Abstract
As death mediating proteases caspases and caspase-3 in particular, have been implicated in neurodegenerative processes, aging and Alzheimer’s disease (AD). However, emerging evidence suggests that in addition to their classical role in cell death caspases have a key role in modulating synaptic function. It is remarkable that active caspases-3 which can trigger widespread damage and degeneration, aggregates in structures as delicate as synapses and persists in neurons without causing acute cell death. Here we evaluate this dichotomy, and discuss the hypothesis that caspase-3 maybe a bifurcation point in cellular signaling, able to orient the neuronal response to stress down either pathological/apoptotic pathways or towards physiological cellular remodeling. We propose that temporal, spatial and other regulators of caspase activity are key determinants of the ultimate effect of caspase-3 activation in neurons. This concept has implications for differential role of caspase-3 activation across the lifespan. Specifically, we propose that limited caspase-3 activation is critical for synaptic function in the healthy adult brain while chronic activation is involved in degenerative processes in the aging brain.
Introduction: Caspases-mediators of cell death or more?
Caspases are a class of proteases instrumental in carrying out many cellular functions including cell differentiation, remodeling and death. Recently there has been an increased focus on evaluating the role of caspases in non-apoptotic processes such as synaptic plasticity, spine atrophy and memory deficits, in addition to their classic role in cell death. Here we address the question of how the physiological state of a cell determines whether caspase activation triggers cell death or modulates plasticity and cognitive functions. We also discuss the different outcomes of caspase activation and their relevance for aging and AD.
The general mechanism of caspase-mediated cell death is highly conserved in different cell types, including neurons from brain structures such as cortex, hippocampus and the cerebellum (1, 2). These cell death mechanisms have been extensively studied and reviewed in the past (3, 4). Caspases are synthesized in cells as inactive zymogens. Upon proteolysis, initiator caspases such as caspase-8, -9, -10, and -2 cleave executioner caspases-3, -6, and -7. These effector or executioner proteases then degrade structural proteins, signaling molecules, and DNA repair enzymes (5–10). Among the executioner caspases, activation of caspase-3 plays an extremely important role in neuronal apoptosis and is considered the terminal event preceding cell death. Caspase-3 is primarily activated by two initiator pathways driven by caspases 9 and 8, also referred to as the intrinsic and extrinsic pathways of cell death respectively (Figure 1).
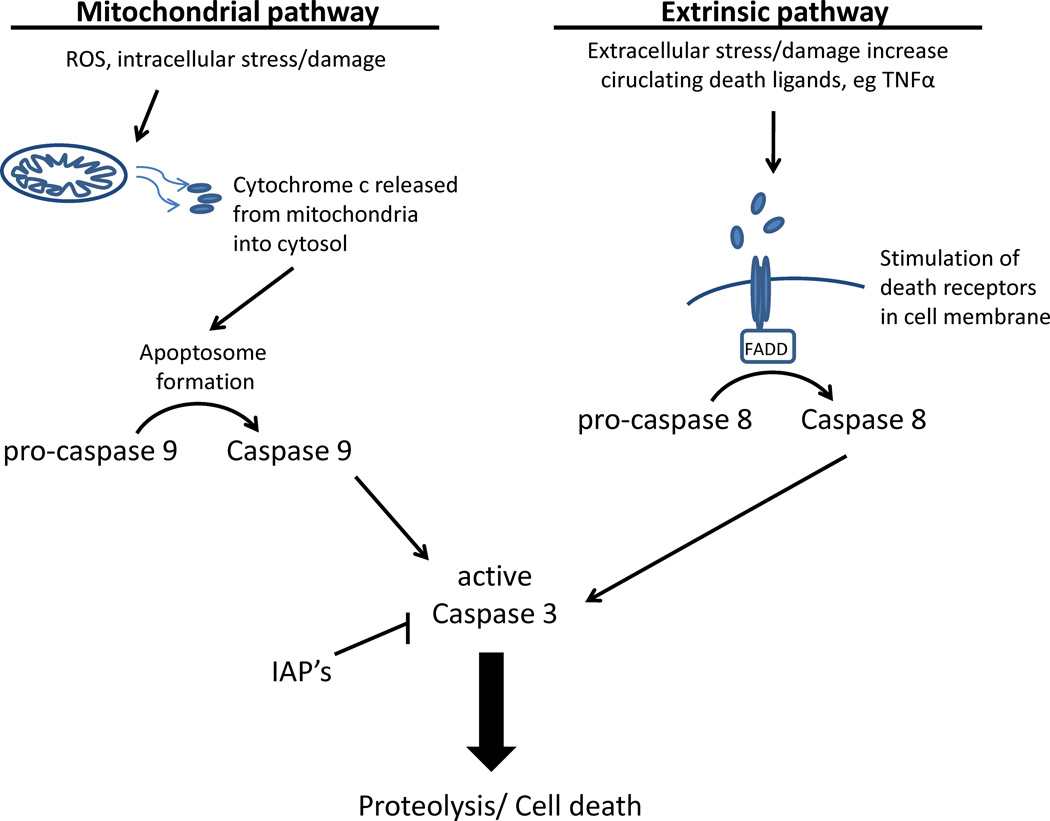
Figure 1. Overview of the intrinsic and extrinsic cell death pathways.
The intrinsic or mitochondrial pathway is triggered by cellular stress signals such as accumulation of ROS, DNA damage and relocalization or activation of proapoptotic proteins. The key event in the intrinsic pathway is mitochondrial cytochrome c release following mitochondrial membrane permeabalization. Cyt c then binds with APAF-1, facilitating formation of the apoptosome that activates procaspase-9 by proteolysis activating downstream effector caspase-3. The extrinsic pathway is initiated by activation of cell surface receptors or death receptors belonging to the tumor necrosis factor (TNF) family. These include FAS receptor (CD95), TNF receptor-1 (TNFR1), DR4, and DR5. The FAS receptor is one of the best-understood death receptors and provides a model for the extrinsic pathway. Ligands bind to these receptors and induce formation of a death-inducing signaling complex (DISC) that includes Fas associated death domain (FADD). This complex cleaves procaspase to active caspase 8, which then leads to activation of caspase-3. Once activated, caspase-3 cleaves a wide variety of substrates in the brain resulting in loss, gain, or change of function for the target protein.
Caspase-3 modulates synaptic function in the adult brain
Caspase-3 activity appears to contribute to apoptosis and connectivity in the early phases of central nervous system development but modulation of synaptic function in the adult brain. Although suppression of apoptosis in the postnatal mammalian brain coincides with decreases in caspase-3 expression (11, 12), active caspase-3 has been detected in neurons and glia from hippocampal slices obtained from adult rats (13). In healthy adult brains caspase-3 appears to have alternative roles which include modulation of synaptic plasticity and some forms of memory.
Caspases modulate synaptic plasticity
Synapse strength is modified in response to neural activity and experience, a crucial process known as synaptic plasticity. Long-term potentiation (LTP) and long-term depression (LTD) are forms of Hebbian synaptic plasticity, characterized by rapid adjustments in the synaptic strength of individual synapses. Recent literature has suggested a non-apoptotic role for caspase-3 in synaptic plasticity and memory. In an elegant work combining pharmacological inhibition of caspase-3 and over expression of endogenous inhibitors, Li et al. (2010) showed that activation of caspase-3 via mitochondria is required for LTD in CA1 hippocampal neurons. This was supported by the absence of LTD in hippocampal slices from caspase-3 knockout mice. The study further demonstrated that Akt1 proteolysis by caspase-3 is necessary for induction of LTD (14). Interestingly, Akt1 is also a substrate of caspase-3 during apoptosis (15). What is then the difference in the caspase-3 activation that leads to different outcomes (i.e. LTD vs. apoptosis)?
Evidence provided by the same group suggests that the intensity and duration of caspase-3 activation determines the final outcome: N-Methyl-D-aspartate (NMDA) induced a transient activation of caspase-3, associated with LTD; whereas higher and persistent levels of active caspase-3 were induced by staurosporine (14), a widely used apoptotic stimulus (16). Supporting the importance of the intensity and duration of caspase-3 activation in distinguishing between the LTD and apoptosis, Jiao and Li (2011) showed that cell death is only induced when neurons are exposed to a high concentration (100 uM) of NMDA for sixty minutes, but not when the treatment is at lower doses (30 uM) or for a shorter periods (10 or 30 minutes). Consistent with these findings, NMDA-induced LTD has been associated with a moderate and transient activation of Bcl-2-associated death promoter (BAD) and BAX, upstream factors involved in the canonical and cell death related activation of caspase-3 (17). Spatial pattern of caspase-3 activation also appears critical in that NMDA induces LTD only by a local (dendritic) and moderate activation of the mitochondrial apoptotic pathway. This allows synaptic changes, including cleavage of Akt1, but prevents cell death (14). In order to fine-tune the caspase-3 activation at specific synaptic sites, a model has been proposed in which caspase-3 is regulated by a mechanism for rapid release and sequestration by its endogenous inhibitor × linked inhibitor of apoptosis protein (XIAP) (18).
In contrast to LTD, Li et al (2010) found that caspase-3 inhibition by Z-DEVD-FMK does not disturb LTP in hippocampus. This concept was supported by another study which reported that Z-VAD-FMK, a pan-caspase inhibitor, has no effect on LTP per se (19). However, the same study also found that Z-VAD-FMK prevented the inhibition of LTP by amyloid β1–42 (Aβ) in CA1 hippocampal neurons (19). Blockade of LTP by Aβ has been postulated as one mechanism underlying memory deficits in Alzheimer’s Disease (AD), but the molecular details underlying Aβ actions remain unknown. The key role of caspase-3 in the inhibition of LTP by Aβ was demonstrated by experiments showing that Aβ had no effect on LTP in caspase-3 knockout mice. It was also observed that cleavage of Akt1 by caspase-3 is an essential step for the inhibition of LTP by Aβ (19). Following the canonical pathway which establishes that Akt inactivates GSK-3β by an N-terminal phosphorylation (Ser-9) in neurons exposed to Aβ, the increased levels of active caspase-3 correlated with a decrease in the levels of p-GSK-3β (Ser-9). As expected, pharmacological inhibition of GSK-3β prevented the Aβ-induced inhibition of LTP, suggesting a serial mechanism in which activation of casapse-3 leads to cleavage of Akt1 which then removes a tonic inhibition of GSK-3β (19). Although it is unknown whether this process is also associated with the role of caspase-3 in LTD (14), overall it appears that caspase-3 activation can modulate LTP and LTD differentially by modulating the Akt/GSK-3β pathway and that caspase-3 activation is essential for normal synaptic function.
Caspases modulate learning and memory processes
In concert with the increasing evidence supporting the role of caspase-3 in synaptic plasticity, it has also become clear that caspase-3 has an important role in learning and memory processes in different animal models. For instance, Huesmann and Clayton (18) reported that caspase-3 activation is required for the development of long-term habituation to a song in birds, and that inhibiting caspase-3 prevented the occurrence of a persisting memory for the song. Similarly, Stephanichev et al. (20) used Z-DEVD-FMK to demonstrate that inhibition of caspase-3 levels in the adult rat brain impairs acoustic startle response, providing strong evidence for a role of caspase-3 in normal cognitive processes in the mature brain (20). Inhibition of caspase activity in the hippocampus has also been shown to block long-term, but not short-term, spatial memory in the water maze task (13). Also, Z-DEVD-FMK impaired memory in the contextual fear conditioning task in wild-type mice (21). These reports are consistent with the emerging hypothesis that caspase-3 activation is required for learning and memory processes.
Role of caspase-3 in the aging brain
While caspase-3 activation is essential for some normal function in the healthy adult brain, in the aged brain the result of caspase-3 activation can often be detrimental. Activation of the caspase cascades and caspase-3 in particular is strongly implicated in several degenerative processes in the aging brain (22–25) and in the pathogenesis of late onset degenerative diseases (26). In addition to causing breakdown of structural proteins such as actin and laminins, caspase-dependent cleavage of specific proteins inactivates survival pathways, such as the phosphatidylinositol-3 kinase/Akt pathways (27, 28) and mitogen-activated protein kinases (MAPKs) pathways. This modulation of various signal transduction pathways by caspases suggests a wider range of function for the proteases.
Caspase-3 and Akt pathway
Akt, also known as PKB, is a serine/threonine kinase regulated by the membrane levels of phosphatidylinositol 3-phosphate (PI3P). Activation of Akt requires a dual mechanism, 1) translocation to the plasma membrane 2) phosphorylation at Thr308 (via PDK1) and Ser473 (via PDK2) (29, 30). Once activated, Akt shows significant anti-apoptotic signaling properties (31). Cell survival by Akt is likely to be mediated by the ability of Akt to promote growth through mTOR (32) and phosphorylate and inactivate several pro-apoptotic molecules including BAD (33), caspase-9 (34) and FOXO (35). Accordingly, blocking Akt signaling has been associated with reduced neuronal survival (36).
In the aging rat brain Akt p-Ser308 levels have been shown to be reduced in the CA1, and increased in the CA3 regions of the hippocampus (27). Similarly, the senescence-accelerated prone 10 (SAMP10) mouse model for early onset of neurodegenerative dementia shows decreased phosphorylation of Akt Ser473 in the hippocampus (28). Furthermore, decreases in levels of brain derived neurotrophic factor (BDNF), as observed in the aging brain, may also interfere with Akt function resulting in increased vulnerability of neurons (37). Akt is also a direct cleavage target for caspase-3 (19). Thus it appears likely that the protective effects of Akt signaling in the mature brain are negated with aging due to activation of caspase-3.
Caspase-3 and MAPK pathway
Although there are many signaling cascades affected by caspases in aging neuronal populations, very few, if any at all, have shown evidence of mediating a possible survival/plasticity switch. If such a switch indeed exists, then it may be critical in providing some insight into the role of caspases in functions other than cell death. The mitogen-activated protein kinases (MAPKs) are especially important in this respect. MAPKs include three subfamilies known as ERKs, JNKs, and p38/HOG1 kinase, each of which contributes differentially to pro- and anti-apoptotic pathways (38). While the JNK and p38 pathways are often associated with induction of apoptosis, the ERK pathway signaling is thought to protect cells from apoptosis (39–41). ERK promotes cell survival during development and tissue homeostasis by phosphorylation and inhibition of caspase 9 which in turn leads to reduced caspase-3 activation (42). However, an increasing number of studies have reported a role of ERK signaling in neuronal degeneration and death (22, 43).
While the ERK cascade promotes cell survival in conditions of unstressed tissue homeostasis, it promotes apoptosis under elevated reactive oxygen species (ROS) or other stress conditions. In particular, non-apoptotic (caspase-independent) neuronal cell death is induced by the activation of the MEK2-ERK2, but not the MEK1-ERK1, pathway, suggesting that the ratio of ERK1/ERK2 might be a critical determinant of cell fate (44). An upstream kinase in the ERK cascade is MEKK1. This full length kinase has a key role in the cell survival signal of ERK and acts by mediating transcription of NFκB. However, activation of caspase-3 leads to cleavage of MEKK1 to form proapototic C terminal fragments. Cardone et al. (34) have also reported that these small length fragments engage a positive feedback loop to initiate caspase cascades. This adds to the evidence that MEKK1 driven ERK pathway may have a significant role in controlling the survival –apoptosis switch in cells. This intriguing hypothesis remains to be confirmed.
In the aging brain, caspase-3 activation is a common convergence points for a number of toxic triggers such as oxidative damage and Aβ. Studies conducted by our group using canine subjects as a model of aging (25) and in human subjects (45) have shown that activated caspases are risk factors for cognitive decline and neurodegeneration. However, we also found that although caspase-3 activation occurs in the aged brain, it is not correlated with neuronal death (25). This is in agreement with what has been reported in transgeneic AD mouse models (46). Our group has been using the aged canine to study the specific effects of lifestyle interventions on brain aging and cognitive function for several years now. Using this model we recently answered, a so far unanswered question about whether it is possible to use life style interventions to prevent age related caspase activation in the brain. Our findings revealed that sustained change in lifestyle consisting of behavioral enrichment, exercise, dietary supplementation with antioxidants and mitochondrial cofactors, or the combination of these interventions dramatically reduce the abundance of cells expressing activated caspase-3. This finding supports the concept that caspase-3 activation may be a focal point for multiple pathways that can be regulated by lifestyle interventions.
Caspases in Alzheimer’s Disease
AD is a neurodegenerative condition characterized by (1) progressive deposition of amyloid β-peptide (Aβ) in plaques, (2) aggregation of paired helical filament tau in neurofibrillary tangles (NFTs), and (3) synaptic degeneration and neuronal loss. Furthermore, caspase-3 levels are higher in AD brains than in age-matched controls (47).
There are multiple pathways by which caspase activation can exacerbate each hallmark of AD pathology and impair cognitive function. For instance, an essential step in Aβ production is cleavage of APP by beta-secretase (BACE), which is upregulated by stress and targeted for lysosomal degradation by golgi-localized, gamma adaptin ear-containing, ARF-binding (GGA3). Recently the Tessler-Lavigne group reported that GGA3 is a degradation target of caspase-3, suggesting that caspase activation would allow accumulation of BACE and therefore increased production of Aβ (48). The role of caspase-cleaved Akt on the effects of Aβ in the brain has also been discussed previously (19). Thus, caspase activation in the AD brain appears to mediate and exacerbate the pathological effects of Aβ. Moreover, the microtubule associated protein tau which forms NFTs is a substrate of caspase-3 and can lead to neurite degeneration by reducing the availability of full length tau for binding to microtubules. Cleavage of tau at Asp421 also generates a positive-feedback loop for neuronal degeneration (49–52). In a recent study by Hyman and co workers (46), it was reported that caspase-3 activation precedes and correlates with cleaved tau and NFT formation. Calcineurin is also cleaved as a consequence of the caspase cascade, constitutively activating it. Because calcineurin is a critical mediator of LTD and spine loss, its increased activation may increase LTD, spine loss and decrease functional connectivity.
A selective accumulation of caspase-3 in the postsynaptic density fractions (PSDs) in AD patients has also been described (53). Synapse-specific elevation of caspase-3 can lead to synapse degeneration, significant because synaptic loss is the pathology most highly correlated to severity of cognitive impairment in AD (54, 55). Caspase-3 activation has not only been found to induce synapse degeneration, but more recently to decrease synaptic plasticity through internalization and dephosphorylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (14).
Homeostatic plasticity describes the scaling of the neuronal output without changing the relative strength of individual synapses, in order to keep neurons firing within an optimal range. It is thought that homeostatic plasticity compensates for Hebbian forms of synaptic plasticity (56). One of the mechanisms of homeostatic plasticity involves modifying miniature excitatory postsynaptic currents (mEPSCs) by scaling up AMPA receptors under activity-deprived conditions and scaling down AMPA receptors during increased network excitability (57, 58). In agreement with the putative roles of caspase-3 in synaptic function, several lines of evidence suggest that caspase-3 may be involved in the mechanism controlling the availability of AMPA receptors in the synapse: 1) it has been shown that caspase-3 activity is critical for the NMDA-induced internalization of AMPA receptors (14); 2) caspase-3 can alter specific properties of AMPA receptors by cleaving subunits such as GluR1 (59) and GluR4 (60); 3) anchoring and/or trafficking of the AMPA receptors can be affected by caspase cleavage of AMPA receptor-associated proteins such as PSD-95 (61), CaMKII and calcineurin A (21), all of which belong to the caspase-3 substrate family. In a recent commentary, (62) the author suggests, that altering the balance between kinase cascades such as Akt and phosphatases such as calcineurin could be critical in determining the outcome of caspase activation (LTD or neurodegeneration). However, AMPA receptors are removed from their post synaptic sites in case of both LTD and neurodegerenation. Evidence for deranged homeostatic synaptic scaling of AMPA receptors was also found in AD mouse models that produce humanized Aβ (63). A similar model for the proteolytic loss-of-function of normal homeostatic processes in AD was recently described in which presenilin knock-out and mutant presenilin mice failed to activate Akt and therefore did not homeostatically scale excitatory synapses (64). The cognitive effects of such alterations remains to be determined, but overall it appears likely that caspases have a critical role in regulation of neuronal dysfunction in neurodegenerative conditions perhaps due to both gain of pathological functions and loss of normal function.
Control of Caspase activation in brain plasticity and neuronal death
It is remarkable that a protease cascade which can trigger widespread damage and degeneration should aggregate in structures as delicate as synapses which underlie cognitive processes and memory formation. A possible explanation is that acquisition of higher cognitive process such as learning, memory, and problem solving necessitate a greater amount of synaptic plasticity, which in turn, requires activation of regulatory molecules such as caspases involved in axonal sprouting and dendritic pruning. In aged cells however, which have higher levels of several misfolded proteins or other cytotoxic structures, activation of caspase-3 is more likely to cause extensive cellular damage and even eventual cell death. Thus under conditions of low cell stress caspase-3 activation primarily modulates plasticity, and under high stress conditions caspase-3 activation likely results in cell degeneration and death. It stands to reason then that a mechanism must exist for determining the specific role of caspase-3 in these different functions. Here we suggest that caspase-3 is actually a bifurcation point in cellular signaling, able to orient the neuronal response to stress down either pathological/apoptotic pathways or towards physiological cellular remodeling (Fig 2).
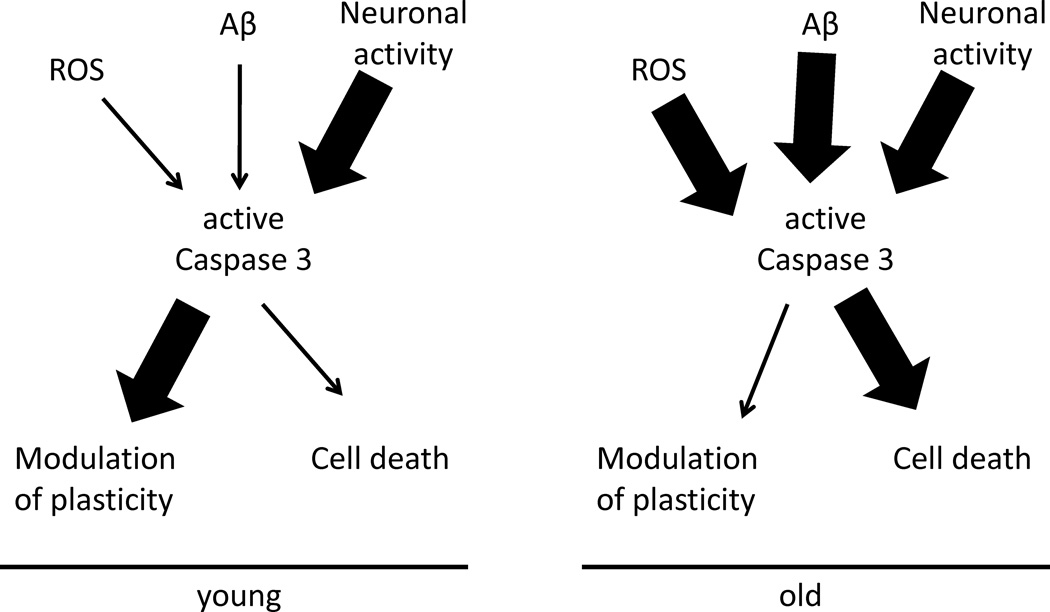
Figure 2. Caspase-3 activation is a bifurcation point in the cellular response to stimuli.
Caspase-3 activation may have different effects in a young healthy brain compared to an aged or diseased brain. Diagram shows how on a background of low cell stress, caspase activation (young) due to a specific pattern of neuronal activity could primarily modulate plasticity. On the other hand, under conditions of high stress such as due to accumulation of reactive oxygen species (ROS) or aggregated amyloid-β (Aβ) due to age, the role of caspase-3 activation would primarily be in cell death pathways, overwhelming normal regulation of plasticity.
Several regulatory mechanisms are likely determinants of caspase activity in the brain. Four of these are discussed here:
Regulated proteolytic activation: Since caspases are produced as precursor proteins (zymogens) and must be activated by proteolytic cleavage, the intrinsic and extrinsic pathways both regulate caspase activation. Such post translational processing may be especially relevant for aging as accumulation of insults such as oxidative damage, stress, and decreased neurotrophic support leads to activation of the intrinsic or caspase 9-dependent pathway. Phosphorylation and inactivation of caspase 9 is considered to be a mechanism used by Akt to promote cell survival (65). Therefore while in the healthy brain Akt promotes cell survival by inactivating caspase 9, increased oxidative damage and accumulation of toxins like Aβ in the aging brain are likely to interfere with regulatory molecules such as Akt and may lead to a positive feedback loop to increase activation of caspase-3. It is also conceivable that activation of phosphatases such as calcinuerin interfere with kinase cascades to determine the outcome of caspase activation.
Transient activation: Recent evidence indicates that active caspase-3 is always present in synaptic terminals but released only transiently to effect a synaptic process essential for memory storage. Neurons may therefore control the duration of proteolytic cleavage enacted by caspases through molecular custodians such as XIAP (18). For instance, temporally limited activation of caspase-3 is essential for PC-12 and neurosphere differentiation (66, 67) However, under condition of stress and injury, regulatory molecules are impaired in function and can lead to chronic activation of caspases in the brain. In agreement with this hypothesis Li et al., (14) also reported that transient activation of caspase-3 induced by NMDA was associated with LTD whereas a higher and persistent level of caspase-3 was induced by the proapoptotic reagent staurosporine.
Spatial activation: Similarly a role of local caspase-3 activity in both synaptic and dendritic degenerative processes showed that glutamate induces caspase-3 activation in cultured hippocampal neurons (68). Subsequent investigations show that limited caspase activation results primarily in proteolytic outcomes of caspases, while widespread caspase activation often results in cell death. Specifically, spatially limited caspase activation limited the destructive capacity of caspase activation to dendrite pruning and synapse elimination (69–72). Furthermore spatially or temporally limited activation of caspase-3 has been reported to downregulate neuronal excitability through GluR1 cleavage (73), and dephosphorylation (21).
Endogenous Inhibitors: Caspase activation is limited by endogenous inhibitors such as the inhibitor of apoptosis proteins (IAPs), characterized by the presence of at least one baculoviral IAP repeat (BIR) domain (74). In drosophila, the caspase inhibitor drosophila/thread inhibitor of apoptosis (DIAP) binds caspases to prevent apoptosis through both caspase degradation (75) and nondegradation pathways (76). DIAP1 in turn is modulated by the ubiquitin-proteasome system (70). Therefore the ratio of DIAP proteins to caspases may determine the ultimate cell fate after caspase activation.
Most studies of caspase-3 regulation have been conducted with regard to cell death or structural remodeling. However, based on emerging evidence, we propose here that the site, duration and intensity of caspase activation are important determinants of whether caspase activation results in cell degeneration or synaptic plasticity (Figure 3).
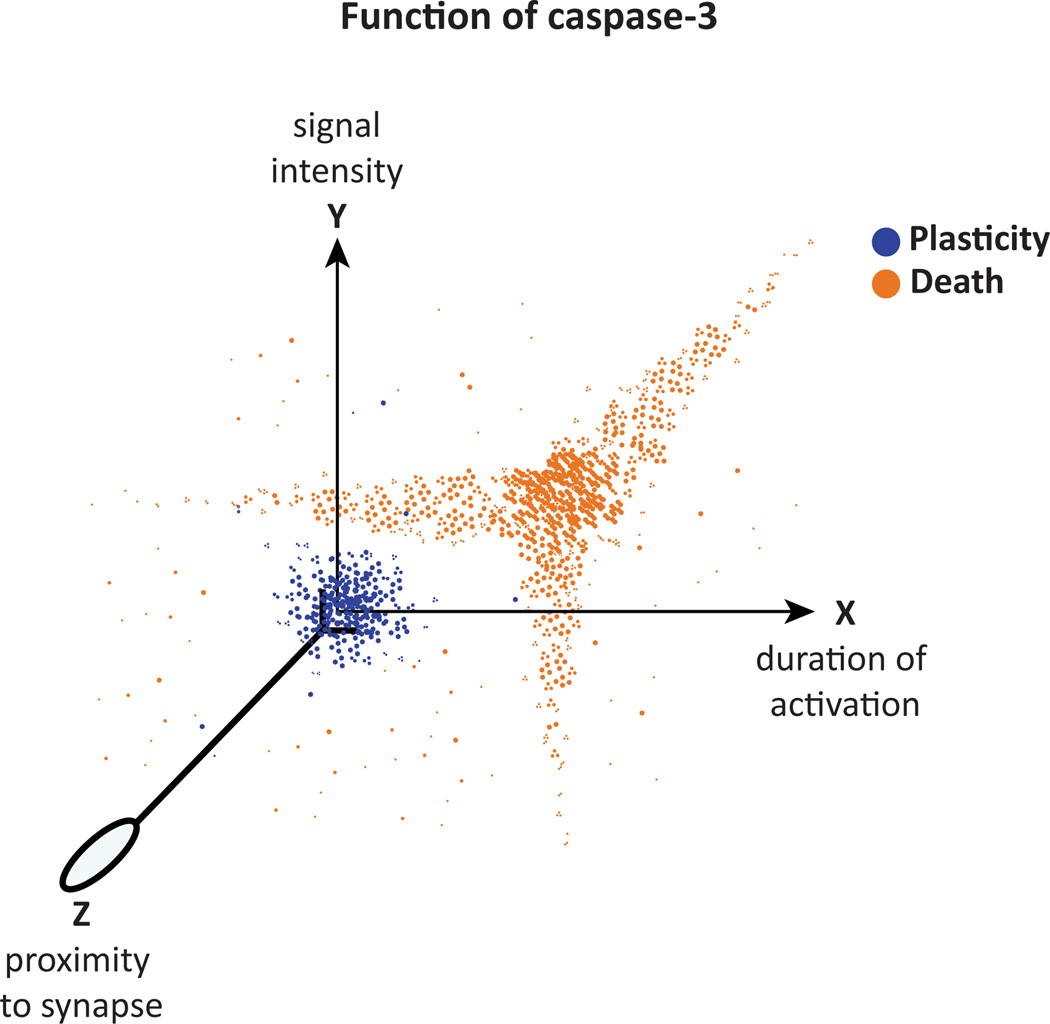
Figure 3. Different physiological states, two different roles of caspase-3 in the brain.
Graph showing variables related with the activation of caspase-3 that determine its function in neuronal circuitries: signal intensity, duration of activation and proximity to the synapse. This model suggests that the function of caspase-3 depends on the context in which it is activated. When signals perceived by neurons are transduced and integrated, caspase-3 activity is modified leading to two possible outcomes (color dots): the enrollment of caspase-3 in synaptic plasticity processes, or its participation in cell death pathways. The model predicts the existence of a threshold of caspase-3 activity that determines its role during any neuronal state; the threshold is determined by the strength and quality of all the variables involved in caspase-3 activation.
Conclusion
The existence of so many different regulatory mechanisms for caspase activity means that caspases can take on multiple different roles depending on the cellular context. Indeed, while the proteolytic activity of caspases remains unchanged, the effect on different molecular targets is largely determined by temporal, spatial, and activation access provided by the various regulators. It appears that a survival/plasticity switch in neuronal cells regulates the ultimate effect of caspase-3 activation in neurons. By regarding caspase-3 as tightly regulated cleavers of specific proteins, we may be able to integrate our understanding of the diverse roles of caspase-3 across lifespan. In cells with widespread caspase activation, caspases initiate apoptosis. In young cells with localized caspase-3 activation, caspases mediate branch pruning and in healthy adults, caspase-3 activation is required for normal maintenance of Hebbian and homeostatic plasticity. Therefore, in neurodegenerative conditions such as AD caspase dysregulation can have a doubly detrimental effect, through both loss of normal plasticity function and induction of synaptic and cellular degradation. Attempting to reduce the pathological effects of caspases through complete caspase inhibition may hence result in a loss of normal physiological caspase functions. In the future, a better understanding of the complex caspase regulatory pathways may suggest the best intervention target, so that the outcome of caspase activation can be directed to a preferred outcome.
Acknowledgement
Funded in part by AG012694-16
References
- 1.Du Y, Bales KR, Dodel RC, Hamilton-Byrd E, Horn JW, Czilli DL, et al. Activation of a caspase 3-related cysteine protease is required for glutamate-mediated apoptosis of cultured cerebellar granule neurons. Proc Natl Acad Sci U S A. 1997;94(21):11657–11662. doi: 10.1073/pnas.94.21.11657. PMCID: 23571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gillardon F, Kiprianova I, Sandkuhler J, Hossmann KA, Spranger M. Inhibition of caspases prevents cell death of hippocampal CA1 neurons, but not impairment of hippocampal long-term potentiation following global ischemia. Neuroscience. 1999;93(4):1219–1222. doi: 10.1016/s0306-4522(99)00292-4. [DOI] [PubMed] [Google Scholar]
- 3.Earnshaw WC, Samejima K, Svingen PA, Basi GS, Kottke T, Mesner PW, et al. Caspase-mediated cleavage of DNA topoisomerase I at unconventional sites during apoptosis. Journal of Biological Chemistry. 1999;274(7):4335–4340. doi: 10.1074/jbc.274.7.4335. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6(11):1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- 5.Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10(1):76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14(1):32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 8.Marks N, Berg MJ. Recent advances on neuronal caspases in development and neurodegeneration. Neurochem Int. 1999;35(3):195–220. doi: 10.1016/s0197-0186(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 9.Salvesen GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Acad Sci U S A. 1999;96(20):10964–10967. doi: 10.1073/pnas.96.20.10964. PMCID: 34227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117(7):855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 11.de Bilbao F, Guarin E, Nef P, Vallet P, Giannakopoulos P, Dubois-Dauphin M. Postnatal distribution of cpp32/caspase 3 mRNA in the mouse central nervous system: an in situ hybridization study. J Comp Neurol. 1999;409(3):339–357. doi: 10.1002/(sici)1096-9861(19990705)409:3<339::aid-cne1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Yakovlev AG, Ota K, Wang G, Movsesyan V, Bao WL, Yoshihara K, et al. Differential expression of apoptotic protease-activating factor-1 and caspase-3 genes and susceptibility to apoptosis during brain development and after traumatic brain injury. J Neurosci. 2001;21(19):7439–7446. doi: 10.1523/JNEUROSCI.21-19-07439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash PK, Blum S, Moore AN. Caspase activity plays an essential role in long-term memory. Neuroreport. 2000;11(12):2811–2816. doi: 10.1097/00001756-200008210-00040. [DOI] [PubMed] [Google Scholar]
- 14.Li Z, Jo J, Jia JM, Lo SC, Whitcomb DJ, Jiao S, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141(5):859–871. doi: 10.1016/j.cell.2010.03.053. PMCID: 2909748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachelder RE, Ribick MJ, Marchetti A, Falcioni R, Soddu S, Davis KR, et al. p53 inhibits alpha 6 beta 4 integrin survival signaling by promoting the caspase 3-dependent cleavage of AKT/PKB. J Cell Biol. 1999;147(5):1063–1072. doi: 10.1083/jcb.147.5.1063. PMCID: 2169339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belmokhtar CA, Hillion J, Segal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20(26):3354–3362. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- 17.Jiao S, Li Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron. 2011;70(4):758–772. doi: 10.1016/j.neuron.2011.04.004. PMCID: 3102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huesmann GR, Clayton DF. Dynamic role of postsynaptic caspase-3 and BIRC4 in zebra finch song-response habituation. Neuron. 2006;52(6):1061–1072. doi: 10.1016/j.neuron.2006.10.033. PMCID: 1847391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jo J, Whitcomb DJ, Olsen KM, Kerrigan TL, Lo SC, Bru-Mercier G, et al. Abeta(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat Neurosci. 2011;14(5):545–547. doi: 10.1038/nn.2785. [DOI] [PubMed] [Google Scholar]
- 20.Stepanichev MY, Kudryashova IV, Yakovlev AA, Onufriev MV, Khaspekov LG, Lyzhin AA, et al. Central administration of a caspase inhibitor impairs shuttle-box performance in rats. Neuroscience. 2005;136(2):579–591. doi: 10.1016/j.neuroscience.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.D'Amelio M, Cavallucci V, Middei S, Marchetti C, Pacioni S, Ferri A, et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci. 2011;14(1):69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- 22.Lesuisse C, Martin LJ. Immature and mature cortical neurons engage different apoptotic mechanisms involving caspase-3 and the mitogen-activated protein kinase pathway. J Cereb Blood Flow Metab. 2002;22(8):935–950. doi: 10.1097/00004647-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Lynch AM, Lynch MA. The age-related increase in IL-1 type I receptor in rat hippocampus is coupled with an increase in caspase-3 activation. Eur J Neurosci. 2002;15(11):1779–1788. doi: 10.1046/j.1460-9568.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- 24.Martin DS, Lonergan PE, Boland B, Fogarty MP, Brady M, Horrobin DF, et al. Apoptotic changes in the aged brain are triggered by interleukin-1beta-induced activation of p38 and reversed by treatment with eicosapentaenoic acid. J Biol Chem. 2002;277(37):34239–34246. doi: 10.1074/jbc.M205289200. [DOI] [PubMed] [Google Scholar]
- 25.Snigdha S, Berchtold N, Astarita G, Saing T, Piomelli D, Cotman CW. Dietary and Behavioral Interventions Protect against Age Related Activation of Caspase Cascades in the Canine Brain. PLoS One. 2011;6(9):e24652. doi: 10.1371/journal.pone.0024652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer's and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898(2):350–357. doi: 10.1016/s0006-8993(01)02018-2. [DOI] [PubMed] [Google Scholar]
- 27.Jackson TC, Rani A, Kumar A, Foster TC. Regional hippocampal differences in AKT survival signaling across the lifespan: implications for CA1 vulnerability with aging. Cell Death Differ. 2009;16(3):439–448. doi: 10.1038/cdd.2008.171. PMCID: 2680608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nie K, Yu JC, Fu Y, Cheng HY, Chen FY, Qu Y, et al. Age-related decrease in constructive activation of Akt/PKB in SAMP10 hippocampus. Biochem Biophys Res Commun. 2009;378(1):103–107. doi: 10.1016/j.bbrc.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272(50):31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 30.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, et al. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17(3):313–325. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 31.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith ED, Tong L, Cotman CW. mTOR is essential for BDNF-dependent cell survival and is down-regulated by prolonged IL-1 treatment in vitro. SFN poster presentation. 2009;878.19 [Google Scholar]
- 33.Condorelli F, Salomoni P, Cotteret S, Cesi V, Srinivasula SM, Alnemri ES, et al. Caspase cleavage enhances the apoptosis-inducing effects of BAD. Mol Cell Biol. 2001;21(9):3025–3036. doi: 10.1128/MCB.21.9.3025-3036.2001. PMCID: 86931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 35.Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, et al. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J Am Soc Nephrol. 2006;17(12):3325–3335. doi: 10.1681/ASN.2006070754. [DOI] [PubMed] [Google Scholar]
- 36.Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29(9):1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong L, Balazs R, Thornton PL, Cotman CW. Beta-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24(30):6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410(6824):37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 39.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271(8):4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 40.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 41.Yang JY, Michod D, Walicki J, Widmann C. Surviving the kiss of death. Biochem Pharmacol. 2004;68(6):1027–1031. doi: 10.1016/j.bcp.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 42.Allan LA, Morrice N, Brady S, Magee G, Pathak S, Clarke PR. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5(7):647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 43.Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, et al. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275(16):12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 44.Bredesen DE, Castro-Obregon S, Rao RV, del Rio G, Chen SF, Poksay KS, et al. Alternative, nonapoptotic programmed cell death - Mediation by arrestin 2, ERK2, AND Nur77. Journal of Biological Chemistry. 2004;279(17):17543–17553. doi: 10.1074/jbc.M312363200. [DOI] [PubMed] [Google Scholar]
- 45.Zhao M, Su J, Head E, Cotman CW. Accumulation of caspase cleaved amyloid precursor protein represents an early neurodegenerative event in aging and in Alzheimer's disease. Neurobiol Dis. 2003;14(3):391–403. doi: 10.1016/j.nbd.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 46.de Calignon A, Spires-Jones TL, Hyman BT. Caspase activation precedes and leads to neurodegeneration in a marine model of Alzheimer's disease. M S-Medecine Sciences. 2010;26(10):787–789. doi: 10.1051/medsci/20102610787. [DOI] [PubMed] [Google Scholar]
- 47.Shimohama S, Tanino H, Fujimoto S. Changes in caspase expression in Alzheimer's disease: Comparison with development and aging. Biochemical and Biophysical Research Communications. 1999;256(2):381–384. doi: 10.1006/bbrc.1999.0344. [DOI] [PubMed] [Google Scholar]
- 48.Tesco G, Koh YH, Kang EL, Cameron AN, Das S, Sena-Esteves M, et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron. 2007;54(5):721–737. doi: 10.1016/j.neuron.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cattaneo A, Fasulo L, Ugolini G. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. Journal of Alzheimers Disease. 2005;7(1):3–13. doi: 10.3233/jad-2005-7102. [DOI] [PubMed] [Google Scholar]
- 50.Fasulo L, Ugolini G, Cattaneo A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. J Alzheimers Dis. 2005;7(1):3–13. doi: 10.3233/jad-2005-7102. [DOI] [PubMed] [Google Scholar]
- 51.Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Torp R, Vitek MP, et al. Caspase-cleavage of tau is an early event in Alzheimer disease tangle pathology. J Clin Invest. 2004;114(1):121–130. doi: 10.1172/JCI20640. PMCID: 437967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc Natl Acad Sci U S A. 2003;100(17):10032–10037. doi: 10.1073/pnas.1630428100. PMCID: 187753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arnold SE, Louneva N, Cohen JW, Han LY, Talbot K, Wilson RS, et al. Caspase-3 Is Enriched in Postsynaptic Densities and Increased in Alzheimer's Disease. American Journal of Pathology. 2008;173(5):1488–1495. doi: 10.2353/ajpath.2008.080434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selkoe DJ, Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. Journal of Neuroscience. 2007;27(11):2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terry RD, Masliah E, Salmon DP, Butters N, Deteresa R, Hill R, et al. Physical Basis of Cognitive Alterations in Alzheimers-Disease - Synapse Loss Is the Major Correlate of Cognitive Impairment. Annals of Neurology. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 56.Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- 57.Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci. 2011;14(3):279–284. doi: 10.1038/nn.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turrigiano GG, Nelson SB. Thinking globally, acting locally: AMPA receptor turnover and synaptic strength. Neuron. 1998;21(5):933–935. doi: 10.1016/s0896-6273(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 59.Mattson MP, Lu CB, Fu WM, Salvesen GS. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3 - Implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Medicine. 2002;1(1):69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- 60.Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20(10):3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu SJ, Gasperini R, Foa L, Small DH. Amyloid-beta decreases cell-surface AMPA receptors by increasing intracellular calcium and phosphorylation of GluR2. J Alzheimers Dis. 2010;21(2):655–666. doi: 10.3233/JAD-2010-091654. [DOI] [PubMed] [Google Scholar]
- 62.Hyman BT. Caspase activation without apoptosis: insight into Abeta initiation of neurodegeneration. Nat Neurosci. 2011;14(1):5–6. doi: 10.1038/nn0111-5. [DOI] [PubMed] [Google Scholar]
- 63.Chang EH, Savage MJ, Flood DG, Thomas JM, Levy RB, Mahadomrongkul V, et al. AMPA receptor downscaling at the onset of Alzheimer's disease pathology in double knockin mice. Proc Natl Acad Sci U S A. 2006;103(9):3410–3415. doi: 10.1073/pnas.0507313103. PMCID: 1413872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pratt KG, Zimmerman EC, Cook DG, Sullivan JM. Presenilin 1 regulates homeostatic synaptic scaling through Akt signaling. Nat Neurosci. 2011;14(9):1112–1114. doi: 10.1038/nn.2893. PMCID: 3164917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278(5338):687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 66.Fernando P, Brunette S, Megeney LA. Neural stem cell differentiation is dependent upon endogenous caspase 3 activity. FASEB J. 2005;19(12):1671–1673. doi: 10.1096/fj.04-2981fje. [DOI] [PubMed] [Google Scholar]
- 67.Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, et al. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer's disease. Am J Pathol. 2001;158(1):189–198. doi: 10.1016/S0002-9440(10)63957-0. PMCID: 1850275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mattson MP, Partin J, Begley JG. Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998;807(1–2):167–176. doi: 10.1016/s0006-8993(98)00763-x. [DOI] [PubMed] [Google Scholar]
- 69.Ivins KJ, Bui ET, Cotman CW. Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis. 1998;5(5):365–378. doi: 10.1006/nbdi.1998.0228. [DOI] [PubMed] [Google Scholar]
- 70.Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51(3):283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Mattson MP, Duan W. "Apoptotic" biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58(1):152–166. [PubMed] [Google Scholar]
- 72.Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9(10):1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- 73.Lu C, Fu W, Salvesen GS, Mattson MP. Direct cleavage of AMPA receptor subunit GluR1 and suppression of AMPA currents by caspase-3: implications for synaptic plasticity and excitotoxic neuronal death. Neuromolecular Med. 2002;1(1):69–79. doi: 10.1385/NMM:1:1:69. [DOI] [PubMed] [Google Scholar]
- 74.Sun C, Cai M, Gunasekera AH, Meadows RP, Wang H, Chen J, et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401(6755):818–822. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 75.Wilson R, Goyal L, Ditzel M, Zachariou A, Baker DA, Agapite J, et al. The DIAP1 RING finger mediates ubiquitination of Dronc and is indispensable for regulating apoptosis. Nat Cell Biol. 2002;4(6):445–450. doi: 10.1038/ncb799. [DOI] [PubMed] [Google Scholar]
- 76.Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KT, et al. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell. 2008;32(4):540–553. doi: 10.1016/j.molcel.2008.09.025. PMCID: 2713662. [DOI] [PMC free article] [PubMed] [Google Scholar]