Abstract
EEG is a primary method for studying temporally precise neuronal processes across the lifespan. Most of this work focuses on Event Related Potentials (ERPs); however, using time-locked time frequency analysis to decompose the EEG signal can identify and distinguish multiple changes in brain oscillations underlying cognition (Bastiaansen et al., 2010). Further this measure is thought to reflect changes in inter-neuronal communication more directly than ERPs (Nunez & Srinivasan, 2006). Although time frequency has elucidated cognitive processes in adults, applying it to cognitive development is still rare. Here, we review the basics of neuronal oscillations, some of what they reveal about adult cognitive function, and what little is known relating to children. We focus on language because it develops early and engages complex cortical networks. Additionally, because time frequency analysis of the EEG related to adult language comprehension has been incredibly informative, using similar methods with children will shed new light on current theories of language development and increase our understanding of how neural processes change over the lifespan. Our goal is to emphasize the power of this methodology and encourage its use throughout developmental cognitive neuroscience.
In the current understanding of cognitive neuroscience, it is widely accepted that human behavior and cognition arise through communications between and within complex neuronal networks (Fuster, 1997; Sauseng & Klimesch, 2008; Varela et al., 2001). Very little is known about how these communications develop over the lifetime for even simple cognitive tasks. The rapid dynamic nature of these processes cannot be captured by slow moving changes in the BOLD signal with fMRI. Human scalp EEG, however, can record activity related to a large number of highly synchronized neurons in the cortex from the scalp. From these data, we can make inferences about how and when large-scale networks are engaged during task performance. Current advances in data analysis tools, processing capabilities and our understanding of systems neuroscience has led to an increased interest in the synchronization and desynchronization of neuronal oscillations underlying the EEG and what they can reveal about human cognition. To date, the bulk of the work on this topic focuses on adult cognition, despite the incredible potential that this method holds for developmental cognitive neuroscience. Expanding this work to children can greatly advance our understanding of how neuronal communication changes with development.
Event Related Potentials (ERPs) Compared to Neuronal Oscillations
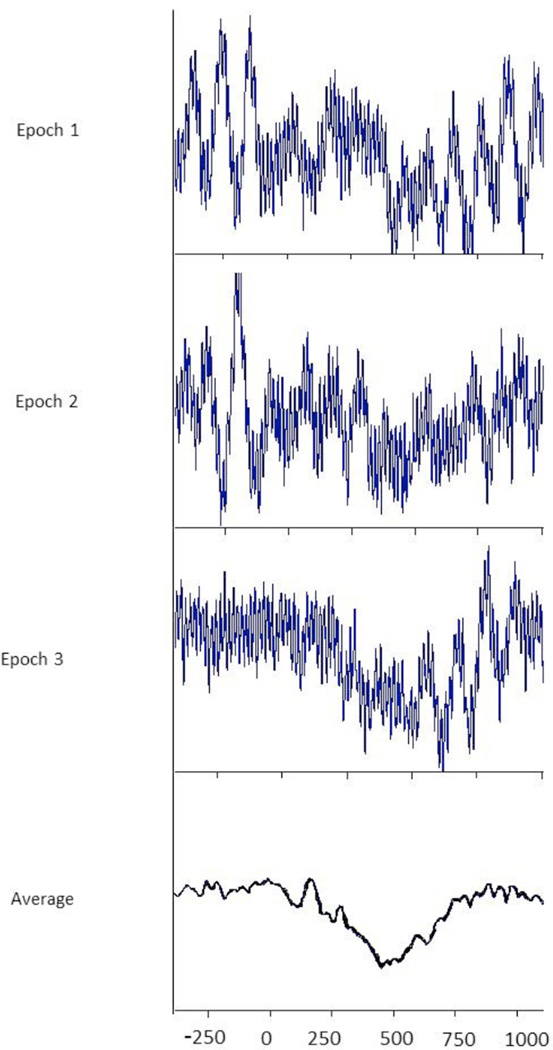
The most common use of EEG to study cognitive functions is through ERPs. ERPs are derived by epoching the ongoing EEG at the point of stimulus presentation then averaging these epochs together to form a stable waveform (See Figure 1). These waveforms contain predictable peaks related to various cognitive functions (e.g. P300, N400). Comparisons of the amplitude, topography and timing of these peaks across conditions result in inferences about underlying differences in neuronal engagement.
Figure 1.
Three epochs from an ongoing EEG and their average
For decades, ERPs have provided a wealth of information about developmental changes in the neuronal underpinnings of cognitive functions. For instance, ERPs have informed our understanding of how infants differentiate phonemes (e.g., Conboy et al., 2008; Rivera-Gaxiola et al., 2012), toddlers learn words (e.g., Torkildsen et al., 2009; Torkildsen et al., 2006; Torkildsen et al., 2008) and toddlers and young children process syntax (e.g., Oberecker & Friederici, 2006; Oberecker et al., 2005). However, these findings only tell one part of the story because, while the averaging method used to calculate the ERP increases the signal-to-noise ratio in the EEG, it also has significant limitations. First, averaging the EEG attenuates or removes important non-stimulus locked changes in oscillatory activity thought to underlie interneuronal communication (Nunez & Srinivasan, 2006). As a result, ERPs only reveal a portion of the changes in the EEG related to stimulus presentation. Second, differences in ERP components can be the result of many factors that are difficult to tease apart. For example, the N400 amplitude is influenced by a word’s concreteness, age of acquisition, and frequency, as well as test language and task differences (i.e., the number of repetitions of a word; Vigliocco et al., 2011). Decomposing the oscillations comprising the ERP and analyzing their underlying frequencies retains time resolution near that of the ERP yet can often better differentiate simultaneous processes that may originate in similar cortical areas to identify these influences (Bastiaansen & Hagoort, 2006; Maguire et al., 2010). As a result, time frequency analysis can compliment and expand upon ERP findings.
How to Measure Changes in Neuronal Oscillations
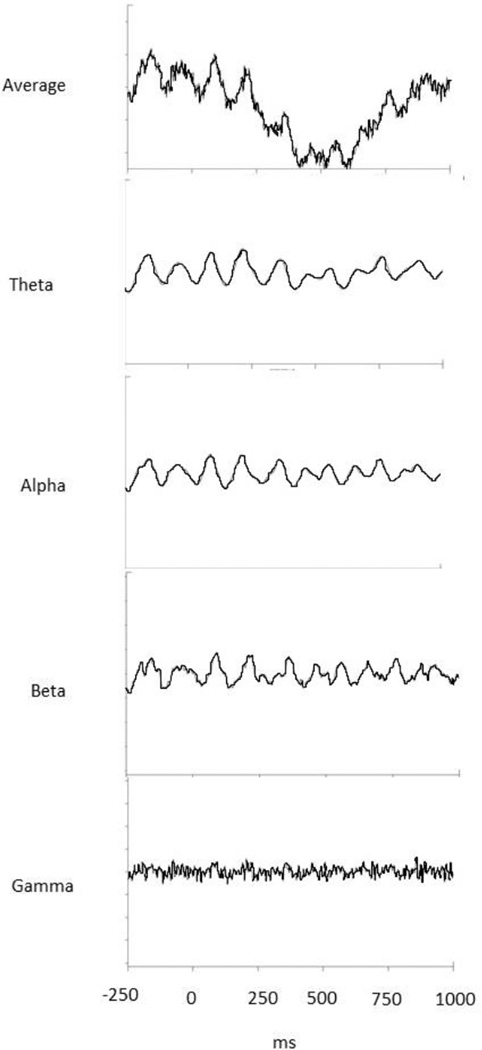
The EEG is generally modeled as overlapping sine waves of different frequencies which can be decomposed into its underlying signals (See Figure 2). Using a time frequency analysis to perform this decomposition, one can derive three important changes in the EEG: (1) magnitude, or amplitude, of the response, (2) frequency, or rate, of the response and (3) phase angle with respect to stimulus onset. Changes in one or more of these EEG characteristics in relation to a stimulus provide information about the underlying neuronal networks. Different time frequency measurements address these potential changes.
Figure 2.
Decomposition of an averaged EEG wave into overlapping sine waves of different frequencies
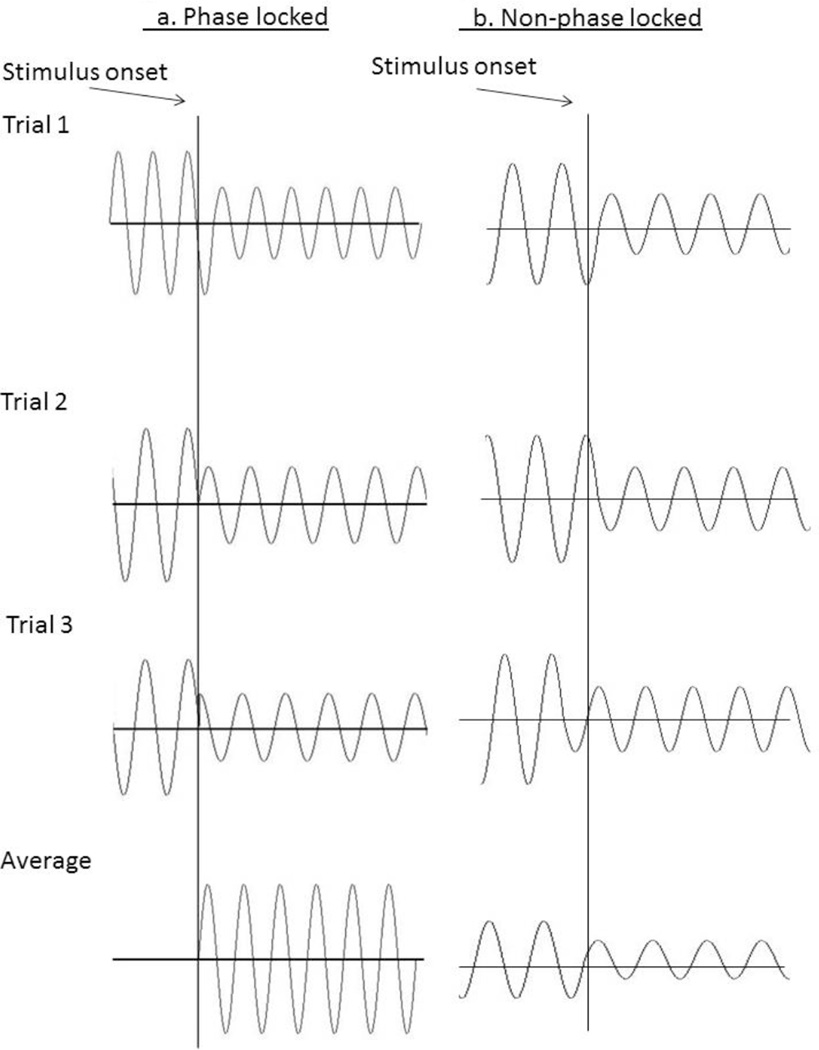
Two common measurements include phase resetting changes (sometimes called ‘evoked’ changes) and magnitude changes (sometimes called ‘invoked’ changes). Phase resetting occurs when the onset of a stimulus results in the ongoing oscillations resetting so they all occur at the same angle (see Figure 3a). Because these oscillations are at the same angle they do not average out in the traditional ERP analysis; instead they remain robust during the averaging process and are the primary source of prominent ERP peaks. Magnitude changes occur when a particular frequency’s amplitude increases in response to a stimulus without corresponding changes in the phase angle. This type of change is thought to relate to the activation of additional neural assemblies firing at the same frequency, or neuronal synchrony, resulting in an increase in the amplitude of the EEG oscillations at that frequency. These changes can be studied at the trial, study, or group level. Regardless of the size of the magnitude increase, because these responses are not phase-locked they may average out or attenuate in the ERP (see Figure 3b). Magnitude changes are often discussed in terms of changes in power. For example, an “increase in theta power” means the magnitude of the theta frequency increased without phase locking. Additional measures of power are coherence within and between frequencies across electrode sites. Although coherence is important to understanding networks in the brain, coherence and power changes address different aspects of cognitive function and are interpreted differently (Weiss & Mueller, 2012); thus, coherence is beyond the scope of this review.
Figure 3.
Example of (a) phase locked EEGs (becoming larger when averaged) and (b) non-phase locked responses (averaging out)
Studying Brain Oscillations Related to Cognitive Development
Using a mix of neuroimaging techniques including fMRI, MEG, and EEG, researchers have begun to create detailed models of the dynamic web of communication that occurs between cortical areas during adult cognition, including processes like language comprehension (Bastiaansen & Hagoort, 2006; Giraud & Poeppel, 2012; Hart et al., 2012; Pulvermüller, 2003; Weiss & Mueller, 2012) and memory encoding and retrieval (Benchenane et al., 2011; Düzel et al., 2010; Jutras & Buffalo, 2010; Klimesch, 1999; Nyhus & Curran, 2010; Stella & Treves, 2011). However, similar work focused on the development of these networks is noticeably lacking1. Developmental models cannot simply be derived from adult data as a number of factors are at play in the developing brain. First, areas of the brain develop at different rates (Li & Lindenberger, 2002; Sowell et al., 2003). So while memory engages the medial temporal lobes and prefrontal cortex in adults, for example, the prefrontal cortex develops quite late in children (Sowell et al., 2003), raising questions of how they compensate in the meantime. Similarly, the dynamics of brain activation change throughout the lifespan, such that younger children engage more cortical areas than adults during the same task. For example, in language comprehension, children show greater bilateralized activity than adults (Holland et al., 2001). Lastly, although adult frequency bands are relatively clearly defined and their part in cognitive processing is generally well accepted, adult and child EEG frequencies differ (see Bell, 1998; Pivik et al., 1993; Stroganova & Orekhova, 2007). For instance, the adult alpha rhythm of 8–13 Hz seems to be analogous to the infant/toddler 6–9 Hz activity (Marshall et al., 2002; Stroganova et al., 1999). Although this may seem like a small difference, some researchers, most notablyKlimesch et al. (1992), argue for a distinction between lower alpha (7–10 Hz), which is more global and related to general attentional demands, and upper alpha (10–13 Hz), which is more localized and related to specific task demands in adults. Currently, it is unknown how the infant/toddler alpha develops to parallel the lower alpha and upper alpha in adults.
Given these developmental factors, we learn little about the origins and development of cortical networks by focusing on adults. Instead, this knowledge requires the direct study of children, ideally in relation to an adult control group. In this way we can index what children are developing towards while accruing more information about adults to inform that literature (Poeppel, 2012). Research in social cognition (see Marshall & Meltzoff, 2011 review) has done such developmental work but the other areas of cognition are less successful. In this paper, we focus on language comprehension, highlighting the largely untapped potential of time frequency analysis of the EEG to address important theoretical questions about semantic and syntactic development. An additional goal of this review is to motivate other researchers to capitalize on the potential of time frequency analysis to study language and cognitive development.
EEG and Language
Language comprehension is a complex process involving multiple dimensions of analysis, including phonology, morphology, semantics, syntax and discourse. Accurate comprehension of spoken language requires the simultaneous identification, retrieval and integration across these domains with millisecond procession. Despite the speed and complexity of this task, children comprehend it relatively effortlessly from a young age. To date we know very little about how the dynamic systems underlying this ability develop. We argue that by using time frequency analysis, researchers could make significant gains in this area. We will first note what little research has used time frequency analyses of the EEG to study language development and the limitations of that work. Our focus in this review is on linguistic processes that have long developmental trajectories, specifically focusing on semantics and syntax. Studying the development of phonological processing using the Mismatch Negativity ERP response in particular has an extensive history (see the work of Kuhl and colleagues; e.g., Kuhl, 2010); however, to our knowledge, phonological development has not been studied using time frequency analysis in EEG.
Time-locked Time Frequency Analysis of the EEG in Children
There is extensive work using ERPs to study syntactic and semantic development; surprisingly there is limited work on semantic development and, based on our review, no work on syntactic development using time frequency analysis of the EEG. These are gaps that we hope this review will help encourage fellow researchers to fill. The work in semantic development generally focuses on atypical language development, most often dyslexia, compared to typical language (Bishop et al., 2010; Coben et al., 2008; Klimesch, Doppelmayr, Wimmer, Gruber, et al., 2001; Klimesch, Doppelmayr, Wimmer, Schwaiger, et al., 2001; Özge et al., 2004; Penolazzi et al., 2008; Spironelli et al., 2008). While this work is promising, the research that has been published to date provides little information about typical development due to the focus on language disorders and delays because the designs and paradigms used to study language disorders are based on cognitive or linguistic abilities identified as problematic in the disorders (i.e., reading or nonword/phonological processing in dyslexia). Research documenting typical development is needed to establish a standard for which to compare children with disordered development (see Bishop et al., 2010 for an example of such a study with auditory discrimination).
In the following sections, we review the adult research using time frequency of the EEG to study semantic and syntactic processing, comparing these findings to those from ERPs using similar paradigms, and then suggest how these findings could apply to and build on our current understanding of semantic and syntactic development. Above we noted the difference between phase-locked and non-phase locked changes in oscillatory activity, which measure different types of neural activity. The studies reviewed below primarily focus on changes in non-phase locked activity, or power.
Time-locked Time Frequency Analysis of the EEG in Adult Semantic Processing
EEG studies of semantic processing commonly focus on the N400 ERP response. The N400 is a measure of semantic retrieval and lexical-semantic integration and is elicited by individual words in isolation or words in context. The N400 amplitude is generally thought to index the effort related to semantic memory retrieval, such that words that are primed by the preceding context (words, sentences, or pictures) exhibit smaller responses than unprimed words. However, as mentioned, various factors related to the specific word stimuli (i.e., concreteness, age of acquisition, frequency) and task (i.e., test language, number of repetitions of a specific word; Vigliocco et al., 2011) influence the N400. Much work has sought to identify the time frequency changes related to the N400 because it is a robust measure that could potentially tease apart competing influences on the N400 and increase our understanding semantic processing (Davidson & Indefrey, 2007; Hald et al., 2006; Maguire et al., 2010; Van Elk et al., 2010; L. Wang et al., 2012). Two frequency bands in particular, theta and gamma, appear to relate to semantic retrieval and integration.
Semantic retrieval
Changes in both the N400 and theta power relate to semantic retrieval (Bastiaansen et al., 2005; Bastiaansen et al., 2008; Shahin et al., 2009). For instance, timing and topographical differences in the N400 and theta are found between open-class words, which carry semantic meaning - such as nouns (dog) and verbs (run) - and closed-class words, which carry syntactic information but limited semantic meaning - such as articles (the) and prepositions (of). Most studies report a larger N400 amplitude for open-class words versus closed-class words between 300 and 400 milliseconds over centro-parietal areas (e.g., Münte et al., 2001; Osterhout et al., 2002). Whether this N400 difference is due to semantic retrieval or other linguistic properties like word length (open-class words are generally longer than closed-class words) or frequency (closed-class words are generally more frequent than open-class words) is difficult to tease apart (Osterhout et al., 2002). AsOsterhout et al. (2002) note in their study of the ERPs related to open- and closed-class words, “it could very well be that the electrophysiological concomitants of these distinctions will become visible only with application of other techniques (e.g. coherence or power analyses) for analyzing the brain’s response to words” (p. 185).
Such work with adults is underway and reveals promising differences. For example, both open- and closed-class words elicit an increase in theta power between 300 and 500 milliseconds after stimulus presentation over midfrontal areas. Interestingly, open-class words result in additional theta increases over left occipital and temporal areas compared to closed-class words, a finding that the authors attribute to greater engagement of the functional networks related to the semantic properties of words with more robust meanings (Bastiaansen et al., 2005). Further, beyond broad categories of open- and closed-class words, the topography of the theta power increase reveals more information about the neural networks underlying specific semantic meanings. For example, words with visual semantic properties (i.e., color or shape) elicit theta increases over left occipital areas (visual cortex) compared to words with auditory semantic properties (i.e., sound terms), which elicit larger theta increases over left temporal areas (auditory cortex; Bastiaansen et al., 2008). Taken together, these findings suggest that the topography of theta power change is sensitive to detailed semantic properties of words and support arguments that semantic processes engage corresponding perceptual processing related to a word’s meaning.
Similar semantic retrieval research in children is surprisingly missing. Such work would inform the current debate about the relationship between perceptual features of specific words, such as how easy they are to form a mental image of or how ‘embodied’ the semantic features are, and how children acquire the word (Kaplan et al., 2008; Maguire et al., 2006a; Maouene et al., 2011; Smolík, 2013). Embodied semantics is the notion that word comprehension engages perceptual processes related to the word’s meaning, being “embodied” such that action verbs engage motor cortex and object nouns engage visual cortex. According to some language development theories, semantic embodiment originates in the earliest stages of lexical acquisition, as children link words to experiences like performing actions and seeing objects (Howell et al., 2005; Maguire et al., 2006b; Meteyard et al., 2010; Smiley & Huttenlocher, 1995). However this is difficult to reconcile with our current understanding that the neural networks sub-serving semantic processing becomes lateralized and specialized into adolescence (Chou et al., 2006; Holland et al., 2001; Szaflarski et al., 2005; Vannest et al., 2009). Changes in neural oscillations might provide an important link between these two seemingly contradictory ideas. In adults changes in beta frequency, a common correlate of motor responses, are observed during semantic processing involving actions, such as verb generation and action verb comprehension (see Weiss & Mueller, 2012 review). The fact that adults reveal similar beta responses when performing a motor response and when processing semantics related to action concepts has been attributed to embodiment, or engaging the perceptual correlates of action word meanings. Thus, while the localization of semantic properties related to word meaning may have a prolonged development because of the speed of cortical specialization, beta changes related to embodied semantics may occur earlier, shedding new light on questions of how embodied semantics, and brain function, develop.
Semantic integration in word associations
In addition to changes in theta indexing the semantic retrieval of individual words, theta also relates to semantic integration between word pairs. Like the N400, there is a larger increase in theta for unrelated compared to related words around 400 milliseconds after the presentation of the second word (Klimesch et al., 1994; Maguire et al., 2010). This is thought to index the increased processing necessary to access an unprimed word compared to a primed word. More importantly, the type of relationship between the words results in different patterns of activation. Specifically, although the N400 does not consistently reveal differences between thematically related words (i.e., egg – chicken) compared to taxonomically related words (i.e., dog – chicken), using time frequency analysis, thematically related words elicit a larger increase in theta over right frontal areas compared to taxonomically related words, which authors attributed to an increased use of memory and experience for identifying thematic relationships (Maguire et al., 2010). As such, theta provides an advantage over the N400 in that theta may differentiate additional cognitive processes related to different types of semantic integration.
The fact that theta is sensitive to different types of semantic relationships in adults may provide developmental researchers with a new tool for studying the formation of semantic relationships in early childhood. In the child ERP literature, an N400 occurs for unrelated word pairs by age 5 (Holcomb et al., 1992; S. Wang et al., 2009), although the latency and amplitude of the effect do not become adult-like until about age 12 (S. Wang et al., 2009). These findings suggest a prolonged development of semantic integration; however, ERP methods cannot inform more specific issues, such as the current debate concerning the relative development of taxonomic and thematic relationships. These two ways of grouping semantic knowledge are important in human cognition for different reasons. Taxonomic relationships are essential for language and the formation of lexical categories (Landau et al., 1988; Markman, 1990). Thematic relationships are necessary for making sense of past experiences and predicting future similar experiences (Lin & Murphy, 2001; Markman, 1981). By studying the neurological underpinnings of how children develop these semantic associations we can shed new light on how these relationships mature.
Semantic integration in a sentence
One of the most consistent ERP findings is an N400 response to semantic errors in a sentence, such as “I like my toast with socks” (Kutas & Hillyard, 1984). Such errors elicit theta and gamma changes related to semantic integration in a natural context. Importantly, the frequencies index different aspects of semantic processing, as observed at the point of a semantic (but not syntactic) error in a sentence. For example, the semantically incongruent word in a sentence results in a theta increase around 300–800 milliseconds after onset (Davidson & Indefrey, 2007; Hagoort et al., 2004; Hald et al., 2006; Wang et al., 2012; but see Penolazzi et al., 2009 for counter arguments2) and a decrease in gamma (Bastiaansen et al., 2010; Hagoort et al., 2004; Hald et al., 2006; Penolazzi et al., 2009; L. Wang et al., 2012). Theta has been interpreted as a measure of semantic retrieval and integration, similar, and likely directly related to, the N400 (Davidson & Indefrey, 2007). The changes in gamma provide a different measure of semantic integration. In fact, gamma is the only frequency band that shows a pattern in which power increases for correct words and decreases for violations. This suggests that gamma increases are part of normal neural activity related to typical language processing, specifically that gamma is sensitive to the semantic predictability of an upcoming word (L. Wang et al., 2012).
Evidence that gamma relates to semantic integration in a sentence in adults is of great import to the child literature, in which the development of sentence processing and integration informs both typical and atypical language theory. Children as young as 19- months-old show an N400 to incongruent words in simple sentences, although the latency of the N400 is longer than in adults (Friedrich & Friederici, 2005). The latency decreases until age 10, when the N400 is adult-like (Atchley et al., 2006; Benau et al., 2011; Hahne et al., 2004; Silva-Pereyra, Klarman, et al., 2005; Silva-Pereyra, Rivera-Gaxiola, et al., 2005). Whether this effect represents the semantic retrieval represented by theta or semantic integration represented by gamma cannot be determined based on the N400 findings alone. Identifying the relative development of these processes is important to understanding language comprehension in a natural context.
Summary
Time frequency studies have identified two frequency bands commonly associated with semantic processing: theta which is associated with lexico-semantic retrieval and integration and gamma which is associated with semantic integration. Changes in these frequency bands appear to be sensitive to different semantic properties than the N400, therefore providing additional and complimentary information to our current understanding of semantic processing. Further, findings from the adult literature address questions that are currently of great interest in language development. Specifically, the power and topography of the theta and gamma changes index (1) processing of semantic properties of individual words, including visual and auditory features, (2) the amount of effort necessary for semantic retrieval, and (3) types of semantic integration, such as differentiating taxonomic compared to thematic word pairs, and indexing word-by-word integration during sentence comprehension. The sensitivity of time frequency analysis, demonstrated by these findings, indicates that using this method in EEG studies with children is vital to our understanding of semantic development.
Syntax
Language comprehension relies on the ability to process each word of a sentence and unify that information into a coherent representation of overall meaning. The rules underlying syntax are vast and complex, but processed seemingly effortlessly by adults and mastered by children by about age five. To target individual elements of syntactic comprehension, researchers typically compare processing of sentences with errors, or violations, to processing of correct sentences. This focus on errors may seem counterintuitive for studying naturalistic language processing because syntactic violations are essentially nonexistent in the adult grammar and uncommon in the child grammar; however, comparing violations to correct grammar provides a window into the neuronal underpinnings of specific syntactic elements that is otherwise unavailable.
Using EEG to study how adults process grammatical errors has been instrumental in understanding adult syntactic comprehension. In applying the findings from adults to children, it is important to note that the literature is diverse in the types of syntactic violations that have been studied, how much they disrupt the syntactic structure of the sentence, how common they are in natural speech, and the age of acquisition of each syntactic element. Further, there is much debate about the effectiveness of ERPs at teasing apart neuronal differences that may underlie processing of different types of syntactic errors (Steinhauer & Drury, 2012). A systematic study of error types in conjunction with a more specific measure of the EEG data, as is found in time frequency analysis, may better address the neural underpinnings of syntactic processing.
Consider two types of errors commonly addressed in the adult ERP literature – phrase structure errors and morphosyntactic errors. Broadly, phrase structure is the grammatical arrangement of word types in sentences, such as the rules specifying the relationships between nouns, verbs, articles and prepositions. To demonstrate, consider the sentence, “Yesterday he walked to school”. In English, nouns precede verbs so an alternation of the two would result in a phrase structure violation, “Yesterday walked he to school”. This is a very noticeable violation that is essentially never made in adult grammar and is unlikely in child grammar. Alternatively, morphosyntactic violations are more subtle and are generally considered to be a naturally occurring stage in development (Wexler, 1998). Morphosyntax is the use of small, meaningful units of language to indicate syntax. In the example above, a morphosyntactic error would be the omission of –ed on the verb when the occurrence of the word yesterday indicates past tense, “Yesterday he walk to school”. While both types of errors demonstrate a violation of the syntactic rules of English, identifying the neural processes underlying morphosyntax is likely more important than information about phrase structure violations for our understanding of language development and, importantly, to apply that knowledge to differences in syntactic processing in developmental language delays and deficits.
The ERP literature identifies three components related to grammatical processing that some argue can differentiate neural engagement for phrase structure and morphosyntactic errors as well as the reanalaysis or repair of sentences containing errors. As shown in Table 1, the Early Left Anterior Negativity (ELAN) associates with phrase structure violations (e.g., Hahne & Friederici, 1999), the Left Anterior Negativity (LAN) associates with morphosyntactic violations (e.g., Gunter & Stowe, 1997) and the P600, the component commonly associated with syntactic processing, indicates the reanalaysis and repair of sentence structure across violations or ambiguous sentences (Friederici, 2002). Additional arguments support dual responses for syntactic violations, where ELAN is followed by the P600 (Friederici, 2002). Importantly, the ERP literature generally treats the broad categories of violations and subtypes as essentially the same in terms of implications for syntactic processing, a practice that has recently encountered controversy (Steinhauer & Drury, 2012).
Table 1.
Summary of Findings from ERP Studies of Syntactic Processing in Adults
| Syntactic process |
Violation type (example) |
Study citation | Frequency findings at critical word temporal and topographic information) |
|
|---|---|---|---|---|
| Phrase structure error | Word category violation (e.g., the boy likes the to eat toast) | Bastiaansen et al. (2010) | Gamma Beta |
Decrease 0–1000msec, right centro-temporal Decrease 0–600msec, left frontal and 1000ms, mid- and right-frontal |
| Theta | No change | |||
| Davidson & Indefrey (2007) | Beta Theta |
Decrease 500–900msec, right temporal No change |
||
| Morphosyntactic error | Agreement violation (e.g., two boy like to eat toast; the boy like to eat toast) | Bastiaansen et al. (2002) | Theta | Increase 300–500msec, left anterior |
| Davidson & Indefrey (2007) | Beta Theta |
No change No change |
||
| Gender violation* (e.g., I saw a darkNEUTER cloudCOMMON on the horizon) | Bastiaansen et al. (2002) | Theta | Increase 300–800msec, right anterior | |
| Sentence integration | Correct sentences | Bastiaansen et al. (2002) | Theta | Increase during sentence, 0–4000msec, bilateral centro-parietal |
| Bastiaansen et al. (2010) | Beta Theta |
Increase during sentence, 200–1000msec, frontal Increase during sentence, 200–1000msec, right centro-parietal |
||
does not occur in English
Recently, researchers have begun to use time frequency analysis in syntactic processing studies with the goal of expanding on and more accurately identifying the similarities and differences between violation types addressed in the ERP literature. These studies (see Table 1) are few in number; however, early associations between frequency bands and syntactic processing are beginning to emerge. Similar to the semantic literature, temporal and topographic differences within frequency bands provide new information that could be vitally important to questions about language development, specifically differentiating subtypes of grammatical errors that show unique developmental trajectories in typical and atypical development. Importantly, all of this work focuses on adults; to our knowledge, no studies report time frequency analyses of EEG data on syntactic processing in children. How research of this kind will inform theories of typical and atypical language development is discussed below.
Phrase structure violations
Phrase structure violations result in two temporally and topographically distinct decreases in beta amplitude at the phrase structure violation (Bastiaansen et al., 2010; Davidson & Indefrey, 2007). Following the violation, beta first decreases at 200 milliseconds in left frontal areas and again at 600 milliseconds postviolation over the mid and right frontal and temporal areas. Supporting the claim that beta changes underlie P600, Davidson and Indefrey (2007) reported an association between the later beta decrease and P600, when analyzed from the same data. The finding of two beta changes in response to phrase structure violations coincides with the dual response proposal in the ERP literature, where both an early response (ELAN) and a later repair or reanalysis attempt (P600) follow phrase structure violations. In fact, the presence of two distinct beta changes following phrase structure violations speak to recent challenges to the dual response proposal that argue that the ELAN and P600 are not distinct components but may instead overlap or share underlying mechanisms (Steinhauer & Drury, 2012). The potential for overlap across components is possible using ERP because each component is calculated using its own baseline; however, the dual response shown by beta is calculated across a longer time window with one baseline, thus eliminating the possibility of overlapping effects. In this way, beta seems to be a more sensitive measure of phrase structure violations.
Extending time frequency analysis to include children would greatly increase our understanding of syntax development because the ELAN/P600 dual response shows a broad and sometimes conflicting developmental trend in child ERPs. ELAN is not shown for phrase structure violations in simple, active sentences (i.e., the lion in the roars) in 24-month-olds but is present in 32-month-olds (Oberecker & Friederici, 2006; Oberecker et al., 2005). For more syntactically complex passive sentences (i.e., the goose was in the fed), six year-olds show no ELAN-like effects, 7–10-year-olds present a later-occurring anterior negativity (suggested to be a precursor to ELAN) and an adult-like ELAN follows phrase structure violations for 13-year-olds (Hahne et al., 2004). Alternatively, P600 consistently follows phrase structure violations for children of all ages and sentence structures (Atchley et al., 2006; Hahne et al., 2004; Oberecker & Friederici, 2006; Oberecker et al., 2005). Thus, while appearing to be more sensitive than P600 to phrase structure development, ELAN is also affected by the syntactic complexity of the sentence and the timing of the anterior negativity considered a precursor to ELAN overlaps that of the standard P600. Considering this, it is difficult to create a developmental timeline of phrase structure acquisition using the available ERP data. Time frequency analysis may be a better measure of phrase structure development due to its ability to examine multiple effects in a longer time window without the concern of overlapping effects.
Morphosyntactic violations
Similar to the LAN ERP component, theta indexes general morphosyntactic processing insofar as theta power increases with morphosyntactic violations in a time window similar to that of LAN (~300–500 milliseconds; Bastiaansen et al., 2002; Davidson & Indefrey, 2007). An important difference between LAN and theta is that theta topography demonstrates sensitivity to different subtypes of morphosyntactic processing while LAN does not. Within the broader category of morphosyntactic violations, changes in theta were assessed to gender violations (i.e., I saw a darkneuter cloudcommon) and quantifier-noun number mismatches (i.e., several boy like to eat toast; Bastiaansen et al., 2002). The timing and amplitude of the theta increase were similar across both morphosyntactic violation subtypes but topography differed; gender violations occurred over the right hemisphere and number violations over the left hemisphere. Theta is not responsive to all morphosyntactic errors, however. There was no theta increase with subject number-verb mismatch (i.e., the boy like to eat toast), which instead elicited a reduction in alpha and beta power (Davidson & Indefrey, 2007). These findings highlight the promise of this methodology for identifying distinctions between morphosyntactic subtypes which will greatly inform developmental theory.
As with ELAN, extending the use of time frequency to study syntactic processing related to LAN in children could clarify some conflicting findings. Like adults, LAN indexes morphosyntactic violations in children (Clahsen et al., 2007; Schipke et al., 2011). LAN is not present for all morphosyntax subtypes and for all ages, however. Some of the null findings of LAN are developmentally appropriate. For instance, LAN was not found following tense violations (i.e., my uncle will watching the movie3) in children ages 30 months, 3-years and 4-years (Silva-Pereyra, Klarman, et al., 2005; Silva-Pereyra, Rivera- Gaxiola, et al., 2005), which is expected because that tense marking is not acquired until age 5 (Wexler, 1998). Other absences of LAN are unexpected. LAN was not found following gender violations up to 8-years of age (Courteau et al., 2012) although gender is acquired around 3-years (e.g., Szagun et al., 2007). Also, LAN was reported for plural violations (i.e. the three dog walked) in 8–12 year olds but not 6–7 year olds (Clahsen et al., 2007). This is unexpected considering that children as young as 3-years demonstrate understanding of plural markers (Kouider et al., 2006) although production of plural markers is still inconsistent (Clark & Nikitina, 2009). Thus, LAN does not appear to be sensitive to the acquisition of different morphosyntactic subtypes. This highlights the fact that the ERP is not capturing all of the neural processing related to language comprehension. Using complimentary time frequency analysis may uncover information to address the gap between behavioral findings and ERP results. For instance, because the topography of theta changes is sensitive to morphosyntactic subtypes in adults, these theta changes in children may index subtle aspects of morphosyntactic development.
A P600-like effect is more consistently found for certain morphosyntactic subtypes in children (Atchley et al., 2006; Clahsen et al., 2007; Courteau et al., 2012; Schipke et al., 2011; Silva-Pereyra, Rivera-Gaxiola, et al., 2005). This finding is unexpected given that P600 following morphosyntactic violations is not regularly reported in the adult literature. The P600 identified in the child data varies widely in timing and localization for different morphosyntactic subtypes; for instance, following a plural violation, 8–12 year old children showed a P600 at 1000 milliseconds over occipital and parietal regions (Clahsen et al., 2007) whereas the P600 following a subject number-verb mismatch (i.e., the boy like to eat toast) in 8–13 year old children occurred 620–720 milliseconds after the stimulus over parietal and centroparietal regions (Atchley et al., 2006). Considering this variability, it is unlikely that in this case the P600 represents general morphosyntactic processing. Because theta differentiates morphosyntactic subtypes in adults, this would provide a vital tool for studying typical syntactic development, in which these subtypes develop at different times, and atypical language development, in which the different subtypes are differentially affected by the disorder.
Syntactic unification
Syntactic unification, the ability to successfully integrate new linguistic input into an ongoing syntactic model, is a key component to language comprehension and early language development that is generally overlooked in the ERP literature. One of the few studies to address this process using ERPs found an N400 amplitude decrease with each word in a sentence (Kutas & Federmeier, 2000), although this decrease was interpreted as relating to increased semantic probability throughout a sentence rather than syntactic unification.
Time frequency analysis is more accurate than ERP at tracking amplitude changes over a longer time span, allowing researchers to identify theta and beta changes during syntactic integration over the course of a sentence (Bastiaansen et al., 2010; Bastiaansen et al., 2002). During the course of a sentence there is a linear increase in theta over centro-parietal areas (Bastiaansen et al., 2002; Bastiaansen et al., 20104). Interestingly, the linear increase in theta was found for both grammatically correct sentences and those with grammatical errors, but not when the words of a sentence were presented in a random order (Bastiaansen et al., 2010). This finding led the authors to conclude that this widely distributed effect over the course of the sentence was due to semantic working memory.
Counter to theta, beta plays a role in syntactic integration specifically. The majority of the research examining an association between beta and syntactic processing comes from coherence studies that are not covered in the current paper (see Weiss & Mueller, 2012 for a review); however time frequency analyses indicate that beta gradually increases as a sentence is silently read (Bastiaansen et al., 2010). This beta increase was disrupted at a grammatical error and was completely absent for words displayed in an ungrammatical way (Bastiaansen et al., 2010), lending support to a syntactic rather than semantic function of beta.
The issue of syntactic unification is integral for our understanding of how children acquire phrase structure concepts and begin to combine words in meaningful ways; however, to our knowledge syntactic unification and integration using time frequency has not been addressed in children. Of particular importance is the contribution of semantic working memory (theta) and syntactic integration (beta) to sentence processing, how the contributions of each change during typical development, and how that may differ in children with atypical language.
Summary
Time frequency analysis of the EEG can shed new light on the neuronal underpinnings of syntactic developmentally important ways. First, the topography of time frequency changes appears to differentiate subtypes of phrase structure and morphosyntactic comprehension. To date, the behavioral and ERP literature related to how these abilities develop is filled with conflicting findings, making this an important area for understanding typical and disordered language development. Second, time frequency allows for the analysis of sentence integration during an ongoing sentence, a process that is poorly understood in typical development. To date, despite its potential, this type of research has not been conducted with children.
Important Considerations
Our goal was to highlight the potential of time frequency analysis to address pressing theoretical questions in language development; however we would be remiss if we did not acknowledge some of the complications of using time frequency analysis, a method still in its infancy. First, as with ERPs, time frequency analysis does not remove the inverse problem. Namely, we cannot definitively determine the underlying brain region supporting a specific cognitive or linguistic process based on the scalp location of the frequency change. It is also important to consider issues of noise when conducting time frequency analysis. As a result, many trials are necessary to produce a robust signalto- noise ratio when studying children.
Time frequency analysis does not have the millisecond temporal sensitivity of ERPs because the frequency of the signal must be calculated within a sampling window (Nunez & Srinivasan, 2005). Importantly, a larger window is better for capturing slow wave activity, such as delta (< 4 Hz), but as the window increases, temporal sensitivity decreases. As a result, temporal and frequency precision must be weighed against one another in the analysis (see Bendat & Piersol, 2011 for description of Nyquist criterion).
Due to how new this methodology is and the amount of data available when using time frequency, identifying the best statistical analysis to uncover condition or group differences of interest can be difficult. Specifically, the data collected during an EEG task includes one data point for each millisecond (depending on your collection rate), for each electrode, for each condition, for each participant. In the ERP analyses, this is condensed by focusing on well-studied peaks that occur over an expected time range and location. In the time frequency literature, no such constraints have been identified. As a result, type I errors are possible when analyzing data without specific predictions regarding frequency bands, time and scalp areas of interest. Fortunately, there are promising new data-driven approaches to the statistical analysis that have the potential to combat some of these concerns, such as Principal Component Analysis (PCA; Ferree et al., 2009; Spence et al., 2011), Spectral Factor Analysis (SFA; Shackman et al., 2010), and Independent Components Analysis (ICA; Makeig et al., 1996). Related to this issue, because this research is so new, it can be difficult to interpret findings resulting from the analyses, as the body of work to reference is still quite small. Although there are some difficulties in moving forward in using time frequency in developmental cognitive neuroscience, this problem highlights the need for more work in this area and the need to study the whole of the developmental trajectory, including adults, as one way of addressing these issues.
Roadmap for Language Development Research
One goal of this paper is to encourage developmental cognitive neuroscientists to add time frequency analysis as a tool towards understanding the development of cortical networks. In addition we want to identify areas where we believe this methodology will be most fruitful in studying language development specifically. Above we attempted to review areas of debate in language development that could be addressed using paradigms from the adult neurolinguistics literature. In semantics this includes questions about the role of embodiment in word learning and how identifying taxonomic and thematic semantic relationships may change with age. In syntax this includes questions about how typical children come to comprehend different syntactic rules, such as phrase structures and morphosyntactic rules, with an eye on how that might differ in children with language disorders. Answering these questions would require adapting previously published studies with adults to developmental populations. Additionally, it is important replicate ERP studies that have provided insights into language development and analyze the EEG data using traditional ERP methods as well as the time frequency methods. This will allow us to expand our understanding of how children’s brains develop to support cognition while grounding new findings in the bedrock of previous research.
Conclusions
The data provided in this review show the promise of time frequency analysis of the EEG to better understand developmental cognitive neuroscience and specifically language development. Similar to ERPs, the methodology is non-evasive and has excellent temporal precision. In addition, it can identify neuronal changes occluded by the averaging technique and tease apart multiple potential changes that may underlie amplitude differences in traditional ERP analyses. Expanding this work to children could usher in a new period of growth in our understanding of the how the dynamic neural networks underlying cognitive development change throughout the lifespan.
Acknowledgments
Role of this funding source
This work was supported by NIH grant 1R03HD064629-01 awarded to the first author and the Callier Center Postdoctoral Fellowship provided to the second author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors of the paper have no financial or other relationship with people or organizations that may inappropriately influence this work within the last three years.
One notable exception is the body of work on changes in the mu frequency in infants and adults in relation to social interactions (see Marshall & Meltzoff, 2011 for a review).
Penolazzi, Angrilli & Job (2009) reported no changes in theta with semantic violations in their sentences. However, a closer inspection of their stimuli indicates that the semantic violations used in Penolazzi et al. (a specific noun setting up the expectation for a given verb, the critical word) are more similar to the world knowledge violations used inHagoort et al. (2004) who did not find any changes in theta with world knowledge violations.
Note that this violation is similar to the phrase structure violations used in Bastiaansen et al. (2010) where the error cannot be determined until the final morpheme in the target word. This complications interpretations of whether Silva-Pereyra et al.’s (2005; 2005b) findings represent phrase structure or morphosyntactic processing.
Bastiaansen et al. (2010) used MEG rather than EEG; however, they frame their results in terms of comparisons to their earlier 2002 EEG findings. As a result, the MEG results are included as they present a full picture of syntactic integration across methodologies and speak directly to the EEG findings.
References
- Atchley RA, Rice ML, Betz SK, Kwasny KM, Sereno JA, Jongman A. A comparison of semantic and syntactic event related potentials generated by children and adults. Brain and Language. 2006;99(3):236–246. doi: 10.1016/j.bandl.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Hagoort P. Oscillatory neuronal dynamics during language comprehension. In: Neuper, Klimesch, editors. Progress in brain research. Vol. 159. 2006. p. 179. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Linden M, Keurs M, Dijkstra T, Hagoort P. Theta responses are involved in lexical-semantic retrieval during language processing. Journal of Cognitive Neuroscience. 2005;17(3):530–541. doi: 10.1162/0898929053279469. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Magyari L, Hagoort P. Syntactic unification operations are reflected in oscillatory dynamics during on-line sentence comprehension. Journal of Cognitive Neuroscience. 2010;22(7):1333–1347. doi: 10.1162/jocn.2009.21283. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Oostenveld R, Jensen O, Hagoort P. I see what you mean: theta power increases are involved in the retrieval of lexical semantic information. Brain and Language. 2008;106(1):15–28. doi: 10.1016/j.bandl.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Bastiaansen M, Van Berkum JJ, Hagoort P. Event-related theta power increases in the human EEG during online sentence processing. Neuroscience Letters. 2002;323(1):13–16. doi: 10.1016/s0304-3940(01)02535-6. [DOI] [PubMed] [Google Scholar]
- Bell M. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. Neuroimaging in child neuropsychiatric disorders. 1998:97–111. [Google Scholar]
- Benau EM, Morris J, Couperus JW. Semantic processing in children and adults: Incongruity and the N400. Journal of psycholinguistic research. 2011;40:225–239. doi: 10.1007/s10936-011-9167-1. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Current opinion in neurobiology. 2011;21(3):475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Bendat JS, Piersol AG. Random data: analysis and measurement procedures. (Vol. 729) John Wiley & Sons; 2011. [Google Scholar]
- Bishop DVM, Hardiman MJ, Barry JG. Lower-frequency event-related desynchronization: a signature of late mismatch responses to sounds, which is reduced or absent in children with specific language impairment. The Journal of Neuroscience. 2010;30(46):15578–15584. doi: 10.1523/JNEUROSCI.2217-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Bitan T, Burman DD, Bigio JD, Cone NE, Cao F. Developmental and skill effects on the neural correlates of semantic processing to visually presented words. Human Brain Mapping. 2006;27(11):915–924. doi: 10.1002/hbm.20231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clahsen H, Luck M, Hahne A. How children process overregularizations: Evidence from event-related brain potentials. Journal of child language. 2007;34:601–622. doi: 10.1017/s0305000907008082. [DOI] [PubMed] [Google Scholar]
- Clark EV, Nikitina TV. One vs. more than one: Antecedents to plural marking in early language acquisition. Linguistics. 2009;47(1):103–139. [Google Scholar]
- Coben R, Clarke AR, Hudspeth W, Barry RJ. EEG power and coherence in autistic spectrum disorder. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology. 2008;119(5):1002. doi: 10.1016/j.clinph.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Conboy BT, Rivera-Gaxiola M, Silva-Pereyra J, Kuhl PK. Event-related potential studies of early language processing at the phoneme, word, and sentence levels. Early language development. 2008;5:23–64. [Google Scholar]
- Courteau É, Royle P, Gascon A, Marquis A, Drury JE, Steinhauer K. Gender Concord and Semantic Processing in French Children: An Auditory ERP Study; Paper presented at the Boston University Conference on Language Development; Boston University: 2012. [Google Scholar]
- Davidson DJ, Indefrey P. An inverse relation between event-related and time–frequency violation responses in sentence processing. Brain Research. 2007;1158:81–92. doi: 10.1016/j.brainres.2007.04.082. [DOI] [PubMed] [Google Scholar]
- Düzel E, Penny WD, Burgess N. Brain oscillations and memory. Current opinion in neurobiology. 2010;20(2):143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Ferree TC, Brier MR, Hart J, Kraut MA. Space–time–frequency analysis of EEG data using within-subject statistical tests followed by sequential PCA. Neuroimage. 2009;45(1):109–121. doi: 10.1016/j.neuroimage.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in cognitive sciences. 2002;6(2):78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friedrich M, Friederici AD. Semantic sentence processing reflected in the event-related potentials of one- and two-year old children. Developmental Neuroscience. 2005;16:1801–1804. doi: 10.1097/01.wnr.0000185013.98821.62. [DOI] [PubMed] [Google Scholar]
- Fuster JnM. Network memory. Trends in neurosciences. 1997;20(10):451–459. doi: 10.1016/s0166-2236(97)01128-4. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Poeppel D. Cortical oscillations and speech processing: emerging computational principles and operations. Nature neuroscience. 2012;15(4):511–517. doi: 10.1038/nn.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter T, Stowe L. In: When syntax meets semantics. Psychophysiology. Mulder G, editor. Vol. 34. 1997. pp. 660–676. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Hald LA, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304(5669):438–441. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Hahne A, Eckstein K, Friederici AD. Brain signatures of syntactic and semantic processes during children's language development. Journal of Cognitive Neuroscience. 2004;16(7):1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Hahne A, Friederici AD. Electrophysiological evidence for two steps in syntactic analysis: Early automatic and late controlled processes. Journal of Cognitive Neuroscience. 1999;11(2):194–205. doi: 10.1162/089892999563328. [DOI] [PubMed] [Google Scholar]
- Hald LA, Bastiaansen M, Hagoort P. EEG theta and gamma responses to semantic violations in online sentence processing. Brain and Language. 2006;96(1):90–105. doi: 10.1016/j.bandl.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hart J, Maguire MJ, Motes M, Mudar RA, Chiang H-S, Womack KB, Kraut MA. Semantic memory retrieval circuit: Role of pre-SMA, caudate, and thalamus. Brain and Language. 2012 doi: 10.1016/j.bandl.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Holcomb PJ, Coffey SA, Neville HJ. Visual and auditory sentence processing: A developmental analysis using event - related brain potentials. Developmental Neuropsychology. 1992;8(2–3):203–241. [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Howell SR, Jankowicz D, Becker S. A model of grounded language acquisition: Sensorimotor features improve lexical and grammatical learning. Journal of Memory and Language. 2005;53(2):258–276. [Google Scholar]
- Jutras MJ, Buffalo EA. Synchronous neural activity and memory formation. Current opinion in neurobiology. 2010;20(2):150. doi: 10.1016/j.conb.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan F, Oudeyer PY, Bergen B. Computational models in the debate over language learnability. infant and child development. 2008;17(1):55–80. [Google Scholar]
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Research Reviews. 1999;29(2):169–195. doi: 10.1016/s0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Wimmer H, Gruber WR, Röhm D, Schwaiger J, Hutzler F. Alpha and beta band power changes in normal and dyslexic children. Clinical Neurophysiology. 2001;112(7):1186–1195. doi: 10.1016/s1388-2457(01)00543-0. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Wimmer H, Schwaiger J, Röhm D, Gruber WR, Hutzler F. Theta band power changes in normal and dyslexic children. Clinical Neurophysiology. 2001;112(7):1174–1185. doi: 10.1016/s1388-2457(01)00545-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Pfurtscheller G, Schimke H. Pre-and post-stimulus processes in category judgement tasks as measured by event-related desynchronization (ERD) Journal of Psychophysiology. 1992 [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalography and clinical Neurophysiology. 1994;91(6):428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Kouider S, Halberda J, Wood J, Carey S. Acquisition of English number marking: The singular-plural distinction. Language Learning and Development. 2006;2(1):1–25. [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67(5):713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Electrophysiology reveals semantic memory use in language comprehension. Trends in cognitive sciences. 2000;4(12):463–470. doi: 10.1016/s1364-6613(00)01560-6. [DOI] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Brain potentials during reading reflect word expectancy and semantic association. Nature. 1984;307(5947):161–163. doi: 10.1038/307161a0. [DOI] [PubMed] [Google Scholar]
- Landau B, Smith LB, Jones SS. The importance of shape in early lexical learning. Cognitive Development. 1988;3(3):299–321. [Google Scholar]
- Li SC, Lindenberger U. Coconstructed functionality instead of functional normality. Behavioral and Brain Sciences. 2002;25(6):761–761. [Google Scholar]
- Lin EL, Murphy GL. Thematic relations in adults' concepts. Journal of Experimental Psychology: General. 2001;130(1):3. doi: 10.1037/0096-3445.130.1.3. [DOI] [PubMed] [Google Scholar]
- Maguire MJ, Brier MR, Ferree TC. EEG theta and alpha responses reveal qualitative differences in processing taxonomic versus thematic semantic relationships. Brain and Language. 2010;114(1):16–25. doi: 10.1016/j.bandl.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Maguire MJ, Hirsh-Pasek K, Golinkoff RM. 14 A Unified Theory of Word Learning: Putting Verb Acquisition in Context. Action Meets Word: How Children Learn Verbs: How Children Learn Verbs. 2006a:364. [Google Scholar]
- Maguire MJ, Hirsh-Pasek K, Golinkoff RM. A Unified Theory of Word Learning: Putting Verb Acquisition in Context. In: Hirsh-Pasek K, Golinkoff RM, editors. Action meets word: How children learn verbs. New York: Oxford University Press; 2006b. pp. 364–391. [Google Scholar]
- Makeig S, Bell AJ, Jung T-P, Sejnowski TJ. Independent component analysis of electroencephalographic data. Advances in neural information processing systems. 1996:145–151. [Google Scholar]
- Maouene J, Sethuraman N, Laakso A, Maouene M. The body region correlates of concrete and abstract verbs in early child language. Cognition, Brian, Behavior: An Interdisciplinary Journal. 2011:449. [Google Scholar]
- Markman EM. Comprehension monitoring. Children's oral communication skills. 1981:61–84. [Google Scholar]
- Markman EM. Constraints children place on word meanings. Cognitive Science. 1990;14(1):57–77. [Google Scholar]
- Marshall PJ, Bar-Haim Y, Fox NA. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology. 2002;113(8):1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Meltzoff AN. Neural mirroring systems: Exploring the EEG mu rhythm in human infancy. Developmental Cognitive Neuroscience. 2011;1(2):110. doi: 10.1016/j.dcn.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meteyard L, Rodriguez Cuadrado S, Bahrami B, Vigliocco G. Coming of age: A review of embodiment and the neuroscience of semantics. Cortex. 2010 doi: 10.1016/j.cortex.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Münte TF, Wieringa BM, Weyerts H, Szentkuti A, Matzke M, Johannes S. Differences in brain potentials to open and closed class words: class and frequency effects. Neuropsychologia. 2001;39(1):91–102. doi: 10.1016/s0028-3932(00)00095-6. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford University Press, USA: 2005. [Google Scholar]
- Nunez PL, Srinivasan R. Electric fields of the brain: the neurophysics of EEG. Oxford University Press, USA: 2006. [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neuroscience & Biobehavioral Reviews. 2010;34(7):1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberecker R, Friederici AD. Syntactic event-related potential components in 24-month-olds' sentence comprehension. Developmental Neuroscience. 2006;17:1017–1021. doi: 10.1097/01.wnr.0000223397.12694.9a. [DOI] [PubMed] [Google Scholar]
- Oberecker R, Friedrich M, Friederici AD. Neural correlates of syntactic processing in two-year-olds. Journal of Cognitive Neuroscience. 2005;17:1667–1678. doi: 10.1162/089892905774597236. [DOI] [PubMed] [Google Scholar]
- Osterhout L, Allen M, McLaughlin J. Words in the brain: lexical determinants of word-induced brain activity. Journal of Neurolinguistics. 2002;15(3):171–187. [Google Scholar]
- Özge A, Toros F, Çömelekoğlu Ü. The role of hemispheral asymmetry and regional activity of quantitative EEG in children with stuttering. Child Psychiatry & Human Development. 2004;34(4):269–280. doi: 10.1023/B:CHUD.0000020679.15106.a4. [DOI] [PubMed] [Google Scholar]
- Penolazzi B, Angrilli A, Job R. Gamma EEG activity induced by semantic violation during sentence reading. Neuroscience Letters. 2009;465(1):74–78. doi: 10.1016/j.neulet.2009.08.065. [DOI] [PubMed] [Google Scholar]
- Penolazzi B, Spironelli C, Angrilli A. Delta EEG activity as a marker of dysfunctional linguistic processing in developmental dyslexia. Psychophysiology. 2008;45(6):1025–1033. doi: 10.1111/j.1469-8986.2008.00709.x. [DOI] [PubMed] [Google Scholar]
- Pivik R, Broughton R, Coppola R, Davidson R, Fox N, Nuwer M. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30(6):547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Poeppel D. The maps problem and the mapping problem: Two challenges for a cognitive neuroscience of speech and language. Cognitive neuropsychology. 2012;29(1–2):34–55. doi: 10.1080/02643294.2012.710600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. The neuroscience of language: on brain circuits of words and serial order. Cambridge University Press: 2003. [Google Scholar]
- Rivera-Gaxiola M, Garcia-Sierra A, Lara-Ayala L, Cadena C, Jackson-Maldonado D, Kuhl PK. Event-related Potentials to an English/Spanish syllabic contrast in Mexican 10–13-month-old infants. ISRN neurology. 2012;2012 doi: 10.5402/2012/702986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W. What does phase information of oscillatory brain activity tell us about cognitive processes? Neuroscience and biobehavioral reviews. 2008;32(5):1001–1013. doi: 10.1016/j.neubiorev.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Schipke CS, Friederici AD, Oberecker R. Brain responses to case-marking violations in German preschool children. NeuroReport. 2011;22(16):850. doi: 10.1097/WNR.0b013e32834c1578. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Maxwell JS, Greischar LL, Davidson RJ. Identifying robust and sensitive frequency bands for interrogating neural oscillations. Neuroimage. 2010;51(4):1319–1333. doi: 10.1016/j.neuroimage.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin AJ, Picton TW, Miller LM. Brain oscillations during semantic evaluation of speech. Brain and cognition. 2009;70(3):259–266. doi: 10.1016/j.bandc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Pereyra J, Klarman L, Lin LJ, Kuhl PK. Sentence processing in 30-month-old children: An event-related potential study. Cognitive Neuroscience and Neuropsychology. 2005;16:645–648. doi: 10.1097/00001756-200504250-00026. [DOI] [PubMed] [Google Scholar]
- Silva-Pereyra J, Rivera-Gaxiola M, Kuhl PK. An event-related brain potential study of sentence comprehension in preschoolers: semantic and morphosyntactic processing. Cognitive Brain Research. 2005;23(2–3):247–258. doi: 10.1016/j.cogbrainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Smiley P, Huttenlocher J. Conceptual development and the child’s early words for events, objects, and persons. Beyond names for things: Young children’s acquisition of verbs. 1995:21–61. [Google Scholar]
- Smolík F. Noun Imageability Facilitates the Acquisition of Plurals: Survival Analysis of Plural Emergence in Children. Journal of psycholinguistic research. 2013:1–16. doi: 10.1007/s10936-013-9255-5. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nature neuroscience. 2003;6(3):309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Spence JS, Brier MR, Hart J, Jr, Ferree TC. Removing an intersubject variance component in a general linear model improves multiway factoring of event - related spectral perturbations in group EEG studies. Human Brain Mapping. 2011 doi: 10.1002/hbm.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spironelli C, Penolazzi B, Angrilli A. Dysfunctional hemispheric asymmetry of theta and beta EEG activity during linguistic tasks in developmental dyslexia. Biological psychology. 2008;77(2):123. doi: 10.1016/j.biopsycho.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Steinhauer K, Drury JE. On the early left-anterior negativity (ELAN) in syntax studies. Brain and Language. 2012;120(2):135–162. doi: 10.1016/j.bandl.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stella F, Treves A. Associative memory storage and retrieval: involvement of theta oscillations in hippocampal information processing. Neural plasticity. 2011;2011 doi: 10.1155/2011/683961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroganova TA, Orekhova EV. EEG and infant states. Infant EEG and event-related potentials. 2007:251–287. [Google Scholar]
- Stroganova TA, Orekhova EV, Posikera IN. EEG alpha rhythm in infants. Clinical Neurophysiology. 1999;110(6):997–1012. doi: 10.1016/s1388-2457(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Human Brain Mapping. 2005;27(3):202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szagun G, Stumper B, Sondag N, Franik M. The acquisition of gender marking by young German-speaking children: Evidence for learning guided by phonological regularities. Journal of child language. 2007;34(03):445–471. doi: 10.1017/s0305000906007951. [DOI] [PubMed] [Google Scholar]
- Torkildsen JvK, Friis Hansen H, Svangstu JM, Smith L, Simonsen HG, Moen I, Lindgren M. Brain dynamics of word familiarization in 20-montholds: Effects of productive vocabulary size. Brain and Language. 2009;108(2):73–88. doi: 10.1016/j.bandl.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Torkildsen JvK, Sannerud T, Syversen G, Thormodsen R, Simonsen HG, Moen I, Lindgren M. Semantic organization of basic-level words in 20-month-olds: An ERP study. Journal of Neurolinguistics. 2006;19(6):431–454. [Google Scholar]
- Torkildsen JvK, Svangstu JM, Hansen HF, Smith L, Simonsen HG, Moen I, Lindgren M. Productive vocabulary size predicts event-related potential correlates of fast mapping in 20-month-olds. Journal of Cognitive Neuroscience. 2008;20(7):1266–1282. doi: 10.1162/jocn.2008.20087. [DOI] [PubMed] [Google Scholar]
- Van Elk M, Van Schie H, Zwaan R, Bekkering H. The functional role of motor activation in language processing: motor cortical oscillations support lexical-semantic retrieval. Neuroimage. 2010;50(2):665. doi: 10.1016/j.neuroimage.2009.12.123. [DOI] [PubMed] [Google Scholar]
- Vannest J, Karunanayaka PR, Schmithorst VJ, Szaflarski JP, Holland SK. Language networks in children: evidence from functional MRI studies. American Journal of Roentgenology. 2009;192(5):1190–1196. doi: 10.2214/AJR.08.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux J-P, Rodriguez E, Martinerie J. The brainweb: phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2(4):229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vigliocco G, Vinson DP, Druks J, Barber H, Cappa SF. Nouns and verbs in the brain: a review of behavioural, electrophysiological, neuropsychological and imaging studies. Neuroscience & Biobehavioral Reviews. 2011;35(3):407–426. doi: 10.1016/j.neubiorev.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu Z, Bastiaansen M. Integration or predictability? A further specification of the functional role of gamma oscillations in language comprehension. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dong X, Ren Y, Yang Y. The development of semantic priming effect in childhood: An event-related potential study. NeuroReport. 2009;20(6):574–578. doi: 10.1097/WNR.0b013e328329f215. [DOI] [PubMed] [Google Scholar]
- Weiss S, Mueller HM. “Too many betas do not spoil the broth”: the role of beta brain oscillations in language processing. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler K. Very early parameter setting and the unique checking constraint: A new explanation of the optional infinitive stage. Lingua. 1998;106(1):23–79. [Google Scholar]