Abstract
Cynomolgus macaques (Macaca fascicularis) are quickly becoming a useful model for infectious disease and transplantation research. Even though cynomolgus macaques from different geographic regions are used for these studies, there has been limited characterization of full-length Major Histocompatibility Complex (MHC) Class I immunogenetics of distinct geographic populations. Here, we identified 48 MHC class I cDNA nucleotide sequences in eleven Indonesian cynomolgus macaques, including 41 novel Mafa-A and Mafa-B sequences. We found seven MHC class I sequences in Indonesian macaques that were identical to MHC class I sequences identified in Malaysian or Mauritian macaques. Sharing of nucleotide sequences between these geographically distinct populations is also consistent with the hypothesis that Indonesia was a source of the Mauritian macaque population. In addition, we found that the Indonesian cDNA sequence Mafa-B*7601 is identical throughout its peptide binding domain to Mamu-B*03, an allele that has been associated with control of SIV viremia in Indian rhesus macaques. Overall, a better understanding of the MHC class I alleles present in Indonesian cynomolgus macaques improves their value as a model for disease research and it better defines the biogeography of cynomolgus macaques throughout Southeast Asia.
Keywords: MHC, Immunogenetics, Indonesia, Macaca fascicularis
Introduction
Indian rhesus macaques (Macaca mulatta) have been widely used as an animal model for studying human diseases because of their extensive similarities to humans (Gibbs et al. 2007). However, the limited availability of rhesus macaques due to high demand and the 1978 ban on exportation from India (Southwick and Siddiqi 1994) has led to a reduced supply of these animals for important scientific research. Investigators have thus turned to closely related macaque species as viable scientific models, including the cynomolgus macaque (Macaca fascicularis). These animals are becoming increasingly valuable as models for the study of infectious diseases, such as AIDS, tuberculosis, and severe acute respiratory syndrome (SARS) (Capuano et al. 2003; Lawler et al. 2006; Wiseman et al. 2007). In addition, cynomolgus macaques are used as models of Alzheimer’s disease, Parkinson’s disease, and human reproduction (Conlee et al. 2004; Emborg 2007; Shively et al. 2007; Wang et al. 2007), and they are often used for transplantation and immunotherapy research (Liu et al. 2007; Wiseman and O’Connor 2007). With the recent increase in demand for cynomolgus macaques in biological research, there is a need to define Major Histocompatibility Complex (MHC) genetics in this species.
Cynomolgus macaques are found throughout Southeast Asia and extensive MHC polymorphism among these geographically-determined populations is evident (Kondo et al. 1993; Bonhomme et al. 2007). Unfortunately, researchers often overlook these differences between regionally defined populations of macaques. Previous studies of MHC class I alleles in both rhesus and cynomolgus macaques from different geographic origins have demonstrated that only a small subset of alleles are shared, while most alleles are unique to animals from a particular region (Krebs et al. 2005; Otting et al. 2007; Karl et al. 2008). In addition, it is reasonable to predict that rare MHC class I alleles present in macaques from different geographic regions are either derived from a common ancestor or arose during secondary contact, while the occurrence of numerous alleles unique to each population reflects the loss of ancestral alleles through genetic drift subsequent to geographic isolation and/or the effects of geographically variable selection pressures.
To our knowledge, there are only a handful of published studies on MHC class I allele sequences in cynomolgus macaques, and animals of Indonesian origin have not been well-examined to date (Uda et al. 2004; Krebs et al. 2005; Uda et al. 2005; Otting et al. 2007; Wiseman et al. 2007). The location of Indonesia, however, makes this population of cynomolgus macaques particularly interesting. The variable sea levels associated with glaciation and deglaciation during the Pleistocene Age in Southeast Asia led to a variety of land mass configurations that could have promoted macaque migration and thus greatly diversified the macaque populations in this region (Voris 2000). Studies of mitochondrial DNA sequences among cynomolgus macaques from five different geographic origins support the notion that this species originated in Southeast Asia (Smith et al. 2007). Therefore, a comparison of the MHC class I cDNA sequences between cynomolgus macaques of Indonesia and other areas in Southeast Asia may foster a better understanding of the biogeography of these animals.
Recent studies of MHC class I and II alleles in cynomolgus macaques of Mauritian origin have revealed uniquely simple MHC immunogenetics (Leuchte et al. 2004; Blancher et al. 2006; O’Connor et al. 2007; Wiseman et al. 2007). The limited MHC diversity observed in this geographically isolated population makes these animals extremely valuable for pathogenesis and transplantation research (Wiseman and O’Connor 2007; Wiseman et al. 2007). It has been suggested that cynomolgus macaques were introduced to Mauritius by European travelers approximately 400 years ago (Sussman and Tattersall 1986), and that both founder effects and subsequent inbreeding led to extensive allele sharing among Mauritian cynomolgus macaques in the polymorphic MHC loci (Krebs et al. 2005). Furthermore, analyses of mitochondrial and Y-chromosomal DNA suggest that the most probable origin of Mauritian macaques is Indonesia, or more specifically, Java or Sumatra (Tosi and Coke 2007). Therefore, we hypothesized that Mafa-A and Mafa-B cDNA sequences would be shared between Indonesian and Mauritian cynomolgus macaques.
To examine whether MHC class I genes found in Indonesian cynomolgus macaques are a central source for other geographic populations of cynomolgus macaques, we characterized 48 full length Mafa-A and Mafa-B cDNA sequences in 11 cynomolgus macaques of Indonesian origin. We found several nucleotide sequences that are shared between Indonesian and Mauritian or Malaysian cynomolgus macaques. In addition, we found that the predicted amino acid translation of one nucleotide sequence would generate an MHC class I molecule with a peptide binding domain identical to Mamu-B*03, an allele associated with low SIV plasma viremia in Indian rhesus macaques (Evans et al. 1999; Loffredo et al. 2007b). This study provides further evidence that Mauritian cynomolgus macaques originated, at least in part, in Indonesia, and it further expands the repertoire of full length MHC class I nucleotide sequences found in cynomolgus macaques, improving the value of this species as a model for biomedical research.
Materials and Methods
Animals
The Washington National Primate Research Center (Seattle, Washington) provided peripheral blood mononuclear cell (PBMC) samples for 12 Indonesian cynomolgus macaques (IN01-IN12). Two of these animals (IN02 and IN04) were obtained from Jakarta, while the remaining animals were bred in captivity at a natural habitat breeding colony on Tinjil Island located off the south coast of west Java in Indonesia. In addition, the Cerus Corporation (Concord, CA) supplied thirty PBMC samples from cynomolgus macaques of Indonesian origin (CE01-CE30). These animals were originally imported from purpose-bred colonies stocked primarily with animals from Sumatra by Primate Products (Miami, FL) and Worldwide Primates, Inc. (Miami, FL). Although knowledge regarding the relatedness of these animals is limited, microsatellite analysis suggests that they are not closely related (data not shown).
RNA/DNA isolation, cDNA synthesis, and cloning of MHC class I cDNAs
RNA and DNA were isolated from IN01-IN12 PBMC using the Qiagen AllPrep DNA/RNA Mini purification kit (Valencia, CA), while RNA and DNA from CE01-CE30 PBMC were obtained using the MagNA Pure LC Instrument (Roche Applied Science, Indianapolis, IN). Complementary DNA (cDNA) was synthesized using the Invitrogen Superscript™ III First-Strand Synthesis System for RT-PCR (Carlsbad, CA). To amplify the MHC class I cDNAs, PCR was executed with high-fidelity Phusion™ polymerase (New England Biolabs, Ipswich, MA) using primers specific for the MHC class I untranslated regions. To analyze both Mafa-A and Mafa-B sequences, different primer combinations were used. We used a common 5′ primer [5′MHC_UTR (5′-GGACTCAGAATCTCCCCAGACGCCGAG)] and locus specific 3′ primers [3′MHC_UTR_A (5′-CAGGAACAYAGACACATTCAGG)] or [3′MHC_UTR_B (5′-GTCTCTCCACCTCCTCAC)]. All cDNA amplifications were run under the same PCR program on an MJ Research Tetrad Thermocycler (Bio-Rad Laboratories, Hercules, CA): initial denaturation at 98°C for 30 seconds; 21–29 cycles of 98°C for 5 seconds, 63°C for 1 second, 72°C for 20 seconds; and a final extension period of 72°C for 5 minutes. Agarose gel electrophoresis was used to determine the lowest cycle number yielding a detectable PCR product. The 1.2 kilobase pair band was excised and purified using the QIAquick PCR Purification Kit (Qiagen, Valencia, CA).
Using either the Invitrogen Zero Blunt® Cloning Kit (Carlsbad, CA) or the Invitrogen Zero Blunt® TOPO® PCR Cloning Kit (Carlsbad, CA), the purified PCR products were ligated into pCR-Blunt vectors. Ligated samples were transformed into chemically competent Escherichia coli Top10 cells (Invitrogen, Carlsbad, CA). Twenty-four to ninety-six colonies per locus (48–192 per animal) were picked and incubated in a shaker for 17–24 hours at 37°C in 1.3 mL of Circle Grow (Qbiogene, Irvine, CA) containing kanamycin (50 μg/mL). DNA was isolated using the Eppendorf® Perfectprep® Plasmid 96 VAC Direct Binding Kit (Brinkmann, Westbury, NY). Sample concentrations were determined using the Nanodrop 1000 (Wilmington, DE). In order to ascertain which clones contained an MHC class I cDNA insert, EcoRI (New England Biolabs, Ipswich, MA) restriction digests were performed.
Sequencing of MHC Class I Alleles
Clones were sequenced in the forward and reverse directions using five primers: T7 (5′-TAATACGACTCACTATAGGG), M13rev (5′-CAGGAAACAGCTATGAC), and internal primers 5′Refstrand_v2 (5′-GCTACTACAACCAGAGCGAGG), 5′Transmembrane (5′-GGAACCTTCCAGAAGTGGG), and 3′Refstrand (5′-CAGAAGGCACCACCACAGC). The DYEnamic ET Terminator Cycle Sequencing Kit (Amersham, Piscataway, NJ) was used to perform sequencing reactions under the following conditions: 30 cycles of 95°C for 20 seconds, 50°C for 15 seconds, and 60°C for 1 minute. Sequencing products were purified using the Agencourt® CleanSEQ® dye-terminator removal kit (Beverly, MA). The purified products were sequenced on an ABI 3730xl (Applied Biosystems, Foster City, CA), and analyzed using CodonCode Aligner (Dedham, MA) and Lasergene (DNASTAR, Madison, WI) software. To ensure authenticity, sequences were given unique names if three or more identical full-length clones could be observed for that particular sequence in a single animal. The identified Mafa-A and Mafa-B nucleotide sequences were submitted to GenBank (accession numbers EU203680-EU203726, and EU046324) and to the IMGT/MHC Non-human Primate Immuno Polymorphism Database-MHC (IPD-MHC) and NHP Nomenclature Committee (Robinson et al. 2003).
MHC class I genotyping
Sequence-specific PCR assays for Mafa-B*4501 and Mafa-B*5101 were designed using MegAlign software (DNASTAR, Madison, WI) and Primer3 (v. 0.4.0) (Rozen and Skaletsky 2000). The primers were optimized for use with the Amplitaq Gold PCR Master Mix at 5 μM B*450101-F (5′-GGTCTCACACAGTCCAGACA), B*450101-R (5′-CTCTGTCCTTCTCCGCTG), B*510101-F (5′-CAAGGACGCCGCACAGT), B*510101-R (5′-GCACCGGCCCTCCAC)]. A set of positive control primers designed to amplify exon 2 and 3 sequences of nearly all MHC class I alleles were run in parallel with identical PCR conditions [5′RefStrand (5′-GCTACGTGGACGACACGC), 3′Short-RSCA (5′-TTCAGGGCGATGTAATCC)]. Samples were run at the following conditions: 96°C for 5 min; 30 cycles of 94 °C for 30s, 65°C for 45s, 72°C for 45s; and a final extension at 72° for 10 min. PCR products were resolved on a 1.8% agarose gel and visualized with SYBR Safe DNA gel stain (Invitrogen, Eugene, OR). PCR products were then excised and purified using Ultrafree-DA Centrifugal Devices (Millipore, Billerica, MA). Purified PCR products were sequenced bidirectionally with the same primers used for the PCR-SSP reaction using the DYEnamic ET Terminator Cycle Sequencing Kit (Amersham, Piscataway, NJ) under the same conditions described above for sequencing MHC class I alleles.
Sequence-specific PCR assays for Mamu-B*03 were performed as described previously (Kaizu et al. 2007).
Microsatellite analysis
Microsatellite analysis of DNA from all 42 Indonesian cynomolgus macaques was performed with a panel of markers spanning the MHC class I B region as previously described (Wiseman et al. 2007; Wojcechowskyj et al. 2007; Karl et al. 2008). Two new primer pairs were used to further define this region of the MHC, P03-129952F (5′-TGGGCAACAACAGTGAAACT), P03-129952R (5′-GTGGGTTAGGGGTCCTGTTT), L13-1494F (5′-AGCAGGTCCTCAGAATCCAA), and L13-1494R (5′-CAGCTACTCGGGACGCTAAG). P03-129952F was labeled with HEX (6-carboxy-2, 4, 4, 5, 7, 7-hexachlorofluorescein) and L13-1494F was labeled with FAM (6-carboxyfluorescein).
Phylogenetic Analysis
The phylogenetic analysis was based on 1031 nucleotide sites from coding regions, aligned by the Clustal X program (Thompson et al. 1997). A neighbor-joining tree (Saitou and Nei 1987) was constructed on the basis of the Maximum Composite Likelihood (MCL) distance using the MEGA 4.0 program (Tamura et al. 2007). The reliability of clustering patterns in the tree was assessed by bootstrapping (Felsenstein 1985); 1000 bootstrap pseudo-samples were used.
Results and Discussion
Summary of MHC class I cDNA nucleotide sequences identified
Indonesia consists of approximately 13,000 islands (Loudon et al. 2006), and it is likely that animals from distant sites have distinct MHC genetic repertoires. Therefore, we chose to identify MHC class I nucleotide sequences from cynomolgus macaques that came from independent sources and different regions within Indonesia, including Jakarta, Tinjil Island, and Sumatra. We sequenced 1,760 MHC class I cDNA clones from eleven cynomolgus macaques of Indonesian origin and identified 48 distinct MHC class I nucleotide sequences, including 19 Mafa-A and 29 Mafa-B cDNAs. Of these 48 sequences, seven were previously reported in GenBank, while the other 41 nucleotide sequences are newly described in this manuscript. The diversity of Mafa-A and Mafa-B cDNA sequences found in such a small cohort of animals is not surprising, since microsatellite analysis of the MHC region showed very little relatedness between these individuals (data not shown). Table 1 lists all 48 cDNA sequences, their accession number, reference animal, and identity to other previously described MHC class I genes. Allele names were designated by the Non-human Primate Immuno Polymorphism Database-MHC (IPD-MHC) nomenclature committee (Robinson et al. 2003).
Table 1.
MHC class I cDNA sequences identified in Indonesian-origin cynomolgus macaques (ICM).
| ICM allele name | ICM Accession # | Reference animal | Previously described identical nucleotide sequence1 |
|---|---|---|---|
| Mafa-A alleles | |||
| Mafa-A1*060102 | EU203689 | IN04 (02367) | |
| Mafa-A1*1002 | EU203687 | IN02 (02326) | Mane-A*19 (pig-tail, EF010520);Mafa-A1*1002(unknown cynomolgus, AM295831); Mamu-A1*1001(Chinese rhesus, AM295894) (1,2) |
| Mafa-A1*1004 | EU203706 | CE19 (13659) | |
| Mafa-A1*1005 | EU203707 | CE28 (13668) | |
| Mafa-A1*1006 | EU203699 | IN12 (04146) | |
| Mafa-A1*1803 | EU203709 | CE29 (13670) | |
| Mafa-A1*2202 | EU203696 | IN07 (04132) | Mafa-A1*2202 (unknown cynomolgus, AM295835) (2) |
| Mafa-A1*4103 | EU203713 | CE16 (13655) | |
| Mafa-A1*6003 | EU203698 | IN10 (04141) | |
| Mafa-A1*6202 | EU203711 | CE12 (13651) | |
| Mafa-A1*6603 | EU203712 | CE12 (13651) | |
| Mafa-A1*7001 | EU203708 | CE28 (13668) | Mafa-A1*7001 (unknown cynomolgus, AM295858) (2) |
| Mafa-A1*780102 | EU203685 | IN01 (01095), IN02 (02326) | |
| Mafa-A1*7802 | EU203705 | CE19 (13659) | |
| Mafa-A1*8701 | EU203710 | CE29 (13670) | |
| Mafa-A1*8801 | EU203686 | IN01 (01095) | |
| Mafa-A1*8901 | EU203697 | IN10 (04141) | |
| Mafa-A1*9001 | EU203700 | IN12 (04146) | |
| Mafa-A2*0532 | EU203688 | IN04 (02367) | |
| Mafa-B alleles | |||
| Mafa-B*0302 | EU203720 | CE29 (13670) | |
| Mafa-B*0702 | EU203704 | IN12 (04146) | |
| Mafa-B*1201 | EU203690 | IN04 (02367), IN10 (04141) | Mafa-B*12 (Malaysian and Mauritian cynomolgus, AB195442) (3,4) |
| Mafa-B*1202 | EU203682 | IN02 (02326) | |
| Mafa-B*3302 | EU046324 | CE16 (13655), CE19 (13659) | |
| Mafa-B*4403 | EU203715 | CE19 (13659) | |
| Mafa-B*4501 | EU203717 | CE28 (13668) | Mafa-B*450101 (Mauritian cynomolgus, AY958143) (5) |
| Mafa-B*5002 | EU203693 | IN04 (02367) | |
| Mafa-B*5101 | EU203718 | CE28 (13668) | Mafa-B*510101 (Mauritian cynomolgus, AY958150) (5) |
| Mafa-B*5601 | EU203714 | CE16 (13655) | |
| Mafa-B*5701 | EU203719 | CE28 (13668) | |
| Mafa-B*5801 | EU203722 | CE12 (13651) | |
| Mafa-B*5802 | EU203683 | IN02 (02326) | |
| Mafa-B*5803 | EU203721 | CE29 (13670) | |
| Mafa-B*5901 | EU203723 | CE12 (13651) | |
| Mafa-B*6602 | EU203716 | CE28 (13668) | |
| Mafa-B*6701 | EU203724 | CE12 (13651) | |
| Mafa-B*6801 | EU203725 | CE12 (13651) | |
| Mafa-B*6901 | EU203726 | CE16 (13655) | |
| Mafa-B*7001 | EU203680 | IN01 (01095) | |
| Mafa-B*7101 | EU203681 | IN02 (02326) | |
| Mafa-B*7201 | EU203684 | IN02 (02326) | |
| Mafa-B*7301 | EU203701 | IN07 (04132) | |
| Mafa-B*7401 | EU203702 | IN07 (04132) | |
| Mafa-B*7501 | EU203703 | IN12 (04146) | |
| Mafa-B*7601 | EU203691 | IN04 (02367) | |
| Mafa-B*7701 | EU203692 | IN04 (02367) | |
| Mafa-B*7801 | EU203694 | IN04 (02367), CE28 (13668) | |
| Mafa-B*7901 | EU203695 | IN04 (02367) | Mamu-B*05 (Indian rhesus, U41827) (6) |
The references for the previously described alleles are shown in parentheses.
MHC class I cDNA sequences identified in this study were compared with other known MHC nucleotide sequences previously identified in other macaque species. Previously named alleles whose sequences are identical to the Indonesian cynomolgus macaque MHC class I cDNA sequences are shown with their accession number. The reference animal used for allele discovery is shown. References listed are: (1) (Lafont et al. 2007), (2) (Otting et al. 2007), (3) (Uda et al. 2005), (4) (Wiseman et al. 2007), (5) (Krebs et al. 2005), (6) (Boyson et al. 1996).
During the process of Mafa-A and Mafa-B sequence discovery, nucleotide sequences were defined as authentic if we could identify three identical full-length clones from a single animal. After we completed the discovery process, we re-examined the nucleotide sequences from each animal to determine if any defined Indonesian MHC class I cDNAs could be detected in two or fewer clones in the other animals examined. This analysis led to the recognition of three additional Mafa-B alleles in IN10, thus identifying 4 Mafa-B alleles shared between IN04 and IN10. All animals and the nucleotide sequences we detected are summarized in Table 2.
Table 2.
Summary of MHC class I cDNA sequences identified in each Indonesian-origin cynomolgus macaque
| Official name | IN01 | IN02 | IN04 | IN07 | IN10 | IN12 | CE12 | CE16 | CE19 | CE28 | CE29 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mafa-A1*060102 | |||||||||||
| Mafa-A1*1002 | |||||||||||
| Mafa-A1*1004 | |||||||||||
| Mafa-A1*1005 | |||||||||||
| Mafa-A1*1006 | |||||||||||
| Mafa-A1*1803 | |||||||||||
| Mafa-A1*2202 | |||||||||||
| Mafa-A1*4103 | |||||||||||
| Mafa-A1*6003 | |||||||||||
| Mafa-A1*6202 | |||||||||||
| Mafa-A1*6603 | |||||||||||
| Mafa-A1*7001 | |||||||||||
| Mafa-A1*780102 | |||||||||||
| Mafa-A1*7802 | |||||||||||
| Mafa-A1*8701 | |||||||||||
| Mafa-A1*8801 | |||||||||||
| Mafa-A1*8901 | |||||||||||
| Mafa-A1*9001 | |||||||||||
| Mafa-A2*0532 | |||||||||||
| Mafa-B*0302 | |||||||||||
| Mafa-B*0702 | |||||||||||
| Mafa-B*1201 | |||||||||||
| Mafa-B*1202 | |||||||||||
| Mafa-B*3302 | |||||||||||
| Mafa-B*4403 | |||||||||||
| Mafa-B*4501 | |||||||||||
| Mafa-B*5002 | |||||||||||
| Mafa-B*5101 | |||||||||||
| Mafa-B*5601 | |||||||||||
| Mafa-B*5701 | |||||||||||
| Mafa-B*5801 | |||||||||||
| Mafa-B*5802 | |||||||||||
| Mafa-B*5803 | |||||||||||
| Mafa-B*5901 | |||||||||||
| Mafa-B*6602 | |||||||||||
| Mafa-B*6701 | |||||||||||
| Mafa-B*6801 | |||||||||||
| Mafa-B*6901 | |||||||||||
| Mafa-B*7001 | |||||||||||
| Mafa-B*7101 | |||||||||||
| Mafa-B*7201 | |||||||||||
| Mafa-B*7301 | |||||||||||
| Mafa-B*7401 | |||||||||||
| Mafa-B*7501 | |||||||||||
| Mafa-B*7601 | |||||||||||
| Mafa-B*7701 | |||||||||||
| Mafa-B*7801 | |||||||||||
| Mafa-B*7901 |
MHC class I cDNA sequences identified in each animal are shown. MHC class I cDNAs identified in three or more clones in a single animal are highlighted in black. MHC class I cDNAs identified in only one
Surprisingly, of the 19 identified Mafa-A sequences, 18 sequences were named based on similarity to alleles previously described for the Mafa-A1 locus. Only one Mafa-A sequence identified in this study was categorized as an allele from the Mafa-A2 locus. These results are in stark contrast to the observation by Otting and colleagues who found that cynomolgus macaques express a diverse set of Mafa-A alleles derived from at least six different loci (Otting et al. 2007). The reduced diversity observed in the current study may be a consequence of different PCR primers used for each study, or it may suggest that selection for high expression of Mafa-A1 alleles has occurred in Indonesian cynomolgus macaques.
Additionally, we found that the number of distinct Mafa-B cDNA nucleotide sequences expressed in each animal ranged from one to six. Unlike the recently characterized Mafa-A loci, distinct Mafa-B loci have not yet been defined in macaques (Otting et al. 2007). Although it has been suggested that the dominant Mafa-B cDNAs would be most highly expressed, knowledge of gene order and gene number of Mafa-B alleles in macaques is still lacking (Otting et al. 2005). Therefore, the range of distinct Mafa-B cDNAs per animal that we observed was likely a consequence of the limited number of clones examined per animal, differential gene expression, and variable gene number per haplotype in macaques.
Phylogenetic analysis of MHC class I cDNA sequences
In the phylogenetic tree of MHC class I nucleotide sequences, the Mafa-A and Mafa-B cDNAs formed distinct clusters, separated by a branch that received highly significant (99%) bootstrap support (Figure 1). Within each of these clusters, sequences identified in Indonesian cynomolgus macaques and Mauritian cynomolgus macaques were intermingled (Figure 1). Indeed, in nine cases, the closest relative of a Mauritian allele was an Indonesian cDNA sequence; and these clustering patterns were generally well supported by bootstrapping (99% in the case of six pairs and >70% for all nine pairs of alleles; Figure 1). The phylogenetic analysis was thus consistent with the hypothesis that Mauritian MHC class I cDNA sequences represent a sample from the Indonesian population of MHC class I cDNA sequences.
Figure 1. Neighbor-joining tree of MHC class I cDNA sequences from cynomolgus macaques from Mauritius (M) and Indonesia (I).
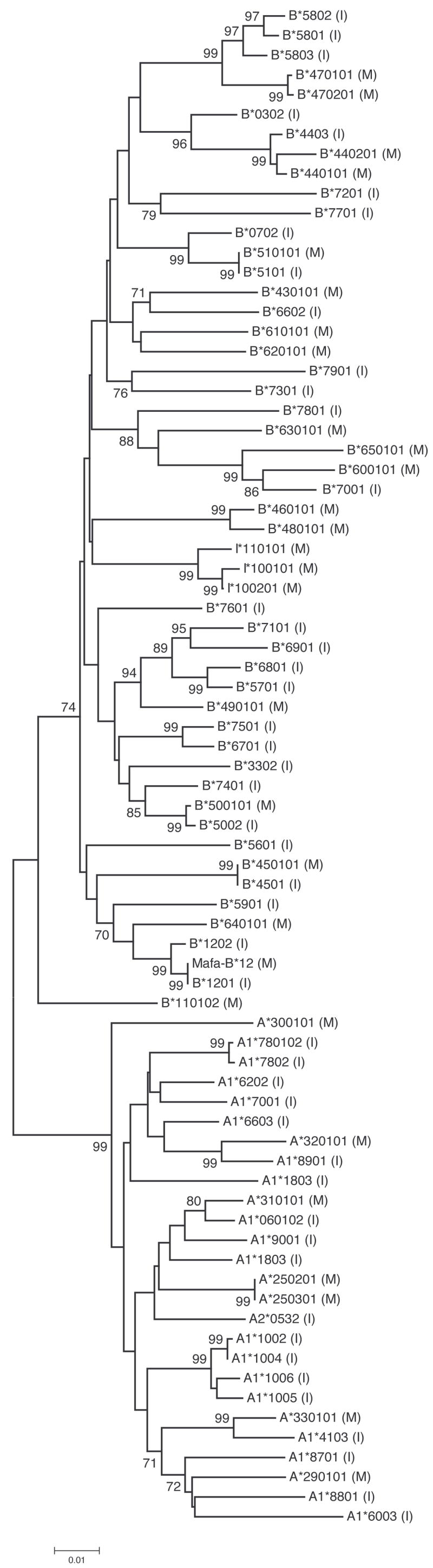
Numbers on the branches represent the percent of bootstrap samples supporting a given branch; only values ≥ 70% are shown.
Sharing of MHC cDNA sequences between different macaque species
Two of the seven previously described Indonesian cynomolgus macaque MHC class I nucleotide sequences were identical to alleles described in other macaque species. Mafa-B*7901 was previously identified as Mamu-B*05 in a rhesus macaque of Indian origin, while Mafa-A1*1002 was previously identified in a rhesus macaque of Chinese origin, a pig-tail macaque of unknown origin, and a cynomolgus macaque from the University of Utrecht of unknown geographic origin (Table 1) (Boyson et al. 1996; Lafont et al. 2007; Otting et al. 2007). In addition, we identified Mafa-B*5601, a sequence that deviates from Mane-B*03 by a single nucleotide synonymous substitution (Lafont et al. 2003). Our observation that approximately 6% (3/48) of the Mafa-A and Mafa-B nucleotide sequences identified in this population of Indonesian cynomolgus macaques are shared with other macaque species is similar to the frequency of MHC class I sequence sharing in other studies (Otting et al. 2007). This infrequent sharing of cDNA sequences between macaque species supports studies suggesting that certain rare MHC class I alleles were likely present in a common ancestor. In contrast, the observation that >90% of the observed MHC class I cDNAs are unique to cynomolgus macaques further suggests that MHC class I alleles are largely species specific, a circumstance that is likely attributable to pathogen-driven selection (Cooke and Hill 2001; Piertney and Oliver 2006). Ultimately, this observation further highlights the importance of a thorough characterization of the MHC of macaques from varying species and geographic origins.
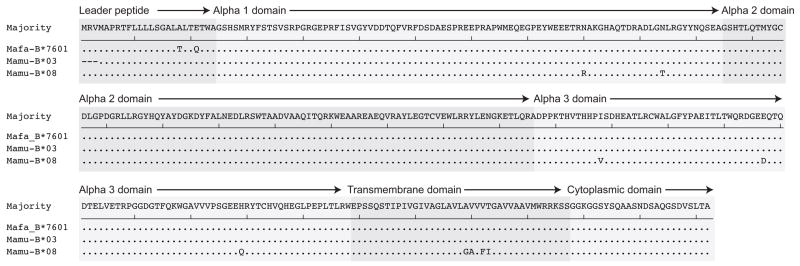
Interestingly, we found that the predicted gene product of Mafa-B*7601 was identical to the Indian rhesus macaque allele Mamu-B*03 throughout the entire polymorphic region of the molecule (Figure 2). The only amino acid differences between these two predicted molecules reside in the leader peptide. Studies have found that Mamu-B*03 is associated with improved control of SIV viremia in Indian rhesus macaques, but the rarity of this allele makes it difficult to study in large cohorts of animals (Evans et al. 1999; Evans et al. 2000; Loffredo et al. 2007b). Additionally, the predicted peptide binding motif of Mamu-B*03 is similar to HLA-B*27, an allele in humans that has been associated with slower disease progression in HIV-infected individuals (Dzuris et al. 2000; Carrington and O’Brien 2003). The predicted alpha 2 domain of Mamu-B*03 is also identical to another Indian rhesus macaque allele associated with improved control of SIV viremia, Mamu-B*08, and it has been hypothesized that these two alleles may restrict similar peptides (Loffredo et al. 2007a; Loffredo et al. 2007b). Therefore, it is reasonable to predict that Mafa-B*7601 may be associated with control of SIV viremia in cynomolgus macaques since it likely binds peptides identical to those bound by Mamu-B*03.
Figure 2. Mafa-B*7601 and Mamu-B*03 share identical peptide binding domains.
Predicted amino acid translations of Mafa-B*7601, Mamu-B*03, and Mamu-B*08 were aligned with MegAlign software (DNASTAR, Madison, WI). Peptide binding domains were predicted based on previous studies of MHC class I alleles in Indian rhesus macaques (Boyson et al. 1996).
Because Mafa-B*7601 may be an important molecule for understanding the role of CD8+ cell-mediated immune control of SIV viremia in macaques, we decided to genotype our expanded cohort of 42 Indonesian cynomolgus macaques for the presence of the Mafa-B*7601 cDNA nucleotide sequence. The methods for typing Mamu-B*03 by PCR-SSP have been established, and we expected this assay would detect Mafa-B*7601. We found that 2/42 (4.8%) animals in our cohort possessed this allele, and these results were confirmed by sequence verification of the positive PCR products (data not shown). The frequency of Mafa-B*7601 is comparable to the frequency of Mamu-B*08 (5.8%), but greater than Mamu-B*03 (0.7%) in Indian rhesus macaques (Kaizu et al. 2007). The higher frequency of Mafa-B*7601 in Indonesian cynomolgus macaques implies that this population of macaques may provide a valuable model for understanding the role of this protective MHC class I molecule in control of SIV viremia.
Sharing of MHC class I cDNA sequences between Indonesian and Malaysian cynomolgus macaques
In this study, we found MHC class I sequences that are identical to or closely related to Mafa-A and Mafa-B sequences previously found in cynomolgus macaques of Malaysian origin. The nucleotide sequence Mafa-B*1201 is identical to Mafa-B*12, an allele that was previously found in Malaysian macaques (Uda et al. 2005). In addition, the Malaysian MHC class I allele Mafa-A*08 differs from the Indonesian allele Mafa-A1*780102 by a single synonymous nucleotide substitution and it differs from the Indonesian allele Mafa-A1*7802 by a single amino acid substitution in the leader peptide (Uda et al. 2004). These three nearly-identical Mafa-A alleles, therefore, are likely capable of presenting identical peptide epitopes to T cells.
Previous studies have identified the co-existence of Y-chromosomal lineages in Malaysian and Sumatran macaques and provided support to the hypothesis that a land bridge existed between Malaysia and Indonesia during the Late Pleistocene age that led to gene flow between these two populations (Voris 2000; Tosi and Coke 2007). Here, our observation that three Indonesian MHC class I nucleotide sequences are identical or nearly-identical to MHC class I nucleotide sequences previously found in Malaysian macaques further supports the notion of gene flow between these two populations.
Sharing of MHC class I cDNA sequences between Indonesian and Mauritian cynomolgus macaques
Based upon phylogenetic studies of Mauritian and Indonesian cynomolgus macaques, we hypothesized that these two geographically distinct populations of macaques would also share MHC class I cDNA sequences (Tosi and Coke 2007). In our analysis, we found three Indonesian cynomolgus macaque MHC class I cDNA sequences that were previously identified in Mauritian cynomolgus macaques; an observation that is consistent with our hypothesis.
We found that Mafa-B*1201 in Indonesian macaques was identical to Mafa-B*12 in Mauritian macaques. From this study, we can now conclude that Mafa-B*1201 is actually shared between three geographically distinct populations of cynomolgus macaques: Indonesian, Mauritian, and Malaysian. This observation is surprising, as MHC class I alleles are rarely found in multiple geographically distinct populations (Krebs et al. 2005; Otting et al. 2007; Karl et al. 2008). In Mauritian macaques, Mafa-B*12, is found on the relatively rare H5 Mauritian MHC haplotype which comprises approximately 3% of the feral population (Wiseman et al. 2007). Interestingly, this allele was found in two animals, IN04 and IN10. In both of these animals, we also identified Mafa-B*5002, which is closely related to Mafa-B*500101 (Figure 1), an allele that is also found on the H5 Mauritian MHC haplotype (Wiseman et al. 2007). This data suggests that these two Mafa-B sequences may be carried on the same ancestral MHC haplotype in Indonesian macaques and it further supports the hypothesis that some Mauritian cynomolgus macaques originated in Indonesia.
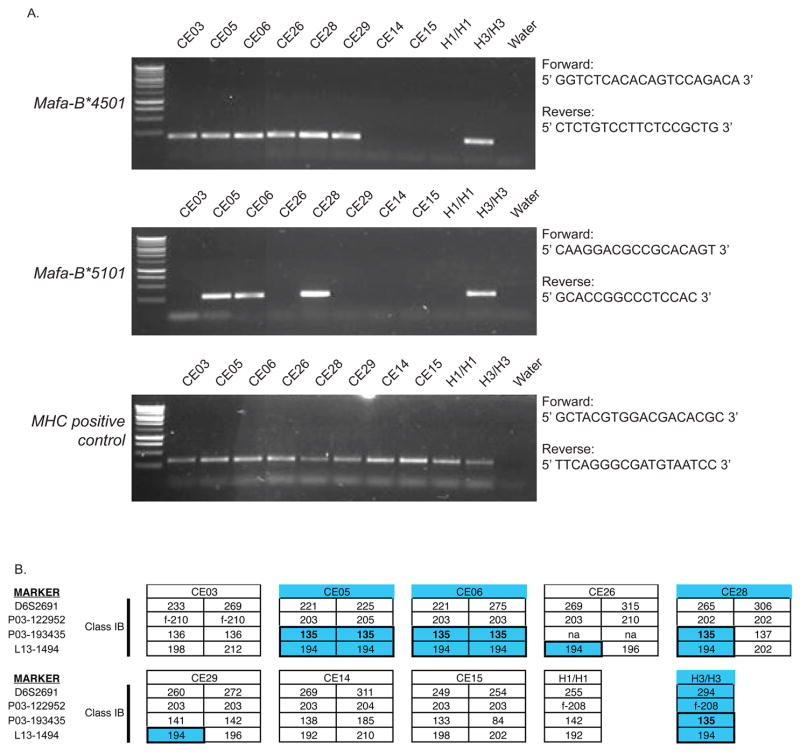
We also identified Mafa-B*4501 and Mafa-B*5101, a pair of MHC class I nucleotide sequences that are identical to Mafa-B*450101 and Mafa-B*510101 found in Mauritian cynomolgus macaques. These two Mafa-B sequences are found on the same common H3 MHC haplotype found in feral Mauritian cynomolgus macaques at a frequency of approximately 15% (Wiseman et al. 2007). To further investigate the frequency of these Mafa-B sequences, we developed PCR-SSP assays to screen the entire larger cohort of 42 Indonesian cynomolgus macaques, and we then sequenced the positive PCR products to verify the identity of each Mafa-B sequence. Surprisingly, we found that six animals express Mafa-B*4501, but only three of these same animals express Mafa-B*5101 (Figure 3A). Unlike feral Mauritian cynomolgus macaques, where the overall presence of recombinant H3 MHC class I haplotypes is fairly low, we found that half of the Indonesian macaques expressing Mafa-B*4501 did not concomitantly express Mafa-B*5101. In contrast, we found no animals expressing Mafa-B*5101 without Mafa-B*4501.
Figure 3. Mafa-B*4501 and Mafa-B*5101 are shared between Indonesian and Mauritian cynomolgus macaques.
(A) PCR-SSP analysis of Mafa-B*4501 and Mafa-B*5101 in 8 Indonesian cynomolgus macaques is shown. In parallel, primers based on sequences in exons 2 and 3 that are conserved in nearly all MHC class I alleles were used as a positive control to verify the cDNA integrity. Samples from H3/H3 and H1/H1 Mauritian cynomolgus macaques were used as positive and negative controls, respectively, for the amplification of Mafa-B*4501 and Mafa-B*5101. (B) Microsatellite analysis of the same 8 Indonesian cynomolgus macaques and two control Mauritian macaques is shown. Microsatellite allele sizes matching those found in an H3/H3 homozygous animal are highlighted in blue. The names of the animals who express both Mafa-B*4501 and Mafa-B*5101 are also highlighted in blue.
We further explored whether Indonesian cynomolgus macaques share MHC haplotypes with Mauritian cynomolgus macaques by typing them with an expanded panel of microsatellite markers that spans the Mafa-B region of the genome (Wojcechowskyj et al. 2007; Karl et al. 2008). Interestingly, we found identical allele sizes for microsatellite markers (P03-193435 and L13-1494) shared between H3-homozygous Mauritian macaques and the Indonesian animals who express both Mafa-B*4501 and Mafa-B*5101 (Figure 3B). The microsatellite allele size of 135bp for marker P03-193435 is only detected in our Indonesian cohort in animals that express Mafa-B*5101. This observation suggests this microsatellite locus is tightly linked to Mafa-B*5101 and may be useful, in addition to the PCR-SSP assay, when screening for animals who possess this Mafa-B cDNA sequence.
Overall, our observation that three Mafa-B sequences are shared between cynomolgus macaques of Indonesian and Mauritian origin provides further evidence that Indonesia is likely a source of the Mauritian cynomolgus macaques. Because we defined MHC class I sequences from a relatively small cohort of Indonesian macaques, it is not surprising that we found a limited number of cDNAs shared between these two geographic populations. It is certainly possible that further exploration in additional Indonesian cynomolgus macaques may reveal a greater extent of Mafa-A and Mafa-B sequence sharing. While there are multiple lines of evidence suggesting that Indonesia is the most likely origin of the Mauritian macaques, other possible geographic origins have been suggested. Specifically, certain MHC class II DRB alleles have been identified in both Filipino and Mauritian cynomolgus macaques. Likewise, it has been observed that variants of non-MHC proteins are shared between cynomolgus macaques of Mauritian and Southeast Asian origin (Kondo et al. 1993; Blancher et al. 2006). Therefore, it is certainly possible that MHC class I cDNA sequences found in Mauritian cynomolgus macaques may also be identified in cynomolgus macaques from other regions in Southeast Asia.
Implications for SIV vaccine research
Several studies in rhesus macaques have demonstrated that CD8+ T cells are important in the immune response to SIV (Schmitz et al. 1999; McMichael and Hanke 2002). To best understand the role that CD8+ T cells play in SIV pathogenesis, it is critical that researchers are aware of the MHC molecules expressed in their experimental animals so that CD8+ T cell responses can be monitored during disease progression. Unfortunately, few studies have examined CD8+ T cell biology in SIV-infected cynomolgus macaques because, until recently, very few MHC class I cDNA sequences were described in this species. In contrast to the well-defined SIV epitopes in Indian rhesus macaques, there have only been two studies published that define SIV-derived CTL peptide epitopes in cynomolgus macaques (Geretti et al. 1997; Negri et al. 2006). Although these studies identify specific SIV peptide sequences, they fail to identify the MHC class I molecule responsible for the epitope restriction. By characterizing full length MHC class I cDNA sequences in cynomolgus macaques of specific geographic origins, SIV researchers can now begin to develop reagents to better study CD8+ T cell responses in SIV-infected cynomolgus macaques.
The presence of certain Mamu-B and HLA-B alleles has also been associated with control of SIV/HIV viremia in Indian rhesus macaques and humans (Carrington and O’Brien 2003; Bontrop and Watkins 2005). Therefore, it seems likely that Mafa-B alleles also exist in cynomolgus macaques that may afford protection from SIV disease progression. Here, we provide data suggesting that the Indonesian MHC class I molecule Mafa-B*7601 may be capable of presenting a similar set of peptides as Mamu-B*03, a MHC class I molecule associated with control of SIV viremia in Indian rhesus macaques. Because Mafa-B*7601 is present at a frequency of about 5% in Indonesian cynomolgus macaques and these animals are known to be susceptible to infection with SHIV89.6p (Shiu-Lok Hu, personal communication), this animal model may be useful for better understanding the role of this MHC molecule in SIV disease.
In addition, an ongoing study examining the association of MHC genetics in Mauritian cynomolgus macaques with resistance to SHIV89.6p infection provides evidence suggesting that the Mafa-B alleles present on the H3 Mauritian MHC haplotype associate with low plasma viremia, while the Mafa-B alleles present on the H5 Mauritian MHC haplotype associate with high plasma viremia (Florese et. al., submitted). Because the Indonesian and Mauritian cynomolgus macaques share these potentially protective and susceptible Mafa-B alleles, the Indonesian population may also be a useful model for examining the influence of MHC genetics on SIV pathogenesis.
In conclusion, the MHC class I cDNA sequences described in this manuscript provide an important addition to the limited immunogenetic information available for Indonesian cynomolgus macaques. Moreover, the identification of Mafa-A and Mafa-B sequences that have been associated with protection and susceptibility to SIV increase the value of Indonesian cynomolgus macaques as an animal model for SIV pathogenesis and vaccine research. The results of this study underscore the importance of additional MHC class I sequence discovery in non-human primates. Identification of shared and unique MHC class I cDNA sequences may be critical for disease research and may help better understand the biogeography of non-human primates.
Acknowledgments
This work was supported by 1 R24 RR021745-01A1. PCR-SSP assay development was supported by NIAID Contract number HHSN266200400088C/N01-AI-40088. Support to C.J.P. was made possible through funding for undergraduate research from the UW-Madison Hilldale Fund, and support to A.L.H. was provided by grant number GM43940 from the NIH. This publication was also made possible in part by grant number P51 RR000167 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), to the Wisconsin National Primate Research Center, University of Wisconsin-Madison. This research was conducted in part at a facility constructed with support from Research Facilities Improvement Program grant numbers RR15459-01 and RR020141-01. This publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
We thank the Washington Primate Research Center for providing blood samples from animals IN01 through 1N12 and the Cerus Corporation for providing PBMC from animals CE01 through CE30. We thank the MHC typing core at the Wisconsin Primate Research Center for typing the Indonesian cohort for Mamu-B*03. We acknowledge Natasja de Groot and the IMGT for assigning uniform allele nomenclature. Finally, we appreciate many members of the O’Connor lab for helpful discussions.
References
- Blancher A, Tisseyre P, Dutaur M, Apoil PA, Maurer C, Quesniaux V, Raulf F, Bigaud M, Abbal M. Study of Cynomolgus monkey (Macaca fascicularis) MhcDRB (Mafa-DRB) polymorphism in two populations. Immunogenetics. 2006;58:269–282. doi: 10.1007/s00251-006-0102-9. [DOI] [PubMed] [Google Scholar]
- Bonhomme M, Blancher A, Jalil MF, Crouau-Roy B. Factors shaping genetic variation in the MHC of natural non-human primate populations. Tissue Antigens. 2007;70:398–411. doi: 10.1111/j.1399-0039.2007.00925.x. [DOI] [PubMed] [Google Scholar]
- Bontrop RE, Watkins DI. MHC polymorphism: AIDS susceptibility in non-human primates. Trends Immunol. 2005;26:227–233. doi: 10.1016/j.it.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Boyson JE, Shufflebotham C, Cadavid LF, Urvater JA, Knapp LA, Hughes AL, Watkins DI. The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J Immunol. 1996;156:4656–4665. [PubMed] [Google Scholar]
- Capuano SVr, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–551. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- Conlee KM, Hoffeld EH, Stephens ML. A Demographic Analysis of Primate Research in the United States. ATLA Supplement. 2004;1:315–322. doi: 10.1177/026119290403201s52. [DOI] [PubMed] [Google Scholar]
- Cooke GS, Hill AV. Genetics of susceptibility to human infectious disease. Nat Rev Genet. 2001;2:967–977. doi: 10.1038/35103577. [DOI] [PubMed] [Google Scholar]
- Dzuris JL, Sidney J, Appella E, Chesnut RW, Watkins DI, Sette A. Conserved MHC class I peptide binding motif between humans and rhesus macaques. J Immunol. 2000;164:283–291. doi: 10.4049/jimmunol.164.1.283. [DOI] [PubMed] [Google Scholar]
- Emborg ME. Nonhuman primate models of Parkinson’s disease. ILAR J. 2007;48:339–355. doi: 10.1093/ilar.48.4.339. [DOI] [PubMed] [Google Scholar]
- Evans DT, Jing P, Allen TM, O’Connor DH, Horton H, Venham JE, Piekarczyk M, Dzuris J, Dykhuzen M, Mitchen J, Rudersdorf RA, Pauza CD, Sette A, Bontrop RE, DeMars R, Watkins DI. Definition of five new simian immunodeficiency virus cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I molecules: evidence for an influence on disease progression. J Virol. 2000;74:7400–7410. doi: 10.1128/jvi.74.16.7400-7410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DT, O’Connor DH, Jing P, Dzuris JL, Sidney J, da Silva J, Allen TM, Horton H, Venham JE, Rudersdorf RA, Vogel T, Pauza CD, Bontrop RE, DeMars R, Sette A, Hughes AL, Watkins DI. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5:1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Geretti AM, Hulskotte EG, Dings ME, van Baalen CA, van Amerongen G, Osterhaus AD. CD8+ cytotoxic T lymphocytes of a cynomolgus macaque infected with simian immunodeficiency virus (SIV) mac32H-J5 recognize a nine amino acid epitope in SIV Gag p26. J Gen Virol. 1997;78:821–824. doi: 10.1099/0022-1317-78-4-821. [DOI] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’brien WE, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Kaizu M, Borchardt GJ, Glidden CE, Fisk DL, Loffredo JT, Watkins DI, Rehrauer WM. Molecular typing of major histocompatibility complex class I alleles in the Indian rhesus macaque which restrict SIV CD8(+) T cell epitopes. Immunogenetics. 2007 doi: 10.1007/s00251-007-0233-7. [DOI] [PubMed] [Google Scholar]
- Karl JA, Wiseman RW, Campbell KJ, Blasky AJ, Hughes AL, Ferguson B, Read DS, O’Connor DH. Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics. 2008;60:37–46. doi: 10.1007/s00251-007-0267-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Kawamoto Y, Nozawa K, Matsybayashi K, Watanabe T, Griffiths O, Stanley M. Population Genetics of Crab-Eating Macaques (Macaca fascicularis) on the Island of Mauritius. American Journal of Primatology. 1993;29:167–182. doi: 10.1002/ajp.1350290303. [DOI] [PubMed] [Google Scholar]
- Krebs KC, Jin Z, Rudersdorf R, Hughes AL, O’Connor DH. Unusually high frequency MHC class I alleles in Mauritian origin cynomolgus macaques. J Immunol. 2005;175:5230–5239. doi: 10.4049/jimmunol.175.8.5230. [DOI] [PubMed] [Google Scholar]
- Lafont BA, Buckler-White A, Plishka R, Buckler C, Martin MA. Characterization of pig-tailed macaque classical MHC class I genes: implications for MHC evolution and antigen presentation in macaques. J Immunol. 2003;171:875–885. doi: 10.4049/jimmunol.171.2.875. [DOI] [PubMed] [Google Scholar]
- Lafont BA, McGraw CM, Stukes SA, Buckler-White A, Plishka RJ, Byrum RA, Hirsch VM, Martin MA. The locus encoding an oligomorphic family of MHC-A alleles (Mane-A*06/Mamu-A*05) is present at high frequency in several macaque species. Immunogenetics. 2007;59:211–223. doi: 10.1007/s00251-007-0190-1. [DOI] [PubMed] [Google Scholar]
- Lawler JV, Endy TP, Hensley LE, Garrison A, Fritz EA, Lesar M, Baric RS, Kulesh DA, Norwood DA, Wasieloski LP, Ulrich MP, Slezak TR, Vitalis E, Huggins JW, Jahrling PB, Paragas J. Cynomolgus macaque as an animal model for severe acute respiratory syndrome. PLoS Med. 2006;3:677–686. doi: 10.1371/journal.pmed.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchte N, Berry N, Kohler B, Almond N, LeGrand R, Thorstensson R, Titti F, Sauermann U. MhcDRB-sequences from cynomolgus macaques (Macaca fascicularis) of different origin. Tissue Antigens. 2004;63:529–537. doi: 10.1111/j.0001-2815.2004.0222.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Noorchashm H, Sutter JA, Naji M, Prak EL, Boyer J, Green T, Rickels MR, Tomaszewski JE, Koeberlein B, Wang Z, Paessler ME, Velidedeoglu E, Rostami SY, Yu M, Barker CF, Naji A. B lymphocyte-directed immunotherapy promotes long-term islet allograft survival in nonhuman primates. Nat Med. 2007;13:1295–1298. doi: 10.1038/nm1673. [DOI] [PubMed] [Google Scholar]
- Loffredo JT, Friedrich TC, Leon EJ, Stephany JJ, Rodrigues DS, Spencer SP, Bean AT, Beal DR, Burwitz BJ, Rudersdorf RA, Wallace LT, Piaskowski SM, May GE, Sidney J, Gostick E, Wilson NA, Price DA, Kallas EG, Piontkivska H, Hughes AL, Sette A, Watkins DI. CD8 T Cells from SIV Elite Controller Macaques Recognize Mamu-B*08-Bound Epitopes and Select for Widespread Viral Variation. PLoS ONE. 2007a;2:e1152. doi: 10.1371/journal.pone.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, Watkins DI. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol. 2007b;81:8827–8832. doi: 10.1128/JVI.00895-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon JE, Howells ME, Guentes A. The Importance of Integrative Anthropology: A Preliminary Investigation Employing Primatological and Cultural Anthropological Data Collection Methods in Assessing Human-Monkey Co-existence in Bali, Indonesia. Ecological and Environmental Anthropology. 2006;2:2–13. [Google Scholar]
- McMichael A, Hanke T. The quest for an AIDS vaccine: is the CD8+ T-cell approach feasible? Nat Rev Immunol. 2002;2:283–291. doi: 10.1038/nri779. [DOI] [PubMed] [Google Scholar]
- Negri DR, Borghi M, Baroncelli S, Macchia I, Buffa V, Sernicola L, Leone P, Titti F, Cara A. Identification of a cytotoxic T-lymphocyte (CTL) epitope recognized by Gag-specific CTLs in cynomolgus monkeys infected with simian/human immunodeficiency virus. J Gen Virol. 2006;87:3385–3392. doi: 10.1099/vir.0.81934-0. [DOI] [PubMed] [Google Scholar]
- O’Connor SL, Blasky AJ, Pendley CJ, Becker EA, Wiseman RW, Karl JA, Hughes AL, O’Connor DH. Comprehensive characterization of MHC class II haplotypes in Mauritian cynomolgus macaques. Immunogenetics. 2007;59:449–462. doi: 10.1007/s00251-007-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N, de Vos-Rouweler AJ, Heijmans CM, de Groot NG, Doxiadis GG, Bontrop RE. MHC class I A region diversity and polymorphism in macaque species. Immunogenetics. 2007;59:367–375. doi: 10.1007/s00251-007-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otting N, Heijmans CM, Noort RC, de Groot NG, Doxiadis GG, van Rood JJ, Watkins DI, Bontrop RE. Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci U S A. 2005;102:1626–1631. doi: 10.1073/pnas.0409084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piertney SB, Oliver MK. The evolutionary ecology of the major histocompatibility complex. Heredity. 2006;96:7–21. doi: 10.1038/sj.hdy.6800724. [DOI] [PubMed] [Google Scholar]
- Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31:311–314. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, Sitruk-Ware RL, Tsong YY, Cline JM. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32:981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Smith DG, McDonough JW, George DA. Mitochondrial DNA variation within and among regional populations of longtail macaques (Macaca fascicularis) in relation to other species of the fascicularis group of macaques. Am J Primatol. 2007;69:182–198. doi: 10.1002/ajp.20337. [DOI] [PubMed] [Google Scholar]
- Southwick C, Siddiqi F. Population Status of Nonhuman Primates in Asia, with Emphasis on Rhesus Macaques in India. American Journal of Primatology. 1994;34:51–59. doi: 10.1002/ajp.1350340110. [DOI] [PubMed] [Google Scholar]
- Sussman RW, Tattersall I. Distribution, Abundance, and Putative Ecological Strategy of Macaca fascicularis on the Island of Mauritius, Southwestern Indian Ocean. Folia Primatology. 1986;46:28–43. [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi AJ, Coke CS. Comparative phylogenetics offer new insights into the biogeographic history of Macaca fascicularis and the origin of the Mauritian macaques. Mol Phylogenet Evol. 2007;42:498–504. doi: 10.1016/j.ympev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Uda A, Tanabayashi K, Fujita O, Hotta A, Terao K, Yamada A. Identification of the MHC class I B locus in cynomolgus monkeys. Immunogenetics. 2005;57:189–197. doi: 10.1007/s00251-005-0782-6. [DOI] [PubMed] [Google Scholar]
- Uda A, Tanabayashi K, Yamada YK, Akari H, Lee YJ, Mukai R, Terao K, Yamada A. Detection of 14 alleles derived from the MHC class I A locus in cynomolgus monkeys. Immunogenetics. 2004;56:155–163. doi: 10.1007/s00251-004-0683-0. [DOI] [PubMed] [Google Scholar]
- Voris H. Maps of Pleistocene sea levels in Southeast Asia: shorelines, river systems and time durations. Journal of Biogeography. 2000;27:1153–1167. [Google Scholar]
- Wang CY, Finstad CL, Walfield AM, Sia C, Sokoll KK, Chang TY, Fang XD, Hung CH, Hutter-Paier B, Windisch M. Site-specific UBITh amyloid-beta vaccine for immunotherapy of Alzheimer’s disease. Vaccine. 2007;25:3041–3052. doi: 10.1016/j.vaccine.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Wiseman RW, O’Connor DH. Major histocompatibility complex-defined macaques in transplantation research. Transplantation Reviews. 2007;21:17–25. [Google Scholar]
- Wiseman RW, Wojcechowskyj JA, Greene JM, Blasky AJ, Gopon T, Soma T, Friedrich TC, O’Connor SL, O’Connor DH. Simian immunodeficiency virus SIVmac239 infection of major histocompatibility complex-identical cynomolgus macaques from Mauritius. J Virol. 2007;81:349–361. doi: 10.1128/JVI.01841-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcechowskyj JA, Yant LJ, Wiseman RW, O’Connor SL, O’Connor DH. Control of Simian Immunodeficiency Virus SIVmac239 Is Not Predicted by Inheritance of Mamu-B*17-Containing Haplotypes. J Virol. 2007;81:406–410. doi: 10.1128/JVI.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]