Abstract
Pseudomonas aeruginosa (PA) is a Gram-negative bacterial pathogen commonly associated with chronic lung infections. Previously, we have identified several PA virulence factors that are important for resistance to the surfactant protein-A (SP-A), a pulmonary innate immunity protein that mediates bacterial opsonization and membrane permeabilization. In this study, we demonstrate that the type IV pilus (Tfp) is important in the resistance of PA to antibacterial effects of SP-A. The Tfp-deficient mutant, ΔpilA, is severely attenuated in an acute pneumonia model of infection in the lungs of wild-type mice, but is virulent in the lungs of SP-A−/− mice. The ΔpilA bacteria are more susceptible to SP-A-mediated aggregation and opsonization. In addition, the integrity of the outer membranes of ΔpilA bacteria is compromised, rendering them more susceptible to SP-A-mediated membrane permeabilization. By comparing Tfp extension and retraction mutants, we demonstrate that the increased susceptibility of ΔpilA to SP-A-mediated opsonization requires the total absence of Tfp from PA cells. Finally, we provide evidence that increased expression of nonpilus adhesin OprH that may serve as SP-A ligand, resulting in increased phagocytosis and preferential pulmonary clearance of ΔpilA.
Keywords: Surfactant protein-A, Pseudomonas aeruginosa, Type IV pilus, Opsonization, Membrane permeabilization, Adhesins
Introduction
Pseudomonas aeruginosa (PA) is an opportunistic Gram-negative bacterial pathogen commonly associated with acute or chronic infection of mechanically damaged (ventilator associated pneumonia), immunocompromised (HIV, malignancies, immunosuppressive drugs) and mechanically obstructed (cystic fibrosis, chronic obstructive pulmonary disease) lungs [1]. In addition to lung infections, PA is also prevalent in contact lens associated keratitis, and a major cause of burn infections [2,3]. The reason for PA prevalence in many infections lies in its ability to elaborate a wide array of virulence factors, to form biofilms [4], as well as its intrinsic high levels of resistance to many antibiotics [5].
Among the virulence factors expressed by PA is the unipolarly-localized surface appendage, type IV pilus (Tfp) [4]. Tfp-deficiency attenuates the ability of PA to induce epithelial cytotoxicity, pneumonia, septicemia and mortality in mice [6,7]. Tfp is important for twitching motility, epithelial adhesion, substratum attachment and biofilm formation, and phage attachment and uptake [4,8]. Tfp is made up of monomers of pilin, encoded by the pilA gene [8,9]. The assembly and disassembly of Tfp are powered by the ATPases PilB and PilT/U, respectively, producing twitching motility [8,10]. These extension and retraction proteins are regulated by the Chp chemosensory system [10]. The asialoGM1 [4,8,9,11] and N-glycan chains [12,13] located on the apical surface of the host epithelium are the main receptors for Tfp.
The lung’s surfactant contains phospholipids and four major surfactant proteins: SP-A, B, C and D [14,15]. The naturally occurring octadecameric SP-A is the most abundant [14,15]. Each monomer contains a N-terminal, triple helical collagen region that binds to eukaryotic receptor (SP-A receptor 210), and a C-terminal carbohydrate recognition domain (CRD) that binds to microbial carbohydrates [14-16]. Binding of CRD domain to microbial carbohydrates aggregates microbes, enhancing their phagocytosis [14,17-21]. In addition to opsonic phagocytosis, SP-A enhances phagocytosis directly [15,22]. SP-A−/− mice are more susceptible to lung infection, with decreased bacterial clearance and reduced macrophage phagocytosis [15,23,24]. Most recently, we and others have shown that SP-A directly permeabilizing microbial membranes [17-21].
Previously, our laboratory has shown that several virulence factors of PA, including LPS, phosphoenolpyruvate transferase, isochorismate synthase, flagellum, and elastase B, confer resistance to SP-A-mediated antimicrobial effects [18-21]. In this current paper, we show that Tfp of PA also plays a major role in resistance to SP-A-mediated phagocytosis and membrane permeabilization.
Materials and Methods
Bacterial Strains and Growth Conditions
The wild-type PA strain PAO1, and its isogenic derivatives ΔpilA, the genetically-complemented strain ΔpilA-comp, ΔchpA, ΔpilG, ΔpilB, ΔpilH, ΔpilT and ΔpilU were generously provided by Professor Joanne Engel (University of California-San Francisco) [10]. The rpoN::ISphoA/hah mutant was purchased from the University of Washington Genome Sciences [25] (Table 1). PA strains were grown in Luria-Bertani (LB) broth and stored at −80°C in 30% glycerol. Before each experiment, bacteria were streaked from frozen stock onto LB agar for 18 hours at 37°C. One colony from this streak was then cultured in 5 ml LB broth to stationary phase (OD 600 nm ~ 3.0).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| P. aeruginosa strains | ||
| PAO1 | Wild-type | 3 |
| PAO1 ΔpilA | In frame deletion of the pilA gene | 3 |
| ΔpilA (comp) | Genetically-complemented ΔpilA mutant |
3 |
| PAO1 – GFP | PAO1 harboring pUCP19-gfp | This study |
| ΔpilA – GFP | ΔpilA harboring pUCP19-gfp | This study |
| ΔchpA | In frame deletion of the chpA gene | 3 |
| ΔpilG | In frame deletion of the pilG gene | 3 |
| ΔpilB | In frame deletion of the pilB gene | 3 |
| ΔpilH | In frame deletion of the pilH gene | 3 |
| ΔpilT CTX-PilU | In frame deletion of the pilT; pilU and 1 kb upstream sequence at attB site |
3 |
| ΔpilU | In frame deletion of the pilU gene | 3 |
| rpoN: :ISphoA/hah | Mutant harboring a ISphoA/hah transposon insertion into the rpoN gene |
University of Washington Genome Sciences |
| Plasmids | ||
| pUCP19-gfp | pUCP19 plasmic expressing a green fluorescence protein |
16 |
Mouse Clearance Assay
Wild-type C3H/HeN (SP-A+/+) mice were purchased from Harlan Laboratory (South Easton, MA). Isogenic SP-A−/− mice were gifts from Dr. Francis McCormack (University of Cincinnati College of Medicine). Mouse experiments complied with the guidelines and were approved by the University of Illinois at Urbana-Champaign Institutional Animal Care and Use Committee (IACUC). SP-A+/+ and SP-A−/− mice (n = 5) were given a single intranasal inoculation of 1 × 107 PAO1 or ΔpilA strain. After 18 hours of infection, mouse lungs were harvested for histology or bacterial enumeration as we have previously described (18-21).
In Vivo Phagocytosis Assay
The phagocytosis rates between different PA strains were compared using the gentamicin exclusion assay as previously described [21]. Briefly, SP-A+/+ and SP-A−/− mice (n = 3) were given a single intranasal inoculation of 1 × 107 PAO1 or ΔpilA cells. After two hours, mouse lungs were lavaged to collect the alveolar macrophages and neutrophils. The macrophages were then incubated in PBS supplemented with 100 μg/ml gentamicin to kill the remaining extracellular bacteria. The macrophages were lysed with 1% Triton X-100 solution and serially diluted for the enumeration of internalized PA. The ratio of CFU counts between SP-A+/+ and SP-A−/− mice was computed for the fold increase of phagocytosis mediated by SP-A.
Purification of Human hSP-A
Discarded lung washings from anonymous alveolar proteinosis patients were generously provided by Professor Francis McCormack (University of Cincinnati College of Medicine). hSP-A was purified as previously described [26]. Briefly, raw lung washings, equilibrated with 1 mM CaCl2, was passed through Sepharose 6B® column ladened with mannose. The captured SP-A was eluted using the elution buffer (2 mM EDTA 5 mM Tris-HCl, pH 7.4). The eluted fractions were dialyzed using the dialysis buffer (150 mM NaCl 5 mM Tris-HCl, pH 7.4) to remove EDTA. The purity of hSP-A preparations was confirmed by Coomasie blue staining.
Murine Macrophage Cell Line and In Vitro Phagocytosis Assay
Murine RAW 264.7 macrophages (ATCC# TIB-71) were maintained in DMEM supplemented with 10% FBS at 5% CO2 and 37°C [21]. The phagocytosis rates between different PA strains were compared using the gentamicin exclusion assay. Briefly, 1 × 106 RAW 264.7 macrophages/ml were plated in 6-well cell culture plates overnight at 37°C incubator with 5% CO2. PA strains were pre-incubated with 12.5, 25 or 50 μg/ml hSP-A in the presence of 2 mM CaCl2 for 1, 6 or 12 hours in a shaker (300 rpm) at 37°C. The resulting mixture was then incubated with the RAW 264.7 cells at a ratio of 10 bacteria:1 macrophage for 1.5 hours. The macrophages were then washed, and incubated in fresh DMEM supplemented with 100 μg/ml gentamicin to kill the remaining extracellular bacteria. The macrophages were lysed with 1% Triton X-100 solution, and serially diluted for enumeration. The ratio of CFU between treated and untreated bacteria was computed for the fold increase of phagocytosis mediated by hSP-A.
Aggregation Assay
PA strains were transformed with the plasmid pUCP19 harboring a GFP gene by electroporation as previously described [21]. Bacterial aggregation were performed using the stationary phase PA incubated with 25 μg/ml hSP-A and 2 mM CaCl2 for one hour in a shaker (300 rpm) at 37°C. Bacterial clusters were enumerated from 10 independent fields under a fluorescence microscope.
Membrane Permeabilization Assay
Membrane permeability effects of hSP-A were measured using both the thiol-specific fluorophore ThioGlo® (Calbiochem, San Diego, CA) and phosphatase substrate Enzyme Labeled Fluorescence-97 (ELF-97®) (Molecular Probes, Carlsbad, CA), as previously described [17,20,21]. Stationary phase PA bacteria were washed, and incubated with either 50 μg/ml hSP-A for 15 minutes at 37°C, or with 50 μg/ml total protein of bronchoalveolar lavage fluids (BALF) from either SP-A+/+ or SP-A−/− mice. For the ThioGlo assay, the bacterial-SP-A mixture was sedimented, and the supernatant was incubated with 10 μM ThioGlo. Fluorescence was measured at excitation wavelength 405 nm and emission wavelength 535 nm. For the ELF-97 assay, the bacterial-SP-A mixture was directly incubated with 100 μM ELF-97. Fluorescence was measured at excitation 355 nm and emission 535 nm every 3 minutes for the duration of 90 - 120 minutes.
Bacterial Viability Assay
Stationary phase PA bacteria were washed, and incubated with either 50 μg/ml hSP-A or 50 μg/ml total protein of BALF from either SP-A+/+ or SP-A−/− mice for 60 minutes or 6 hours at 37°C. The bacterial were then stained with a combination of SYTO 9® and propidium iodide from the commercial bacterial viability kit, LIVE/DEAD BacLight® (Molecular Probes, Carlsbad, CA). The number of dead and live bacteria was counted in 10 independent high power fields (400×) using a fluorescent microscope.
Elastase Assay
Elastase production in the culture supernatant of stationary phase PA was evaluated using the Sensolyte™ Red Protease Assay Kit (AnaSpec, Inc, San Jose, CA). Fluorescence was measured at excitation 546 nm and emission 575 nm.
Pyocyanin Assay
Pyocyanin in the bacterial-free supernatant of stationary phase PA cultured in LB broth or a low phosphate medium (20 mM succinic acid, 40 mM NH4Cl, 2 mM K2SO4, 0.014 g NAH2PO4, 1 M MOPS, 40 mM MgCl2, 100 mM CaSO4, 100 mM ZnCl2, 30 mM MnCl2, 30 mM Fe(NO3)3) were measured at OD 690 nm [20].
SDS Lysis Assay
To assess the membrane stability of PA strains, bacterial cell lysis was performed with 0.25% SDS as previously described [19]. Stationary phase PA bacteria (OD 600 nm 3.0) were washed, and resuspended in PBS with 0.25% SDS. The OD 600 nm was measured every 10 minutes for 1 hour.
LPS Analysis
LPS was purified from stationary phase PA strains as follows: the bacteria were sedimented, and resuspended in 200 μl lysis buffer (2 g SDS, 4 ml 2-mercaptoethanol, 0.003 g bromophenol blue, 1 M Tris-Hcl pH6.8) and boiled for 10 min. 3 μl of 20 μg/μl Proteinase K was added, and mixture was incubated for 1 hour at 60°C. The resulting mixture was separated in a SDS-PAGE gel. The resolved gel was incubated with a fixing solution (25% v/v isopropyl alcohol, 7% v/v acetic acid) overnight at 4°C, oxidized (0.7% periodic acid, 2.7% ethanol, 0.3% v/v acetic acid) for 5 min with gentle agitation, and washed with water for 30 min 3 times. The gel was then placed in staining solution (4% v/v 1 M NaOH, 5% v/v NH4OH, 2% AgNO3) and shaken for 10 minutes, followed by four 10-minute washes in water. Finally, the gel was developed (0.02% citric acid, 0.05% v/v formaldehyde) for 20 minutes. The development was stopped using a stop solution (0.8% v/v acetic acid).
Transmission Electron Microscopy (TEM)
PA strains were grown to OD 600 nm ~3.0 in LB broth at 37°C. Bacteria were washed with PBS, and subsequently fixed using the Karnovsky fixative. TEM was performed at the University of Illinois Material Research Laboratory.
Ligand Blot
Biotinylated SP-A was used to identify potential receptors in PA strains as previously described [16]. Briefly, stationary phase bacteria were ruptured under 14,000 psi in a French Press. Membranes were isolated in 100 mM sodium carbonate and centrifuged at 115,000 g. Isolated membranes were solubilized using 50 mM Tris pH 7.4, mixed with loading buffer, and boiled for 5 minutes, and resolved using 15% SDS-PAGE gels. Gels were either stained using Coomassie blue or transferred to PVDF membranes. Membranes were blocked for 2 hours, and subsequently incubated with 3 μg/ml biotinylated SP-A. After three washings, streptavidin-conjugated HRP was added. Signal was detected using commercially available Western blot stain and substrate. Ligand blot signal was analyzed with the Image J software. Corresponding band in gels stained with Coomassie blue to the signal detected in PVDF membrane, were sent for analyses at the University of Illinois LC-MS/MS core facility.
RESULTS
Type IV Pilus is Important in Resistance to SP-A-Mediated Lung Clearance
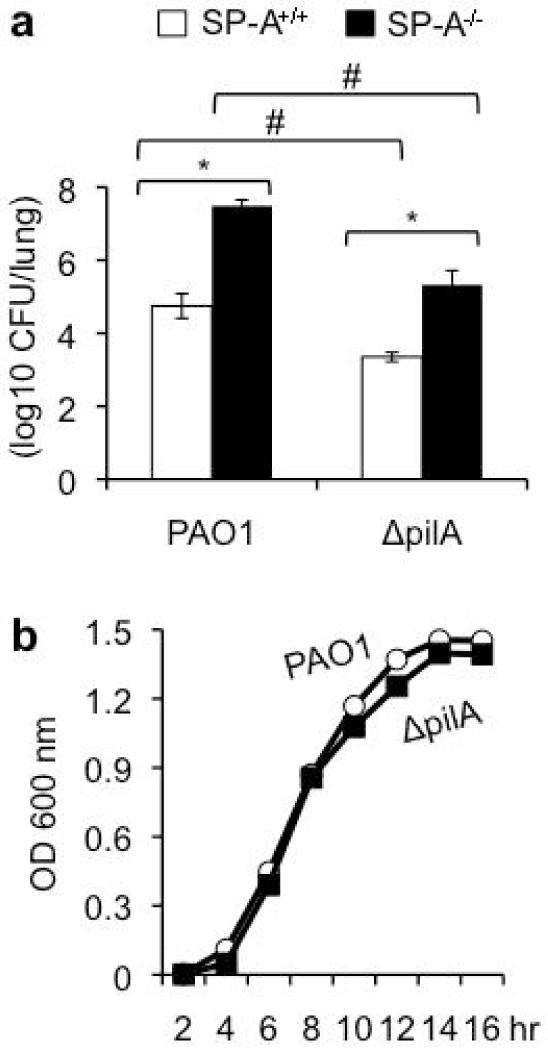
To determine the contribution of Tfp-mediated resistance to SP-A, we compared the virulence of the wild-type PA PAO1 and the isogenic ΔpilA mutant in a mouse model of acute pneumonia. Eighteen hr after intranasal inoculation with PAO1 or ΔpilA, SP-A+/+ mice showed signs of infection and respiratory distress but were not moribund. In contrast, PAO1-infected SP-A−/− mice were moribund and had to be euthanized (data not shown). The number of viable PAO1 or ΔpilA bacteria in SP-A−/− were 2.74 log and 1.97 log higher than in SP-A+/+ mice, respectively (Fig. 1a). The number of ΔpilA bacteria in SP-A−/− mice was statistically indistinguishable when compared to the number of PAO1 the SP-A+/+mice, suggesting that ΔpilA was more virulence during lung infection in the absence of SP-A. However, the viable counts of ΔpilA were 1.4 log lower than PAO1 in SP-A+/+ mice, and was also 2.16 log lower than PAO1 in SP-A−/− mice. Because the relative decrease of bacterial load between PAO1 and ΔpilA from SP-A−/− mice to SP-A+/+ mice is only 0.77 log, this suggests that ΔpilA is in general less virulent. In addition, other lung innate immune factors within alveolar space may compensate for the absence of SP-A. Thus, a straightforward link between Tfp and SP-A cannot be easily established (Fig. 1a). The ΔpilA showed similar growth kinetics to that of PAO1, suggesting that the difference in mouse clearance was not due to the growth defects in the former (Fig. 1b).
Fig. 1.
ΔpilA bacteria are more susceptible to clearance by SP-A. (a) Respiratory tract infections with wild-type PAO1 versus ΔpilA were performed by intranasal inoculation of anesthetized SP-A+/+ or SP-A−/− mice. Mouse lungs were harvested 18 hours post infection for bacterial enumeration. Data are the mean CFU ± SE (n = 5 per group). *p < 0.05 when comparing the bacterial loads between SP-A+/+ versus SP-A−/− infected by PAO1 or ΔpilA. #p < 0.05 when comparing the bacterial loads between PAO1 versus ΔpilA infecting the SP-A+/+ or SP-A−/− mice. (b) The growth kinetics of PAO1 and ΔpilA bacteria were determined by measuring OD 600nm. The experiments were performed three times independently in triplicates. The representative growth curve from one out of three independent experiments is shown.
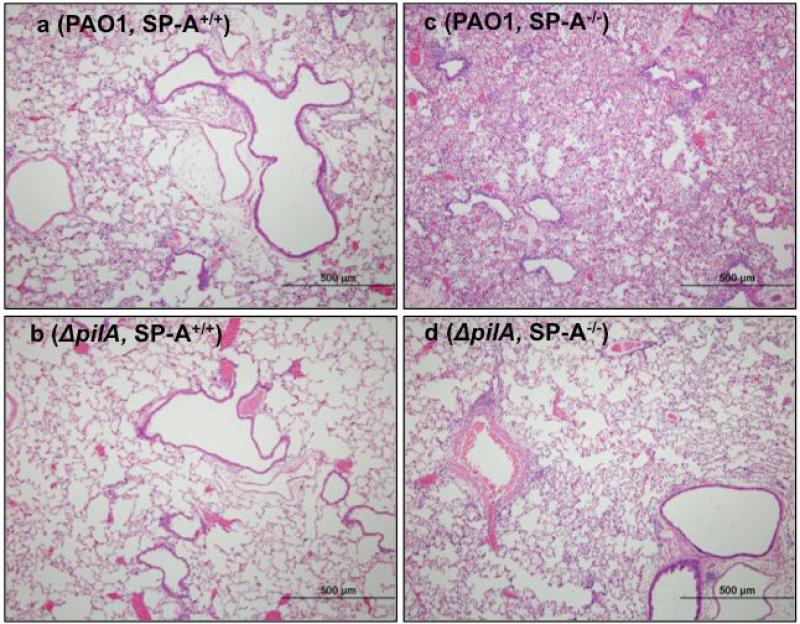
The aforementioned quantitative observations were supported by histopathology (Fig. 2). In the absence of bacterial infection, histopathological features of SP-A−/− mouse lungs were indistinguishable when compared to the lungs of SP-A+/+ mice (data not shown). PAO1 caused moderate bronchopneumonia (Fig. 2a) whereas the ΔpilA mutant only caused mild bronchopneumonia in the lungs of SP-A+/+ mice (Fig. 2b). In contrast, PAO1 caused severe bronchopneumonia in SP-A−/− mice, with large amounts of pulmonary infiltrates and consolidation (Fig. 2c). Importantly, ΔpilA caused more severe pulmonary infiltrate in SP-A−/− mice (Fig. 2d) than the SP-A+/+ mice (Fig. 2b). The severity of ΔpilA-mediated bronchopneumonia in SP-A−/− lungs (Fig. 2d) were similar to that caused by PAO1 in SP-A+/+ lungs (Fig. 2a), suggesting that the virulence levels of ΔpilA in mouse lungs devoid of SP-A was similar to PAO1 in mouse lungs with sufficient SP-A. Collectively, these results indicate that Tfp plays an protective role against anti-PA activity mediated by SP-A.
Fig. 2.
Histopathology of PA-infected lungs. SP-A+/+ and SP-A−/− mice were infected with PAO1 or ΔpilA as described in Fig. 1. Representative H&E-stained lung sections from SP-A+/+ and SP-A−/− mice (n = 5) 18-hour post intranasal instillation of PAO1 (a, c) and ΔpilA (b, d) bacteria.
Type IV Pilus is Important for Resistance of SP-A-Mediated Phagocytosis
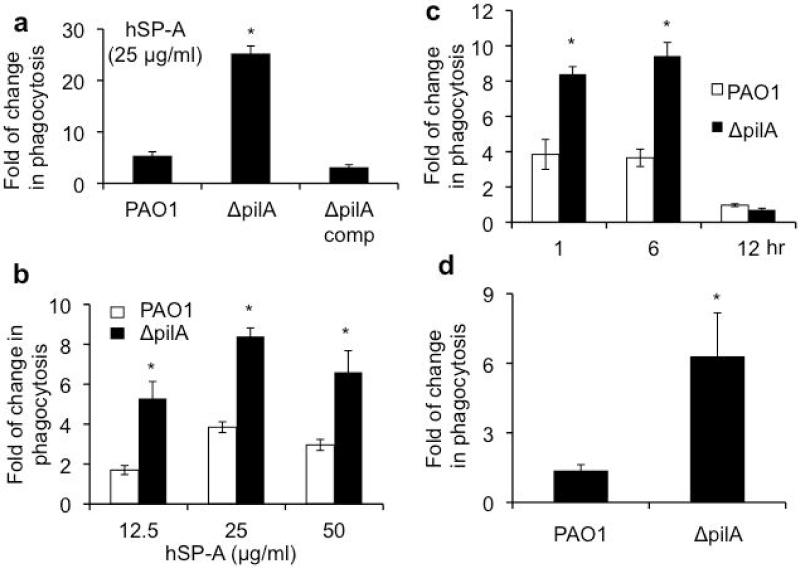
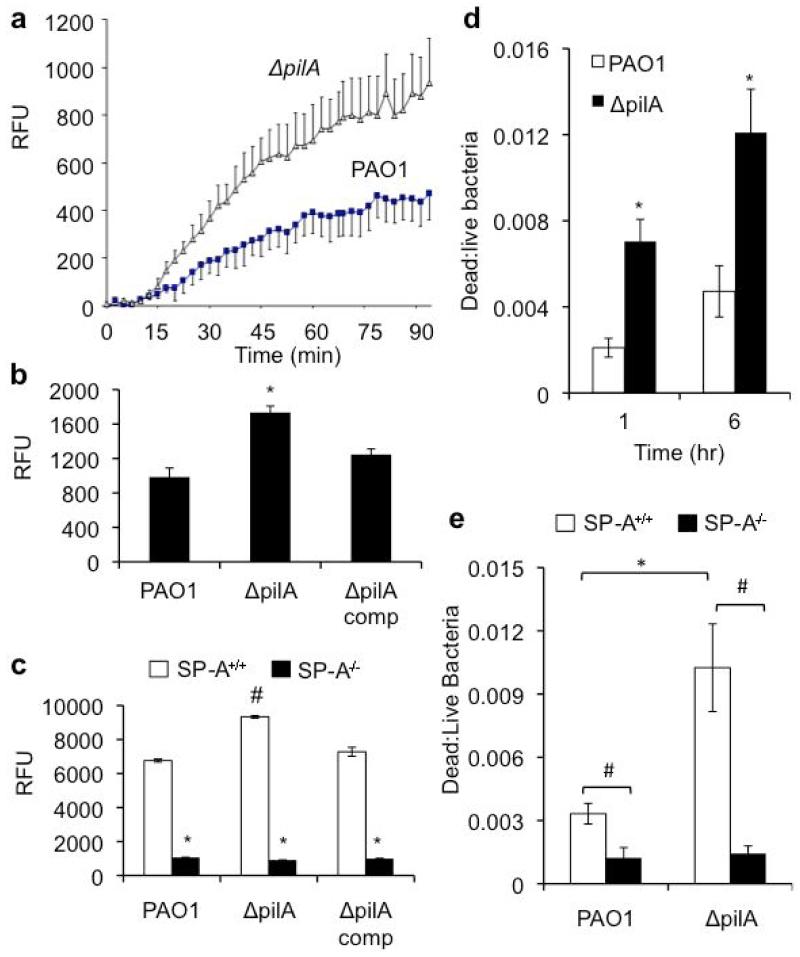
To decipher the mechanism of Tfp resistance in SP-A-mediated lung clearance, we examined whether ΔpilA bacteria were more susceptible to SP-A-mediated opsonization. In the presence of 25 μg/ml SP-A, ΔpilA bacteria were phagocytized 5 times more efficiently than the wild-type PAO1 (Fig. 3a). The genetically-complemented ΔpilA(comp) bacteria were as resistant to SP-A-mediated opsonization as PAO1 (Fig. 3a). These results were confirmed by studies using different concentrations of SP-A, which consistently showed that, in the presence of SP-A, ΔpilA was more susceptible to phagocytosis by macrophages than PAO1 (Fig. 3b). We also examined the phagocytosis of ΔpilA in a time-dependent manner. Again, ΔpilA was more susceptible to SP-A-mediated opsonization at 1 and 6 hours after exposure to RAW 264.7 macrophages (Fig. 3c). However, prolonged exposure (12 hours) abolished phagocytosis, most probably because both PAO1 and ΔpilA bacteria degraded SP-A by secreting exoproteases, as we have previously described [20,21]. These observations were confirmed by an in vivo phagocytosis assay, which showed that ΔpilA was 4.5 times more susceptible to SP-A-mediated opsonization than PAO1 (Fig. 3d). The in vivo phagocytosis assay measures the total phagocytosis activity involving both alveolar macrophages and infiltrating neutrophils responding to PA infection. Collectively, these results indicate that Tfp is important for resistance to SP-A-mediated opsonization.
Fig. 3.
The ΔpilA mutant is more susceptible to SP-A-mediated opsonization. (a-c) RAW 264.7 macrophages were infected with either PAO1 or ΔpilA in the presence or absence of hSP-A. The ratio of ingested bacteria was expressed as fold increase in phagocytosed bacteria due to the effect of hSP-A. (a) Phagocytosis of PAO1, ΔpilA and genetically complemented ΔpilA(comp) in the presence of 25 μg/ml hSP-A. (b) Phagocytosis of PAO1 and ΔpilA in the presence of different concentrations of hSP-A. (c) Time dependent phagocytosis of PAO1 versus ΔpilA in the presence of 25 μg/ml hSP-A. (d) In vivo phagocytosis of PAO1 versus ΔpilA in SP-A+/+ and SP-A−/− mice (n = 3). All phagocytosis experiments were independently performed three times in triplicates. The mean ± standard deviation from one representative experiment is shown. *p < 0.05 when comparing the number of phagocytosed ΔpilA against PAO1.
Type IV Pilus is Important for Resistance to SP-A-Mediated Aggregation
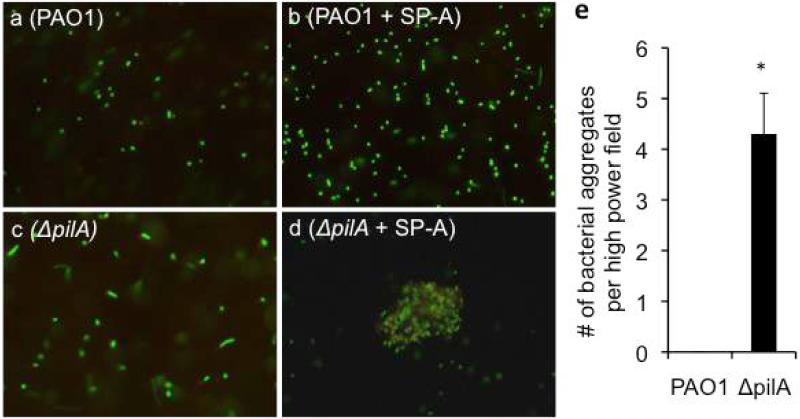
One of the mechanisms by which SP-A enhances microbial phagocytosis is by increasing their aggregation, allowing more efficient phagocytosis [22]. We compared the aggregation of wild-type PAO1 versus ΔpilA by SP-A in vitro. The ΔpilA mutant bacteria were aggregated by SP-A 4-fold higher than PAO1 per high power field (Fig. 4a-e). Overall, these results show that Tfp confers resistance to SP-A-mediated aggregation.
Fig. 4.
ΔpilA bacteria are more susceptible to SP-A-mediated aggregation. GFP-expressing PAO1 and ΔpilA bacteria were incubated with 25 μg/ml hSP-A for 1 hour, and examined under a confocal fluorescence microscope. (a) PAO1 without hSP-A. (b) PAO1 with hSP-A. (c) ΔpilA without hSP-A. (d) ΔpilA with hSP-A. (e) The number of aggregates was averaged from 10 independent high power field. *p < 0.05 when comparing the number aggregates in ΔpilA against PAO1 .
Type IV Pilus is Important for Resistance to SP-A-Mediated Membrane Permeabilization
Apart from its ability to opsonize and facilitate the phagocytosis of microbes by macrophages, SP-A is also capable of directly killing bacteria by membrane permeabilization [17-19]. We examined the susceptibility of ΔpilA to SP-A-mediated membrane permeabilization, by measuring the diffusion of an impermeable phosphatase substrate ELF97 into PA cells, and by measuring the leakage of thiol-containing proteins. After 90 minutes of exposure, the ELF97 assay indicates that ΔpilA bacteria were permeabilized 2.1 fold higher than the PAO1 (Fig. 5a). In addition, the leakage of thiol-containing proteins increased by 1.81 fold relative to PAO1 (Fig. 5b). To confirm our in vitro observations, we compared the ability of BALF of SP-A+/+ and SP-A−/− mice to permeabilize the membrane of PAO1 and ΔpilA (Fig 5c). The ΔpilA bacteria were more susceptible to BALF from SP-A+/+ mice than PAO1. In contrast, BALF from SP-A−/− only possessed low levels of membrane permeabilizing capability, suggesting that SP-A is an important membrane permeabilizing protein within the BALF. To determine whether increased susceptibility to membrane permeability leads to direct bacterial killing, we examined the bacterial viability after exposure to hSP-A and to BALF. Although both the hSP-A and the BALF from SP-A+/+ mice only cause low levels of PA killing, the ΔpilA was consistently shown to be significantly more susceptible to direct killing by hSP-A (Fig 5d) and BALF of SP-A+/+ mice (Fig 5e). These results suggest that SP-A-mediated membrane permeabilization contribute minimally to PA clearance.
Fig. 5.
ΔpilA are more susceptible to SP-A-mediated membrane permeabilization. (a) ELF-97® assay. PAO1 and ΔpilA were preincubated with 50 μg/ml hSP-A for 15 minutes before the addition of ELF-97®. Absorbance was measured every 3 minutes at excitation wavelength 355 nm and emission wavelength 535 nm, for a total of 90 minutes. (b) In vitro ThioGlo® assay. PAO1, ΔpilA and ΔpilA comp were preincubated with 50 μg/ml hSP-A for 15 minutes. The bacterial-free supernatants were then mixed with ThioGlo®. Absorbance was measured at excitation wavelength 405 nm and emission wavelength 535 nm. *p < 0.05 when comparing the relative fluorescence unit (RFU) of ΔpilA against PAO1 and ΔpilA comp. (c) Ex vivo ThioGlo® assay. PAO1, ΔpilA and ΔpilA comp were preincubated with 50 μg/ml total protein of BALF from either SP-A+/+ mice or SP-A−/− mice for one hour. The bacterial free supernatants were then mixed with ThioGlo®. Absorbance was measured at excitation wavelength 405 nm and emission wavelength 535 nm. #p < 0.05 when comparing the RFU of ΔpilA against PAO1 and ΔpilA in BALF from SP-A+/+ mice. *p < 0.05 when comparing the RFU of each PA strain in BALF from SP-A+/+ versus SP-A−/− mice. (d) In vitro PA killing by hSP-A. PAO1 and ΔpilA were preincubated with 50 μg/ml hSP-A for one or six hour(s). The bacteria were then stained with a mixture of SYTO 9® and propidium iodide for 15 minutes. The ratio of dead to live bacteria is counted in 10 high power fields. *p < 0.05 when comparing the ratio of dead to live ΔpilA versus PAO1 bacteria in the presence of hSP-A. (e) Ex vivo PA killing by mouse SP-A within BALF. PAO1 and ΔpilA were preincubated with 50 μg/ml total protein of BALF from either SP-A+/+ mice or SP-A−/− mice for one hour. Bacterial viability was determined as in d. #p < 0.05 when comparing the ratio of dead to live bacteria between those treated with BALF from SP-A+/+ versus SP-A−/− mice. *p < 0.05 when comparing the ratio of dead to live PAO1 versus ΔpilA bacteria treated with BALF from SP-A+/+ mice. Experiments were performed independently three times in triplicates (for ELF and ThioGlo assays) or decuplicate (for Live Dead assay). Data from one typical experiment are shown.
Type IV Pilus-Mediated Resistance to SP-A-Mediated Phagocytosis is Independent of the Presence of the Appendages on the Cell Surface
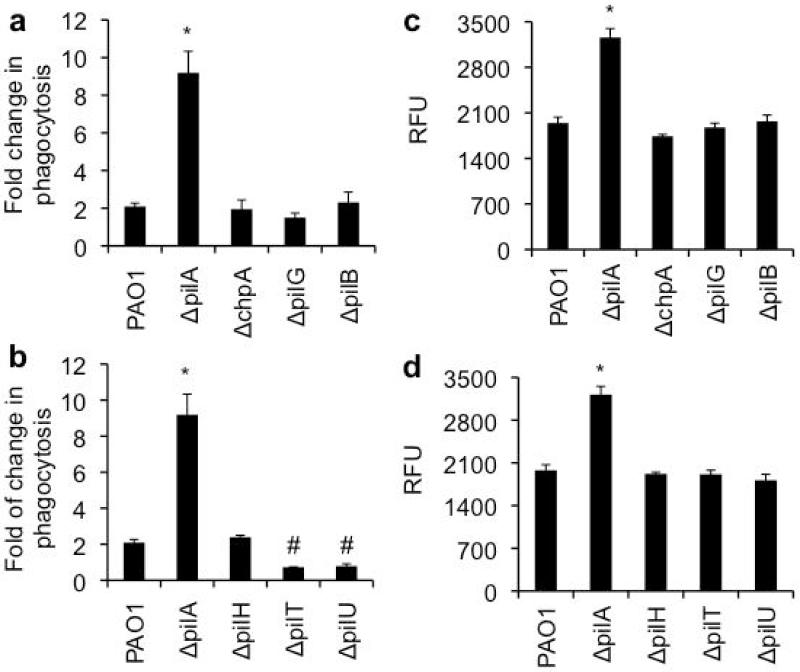
Compared to other PA mutants we have studied previously (15, 16, 36, 37), ΔpilA is uniquely more susceptible to both SP-A-mediated opsonization and membrane permeabilization. We used both the pilin extension (ΔchpA, ΔpilG and ΔpilB) and the retraction (ΔpilH, ΔpilT and ΔpilU) mutants to determine if the presence of Tfp on the cell surface of PA is required for resistance to both SP-A-mediated opsonization and membrane permeabilization. As shown in Fig. 6a, the mere absence of Tfp on the bacterial surface in the extension mutants (ΔchpA, ΔpilG, ΔpilB) does not increase susceptibility to SP-A-mediated phagocytosis. Interestingly, the retraction mutants, ΔpilT and ΔpilU, which are hyperpiliated, are more resistant to SP-A-mediated opsonization (Fig. 6b). Both extension and retraction mutants were as resistant to SP-A-mediated membrane permeabilization as PAO1 (Fig. 6c-d). Collectively, these results suggest that the presence of pilin anchoring the membranes is adequate to confer resistance to SP-A-mediated phagocytosis. In addition, expression of additional Tfp (e.g., ΔpilT and ΔpilU mutants) offers in increased resistance to SP-A-mediated phagocytosis.
Fig. 6.
The susceptibility of Tfp extension and retraction mutants to SP-A-mediated opsonization. (a-b) RAW 264.7 macrophages were infected with PAO1, ΔpilA, the extension or retraction mutants in the presence or absence of hSP-A. The ratio of ingested bacteria between those exposed to hSP-A versus those unexposed was expressed as fold increase in phagocytosis. (a) Phagocytosis of PAO1, ΔpilA and extension mutants ΔchpA, ΔpilG, ΔpilB. (b) Phagocytosis of PAO1, ΔpilA and retraction mutants ΔpilH, ΔpilT, ΔpilU. *p < 0.05 when comparing phagocytosis of PAO1 versus ΔpilA mutant. #p < 0.05 when comparing phagocytosis of PAO1 versus ΔpilT and ΔpilU mutants. All experiments were independently performed three times in triplicates. The mean ± standard deviation from one representative experiment is shown. (c-d) Membrane permeabilization of PA strains by hSP-A. (c) Membrane permeabilization of PAO1, ΔpilA and extension mutants ΔchpA, ΔpilG, ΔpilB. (d) Membrane permeability of PAO1, ΔpilA and retraction mutants ΔpilH, ΔpilT, ΔpilU. All experiments were independently performed three times in triplicates. The mean ± standard deviation from one representative experiment is shown.
Increased Susceptibility of ΔpilA to SP-A-Mediated Phagocytosis is Probably Due to A Compensatory Increase in Nonpilus Adhesins
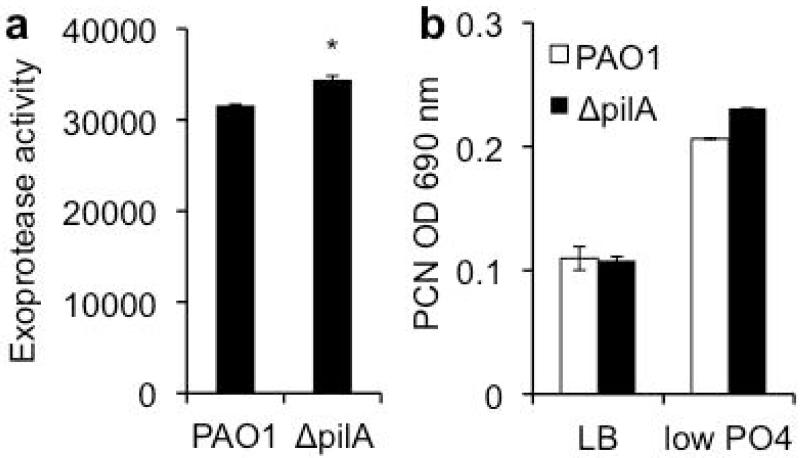
Previous work in our laboratory has shown PA confers resistance to SP-A-mediated phagocytosis by increasing the degradation of SP-A through elastase B [20,21], through mechanisms regulated by both flagellum and quorum-sensing. Flagellar-deficient mutants are deficient in quorum sensing, decreasing their ability to produce adequate elastase B to degrade SP-A. However, ΔpilA produced wild-type levels of both quorum-sensing regulated exoprotease activities (Fig. 7a) and the redox-active secondary metabolite pyocyanin (Fig. 7b). Furthermore, the ΔpilA lost its susceptibility to SP-A-mediated phagocytosis after 12 hours (Fig. 3c), suggesting that SP-A was degraded. Thus, the lack of quorum-sensing and SP-A degradation is not the cause of enhanced opsonization of ΔpilA by SP-A.
Fig. 7.
Resistance to SP-A-mediated phagocytosis is independent of quorum sensing. (a) Exoprotease activity of PAO1 versus ΔpilA. (b) Pyocyanin production of PAO1 versus ΔpilA.
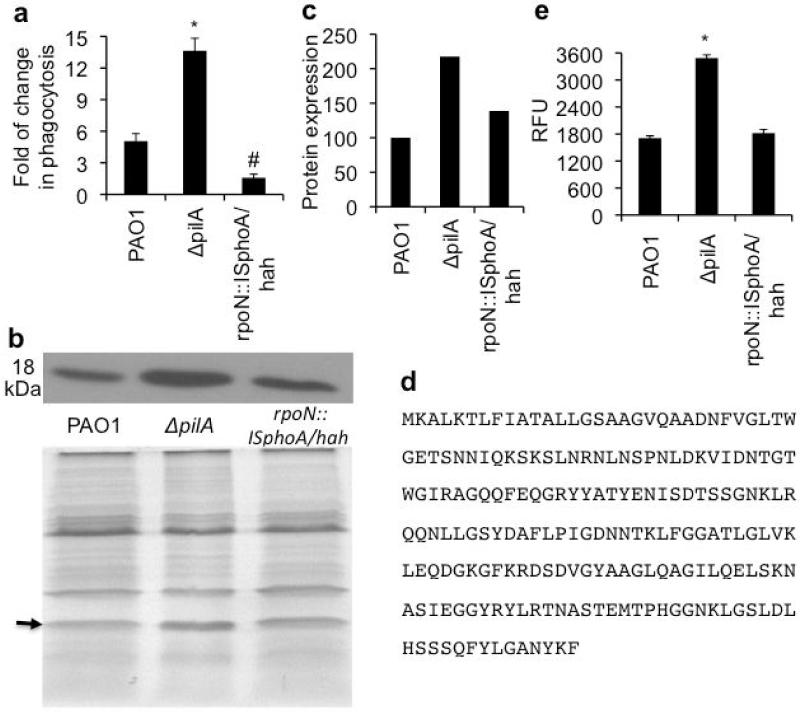
Previous studies have shown that ΔpilA has higher binding affinity to host epithelial cells than the hyperpiliated mutants ΔpilT and ΔpilU [27-30]. This is speculated to be caused by over-expression of alternative “nonpilus adhesins” in ΔpilA. The alternative sigma factor RpoN has been shown to regulate the expression of these adhesins [27-30]. We hypothesized that one or more of these nonpilus adhesins may serve as ligands for binding to SP-A. We compared the phagocytosis of ΔpilA versus rpoN::ISphoA/hah in the presence of SP-A. Fig. 8a shows rpoN::ISphoA/hah, which lacks the adhesins, is more resistant to SP-A-mediated phagocytosis than both with-type PAO1 and ΔpilA. Importantly, ligand blot analysis shows that the ΔpilA overexpresses a protein of ~ 18 kDa compared to both PAO1 and rpoN::ISphoA/hah (Fig. 8b, c). LCMS/MS and micro-sequencing analyses indicate that this putative adhesin is the outer membrane protein H1 precursor OprH (PA1178) (Fig. 8d). Finally, rpoN::ISphoA/hah does not exhibit increased susceptibility to SP-A-mediated membrane permeabilization, suggesting that these nonpilus adhesins are dispensable against the pore-forming function of SP-A (Fig. 8e).
Fig. 8.
Resistance to SP-A-mediated phagocytosis is regulated by RpoN. (a) Comparison of bacterial phagocytosis by RAW 264.7 macrophages between PAO1, ΔpilA and rpoN::ISphoA/hah in the presence or absence of 25 μg/ml hSP-A. All experiments were independently performed three times in triplicates. The mean ± standard deviation from one representative experiment is shown. *p < 0.05 when comparing PAO1 versus ΔpilA. #p < 0.05 when comparing PAO1 versus rpoN::ISphoA/hah. (b) Ligand blot analysis of nonpilus adhesins in PAO1, ΔpilA and rpoN::ISphoA/hah. (c) Image J analysis of the ligand blot. Protein expression was normalized to PAO1 as 100%. (d) Protein sequence of OprH. (e) Comparison of membrane permeabilization between PAO1, ΔpilA and rpoN::ISphoA/hah. The experiments were independently performed three times in triplicates. The mean ± standard deviation from one representative experiment is shown. *p < 0.05 when comparing PAO1 versus ΔpilA and rpoN::ISphoA/hah.
ΔpilA Has Reduced Membrane Stability, Rendering it Susceptible to SP-A-Mediated Membrane Permeabilization
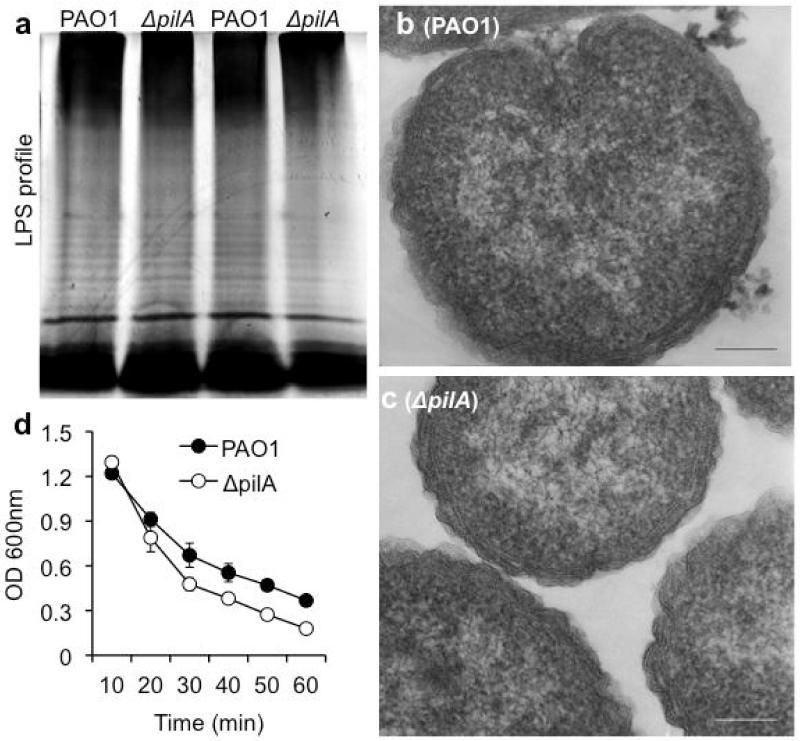
Next, we examined the potential mechanisms of increased susceptibility of ΔpilA to SP-A-mediated membrane permeabilization. Previously, we have shown that the loss of LPS [18-19] and flagellum [19-21] destabilizes membrane integrity, rendering them more susceptible to SP-A-mediated membrane permeabilization. In addition, Abeyrathne et al have shown that the O-antigen ligase mutant, ΔwaaL, which is unable to attach O-antigen to the core polysaccharide of LPS, has decreased or absent Tfp and flagella [31]. We examined whether the absence of Tfp destabilizes the membranes, through reduced expression of LPS. Qualitative analyses of both the LPS and the TEM images of bacterial membranes show no discernible differences between ΔpilA and PAO1 (Fig. 9a-c). However, ΔpilA was more slightly more susceptible to lysis by 0.25% SDS, suggesting that the loss of pilin modestly compromises the integrity of the outer membranes in the mutant bacteria (Fig. 9d).
Fig. 9.
ΔpilA is more susceptible to SDS-mediated cell lysis. (a) LPS analysis of PAO1 and ΔpilA as visualized using silver stain. (b & c) TEM of PAO1 and ΔpilA, respectively. Bar = 100 nm. (d) SDS lysis assay. PAO1 and ΔpilA bacteria were incubated in 0.25% SDS solution. OD 600nm was measured every 10 minutes. Bacterial lysis experiments were independently performed three times in triplicates. The mean from one representative experiment is shown.
Discussion
SP-A is a major pulmonary innate immunity protein that mediates microbial clearance through opsonization and membrane permeabilization. However, little is known about the mechanisms elaborated by microbial pathogens to confer resistance or susceptibility to SP-A. Previously, we have demonstrated that several PA factors—LPS, flagellum, isochorismate synthase, phosphoenolpyruvate phosphotransferase—confer resistance to SP-A-mediated membrane permeabilization [18-21]. Flagellum also regulates quorum sensing-mediated expression of elastase B that degrades and disables SP-A-mediated opsonization during phagocytosis [19-21]. In this study, we found that Tfp of PA is uniquely important for resistance to SP-A-mediated opsonization and membrane permeabilization. Several lines of evidence support this argument: (i) ΔpilA is preferentially cleared in the lungs of SP-A+/+ mice compared to SP-A−/− mice; (ii) ΔpilA is more susceptible to SP-A-mediated aggregation and opsonization; (iii) ΔpilA is more susceptible to SP-A-mediated membrane permeabilization; and (iv) the genetically complemented ΔpilA (comp) strain, which carries a copy of the wild-type pilA gene in trans, has restored resistance to SP-A-mediated opsonization and membrane permeabilization.
At face value, our current report seems to contradict previous findings, which show that Tfp deficient PA strains, including ΔpilA, are more resistant to phagocytosis by macrophages [32-34]. However, this discrepancy can be explained because of different experimental context. Firstly, these previously published studies were pertaining to nonopsonin-mediated, fibronectin-dependent phagocytosis. Secondly, the number of phagocytized bacteria was quantified using a semi-quantitative fluorescent technique, rather than the quantitative method we used with the gentamicin exclusion assay. However, gentamicin exclusion assay, which has been used for decades to quantify microbial phagocytosis, has its disadvantage. Because phagocytes ingest both individual and SP-A aggregated bacteria, we cannot rule the possibility that vigorous pipetting and vortexing procedures used during serial dilution plating may not completely disaggregate bacteria. However, even if this were the case, ΔpilA, which is more susceptible to than PAO1 to SP-A-mediated aggregation, would be undercounted. This would mean that the actual difference in phagocytic factor between ΔpilA and PAO1 would be even higher, further supporting our results showing that ΔpilA is more susceptible to SP-A-mediated opsonization and phagocytosis.
A careful examination of data presented in Figures 3A and 3B indicate that there is variation in the efficiency of phagocytosis in different sets of experiments. This is not unusual, due to the fact that bacteria and macrophage cultures are not “identical” from one set of experiments to the next. For example, the number of bacterial cells used in each experiments will certainly differ slightly from one experiment to the next one. Another contributing factor to the variation in phagocytic efficiency is that different batches of SP-A preparations, sometimes are derived from lung washing from different alveolar proteinosis patients, may have different antimicrobial activity and potency. Nevertheless, in each experiment described, ΔpilA is consistently more susceptible to SP-A-mediated opsonization and membrane permeabilization.
Detailed genetic analyses of the PA mutants defective in retraction and extension of Tfp reveal rather unexpected mechanisms by which the pilus mediates resistance to SP-A-mediated phagocytosis. For example, the extension gene mutants (ΔchpA, ΔpilG and ΔpilH) did not show increased susceptibility to SP-A-mediated phagocytosis. These results suggest that a total absence of pilA both intracellularly and extracellularly is required to render PA becoming susceptible to SP-A-mediated opsonization. We postulate that the absence of pilA causes a compensatory increase of alternative ligands that actually interacts with SP-A. This is supported by previous studies showing that Tfp-deficient PA mutants (e.g., ΔpilA) have higher adhesion to epithelial cells than hyperpiliated Tfp extension mutants strains ΔpilT and ΔpilU, suggesting that Tfp-independent adhesins are responsible for increased binding to epithelial cells [7,27]. Importantly, both hyperpiliated Tfp mutants ΔpilT and ΔpilU are significantly more resistant than the wild-type PAO1 to SP-A-mediated phagocytosis. These observations suggest that additional Tfp expressed by both ΔpilT and ΔpilU mutants may have masked the putative ligands, decreasing SP-A-mediated binding, aggregation and subsequent opsonization and enhanced phagocytosis by phagocytes.
Previous work has shown that the alternative sigma factor, RpoN, positively regulates both pilin and flagellin production through their respective two-component systems PilRS and FleQR [30,35]. Thus, RpoN deficiency results in the loss of both Tfp and flagellum in PA. Interestingly, ΔpilA has been used as a model to study the expression of alternative nonpilus adhesins. In contrast, rpoN mutation has been used as a model for the lack of nonpilus adhesins [28-30]. We speculate that the absence of Tfp causes RpoN to magnify the production of nonpilus adhesins, which serve as ligands for SP-A. This argument is supported by our analyses showing that ΔrpoN is even more resistant to SP-A-mediated phagocytosis than PAO1. Several studies have tried to determine the identity of these nonpilus adhesins, with most of them focusing on those that bind mucins [36-39]. Other studies focus on the role of nonpilus adhesins in nonopsonic phagocytosis [28,29,39]. However, the identity of these adhesins remains elusive. Another complication is the uncertainty whether the alternative adhesins associated with binding to mucin are the same as those conferring susceptibility to SP-A-mediated phagocytosis. By using ligand blot, we have identified a putative non-pilus adhesin that binds SP-A. LC-MS/MS and micro-sequenicng analyses show that the SP-A ligand is the 18 kDa outer membrane protein H1 precursor OprH. OprH expression is governed by low magnesium environment through the PhoP-PhoQ two component regulatory system (40,41). Interestingly, OprH is noted to have direct interaction with LPS, which is bound by the CRD domain of SP-A (40). Another outer membrane protein, P2 of Hemophilus influenzae, has been shown to be a receptor of SP-A (42). We are currently constructing nonpolar deletion mutant and study the genetic, molecular and cellular interactions of OprH with Tfp, RpoN, and SP-A. Furthermore, we are examining the sequence and functional homology between OprH and H. influenzae P2.
Tfp interacts with glycoconjugates (e.g., asialoGM1) on the host epithelium [4]. Similarly, SP-A also interacts with glycoconjugates through its CRD domain. Thus, another possible mechanism of preferential clearance of ΔpilA mutant from mouse lungs is that SP-A may have blocked Tfp receptors, and preventing the binding of PA to the airway epithelium of wild-type mice. In contrast, in SP-A−/− mice, these Tfp receptors will be exposed, allowing better binding and colonization of PA to the lungs devoid of SP-A. Future studies will include performing comparative in vivo binding of wild-type versus various Tfp mutants to the lung epithelium of SP-A+/+ versus SP-A−/− mice.
Our experimental evidence demonstrates that Tfp also mediates resistance to SP-A-mediated membrane permeabilization. Following exposure to hSP-A and to the BALF from SP-A+/+ mice, the integrity of the outer membranes is compromised, rendering the ΔpilA bacteria more permeable to the phosphotase substrate ELF97 into the cells and increased leakage of thiol containing intracellular proteins out of the cells. Previously, we have shown the the wild-type strain PAO1 is highly resistant to direct killing by SP-A. Although ΔpilA is more susceptible to SP-A-mediated membrane permeabilization, this only results in small increase in direct killing of the bacteria, suggesting that the pore forming process may be transient. Qualitative LPS analysis and TEM examination of membrane structures did not reveal any gross alterations in ΔpilA. However, ΔpilA bacteria have reduced membrane stability as demonstrated by a modest increase of cell lysis by 0.25% SDS.
In conclusion, we have shown that Tfp is important for resistance to SP-A-mediated opsonization and membrane permeabilization. We provide evidence that Tfp may camouflage nonpilus adhesin, and prevent the binding of SP-A to these ligands, reducing the opsonization and phagocytosis by macrophages. In addition, Tfp is necessary to stabilize bacterial membranes, rendering PA more resistant to SP-A-mediated membrane permeabilization. Adjunctive treatment regimen aimed at inhibiting Tfp may help to improve the clearance of PA by augmenting the efficiency of SP-A in killing this important respiratory pathogen.
Acknowledgments
We thank Ms. Jennifer Ida for critical reading of the manuscript. We thank Professors Jeff Whitsett and Tom Korfhagen (Cincinnati Children Hospital) for the gift of the Swiss Black SP-A−/− mice, and Professor Frank McCormack (University of Cincinnati College of Medicine) for the C3H SP-A−/− mice and alveolar proteinosis lavage. We thank Professor Joanne Engel for providing PA mutants. This work is supported in part by NIH grant HL090699 and an American Lung Association DeSouza Research Award to GWL. This investigation was conducted in a facility constructed with the support from Research Facilities Improvement Program Grant Number C06 RR 16515-01 from the National Center for Research Resources, National Institutes of Health.
References
- 1.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: A review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 3.Bielecki P, Glik J, Kawecki M, Martins dos Santos VA. Towards understanding Pseudomonas aeruginosa burn wound infections by profiling gene expression. Biotechnol Lett. 2008;30:777–790. doi: 10.1007/s10529-007-9620-2. [DOI] [PubMed] [Google Scholar]
- 4.Lau GW, Hassett DJ, Britigan BE. Modulation of lung epithelial functions by Pseudomonas aeruginosa. Trends Microbiol. 2005;13:389–397. doi: 10.1016/j.tim.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Breidenstein EB, de la Fuente-Nunez C, Hancock RE. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comolli JC, Hauser AR, Waite L, Whitchurch CB, Mattick JS, Engel JN. Pseudomonas aeruginosa gene products pilt and pilu are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect Immun. 1999;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig L, Pique ME, Tainer JA. Type IV pilus structure and bacterial pathogenicity. Nat Rev Microbiol. 2004;2:363–378. doi: 10.1038/nrmicro885. [DOI] [PubMed] [Google Scholar]
- 9.Hansen JK, Forest KT. Type IV pilin structures: Insights on shared architecture, fiber assembly, receptor binding and type ii secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand JJ, West JT, Engel JN. Genetic analysis of the regulation of type IV pilus function by the Chp chemosensory system of Pseudomonas aeruginosa. J Bacteriol. 2010;192:994–1010. doi: 10.1128/JB.01390-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comolli JC, Waite LL, Mostov KE, Engel JN. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect Immun. 1999;67:3207–3214. doi: 10.1128/iai.67.7.3207-3214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bucior I, Mostov K, Engel JN. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect Immun. 2010;78:939–953. doi: 10.1128/IAI.01215-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucior I, Pielage JF, Engel JN. Pseudomonas aeruginosa pili and flagella mediate distinct binding and signaling events at the apical and basolateral surface of airway epithelium. PLoS Pathog. 2012;8:e1002616. doi: 10.1371/journal.ppat.1002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: Structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol. 2007;9:1871–1879. doi: 10.1111/j.1462-5822.2007.00953.x. [DOI] [PubMed] [Google Scholar]
- 16.Sever-Chroneos Z, Krupa A, Davis J, Hasan M, Yang CH, Szeliga J, Herrmann M, Hussain M, Geisbrecht BV, Kobzik L, Chroneos ZC. Surfactant protein a (SP-A)-mediated clearance of Staphylococcus aureus involves binding of sp-a to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class a. J Biol Chem. 2011;286:4854–4870. doi: 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of gram-negative bacteria by increasing membrane permeability. The J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Chen Y, Potvin E, Sanschagrin F, Levesque RC, McCormack FX, Lau GW. Comparative signature-tagged mutagenesis identifies Pseudomonas factors conferring resistance to the pulmonary collectin SP-A. PLoS Pathog. 2005;1:259–268. doi: 10.1371/journal.ppat.0010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, McCormack FX, Levesque RC, O’Toole GA, Lau GW. The flagellum of Pseudomonas aeruginosa is required for resistance to clearance by surfactant protein a. PLoS One. 2007;2:e564. doi: 10.1371/journal.pone.0000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang Z, Hao Y, Hwang S, Zhang S, Kim E, Akinbi HT, Schurr MJ, Irvin RT, Hassett DJ, Lau GW. The Pseudomonas aeruginosa flagellum confers resistance to pulmonary surfactant protein-A by impacting the production of exoproteases through quorum-sensing. Mol Microbiol. 2011;79:1220–1235. doi: 10.1111/j.1365-2958.2010.07516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuang Z, Hao Y, Walling BE, Jeffries JL, Ohman DE, Lau GW. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One. 2011;6:e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Iwaarden F, Welmers B, Verhoef J, Haagsman HP, van Golde LM. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- 23.Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 24.Giannoni E, Sawa T, Allen L, Wiener-Kronish J, Hawgood S. Surfactant proteins A and D enhance pulmonary clearance of Pseudomonas aeruginosa. Am J Respir Cell Mol Biol. 2006;34:704–710. doi: 10.1165/rcmb.2005-0461OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA. Neutrophil extracellular trap (net)-mediated killing of Pseudomonas aeruginosa: Evidence of acquired resistance within the cf airway, independent of cftr. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suwabe A, Mason RJ, Voelker DR. Calcium dependent association of surfactant protein A with pulmonary surfactant: Application to simple surfactant protein a purification. Arch Biochem Biophys. 1996;327:285–291. doi: 10.1006/abbi.1996.0123. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz E, Chemotti DC, Vandal K, Tessier PA. Toll-like receptor 2 represses nonpilus adhesin-induced signaling in acute infections with the Pseudomonas aeruginosa pilA mutant. Infect Immun. 2004;72:4561–4569. doi: 10.1128/IAI.72.8.4561-4569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mork T, Hancock RE. Mechanisms of nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1993;61:3287–3293. doi: 10.1128/iai.61.8.3287-3293.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahenthiralingam E, Speert DP. Nonopsonic phagocytosis of Pseudomonas aeruginosa by macrophages and polymorphonuclear leukocytes requires the presence of the bacterial flagellum. Infect Immun. 1995;63:4519–4523. doi: 10.1128/iai.63.11.4519-4523.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramphal R, Koo L, Ishimoto KS, Totten PA, Lara JC, Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991;59:1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abeyrathne PD, Daniels C, Poon KK, Matewish MJ, Lam JS. Functional characterization of WaaL, a ligase associated with linking o-antigen polysaccharide to the core of Pseudomonas aeruginosa lipopolysaccharide. J Bacteriol. 2005;187:3002–3012. doi: 10.1128/JB.187.9.3002-3012.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DJ, Cox D, Li J, Greenberg S. Rac1 and cdc42 are required for phagocytosis, but not nf-kappab-dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. The J Biol Chem. 2000;275:141–146. doi: 10.1074/jbc.275.1.141. [DOI] [PubMed] [Google Scholar]
- 33.Kelly NM, Kluftinger JL, Pasloske BL, Paranchych W, Hancock RE. Pseudomonas aeruginosa pili as ligands for nonopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun. 1989;57:3841–3845. doi: 10.1128/iai.57.12.3841-3845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahenthiralingam E, Campbell ME, Speert DP. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Potvin E, Sanschagrin F, Levesque RC. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Rev. 2008;32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 36.Arora SK, Ritchings BW, Almira EC, Lory S, Ramphal R. Cloning and characterization of Pseudomonas aeruginosa FliF, necessary for flagellar assembly and bacterial adherence to mucin. Infect Immun. 1996;64:2130–2136. doi: 10.1128/iai.64.6.2130-2136.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy MS. Binding between Pseudomonas aeruginosa adhesins and human salivary, tracheobronchial and nasopharyngeal mucins. Biochem Mol Biol Int. 1996;40:403–408. doi: 10.1080/15216549600201902. [DOI] [PubMed] [Google Scholar]
- 38.Plotkowski MC, Tournier JM, Puchelle E. Pseudomonas aeruginosa strains possess specific adhesins for laminin. Infect Immun. 1996;64:600–605. doi: 10.1128/iai.64.2.600-605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carnoy C, Scharfman A, Van Brussel E, Lamblin G, Ramphal R, Roussel P. Pseudomonas aeruginosa outer membrane adhesins for human respiratory mucus glycoproteins. Infect Immun. 1994;62:1896–1900. doi: 10.1128/iai.62.5.1896-1900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edrington TC, Kintz E, Goldberg JB, Tamm LK. Structural basis for the interaction of lipopolysaccharide with outer membrane protein h (OprH) from Pseudomonas aeruginosa. J Biol Chem. 2011;286:39211–39223. doi: 10.1074/jbc.M111.280933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macfarlane ELA, Kwasnicka A, Ochs MM, Hancock REW. PhoP±PhoQ homologues in Pseudomonas aeruginosa regulate expression of the outer-membrane protein OprH and polymyxin B resistance. Mol Microbiol. 1999;34:305–316. doi: 10.1046/j.1365-2958.1999.01600.x. [DOI] [PubMed] [Google Scholar]
- 42.McNeely TB, Coonrod JD. Aggregation and opsonization of Type A but not Type B Hemophilus influenzae by surfactant protein A. Am J Respir Cell Mol Biol. 1994;11:114–122. doi: 10.1165/ajrcmb.11.1.8018334. [DOI] [PubMed] [Google Scholar]