Abstract
The pace of pathogen discovery is increasing dramatically. This reflects not only factors that enable the appearance and globalization of new microbial infections but also improvements in methods for ascertainment. New molecular diagnostic platforms; investments in pathogen surveillance in wildlife, domestic animals and humans; and the advent of social media tools that mine the world wide web for clues to outbreaks of infectious disease are proving invaluable in early recognition of threats to public health. Additionally, models of microbial pathogenesis are becoming more complex, providing insights into the mechanisms by which microorganisms can contribute to chronic illnesses like cancer, peptic ulcer disease and mental illness. Here we review the contributions of each of these elements to infectious disease emergence and strategies for addressing the challenges of pathogen surveillance and discovery.
Introduction
When the H1N1 influenza virus struck in 1918, little was known about how infectious diseases emerge or their routes of transmission. Indeed, even the identity of the causative agent as a virus (i.e. capable of passing through a filter) rather than the bacterium Hemophilus influenza championed by some leading microbiologists, was in dispute until late in the course of the pandemic1. The 1918 virus had a case-fatality rate estimated at 10-20%, spread to 6 continents, infected ~500 million people, and killed approximately 3% of the world's population 2,3. The SARS coronavirus pandemic of 2003, the first pandemic of the 21st century, had case-fatality rate near 10% but infected far fewer people (8,096)4. Transmissibility as estimated by the R0 (the number of cases generated through contact with an infected individual) is similar for influenza (2-3)5 and SARS (2-5)6,7. However, the global response to SARS was facilitated by advances in epidemiology and microbiology that enabled rapid containment and identification of the causative agent as a novel coronavirus. The discovery process is faster still today. Whereas two large teams invested several days in characterizing SARS coronavirus genetic material amplified in tissue culture by using classical dideoxy sequencing techniques8,9, high throughput sequencing platforms employed in more recent outbreaks such as LuJo in South Africa have allowed identification of novel agents in clinical materials in 48 to 72 hours10.
As a consequence of globalization of travel and trade, infectious agents are expanding in geographic range and appearing in new contexts. Thus, clinicians and public health officials must be prepared to detect and respond to the unexpected. The ongoing development of new antimicrobial drugs, therapeutic antibodies, vaccines and probiotics means that early and accurate diagnosis can have profound implications for medical management and public health. This is particularly true for viral infections where, until recently, opportunities for effective intervention were limited to HIV, hepatitis C virus and herpesvirus infections. Surveillance and discovery efforts are bearing fruit in chronic disorders and in studies of normal physiology as well as in the investigation of acute diseases like pneumonia, diarrhea, meningitis/encephalitis and hemorrhagic fevers. The links between Helicobacter pylori and peptic ulcer11, human papillomavirus and cervical cancer12 and polyomaviruses and Merkel cell carcinoma13 are prominent examples in which microorganisms contribute to the pathogenesis of disorders that were idiopathic only a few years ago. Insights into the role of the human microbiome in nutrition, allergies and autoimmunity have led to implementation of the same surveillance and discovery platforms employed to investigate classic infectious diseases 14,15. Finally, although there have been no recent examples of bioterrorism, the risk has only increased with political instability and the accessibility of synthetic genomics that enable the creation or recreation of virulent pathogens.
In this essay, I will discuss factors that contribute to the emergence (and reemergence) of infectious diseases, the evolution of strategies and tools for pathogen surveillance and discovery, and future prospects for the field. To guide the reader we provide a time line of events and innovations described in the text (See Timeline)
Factors in microbial emergence
Globalization of travel and trade
Travel and trade are increasingly global. For instance, the number of international airline flights has nearly doubled over the past 15 years from just under 500,000 in 1996 to close to 850,000 in 2011 (U.S. Bureau of Transportation Statistics: www.bts.gov/). John F. Kennedy airport for example, one of two international airports in the greater New York metropolitan area, USA, hosts nonstop flights to 100 international destinations, and serves nearly 12 million international customers annually (http://www.bts.gov/press_releases/2012/bts017_12/html/bts017_12.html). Similar data apply worldwide for airports serving large urban centers. This means that an infected individual or mosquito can rapidly travel around the world in less than 24 hours, making it not surprising that air travel has been implicated in the global dissemination of HIV, SARS coronavirus, West Nile virus, chikungunya virus, influenza virus and M. tuberculosis16-18.
Indeed it is perhaps more remarkable that so few outbreaks of infectious disease have been attributed to air travel, especially given that the transportation of plants and animals has continued to grow dramatically with the development of global agribusinesses and urbanization. Whereas the global population and food production have increased at similar rates (74% and 100%) since 1975, the international food trade has burgeoned by more than 200% (United Nations: http://esa.un.org/unpd/wpp/Excel-Data/population.htm, http://faostat.fao.org/). A hundred years ago, most fresh food was produced and consumed in a radius of a few kilometres, it is now not unusual for individuals to consume plants and animals harvested thousands of kilometres away19.
Agricultural practices
Contamination of meat has impacted international trade of livestock on a number of occasions20, with examples including agents that can threaten humans such as prions, influenza viruses and Rift Valley fever virus, or agents that threaten the livestock themselves such as foot and mouth disease virus and Schmallenberg virus21. However, bacteria, viruses and parasites, particularly those present in faeces, can also contaminate fruits and vegetables to cause disease in humans and other animals, resulting in costly food recalls and affecting consumer demand22,23. High-density farming of livestock, poultry and fish is frequently associated with the use of antibiotics as growth promoters that results in the emergence of antibiotic-resistant bacteria24,25.
Centralization of food production and processing, particularly of ground meat or raw fruits and vegetables, has resulted in outbreaks of infectious diseases that may be distributed over large geographic areas. Furthermore, illegal trafficking in wildlife with pets or food, estimated to exceed US $10 Billion and US $15 Billion per annum in sales, respectively, is difficult to monitor (U.S. Department of State, Wildlife Trafficking, http://www.state.gov/e/oes/env/wlt/index.htm). Nonetheless, there is evidence that these activities are associated with the introduction of microorganisms into new environs that may pose threats to public health. Analysis of bushmeat from bats, rodents and primates that was confiscated in major ports has revealed evidence of foamy viruses, herpesviruses and pathogenic bacteria26. Imported pets have been linked to outbreaks of human infection with poxviruses and salmonella, as well other pathogens26.
Although plant pathogens do not infect humans or other animals, infection of food crops can have dire economic consequences. Recently, with greater appreciation of the importance of pollinators in food production and the recognition of colony collapse disorder, increased attention has been directed towards the potential for emerging infections of honeybees (Apis mellifera) and other pollinators, particularly with regards to viruses, fungi and external parasites27. Mariculture (ocean aquaculture) is also at risk of emerging infectious diseases, as demonstrated by recent reports of novel viruses in farmed salmon28-30. Attention has also increasingly focused on the role of land use dynamics in infectious disease emergence31. Deforestation and the expansion of agriculture and the extractive industries, particularly in tropical regions with high wildlife biodiversity, has led directly or indirectly to the emergence of HIV/AIDS, Nipah virus and filoviruses32,33.
Climate change and mass migration
Global warming is already extending the geographic range of mosquitos and ticks that harbor and transmit Plasmodium sp. and arboviruses, resulting in outbreaks of malaria, dengue and yellow fever in new locations34. Recent examples in North America include the appearance of dengue fever in Florida in 2010 35,36 and a surge in cases of West Nile encephalitis in Texas in 201237. Mass migration (due to war, natural disaster, poverty and desertification) can lead to increases in the population density, not only of humans, but also of disease vectors like rodents and ectoparasites that carry pathogenic viruses and bacteria. These factors, along with poor sanitation, malnutrition, lack of access to vaccines, and exposure to contaminated food and water create a perfect storm for the emergence and transmission of infectious diseases38,39.
Laboratory analyses
Culture, once the mainstay for detection of organisms in the laboratory, is still emphasized in some public health organizations and remains vital to clinical microbiology, chiefly as a tool for testing the utility of drugs. However, genetic methods have moved to the forefront in microbial surveillance and diagnostics40-44. The foundation for most of these methods is the polymerase chain reaction (PCR) developed in 1983 by Kary Mullis (See Timeline). PCR requires minimal equipment and operator training, can be completed in minutes rather than days, is less expensive, and has been adapted to portable instruments that can be used in the field in developing countries or near a patient's bedside. Furthermore, like other genetic methods, PCR may succeed in detecting an organism in instances where fastidious requirements confound cultivation. Most PCR assays approved for clinical applications test for the presence of a single type of bacterium or virus. Such assays, described as singleplex, are used to screen for any evidence of infection (e.g., hepatitis B virus in blood products used for transfusion) or to quantitate microorganism levels when assessing response to therapy (e.g. HIV burden in serum or plasma of subjects receiving anti-retroviral medication).
Multiplex Assays
Multiplex PCR, initially implemented for screening human genetic polymorphisms has been extended to microbiology wherein assays have been developed that allow simultaneous screening for the presence of up to 30 different microorganisms40. Such assays are particularly important for differential diagnosis in medicine where many distinct infectious agents may be implicated in diseases like pneumonia, diarrhea or meningitis/encephalitis. Thus, although multiplex PCR assays are rare outside of research and public health laboratories, there is reason to believe that they will ultimately gain wider acceptance.
An even broader platform is DNA microarrays where millions of genetic probes are bound to glass or silicon wafers and tested for their capacity to bind complementary sequences in clinical and environmental samples. Binding is typically detected through the measurement of fluorescent molecules attached to nucleic acid amplified from sample extracts. Such microarrays have the potential to survey the entire known microbial world; however, implementation has been hampered by low sensitivity and cumbersome processing. Recent prototypes have been developed that suggest it may be possible to circumvent these obstacles through use of portable devices that employ nanofluidics and electronic nanocircuitry45,46.
Genomics and Metagenomics
The most disruptive advances in microbial surveillance have been achieved in DNA sequencing. The emergence of high throughput sequencing over the past decade has enabled the discovery of new microorganisms, rapid resolution of the causes of outbreaks of infectious diseases, and the development of metagenomics, a field wherein investigators inventory the complex microbial communities found in humans, domesticated animals, wildlife, plants and various environments. Although initially confined to specialized laboratories due to high costs for instruments and supplies costs along with requirements for sophisticated personnel, technical improvements have increased access for a broader segment of the research community. One index to the evolution of sequencing technology is the per-base cost which decreased from $5,000 per megabase in 2001 with classical dideoxy methods, to $15 per megabase in 2008 with pyrosequencing (http://www.genome.gov/sequencingcosts), to $0.5 per megabase in 2012 with the Illumina platform47. Another is the time required for obtaining sequence data. Whereas the SARS coronavirus genome was sequenced over the course of a week by a large team in 20038 a single investigator could sequence that same genome in a few hours in 2012. Recent examples of the power of advancements in methods for genomic sequencing include reports on the evolution of influenza48, hepatitis C49 and human immunodeficiency viruses50, the human origin of livestock associated methicillin-resistant Staphyloccocus aureus 51and the spread of antibiotic resistant Klebsiella pneumoniae between and in health care institutions52.
Metagenomic analyses40,53,54 have revealed dynamic relationships between microorganisms and hosts that influence normal physiological processes like digestion55,56 and immune response57, may be factors in the pathogenesis of autoimmune diseases57 and cancer 54, and likely contribute to global climate regulation through effects on marine plankton58. The challenge now is not in obtaining sequence data but in analyzing it. Millions of sequence reads must be assembled into continuous strings of genetic information, and identified as originating in a microorganism or a host by using algorithms that compare the sequences obtained for similarity to those already catalogued in existing databases. Few investigators now have the in-house processing power and expertise required for these types of analyses; however, access to large computer clusters can be achieved through cloud computing and high-throughput sequencing software that is rapidly becoming more user friendly.
Microbial surveillance and forecasting
Passive and active surveillance
Surveillance is broadly divided into the categories of passive and active. Whereas passive surveillance employs data that already exist or are collected routinely, active surveillance implies a new investment in and/or processes for microorganism collection and analysis. A classic example of passive surveillance is the concept of reportable diseases. Most regional and national public health authorities maintain lists of infectious diseases and laboratory tests indicative of infection that must be reported (CDC National Notifiable Diseases Surveillance System, http://wwwn.cdc.gov/nndss/script/downloads.aspx) These include infections characterized by human-to-human transmission, such as sexually transmissible diseases (e.g., syphilis or gonorrhea) and vaccine-preventable diseases (e.g., measles), as well as those where detection indicates the presence in the environment of an infectious agent that poses a substantial threat to public health, such as hemorrhagic fever viruses or highly pathogenic bacteria like Yersinia pestis, the agent of the bubonic plague. On the non-human side, agricultural authorities monitor infections like foot and mouth disease, which have important economic implications. Passive surveillance is informative and inexpensive, but may underestimate the true frequency of an agent or a disease. Furthermore, by definition it cannot detect risk prior to onset of symptoms.
Surveillance Using Social Media
Largely due to the influence of Joshua Lederberg59, a pioneer in microbial genetics and the use of computers for communication as well as data analysis, internet based infectious disease surveillance is well established. ProMED-mail (Programme for Monitoring Emerging Infectious Diseases), created in 1994, provides continuous free email updates on new or evolving outbreaks and epidemics60. Submissions from a grassroots network of readers are curated by a panel of experts who post submissions with commentary in 5 languages to a listserve comprising more than 60,000 subscribers in 185 different countries. GPHIN (Global Public Health Intelligence Network)61 scans news services across the globe in 9 languages for information concerning outbreaks. Unlike ProMED-mail, GPHIN is a fee-based, private subscription, and does not systematically validate its posts, although the WHO began providing verification services when GPHIN was added to the Global Outbreak Alert Response Network (GOARN) in 2000.
HealthMap62 is a hybrid of the passive and active surveillance strategies employed respectively by ProMED-mail and GPHIN. It integrates reports from news media, ProMED-mail and official documents into a user-friendly map that displays real-time updates of disease emergence. HealthMap also allows for public submission of geo-referenced observations or “crowd sourcing” of apparent disease occurrence via its website or a number of smart phone based apps such as Outbreaks Near Me. Google Flu Trends (http://www.google.org/flutrends/) is similar but aggregates search data to estimate global influenza activity.
InSTEDD (Innovative Strategies to Emergencies, Diseases and Disasters), proposed during Larry Brilliant's talk at the 2006 TED Conference “One Wish to Change the World”, develops open source tools to improve global information collection and exchange (http://www.instedd.org/). What is anticipated but not yet achieved are systems that aggregate medical service utilization data, prescription and over-the-counter drug purchases, and other chatter that could promote situational awareness and focus epidemiological investigation. Zoonoses, infections that originate in wildlife or domestic animals, account for more than 70% of emerging infectious diseases63; thus, to be proactive, a substantive surveillance system64 for humans must also include nonhuman animal surveillance.
Modeling infectious disease emergence
Quantitative analyses of emerging and re-emerging infectious diseases have enabled the identification of geographic hotspots of infectious disease emergence and of underlying drivers (primarily human activities) that facilitate the process31,65-68. The recent development of high quality, global scale data sets of human demographics, agricultural production, land-use change, travel and trade patterns, climate, and wildlife distribution has greatly improved the resolution and specificity of predictive modeling38. This has resulted in substantive advances in risk analyses from the original hot spots maps38 (Fig. 1). These risk algorithms are being used to focus passive and active surveillance programmes on sites, populations, professions, and species of domestic animals and wildlife where there is an increased probability of known or novel high threat pathogen emergence.
Figure 1. Hot spots of outbreaks for recently emerging and re-emerging infectious diseases.
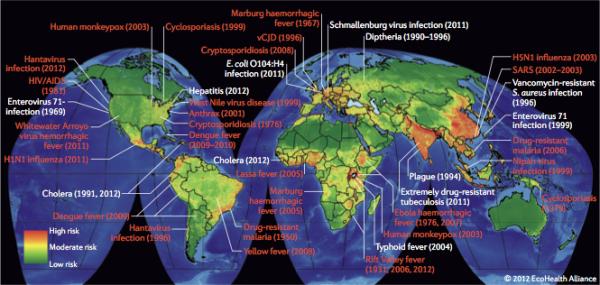
Green: low density of outbreaks; red: high density of outbreaks. Zoonotic infections are highlighted in red text. Ref. 112 and unpublished data from Peter Daszak (EcoHealth Alliance)[Will ask author for permissions for this data and for the use of the maps].
An example is the United States Agency for International Development's Emerging Pandemic Threats programme (http://avianflu.aed.org/eptprogram/) wherein hot spots models are used to prioritize regions and countries for investments in surveillance, laboratory diagnostics, outbreak response, and collaboration in 20 countries in Africa, South America, and Asia. Algorithms used to build hot spots models are continuously tested and modified in light of experimental data derived from human, domestic animal and wildlife sample analysis.
Proof of causation and mechanisms of pathogenesis
Finding footprints of a microorganism is only the first step in establishing a causative role in disease. In some instances the connection is immediately apparent because precedent supports plausibility, for example, finding a new type of ebolavirus in an individual with a hemorrhagic fever, or a new stain of Vibrio cholera in a diarrhea outbreak. However, in others the link is more tenuous. Host factors can have a profound impact on susceptibility to infection and consequences thereof. Morbidity and mortality can be high with otherwise innocuous agents in individuals with immunological deficits, whether due to genetic mutations, age, malnutrition, infection (e.g. HIV/AIDS) or complications of cancer treatment or transplantation.
Mechanisms of disease can vary (Box 1). Microorganisms may cause damage at the site of infection as a direct result of replication, or as an indirect effect of innate or adaptive immune responses to microbial gene products. They may also induce neoplasia through interference with cell cycle controls. Although not yet confirmed in human disease, work in animal models indicates that viruses can reduce production of hormones or neurotransmitters vital to normal physiology without apparent cell or organ damage69. Linkage to infection is facilitated in each of the foregoing examples because the microorganism, its nucleic acid and/or protein are found at the site of pathology. More difficult to recognize are instances where expression of microbial toxins have remote effects, or infection induces immune responses to the microorganism that break tolerance to self. Clostridium bacteria, for example, can infect the skin or gastrointestinal tract and produce toxins that act on the nervous system to cause spasm (C. tetani)70 or flaccid paralysis (C. botulinum)71. Streptococcal infection of the skin or oropharynx can result in autoimmunity, culminating in cardiac damage (rheumatic heart disease) and brain dysfunction (Sydenham chorea)72.
The best-established criteria for proof of causation were formulated by Loeffler and Koch in the 1880s. Popularly known as Koch's postulates,73 they require that an agent be present in every case of the disease, specific for the disease and sufficient to reproduce the disease after culture and inoculation into a naïve host. Rivers74 modified them by acknowledging the significance of neutralizing antibodies to an agent as evidence of infection. Fredericks and Relman75 noted that pathogens may be recognized by molecular methods before they can be cultured, and allowed as evidence the presence of microbial sequences as well as of infectious microorganisms. Thus, although Koch's postulates remain the gold standard, they need not be fulfilled to implicate an agent. Indeed, a focus on Koch's postulates may impede the successful discovery and response to emerging pathogens and models for infectious disease.
Through the discovery and characterization of nearly 500 viruses, we have developed a three level scoring system for establishing levels of confidence in strength of association from possible to definitive (Box 2). Poor design or execution of pathogen discovery projects can lead to spurious links between infectious agents and diseases that result in inappropriate and potentially dangerous treatments or rejection of health promoting interventions like vaccines40. The effort required to break these links can be greater than that invested in building them, particularly in disorders where the prognosis is grim and/or treatment options are limited. Based on experience with amyotrophic lateral sclerosis (enteroviruses), mental illness (bornaviruses), autism (MMR vaccine) and myalgic encephalomyelitis/chronic fatigue syndrome (XMRV and pMLV), we have developed a strategy for trying and acquitting microorganisms that addresses social as well as scientific considerations (Box 3).
Progress in Microbial Detection: Field Cases
Over the last three decades, innovations in genetic and information technologies have enhanced and expedited the rate of detection and solution of outbreaks of infectious disease. The following examples illustrate this progress in methods for acquiring public health intelligence.
In 1976, the Centers for Disease Controls was alerted that 11 war veterans had died from pneumonia after returning from the US Bicentennial Convention of the American Legion in Philadelphia 76. A case definition was established76,77, and Pennsylvania health officials were notified of a potential statewide epidemic. Public-health personnel searched hospitals, news reports and obituaries, while a telephone hotline was established to accept tips from the general public. Ultimately, 221 cases were identified. All were individuals who had visited the convention hotel lobby or walked along the adjacent street. Initial efforts to culture an infectious agent failed76,78. Histological staining of lungs of individuals with Legionnaire's disease revealed inflammation but no microorganisms.
The breakthrough came when Joseph McDade, a rickettsia expert at the CDC, recognized liver disease in some victims and in guinea pigs inoculated with extracts from patients. He inoculated embryonated chicken eggs with livers extracts from guinea pigs, and then reproduced the disease by inoculating additional guinea pigs with the extracts from the embryonic chicken eggs. This enrichment through passage in these model systems resulted in the discovery of a novel fastidious gram-negative bacterium, Legionella pneumophila.
The 4 months that elapsed between the onset of the outbreak in Philadelphia and the identification of the causative agent would not be required today. Whereas almost 2 weeks passed before the CDC was notified in 1976, alerts are now distributed at near real-time with services like ProMED-mail and HealthMap. Additionally, access to an electronic registry of the convention's guests would obviate the need for a statewide, grassroots search for cases. Culture-independent methods of pathogen discovery would also enhance response time.
Recent improvements in surveillance and microbial forensic science were illustrated by the 2011 outbreak in Europe of a Shiga-toxin producing Escherichia coli O104:H4 illustrates 79,80. In May, Germany's national public-health agency—the Robert Koch Institute—sent representatives to investigate a cluster of hemolytic–uremic syndrome associated with bloody diarrhea in Hamburg. By the time the outbreak resolved, more than 3,000 people were infected and 40 died. Economic losses were substantive, particularly in Spain, where early inaccurate links to cucumbers from Spain led to a import ban on produce81,82.
The initial clues to the identity of the casual agent were obtained by using PCR83,84. Genomic characterization was rapidly achieved using high-throughput sequencers85. Within 3 days of receipt of a clinical sample, sequence data was released into the public domain for global, crowd-sourced bioinformatics analysis86. Genome assembly was completed in the next 24 hours, which enabled the development of specific diagnostic tests and insights into pathogenesis and phylogenetic origin of the bacterium. Integration of laboratory findings with patient surveys ultimately led to implication of bean sprouts from a single farm in Lower Saxony79.
Another comparison involves the discovery of Borna disease virus (BDV)87 and the related but distinct avian bornavirus. Borna disease, named for a town in Saxony where a characteristic fatal meningoencephalitis was described in horses, has been known in the veterinary literature since the 1700s88-90. The transmissibility of disease was first demonstrated in 1920s; however, nearly 60 years were required to establish methods for viral culture and another 10 years were needed for classification as a novel non-segmented, negative-strand RNA virus91. The capacity to culture the virus led to serological assays that provided evidence of a connection to human neuropsychiatric diseases in 198392.
Intrigued by the potential importance of BDV in human disease and challenged by the failure of efforts to characterize the virus by electron microscopy or to isolate BDV nucleic acids, I initiated a subtractive cloning project that culminated in identification of the first BDV sequences in 199093. An additional 4 years were required to determine its genomic organization94,95. With specific cloned reagents in hand, I reasoned that it would be straightforward to determine whether BDV was indeed a human pathogen; however, the issue lingered until 2011 when blinded multicenter analyses, molecular and serologic, ultimately revealed no evidence of human infection 96.
By contrast, avian bornavirus (ABV), the causative agent of proventricular dilatation disease (PDD), a wasting syndrome in psittacine birds (parrots), was identified in only a few days.97,98 The breakthrough was enabled by access to genome databases and culture-independent methods for pathogen discovery, including viral microarrays and high-throughput sequencing. Subsequently, ABV PCR assays and serology have allowed for the investigation and containment of outbreaks in aviaries99.
Future prospects
Although we will continue to see instances where classical approaches to microorganism hunting like culture and pursuit of Koch's postulates will succeed, pathogen discovery has evolved from a whodunit exercise performed by solitary investigators to team efforts of microbiologists, cellular and systems biologists, geographers, mathematicians and other specialists. Models of disease have expanded from simple one-to-one relationships between organ damage and the presence of a single agent therein to consider more complex mechanisms that may enable recognition of links between microorganisms and mental illness, obesity, vascular disease, cancer and autoimmunity.
Growth in international travel and trade has led to the globalization of infectious diseases. It has also fostered a new appreciation of the relationship between land use, particularly in the developing world, and the appearance of zoonoses. Globalization of risk across national and species boundaries has promoted the development of international health regulations100 that emphasize technology transfer and data sharing, as well as programmes that proactively survey, not only humans, but the entire animal kingdom for insights into potential threats to public health and economic welfare.
The integration of human and animal medicine, the advent of tools for rapid and efficient molecular characterization of microorganisms and hosts, and emphasis on the use of social media to promote early detection of risk promises the potential for development of a truly global immune system.
Box 1. Mechanisms of microbial pathogenesis.
Direct damage at site of replication due to cell lysis, apoptosis or autophagy
Indirect damage at site of replication due to expression of proteins that serve as targets for humoral or cell-mediated immune responses
Elaboration of toxins or other products that have deleterious local or systemic effects
Induction of expression of host cytokines and chemokines that have deleterious local or systemic effects
Abrogation of tolerance for self, resulting in autoimmunity
Immunosuppression resulting in opportunistic infection
Neoplasia
Disturbance of differentiated cell functions
Disruption of embryogenesis
Box 2. Levels of Certainty in Pathogen Discovery.
Level 1. Possible causative relationship
The initial clue in pathogen discovery is evidence of exposure to a microorganism in one or more individuals with disease. This evidence may be the presence of a microorganism in blood, other body fluids, faeces or tissues that can be grown on media or in cultured cells or animals. It may also be a nucleic acid or protein component of a microorganism, a specific adaptive immune response to a microorganism, or visualization by light, immuno- or electron microscopy. Keys features of level 1 include:
Microorganism is isolated and cultured
Microbial nucleic acids are detected by polymerase chain reaction, DNA microarray or sequencing
Microbial proteins are detected using immunological methods or mass spectroscopy
Antibodies to a microorganism are detected using immunological methods
Morphological evidence of the presence of a microorganism is found by microscopy or electron microscopy
Level 2. Probable causal relationship
More confidence in the clinical significance of the association is achieved when a causal relationship is biologically plausible. Evidence of biological plausibility may include the presence of microbial nucleic acid, protein or microorganism-specific antibody in or adjacent to cells showing signs of disease, or precedent for a similar disease due to a similar agent in either the same or a similar host. The strength of the association is increased when the concentration of the microorganism (or nucleic acid, protein or antibody) is high, the antibody response indicates recent exposure (IgM and/or a recent increase in IgG titre), and there is evidence of infection in other individuals, all of whom have disease. However, a microorganism can be implicated in disease without a robust immune response, particularly in chronic infections.
Different microorganisms can cause similar diseases. Although clusters of disease are the ideal proving ground, many opportunities for discovery involve only one or a few cases; indeed, clusters may not be appreciated as such until details of common exposure (e.g., through travel, food, water, intermediary hosts) become apparent. Keys features of level 2 include:
Microbial burden (levels of an infectious bacterium, virus, fungus, parasite or associated nucleic acids or protein) is high
Antibody response is consistent with recent exposure (presence of IgM or an increased IgG titre)
Evidence of infection is found in more than one individual with disease
Location of a microorganism, its nucleic acid or proteins, or immune response to the microorganism is correlated with disease
Similar diseases are known to occur with exposure to similar microorganisms in the same or other hosts
Level 3. Confirmed causal relationship
Proof of causation can be achieved through fulfillment of Koch's postulates or by mitigation or prevention of disease through use of a microorganism-specific drugs, antibodies or vaccines. Although not formally required we insist on replication of results by independent investigators. Keys features of level 3 include:
Koch's postulates are met
Drugs mitigate or prevent disease and reduce levels of the microorganism, its nucleic acid or proteins, or the immune response to the microorganism
Vaccines mitigate or prevent disease
Box 3. The road to pathogen de-discovery.
Step 1. Initial Finding
Report links disease or syndrome to an infectious agent, toxin or other factor.
Step 2. Failure to reproduce
Independent efforts by other investigators fail to confirm the statistically significant association described in the initial report. Members of the scientific community begin to question validity of the association but cannot exclude the possibility that the apparent difference in results may reflect variability in the samples or assays employed.
Step 3. Plausible doubt
A biologically plausible explanation is developed that accounts for findings in the initial report that may have led to misinterpretation. Mainstream scientific community rejects validity of the association. However, some investigators, patients and advocates might continue to believe the merits of the initial report, necessitating Step 4.
Step 4. Comprehensive De-discovery
A well-powered, blinded analysis of samples from well-characterized subjects—that includes the investigators responsible for the initial report and uses a strategy approved in advance by the investigators, representatives of patients and patient advocacy groups—fails to replicate the findings of the initial report.
Acknowledgements
The author thanks Nsikan Akpan for assistance with the manuscript; Peter Daszak, William Karesh, Cadhla Firth, Mady Hornig and Eddie Holmes for thoughtful comments; and the National Institutes of Health (AI57158), United States Agency for International Development (PREDICT) and the Defense Threat Reduction Agency for financial support.
Footnotes
Timeline for microbial surveillance and disease. Major events in microbial discovery, as listed in this review.
References
- 1.Taubenberger JK, Hultin JV, Morens DM. Discovery and characterization of the 1918 pandemic influenza virus in historical context. Antiviral therapy. 2007;12:581–591. [PMC free article] [PubMed] [Google Scholar]
- 2.Durand JD. Historical estimates of world population, an evaluation. 1974 [Google Scholar]
- 3.Taubenberger JK, Morens DM. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. doi:10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. http://www.who.int/csr/sars/country/table2004_04_21/en/index.html.
- 5.Mills CE, Robins JM, Lipsitch M. Transmissibility of 1918 pandemic influenza. Nature. 2004;432:904–906. doi: 10.1038/nature03063. doi:10.1038/nature03063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley S, et al. Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–1966. doi: 10.1126/science.1086478. doi:10.1126/science.1086478. [DOI] [PubMed] [Google Scholar]
- 7.Lipsitch M, et al. Transmission dynamics and control of severe acute respiratory syndrome. Science. 2003;300:1966–1970. doi: 10.1126/science.1086616. doi:10.1126/science.1086616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marra MA, et al. The Genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. doi:10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 9.Rota PA, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. doi:10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 10.Briese T, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS pathogens. 2009;5:e1000455. doi: 10.1371/journal.ppat.1000455. doi:10.1371/journal.ppat.1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurata JH, Nogawa AN. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. Journal of clinical gastroenterology. 1997;24:2–17. doi: 10.1097/00004836-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. doi:10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 13.Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Current opinion in oncology. 2012;24:141–149. doi: 10.1097/CCO.0b013e32834fc9fe. doi:10.1097/CCO.0b013e32834fc9fe. [DOI] [PubMed] [Google Scholar]
- 14.Blumberg R, Powrie F. Microbiota, disease, and back to health: a metastable journey. Science translational medicine. 2012;4:137rv137. doi: 10.1126/scitranslmed.3004184. doi:10.1126/scitranslmed.3004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. doi:10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosseini P, Sokolow SH, Vandegrift KJ, Kilpatrick AM, Daszak P. Predictive power of air travel and socio-economic data for early pandemic spread. PLoS One. 2010;5:e12763. doi: 10.1371/journal.pone.0012763. doi:10.1371/journal.pone.0012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowdall NP, Evans AD, Thibeault C. Air Travel and TB: an airline perspective. Travel medicine and infectious disease. 2010;8:96–103. doi: 10.1016/j.tmaid.2010.02.006. doi:10.1016/j.tmaid.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Mangili A, Gendreau MA. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365:989–996. doi: 10.1016/S0140-6736(05)71089-8. doi:10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maki DG. Don't eat the spinach--controlling foodborne infectious disease. N Engl J Med. 2006;355:1952–1955. doi: 10.1056/NEJMp068225. doi:10.1056/NEJMp068225. [DOI] [PubMed] [Google Scholar]
- 20.Newell DG, et al. Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. International journal of food microbiology. 2010;139(Suppl 1):S3–15. doi: 10.1016/j.ijfoodmicro.2010.01.021. doi:10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Food Safety Authority publishes its second report on the Schmallenberg virus. Euro surveillance : bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 2012;17 [PubMed] [Google Scholar]
- 22.Beuchat LR. Surface decontamination of fruits and vegetables eaten raw : a review. Food Safety Unit, World Health Organization; 1998. [Google Scholar]
- 23.Berger CN, et al. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environmental microbiology. 2010;12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x. doi:10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Alvarez L, Dawson S, Cookson B, Hawkey P. Working across the veterinary and human health sectors. The Journal of antimicrobial chemotherapy. 2012;67(Suppl 1):i37–i49. doi: 10.1093/jac/dks206. doi:10.1093/jac/dks206. [DOI] [PubMed] [Google Scholar]
- 25.McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34(Suppl 3):S93–S106. doi: 10.1086/340246. doi:10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 26.Smith KM, et al. Zoonotic viruses associated with illegally imported wildlife products. PLoS One. 2012;7:e29505. doi: 10.1371/journal.pone.0029505. doi:10.1371/journal.pone.0029505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox-Foster DL, et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science. 2007;318:283–287. doi: 10.1126/science.1146498. doi:10.1126/science.1146498. [DOI] [PubMed] [Google Scholar]
- 28.Finstad OW, Falk K, Lovoll M, Evensen O, Rimstad E. Immunohistochemical detection of piscine reovirus (PRV) in hearts of Atlantic salmon coincide with the course of heart and skeletal muscle inflammation (HSMI). Veterinary research. 2012;43:27. doi: 10.1186/1297-9716-43-27. doi:10.1186/1297-9716-43-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovoll M, et al. A novel totivirus and piscine reovirus (PRV) in Atlantic salmon (Salmo salar) with cardiomyopathy syndrome (CMS). Virol J. 2010;7:309. doi: 10.1186/1743-422X-7-309. doi:10.1186/1743-422X-7-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palacios G, et al. Heart and skeletal muscle inflammation of farmed salmon is associated with infection with a novel reovirus. PLoS One. 2010;5:e11487. doi: 10.1371/journal.pone.0011487. doi:10.1371/journal.pone.0011487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karesh WB, et al. The Ecology of Zoonoses: Their Natural and Unnatural Histories. Lancet (In review) doi: 10.1016/S0140-6736(12)61678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chua KB, et al. Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. doi:10.1016/S0140-6736(99)04299-3. Lancet. 1999;354:1257–1259. doi: 10.1016/S0140-6736(99)04299-3. [DOI] [PubMed] [Google Scholar]
- 33.Pulliam JR, et al. Agricultural intensification, priming for persistence and the emergence of Nipah virus: a lethal bat-borne zoonosis. Journal of the Royal Society, Interface / the Royal Society. 2012;9:89–101. doi: 10.1098/rsif.2011.0223. doi:10.1098/rsif.2011.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shuman EK. Global climate change and infectious diseases. N Engl J Med. 2010;362:1061–1063. doi: 10.1056/NEJMp0912931. doi:10.1056/NEJMp0912931. [DOI] [PubMed] [Google Scholar]
- 35.Locally acquired Dengue--Key West, Florida, 2009-2010. MMWR. Morbidity and mortality weekly report. 2010;59:577–581. [PubMed] [Google Scholar]
- 36.Adalja AA, Sell TK, Bouri N, Franco C. Lessons learned during dengue outbreaks in the United States, 2001-2011. Emerg Infect Dis. 2012;18:608–614. doi: 10.3201/eid1804.110968. doi:10.3201/eid1804.110968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roehr B. Texas records worst outbreak of West Nile virus on record. BMJ. 2012;345:e6019. doi: 10.1136/bmj.e6019. doi:10.1136/bmj.e6019. [DOI] [PubMed] [Google Scholar]
- 38.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morse SS. Factors and determinants of disease emergence. Rev Sci Tech. 2004;23:443–451. doi: 10.20506/rst.23.2.1494. [DOI] [PubMed] [Google Scholar]
- 40.Lipkin WI. Microbe hunting. Microbiology and molecular biology reviews : MMBR. 2010;74:363–377. doi: 10.1128/MMBR.00007-10. doi:10.1128/MMBR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casas I, Tenorio A, Echevarria JM, Klapper PE, Cleator GM. Detection of enteroviral RNA and specific DNA of herpesviruses by multiplex genome amplification. J Virol Methods. 1997;66:39–50. doi: 10.1016/s0166-0934(97)00035-9. [DOI] [PubMed] [Google Scholar]
- 42.Nichol ST, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 43.Shirato K, et al. Diagnosis of human respiratory syncytial virus infection using reverse transcription loop-mediated isothermal amplification. J Virol Methods. 2007;139:78–84. doi: 10.1016/j.jviromet.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tumpey TM, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 45.Rosenstein JK, Wanunu M, Merchant CA, Drndic M, Shepard KL. Integrated nanopore sensing platform with sub-microsecond temporal resolution. Nat Methods. 2012;9:487–492. doi: 10.1038/nmeth.1932. doi:10.1038/nmeth.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaittanis C, Santra S, Perez JM. Emerging nanotechnology-based strategies for the identification of microbial pathogenesis. Advanced drug delivery reviews. 2010;62:408–423. doi: 10.1016/j.addr.2009.11.013. doi:10.1016/j.addr.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loman NJ, et al. Performance comparison of benchtop high-throughput sequencing platforms. Nature biotechnology. 2012;30:434–439. doi: 10.1038/nbt.2198. doi:10.1038/nbt.2198. [DOI] [PubMed] [Google Scholar]
- 48.Bhatt S, Holmes EC, Pybus OG. The genomic rate of molecular adaptation of the human influenza A virus. Molecular biology and evolution. 2011;28:2443–2451. doi: 10.1093/molbev/msr044. doi:10.1093/molbev/msr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bull RA, et al. Sequential bottlenecks drive viral evolution in early acute hepatitis C virus infection. PLoS pathogens. 2011;7:e1002243. doi: 10.1371/journal.ppat.1002243. doi:10.1371/journal.ppat.1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poon AF, et al. Reconstructing the Dynamics of HIV Evolution within Hosts from Serial Deep Sequence Data. PLoS computational biology. 2012;8:e1002753. doi: 10.1371/journal.pcbi.1002753. doi:10.1371/journal.pcbi.1002753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fitzgerald JR. Human origin for livestock-associated methicillin-resistant Staphylococcus aureus. mBio. 2012;3:e00082–00012. doi: 10.1128/mBio.00082-12. doi:10.1128/mBio.00082-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Snitkin ES, et al. Tracking a Hospital Outbreak of Carbapenem-Resistant Klebsiella pneumoniae with Whole-Genome Sequencing. Science translational medicine. 2012;4:148ra116. doi: 10.1126/scitranslmed.3004129. doi:10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011;365:347–357. doi: 10.1056/NEJMra1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Streit WR, Daniel R. Metagenomics : methods and protocols. Humana Press; 2010. [Google Scholar]
- 55.Faust K, Raes J. Microbial interactions: from networks to models. Nature reviews. Microbiology. 2012;10:538–550. doi: 10.1038/nrmicro2832. doi:10.1038/nrmicro2832. [DOI] [PubMed] [Google Scholar]
- 56.Claesson MJ, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. doi:10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 57.Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell host & microbe. 2008;3:417–427. doi: 10.1016/j.chom.2008.05.001. doi:10.1016/j.chom.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Danovaro R, et al. Marine viruses and global climate change. FEMS microbiology reviews. 2011;35:993–1034. doi: 10.1111/j.1574-6976.2010.00258.x. doi:10.1111/j.1574-6976.2010.00258.x. [DOI] [PubMed] [Google Scholar]
- 59.Microbial Evolution and Co-Adaptation: A Tribute to the Life and Scientific Legacies of Joshua Lederberg: Workshop Summary. National Academies Press; US: 2009. [PubMed] [Google Scholar]
- 60.Madoff LC. ProMED-mail: an early warning system for emerging diseases. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2004;39:227–232. doi: 10.1086/422003. doi:10.1086/422003. [DOI] [PubMed] [Google Scholar]
- 61.Mykhalovskiy E, Weir L. The Global Public Health Intelligence Network and early warning outbreak detection: a Canadian contribution to global public health. Canadian journal of public health. Revue canadienne de sante publique. 2006;97:42–44. doi: 10.1007/BF03405213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freifeld CC, Mandl KD, Reis BY, Brownstein JS. HealthMap: global infectious disease monitoring through automated classification and visualization of Internet media reports. Journal of the American Medical Informatics Association : JAMIA. 2008;15:150–157. doi: 10.1197/jamia.M2544. doi:10.1197/jamia.M2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woolhouse ME, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg Infect Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. doi:10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karesh WB CR. One world--one health. Clin Med. 2009 Jun;9:259–260. doi: 10.7861/clinmedicine.9-3-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lederberg J, Shope RE, Oaks SC, editors. Emerging Infections: Microbial Threats to Health in the United States. National Academy Press; 1992. [PubMed] [Google Scholar]
- 66.Morse SS. Factors in the emergence of infectious disease. Emerg Infect Dis. 1995;1:7–15. doi: 10.3201/eid0101.950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daszak P, Cunningham AA, Hyatt AD. Emerging infectious diseases of wildlife -threats to biodiversity and human health. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 68.Smolinski MS, Hamburg MA, Lederberg J, editors. Microbial threats to health: emergence, detection, and response. The National Academies Press; 2003. p. 398. [PubMed] [Google Scholar]
- 69.Lyte M. Microbial endocrinology and infectious disease in the 21st century. Trends in microbiology. 2004;12:14–20. doi: 10.1016/j.tim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Bruggemann H, et al. The genome sequence of Clostridium tetani, the causative agent of tetanus disease. Proc Natl Acad Sci U S A. 2003;100:1316–1321. doi: 10.1073/pnas.0335853100. doi:10.1073/pnas.0335853100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Segelke B, Knapp M, Kadkhodayan S, Balhorn R, Rupp B. Crystal structure of Clostridium botulinum neurotoxin protease in a product-bound state: Evidence for noncanonical zinc protease activity. Proc Natl Acad Sci U S A. 2004;101:6888–6893. doi: 10.1073/pnas.0400584101. doi:10.1073/pnas.0400584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Allos BM. Association between Campylobacter infection and Guillain-Barre syndrome. J Infect Dis. 1997;176(Suppl 2):S125–128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 73.Koch R. Ueber bakteriologische Forschung, Verhandl. des X. Interna. Med. Congr., Berlin 1890., Berlin, Germany., 1891.
- 74.Rivers TM. Viruses and Koch's Postulates. J Bacteriol. 1937;33:1–12. doi: 10.1128/jb.33.1.1-12.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fraser DW. The challenges were legion. The Lancet infectious diseases. 2005;5:237–241. doi: 10.1016/S1473-3099(05)70054-2. doi:10.1016/S1473-3099(05)70054-2. [DOI] [PubMed] [Google Scholar]
- 77.Fraser DW, et al. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. doi:10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 78.McDade JE, et al. Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. doi:10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 79.Frank C, et al. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med. 2011;365:1771–1780. doi: 10.1056/NEJMoa1106483. doi:10.1056/NEJMoa1106483. [DOI] [PubMed] [Google Scholar]
- 80.Karch H, et al. The enemy within us: lessons from the 2011 European Escherichia coli O104:H4 outbreak. EMBO molecular medicine. 2012;4:841–848. doi: 10.1002/emmm.201201662. doi:10.1002/emmm.201201662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.BBC E. coli cucumber scare: Spain angry at German claims. http://www.bbc.co.uk/news/world-europe-13605910.
- 82.Reuters Germany says beansprouts may be behind E.coli. http://www.reuters.com/article/2011/06/05/us-ecoli-idUSTRE7511UX20110605.
- 83.Bielaszewska M, et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. The Lancet infectious diseases. 2011;11:671–676. doi: 10.1016/S1473-3099(11)70165-7. doi:10.1016/S1473-3099(11)70165-7. [DOI] [PubMed] [Google Scholar]
- 84.Scheutz F, et al. Characteristics of the enteroaggregative Shiga toxin/verotoxin-producing Escherichia coli O104:H4 strain causing the outbreak of haemolytic uraemic syndrome in Germany, May to June 2011. Euro surveillance : bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 2011;16 doi: 10.2807/ese.16.24.19889-en. [DOI] [PubMed] [Google Scholar]
- 85.Mellmann A, et al. Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PLoS One. 2011;6:e22751. doi: 10.1371/journal.pone.0022751. doi:10.1371/journal.pone.0022751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rohde H, et al. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med. 2011;365:718–724. doi: 10.1056/NEJMoa1107643. doi:10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- 87.Hornig M, Briese T, Lipkin WI. Borna disease virus. J Neurovirol. 2003;9:259–273. doi: 10.1080/13550280390194064. doi:10.1080/13550280390194064. [DOI] [PubMed] [Google Scholar]
- 88.Abildgaard P. Pferde- und Vieharzt in einem kleinen Auszüge. Johann Heinrich Schubothe; 1795. [Google Scholar]
- 89.Trichtern V. Pferd-Anatomie, oder Neu-auserlesen-vollkommen- verbessert-und ergänztes Roß-Artzeney-Buch. Adam Jonathan Felßecker; 1716. [Google Scholar]
- 90.von Sind J. Der im Feld und auf der Reise geschwind heilende Pferdearzt, welcher einen gründlichen Unterricht von den gewöhnlichsten Krankheiten der Pferde im Feld und auf der Reise wie auch einen auserlesenen Vorrath der nützlichsten und durch die Erfahrung bewährtesten Heilungsmitteln eröffnet. Heinrich Ludwig Brönner; 1767. [Google Scholar]
- 91.Lipkin W, Briese T. In: Fields Virology. Knipe DM, Howley RM, editors. Lippincott, Williams & Wilkins; 2007. pp. 1829–1851. [Google Scholar]
- 92.Rott R, et al. Detection of serum antibodies to Borna disease virus in patients with psychiatric disorders. Science. 1985;228:755–756. doi: 10.1126/science.3922055. [DOI] [PubMed] [Google Scholar]
- 93.Lipkin WI, Travis GH, Carbone KM, Wilson MC. Isolation and characterization of Borna disease agent cDNA clones. Proc Natl Acad Sci U S A. 1990;87:4184–4188. doi: 10.1073/pnas.87.11.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Briese T, de la Torre JC, Lewis A, Ludwig H, Lipkin WI. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc Natl Acad Sci U S A. 1992;89:11486–11489. doi: 10.1073/pnas.89.23.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Briese T, et al. Genomic organization of Borna disease virus. Proc Natl Acad Sci U S A. 1994;91:4362–4366. doi: 10.1073/pnas.91.10.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hornig M, et al. Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Molecular psychiatry. 2012;17:486–493. doi: 10.1038/mp.2011.179. doi:10.1038/mp.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Honkavuori KS, et al. Novel borna virus in psittacine birds with proventricular dilatation disease. Emerg Infect Dis. 2008;14:1883–1886. doi: 10.3201/eid1412.080984. doi:10.3201/eid1412.080984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kistler AL, et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: identification of a candidate etiologic agent. Virol J. 2008;5:88. doi: 10.1186/1743-422X-5-88. doi:10.1186/1743-422X-5-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kistler AL, Smith JM, Greninger AL, Derisi JL, Ganem D. Analysis of naturally occurring avian bornavirus infection and transmission during an outbreak of proventricular dilatation disease among captive psittacine birds. J Virol. 2010;84:2176–2179. doi: 10.1128/JVI.02191-09. doi:10.1128/JVI.02191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alison K, Hottes BR, Fran Sharples Rapporteurs. Biosecurity Challenges of the Global Expansion of High-Containment Biological Laboratories. National Research Council; Washington, DC: 2012. [PubMed] [Google Scholar]


