Abstract
Recent advances in our understanding of the community structure and function of the human microbiome have implications for the potential role of probiotics and prebiotics in promoting human health. A group of experts recently met to review the latest advances in microbiota/microbiome research and discuss the implications for development of probiotics and prebiotics, primarily as they relate to effects mediated via the intestine. The goals of the meeting were to share recent advances in research on the microbiota, microbiome, probiotics, and prebiotics, and to discuss these findings in the contexts of regulatory barriers, evolving healthcare environments, and potential effects on a variety of health topics, including the development of obesity and diabetes; the long term consequences of exposure to antibiotics early in life to the gastrointestinal (GI) microbiota; lactose intolerance; and the relationship between the GI microbiota and the central nervous system, with implications for depression, cognition, satiety, and mental health for people living in developed and developing countries. This report provides an overview of these discussions.
Keywords: microbiome, probiotics, prebiotics, intestinal microbiota, health disorders
Introduction
Major advances have occurred in our understanding of the composition and metabolic capabilities of microbial communities in the human body, gains made from revolutionary advances in DNA sequencing, metagenomic analytical techniques, and computational biology. These strides have greatly increased our understanding of the bacterial genomes present in these microbial communities, the boundaries of normal variation, and how variations in microbial composition are associated with pathology and disease. Indeed, the number of published studies on microbiome-related research has increased four-fold between 2005 and 2012. Much remains to be learned, however, about how to translate this information to probiotic or prebiotic interventions that may modify the microbiome and promote human health.
A one-day conference, “Probiotics, Prebiotics, and the Host Microbiome,”1 hosted by the New York Academy of Sciences, the Sackler Institute for Nutrition Science, and the International Scientific Association for Probiotics and Prebiotics (ISAPP), including experts in the fields of microbiome research, probiotics, and prebiotics, met on 12 June 2013 in New York City to review the latest research on these topics, discuss the implications for public health, and increase communication and collaboration. The conference was divided into five sessions and included oral and visual presentations, as well as a panel discussion.
Putting probiotics, prebiotics, and the microbiome into translational context
The conference opened with a presentation by John Hutton (University of York, United Kingdom) on the economic challenges associated with probiotic- and prebiotic-based interventions. Hutton said that during the past 20 years economic evaluation has become widespread practice in the pharmaceutical sector, including cost versus benefit analyses to aid decision making about utilization and reimbursement of disease treatments.2 In fact, health technology assessment (HTA), including the demonstration of the cost-effectiveness of new products, has become an essential tool for gauging the value of drug-based approaches worldwide. The economic evaluation methods used in comprehensive HTA consider a number of elements, including clinical (e.g., efficacy and safety), economic, social (equity of access), and political (incentive for innovation) aspects.
The value of nutrition for achieving improved health outcomes has become recognized. Consequently, nutritional products are now considered to be a health technology. However, the economic evaluation methods used in HTA are more difficult to apply in the context of nutrition, where reduction of disease risk is the most frequent aim. It appears unlikely that HTA methods can be used to bridge the boundary between drug and food (including probiotics and prebiotics) in the immediate future because of the difference in endpoints needed for supporting data in each category. Additional clarity is needed on the business model for economic evaluation of general public and disease prevention benefits, compared to disease treatment claims. For disease-specific interventions, higher evidential standards will be expected in jurisdictions where healthcare system funding is sought. For more general interventions to change dietary behavior, as with many public health policies, the benefits in terms of disease-risk reduction may not be realized until well into the future. HTA in this context relies on modeling and projections of costs and benefits, and is subject to many uncertainties that can only be reduced by the collection of long-term epidemiological data linking interventions with lifetime health outcomes. However, some HTA agencies, such as the National Institute for Health and Clinical Excellence (NICE) in England and Wales, are developing methods to address the analytical and data issues.
Assessment of the economic feasibility of probiotic or prebiotic interventions is further complicated by the variability in products, intervention protocols, local study procedures, populations targeted and trial outcomes. These differences must be addressed across centers to allow advancement of tools in the domain of probiotics and prebiotics. Going forward, the approach described by Whitehead et al.3 for assessing the economic viability of a nutritional intervention for irritable bowel syndrome (IBS) might serve as a good model for evaluating probiotic- or prebiotic-based interventions.a In order for economic evaluation of probiotics and prebiotics as nutritional interventions to succeed, quality data (especially involving disease interventions), behavioral changes by individuals, and economic drivers must become part of the overall health outcome process.
Programming the microbiome
Metagenomic studies of the human intestinal microbiome reveal that the human gut carries on average about 540,000 microbial genes, representing the dominant microbes in this ecosystem.4 Approximately 55% of these genes constitute the core metagenome (i.e., are genes shared among at least 50% of individuals), while many other genes appear to be unique and/or present in less than 20% of individuals.
The second conference session, moderated by David Mills (University of California, Davis), had presentations on the early development of the intestinal microbiota and how fluctuations in the human microbiome can correlate with changes in human health. The aim of the session was to provide an overview of the initial programming of the intestinal microbiota and how environmental factors or therapeutics can alter the composition and contribute to various metabolic disease states.
David Relman (Stanford University School of Medicine) opened the session with a perspective as an infectious disease specialist and clinical researcher interested in variation in microbial diversity patterns as a function of time (microbial succession) and in response to perturbation. Relman described the initial acquisition and development of the gut microbiota during the early weeks of life, and emphasized its significant role in human health and disease. In addition to contributing to food digestion and nutrition and the regulation of metabolism, the gut microbiota is involved in development and terminal maturation of host mucosa, regulation of immune system target recognition and responses, and resistance to colonization and invasion by pathogens. Humans are born essentially devoid of an intestinal microbiota, but this highly important ecological system is soon acquired after birth and eventually comprises over 90% of the cells of the human adult. Despite the advances in tools and techniques for studying the human gut microbiome, a number of questions remain about its early acquisition and succession.
Relman and colleagues study the assembly of the gut, oral, and skin microbiota of infants during the first several years of life to better understand the development of habitat specificity and the factors underpinning compositional variation during this critical timespan. Multiple factors appear to influence the early succession of the gut microbiota in individual infants, including mode and timing of birth, host genetics, diet, environmental exposures (other humans, animals, medications), and health status. Evidence from a number of studies indicates that the fecal microbiota of the vaginally-delivered newborn resembles that of the maternal gastrointestinal tract or vagina, while that of Caesarian-delivered newborns resembles that of the maternal skin.5, 6 Various taxonomic groups are acquired early during a window of permissivity and persist over time, possibly undergoing shifts in population from dominant to subdominant, while other taxonomic groups may be acquired later or disappear altogether (Fig. 1). Recent studies using cultivation-independent evaluation of the fecal microbiota of premature infants have revealed a low level of diversity, high inter-individual variability, and a capacity for abrupt temporal shifts in species- and strain-level composition. The long-term effects of these differences are not fully understood. A key emerging question in the field is how these early life differences in microbiota development influence health through childhood and later in life.
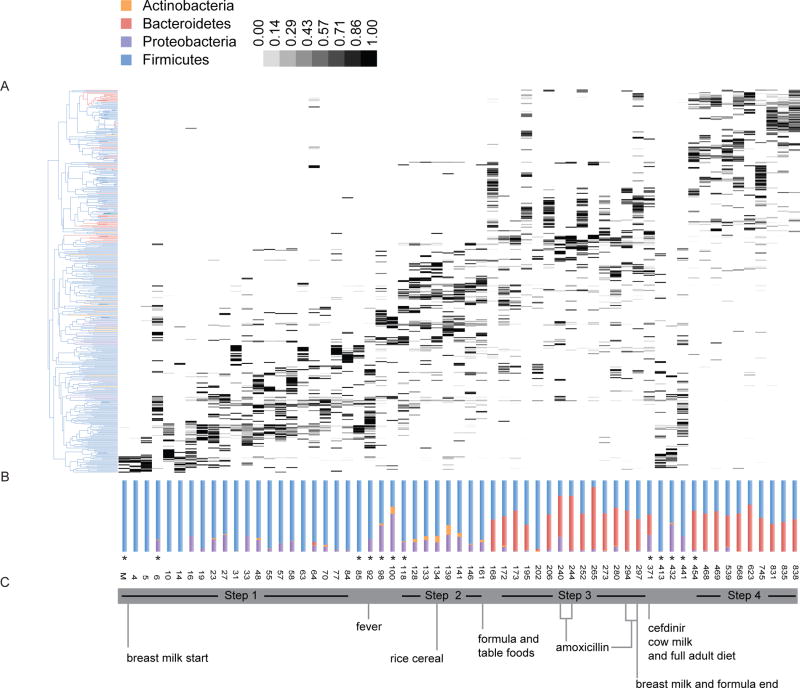
Figure 1.
Relative changes in community structure and composition of the intestinal microbiota in early life. (A) Vertical lanes correspond to sample days and gray-shaded boxes represent the relative abundance of different taxonomic groups. (B) Relative abundances of major bacterial phyla represented in each sample. (C) Significant events with relevance to the infant’s diet that may have influence on microbiota changes. From Koenig JE, et al.54
Evaluation of the compositional structure of the infant gut microbiota over time indicates that ecological states persist for days or weeks, followed by abrupt transitions to alternative states. These distinctive, successive equilibrium states are observed in both preterm and term babies, although the composition of their communities differ significantly at the earliest days of life. Periods of stable phylogenetic composition at the taxonomic level of genus and species may belie underlying shifts in the abundances of strains with different functional potential.7 Bifidobacteria eventually appear in most babies, typically detected between 1 and 2 months of life. In general, members of enterobacteria and bifidobacteria appear early on, Bacteroides spp. at about the middle of the first year, and butyrate-producing Faecalibacterium and Roseburia towards the end of the first year of life. Considerable intra- and interpersonal variation in fecal bacterial community structures is observed during the first year of life, and intrapersonal variation decreases as a function of age.5 The emergence of an adult-like microbiota pattern occurs by the third year.8 While changes in diet appear to be associated with major shifts in the structure of the infant microbial communities, the relative role of other variables (e.g., medications, nutrition, phages, parasitic burden) is not well understood.
Martin Blaser (New York University School of Medicine) is leading a research effort on the developmental pathway for the microbiome during early life exploring how perturbations may influence later risk of various disorders.9 During his presentation, “Impact of antibiotic exposures on the developing microbiota”, Blaser reminded the audience that medical interventions can affect the composition of the microbiota, with potential effects on metabolic and immunologic development. Examples include antibiotics administered to mothers or infants, Caesarian delivery, and use of basic infant formula. The collective effects of these exposures, over time and following transmission from exposed mothers to infants, may reduce the diversity of the gut microbiota. Low-dose antibiotics have been used to enhance weight gain and growth of livestock, so early exposure to antibiotics could influence the risk of obesity, metabolic syndrome and associated conditions in humans.10 A striking correlation is observed when comparing geographic distribution patterns in the United States for rates of obesity and antibiotic use (Fig. 2), although the causal relationship is unknown.
Figure 2.
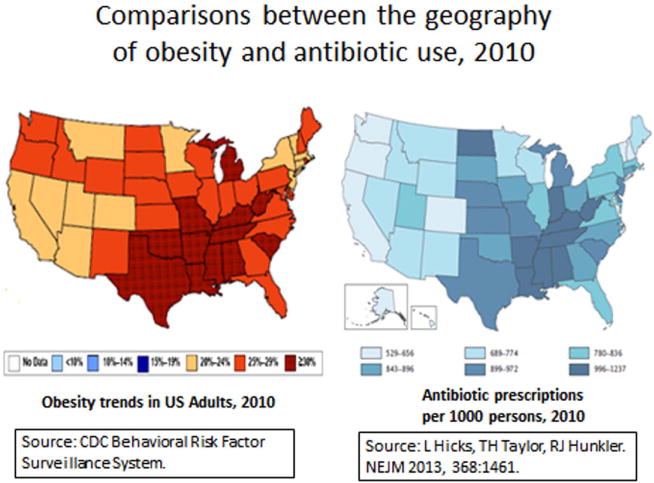
Comparisons between the geographical distribution of obesity (left) and antibiotic use in the United States, 2010 (right). Obesity trend data were from the CDC Behavioral Risk Factor Surveillance System (http://www.cdc.gov/brfss/). Antibiotic prescriptions were from a national survey of out-patient pharmacy records, as described by Hicks, et al.55
To study the effects of early microbiome perturbation, Blaser and colleagues have developed animal models to evaluate the effects of continuous sub-therapeutic antibiotic treatment from pre-weaning to adulthood (STAT model) or pulsed antibiotic treatment for 3–5 day periods at developmental stages of late pre-weaning, weaning, and adulthood (PAT model) on gut microbiota composition and other developmental factors. These studies have shown short-term changes in the microbiota composition of the ileum and colon, and liver adiposity and lipogenesis gene expression, as well as long-term effects on host morphometry, metabolism, and immune cell populations.9 The results suggest that antibiotic exposures in early life not only affect the developing microbiota, but may also affect the risk of obesity, metabolic syndromes, and autoimmune diseases.
A number of other reports have drawn attention to an association between alterations in the intestinal microbiota and obesity or insulin resistance. In his presentation titled, “When the programming goes awry: diabetes, obesity, and beyond,” Patrice D. Cani (Université catholique de Louvain, Belgium) discussed how disruptions in the programming of gut microbiota may contribute to the development of obesity and type 2 diabetes (T2D). While a number of studies have associated changes in the gut microbiome with metabolic diseases, the causality remains to be proven in humans. Cani described studies into the mechanism underlying this association, and specifically the concept of metabolic endotoxemia—that is, an increase in plasma lipopolysaccharide (LPS) levels—as one of the triggering factors that can lead to the development of metabolic inflammation and insulin resistance associated with obesity.11 High-fat diets have been shown to alter the gut microbiota composition. Cani and colleagues have previously demonstrated that mice treated with antibiotics are resistant to diet-induced metabolic endotoxemia, fat mass development, metabolic inflammation and insulin resistance.12 Similarly, several studies have demonstrated that germ-free (GF) mice are protected against glucose intolerance, metabolic inflammation, and insulin resistance, which suggests that the microbiota may be involved.13, 14 The microbiota of high-fatfed mice or obese (ob/ob) mutant mice can also transfer the obesity/T2D phenotype to ex-GF mice.13, 15 Other studies have shown that antibiotic treatment protects mice from this diet-induced obesity,12, 16 while prebiotics lead to reduced body-weight gain and fat deposition and protect against hepatic steatohepatitis in obese and T2D rats.17 Cani and colleagues have shown that nutritional or genetic-induced obesity and type 2 diabetic rodents display gut barrier dysfunctions leading to the leakage of LPS and possibly other microbiota-derived factors.12 They found that gut microbiota metabolites can interact with the endocannabinoid system,18 as well as with enteroendocrine L-cells to alter gut permeability (Fig. 3), possibly through production of GLP-1, PYY, and GLP-2.19
Figure 3.
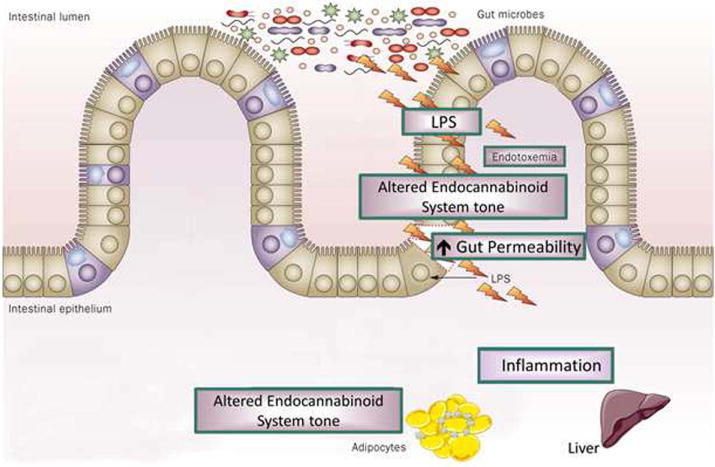
Interactions between gut microbiota and the endocannabinoid system: impact on gut barrier function and metabolic inflammation. Obesity (nutritional or genetic) is associated with changes in the gut microbiota composition and pathophysiological changes whereby the endocannabinoid system tone is altered. This phenomenon is associated with the development of gut permeability, metabolic endotoxemia, metabolic inflammation and altered adipose tissue metabolism (adipogenesis). From Delzenne NM, et al.56
Additional studies have used prebiotics and probiotics to identify novel mechanisms of bacterial interaction with the host that control gut permeability and metabolism during obesity and T2D. They showed that levels of Akkermansia muciniphila are decreased in the gut microbiota of mice fed high-fat diets and in ob/ob mice, while feeding the mice prebiotics (inulin-type fructans) restores these populations.20 Transfer of A. muciniphila to high-fat diet–induced obese mice led to decreased fat mass gain, increased fat oxidation, and restored gut barrier function in the colon. Viable, but not heat-killed A. muciniphila, increased mucus layer thickness, decreased plasma LPS levels, and counteracted the diet-induced metabolic disturbances.20 These studies demonstrate that the gut microbiota influences energy homeostasis; that bacterial compounds contribute to low-grade inflammation and gut permeability in obesity and T2D; and that certain types of bacteria within the gut microbiota, such as A. muciniphila, may play a role in development of these obesity-related conditions.
Another perspective on the role of the gut microbiome in the development of metabolic disorders was presented by Max Nieuwdorp (University of Amsterdam, Netherlands). Nieuwdorp and collaborators have been investigating the role of the gut microbiota in health and disease using fecal transplantation. While fecal transplantation has been practiced for centuries, since 1958 only 500 case reports exist on treatment of patients with recurrent Clostridium difficile infection, IBS, inflammatory bowel disease (IBD), or multiple sclerosis.22 Modern procedures involve bowel lavage for 4–6 hours followed by either colonoscopy or duodenal infusion of fecal homogenates prepared from healthy screened donors (following a questionnaire on bowel habits, travel history, medications, and screened for an extensive list of fecal and blood-borne pathogens). A recent study by Van Nood et al.21 showed a 92% cure rate, with an increase in microbiota diversity for over 6 months, in patients with recurrent C. difficile infection.
Nieuwdorp and collaborators then conducted a randomized controlled trial in obese subjects to investigate the effects of fecal transplantation on insulin resistance using hyperinsulinemic euglycemic clamp techniques and evaluation of gut microbiota composition.22 Subjects were randomized to receive fecal microbiota (FM) from homogenates of their own feces (autologic) or from healthy, lean donors (allogenic). Results showed a significant improvement in insulin sensitivity in subjects receiving allogenic FM lasting for up to 6 weeks, but no change in those receiving autologic FM. Infusion of allogenic FM also led to changes in the composition of the fecal and small intestinal microbiota (mostly butyrate producers) and a reduction in fecal short chain fatty acids (SCFA) butyrate and propionate. No change was observed in food intake or weight among groups; this is interesting, as obesity can result from increased food intake as well as altered nutrient content. A more striking improvement in insulin sensitivity was observed in one subject following FM transplant from a specific allogenic donor. This result correlated with higher levels of Eubacterium hallii (anaerobic Gram-positive Firmicute) in the small intestine. Recognizing that other studies have shown that certain groups within the gut microbiota may have diagnostic and clinical value in predicting T2D in obese patients (e.g., Roseburia species, Faecalibacterium prausnitzii, Lactobacillus gasseri),23 Nieuwdorp et al. have since investigated E. hallii and generated data suggesting that four weeks of daily gavage with cultured E. hallii in male db/db mice is safe and has beneficial effects on glucose metabolism, most likely through altered fecal SCFA production.24 Additional studies are planned to evaluate the safety and potential benefits of E. hallii for improving insulin sensitivity in humans.
Collectively, these studies suggest that the gut microbiota per se and certain bacterial products in particular, play a role in the development of obesity and changes in insulin sensitivity. Future research needs to confirm causality in humans and clinical relevance with respect to gut microbiota, as well as explore mechanisms of action and use of probiotics or prebiotics.
Translating research to transform healthcare
Public health is confronted with complex systems that have outcomes with multiple determinants that interact in obscure ways. As a result, changes to public health systems often lead to unintended consequences. The third session, moderated by Gregor Reid (Western University and Lawson Health Research Institute, Canada), consisted of a keynote presentation by Harry Burns (Chief Medical Officer for Health, Scotland) and a panel discussion that considered potential pathways and issues involved with translating research advances to influence public health policy. The goals of the session were to understand the factors involved with influencing a healthcare system—at the country, state/province, or local community level—and discuss how best to apply this knowledge in transforming research findings on probiotics and (to a lesser extent) prebiotics to change public health policy.
In his keynote lecture, Burns provided examples of how a quality improvement model under his leadership led to significant reductions in hospital infection rates (e.g., ~90% reduction in C. difficile), hospital mortality rates, infant mortality rates, and improved clinical record keeping. A key reason stated for the success of this model was the involvement and complete buy-in from hospital personnel and other key constituents. One example of this collaboration is the Early Years Collaborative (EYC),25 whose objective is to support and drive practical action under a broader partnership program aimed at delivering a shared commitment to give children the best start in life and to improve the life chances of children, young people, and families at risk. The EYC effort aims to (1) deliver measurable improvement in outcomes and reduce inequalities for Scotland’s vulnerable children; (2) put Scotland on course to shift the balance of public services towards early intervention and prevention by 2016; and (3) sustain this change to 2018 and beyond. The strategy used for implementing such changes involves the following actions: understand the problem, build the will for change, execute the change, and drive the change with data.
Their main tactical approach is to design an intervention based upon sound rationale (whether medical, scientific, or social), test it in a real situation, measure and modify it, then continue until the implementation is optimized. By building the will of all the change makers, objectives can be met and tangible improvements achieved.
Panel discussion
In translating these learnings to probiotics, Burns and an expert panel including Rowena Pullan (Pfizer Consumer Health Care), Bruno Pot (Institut Pasteur de Lille), and David Mills (University of California, Davis) restated the importance of public–private partnerships to drive public policy change. And while the strength of evidence for probiotics is clear in a number of applications26 (such as treating necrotizing enterocolitis in premature infants,27 preventing antibiotic-associated diarrhea,28 improving urogenital health in women,29 and countering infection and allergy related to respiratory health30, 31), these data need to be collated, presented to changemakers, and used as a means to shift medical practice. This is currently a major challenge in the United States, where any product (including yogurt) being tested to treat, prevent, or cure disease must be investigated as a drug, while fewer barriers exist in Canada and other countries.
General concern was expressed by the audience about the regulatory view of probiotic food research and how it can be used to substantiate market approval and claims. The regulatory approval path for probiotic foods is complicated, particularly going through the FDA. Pot responded to questions about the situation with probiotics at the European Food Safety Authority (EFSA). He explained that the EFSA position on probiotics is inconsistent with precedent established by other dietary substances. For example, the data on the health benefits of probiotics are more extensive and of higher quality (i.e., evaluating non-essential endpoints) than data for most vitamins and minerals as health supplements that have been accepted by the EFSA. Yet, no probiotic claims have been approved by this government agency. The EFSA recently determined that the use of the words probiotic and prebiotic on foods is an implied health claim, and as such is not allowed despite mounting scientific evidence of beneficial effects. There are clear challenges with the regulatory framework in the United States and the European Union, but those involved with probiotics and prebiotics must continue to do quality research and communicate the outcomes of that science in more efficient and effective ways.
A large gap currently exists in frontline health care providers’ understanding of probiotics, prebiotics and the human microbiome, and certainly on how to interpret the vast data sets from omics studies. The benefits of advances in microbiome research and probiotics will only be realized when new technologies from omics are integrated into medical diagnostics, and when medical students, physicians, and administrators are educated on the health benefits and cost-savings potential from probiotics. Clear messages need to be crafted and directed at key target audiences, such as consumers, politicians, doctors, pharmacists, and others. One new initiative, Gut Microbes for Health,32 aims to bridge this information gap with clinicians through annual meetings and an interactive website with basic information on probiotics/prebiotics and the gut microbiome. Lessons might be learned from similar initiatives in the clinical field, such as the European PathoNGenTrace project, which ames to extract useful functional (clinically relevant) information from the whole genome sequence.b
David Relman inquired about the cost to collect the data needed for regulatory approval. Cost savings in healthcare are difficult to evaluate. Burns pointed out that although governments value cost-saving interventions, changes are often adopted simply because they improve well-being and they are the right thing to do. In general, the public is not impressed by cost-effectiveness.
Impact of gut bacteria on brain development, circuitry, and behavior
While many general press articles on gut microbes discuss the apparent link to obesity, there has also been a marked increase in discussions of gut–brain interactions. Jane Foster (McMaster University, Canada) has been using conventional and GF mice to study the gut–brain axis, which involves the complex interplay between the autonomic and enteric nervous systems, pituitary and gut hormones, and the gut inflammatory systems (Fig. 4). Her research uses a well-established behavioral test—the elevated plus maze (EPM)— to examine exploratory behavior and is used to assess anxiety levels. Typically, conventional mice spend most time in closed-arm areas (harm avoidance) of the EPM compared to open-arm areas. Extending from earlier studies observing that GF mice show enhanced stress-reactivity,33 Foster et al. found that GF mice demonstrated reduced anxiety-like behavior in the EPM (more time in open arms, both in duration and numbers of visits), compared with specific pathogen–free (SPF) mice.34, 35 The reduced anxiety behavior persisted when GF mice were colonized with SPF microflora during adulthood (ex-GF), but not when colonized at an earlier age, demonstrating that gut–brain interactions influence central nervous system wiring early in life. The low anxiety–like phenotype was accompanied by long-term changes in plasticity-related genes in the hippocampus and amygdala.
Figure 4.
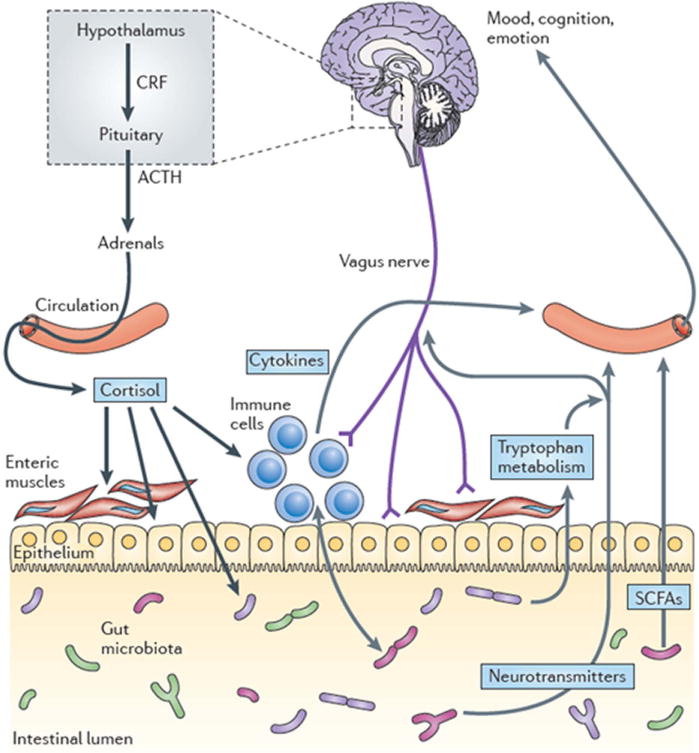
Pathways involved in bidirectional communication between the gut microbiota and the brain. Multiple pathways exist through which the gut microbiota can modulate the gut–brain axis. They include endocrine (cortisol), immune (cytokines) and neural (vagus and enteric nervous system) pathways. The brain recruits these same mechanisms to influence the composition of the gut microbiota, for example, under conditions of stress. The hypothalamus–pituitary– adrenal axis regulates cortisol secretion, and cortisol can affect immune cells, alter gut permeability and barrier function, and change gut microbiota composition. Conversely, the gut microbiota and probiotic agents can alter the levels of circulating cytokines, and this can have a marked effect on brain function. Both the vagus nerve and modulation of systemic tryptophan levels are strongly implicated in relaying the influence of the gut microbiota to the brain. In addition, short-chain fatty acids (SCFAs) are neuroactive bacterial metabolites of dietary fibers that can also modulate brain and behavior. ACTH, adrenocorticotropic hormone; CRF, corticotropin-releasing factor. From Cryan and Dinan.57
The researchers have also been examining the interplay of leptin and central circuits for stress-reactivity and feeding in the presence or absence of the gut microbiota. They found that leptin-insufficiency in GF mice leads to long-term changes in the expression levels of the brain’s leptin receptors, as well as certain peptides such as neuropeptide Y (NPY), which influence corticosterone levels and thus plays pivotal roles in the stress response. The response of different strains of mice and the role of serotonin or other feedback mechanisms are unclear. While many questions remain, continued work in this exciting area may shed light on how developmental factors and the intestinal microbiota influence the interface between metabolic diseases and mood disorders.
Gary Frost (Imperial College London, United Kingdom) reported on another area of study regarding the effects of gut microbiota on brain activity, namely, the effect of dietary prebiotics and associated production of SCFAs such as acetate, propionate, and butyrate on hypothalamic neuronal activity and obesity. It is widely believed that the increasing incidence of obesity is associated with low consumption of fermentable fibers and high intake of dietary carbohydrates. Frost and colleagues are investigating the mechanisms involved in the decrease in body weight and improved insulin sensitivity that occurs with increased intake of non-absorbable fermentable carbohydrate. Feeding mice high levels of non-absorbable fermentable carbohydrate leads to decreased adiposity and increased levels of plasma GLP-1, one of several anorectic gastrointestinal hormones produced by enteroendocrine L cells in the colon, and capable of suppressing neuronal activity. Animal studies have shown that enteroendocrine L cells contain G protein–coupled SCFA receptors and release gut hormones such as GLP1 and PYY.36 Using a primary colonic cell model, Frost et al. found that SCFAs stimulate PYY release from human primary L cells. In other studies, they demonstrated that acetate decreases appetite in mice following intraperitoneal administration, is capable of crossing the blood–brain barrier, and has a direct effect on the hypothalamic arcuate nucleus stimulating anorectic signals. These observations are consistent with the hypothesis that SCFAs have positive biological effects involved with improved appetite regulation and glucose homeostasis. Additional studies are planned to evaluate the effects of direct colonic delivery of SCFAs (e.g., propionate esters) on release of gut hormones and appetite regulation in humans.
Helen E. Raybould (University of California, Davis) described her group’s work on the gut–brain axis as it relates to regulation of GI function and food intake. Luminal chemosensors found on enteroendocrine cells that line the intestine transmit signals to the central nervous system (CNS) via the vagal afferent neurons, which in turn influence GI function through parasympathetic pathways and eating behavior through effects on higher brain centers. Studies were designed to evaluate changes in microbiota and gut epithelial function that may be connected with hormonal pathways. These pathways, which influence food intake and obesity, were assessed using Sprague-Dawley rats fed a high-fat diet to induce either an obesity-prone (DIO-P) or obesity-resistant (DIO-R) phenotype.37 Using bacterial 16S rRNA measurements, they showed a decrease in total bacterial density and an increase in the relative proportion of Clostridiales in high-fat–fed rats regardless of phenotype, while an increase in Enterobacteriales was only seen in the microbiota of DIO-P rats. The DIO-P group also exhibited increases in intestinal permeability, which resulted in elevated plasma LPS levels, and Toll-like receptor 4 activation. The data suggest that DIO-P rats are unable to transmit signals to the brain that involve cholecystokinin (CCK) regulation of vagal afferent neuron transmissions to communicate satiety in response to food intake. Further studies show that, compared with a normal diet, paracellular permeability, transcellular permeability, and mucosal inflammation are increased by feeding a Western diet and these changes can be prevented by feeding Bifidobacterium infantis and prebiotic bovine milk oligosaccharides (MOs). Collectively, these results suggest that consumption of a high-fat diet may induce changes in the gut microbiota and increase low-grade inflammation that ultimately contributes to the development of diabetes and obesity. Additional investigations are expected to clarify how specific prebiotic/probiotic combinations may modulate gut function and inflammatory responses to delay the onset of, or even prevent, diabetes and obesity.
Reaching people in need with probiotics and prebiotics
The final session, moderated by Ruth Ley (Cornell University), consisted of a series of short presentations with a focus on translating scientific innovations in pro- and prebiotics to reach the people with the greatest needs. Andrew Serazin (Matatu, LLC) set the stage by presenting the challenges involved in these activities. Studies into the microbiome are one of the most active areas of the life sciences today, according to Serrazin, since the isolation of restriction enzymes nearly forty years ago. Shifting consumer preferences and dietary patterns, at least in the United States, underlie drastic changes in consumption of major nutrient classes with concurrent rises in chronic disease. Escalating healthcare costs and demands of an aging population have led to a growing preference for self-treatment or prevention of disease including wellness approaches. To be successful in developing widely distributed probiotic and prebiotic products, the surge of scientific inquiry into the structure and function of the microbiome must be matched by a focused and transparent effort to engage industry, health policy makers, and the general public.
Extending such products to people in need in emerging economies will present a whole new set of challenges. Most of the world’s 7 billion people are currently experiencing significant alterations in disease burdens, dietary patterns, and lifestyle. This has been dramatically documented in countries such as India and South Africa, where it is common to find high levels of both malnutrition-induced stunting and obesity in the same population, which is indicative of nutritional deficits that manifest in opposite forms.
Products derived from advances in our understanding of the microbiome represent an entirely new field at the union of nutrition and medicine, and their applications are likely to be profound in meeting future challenges in food and nutrition. Successful translation of advances made in microbiome research to probiotic and prebiotic products will require the following: (1) an open, engaged, and realistic research community with clear goals, including sharing of potential benefits through commitment to global access; (2) recognition that a broad number of foods and ingredients shape microbiome structure and function and in turn can impact health of consumers; (3) regulation based on meaningful biomarkers and defined outcomes; and (4) trusted products with clear health benefit to consumers.
Gregor Reid (Western Heads East) partnered with Patricia Hibberd (Harvard Medical School and Massachusetts General Hospital) in a presentation titled, “From yogurt to vaccine for the developing world.” Reid explained that a striking relationship exists when comparing global longevity patterns and mortality rates due to malnutrition (Fig. 5). Countries in Africa tend to have among the lowest life expectancy and the highest rates of malnutrition-associated deaths. High rates of malnutrition and mortality also appear to occur in countries with low milk consumption rates, leading to the question of whether milk-based probiotic interventions could be used to prevent, or treat, major causes of morbidity and mortality in the developing world. Reid described a project (Western Heads East) run by students, staff, and faculty at Western University in Canada that established community kitchens in Tanzania, Kenya and Rwanda to prepare yogurt supplemented with L. rhamnosus GR-1 and other local micronutrients. The aim of the project is to provide communities suffering high rates of HIV and malnutrition with a source of quality nutrition augmented by beneficial bacteria.
Figure 5.
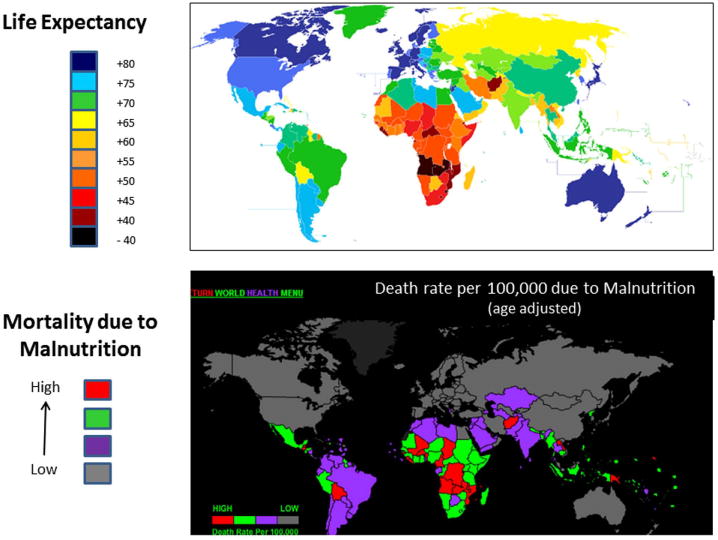
Comparison of worldwide distribution of life expectancy patterns with rates of malnutrition-related mortality. Countries with high death rates due to malnutrition are typically associated with low life expectancy. From Wikipedia: http://en.wikipedia.org/wiki/File:Esperanza_de_vida.PNG; and World Health Rankings for death rates: http://www.worldlifeexpectancy.com/cause-of-death/malnutrition/by-country/.
Preliminary studies with L. rhamnosus GR-1 have shown benefits for improving gut barrier function and weight gain, reductions in fungal infections and diarrhea episodes, and increases in overall energy levels. These observations led Reid and collaborators to conduct a randomized, double-blind, controlled trial in which HIV patients who had no prior antiretroviral treatment were given yogurt fortified solely with either micronutrients (control group) or with both micronutrients and L. rhamnosus GR-1 (probiotic group) for 4 weeks. Although the results from this pilot study did not show improvements in CD4+ T cell counts due to probiotics, there were unexpected social benefits, such as economic empowerment of women involved with the community kitchen efforts. Additional studies are underway to evaluate other possible benefits of L. rhamnosus GR-1, including improved tolerance of HIV medications, binding of aflatoxin B1, and reductions in blood levels of certain toxic metals (mercury, arsenic, lead, and cadmium). Reid concluded by saying that this grass-roots community effort and associated research should serve as a model to encourage others to help reduce malnutrition and improve health in other underdeveloped areas around the world.
Patricia Hibberd (Harvard Medical School and Massachusetts General Hospital) discussed the use of probiotics to enhance immunizations in resource-limited settings. The World Health Organization’s (WHO) Expanded Program on Immunizations (EPI) has helped increase childhood immunizations against diseases such as polio, diphtheria, pertussis, measles, and tetanus. While such efforts have saved an estimated 20 million children’s lives over the last 20 years, in 2011 over 23 million children received no immunizations at all. A key reason for this is that gaps exist in the cold-chain distribution systems needed to preserve the potency of the vaccines. Hibberd described her and collaborators’ efforts to create vaccine-delivery vehicles by genetically engineering the probiotic bacterium Bacillus subtilis to display vaccine antigens; B. subtilis was chosen because it can withstand extreme environmental conditions. Hibberd and colleagues have created non-injectable, thermo-stable vaccines for tetanus and rotavirus, with plans to extend to pertussis, diphtheria, and other major causes of childhood diseases. The engineered vaccines are stable at 45 °C without refrigeration for more than 1 year, and have been shown in animal safety and immunogenicity studies to be safe and capable of producing protective levels of antibodies when administered intranasally, sublingually, or transdermally.38, 39 Plans are underway to conduct testing of the B. subtilis vaccine platform in humans under an IND, which could eventually lead to a viable approach for providing childhood vaccines that do not require the cold chain, needles, or administration by skilled personnel, in resource-limited settings.
Lactose intolerance continues to be a problem for over 40 million people in the United States. Research by Dennis Savaiano (Purdue University), Andrea Azcaarate-Peril (UNC Microbiome Center), and Todd Klaenhammer (North Carolina State University) is evaluating the clinical effects of feeding a highly purified, short-chain galactooligosaccharide (GOS/RP-G28) on lactose intolerance and changes in the composition of the colonic microbiota (using terminal restriction fragment length polymorphisms (TRFLP) and 16S rRNA pyrosequencing). In a randomized, double-blinded study, they fed GOS/RP-G28 to lactose-intolerant adults for 36 days and collected stool samples at the start of the study (day 0), after GOS treatment (day 36), and 30 days after GOS feeding was stopped (day 66); consumption of dairy products was encouraged in both placebo and intervention groups after day 36. Lactose digestion and overall symptoms of lactose intolerance improved in subjects fed GOS/RP-G28 compared to a placebo group; subjects on GOS were six times more likely to claim they were lactose tolerant post-treatment. When compared to the placebo group, subjects fed GOS/RP-G28 showed only minor changes in microbiota composition on day 36, but statistically significant major shifts in the microbiota occurred at day 66. Changes in the microbiota by day 66 included increased abundance of Faecalibacterium prausnitzii, Lactobacillales, and Roseburia spp. within the Firmicutes phyla; increases in (Lac+) Oscillibacter and Dorea spp.; and a reduction in some Clostridia class members. Expanded studies are planned to confirm these changes in the fecal microbiota of lactose-intolerant individuals that were clinically responsive to dietary adaptation to GOS/RP-G28.
Joël Doré (Institut National de la Recherche Agronomique, France) provided an excellent overview of research and clinical studies on the anti-inflammatory properties of F. prausnitzii, and described studies conducted by his group aimed at evaluating the mechanisms of crosstalk between F. prausnitzii and host cells that may underlie its role as a mutualistic commensal. He set the stage by pointing out that research studies conducted during the past decade have demonstrated an association between certain chronic immune diseases and dysbiosis of the intestinal microbiota40–42 A recurrent theme in many of these studies is the observation that such chronic immune disorders are associated with the presence of low-grade inflammation on the host side and a reduction of some important antimicrobial commensal species on the microbiome side. Most likely, other factors such as diet, genetic predisposition, environment, and lifestyle also contribute to the low-grade inflammatory state and changes in microbiota composition (Fig. 6). F. prausnitziii is one example of a potentially beneficial intestinal commensal, based on anti-inflammatory properties demonstrated in preclinical studies.41 Levels of F. prausnitzii are found in low abundance in patients with Crohn’s disease,40 colorectal cancer, obesity,43 or IBS.44 In one study, administration of F. prausnitzii provided protection from endoscopic inflammation relapse 6 months after surgery in 20 patients with active CD requiring ileocecal resection.41
Figure 6.
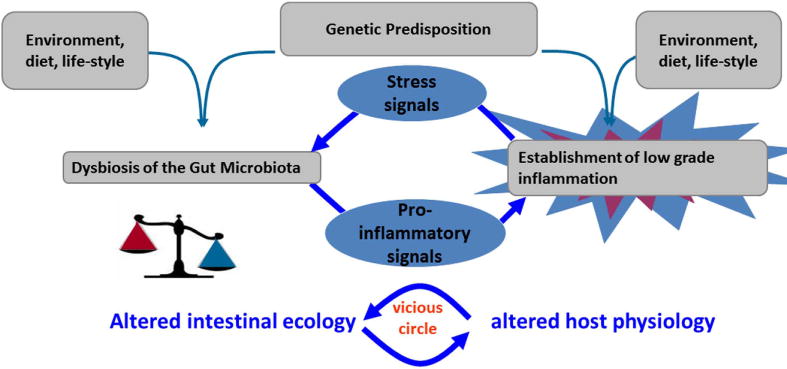
Alterations of the gut microbiota and low-grade inflammation may contribute to a cycle of events that induces a chronic state in immune-mediated diseases. Interventions that target the combined modulation of gut microbiota and inflammation may be the most effective way to manage such conditions.
Doré and colleagues are also investigating bacteria–cell crosstalk using a functional metagenomics approach to better understand how altered intestinal ecology may contribute to a chronic immune condition. Human cell lines have been engineered with stably transfected reporter genes, allowing them to assess modulation of transcription regulators such as NF-κB, AP-1, or PPAR-γ, or production of proteins such as TSLP, TGF-β, or Fiaf. High-throughput screening of interactions between over 20,000 metagenomic clones bearing large genomic inserts of culturable and non-cultured bacteria and human cells have allowed the identification of several bioactive clones that modulate cellular activities with relevance to immune response, proliferation, or metabolism. The genes involved are identified and relevant bioactive signal molecules are identified using biochemical or genetic approaches. Results from such studies may help unravel mechanisms by which commensal bacteria modulate cellular functions which may lead to exploration of ways to favorably modulate probiotic–host interactions.
In the closing presentation, Fred Degnan (King & Spalding, LLP) discussed the perspectives of the U.S. FDA on clinical study requirements as it relates to regulatory classifications for probiotic-containing products. The investigation of a probiotic product can fall along a continuum of regulatory classifications, including “drug,” “food,” “medical food,” “food additive,” or “dietary supplement.” There are implications for each classification in terms of the nature and degree of regulatory requirements and, ultimately, for claim substantiation and market access.
As a general rule, the FDA determines the degree of regulation for clinical trials and assigns product classifications based on the intended use for a given product. The intended use of a product can be determined by a number of factors, including claims, labeling, promotions, and by endpoints of clinical investigation. Based primarily on these types of communications, the product will be deemed a drug, food, dietary supplement, or medical food. The various claim structure for these different products can be summarized as follows:
Drug/Biological product: Focus is on the cure, treatment, mitigation, or prevention of disease, although these products can also affect the structure or function of the human body (biological products are drugs derived from live microorganisms).
-
Foods or dietary supplements:
○ Health or “qualified” health claim: Characterizes the relationship between a nutrient, dietary supplement, or food, and the reduction in risk of a disease or health-related condition. An approved health claim must be supported by “significant scientific agreement”, while “qualified” health claims are based on “emerging” scientific evidence. Both must be pre-reviewed by the FDA via a petition process or based on statements by an authoritative body.
○ Structure/function claim: Describes the effect of a food, food component, nutrient or dietary ingredient on the “structure or any function of the body”. May not imply or express usefulness in the cure, mitigation, treatment or prevention of disease.
Medical food: For a patient under medical supervision for the “dietary management of a disease or condition for which distinctive nutritional requirements have been established by medical evaluation” and which cannot be addressed by the diet alone.
A clinical study involving an FDA-regulated article can evoke certain requirements. If a company or individual wishes to conduct a human study on a probiotic intended for prevention or treatment of a disease, they will be required to follow a more rigorous regulatory pathway. Typically, this will require filing an Investigational New Drug (IND) application (21 CFR Part 312), which must be submitted before initiating studies in humans. The IND will be expected to contain extensive information for the review process, including, but not limited to, a description of Institutional Review Board review (21 CFR Part 56) and informed consent (21 CFR Part 50); how the product is made, to ensure that safe, high-quality manufacturing processes are used; and, often, data from preclinical animal toxicology studies to demonstrate that it is safe to proceed with human clinical studies. As in other countries, the regulatory system has been set up so that only a drug, not a food, can treat, prevent, or cure disease. Questions were raised during the meeting as to why this bureaucratic distinction still remains in place today.
Guidance issued by the FDA in October, 2010 on “Determining whether human research studies can be conducted without an IND”45 had particular relevance for studies involving probiotics. This document included language suggesting that an IND would be required for studies in which a live organism (e.g., virus, bacterium, or fungus) is administered to subjects to study “the pathogenesis of disease” or “the host response to the organism.” The strictest interpretation of this statement could be that any probiotic investigation would require an IND. Other guidance by the FDA issued in February 2012 focuses on clinical trials with live biotherapeutic agents,46 with specific reference to language being “applicable” to the prevention, treatment, or cure of disease. However, this document acknowledges that a basis exists for conducting human studies on food (including dietary supplements) that do not require the same IND application process as drug studies; such studies will need to avoid drug-type endpoints. The intended use will dictate regulation, but examples of appropriate food targets include human studies intended to establish health claims, structure/function claims, or medical food claims. In conclusion, to avoid FDA imposition of a requirement for an IND, Degnan recommended (1) to conceive and design studies based on intended use and clear understanding of regulatory categories; (2) to use caution in documenting/substantiating non-biological product use; and (3) to consult with FDA (CFSAN) with your research plan to study food and on questions regarding food status.
Conclusions
The conference “Probiotics, Prebiotics, and the Host Microbiome” provided a forum in which recent developments and the potential benefits of translating research advances in the human microbiome, probiotics, and prebiotics into robust nutritional and therapeutic applications to promote health were examined. The number of genes in the microbiome is estimated to be more than 300-fold higher than the total number of human genes, which highlights the existence of a highly complex microbiota ecosystem with the potential for profound effects on metabolism and immune function. Significant advances have been achieved and further studies will greatly enhance our understanding of the human microbiota and its role in health and disease development.
It is well-established that the intestinal microbiota plays a critical role in gastrointestinal development and function while regulating host inflammatory responses and immune homeostasis.47, 48 A rapidly growing body of evidence now also indicates that the microbiota acts as a metabolically active organ, capable of interacting with several host systems beyond the gastrointestinal tract, including the brain, urogenital tract, and respiratory tract.49–51 Recent research suggests the gut microbiota is capable of influencing fat storage and metabolism,52, 53 which may position it as a key target in the fight against obesity in conjunction with dietary, exercise, and other interventions. Disruptions in the early programming of the gut microbiota or alterations of adult-like microbiota may contribute to the development of obesity and T2D. Proposed mechanisms include effects on hormone-based satiety, energy salvage, appetite regulation, and LPS-induced metabolic endotoxemia. Probiotics and prebiotics that target specific changes in the microbiota and its crosstalk with the host to improve lipid metabolism and insulin resistance represent exciting potential options for improving the management of what is arguably the 21st century’s major public health issue.
The gut–brain axis is a highly complex system comprised of interactions that involve the autonomic and enteric nervous systems, pituitary and gut hormones, and gut inflammatory systems that are capable of influencing nerve function and pathways, and ultimately behavior. Central nervous system wiring may be influenced by gut–brain interactions early in life, and alterations in the microbiome may influence behaviors related to specific disease conditions. Examples include the possibilities of influencing the microbiota with probiotics to modulate cholecystokinin (CCK) output, which in turn regulates vagal afferent neuron transmissions involved in communicating satiety and avoiding obesity, or providing prebiotics to encourage fermentative production of SCFAs that stimulate release of gut hormones, which in turn influence hypothalamic neuronal activity involved with appetite regulation.
Many questions remain regarding the development of the microbiota in the young infant, and wether probiotic/prebiotic interventions at this time would be effective in supporting the development of a lifelong microbiota for health. Much needs to be learned about how the microbiota is assembled, what influences community structure succession, and which factors contribute to its long term stability in both health and illness. It seems likely that the future roles of probiotics and prebiotics will go beyond traditional gastrointestinal illnesses, particularly as the role of the microbiota and the CNS and other organs is better understood. Novel applications in the future may include chronic immune disorders, and anxiety-like behaviors or psychiatric illness. The development of such products is certain to face increased scrutiny over costs and benefits to support decisions about utilization and reimbursement for disease management. Influencing public health policy to more effectively adopt the use of such products will require clear understanding and communication of the health benefits, building the will for change with providers and policy makers, executing the changes in policy, and driving these changes with strong, reproducible data.
As we get closer to understanding the potential mechanisms by which particular probiotic organisms interact with the microbiota, it will be a missed opportunity if the quality of probiotic research studies does not improve to meet the needs for either evidence-based medicine or nutrition. Standardization of probiotic/prebiotic study methods and protocols, clear understanding of the characteristics, purity, and stability of test agents, and accurate and balanced reporting of study results are urgently needed. In parallel, it will be important to educate healthcare professionals, regulatory authorities, and the public to understand the appropriate use and documented safety and benefits of probiotic or prebiotic products. Presentations and discussions during the conference reiterated that there is no opting out of this paradigm shift, but rather a matter of when and how the innovations from microbiota/microbiome and probiotic/prebiotic research will become part of everyday life. For those in the field, the regulatory antiquity and recalcitrance of many physicians to move from a pharmaceutical-based patient-management perspective to a more holistic one that includes recommendations of food and supplement-based products to both general and patient populations remain among the impediments to progress. Meanwhile, regulatory issues on pro- and prebiotics remain a point of concern. High quality human research conducted on the general population is required to convince regulators of the legitimacy of health benefits. Many convincing probiotic studies have been done on populations that are outside the scope of restrictive regulatory categories, and not considered relevant to foods or supplements. However, the safety and lack of side effects for these products is a strong plus. Thus, it is incumbent upon those in the field to help strengthen the body of evidence and merge the knowledge in a manner that allows consumers and patients to reap the benefits sooner rather than later.
Acknowledgments
The conference “Probiotics, Prebiotics, and the Host Microbiome: The Science of Translation“ was jointly presented by The Sackler Institute for Nutrition Science at the New York Academy of Sciences and The International Scientific Association for Probiotics and Prebiotics in New York, New York, 12 June 2013. The conference was also generously supported (in part) by Award Number R13AT007899 from the National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health (NIH). Co-funding in support of Travel Fellowships to foster the participation of early career and underrepresented minority investigators in this conference was provided by the following: NIH Division of Nutrition Research Coordination (DNRC), the Trans-NIH Probiotics/Prebiotics and Microbiome Working Group (trans-NIH PPWG/T-MWG), the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the US Government. The authors also want to thank Juliet Ansell, Harry Burns, Megan Enos, Gary Frost, David Mills, Rowena Pullan, Helen Reybould, and Victoria Ruiz for their participation during the conference.
Footnotes
These economic evaluations, however, are useful only for health effects that have scientifically been sufficiently substantiated; for IBS, for example, see Hungin et al., 2013. Alimentary Pharmacology & Therapeutics. doi: 10.1111/apt.12460.
Conflicts of interest
T.K. is a consultant for Ritter Pharmaceuticals and helped plan the microbiome aspects of the project reported on page 17. M.E.S. consults with numerous food and dietary supplement companies conducting business in the probiotic industry. She does not have any ownership role or serve on governing boards for any company. The other authors declare no conflicts of interest.
References
- 1.Probiotics, Prebiotics, and the Host Microbiome: The Science of Translation. 2013 doi: 10.1111/nyas.12303. http://www.nyas.org/events/Detail.aspx?cid=c60ea8d5-44f0-4aaa-a8ff-3e5f008186f6. [DOI] [PMC free article] [PubMed]
- 2.Trueman P, Hurry M, Bending M, et al. The feasibility of harmonizing health technology assessments across jurisdictions: a case study of drug eluting stents. Int J Technol Assess Health Care. 2009;25:455–62. doi: 10.1017/S0266462309990389. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead SJ, Lee L, Fang Z, et al. Can faecal calprotectin reduce the demand for colonoscopy in patients with Irritable Bowel Syndrome? Gut. 2010;59 [Google Scholar]
- 4.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer C, Bik EM, DiGiulio DB, et al. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morowitz MJ, Denef VJ, Costello EK, et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc Natl Acad Sci U S A. 2011;108:1128–33. doi: 10.1073/pnas.1010992108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho I, Yamanishi S, Cox L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–6. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trasande L, Blustein J, Liu M, et al. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013;37:16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 13.Rabot S, Membrez M, Bruneau A, et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 2010;24:4948–59. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- 14.Backhed F, Manchester JK, Semenkovich CF, et al. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Backhed F, Fulton L, et al. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho BM, Guadagnini D, Tsukumo DM, et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia. 2012;55:2823–34. doi: 10.1007/s00125-012-2648-4. [DOI] [PubMed] [Google Scholar]
- 17.Daubioul C, Rousseau N, Demeure R, et al. Dietary fructans, but not cellulose, decrease triglyceride accumulation in the liver of obese Zucker fa/fa rats. J Nutr. 2002;132:967–73. doi: 10.1093/jn/132.5.967. [DOI] [PubMed] [Google Scholar]
- 18.Muccioli GG, Naslain D, Backhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 22.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6. e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 24.de Vos WM, Nieuwdorp M. Genomics: A gut prediction. Nature. 2013;498:48–9. doi: 10.1038/nature12251. [DOI] [PubMed] [Google Scholar]
- 25.Early Years Collaborative. The Scottish Government. http://www.scotland.gov.uk/Topics/People/Young-People/Early-Years-and-Family/early-years-collaborative.
- 26.Deshpande G, Rao S, Patole S. Progress in the field of probiotics: year 2011. Curr Opin Gastroenterol. 2011;27:13–8. doi: 10.1097/MOG.0b013e328341373e. [DOI] [PubMed] [Google Scholar]
- 27.Bernardo WM, Aires FT, Carneiro RM, et al. Effectiveness of probiotics in the prophylaxis of necrotizing enterocolitis in preterm neonates: a systematic review and meta-analysis. J Pediatr (Rio J) 2013;89:18–24. doi: 10.1016/j.jped.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Goldenberg JZ, Ma SS, Saxton JD, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2013;5:CD006095. doi: 10.1002/14651858.CD006095.pub3. [DOI] [PubMed] [Google Scholar]
- 29.Macklaim JM, Cohen CR, Donders G, et al. Exploring a road map to counter misconceptions about the cervicovaginal microbiome and disease. Reprod Sci. 2012;19:1154–62. doi: 10.1177/1933719112446075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Q, Lu Z, Dong BR, et al. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2011:CD006895. doi: 10.1002/14651858.CD006895.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Isolauri E, Rautava S, Salminen S. Probiotics in the development and treatment of allergic disease. Gastroenterol Clin North Am. 2012;41:747–62. doi: 10.1016/j.gtc.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Gut Microbiota for Health. http://gmfh-qa.fuzu.me/
- 33.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neufeld KM, Kang N, Bienenstock J, et al. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23:255–64. e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 35.Neufeld KA, Kang N, Bienenstock J, et al. Effects of intestinal microbiota on anxiety-like behavior. Commun Integr Biol. 2011;4:492–4. doi: 10.4161/cib.4.4.15702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tazoe H, Otomo Y, Kaji I, et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol. 2008;59(Suppl 2):251–62. [PubMed] [Google Scholar]
- 37.de La Serre CB, Ellis CL, Lee J, et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Belitsky BR, Brinker JP, et al. Development of a Bacillus subtilis-based rotavirus vaccine. Clin Vaccine Immunol. 2010;17:1647–55. doi: 10.1128/CVI.00135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S, Belitsky BR, Brown DW, et al. Efficacy, heat stability and safety of intranasally administered Bacillus subtilis spore or vegetative cell vaccines expressing tetanus toxin fragment C. Vaccine. 2010;28:6658–65. doi: 10.1016/j.vaccine.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 40.Sokol H, Seksik P, Furet JP, et al. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–9. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- 41.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–6. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalliomaki M, Collado MC, Salminen S, et al. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 43.Furet JP, Kong LC, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajilic-Stojanovic M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 45.Guidance for Industry: Investigational New Drug Applications (INDs) http://www.fda.gov/downloads/Drugs/Guidances/UCM229175.pdf.
- 46.Guidance for Industry: Early Clinical Trials with Live Biotherapeutic Products. http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/General/UCM292704.pdf.
- 47.Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–20. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 48.Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–1051S. doi: 10.1093/ajcn/69.5.1046s. [DOI] [PubMed] [Google Scholar]
- 49.Schippa S, Iebba V, Santangelo F, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) allelic variants relate to shifts in faecal microbiota of cystic fibrosis patients. PLoS One. 2013;8:e61176. doi: 10.1371/journal.pone.0061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid G, Younes JA, Van der Mei HC, et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 51.Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667–72. doi: 10.1016/j.coph.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- 53.Velagapudi VR, Hezaveh R, Reigstad CS, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–12. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koenig JE, Spor A, Scalfone N, et al. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4578–85. doi: 10.1073/pnas.1000081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hicks LA, Taylor TH, Jr, Hunkler RJ. U.S. outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368:1461–2. doi: 10.1056/NEJMc1212055. [DOI] [PubMed] [Google Scholar]
- 56.Delzenne NM, Neyrinck AM, Backhed F, et al. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639–46. doi: 10.1038/nrendo.2011.126. [DOI] [PubMed] [Google Scholar]
- 57.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–12. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]