (19) | 157.2 (79) | 194.1 (98) |
| monkey N | 55.5 (30) | 122.1 (67) | 177.6 (97) | 70 (35) | 99.5 (50) | 169.5 (85) |
| monkey P | 182.8 (58) | 31.7 (10) | 214.5 (69) | 75.2 (32) | 88.1 (37) | 163.3 (69) |
| monkey R | 27.1 (22) | 44.7 (37) | 71.9 (59) | 133.8 (55) | 25.6 (10) | 159.4 (65) |
| monkey S | 72.5 (34) | 26.9 (12) | 99.4 (46) | 85.3 (31) | 77 (28) | 162.4 (58) |
| Left MSTl | Right MSTl | |||||
| partial | complete | total | partial | complete | total | |
| monkey Q | 83.7 (36) | 148.8 (63) | 232.4 (99) | 53.5 (19) | 228.3 (81) | 281.8 (100) |
| monkey N | 47.1 (31) | 90.4 (60) | 137.6 (91) | 78.2 (39) | 80 (40) | 158.1 (79) |
| monkey P | 96.3 (36) | 92.6 (35) | 188.9 (70) | 15.7 (9) | 47.2 (26) | 62.8 (35) |
| monkey R | 55.6 (39) | 43 (30) | 98.6 (69) | 135.4 (47) | 83.9 (29) | 219.2 (76) |
| monkey S | 0 (0) | 0 (0) | 0 (0) | 20.9 (18) | 0 (0) | 20.9 (18) |
| Left MT | Right MT | |||||
| partial | complete | total | partial | complete | total | |
| monkey Q | 0 (0) | 26.9 (100) | 26.9 (100) | 26 (50) | 26.4 (50) | 52.4 (100) |
| monkey N | 13.6 (36) | 9.9 (26) | 23.4 (63) | 20.8 (49) | 21.4 (51) | 42.2 (100) |
| monkey P | 21.8 (28) | 25.9 (33) | 47.7 (60) | 29.0 (51) | 8.9 (16) | 37.9 (67) |
| monkey R | 3.2 (5) | 0 (0) | 3.2 (5) | 52.8 (74) | 2.6 (4) | 55.4 (78) |
| monkey S | 3.3 (7) | 0 (0) | 3.3 (7) | 54.6 (97) | 0 (0) | 54.6 (97) |
Values are areas in mm2. The proportions of the three subregions affected by the lesions are given in parentheses (%). “Partial” refers to areas that have sustained damage to some but not all layers of the cortex. “Complete” refers to areas that have sustained damage to all layers of the cortex. “Total” refers to areas that have sustained any damage to any layer of the cortex (“partial + complete”).
Ocular Following Responses (OFRs)
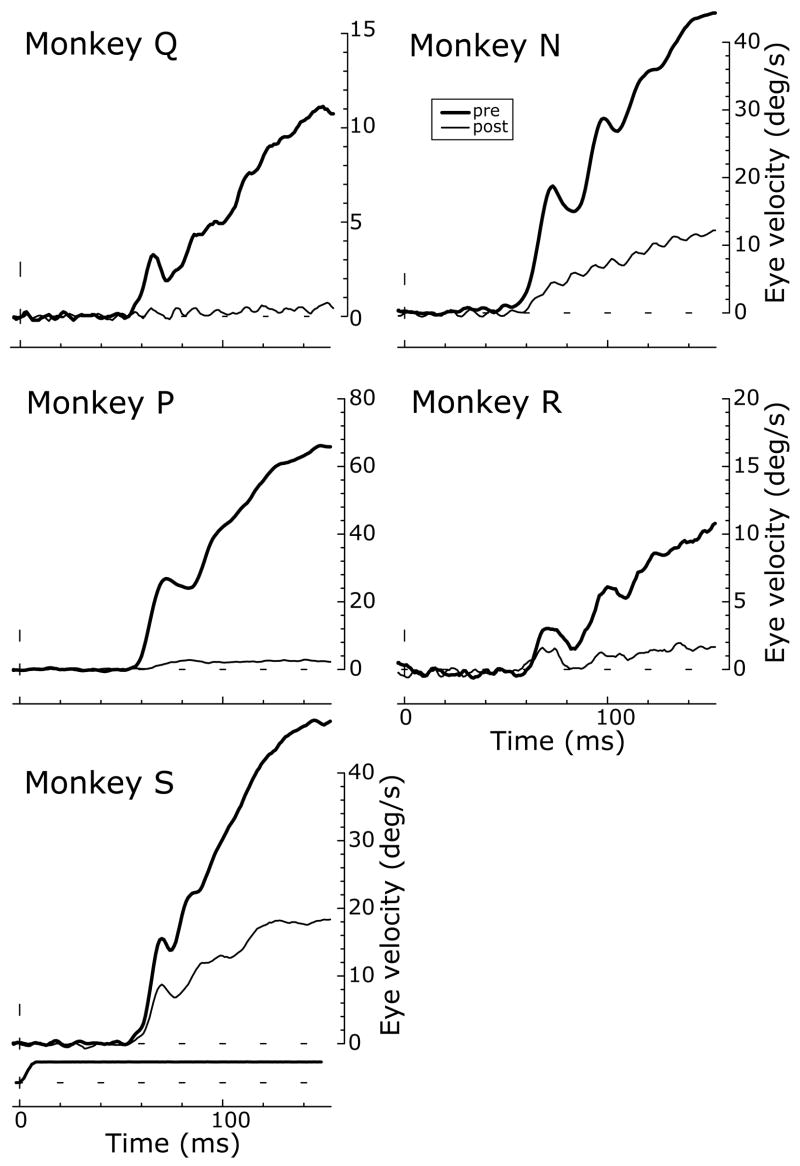
The initial OFRs recorded prior to the injections of ibotenic acid were like those described by Miles et al. (1986) in all essentials, with onset latencies of 55–60ms and mean eye velocity temporal profiles that showed an initial brisk increase followed by a slight hesitation and perhaps a second transient increase before giving way to a more gradual and sustained buildup: see the thick traces in Fig. 2. After the injections, the mean eye velocity temporal profiles showed varying degrees of attenuation: see the thin traces in Fig. 2. Onset latencies before and after the injections were not significantly different in the three monkeys (N, R, S) whose post-injection responses were large enough to permit measurements of latency (Student’s t-test, p>0.05).
Figure 2.
Deficits in OFRs after ibotenic acid injections: sample temporal profiles (data for each of the five monkeys). Mean eye velocity (°/s) over time (ms) in response to multiple presentations of 80°/s downward ramps before (thick traces) and after (thin traces) the injections. Upward deflections denote downward eye movements. Bottom left panel also includes the stimulus velocity profile.
The quantitative effects of the injections on the initial (open-loop) OFRs, based on the change in eye position over the time period 50–100 ms (measured from stimulus onset), are summarized in Fig. 3. For each of the 5 monkeys and the 4 cardinal directions of stimulus motion, Fig. 3 shows histograms indicating the mean percentage reductions in the OFR measures (averaged across the 5 different speeds). These measures indicated that all monkeys exhibited significant impairments in their initial OFRs after the injections (Student’s t-test, p<0.05), the mean percentage reductions for a given direction of motion ranging from 37% to 97%. There were some directional asymmetries in the deficits and we quantified them by computing the right-left, up-down, horizontal-vertical differences, and these data are also shown in Fig. 3. The largest consistent directional asymmetries involved relative sparing of the rightward responses in monkeys N, S, and P, the deficits to leftward motion exceeding those to rightward motion on average by 28%, 18%, and 11%, respectively. The vertical deficits were larger than the horizontal in monkeys Q, N, P, and S, on average by 6–21%; the fifth monkey, R, showed the reverse asymmetry, the horizontal deficits exceeding the vertical, on average by 30%. The graph in Fig. 4 plots the percentage reductions in the OFR measures (averaged across all 4 cardinal directions) against speed for each of the five monkeys, and indicates a tendency for the deficits to be somewhat greater for the data obtained with stimuli moving at the higher speeds.
Figure 3.
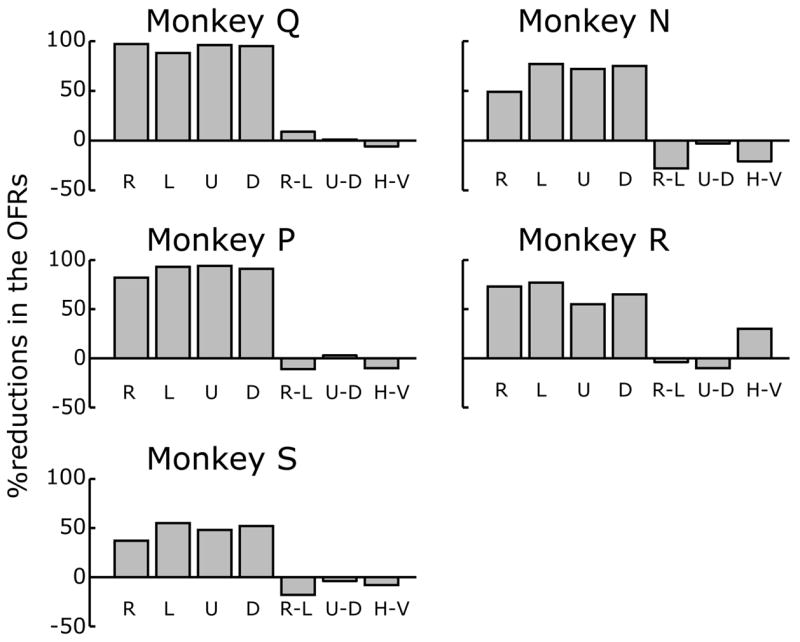
Deficits in OFRs after ibotenic acid injections (histograms for each of the five monkeys). R, L, U, D: percentage reductions in the OFRs (based on the changes in eye position over the time period 50–100 ms) to right-, left-, up- and down-ward motion averaged for 5 speeds ranging from 10°/s to 160°/s. R-L, U-D, H-V: differences between the mean percentage reductions to rightward and leftward, upward and downward, horizontal (R+L) and vertical (U+D), respectively.
Figure 4.
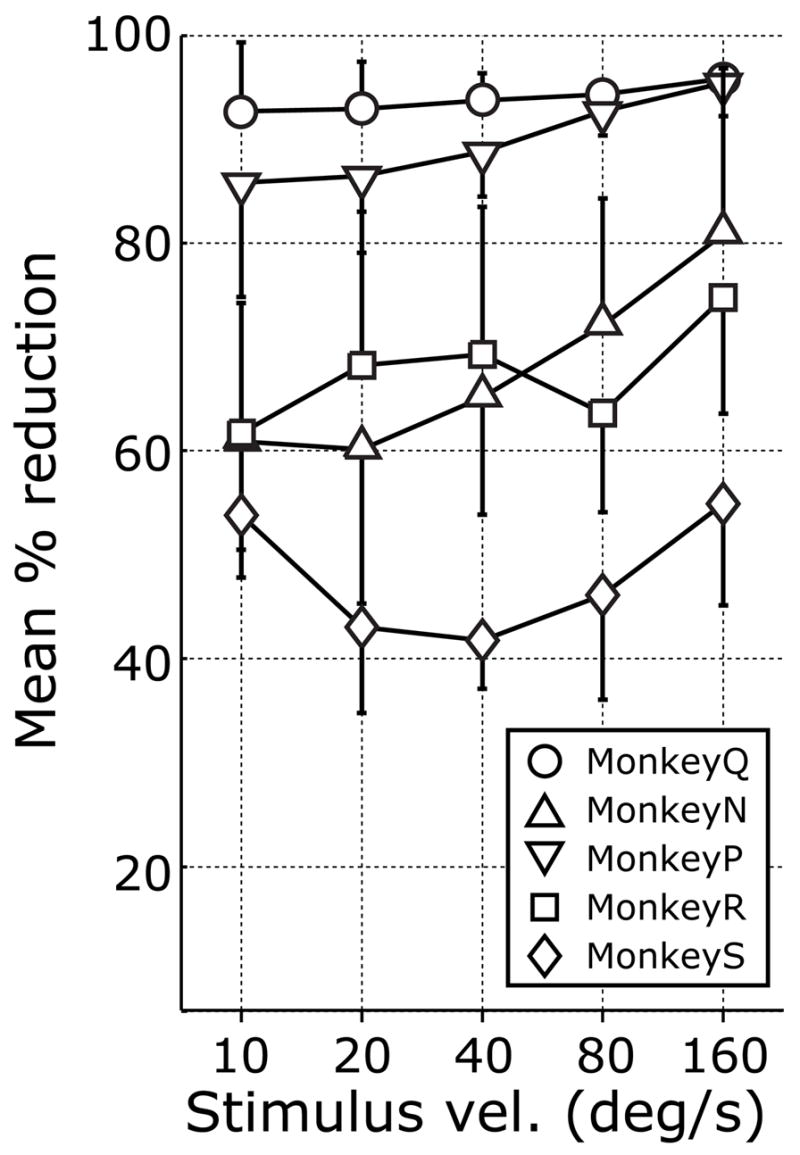
Deficits in OFRs after ibotenic acid injections: dependence on stimulus speed (data for each of the five monkeys). Ordinate: Percentage reduction in the OFRs for each monkey, based on the mean changes in eye position (in degrees, over the time period 50–100 ms measured from onset of the stimulus ramps) averaged for all four cardinal directions. Abscissa: stimulus speed (°/s). Error bars, 1 SD. Symbols: circles, monkey Q; triangles, monkey N; inverted triangles, monkey P; squares, monkey R; diamonds, monkey S.
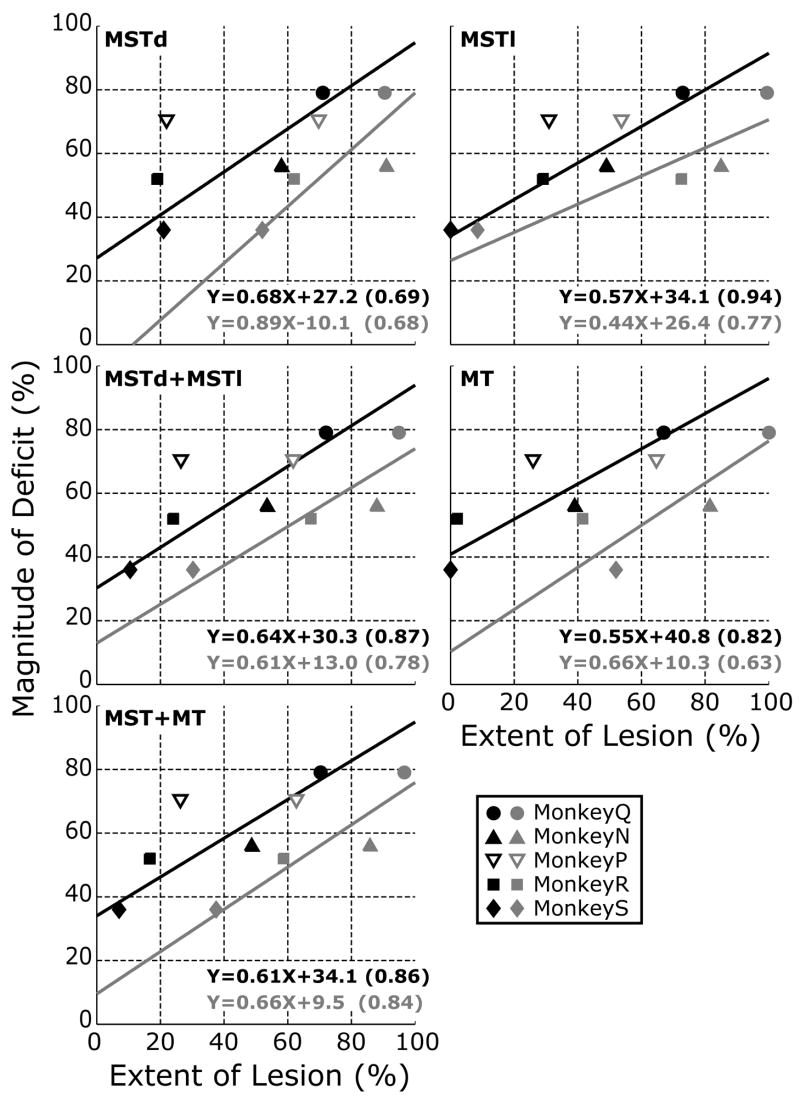
Scrutiny of Table 2 and Fig. 3 suggested that there was a general tendency for the animals with the larger lesions to show larger deficits in their OFRs. We examine this further in Fig. 5, in which the mean percentage reductions in the OFR measures (averaged across all stimulus speeds and directions) for each of the 5 monkeys are plotted against the percentage extents of their lesions in each one of five subdivisions within the STS: MSTd, MSTl, MSTd+MSTl, MT, and MSTd+MSTl+MT. Each monkey has two data entries in each of the graphs, the grey symbols showing the data when the extents of the lesions were based on the percentage of each subdivision that had been damaged partially or completely (i.e., the total lesion listed in Table 2), and the black symbols showing the data when the extents of the lesions were based only on the percentage of each subdivision in which the damage was complete. These plots indicate that there was a general tendency for the average OFR deficit to co-vary with both estimates of the percentage extent of the morphological damage in the various subdivisions of the STS. However, the magnitude of the deficit in monkey P, which had previously been used for recordings in the NOT and the DLPN, was markedly greater than expected from the size of its cortical lesions and the data from the other 4 monkeys (see the open inverted triangles in Fig. 5). When the data of monkey P were excluded, linear regressions of these average OFR deficits on the extent of the lesions in each of the various subgroupings within the STS always showed significant positive slopes with r2 values ranging from 0.51 to 0.78 when based on the total extent of the lesions (grey data and lines in Fig. 5) and from 0.57 to 0.91 when based only on the extent of the complete lesions (black data and lines in Fig. 5).2 The positive correlation between the size of the lesion and the magnitude of the deficit was greatest for the complete lesions in MSTl.
Figure 5.
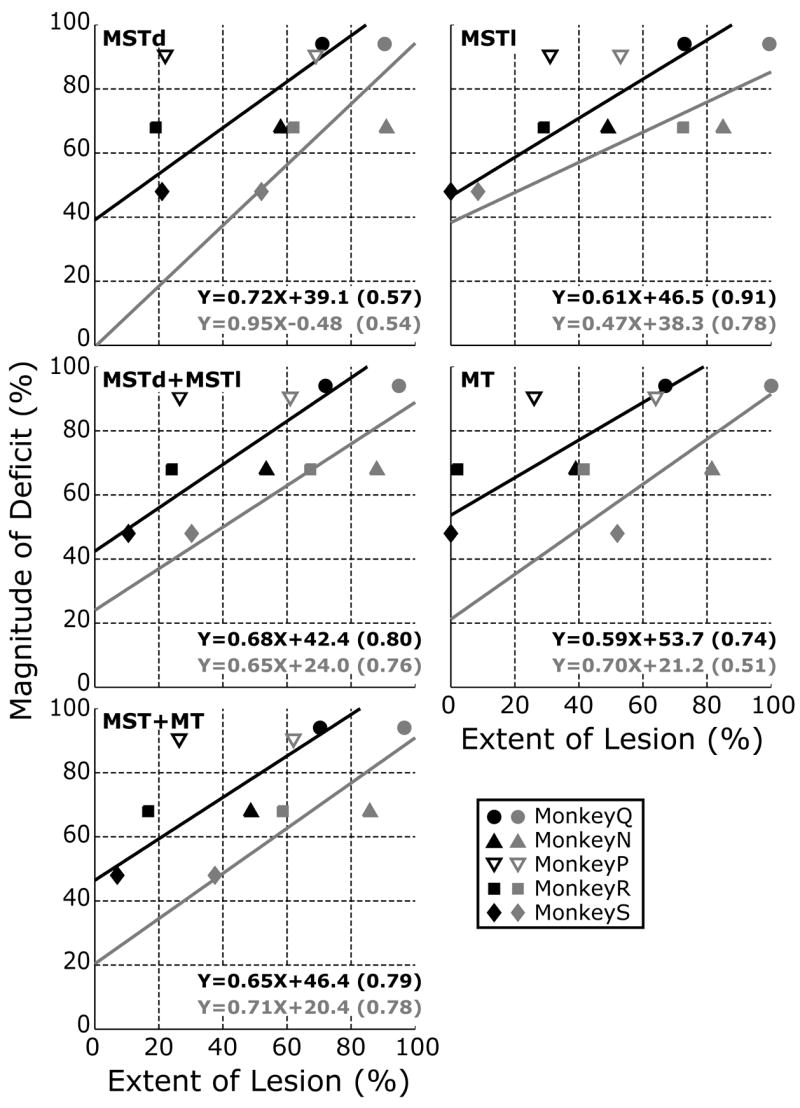
Deficits in OFRs after ibotenic acid lesions: dependence on the estimated extent of the lesions in MSTd, MSTl and MT (data for each of the five monkeys). Ordinates: Percentage reduction in the OFRs for each monkey, based on the mean changes in eye position (in degrees, over the time period 50–100 ms measured from onset of the stimulus ramps) averaged over all stimulus directions and speeds. Abscissas: Percentage of each designated subregion of the STS showing morphological damage in any layer (grey symbols: complete+partial lesions) or all layers (black symbols: complete lesions). Lines are best-fit linear regessions, (taking into account the variance of both X and Y) whose coefficients are shown at the bottom right in each panel (Coefficients of Determination in parentheses). Data in open symbols (monkey P) were excluded from the regressions.
Disparity Vergence Responses (DVRs)
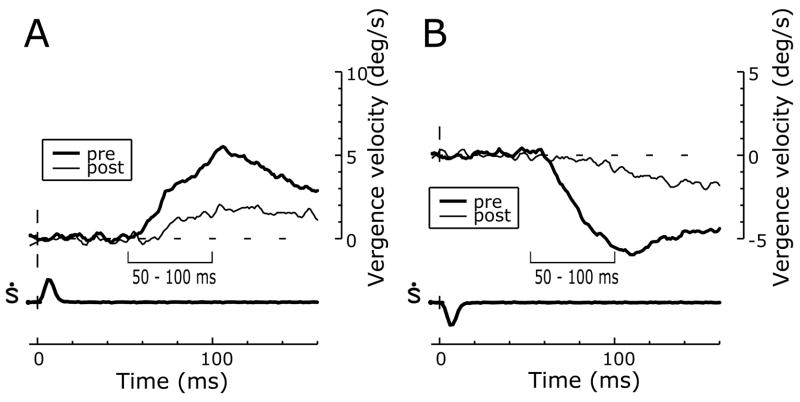
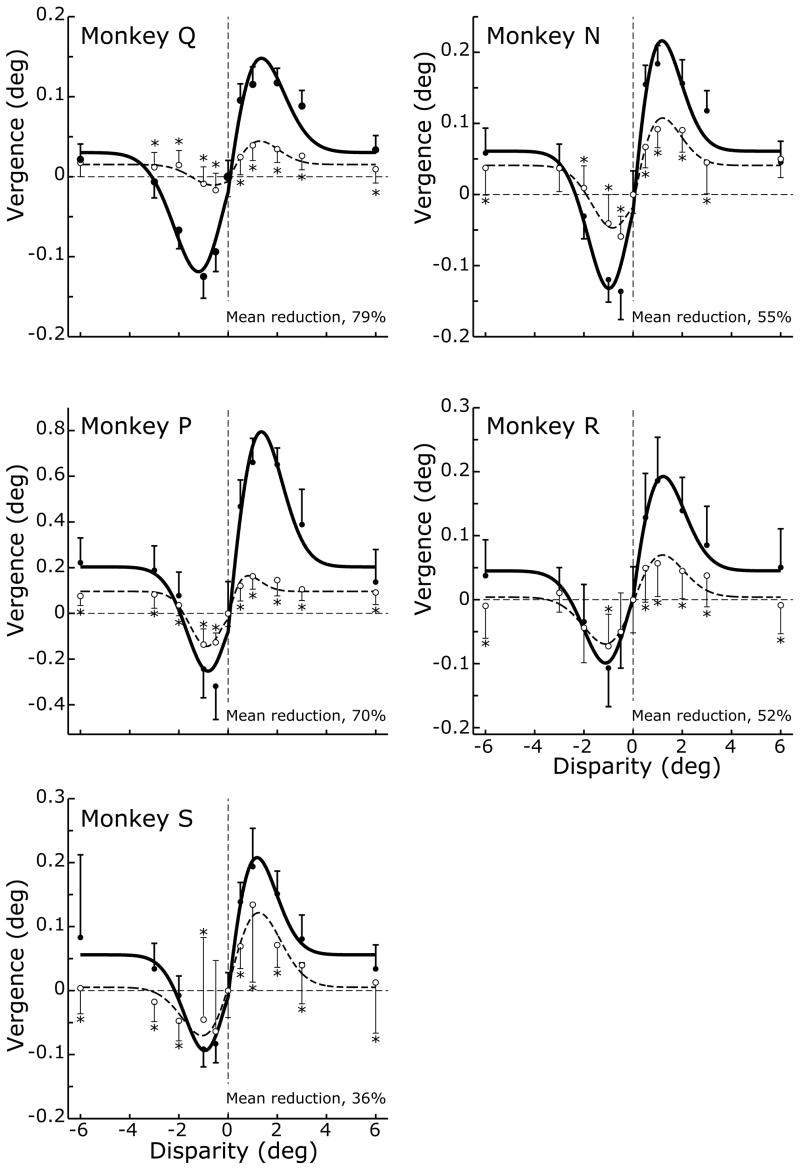
Prior to the ibotenic acid injections, horizontal disparity steps applied to large-field, correlated, random-dot patterns elicited initial DVRs that were, in all essentials, like those described by previous authors (Busettini et al., 1996; Masson et al., 1997; Busettini et al., 2001; Takemura et al., 2001) with onset latencies of 55–60ms and mean vergence velocity profiles that showed an initial transient followed by a smaller sustained response: see the thick traces in Fig. 6, which show the convergent responses to 1° crossed-disparity steps (A) and the divergent responses to 1° uncrossed-disparity steps (B) for monkey N. In addition, disparity tuning curves, describing the dependence of the change-in-vergence-position measures (for the time period 50–100 ms from stimulus onset) on the amplitude of the disparity steps, approximated the derivative of a Gaussian, showing a central servo range over which crossed disparities resulted in convergent responses and uncrossed disparities resulted in divergent responses (filled symbols in Fig. 7). This servo range was quite narrow and responses peaked with disparities of <2° before declining back to a non-zero asymptote as disparities exceeded ~4°, the so-called “default” response to uncorrelation (Busettini et al., 1996; Busettini et al., 2001). The disparity tuning curves were fitted with the following expression from Busettini et al (2001):
| (1) |
Figure 6.
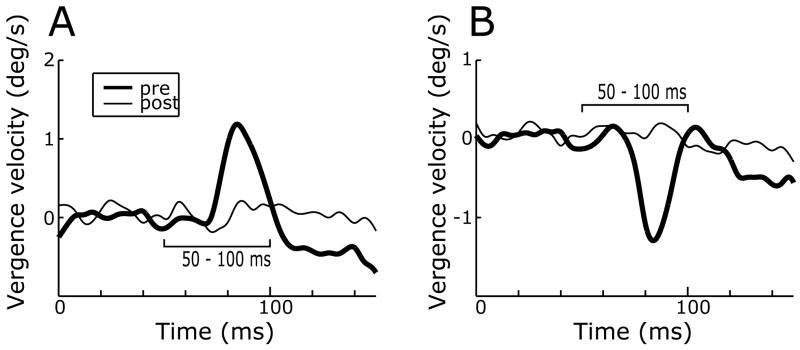
Deficits in DVRs after ibotenic acid injections: sample temporal profiles (monkey Q). Mean vergence velocity (°/s) over time (ms) in response to multiple presentations of 1° crossed (A) and uncrossed (B) disparity steps before (thick traces) and after (thin traces) the injections. Disparity steps were applied at zero on the time scale. Upward deflections denote convergent eye movements. The time bars, 50–100 ms, indicate the period over which the response measures were made.
Figure 7.
Deficits in DVRs after ibotenic acid injections: disparity tuning curves (data for each of the five monkeys). Ordinates: vergence responses, based on the mean changes in horizontal vergence position (in degrees, over the time period 50–100 ms measured from onset of the disparity step) before (closed symbols) and after (open symbols) the injections. Abscissas: disparity step (in degrees). Curves are the least-squares best fits obtained using Expression 1, and the data given at the bottom right in each panel are the mean percentage reductions in Ap-p (peak-to-peak amplitude) based on values in Table 3. Error bars, 1 SD. Asterisks indicate post-injection data for which p < 0.05.
The first term in Expression 1 accounts for the non-zero asymptote and is an exponential function with an asymptotic level, A, and a space constant, B. The value of B was fixed at 0.46, based on the space constants found by Busettini et al (2001) using the orthogonal responses to vertical disparity steps. The second term in Expression 1 is a Gabor function, in which σ is the Gaussian width, f and φ are the spatial frequency and phase of the cosine term, and G is a gain factor. Because the data were usually not symmetrical about zero, we incorporated a parameter, D, to allow the peak of the Gaussian to shift (cf., Busettini et al., 2001). It is clear from Fig. 7 that Expression 1 provided a good fit to our pre-injection data (closed symbols, continuous lines) and the best-fit parameters for these curves are listed in Table 3 (“pre”), together with an estimate of the goodness of fit (r2), the values of which ranged from 0.96 to 0.99.
Table 3.
Dependence of the mean DVRs on the magnitude of the disparity steps before and after the ibotenic acid injections: Best-fit parameters when the data were fitted with Expression 1.
| A | G | σ | D | f | φ | Ap-p | r2 | ||
|---|---|---|---|---|---|---|---|---|---|
| monkey Q | pre | 0.03 | 1.24 | 1.29 | −0.017 | 0.022 | 269.0 | 0.27 | 0.97 |
| post | 0.02 | 0.40 | 1.02 | 0.307 | 0.017 | 270.9 | 0.06 | 0.92 | |
| monkey N | pre | 0.06 | 2.53 | 1.09 | 0.012 | 0.017 | 269.5 | 0.35 | 0.96 |
| pos | 0.04 | 0.14 | 1.26 | 0.042 | 0.138 | 264.4 | 0.16 | 0.95 | |
| monkey P | pre | 0.20 | 6.97 | 1.12 | 0.307 | 0.017 | 271.2 | 1.05 | 0.97 |
| post | 0.10 | 0.63 | 0.82 | −0.310 | 0.077 | 258.8 | 0.31 | 0.96 | |
| monkey R | pre | 0.05 | 1.88 | 1.18 | 0.038 | 0.017 | 270.3 | 0.29 | 0.99 |
| pos | 0.00 | 0.68 | 1.17 | 0.001 | 0.023 | 269.5 | 0.14 | 0.92 | |
| monkey S | pre | 0.06 | 1.87 | 1.08 | 0.094 | 0.020 | 270.3 | 0.30 | 0.98 |
| post | 0.01 | 1.39 | 1.15 | 0.220 | 0.016 | 271.4 | 0.19 | 0.94 | |
An iterative procedure with a least-squares criterion was used to obtain the best fits with each of the parameters resolved to the number of decimal places shown. Ap-p is the peak-to-peak amplitude of Expression 1. r2 is the Coefficient of Determination. A, D, G, σ, φ, and Ap-p are in degrees, and f is in cycles/o. Disparity tuning curves based on these best-fit parameters are shown in Fig. 7. pre: pre-injection data. post: post-injection data.
After bilateral injections of ibotenic acid in the STS there was a significant attenuation of the DVRs to many crossed- and uncrossed-disparity steps (Student’s t-test, P<0.05): see the thin traces in Fig. 6, and the open symbols tagged with an asterisk in Fig. 7. The disparity tuning curves had the same general form after the injections as before and were again well fitted by Expression 1 (dashed lines in Fig. 7), r2 values ranging from 0.92 to 0.96; the best-fit parameters are listed in Table 3 (“post”). The impact of the injections is perhaps best appreciated from the changes in the peak-to-peak amplitudes (Ap-p) of the best-fit curves in Fig. 7, which are also listed in Table 3. The percentage reductions in Ap-p after the injections, which are given at the bottom right of each graph in Fig. 7, averaged 58.6% (range, 36–79%) and were often correlated with the extent of the morphological damage in the various subdivisions of the STS. This can be seen in Fig. 8, in which the percentage reductions in Ap-p are plotted against the percentage extents of the lesions in the five subregions within the STS and which is organized exactly like Fig. 5. Once again, monkey P (open symbols) was an outlier with a greater deficit than expected from the size of its lesions, and when its data were excluded, linear regressions of the reductions in Ap-p on the extent of the lesions in each of the various subdivisions of the STS always showed significant positive slopes, with r2 values ranging from 0.63 to 0.84 when based on the total extent of the lesions (grey symbols and lines) and from 0.69 to 0.94 when based only on the extent of the complete lesions (black symbols and lines). The correlation between the size of the lesion and the magnitude of the deficit was greatest for the complete lesions in MSTl, cf., the OFR. It is also very interesting that there was a highly significant correlation between the magnitudes of the OFR and DVR deficits documented in Figs. 5 and 8 (correlation coefficient, 0.998, when data of monkey P excluded).
Figure 8.
Deficits in DVRs after ibotenic acid lesions: dependence on the estimated extent of the lesions in MSTd, MSTl and MT (data for each of the five monkeys). Ordinates: Percentage reduction in the DVRs for each monkey, based on the changes in the peak-to-peak amplitudes (Ap-p) of the least-squares best fits obtained with Expression 1 shown in Fig. 7 (and whose parameters are listed in Table 3). Abscissas: Percentage of each designated subregion of the STS showing morphological damage in any layer (grey symbols: complete+partial lesions) or all layers (black symbols: complete lesions). Lines are best-fit linear regessions, (taking into account the variance of both X and Y) whose coefficients are shown at the bottom right in each panel (Coefficients of Determination in parentheses). Data in open symbols (monkey P) were excluded from the regressions.
Radial Flow Vergence Responses (RFVRs)
Radial-flow steps applied to large-field random-dot patterns generally elicited vergence eye movements at short latency that were, in all essentials, like the RFVRs described by previous authors (Busettini et al., 1997; Inoue et al., 1998a; Yang et al., 1999): transient convergent responses to expanding (centrifugal) steps and transient divergent responses to contracting (centripetal) steps with onset latencies of 60–70ms. Sample temporal response profiles (means) obtained from monkey P with 5% steps can be seen in Fig. 9 (thick traces), the data obtained with expansions being shown in A and with contractions in B. However, response amplitudes were small (the peak eye velocity was often less than 2°/s) and in two cases—monkey R with centrifugal steps and monkey S with centripetal steps—failed to reach our response criterion (twice the SD of the baseline noise). The ibotenic acid injections in the STS always resulted in significant attenuation of the RFVRs and this was so severe in the cases illustrated in Fig. 9 that it is difficult to distinguish the post-injection responses (thin traces) from the baseline noise. The pre- and post-lesion RFVRs were assessed quantitatively using the change in vergence position over the time period 50–100 ms from stimulus onset and these measures are listed in Table 4, together with the percentage reduction in these measures resulting from the lesions (two rightmost columns). The reductions in the RFVRs after the injections ranged from 51% to 125% (mean reduction, 84%) and all were statistically significant (Student’s t-test, p<0.05).3 The magnitude of these lesion effects was poorly correlated with the extent of the damage in the various subdivisions of the STS, whether considering all areas affected by the lesion (“total” lesion) or only the areas with complete lesions, and the r2 values never exceeded 0.18. Unfortunately, the marginal amplitude of the RFVRs even before the injections raises the possibility that noise obscured any relationship between the magnitude of the deficits and the size of the lesions in the various subregions. In addition, noise might have been a major factor in our finding that there was no significant correlation between the RFVR deficits and the deficits in the OFR and DVR documented in Figs. 5 and 8 (correlation coefficients, 0.114 and 0.115, respectively, when data of monkey P excluded).
Figure 9.
Deficits in RFVRs after ibotenic acid injections: sample temporal profiles (monkey P). Mean horizontal vergence velocity (°/s) over time (ms) in response to multiple presentations of 2% expansions (A) and contractions (B) before (thick traces) and after (thin traces) the injections. Radial flow steps were applied at zero on the time scale. Upward deflections denote convergent eye movements. The time bars, 50–100 ms, indicate the period over which the response measures were made.
Table 4.
The vergence responses to expanding and contracting radial optic flow before and after the ibotenic acid injections.
| Pre-lesion | Post-lesion | Percentage reduction | ||||
|---|---|---|---|---|---|---|
| Expand | Contract | Expand | Contract | Expand | Contract | |
| Monkey Q | 14.8 | −4.5 | 1.0 | −0.9 | 93** | 80** |
| Monkey N | 13.9 | −11.7 | 6.8 | −4.7 | 51* | 60** |
| Monkey P | 19.3 | −19.3 | 2.2 | 4.9 | 89** | 125** |
| Monkey R | - | −26.0 | - | 4.2 | - | 116** |
| Monkey S | 41.1 | - | 18.9 | - | 54** | - |
The pre- and post-lesion entries are the change-in-vergence position measures (over the time period 50–100 ms from stimulus onset) in degrees*10−3, a positive sign indicating convergence and a negative sign indicating divergence. Blank entries indicate that the response failed to exceed twice the SD of the baseline noise. Reductions exceeding 100% indicate reversal of the RFVR. Asterisk denotes p < 0.05; double asterisk denotes p ≤ 0.001.
Optokinetic and smooth pursuit eye movements
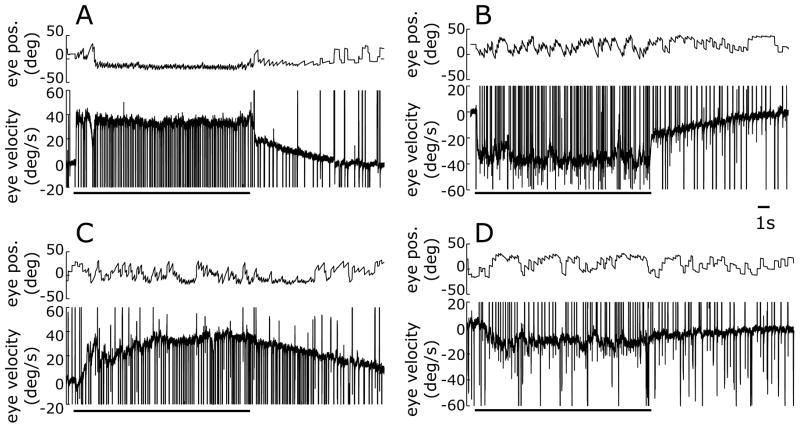
Optokinetic and smooth pursuit eye movements were recorded in two monkeys (P and S) before and after the ibotenic acid injections. Prior to injection, optokinetic nystagmus (OKN) was as described by previous authors (e.g., Cohen et al., 1977; Lisberger et al., 1981): Slow-phase eye velocity showed an initial rapid rise (OKNe) and, if eye speed fell short of stimulus speed at the end of this phase, then this was followed by a more gradual buildup in eye speed (OKNd); after the stimulus was blanked, slow-phase eye velocity showed an initial rapid drop followed by a more gradual decline as eye speed slowly fell back towards zero (optokinetic afternystagmus, OKAN). This pattern of responses is evident in the sample data shown in Fig. 10A, B that were obtained from monkey P prior to the ibotenic acid injections. There is substantial evidence (see Lisberger et al., 1981, for review) indicating that the optokinetic responses are generated by two neural mechanisms, one with brisk dynamics that is responsible for the rapid changes in slow-phase eye velocity (OKNe and the rapid drop after blanking), and the other with sluggish dynamics that is responsible for the more gradual changes (OKNd and OKAN). A previous study showed that both of these components were reduced to varying degrees by unilateral chemical lesions in MST (Dürsteler and Wurtz, 1988): the slow buildup was impaired only for motion towards the side of the lesion and the initial rapid rise was impaired for motion towards or away from the side of the lesion. Our observations were consistent with this, as indicated by the sample data shown in Fig. 10C, D that were obtained from monkey P after bilateral ibotenic acid injections: the brisk responses were severely attenuated to both rightward and leftward motion whereas the sluggish responses were only impaired with the leftward motion (Fig. 10D), perhaps reflecting our finding that the total extent of the lesion in the left MSTl was about twice that in the right MSTl in this animal (Table 2). However, a complication here is that monkey P had been used for prior recordings in the left NOT which might normally contribute to the slow buildup response to the leftward motion. The initial eye acceleration, based on the change in eye velocity over the first 100 ms of the response, which is a measure of the brisk component, was reduced in monkey P by 74% ± 34% (n=3) and 99% ± 8% (n=3) with rightward and leftward motion, respectively; these reductions were 18% ± 17% (n=3) and 56% ± 21% (n=3) in monkey S. The average velocity of the last five slow phases of the OKN (the final OKN), which is a measure of the combined effects of the brisk and sluggish components, was reduced after the injections in monkey P by 11% ± 17% (n=3) and 73% ± 12% (n=3) with the rightward and leftward motion, respectively, but showed little change in monkey S, the corresponding changes being -5% ± 10% (n=3) and 1% ± 6% (n=3), respectively. The effect of the injections on the average velocity of the first three slow phases of the OKAN (the initial OKAN), which is a measure of the sluggish component, were more variable, only the responses of monkey P to leftward motion stimuli showing a reduction—of 56% ± 14% (n=3). All remaining measures of initial OKAN were increased after the lesion: by 70% ± 34% (n=3) in monkey P with rightward motion, and by 87% ± 35% (n=3) and 71% ± 18% (n=3) in monkey S with rightward and leftward motion, respectively. Nonetheless, the rapid drop in eye velocity after blanking [(1-(initial OKAN/final OKN)) × 100%] was consistently smaller after the injections by 72% ± 12% (n=3) and 44% ± 41% (n=3) in monkey P with rightward and leftward motion, respectively, and by 70% ± 21% (n=3) and 44% ± 12% (n=3) in monkey S with rightward and leftward motion, respectively.
Figure 10.
Deficits in the optokinetic responses after ibotenic acid injections: sample temporal profiles (monkey P). A, B: data obtained before the injections. C, D: data obtained after the injections. In each panel, upper traces show horizontal eye position (in degrees) and lower traces show horizontal eye velocity (°/s) over time (ms) in response to rightward (A and C) and leftward (B and D) motion of a random-dot pattern at 40°/s. Upward deflections denote eye movements in the direction of stimulus motion. Thick lines indicate the duration of the illumination of the already-moving random dot pattern.
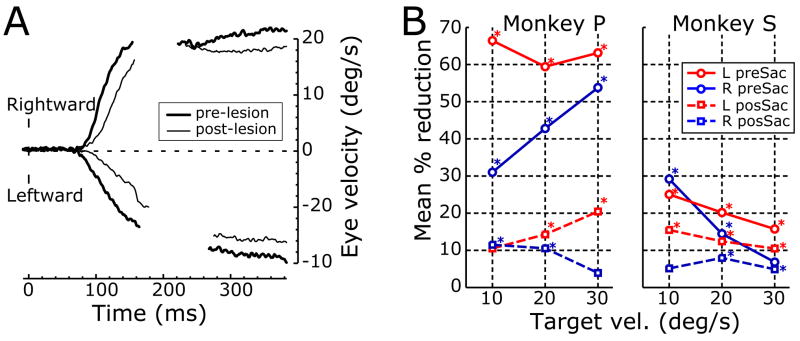
Previous studies that placed chemical lesions in MST and/or MT of one hemisphere reported two types of deficits in the pursuit eye movements elicited by discrete moving targets: impaired initiation of pursuit to target motion in any direction and impaired maintenance of pursuit when the target motion was towards the side of the lesion (Newsome et al., 1985; Dürsteler et al., 1987; Dürsteler and Wurtz, 1988; Yamasaki and Wurtz, 1991). We too observed significant deficits in the initiation and maintenance of pursuit after bilateral chemical lesions in STS, as can be seen in Fig. 11A, which shows sample pre- (thick traces) and post-injection (thin traces) response profiles obtained from monkey P with 20°/s leftward and rightward target motion. However, the deficits were often quite modest. This is evident from the percentage reduction in our quantitative measures of the pre-saccadic and post-saccadic tracking (based on the changes in eye position over the time intervals 100–150 ms and 300–350 ms, respectively, measured from the onset of target motion), which are plotted separately for monkeys P and S in Fig. 11B.
Figure 11.
Deficits in smooth pursuit eye movements after ibotenic acid injections: sample temporal profiles (monkey P) and response measures (monkeys, P and S). A: Mean eye velocity (°/s) over time (ms) in response to multiple presentations of 20°/s leftward and rightward ramps before (thick traces) and after (thin traces) the injections; upward deflections denote rightward eye movements and gaps are due to deletions of saccadic intrusions. B: Percentage reductions in the pursuit responses, based on the mean changes in eye position over i) the time period 100–150 ms (continuous lines: pre-saccadic, open-loop responses), and ii) the time period 300–350 ms (dashed lines: post-saccadic, closed-loop responses), both measured from the onset of the stimulus ramps; red, leftward ramps; blue, rightward ramps; abscissa: stimulus speed (°/s).
Other oculomotor parameters
After ibotenic acid injections in the STS of one hemisphere all monkeys showed a mild nystagmus when placed in the dark, with slow phases directed away from the side of injection. After injections in both hemispheres a nystagmus was still evident in the dark but now it was vertical in 4 monkeys (upward slow phases in monkeys Q, P, and R; downward slow phases in monkey N) and rightward in monkey S. However, in all cases, the nystagmus was largely suppressed when monkeys viewed the stationary random-dot test patterns. This was evident from the ocular drift speeds averaged over the critical 50-ms time period commencing with the onset of the OFR stimuli,4 the mean values of which are plotted for all subjects in Fig. 12A. A further important point is that only in one monkey (Q) was the mean ocular drift speed increased significantly after the injections and then by only a small amount, 0.6°/s. In two monkeys (P, R), the mean drift speed (and its variance) were actually slightly lower after the injections.
Figure 12.
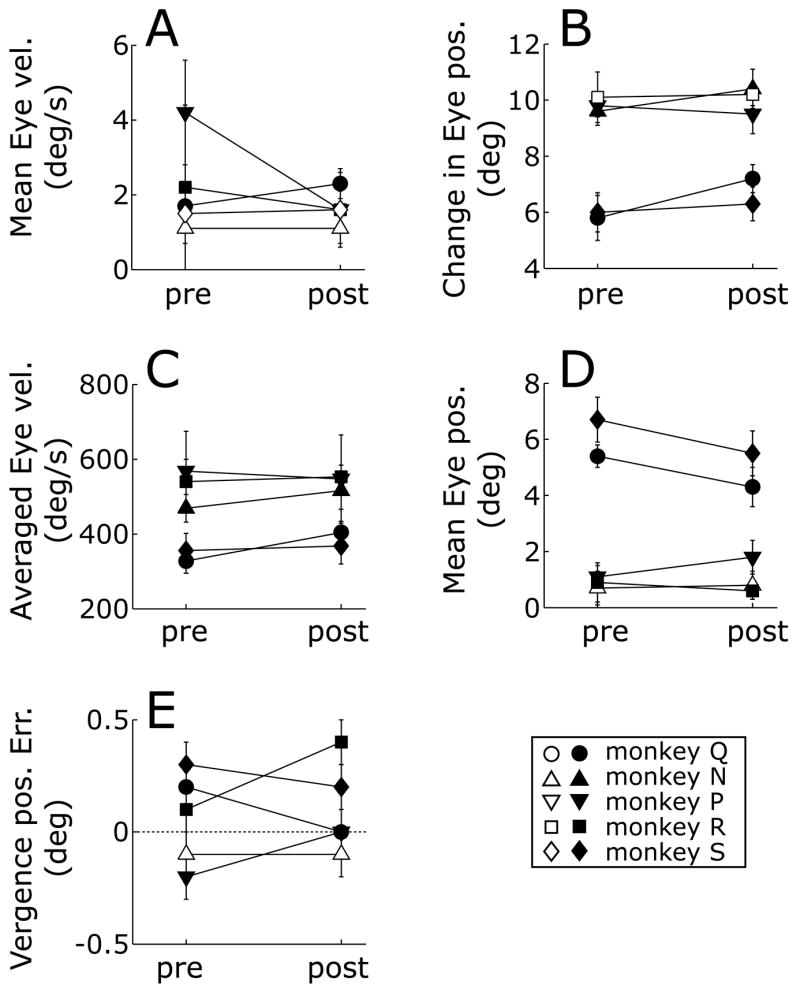
General oculomotor characteristics before (pre) and after (post) ibotenic acid injections. A: Mean ocular drift during the 50-ms period commencing with the onset of the visual stimulus; values are mean drift speeds in °/s (±1 SD) of the velocity vector. B: Mean amplitude of the leftward centering saccades in degrees (±1 SD). C: Mean peak velocity of the leftward centering saccades in °/s (±1 SD). D: Mean eccentricity of gaze in degrees (±1 SD) in the OFR paradigm during the 50-ms period commencing with the onset of the visual stimulus. E: Mean vergence error in degrees (±1 SD) in the RFVR paradigm during the 50-ms period commencing with the onset of the visual stimulus; a positive sign indicates over-convergence. Filled symbols denote that the differences between the means before and after the injections were statistically significant at the 0.05 level (Student’s t-test).
After the injections, the centering saccades showed very modest increases in amplitude and peak velocity in 4/5 monkeys, and these were generally statistically significant: see the average data for each of the 5 monkeys plotted in Fig. 12B,C. The “pre-lesion minus post-lesion” differences in the average amplitudes and peak velocities ranged from −1.4° to 0.3° and −76°/s to 21°/s, respectively. A related concern is how well the stimulus patterns were centered on the retina when the stimuli were applied and this was checked by measuring the angular distance between the fovea and the screen center (“retinal eccentricity”) during the 50-ms time period starting with the onset of the stimulus. In the OFR paradigm, estimated retinal eccentricities were actually slightly smaller for 3/5 monkeys after the injections (mean reduction, 0.9° in those monkeys) and the increases in the other two monkeys amounted to only 0.1° and 0.7°: see Fig. 12D. After the injections, 4/5 monkeys showed small (0.1–0.3°) but statistically significant changes in the mean vergence error during the 50-ms time period starting with the onset of the stimuli: see Fig. 12E. However, only in one case (monkey R) did this result in an increase in the mean vergence error.
Discussion
A family of short-latency reflexes mediated by the STS
Bilateral ibotenic acid injections in the STS of five monkeys caused morphological changes in the three subregions, MSTd, MSTl and MT, and resulted in significant deficits in the initial OFRs, DVRs and RFVRs of all animals. We also examined OKN and pursuit eye movements in two of the monkeys and, in line with previous studies that lesioned MT and MST (Newsome et al., 1985; Dürsteler et al., 1987; Dürsteler and Wurtz, 1988; Yamasaki and Wurtz, 1991), observed significant deficits in both types of tracking. The magnitudes of the deficits in the OFR and DVR in a given animal were directly related to the percentage extent of the morphological damage in the three subregions of the STS (Figs. 5 and 8).5 In fact, the correlation between the size of the lesion and the magnitude of the deficit in the OFR and DVR was highest for MSTl (r2, 0.91 and 0.94, respectively). However, the percentage extent of the lesions in the three subregions often co-varied, rendering the relative contribution of any given subregion to the various deficits uncertain. Notably, it is not possible to attribute any particular deficit solely to damage in one particular subregion. Further, we are unable to determine whether the cortical regions that sustained only partial damage contributed to the deficits. Yet another potential complication is that others who have used ibotenic acid injections in STS presented evidence suggesting that, “the area affected by the injection might be somewhat larger than indicated by the area of cell death” (Dürsteler et al., 1987). These reservations notwithstanding, insofar as MST sustained significant morphological damage in all injected hemispheres, our findings are consistent with our earlier suggestions that neurons in MST have a causal role in the genesis of all three kinds of reflex eye movements. However, we must also concede that the damage to MT might have contributed significantly to the observed deficits, not least because MT is a major source of inputs to MST (Maunsell and van Essen, 1983b; Ungerleider and Desimone, 1986). The deficits in the DVR and the OFR were highly correlated—consistent with a common distributed organization within the STS—though the former were generally less severe than the latter, perhaps suggesting that the DVR is more heavily reliant on other cortical areas. As already pointed out, the marginal amplitude of the RFVRs even prior to the injections means that noise might have obscured any clear relationship between the RFVR deficits and the size of the lesions or the deficits in the other two reflexes.
Secondary effects?
A major concern was the possibility that these deficits might be secondary to deficits in other aspects of the monkeys’ oculomotor performance that are known to be critical for the normal functioning of the three reflexes.
Post-saccadic enhancement
All test stimuli were applied in the immediate wake of a centering saccade to take advantage of post-saccadic enhancement (Kawano and Miles, 1986; Busettini et al., 1996; Busettini et al., 1997) and there is evidence that MST contributes to this enhancement, at least in the case of the OFR (Takemura and Kawano, 2006). If the STS lesions reduced this enhancement then this alone would be expected to reduce the gain of the responses. In addition, the post-saccadic enhancement of the OFR and DVR is known to be due in part to the visual reafference caused by the antecedent saccade sweeping the image of the background across the retina (Kawano and Miles, 1986; Busettini et al., 1996), hence any effects of the lesions on the speed and amplitude of the centering saccades might also have had an impact on the magnitude of this visual reafference and the associated enhancement. Previous studies reported that STS lesions had little impact on saccades to stationary targets (Newsome et al., 1985; Dürsteler and Wurtz, 1988). The centering saccades in the present study were on average slightly larger and faster in 4/5 monkeys after the lesions (Fig. 12B, C), and the study of Kawano & Miles (1986) on the OFR suggests that this would actually work to increase the component of the post-saccadic enhancement of the OFR due to visual reafference, albeit only slightly. No quantitative information is available regarding the dependence of the post-saccadic enhancement of the DVR and the RFVR—or the non-visual component of the post-saccadic enhancement of the OFR—on the parameters of the saccade. However, we think it unlikely that these factors made more than a minor contribution to the observed impairments of the three tracking mechanisms and they might even have had the converse effect.
Retinal eccentricity
The slight changes in the amplitudes of the centering saccades raise another potential concern: How well were the stimulus patterns centered on the retina when the stimuli were applied? Given the large size of the stimulus patterns (90°×90°), we think it very unlikely that the slight, inconsistent changes in the retinal eccentricity after the injections (ranging from a decrease of 1.2° to an increase of 0.7°) would have had much impact on the OFRs or DVRs. In the case of the OFR, it is known that the major visual drive comes from the central 40° of the visual field (Miles et al., 1986). A recent study on humans indicated that increasing the retinal eccentricity of the focus of expansion/contraction reduced the magnitude of the RFVR at most 5% per degree (Miles et al., 2004). If we assume that the situation is very similar in the monkey and further that the retinal eccentricity during the RFVR experiments was similar to that during the OFR experiments (Fig. 12D) then we can estimate the likely impact on the RFVRs. The estimated post-saccadic eccentricity of gaze was actually reduced after the lesions in monkeys Q, R and S by 1.1°, 0.3°, and 1.2°, respectively (which would work to increase the RFVR at most by 5.5%, 1.5%, 6%, respectively), and increased in monkeys N and P by 0.1° and 0.7°, respectively (which would work to decrease the RFVR at most by 0.5% and 3.5%, respectively).
Post-saccadic drift
The effects of the lesions on the ocular drift during the time the stimuli were being applied were invariably minor (Fig. 12A). Only monkey Q showed a significant increase and this was very small (mean increase, 0.6°/s). This might have made a very minor contribution to the unusually large deficits seen in this monkey.
Vergence errors and fixation disparity
The OFR is known to be sensitive to binocular disparity (Takemura et al., 2000; Masson et al., 2001; Yang and Miles, 2003) and so would be influenced by any effect of the lesions on the vergence error such as those documented in Fig. 12E. In addition, the gain of the OFR is known to be directly dependent on the vergence angle, independent of binocular disparity (Busettini et al., 1991; Inoue et al., 1998b). Based on the measured vergence angle for every trial, we estimated that the effect of the lesions on fixation disparity6 and vergence angle7 per se would have operated to decrease the OFR of monkeys Q and P on average by 18% and 15%, respectively, and increase the OFR of monkeys N, R and S on average by 1%, 16%, and 3%, respectively. A recent study on humans reported that the amplitude of the initial RFVR was linearly dependent on the vergence angle with an average sensitivity of 15%/m−1 for expanding flow and 22%/m−1 for contracting flow (Yang et al., 1999). If we assume that the monkey’s RFVR has a similar dependence then, based on the measured vergence angle for every trial, we estimated that the effect of the lesions on vergence angle would have operated to decrease the RFVR of monkeys Q and S on average by 2.0% and 1.3%, respectively, and increase the RFVR of monkeys P and R on average by 1.8% and 2.8%, respectively. It is not known if the RFVR is sensitive to binocular disparity, though it is known to be driven very successfully by monocular stimuli (Busettini et al., 1997). The slight effects of the STS lesions on the fixation disparity would be expected to result in an offset in the disparity steps used to elicit the DVR and hence might be expected to cause slight horizontal shifts without influencing the general form and amplitude of the disparity tuning curves like those in Fig. 7.
We conclude that most of the deficits in the OFR, DVR and RFVR after ibotenic acid injections in the STS were primary and not secondary to changes in other oculomotor parameters such as post-saccadic enhancement, ocular drift, vergence errors or fixation disparity.
Population coding and multiplexing
In a recent study, we showed that the summed activity of the disparity-sensitive cells in MST effectively encodes the initial DVR even though there is no hint of this vergence information at the level of the individual cells, indicating that the representation of the DVR in MST is an emergent property of the overall activity: population coding (Takemura et al., 2001; Takemura et al., 2002). Quantitative analyses indicated that the projection from MST to the next stage in the processing of the vergence drive signal must include contributions from the full spectrum of disparity-selective cells and raised the possibility that it involves merely a random selection of those cells. That the DVR (and OFR) deficits in the present study involved a simple attenuation directly related to the size of the lesion is consistent with reliance on a randomly distributed population of disparity-selective (and motion-selective) cells. Unfortunately, no population data are yet available for the neurons in MST that are known to discharge in relation to the OFR (Kawano et al., 1994; Takemura et al., 2000) and the RFVR (unpublished observations).
Acknowledgments
We thank Y. Inoue for her support during the early phase of this work. We are grateful to M. Okui, A. Muramatsu and T. Takasu for skillful technical assistance; and K. Nagatsuka for secretarial help. This research was supported by Special Coordination Funds for Promoting Science & Technology, by JST-ERATO Shimojo project and by JSPS.KAKENHI (16GS0312) and MEXT.KAKENHI (17022019).
Footnotes
All neuronal recordings in both hemispheres of monkey R and in the left hemisphere of monkey Q were done with flexible microelectrodes introduced through the guide tubes subsequently used for the ibotenic acid injections. An accurate reconstruction of these electrode tracks was not attempted.
Note that both parameters in these regressions—the magnitude of the OFR deficits (Y) and the extent of the lesions (X)—were dependent variables, and in order to take into account the variance of X as well as Y, the slopes given in Fig. 5 are the geometric means of the slope of Y on X and 1/(slope of X on Y).
Reductions exceeding 100% indicate response reversal but we assume that these reversals probably reflected noise, given that even the pre-injection responses were very small.
Vertical eye movements were recorded only in the OFR paradigm, so only in this paradigm were we able to assess the true ocular drift speed, and then only for the right eye.
Excluding the data of monkey P.
The dependence of the OFR on disparity was known for monkeys Q, P and N because they had been subjects in the study of Takemura et al. (2000). For monkeys R and S we used the data from the other three monkeys to estimate a mean curve describing dependence of the OFR on disparity.
We assumed a mean sensitivity of 10% per degree (Inoue et al., 1998b).
References
- Busettini C, Miles FA, Schwarz U. Ocular responses to translation and their dependence on viewing distance. II. Motion of the scene. J Neurophysiol. 1991;66:865–878. doi: 10.1152/jn.1991.66.3.865. [DOI] [PubMed] [Google Scholar]
- Busettini C, Miles FA, Krauzlis RJ. Short-latency disparity vergence responses and their dependence on a prior saccadic eye movement. J Neurophysiol. 1996;75:1392–1410. doi: 10.1152/jn.1996.75.4.1392. [DOI] [PubMed] [Google Scholar]
- Busettini C, Masson GS, Miles FA. Radial optic flow induces vergence eye movements with ultra-short latencies. Nature. 1997;390:512–515. doi: 10.1038/37359. [DOI] [PubMed] [Google Scholar]
- Busettini C, Fitzgibbon EJ, Miles FA. Short-latency disparity vergence in humans. J Neurophysiol. 2001;85:1129–1152. doi: 10.1152/jn.2001.85.3.1129. [DOI] [PubMed] [Google Scholar]
- Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977;270:321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Ungerleider LG. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J Comp Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J Neurophysiol. 1991;65:1329–1345. doi: 10.1152/jn.1991.65.6.1329. [DOI] [PubMed] [Google Scholar]
- Dürsteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60:940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- Dürsteler MR, Wurtz RH, Newsome WT. Directional pursuit deficits following lesions of the foveal representation within the superior temporal sulcus of the macaque monkey. J Neurophysiol. 1987;57:1262–1287. doi: 10.1152/jn.1987.57.5.1262. [DOI] [PubMed] [Google Scholar]
- Eifuku S, Wurtz RH. Response to motion in extrastriate area MSTl: disparity sensitivity. J Neurophysiol. 1999;82:2462–2475. doi: 10.1152/jn.1999.82.5.2462. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Silver staining of myelin by means of physical development. Neurol Res. 1979;1:203–209. doi: 10.1080/01616412.1979.11739553. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Takemura A, Suehiro K, Kodaka Y, Kawano K. Short-latency vergence eye movements elicited by looming step in monkeys. Neurosci Res. 1998a;32:185–188. doi: 10.1016/s0168-0102(98)00072-8. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Takemura A, Kawano K, Kitama T, Miles FA. Dependence of short-latency ocular following and associated activity in the medial superior temporal area (MST) on ocular vergence. Exp Brain Res. 1998b;121:135–144. doi: 10.1007/s002210050445. [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980;20:535–538. doi: 10.1016/0042-6989(80)90128-5. [DOI] [PubMed] [Google Scholar]
- Kawano K, Miles FA. Short-latency ocular following responses of monkey. II. Dependence on a prior saccadic eye movement. J Neurophysiol. 1986;56:1355–1380. doi: 10.1152/jn.1986.56.5.1355. [DOI] [PubMed] [Google Scholar]
- Kawano K, Shidara M, Watanabe Y, Yamane S. Neural activity in cortical area MST of alert monkey during ocular following responses. J Neurophysiol. 1994;71:2305–2324. doi: 10.1152/jn.1994.71.6.2305. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Miles FA, Optican LM, Eighmy BB. Optokinetic response in monkey: underlying mechanisms and their sensitivity to long-term adaptive changes in vestibuloocular reflex. J Neurophysiol. 1981;45:869–890. doi: 10.1152/jn.1981.45.5.869. [DOI] [PubMed] [Google Scholar]
- Masson GS, Busettini C, Miles FA. Vergence eye movements in response to binocular disparity without depth perception. Nature. 1997;389:283–286. doi: 10.1038/38496. [DOI] [PubMed] [Google Scholar]
- Masson GS, Busettini C, Yang DS, Miles FA. Short-latency ocular following in humans: sensitivity to binocular disparity. Vision Research. 2001;41:3371–3387. doi: 10.1016/s0042-6989(01)00029-3. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J Neurophysiol. 1983a;49:1148–1167. doi: 10.1152/jn.1983.49.5.1148. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, van Essen DC. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci. 1983b;3:2563–2586. doi: 10.1523/JNEUROSCI.03-12-02563.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles FA. The neural processing of 3-D visual information: evidence from eye movements. The European Journal of Neuroscience. 1998;10:811–822. doi: 10.1046/j.1460-9568.1998.00112.x. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K. Short-latency ocular following responses of monkey. III. Plasticity. J Neurophysiol. 1986;56:1381–1396. doi: 10.1152/jn.1986.56.5.1381. [DOI] [PubMed] [Google Scholar]
- Miles FA, Kawano K, Optican LM. Short-latency ocular following responses of monkey. I. Dependence on temporospatial properties of visual input. J Neurophysiol. 1986;56:1321–1354. doi: 10.1152/jn.1986.56.5.1321. [DOI] [PubMed] [Google Scholar]
- Miles FA, Busettini C, Masson GS, Yang D-S. Short-latency eye movements: evidence for rapid, parallel processing of optic flow. In: Vaina LM, Beardsley SA, Rushton S, editors. Optic Flow and Beyond. Dordrecht: Kluwer Academic Press; 2004. pp. 79–107. [Google Scholar]
- Newsome WT, Wurtz RH, Dürsteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JP, Wurtz RH. The role of disparity-sensitive cortical neurons in signalling the direction of self-motion. Nature. 1990;348:160–162. doi: 10.1038/348160a0. [DOI] [PubMed] [Google Scholar]
- Roy JP, Komatsu H, Wurtz RH. Disparity sensitivity of neurons in monkey extrastriate area MST. J Neurosci. 1992;12:2478–2492. doi: 10.1523/JNEUROSCI.12-07-02478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara M, Kawano K. Role of Purkinje cells in the ventral paraflocculus in short-latency ocular following responses. Exp Brain Res. 1993;93:185–195. doi: 10.1007/BF00228385. [DOI] [PubMed] [Google Scholar]
- Takemura A, Kawano K. The role of the cortical area MST in modulating the gain of the ocular following responses. Soc for Neurosci Abstract Viewer/Itinerary Planner Outline. 2004:186.9. [Google Scholar]
- Takemura A, Kawano K. Neuronal responses in MST reflect the post-saccadic enhancement of short-latency ocular following responses. Exp Brain Res. 2006;173:174–179. doi: 10.1007/s00221-006-0460-4. [DOI] [PubMed] [Google Scholar]
- Takemura A, Inoue Y, Kawano K. The effect of disparity on the very earliest ocular following responses and the initial neuronal activity in monkey cortical area MST. Neurosci Res. 2000;38:93–101. doi: 10.1016/s0168-0102(00)00149-8. [DOI] [PubMed] [Google Scholar]
- Takemura A, Kawano K, Quaia C, Miles FA. Population coding in cortical area MST. Annals of the New York Academy of Sciences. 2002;956:284–296. doi: 10.1111/j.1749-6632.2002.tb02827.x. [DOI] [PubMed] [Google Scholar]
- Takemura A, Inoue Y, Kawano K, Quaia C, Miles FA. Single-unit activity in cortical area MST associated with disparity-vergence eye movements: evidence for population coding. J Neurophysiol. 2001;85:2245–2266. doi: 10.1152/jn.2001.85.5.2245. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Saito H. Analysis of motion of the visual field by direction, expansion/contraction, and rotation cells clustered in the dorsal part of the medial superior temporal area of the macaque monkey. J Neurophysiol. 1989;62:626–641. doi: 10.1152/jn.1989.62.3.626. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie M, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Desimone R. Cortical connections of visual area MT in the macaque. J Comp Neurol. 1986;248:190–222. doi: 10.1002/cne.902480204. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JH. Two-dimensional maps of the cerebral cortex. J Comp Neurol. 1980;191:255–281. doi: 10.1002/cne.901910208. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Maunsell JH, Bixby JL. The middle temporal visual area in the macaque: myeloarchitecture, connections, functional properties and topographic organization. J Comp Neurol. 1981;199:293–326. doi: 10.1002/cne.901990302. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Visual receptive fields of striate cortex neurons in awake monkeys. J Neurophysiol. 1969;32:727–742. doi: 10.1152/jn.1969.32.5.727. [DOI] [PubMed] [Google Scholar]
- Yamasaki DS, Wurtz RH. Recovery of function after lesions in the superior temporal sulcus in the monkey. J Neurophysiol. 1991;66:651–673. doi: 10.1152/jn.1991.66.3.651. [DOI] [PubMed] [Google Scholar]
- Yang D, Fitzgibbon EJ, Miles FA. Short-latency vergence eye movements induced by radial optic flow in humans: dependence on ambient vergence level. J Neurophysiol. 1999;81:945–949. doi: 10.1152/jn.1999.81.2.945. [DOI] [PubMed] [Google Scholar]
- Yang DS, Miles FA. Short-latency ocular following in humans is dependent on absolute (rather than relative) binocular disparity. Vision Research. 2003;43:1387–1396. doi: 10.1016/s0042-6989(03)00146-9. [DOI] [PMC free article] [PubMed] [Google Scholar]