Abstract
Direct comparison and ranking of vaccine formulations in pre-clinical studies will expedite the identification of cancer vaccines for clinical trials. Two human ErbB-2 (Her-2) vaccines, naked DNA and whole cell vaccine, were tested side-by-side in wild type and Her-2 transgenic mice. Both vaccines can induce humoral and cellular immunity to the entire repertoire of Her-2 epitopes. Mice were electro-vaccinated i.m. with a mixture of pGM-CSF and pE2TM, the latter encodes Her-2 extracellular and transmembrane domains. Alternatively, mice were injected i.p. with human ovarian cancer SKOV3 cells that have amplified Her-2. In wild type mice, comparable levels of Her-2 antibodies (Ab) were induced by these two vaccines. However, T cell immunity and protection against Her-2+ tumors were superior in DNA vaccinated mice. In BALB Her-2 transgenic (Tg) mice, which were tolerant to Her-2, DNA and cell vaccines were administered after regulatory T cells (Treg) were removed by anti-CD25 mAb. Again, comparable levels of Her-2 Ab were induced, but DNA vaccines rendered greater anti-tumor activity. In B6xDR3 Her-2 Tg mice that expressed the autoimmune prone HLA-DR3 allele, higher levels of Her-2 Ab were induced by SKOV3 cell than by Her-2 DNA. But anti-tumor activity was still more profound in DNA vaccinated mice. Therefore, Her-2 DNA vaccine induced greater anti-tumor immunity than cell vaccine, whether mice were tolerant to Her-2 or susceptible to autoimmunity. Through such side-by-side comparisons in appropriate pre-clinical test systems, the more effective vaccine formulations will emerge as candidates for clinical trials.
Keywords: Her-2, Neu, DNA vaccine, Cancer vaccine
Introduction
Vaccination against molecules critical to tumor cell survival is an attractive approach in cancer management. Of the candidate molecules, ErbB-2/Her-2/neu is the first and most convincing target with the demonstrated efficacy of Trastuzumab (Herceptin), a humanized murine mAb (4D5) in breast cancer patients [24]. ErbB-2/Her-2/neu, a member of the ErbB receptor tyrosine kinase family, is weakly to moderately expressed in normal adult tissues. Dysregulated signal transduction from overexpressed or mutated Her-2 leads to cellular immortalization, neoplastic transformation and tumor progression [8]. Her-2 is overexpressed in 20–30% of human breast cancers and is correlated with more aggressive disease and reduced survival [11, 26–28]. Trastuzumab, which binds to Her-2, shows clear activity in metastatic Her-2+ breast cancer, or in the adjuvant setting for patients with less advanced disease [24], supporting the role of Ab in controlling tumor cells with Her-2/neu addiction [32]. Mechanisms of trastuzumab activity may include down-modulation of Her-2, disruption of downstream signaling [16, 35], or induction of Ab dependent cell mediated cytotoxicity (ADCC). In addition, immune priming to Her-2 or other tumor-associated antigens may be initiated via trastuzumab-mediated tumor cell destruction. However, eventual tumor cell evasion under trastuzumab treatment suggests escape from Her-2/neu addiction and warrants further investigation of Her-2 targeted immunotherapy.
Her-2 vaccination can induce both humoral and cellular immunity, thus controlling tumor growth via conventional immunological mechanisms in addition to modulating oncogenic activity of Her-2. In general, cancer vaccines fall under the categories of genetic vaccine, such as naked DNA, viral or bacterial vaccine, and non-genetic vaccines such as peptide, protein or cell vaccines. It is a challenging task to select the appropriate vaccine formulation for clinical trials and requires major investment. Several Her-2 genetic vaccines are under clinical investigation, such as Her-2 DNA developed in our lab [11, 30, 31], Her-2 adenoviral (NCT00197522; http://www.clinicaltrials.gov) or vaccinia viral vaccines (NCT00485277). Of the non-genetic vaccines, Her-2 peptides [4], GM-CSF or CD80-transfected allogeneic cell vaccines [5, 23], and Her-2 transfected dendritic cells [2] have been tested. Pre-clinical ranking of different vaccine formulations will expedite the selection of vaccine candidates for clinical testing.
In this study, we compare the induction Her-2 specific anti-tumor immunity using Her-2 DNA genetic vaccine versus SKOV3 cells, a cell vaccine with amplified Her-2 expression. SKOV3, a Her-2 positive xenogeneic cell line is chosen because it does not express cross-reactive antigens with the mouse tumors, so that comparison of Her-2 specific anti-tumor immunity between these two formulations is valid. As xenogenic cells, the foreign antigens on SKOV3 cells may also serve as an adjuvant to amplify immune response to Her-2. Both formulations induce humoral and cellular immunity to the complete repertoire of Her-2 epitopes and can be administered repeatedly. Both formulations have demonstrated efficacy in pre-clinical studies [9, 17, 31, 34]. Direct comparison as described will provide a rational basis for ranking cancer vaccine formulations.
Materials and methods
Mice
All animal procedures were conducted in accordance with accredited institution guidelines and the US Public Health Service Policy on Humane Care and Use of Laboratory Animals (http://grants.nih.gov/grants/olaw/olaw.htm#pol).
C57BL/6 and BALB/c female mice were purchased from Charles River Laboratory (Frederick, MD). Heterozygous C57BL/6 Her-2 Tg mice (B6 Her-2 Tg), which expressed the full-length, wild type human ErbB-2 (Her-2) under the whey acidic protein (WAP) promoter were generated in our laboratory and have been maintained by breeding with normal B6 mice [19]. Dr. Michael Kershaw (Peter MacCallum Cancer Center, East Melbourne, Australia) back-crossed B6 Her-2 Tg mice into BALB/c background for nine generations, and those mice were further back-crossed to >13 generations in our animal facility to establish syngeneic, heterozygous BALB/c Her-2 Tg (BALB Her-2 Tg) mice.
HLA-DR3 mice provided by Dr. Chella David (Mayo Clinic, Rochester, MN) were generated by introduction of the HLA-DRA/DRB1*0301 transgene into class II-negative Ab0 mice and backcrossed to B10 mice, resulting in DR3 transgenic Ab0 B10 mice [10, 13, 29]. B6xDR3 Her-2 Tg F1 (B6xDR3 Her-2 Tg) mice were produced by mating HLA-DR3 males with B6 Her-2 Tg females. Expression of DR3 was verified by staining peripheral blood leukocytes (PBL) with mouse mAb L243 to human HLA-DR (BD Biosciences, San Jose, CA).
Cell lines and reagents
All tissue culture reagents were purchased from Invitrogen (Carlsbad, CA) unless otherwise specified. Cell lines were maintained in vitro in DMEM supplemented with 5% cosmic calf serum (HyClone, Logan, UT), 5% fetal bovine serum (Hyclone, Logan, UT), 2 mM L-glutamine, 0.1 mM MEM non-essential amino acids, 100 units/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, 0.5 mM sodium pyruvate, and 4 μg/ml insulin (Sigma). The murine cell line D2F2 was established from a mammary tumor that arose in a prolactin-induced BALB/c hyperplastic alveolar nodule line, D2 [15]. D2F2 cells were co-transfected with pRSV/neo and pCMV/neu encoding wild type rat neu to generate D2F2/neu. D2F2/E2 cells were generated by co-transfection with pRSV/neo and pCMV/E2 encoding human ErbB-2 (Her-2) [9, 31]. EL4/E2 cells were generated by co-transfecting EL4, a C57BL/6 thymoma, with pRSV/neo and pCMV/E2 [21]. Stable clones were selected, and maintained in supplemented DMEM containing 0.8 mg/ml of G418 (Geneticin, Invitrogen). EO771/E2 was generously provided by Dr. Daniel Allendorf (James Graham Brown Cancer Center, Louisville, Kentucky) and was generated by transfecting C57BL/6 mammary tumor EO771 with full-length Her-2. EO771/E2 cells were maintained in RPMI 1640 supplemented with 20% fetal bovine serum, L-glutamine, non-essential amino acids, penicillin, streptomycin, HEPES and 0.1 mg/ml of G418. The murine tumor line TUBO was cloned from a spontaneous mammary tumor in a BALB NeuT female transgenic expressing a transforming rat neu under the MMTV promoter [25]. TUBO cells grow progressively in wild type BALB/c mice and give rise to tumors which are histologically similar to autochthonous tumors seen in BALB NeuT females [25].
Antigen presenting cells (APC) 3T3/KB and 3T3/NKB were generated as previously described [9]. Briefly, BALB/c NIH 3T3 fibroblasts were co-transfected with Kd, B7.1 (3T3/KB) and neu (3T3/NKB) or Her-2 (3T3/EKB) and stable clones were selected. 3T3/KB cells were maintained in supplemented DMEM medium containing 0.6 mg/ml G418 and 7.5 μg/ml puromycin. 3T3/EKB and 3T3/NKB cells were maintained in supplemented DMEM medium with 0.6 mg/ml G418 and 0.6 mg/ml of Zeocin. TC-1/E2 cells were generated by transfecting C57BL/6 TC-1 cells (generously provided by Dr. T. C. Wu, The Johns Hopkins University, Baltimore, MD) with pMSCV/puro and pCMV5/E2. TC-1 cells were derived by transforming lung epithelial cells with human papilloma virus-16 E6, E7 and ras oncogene [14] and the cells expressed endogenous Kb and CD80 (B7.1) [9]. Stable TC-1/E2 clones were selected, and maintained in supplemented DMEM containing 7.5 μg/ml puromycin.
SKOV3 (ATCC) is a human ovarian cancer cell line with amplified Her-2 [12]. The cells were maintained in DMEM supplemented with 10% cosmic calf serum, 2 mM L-glutamine, 0.1 mM sodium pyruvate, 100 units/ml penicillin and 100 μg/ml streptomycin.
Immunization
pCMV/E2TM encoding the extracellular and transmembrane domains of Her-2 was previously described [9, 10]. pEFBos/GM-CSF (pGM-CSF) encoding murine GM-CSF was provided by Dr. N. Nishisaki at Osaka University, Osaka, Japan. Mice received DNA electro-vaccination as we previously described [9]. Briefly, 50 μg of each plasmid DNA in a total volume of 50 μl was injected into the quadriceps muscle and followed immediately by square wave electroporation over the injection site using a BTX830 (BTX Harvard Apparatus, Holliston, MA). A tweezer electrode was used to deliver 8 pulses at 100 V for 25 ms per pulse. Wild type mice were immunized twice and transgenic mice four times, every 2 weeks.
Confluent SKOV3 cells were detached from monolayer cultures with trypsin and washed three times with serum free DMEM. Mice were vaccinated by injecting i.p. 2 × 106 live cells, every 2 weeks, two or four times as in DNA vaccination.
T cell depletion
The hybridoma lines PC61 and 2.43 (ATCC, Manassas, VA), which produce rat mAb to mouse CD25 and mouse CD8, respectively, were propagated in SCID mice. To deplete CD25hi regulatory T cells (Tregs), mice were injected once i.p. with 0.5 mg of PC61 mAb. To deplete CD8+ T cells, mice were treated i.p. with 0.5 mg of 2.43 mAb 1 week before tumor cell challenge and then weekly until the completion of the experiment. Depletion of specific T cell subsets in PBL was verified by FACS analysis (data not shown).
Tumor challenge
Mice were inoculated s.c. in the flank with 2 × 105 EL4/E2, EO771/E2, TUBO, D2F2/neu or D2F2/E2 cells. Tumor growth was monitored by weekly palpation, and tumor diameter was measured weekly in two perpendicular dimensions with a caliper. Mice were sacrificed when any one dimension of the tumor reached 20 mm. Difference in tumor incidence was analyzed by the log-rank test.
Measurement of Her-2 or neu Ab by flow cytometry
Her-2 and neu Ab levels were determined as previously described [20]. Briefly, 3T3/EKB or 3T3/NKB cells were incubated with serially diluted mouse sera and PE-conjugated goat anti-mouse IgG Fcγ was the secondary Ab (Jackson ImmunoResearch, West Grove, PA). Anti-Her-2 mouse mAb TA-1 or anti-neu mouse mAb, clone 7.16.4 (EMD Chemicals, Inc, San Diego, CA) were used to generate standard binding cures. The concentrations of Her-2 and neu IgG in the test sera were calculated by regression analysis [20]. To determine the Her-2 IgG isotype, 3T3/EKB cells were incubated with immune sera as the primary Ab, followed by FITC-conjugated goat anti-mouse IgG1, IgG2c, or IgG3 (Jackson ImmunoResearch, West Grove, PA). Flow cytometric analysis was performed with a FACScalibur (Becton Dickinson, Mountain View, CA), and the results were expressed as mean channel fluorescence. Differences in Ab levels were analyzed by Student’s t test.
Measurement of T cell response by IFN-γ ELISPOT assay
Her-2 and neu T cell levels were enumerated by IFN-γ ELI-SPOT assay as previously described [9]. Briefly, 96-well HTS IP plates (Millipore, Bedford, MA), were pre-coated with 2.5 μg/ml rat anti-mouse IFN-γ (clone R4-6A2, BD Biosciences, San Jose, CA). Immune spleen cells (SC) or peripheral blood lymphocytes (PBL) were added to the wells in the presence of TC-1/E2, TC-1/Neu, 3T3/EKB or 3T3/NKB at a 1:10 APC:lymphocyte ratio. Control wells had medium, TC-1 or 3T3/KB cells. Following 48 h incubation, cells were removed, and captured IFN-γ was detected with biotinylated rat anti-mouse IFN-γ (clone XMG1.2, BD Biosciences), avidin-HRP and the substrate 3-amino-9-ethylcarbazole. The visualized cytokine spots were enumerated with the ImmunoSpot analyzer (CTL, Cleveland, OH), and the results were expressed as the number of cytokine-producing cells per 106 PBL or SC. Data were analyzed using the Student’s t test.
Results
Induction of anti-Her-2 immunity in wild type mice
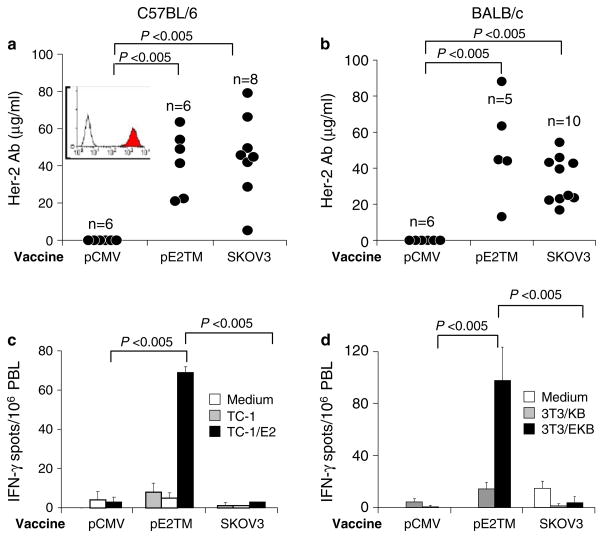
To compare the activity of DNA versus cell vaccine, C57BL/6 or BALB/c mice were immunized twice, 2 weeks apart, by i.m. electro-vaccination with pE2TM and pGM-CSF or by i.p. injection with 2 × 106 SKOV3 cells that expressed amplified Her-2. Expression of Her-2 on SKOV3 cells is shown in Fig. 1a, inset. The control group received blank pCMV vector. At 2 weeks after the second vaccination, sera were collected and Her-2 specific Ab levels were measured by the binding to Her-2 transfected 3T3/EKB cells, using flow cytometry and regression analysis, as we previously described [20]. In C57BL/6 mice, 45 ± 22 and 42 ± 17 μg/ml of Her-2 Ab were induced by DNA and cell vaccine, respectively (Fig. 1a). In BALB/c mice, an average of 51 ± 28 and 34 ± 12 μg/ml Her-2 Ab were induced by DNA and cell vaccine, respectively (Fig. 1b). Therefore, comparable levels of Her-2 Ab were induced by DNA and cell vaccine in the two mice strains.
Fig. 1.
Induction of anti-Her-2 immunity by DNA versus cell vaccine in wild type mice. C57BL/6 (a, c) or BALB/c (b, d) mice were immunized twice, 2 weeks apart, by i.m. electro-vaccination with pE2TM and pGM-CSF or by i.p. injection with 2 × 106 SKOV3 cells. Control mice received blank vector pCMV. The inset shows Her-2 expression on SKOV3 cells measured by anti-Her-2 mAb TA-1 (shaded histogram). Normal mouse IgG control is shown in open histogram. a, b Anti-Her-2 IgG levels in individual mice were measured by flow cytometry 2 weeks after second vaccination as described in “Materials and methods”. c, d PBL were collected 2 weeks after second vaccination and cells from each group were pooled. Her-2-specific T cells were enumerated by IFN-γ ELISPOT essay as described in “Materials and methods”
Her-2 reactive T cells in immunized mouse PBL were measured by IFN-γ ELISPOT assay after in vitro stimulation with the engineered, Her-2+ antigen presenting cells. PBL were pooled from all mice in each test group. We showed previously that this assay system measured the activity of both CD4 and CD8 T cells [10]. In C57BL/6 and BALB/c mice, pE2TM induced 69 ± 3 and 98 ± 39/106 PBL IFN-γ secreting Her-2-reactive T cells, respectively (Fig. 1c, d). SKOV3 immunized mice produced no detectable Her-2 T cell response in either strain (Fig. 1c, d). Therefore, in normal mice, two time vaccination with Her-2 DNA or cell vaccine induced significantly different levels of Her-2 T cells but comparable levels of Her-2 Ab.
Induction of anti-tumor immunity in wild type mice
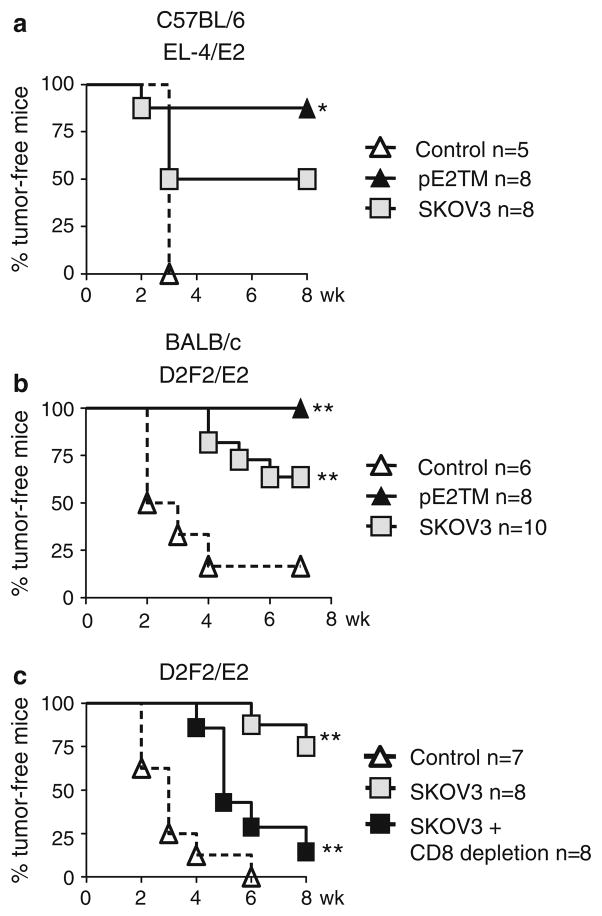
To measure anti-tumor activity, immunized C57BL/6 mice were challenged with Her-2 transfected EL4/E2 cells after two vaccinations, 88% (7/8) of pE2TM immunized and 50% (4/8) of SKOV3 immunized mice rejected the tumor (Fig. 2a). Tumor growth inhibition by pE2TM vaccination was significant when compared to mice in the control group. EL4/E2 tumor growth was also reduced in SKOV3 immunized mice, but the difference did not reach statistical significance. In BALB/c mice, pE2TM vaccination provided 100% (8/8) protection and SKOV3 vaccination provided 60% (6/8) protection from D2F2/E2 tumor (Fig. 2b), both were significantly different than that in control group and complete protection was achieved only by DNA vaccine.
Fig. 2.
Protection against tumor growth by DNA versus cell vaccines in wild type mice. C57BL/6 or BALB/c mice were immunized with pE2TM and pGM-CSF or with SKOV3 cells twice, as described in Fig. 1 and mice were challenged s.c. with 2 × 105 Her-2-transfected tumor cells at 2 weeks after the last vaccination. Tumor growth is expressed as % tumor-free mice. The number of mice per group is indicated on the graphs. a Immunized C57BL/6 mice were challenged with EL4/E2 tumors, b Immunized BALB/c mice were challenged with D2F2/E2 tumors. c SKOV3 immunized BALB/c mice were challenged with D2F2/E2 tumors. Some of the immunized mice were depleted of CD8+ T cells using mAb 2.43. * P < 0.05, ** P < 0.005 compared to vector–control mice by log-rank test
We previously described the critical role of T cells induced by DNA vaccination in the rejection of Her-2 transfected tumors [22]. It was of interest to determine if anti-Her-2 CD8+ T cells contributed to tumor rejection in SKOV3 immunized mice. A total of 16 BALB/c mice were challenged with D2F2/E2 tumor cells after two time immunizations with SKOV3 cells. SKOV3 vaccine prevented the growth of D2F2/E2 tumor in six of eight (75%) immunized mice (Fig. 2c), consistent with results in Fig. 2b. Eight of the immunized mice received mAb 2.43 at 1 week before tumor inoculation to deplete CD8 T cells and mAb treatment continued weekly until the completion of the experiment. In these CD8+ T cell depleted mice, one mouse rejected the tumor challenge and tumor growth was significantly delayed in the others (P < 0.005). Therefore, in mice immunized with the cell vaccine, CD8+ T cells also contributed to tumor rejection. The delay of tumor growth in SKOV3 immunized, CD8 depleted mice, suggested partial protective effects of Her-2 Ab or other effector cells.
Anti-tumor activity induced by vaccination with heterologous DNA or cell vaccine
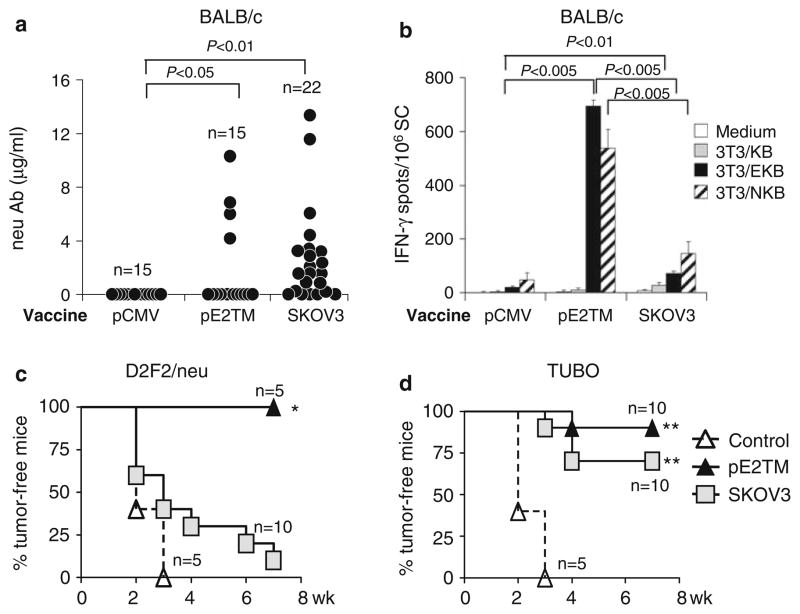
We previously showed that human Her-2 or rat neu DNA immunization resulted in cross-reactive T cells that recognized both cognate and heterologous antigens, although the immune sera only bound cognate antigen. Induction of such cross-reactive T cells may overcome the tolerance to tumor-associated self-antigens and heterologous antigen is often used to trigger experimental autoimmunity [1, 18]. Immune cross-reactivity between rat neu and human Her-2 was tested. BALB/c mice were immunized twice with pE2TM and pGM-CSF or SKOV3 cells. Low levels of cross-reactive Ab to rat neu were detected in 4/15 (27%) DNA-immunized mice and in 15/22 (68%) SKOV3 immunized mice (Fig. 3a). Therefore, Her-2 Ab reactive to neu was induced more frequently by SKOV3 than by DNA vaccine, but the level of binding activity was very low.
Fig. 3.
Cross-protection against neu-expressing tumors by pE2TM or SKOV3 vaccination. BALB/c mice were immunized with pE2TM or SKOV3 as described. a Anti-neu IgG levels in individual mice were measured 2 weeks after second vaccination by flow cytometry. b Immune splenocytes were collected at the same time. Neu-reactive T cells were enumerated by IFN-γ ELISPOT essay after in vitro stimulation with 3T3/NKB cells. Mice were inoculated s.c. with 2 × 105 D2F2/neu (c) or TUBO (d) tumor cells. There were five to ten mice per group. *P < 0.05, ** P < 0.001 compared to vector-immunized
Cross-reactive T cells were enumerated by IFN-γ ELISPOT assay after in vitro stimulation with 3T3/NKB cells that expressed rat neu, Kd and CD80. Two time vaccinations with pE2TM or SKOV3 cells induced Her-2-specific, IFN-γ secreting T cells at the frequency of 694 ± 23 and 71 ± 9/106 splenocytes, respectively (Fig. 3b, solid bars). Her-2 immune T cells that recognized heterologous neu were found in pE2TM and SKOV3 immune mouse spleens at the frequency of 538 ± 70 and 147 ± 42/106 splenocytes, respectively (Fig. 3b, hatched bars). Control 3T3/KB cells, which expressed Kd and CD80, did not activate T cells. Therefore, DNA vaccination induced stronger T cell response with corresponding recognition of heterologous neu.
Cross-protection against neu-expressing tumors was tested by s.c. inoculation of TUBO or D2F2/neu cells. TUBO was derived from a spontaneous mammary tumor in a BALB NeuT mouse expressing a transforming neu [25]. D2F2/neu are D2F2 cells transfected with rat neu, but without neu addiction [9]. Immunization with pE2TM protected 5/5 (100%) mice from D2F2/neu and 9/10 (90%) mice from TUBO tumor (Fig. 3c, d, respectively). Immunization with SKOV3 protected 1/10 (10%) mice from D2F2/neu and 7/10 (70%) mice from TUBO tumor. We have shown previously that rejection of D2F2/neu is critically dependent on T cells, whereas Ab and T cells are both effective against TUBO [33]. These results indicate that the modest level of neu-reactive T cells induced by SKOV3 cell vaccine were insufficient to protect mice from D2F2/neu. Rejection of TUBO cells in SKOV3 cell vaccinated mice may be due to combined activity of neu-reactive Ab and modest T cell response.
Induction of anti-Her-2 immunity in Her-2 Tg mice
To test vaccine efficacy in mice which expressed Her-2, DNA and cell vaccines were administered to Her-2 Tg mice that expressed wild type human c-ErbB-2 under the whey acidic protein promoter [19]. Her-2 Tg mice were bred into C57BL/6 or BALB/c background by >13 generations of back-crosses. Although these mice do not develop spontaneous tumors, they exhibit significant tolerance to Her-2, which is expressed as a self antigen in the cerebellum and lactating mammary gland.
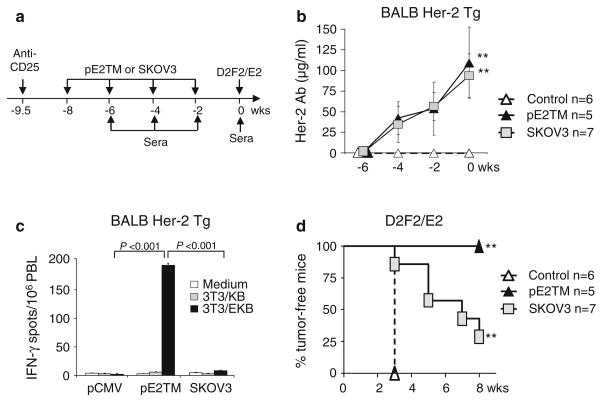
We reported previously that Her-2 Ab response was amplified by depletion of Treg and by repeated vaccination [10]. To induce Her-2 response in BALB Her-2 Tg, CD4+CD25hi Tregs were depleted by i.p. injection with 0.5 mg of CD25 mAb, PC61 (Fig. 4a). pE2TM and SKOV3 induced comparable levels of Her-2 IgG after each vaccination (Fig. 4b). After the fourth vaccination, 109 ± 43 and 94 ± 26 μg/ml Her-2 Ab were detected in DNA and SKOV3 immunized mice (Fig. 4b). It is noted that two time vaccination of wild type mice without Treg depletion (Fig. 1) induced comparable levels of Her-2 Ab as three time vaccination of Treg depleted Her-2 transgenic mice (Fig. 4), showing immune tolerance in Her-2 Tg.
Fig. 4.
Induction of anti-Her-2 immunity by DNA versus cell vaccines in BALB Her-2 Tg mice. a BALB Her-2 Tg mice were injected i.p. with 0.5 mg anti-CD25 mAb, PC61, 10 days before mice were immunized by i.m. electro-vaccination with blank vector, pE2TM and pGM-CSF or by i.p. injection with 2 × 106 SKOV3 cells. The vaccination was repeated three times every 2 weeks. b Her-2 Ab was measured by flow cytometry at 2 weeks after each vaccination. c PBL were collected 2 weeks after the last vaccination and cells from each group were pooled. Her-2-specific T cells were enumerated by IFN-γ ELISPOT essay as described in “Materials and methods”. d At 2 weeks after the last immunization, mice were challenged s.c. with 2 × 105 D2F2/E2 tumor cells. ** P < 0.005 compared to vector-immunized group
T cell response to Her-2 was measured 2 weeks after the fourth vaccination by incubating pooled PBL from immunized mice with 3T3/EKB cells. 3T3/KB cells were used as controls. pE2TM and SKOV3 vaccinated mice generated 193 ± 4 and 8 ± 1 IFN-γ secreting cells per million PBL, respectively (Fig. 4c), showing significantly greater T cell activation by DNA vaccination. SKOV3 cells transfected with GM-CSF showed comparable vaccine activity as untreated SKOV3 cells in mice (not shown).
Immunized mice were challenged with D2F2/E2 cells, 2 weeks after the last immunization (Fig. 4d). All mice immunized with pE2TM rejected D2F2/E2 tumor, but only 29% (2/7) of SKOV3 immunized mice rejected tumor. Therefore, DNA vaccine provided significantly greater protection than cell vaccine in BALB Her-2 Tg mice, although comparable levels of Her-2 Ab were induced.
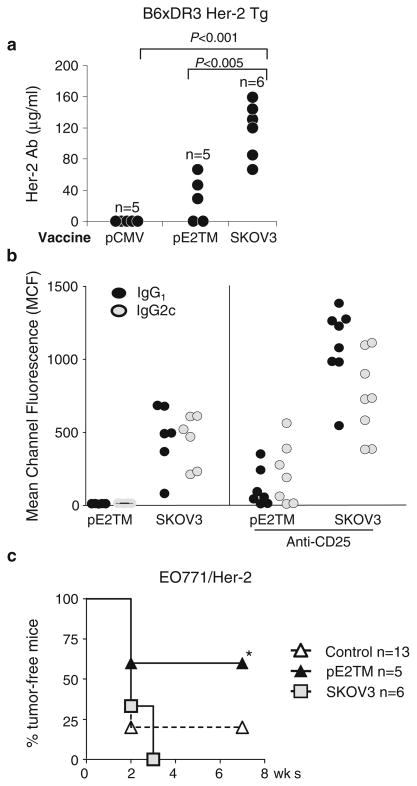
Humans expressing HLA-DR3 and mice expressing transgenic HLA-DR3 are prone to developing autoimmune diseases and may have a more robust immune response to self-Her-2 [3, 6, 7]. To test the impact of HLA-DR3, B6 Her-2 Tg mice were mated with HLA-DR3 transgenic mice to generate B6xDR3 Her-2 Tg mice. Tregs were depleted with CD25 mAb 10 days before the first vaccination and mice were vaccinated four times at 2-week intervals with pE2TM or SKOV3 cells. Immunization with SKOV3 cells induced 117 ± 35 μg/ml of Her-2 Ab, but pE2TM only induced 35 ± 28 μg/ml (Fig. 5a). Both vaccines induced IgG1 and IgG2c, indicating the activation of both Th1 and Th2 CD4+ T cells (Fig. 5b).
Fig. 5.
Induction of anti-Her-2 immunity by DNA versus cell vaccines in B6xDR3 Her-2 Tg mice. B6xDR3 Her-2 Tg mice were depleted of CD4+CD25hi Tregs and immunized four times, as in Fig. 4. a Her-2 Ab levels were measured at 2 weeks after the last vaccination. b IgG1 and IgG2c Her-2 Ab induced by DNA and cell vaccines were measured by flow cytometry as described in “Materials and methods”. c At 2 weeks after the last vaccination, mice were inoculated s.c. with 2 × 105 EO771/E2 tumor cells. * P < 0.05 compared to vector or SKOV3 immunized groups
Anti-tumor activity was assessed by challenging mice with EO771/E2 (Fig. 5c), a C57BL/6 mouse mammary tumor transfected with Her-2. DNA vaccination protected 3/5 (60%) mice (P < 0.05), but SKOV3 vaccination showed no protective effect. Therefore, in B6xDR3 Her-2 Tg mice that express HLA-DR3 with high propensity to respond to self-antigens, SKOV3 cells induced higher levels of Her-2 Ab. Although T cell response was below detection by ELI-SPOT assay (data not shown), greater protection against tumor growth induced by DNA vaccine may suggest low level T cell response. The role of innate immunity such as NK or NKT cells in tumor rejection, however, cannot be ruled out.
Discussion
pE2TM, a plasmid DNA encoding Her-2 extracellular and transmembrane domains, and SKOV3, a human ovarian cancer cell line overexpressing Her-2, induced comparable levels of anti-Her-2 Abs in wild type and BALB Her-2 Tg mice. We have previously shown that DR3 expression did not alter reactivity to Her-2 DNA vaccines although reactivity to mouse thyroglobulin was strikingly more pronounced [10], demonstrating independent genetic regulation of immune reactivity to Her-2 versus other self-antigens. These results were verified by the current findings that vaccination efficacy did not improve in mice that expressed DR3. Importantly, DNA vaccination invariably induced greater protection against Her-2 transfected mouse mammary tumors which are not addicted to neu, but can be recognized by anti-neu Ab and T cells. These results are consistent with our previous findings that rejection of Her-2 transfected tumors by DNA vaccination was mediated primarily by T cells, with lesser contribution by Abs [9, 30]. The reduction of anti-tumor activity after CD8 T cell depletion further supported CTL activity in D2F2/E2 tumor rejection.
In SKOV3 cells, amplified Her-2 was presented among xenogeneic antigens which may amplify Her-2 immune response, but immune reactivity to human antigens was not likely to interact with mouse antigens. Therefore, rejection of Her-2 positive mouse tumors by SKOV3 immunization was primarily a result of Her-2 immunity, although the contribution of innate immunity could not be ruled out. Still pE2TM induced greater T cell response (Fig. 1c, d) and anti-tumor activity (Fig. 2). Recombinant protein expressed in the muscle by DNA electroporation lasted several weeks to provide continuous immune stimulation (not shown). On the other hand, SKOV3 cells injected into the mice was a short-term, bolus antigen stimulation. Our results would suggest that sustained antigen expression from DNA vaccine resulted in stronger T cell response.
We have compared, side-by-side, the efficacy of Her-2 DNA and xenogeneic cell vaccines in wild type and Her-2 Tg mice. These two vaccines induced comparable levels of Her-2 Ab except in mice expressing HLA-DR3, but DNA was superior to cell vaccine at inducing cellular immunity and protection against tumors.
Acknowledgments
This study was supported by NIH CA76340, CA125680 (WZW), Department of Defense W81XWH-04-1-0546 (WZW) and GM 58905-7 (IMSD). The authors wish to thank David Shim and Andi Cani for their technical assistance. We also thank Serene Lane and Laura Baksic for their expert care of the experimental animals.
Contributor Information
Paula J. Whittington, Department of Immunology and Microbiology, School of Medicine, Wayne State University, Detroit, MI 48201, USA
Olga Radkevich-Brown, Karmanos Cancer Institute, School of Medicine, Wayne State University, 110 E. Warren Ave., Detroit, MI 48201, USA.
Jennifer B. Jacob, Karmanos Cancer Institute, School of Medicine, Wayne State University, 110 E. Warren Ave., Detroit, MI 48201, USA
Richard F. Jones, Karmanos Cancer Institute, School of Medicine, Wayne State University, 110 E. Warren Ave., Detroit, MI 48201, USA
Amy M. Weise, Karmanos Cancer Institute, School of Medicine, Wayne State University, 110 E. Warren Ave., Detroit, MI 48201, USA
Wei-Zen Wei, Email: weiw@karmanos.org, Department of Immunology and Microbiology, School of Medicine, Wayne State University, Detroit, MI 48201, USA. Karmanos Cancer Institute, School of Medicine, Wayne State University, 110 E. Warren Ave., Detroit, MI 48201, USA.
References
- 1.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clin Immunol. 2000;94:75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 2.Czerniecki BJ, Roses RE, Koski GK. Development of vaccines for high-risk ductal carcinoma in situ of the breast. Cancer Res. 2007;67:6531–6534. doi: 10.1158/0008-5472.CAN-07-0878. [DOI] [PubMed] [Google Scholar]
- 3.de Vries RR, Huizinga TW, Toes RE. Redefining the HLA and RA association: to be or not to be anti-CCP positive. J Autoimmun. 2005;25(Suppl):21–25. doi: 10.1016/j.jaut.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5:1289–1297. [PubMed] [Google Scholar]
- 5.Dols A, Smith JW, Meijer SL, et al. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther. 2003;14:1117–1123. doi: 10.1089/104303403322124828. [DOI] [PubMed] [Google Scholar]
- 6.Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3:104–110. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 7.Harley JB, Moser KL, Gaffney PM, et al. The genetics of human systemic lupus erythematosus. Curr Opin Immunol. 1998;10:690–696. doi: 10.1016/s0952-7915(98)80090-3. [DOI] [PubMed] [Google Scholar]
- 8.Hung MC, Lau YK. Basic science of HER-2/neu: a review. Semin Oncol. 1999;26:51–59. [PubMed] [Google Scholar]
- 9.Jacob J, Radkevich O, Forni G, et al. Activity of DNA vaccines encoding self or heterologous Her-2/neu in Her-2 or neu transgenic mice. Cell Immunol. 2006;240:96–106. doi: 10.1016/j.cellimm.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Jacob JB, Kong YM, Meroueh C, et al. Control of Her-2 tumor immunity and thyroid autoimmunity by MHC and regulatory T cells. Cancer Res. 2007;67:7020–7027. doi: 10.1158/0008-5472.CAN-06-4755. [DOI] [PubMed] [Google Scholar]
- 11.Kiessling R, Wei WZ, Herrmann F, et al. Cellular immunity to the Her-2/neu protooncogene. Adv Cancer Res. 2002;85:101–144. doi: 10.1016/s0065-230x(02)85004-7. [DOI] [PubMed] [Google Scholar]
- 12.King BL, Carter D, Foellmer HG, et al. Neu proto-oncogene amplification and expression in ovarian adenocarcinoma cell lines. Am J Pathol. 1992;140:23–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Kong YM, Lomo LC, Motte RW, et al. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1*0301 (DR3) gene. J Exp Med. 1996;184:1167–1172. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 15.Mahoney KH, Miller BE, Heppner GH. FACS quantitation of leucine aminopeptidase and acid phosphatase on tumor-associated macrophages from metastatic and nonmetastatic mouse mammary tumors. J Leukoc Biol. 1985;38:573–585. doi: 10.1002/jlb.38.5.573. [DOI] [PubMed] [Google Scholar]
- 16.Montgomery RB, Makary E, Schiffman K, et al. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- 17.Nanni P, Nicolson GL, De Giovanni C, et al. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194:1195–1205. doi: 10.1084/jem.194.9.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldstone MB. Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Top Microbiol Immunol. 2005;296:1–17. doi: 10.1007/3-540-30791-5_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piechocki MP, Ho YS, Pilon S, et al. Human ErbB-2 (Her-2) transgenic mice: a model system for testing Her-2 based vaccines. J Immunol. 2003;171:5787–5794. doi: 10.4049/jimmunol.171.11.5787. [DOI] [PubMed] [Google Scholar]
- 20.Piechocki MP, Pilon SA, Wei WZ. Quantitative measurement of anti-ErbB-2 antibody by flow cytometry and ELISA. J Immunol Methods. 2002;259:33–42. doi: 10.1016/s0022-1759(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 21.Pilon SA, Kelly C, Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol. 2003;170:1202–1208. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- 22.Pilon SA, Piechocki MP, Wei WZ. Vaccination with cytoplasmic ErbB-2 DNA protects mice from mammary tumor growth without anti-ErbB-2 antibody. J Immunol. 2001;167:3201–3206. doi: 10.4049/jimmunol.167.6.3201. [DOI] [PubMed] [Google Scholar]
- 23.Reilly RT, Gottlieb MB, Ercolini AM, et al. HER-2/neu is a tumor rejection target in tolerized HER-2/neu transgenic mice. Cancer Res. 2000;60:3569–3576. [PubMed] [Google Scholar]
- 24.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 25.Rovero S, Amici A, Carlo ED, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 26.Sjogren S, Inganas M, Lindgren A, et al. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 27.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 28.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 29.Strauss G, Vignali DA, Schonrich G, et al. Negative and positive selection by HLA-DR3(DRw17) molecules in transgenic mice. Immunogenetics. 1994;40:104–108. [PubMed] [Google Scholar]
- 30.Wei WZ, Jacob J, Radkevich-Brown O, et al. The “A, B and C” of Her-2 DNA vaccine development. Cancer Immunol Immun-other. 2008;57(11):1711–1717. doi: 10.1007/s00262-008-0464-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei WZ, Shi WP, Galy A, et al. Protection against mammary tumor growth by vaccination with full-length, modified human ErbB-2 DNA. Int J Cancer. 1999;81:748–754. doi: 10.1002/(sici)1097-0215(19990531)81:5<748::aid-ijc14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Weinstein IB, Joe A, Felsher D. Oncogene addiction. Cancer Res. 2008;68:3077–3080. doi: 10.1158/0008-5472.CAN-07-3293. [DOI] [PubMed] [Google Scholar]
- 33.Whittington PJ, Piechocki MP, Heng HH, et al. DNA vaccination controls Her-2+ tumors that are refractory to targeted therapies. Cancer Res. 2008;68(18):7502–7511. doi: 10.1158/0008-5472.CAN-08-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolpoe ME, Lutz ER, Ercolini AM, et al. HER-2/neu-specific monoclonal antibodies collaborate with HER-2/neu-targeted granulocyte macrophage colony-stimulating factor secreting whole cell vaccination to augment CD8+ T cell effector function and tumor-free survival in Her-2/neu-transgenic mice. J Immunol. 2003;171:2161–2169. doi: 10.4049/jimmunol.171.4.2161. [DOI] [PubMed] [Google Scholar]
- 35.Yakes FM, Chinratanalab W, Ritter CA, et al. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]