Abstract
We present the case of 53 year old Caucasian male smoker with remote history of left lower extremity deep venous thrombosis (DVT) and a strong family history of thrombosis, who presented to the Center for Wound Healing at MedStar Georgetown University Hospital with spontaneous left leg ulceration. Prothrombotic evaluation revealed homozygosity for the Factor V Leiden (FVL) mutation.
Therapeutic anticoagulation was commenced with warfarin (Coumadin) and the patient underwent successful debridement and apligraf followed by split-thickness skin graft (STSG) of two wounds. He had an uneventful post-operative course and on the 27th postoperative day the grafts were 95% intact. However, by post-operative day 41 there was 10% graft loss, and over the subsequent 2 weeks both grafts necrosed. On further questioning, it transpired that the patient had discontinued his warfarin on post-operative day 37 because he thought it was no longer necessary.
The patient is enrolled in the WE-HEAL study, and at the time of the original graft, residual skin fragments from the STSG were transplanted onto a nude mouse for development of an animal model of wound healing. The mouse graft was successful and was harvested at post-operative day 87 for pathological examination.
We review the mechanisms by which prothrombotic states, particularly FVL mutation can contribute to skin graft failure and delayed wound healing. This case highlights the importance of considering prothrombotic conditions in patients with spontaneous leg ulcerations and the impact of therapeutic anticoagulation on healing. It further allows us to demonstrate efficacy of our animal model in which we are utilizing residual fragments of STSG tissue for transplant onto nude mice for manipulation in the laboratory.
Keywords: Factor V Leiden, hypercoagulable state, prothrombotic state, leg ulcer, animal model
CASE
A 53 year old Caucasian male presented to the Center for Wound Healing at MedStar Georgetown University Hospital (MGUH) with a 4 month history of spontaneous and painful left leg ulceration. About 4 months prior to the current presentation the patient developed an unusual sore on the medial left ankle which he thought was a spider bite. It was painful but eventually healed spontaneously. Then 3–4 weeks later he developed two lesions on the anterior shin and lateral side of the left ankle that began as small dark necrotic areas and over a period of a few weeks broke down. He was evaluated at an outside wound center and underwent bedside debridement in the clinic. The wound did not heal and he subsequently presented to our facility. Past medical history was notable for a previous left lower extremity deep venous thrombosis at the time of an arthroscopic knee surgery 14 years prior to the current presentation. He has a strong family history of thrombosis with his mother and sister also suffering deep venous thrombi. At the time of the initial thrombosis, the patient was evaluated by a hematologist and found to have homozygous Factor V Leiden R506Q mutation (FVL). He recalls being anticoagulated for approximately 6 months but then remained well without anticoagulation until the current presentation. He is a lifelong smoker with a 24 pack-year exposure history.
PHYSICAL EXAM
Physical examination at the initial presentation to MGUH revealed a healthy appearing Caucasian male in no acute distress. His body mass index was 24.64. Blood pressure was 154/84 and he reported a visual analogue pain score of 8/10. Cardiovascular, respiratory and abdominal examinations were unremarkable. Examination of the lower extremities revealed bilateral lower extremity swelling, worse on the left, with three ulcerations of the left lower extremity extending to subcutaneous tissue measuring 9.3×6.3cm (58.59cm2), 1.2cm×1.4cm (1.68 cm2) and 3.2×7.1cm (22.72cm2) respectively (Figure 1a). He was also noted to have an erythematous tender cord on the right thigh. Pulses were intact.
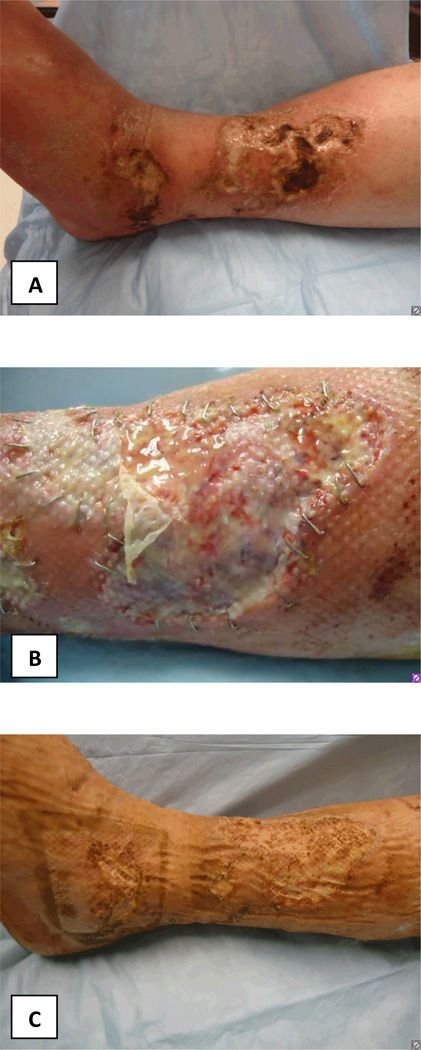
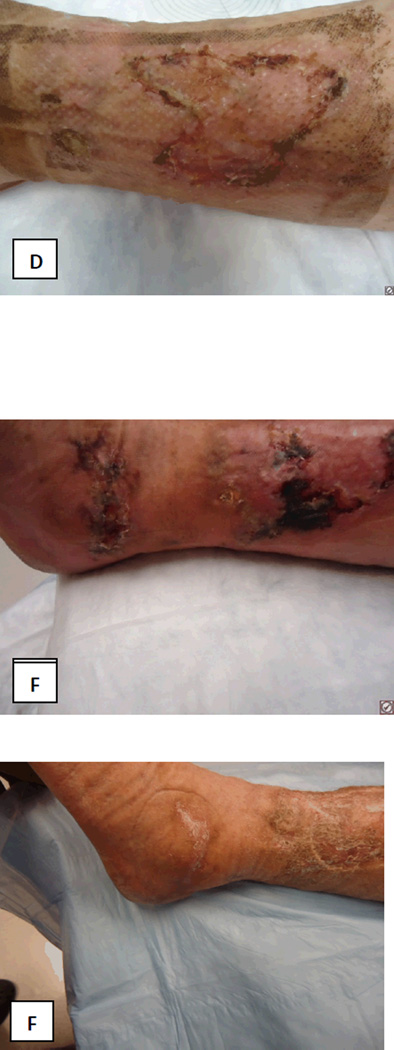
FIGURE 1.
Photographs of patients leg wounds A: At presentation; B: After initial debridement and apligraf placement; C: Prior to STSG demonstrating healthy wound bed; D: At postoperative day 25 after STSG; E: Postoperative day 52 demonstrating necrosis of the STSG; F: Improvement of wounds following reinstitution of anticoagulation therapy.
LABORATORY DATA
Laboratory evaluation at the time of presentation is tabulated in Table 1. Autoimmune screen was unremarkable, but prothrombotic work up was notable for the presence of homozygous FVL mutation, and heterozygosity for the PAI-1 and MTHFR mutations.
Table 1.
Hematologic parameters and autoimmune profile at presentation
| Patient result | Normal Range | |
|---|---|---|
| White Blood Cell Count | 10.6 k/uL | 4.0–10.8 k/uL |
| Differential | 57% neutrophils 33% lymphocytes 10% monocytes |
|
| Hemoglobin | 14.3 g/dL | 12.5–16.5 g/dL |
| Hematocrit | 43.0% | 41–53% |
| Platelet count | 348 k/uL | 145–400 k/uL |
| Sodium | 138 mmol/L | 137–145 mmol/L |
| Potassium | 3.7 mmol/L | 3.5–5.1 mmol/L |
| BUN | 18 mg/dL | 9–20 mg/dL |
| Creatinine | 0.9 mg/dL | 0.66–1.50 mg/dL |
| Alkaline phosphatase | 89 units/L | 38–126 units/L |
| AST | 11 units/L | 3–34 units/L |
| ALT | 25 units/L | 15–41 units/L |
| ANA immunofluorescence | Negative | Negative |
| dsDNA antibodies | Negative | Negative |
| Anti-neutrophil cytoplasmic antibodies (ANCA) | Negative | Negative |
| Sjogren’s Antibodies (SSA and SSB) | Negative | Negative |
| Sm Antibody | Negative | Negative |
| RNP Antibody | Negative | Negative |
| Thyroid Stimulating Hormone | 0.863 IU/mL | 0.40–4.0 IU/mL |
| Uric Acid | 5.7 mg/dL | 3.5–7.2 mg/dL |
| Hemoglobin A1c | 5.9 % | 4.2–5.6% |
| Beta-2 glycoprotein I antibodies IgG | <9 units/mL | <20.0 units/mL negative |
| Beta-2 glycoprotein I antibodies IgA | <9 units/mL | <20.0 units/mL negative |
| Beta-2 glycoprotein I antibodies IgM | <9 units/mL | <20.0 units/mL negative |
| Anti-cardiolipin antibodies IgG | <9 units/mL | <15 units/mL negative |
| Anti-cardiolipin antibodies IgA | <9 units/mL | <12 units/mL negative |
| Anti-cardiolipin antibodies IgM | <9 units/mL | <13 units/mL negative |
| Lupus Anticoagulant Ratio | 1.2 | 1.2–1.5 Weakly positive 1.5–2.0 Moderately positive >2.0 Strongly positive |
| Sedimentation Rate | 20mm/hr | 0–16 mm/hr |
| C-Reactive Protein | 6.23 mg/L | 0.00–3.00 mg/L |
| Human Immunodeficiency Virus 1 and 2 | Negative | Negative |
| Hepatitis B surface antibody | Negative | Negative |
| Hepatitis C antibody | Negative | Negative |
| Plasminogen Activator Inhibitor Mutation |
4G/5G Heterozygous |
5G/5G |
| Factor V Leiden (R506Q) Mutation |
R506Q Homozygous |
Negative |
| Methyl-tetrahydrofolate reductase (MTHFR) C677T mutation |
C677T Heterozygous |
Negative |
| Prothrombin gene (G20210A) mutation | Negative | Negative |
| Protein S activity | 102% | 60–145% |
| Protein C activity | 149% | 74–151% |
| Antithrombin III activity | 61% | 75–135% |
| Plasma Homocysteine | 13.3 µmol/L | 0–15.0 µmol/L |
CLINICAL COURSE
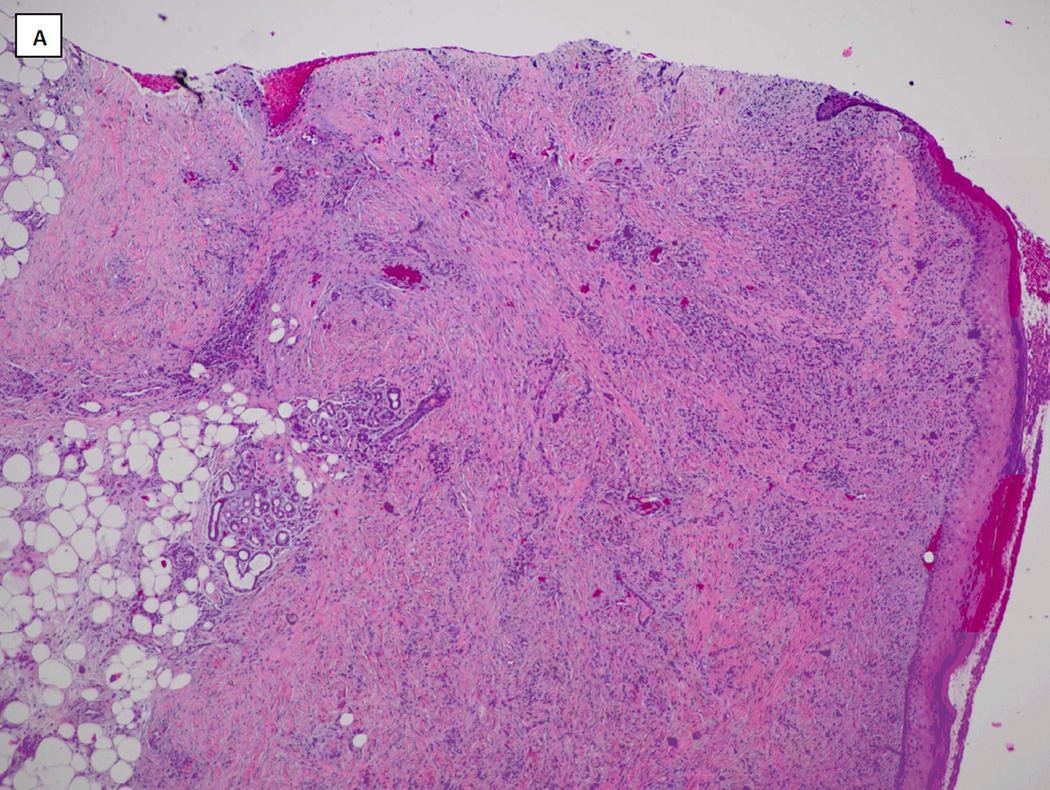
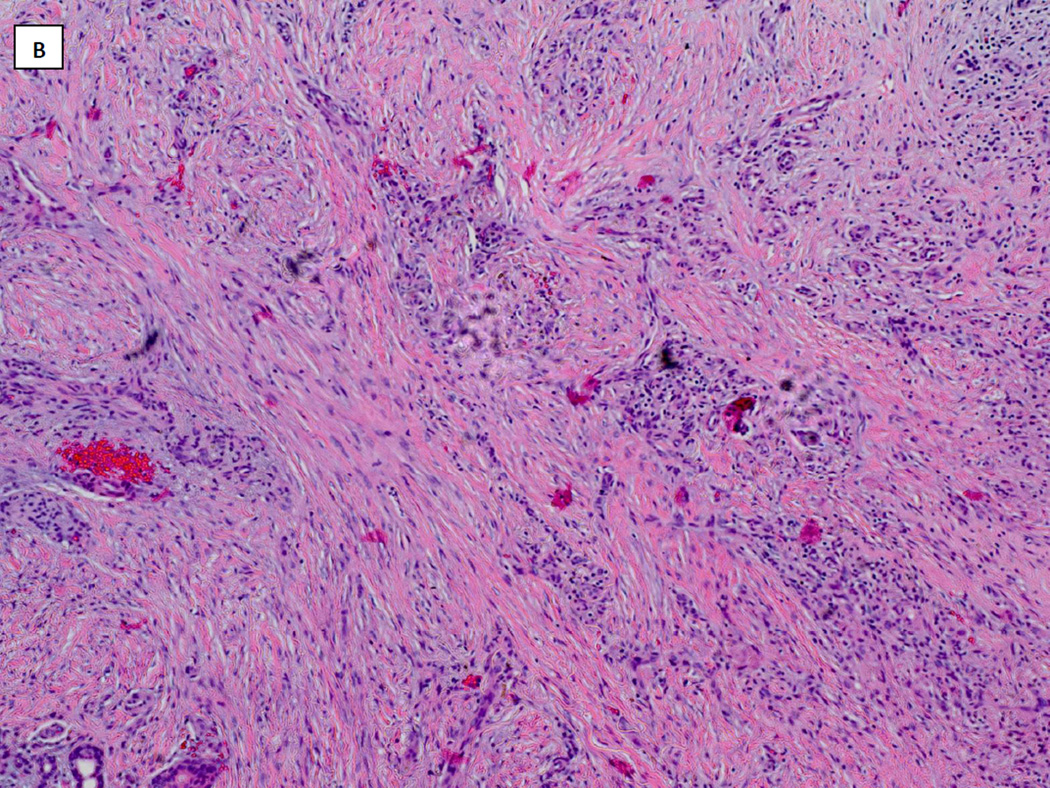
Venous ultrasound revealed acute thrombosis of the greater saphenous vein in the mid-thigh on the right leg, and chronic thrombosis of the mid femoral, distal femoral and popliteal veins on the left leg. Lower extremity arterial dopplers were within normal limits with ankle-brachial pressure indices of 1.28 on the right and 0.95 on the left. He was commenced on therapeutic anticoagulation with enoxaparin (Lovenox®, Sanofi). He underwent surgical debridement and Apligraf® (Organogenesis Inc., Canton, MA) placement and was transitioned to warfarin (Coumadin®, Bristol-Myers Squibb) post-operatively. Pathology from the initial debridement showed skin with ulceration, inflammatory granulation tissue and dermal fibrosis (FIGURE 2). He demonstrated good improvement with the Apligraf® (FIGURE 1B and C) and six weeks later was again transitioned to enoxaparin for split-thickness skin grafting (STSG) of the remaining two wounds. Post-operatively he was transitioned back to warfarin and the wounds demonstrated 95% graft take at the 25th postoperative day (FIGURE 1D). However, by post-operative day 41 there was 10% graft loss and over the subsequent 2 weeks both grafts necrosed (FIGURE 1E). On further questioning, it transpired that the patient had discontinued his warfarin on post-operative day 37 because he thought it was no longer necessary. The patient was re-initiated on enoxaparin and transitioned to warfarin. His wounds slowly healed by secondary intention and at the last visit the wounds were completely healed (FIGURE 1F and FIGURE 3).
FIGURE 2.
Hematoxylin and Eosin stained histopathology specimen from wound biopsy specimen taken at wound debridement demonstrating skin ulceration with inflammatory granulation tissue and dermal fibrosis (A: x4, B: x10).
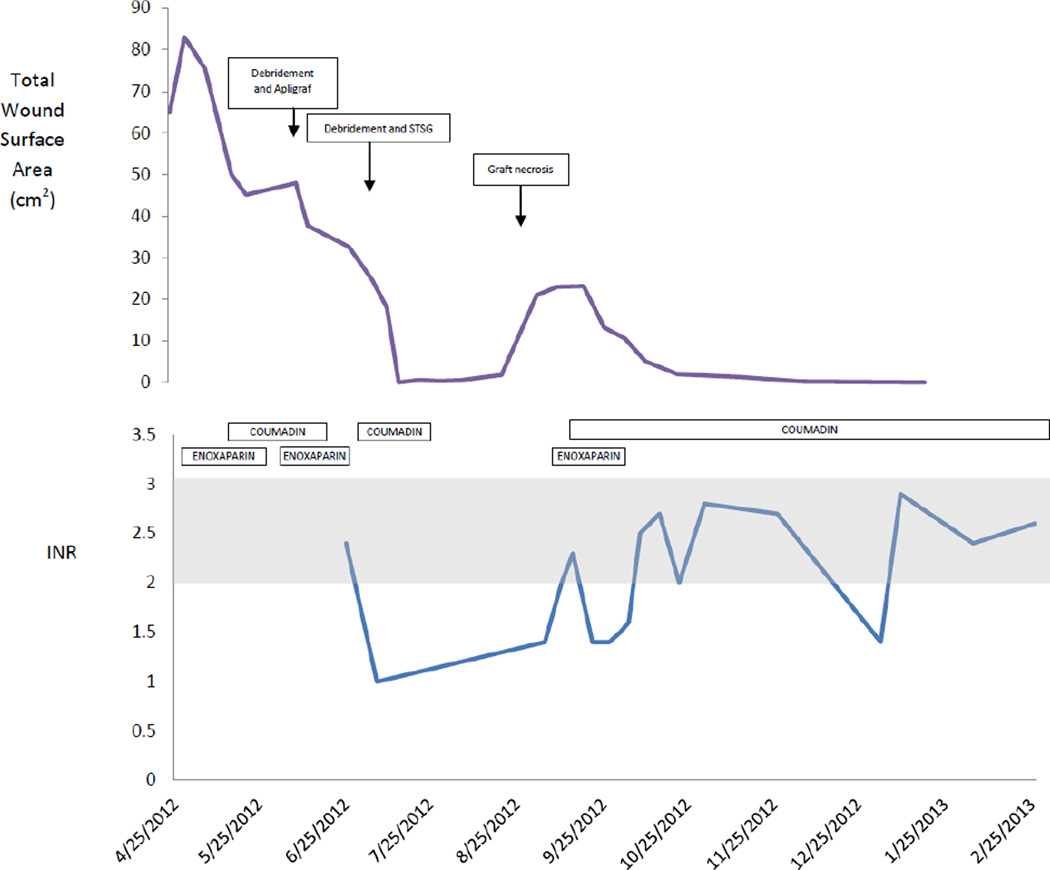
FIGURE 3.
Graph demonstrating timeline of total wound surface area (top panel) with surgical interventions superimposed and anticoagulation therapy superimposed on laboratory results from international normalized ratio (INR) testing (bottom panel, therapeutic INR 2-3 shown in shaded area). The covariation of wound size and graft necrosis with therapeutic anticoagulation is clearly demonstrated.
CORRELATIVE MOUSE MODEL
The patient presented is also enrolled in the WE-HEAL study, an IRB approved specimen and data biorepository (IRB 2011-055, and NCT 01352078) in which patients can elect to donate residual tissue from STSG and wound debridement for use in research. Under a protocol approved by the Georgetown University Animal Care and Use Committee (GUACUC 2011-050) samples collected through the WE-HEAL study may also be used for research to develop a correlative animal model for studying human wound healing. Currently available mouse models for studying human wound healing are limited because wound contraction contributes to wound shrinkage independent of epithelialization. We have developed a model in which human skin, either collected from elective abdominoplasty or from residual skin discarded after STSG harvest, is transplanted onto an athymic nude (nu/nu) mouse and allowed to engraft for several months prior to wounding experiments. As a result of enrollment in the WE-HEAL study, this patient’s residual STSG was therefore transplanted onto a nude mouse, coincidentally providing a correlative animal model.
Method of mouse grafting
In this case, an athymic nude (nu/nu) mouse (Harlan Laboratories) which had been allowed to acclimatize for 14 days in the Georgetown University Division of Comparative Medicine (DCM) rodent barrier facility was used for transplantation. The mouse was anesthetized using inhalation of 1–3% isofluorane in oxygen and the skin was sterilized using povidone iodine and isopropyl alcohol. Full thickness skin was removed from two 1.5cm diameter graft beds on either side of the mouse flank and the residual human STSG skin 1cm diameter was placed on the wound bed. The graft was secured using steri-strips and dressed using a Telfa non-adherent dressing (Tyco Healthcare, MA, USA) and a compressive wrap (Coban, 3M Health Care, Neuss, Germany). Post-operatively the mouse was housed in an individual cage to minimize disturbance of the xenograft. Dressings were changed on post-operative days 7 and 14, at which time the steri-strips were removed.
Results of mouse xenograft
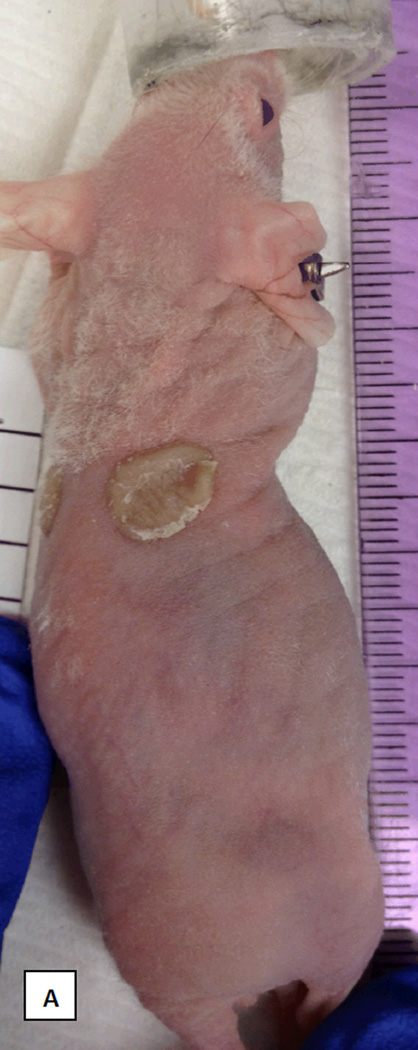
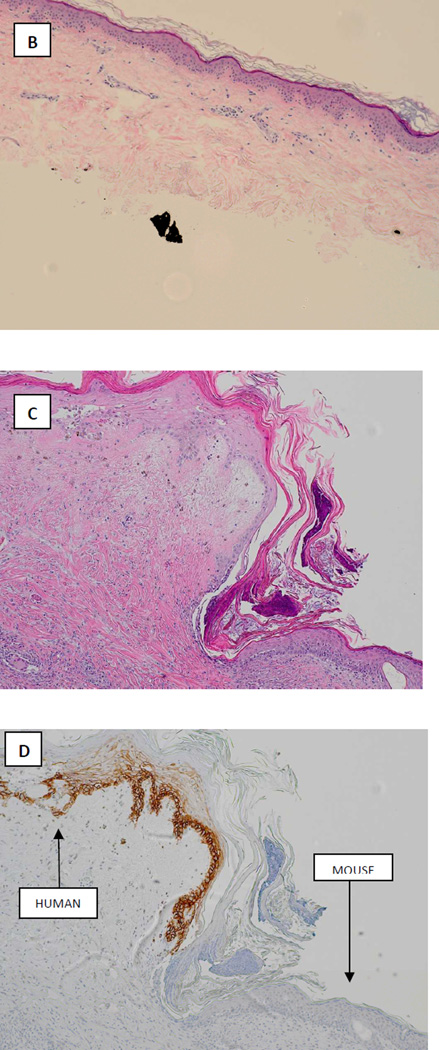
Engraftment of the human STSG on the mouse was successful (FIGURE 4A) and in contrast to the graft on the patient which underwent necrosis on post-operative days 41–52 the graft on the mouse remained intact at 87 days postoperatively. Histopathology of the STSG prior to xenografting and the STSG at the time of harvest are shown in FIGURE 4 B-D.
FIGURE 4.
Correlative mouse xenograft model. A: photograph demonstrating successful engraftment of patient skin onto nude mouse at day 31. B: Hematoxyllin and Eosin staining of xenografted tissue prior to transplantation (x4). C: Hematoxyllin and Eosin staining of xenograft after harvest at 87 days post-transplant (x10). D: Staining for human major histocompatability complex-1 (MHC-1) in the harvested graft clearly demonstrating viable epithelial human tissue engrafted adjacent to mouse tissue (x10).
DISCUSSION
Chronic leg ulcers that have failed to heal after 3 months of appropriate wound care affect approximately 6.5 million people in the US with a prevalence of 1% and costs estimated at $25 billion per year 1. In addition to the financial costs, these wounds significantly impact mortality 2 and cause considerable pain, affecting patient reported psychosocial wellbeing and quality of life 3, 4.
Inherited prothrombotic states are well recognized to be associated with venous leg ulceration and non-healing lower extremity wounds5–8, with the prevalence of thrombophilia of 41% in a population of patients presenting to a hospital-based leg ulcer clinic8. The FVL mutation is highly prevalent in patients with post-thrombotic venous leg ulcers with various rates reported in the literature including 23%9, 36%10 and 41%11, dependent on the racial and geographic region being studied. Data additionally shows an increased incidence of prothrombotic states including FVL mutation in patients presenting with vasculitic leg ulcers12 and livedoid vasculopathy13–15 suggesting a synergy between vascular wall abnormalities and prothrombotic states contributing to tissue injury16.
Mechanisms of thrombosis and delayed healing in patients with FVL mutation
FVL mutation is common accounting for 40–50% of cases of inherited thrombophilia17. The prevalence of the FVL mutation is approximately 5.3% in Caucasians, 2.2% in Hispanic Americans, and 1.2% in African Americans18 and approximately 1% of patients with FVL mutation are homozygous for the mutation.
The mechanisms by which FVL mutation contributes to hypercoagulability can be understood by reviewing the role of factor V in the coagulation cascade. Circulating factor V is an inactive molecule which is activated by thrombin to factor Va. Factor Va then serves as a cofactor in the conversion of prothrombin to thrombin. Factor Va is inactivated by enzymatic cleavage of its heavy chain in a two step process by activated protein C. Cleavage of the protein at Arg506 results in exposure of cleavage sites facilitating subsequent cleavage at Arg306 and Arg 679. The FVL mutation involves replacement of guanine by adenine at nucleotide 1691 (G1691) resulting in replacement of arginine at position 506 in factor V by glutamine and rendering factor Va resistant to cleavage at position 506 by activated protein C and thus delayed inactivation. Delayed inactivation of factor Va results in more factor Va within the prothrombinase complex increasing thrombin generation and thus activating coagulation. Additionally normal factor V cleaved at position 506 synergistically acts as a cofactor with protein S supporting the role of activated protein C in degradation of factor VIIIa and factor Va, in the absence of this cleavage product the anticoagulant effect of activated protein C is reduced.
The major clinical manifestation of FVL is venous thromboembolic disease. The prevalence of FVL heterozygosity in patients with a first confirmed deep venous thrombosis or pulmonary embolism is 12%19, and in otherwise healthy men over 60 who develop a DVT the prevalence of FVL is 25.8%.
Synergistic effect of FVL with other prothrombotic states
FVL mutation may be seen in association with other protrhombotic defects including prothrombin gene mutation and deficiencies of protein C, S, and anti-thrombin III. Carriers of two defects have a higher risk of thrombosis than those with one; for example, FVL heterozygotes have an odds ratio of thrombosis of 4.9, prothrombin gene mutation heterozygotes have an odds ratio of 3.8, but those individuals heterozygous for both mutations have an odds ratio of 20.0 for thrombosis20.
Smoking and venous thromboembolic disease
The association between smoking and venous thromboembolic disease is unclear, some studies have shown increased risk of thromboembolism in smokers21, 22, while others suggest only a modest increased risk23. No studies have investigated the associations between prothrombotic states, smoking and STSG failure. However, it is possible the combined effect of the two prothrombotic states with cigarette smoking contributed to thrombosis and graft loss in this case.
Warfarin-associated skin necrosis
Warfarin-induced skin necrosis has been reported in association with FVL mutation24, 25. This syndrome is caused by a transient prothrombotic state resulting from the acute drop in protein C activity, and increased generation of thrombin during the early phases of warfarin therapy26. In the case reported here, necrosis of the STSG coincided with cessation of warfarin therapy suggesting that the primary hypercoagulable state was more likely to be the cause of the skin necrosis. However, it is essential that patients with primary hypercoagulable states are bridged with another agent during the initiation of warfarin therapy.
Utility of the human-mouse xenograft model for studying wound healing
Mouse models of human skin healing are limited for many reasons, not least because wound contraction contributes to surface area shrinkage independent of epithelialization27–29. To overcome this challenge, we have developed a model for studying human wound healing in vivo using normal human skin engrafted onto athymic nude mice. Taking this model one step further, to try to develop a correlative model for patients with delayed wound healing, we have been performing xenografts with residual STSG from patients enrolled in the WE-HEAL study. Our hypothesis was that we would see correlation between graft failures on the human and mouse in certain disease states. However, what was striking in this case was that the STSG failed in the patient but was maintained in the mouse. This confirms our suspicion that in patients with coagulopathy, activation of the coagulation cascade in the circulation, as opposed to primary skin pathology, contributes to the graft loss.
CONCLUSIONS
A thorough pre-operative history and examination should be completed in all patients with delayed wound healing, particularly patients undergoing split-thickness skin grafting, to identify prior thrombotic events. Hematologic evaluation should be completed prior to operative intervention when indicated.
We report a novel mouse model in which residual human skin from split-thickness skin grafts may be successfully transplanted onto an athymic nude mouse. This model will be a useful tool for studying not only patients with intrinsic skin diseases but also patients in whom serologic factors (such as abnormalities of the coagulation cascade, or autoimmune diseases) play a role in the disease pathogenesis.
KEY POINTS.
Spontaneous leg ulceration may occur in the setting of prothrombotic states and prothrombotic states may contribute to delayed wound healing and split-thickness skin graft failure.
We report a case of a patient with Factor V Leiden (FVL) homozygous mutation, who was non-compliant with his anticoagulation post-operatively and experienced necrosis of his split-thickness skin graft.
We review the pathologic mechanisms by which FVL mutation can contribute to thrombosis and delayed wound healing.
Thorough pre-operative history and examination should be completed in all patients undergoing split-thickness skin graft or with delayed wound healing to identify prior thrombotic events. Hematologic evaluation should be completed prior to operative intervention when indicated.
We report a novel mouse model in which residual human skin from split-thickness skin grafts may be successfully transplanted onto an athymic nude mouse. This model may prove a useful tool for studying patients in whom serologic factors (such as abnormalities of the coagulation cascade or autoimmune diseases) play a role in the disease pathogenesis.
ACKNOWLEDGEMENTS
Dr. Shanmugam is currently supported by award numbers KL2RR031974 and UL1TR000101 (previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA). Sean McNish, Drs. Shanmugam, Attinger, Tassi and Wellstein are supported by award R01NR013888 from the National Institute of Nursing Research.
These studies were conducted in part at the Lombardi Comprehensive Cancer Center Histopathology and Tissue Shared Resource which is supported in part by the National Cancer Institute grant P30CA051008.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Cancer Institute or the National Institutes of Health.
REFERENCES
- 1.Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair and Regeneration. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escandon J, Vivas AC, Tang J, Rowland KJ, Kirsner RS. High mortality in patients with chronic wounds. Wound Repair and Regeneration. 2011;19(4):526–528. doi: 10.1111/j.1524-475X.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 3.Price P, Harding K. The impact of foot complications on health-related quality of life in patients with diabetes. J Cutan Med Surg. 2000;4(1):45–50. doi: 10.1177/120347540000400112. [DOI] [PubMed] [Google Scholar]
- 4.Price P, Harding K. Cardiff Wound Impact Schedule: the development of a condition-specific questionnaire to assess health-related quality of life in patients with chronic wounds of the lower limb. International Wound Journal. 2004;1(1):10–17. doi: 10.1111/j.1742-481x.2004.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiszniewski A, Bykowska K, Bilski R, Jaśkowiak W, Proniewski J. Prevalence rate for inherited thrombophilia in patients with chronic and recurrent venous leg ulceration. Wound Repair and Regeneration. 2011;19(5):552–558. doi: 10.1111/j.1524-475X.2011.00716.x. [DOI] [PubMed] [Google Scholar]
- 6.Calistru A, Baudrier T, Goncalves L, Azevedo F. Thrombophilia in venous leg ulcers: A comparitive study in early and later onset. Indian Journal of Dermatology, Venereology and Leprology. 2012;78(3):406. doi: 10.4103/0378-6323.95477. [DOI] [PubMed] [Google Scholar]
- 7.Bradbury A, MacKenzie R, Burns P, Fegan C. Thrombophilia and chronic venous ulceration. Eur J Vasc Endovasc Surg. 2002;24(2):97–104. doi: 10.1053/ejvs.2002.1683. [DOI] [PubMed] [Google Scholar]
- 8.MacKenzie RK, Ludlam CA, Ruckley CV, Allan PL, Burns P, Bradbury AW. The prevalence of thrombophilia in patients with chronic venous leg ulceration. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2002;35(4):718–722. doi: 10.1067/mva.2002.121749. [DOI] [PubMed] [Google Scholar]
- 9.Maessen-Visch MB, Hamulyak K, Tazelaar DJ, Crombag NHCMN, Neumann HAM. The Prevalence of Factor V Leiden Mutation in Patients With Leg Ulcers and Venous Insufficiency. Arch Dermatol. 1999 Jan 1;135(1):41–44. doi: 10.1001/archderm.135.1.41. 1999. [DOI] [PubMed] [Google Scholar]
- 10.Gaber Y, Siemens HJ, Schmeller W. Resistance to activated protein C due to factor V Leiden mutation: high prevalence in patients with post-thrombotic leg ulcers. British Journal of Dermatology. 2001;144(3):546–548. doi: 10.1046/j.1365-2133.2001.04081.x. [DOI] [PubMed] [Google Scholar]
- 11.Hafner J, Kühne A, Schär B. Factor V leiden mutation in postthrombotic and non-postthrombotic venous ulcers. Archives of Dermatology. 2001;137(5):599–603. [PubMed] [Google Scholar]
- 12.Mekkes JR, Loots MA, van der Wal AC, Bos JD. Increased incidence of hypercoagulability in patients with leg ulcers caused by leukocytoclastic vasculitis. Journal of the American Academy of Dermatology. 2004;50(1):104–107. doi: 10.1016/s0190-9622(03)00881-8. [DOI] [PubMed] [Google Scholar]
- 13.Hairston BR, Davis M, Pittelkow MR, Ahmed I. Livedoid Vasculopathy: Further Evidence for Procoagulant Pathogenesis. Arch Dermatol. 2006 Nov 1;142(11):1413–1418. doi: 10.1001/archderm.142.11.1413. 2006. [DOI] [PubMed] [Google Scholar]
- 14.Calamia K, Balabanova M, Perniciaro C, Walsh J. Livedo (livedoid) vasculitis and the factor V Leiden mutation: additional evidence for abnormal coagulation. Journal of the American Academy of Dermatology. 2002 Jan;46(1):133–137. doi: 10.1067/mjd.2002.117718. [DOI] [PubMed] [Google Scholar]
- 15.Biedermann T, Flaig MJ, Sander CA. Livedoid vasculopathy in a patient with factor V mutation (Leiden) Journal of Cutaneous Pathology. 2000;27(8):410–412. doi: 10.1034/j.1600-0560.2000.027008410.x. [DOI] [PubMed] [Google Scholar]
- 16.Shanmugam V, Steen V, Cupps T. Lower extremity ulcers in connective tissue disease. Isr Med Assoc J. 2008 Jul;10(7):534–536. [PubMed] [Google Scholar]
- 17.Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369(6475):64–67. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 18.Ridker P, Miletich J, Hennekens C, Buring J. Ethnic distribution of factor v leiden in 4047 men and women: Implications for venous thromboembolism screening. JAMA. 1997;277(16):1305–1307. [PubMed] [Google Scholar]
- 19.Ridker PM, Hennekens CH, Lindpaintner K, Stampfer MJ, Eisenberg PR, Miletich JP. Mutation in the Gene Coding for Coagulation Factor V and the Risk of Myocardial Infarction, Stroke, and Venous Thrombosis in Apparently Healthy Men. New England Journal of Medicine. 1995;332(14):912–917. doi: 10.1056/NEJM199504063321403. [DOI] [PubMed] [Google Scholar]
- 20.Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism--pooled analysis of 8 case-control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809–816. [PubMed] [Google Scholar]
- 21.Holst AG, Jensen G, Prescott E. Risk Factors for Venous Thromboembolism: Results From the Copenhagen City Heart Study. Circulation. 2010 May 4;121(17):1896–1903. doi: 10.1161/CIRCULATIONAHA.109.921460. 2010. [DOI] [PubMed] [Google Scholar]
- 22.Pomp ER, Rosendaal FR, Doggen CJM. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. American Journal of Hematology. 2008;83(2):97–102. doi: 10.1002/ajh.21059. [DOI] [PubMed] [Google Scholar]
- 23.Blondon M, Wiggins K, McKnight B, et al. The association of smoking with venous thrombosis in women. A population-based, case-control study. Thromb Haemost. 2013;109(5) doi: 10.1160/TH12-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makris M, Bardhan G, Preston F. Warfarin induced skin necrosis associated with activated protein C resistance. Thromb Haemost. 1996;75(3):523–524. [PubMed] [Google Scholar]
- 25.McGehee WG, Klotz TA, Epstein DJ, Rapaport SI. Coumarin Necrosis Associated with Hereditary Protein C Deficiency. Annals of Internal Medicine. 1984;101(1):59–60. doi: 10.7326/0003-4819-101-1-59. [DOI] [PubMed] [Google Scholar]
- 26.Vigano D'Angelo S, Comp PC, Esmon CT, D'Angelo A. Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. The Journal of Clinical Investigation. 1986;77(2):416–425. doi: 10.1172/JCI112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Ge J, Tredget EE, Wu Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat. Protocols. 2013;8(2):302–309. doi: 10.1038/nprot.2013.002. [DOI] [PubMed] [Google Scholar]
- 28.Wong V, Sorkin M, Glotzbach J, Longaker M, Gurtner G. Surgical Approaches to Create Murine Models of Human Wound Healing. J Biomed Biotechnol. 2011:969618. doi: 10.1155/2011/969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansell DM, Holden KA, Hardman MJ. Animal models of wound repair: Are they cutting it? Experimental Dermatology. 2012;21(8):581–585. doi: 10.1111/j.1600-0625.2012.01540.x. [DOI] [PubMed] [Google Scholar]