Abstract
Venous leg ulcers affect millions of patients worldwide and are a tremendous financial burden on our healthcare system. The hallmark of venous disease of the lower extremities is venous hypertension, and compression is the current mainstay of treatment. However, many patients are noncompliant, in part because of the complexity of the dressings and the difficulties with application and removal. The aim of our study was to determine an effective compression dressing regimen for patients with venous leg ulcers who require changing the ulcer primary dressing twice daily. We used two layers of a latex free tubular elastic bandage for compression. The primary endpoint of our study was increased wound healing rate and our secondary endpoint was complete wound closure. All active study subjects had positive healing rates at week 4 and week 8. Two subjects achieved complete wound closure by week 8. We conclude that compression with a latex-free tubular elastic bandage can be safely used in patients with venous leg ulcers requiring frequent dressing changes. This type of compression allows for daily inspection of wounds, dressing changes at home, flexibility in the context of clinical trials, and is a compromise for patients that are intolerant to compression dressings.
Keywords: compression dressing, compression bandages, ulcer, venous leg ulcer, wound healing, wound healing rate, venous insufficiency, venous hypertension
INTRODUCTION
It is estimated that approximately 1.2 million adults in the United States have venous leg ulcers (VLUs). Venous ulcers are characterized by loss of epithelium (epidermis) plus variable levels of dermal and subcutaneous tissues. The ulcers occur near or above the malleoli of the distal lower extremities especially on the medial side (1). Once VLU heal, their recurrence rate is as high as 72% overall, (2) and 21% at one year (3). This high recurrence rate, coupled with the long ulcer duration of more than a year in more than half of patients, helps explain the high prevalence of venous ulcers (2). This high prevalence is accompanied by substantial costs to our healthcare system and by personal socioeconomic consequences (4). In the United Sates, the annual estimated cost of ulcer treatment is up to 2.5 billion (2). These types of wounds represent the late effects of chronic venous insufficiency and venous hypertension.
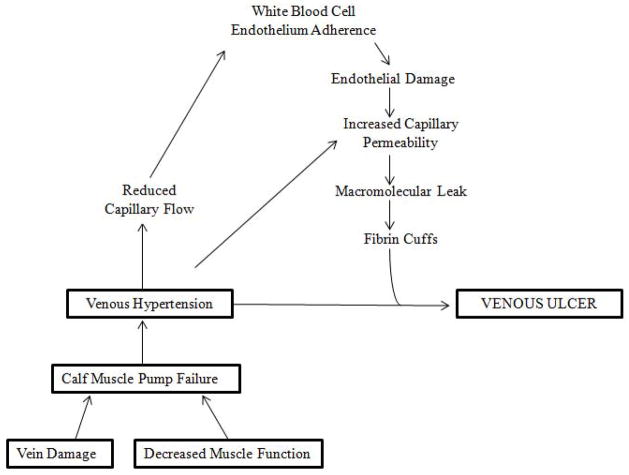
Venous insufficiency results in prolonged venous hypertension, particularly in the lower half of the calf (1). Venous hypertension, refers to sustained elevation of ambulatory venous pressure. It is known that venous hypertension is the result of dysfunction of the calf muscle pump and generally includes the structural and functional damage to the veins (Figure 1). The calf muscle pump comprises the calf muscles, the deep venous system, superficial venous system, and the perforating/communicating vein system. In a diseased venous system and faulty calf muscle pump unit, the predicted fall in venous pressure upon ambulation or leg exercise does not occur (1). Venous hypertension is therefore a misnomer because the venous pressure does not actually increase above baseline (1). It has been shown that there is a direct correlation between ambulatory venous pressure and the development of venous ulceration. In a study by Shull et al, patients with an ambulatory venous pressure (AVP) greater than 60 mm Hg had a 66% chance of developing an ulcer, while no patients with a AVP below 40 mmHg developed ulcers (5).
Figure 1. Pathophysiology of Venous Ulcers.
Diagrammatic representation of the pathogenesis of venous ulceration and the proposed hypothesis linking venous hypertension to the formation of ulcers.
While the mechanisms causing venous hypertension are fairly well worked out, the pathogenic steps leading from venous hypertension to ulceration are still unknown (4). Over the last several decades, numerous hypotheses have emerged to explain the development of venous ulceration. One such hypothesis by Browse and colleagues suggested that unrelieved venous pressure leads to leakage of fibrinogen into the dermis, with the consequent formation of a pericapillary fibrin layer (6–11) (Figure 1). It was proposed that this layer of fibrin interferes with the normal flux of oxygen and nutrients between blood and tissue. This interference, in turn would lead to tissue anoxia and subsequent necrosis (6–11). Decreased transcutaneous oxygen tension in limbs with venous ulcers and the presence of pericapillary fibrin in the skin adjacent to the ulcers has been described (12). However, it was later found that the correlation between the presence of pericapillary fibrin with venous ulcers and impaired healing was not convincing (8). Indeed confocal microscopy has indicated that large gaps exist within the pericapillary fibrin layer (13). These observations lend credence to an alternative trap hypothesis, whereby the fibrin has a more functional rather than structural role (14). The trap hypothesis suggests that fibrin, fibronectin in the fibrin layer, bind to growth factors and other proteins and matrix components critical to healing. Moreover, the leakage of fluid from blood vessels may bind those wound healing components (14). Others have suggested that trapping of white blood cells may damage endothelial cells (15).
Treatment of a non-healing venous ulcer first requires an accurate diagnosis, followed by the identification and correction of contributing systemic factors (1). In the absence of cellulitis or severe bacterial colonization of the wound, which generally require systemic antibiotics, the overall treatment is directed toward improving venous hypertension, and decreasing leg edema.
Venous ulcers are treated with a primary wound dressing that can absorb excess exudate, fill vacant space, and provide a moist environment for optimal healing (16). VLUs can be quite exudative, especially when the legs are edematous and compression is being initially applied. If the wound dressings cannot keep up with the amount of exudate draining from the ulcer, the surrounding area will become macerated. This will lead to delayed wound healing and increased risk of infection. Thus, in our experience, treatment of VLUs often requires daily inspection and dressing changes. Many compression bandages, such as Unna boot (short-stretch bandages) and three or four layer bandages (long stretch bandages), don’t allow for frequent inspection or dressing changes.
Compression therapy is the established treatment for venous ulcers (17). Compression may improve venous return, reduce edema, stimulate healthier granulation tissue within venous ulcers (18). There are several types of compression bandages including, in particular short and long stretch bandages. Elastic stockings are best applied after complete wound closure has taken place. All compression bandages have pros and cons. The majority of bandages are difficult for a patient to apply on their own, and some of them require a well-trained physician or nurse. This difficulty leads to more doctor visits and helps contribute to the healthcare costs for caring for such wounds. Importantly, in the context of testing a topical agent to be applied more than once or twice a week, compression bandages may become an obstacle. Thus, there is a need for a compression bandage regimen that patients can apply on their own, and which still provides some pressure to help reverse the effects of venous hypertension. The results of this study represent a compromise in terms of delivering an ideal compression to the legs.
METHODS
This was an investigator-initiated clinical trial. The trial was approved by the Institutional Review Board (IRB) at Roger Williams Medical Center. The subjects enrolled in our study were recruited from our wound healing clinic and referrals from the community. Written informed consent was obtained for all subjects prior to study entry.
The inclusion criteria were the following: 1) patients 18 years or older; 2) non-healing venous leg ulcers ≥ 2 cm2 in size; 3) ankle to brachial index (ABI) ratio >0.7; 4) venous disease including lipodermatosclerosis, dependent peripheral edema, dermatitis, and/or hyper-pigmentation; 5) female patients of reproductive age having a negative pregnancy test within 1 week of study entry and using adequate birth control methods.
The exclusion criteria were the following: 1) known allergy to the hydrofiber dressing; (Aquacel, ConvaTec, Princeton, NJ) or components; 2) those taking systemic corticosteroids > 20mg/day; 3) involvement in another experimental drug trial within a month prior to the study; 4) clinical evidence of infection or cellulitis in or around the ulcer; 5) history of medical noncompliance; 6) inability to understand the study or provide written informed consent; 7) pregnancy; and 8) a presence of peripheral arterial insufficiency, uncontrolled congestive heart failure (CHF), vasculitis, uncontrolled diabetes mellitus, or severe contact dermatitis.
This was an investigator-initiated, single-center, nonrandomized, open-label, pilot study examining an effective way to apply compression in the home setting to patients with VLUs changing the ulcer primary dressing twice daily. The study was designed to have a screening visit, followed by a 1–2 week run, 8-week open label treatment phase, and 1 follow up visit 4 weeks after completion of the study. A total of 11 clinic visits were conducted over a 14 week period.
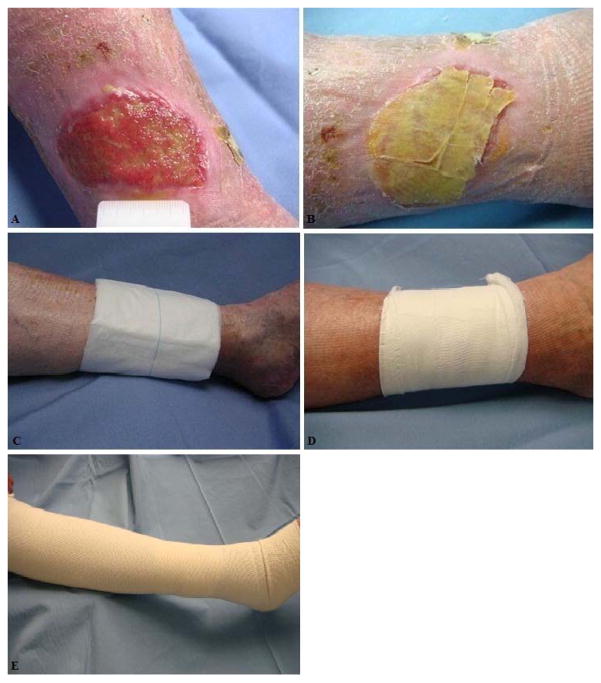
Study subjects were treated with the following topical treatment regimen: a primary dressing, which consisted of a hydrofiber (Aquacel) impregnated with normal saline, an absorbent pad (Allevyn, Smith and Nephew, London, UK) as a secondary dressing, a gauze wrap to stabilize the secondary dressing (which is wrapped from the bottom of the toes to just below the knees). This is followed by the application of compression using two layers of a latex-free tubular elastic bandage (SurgiGrip, Derma Sciences, Princeton, NJ) (Figure 2). The amount of exudate from a wound is dynamic and different for every patient. We believe that a moist wound environment is optimal for wound healing. Therefore, our standard of practice is to impregnate the hydrofiber dressing with normal saline prior to application on wounds. This ensures that the hydrofiber is not drying the wound bed, and is maintaining a moist wound environment while still absorbing the exudate.
Figure 2. Pictorial Description of Study Dressing.
A) VLU with no dressing. B) Calcium alginate primary dressing impregnanted with normal saline. C) Absorbant pad placed over primary dressing (secondary dressing). D) Gauze wrap used to stabilize the secondary dressing. E) Latex-free tubular elastic stocking.
At the screening visit, a complete medical history and Doppler studies were performed to determine the ABI. The following were done at the screening visit and all additional study visits: computerized ulcer planimetry, obtaining the history of concomitant medications, evaluation of adverse events, cleansing of the wound, wound photographs, and wound evaluation.
After all screening tests and evaluations were performed; a 1–2 week run in phase took place in which results from the screening visit were analyzed. Following the run in phase, subjects who met the inclusion criteria for the study began the treatment phase. On Day 1 of the study, subjects were given wound supplies and dressings for a week and instructed on how to change their dressings at home. They were then seen weekly for 8 weeks and 1 month later for a follow up visit. Interim visits were scheduled in the event of a follow-up for an adverse event. Subjects were withdrawn from the study if they were noncompliant with dressing changes, intolerant to the primary or secondary dressing, intolerate to compression, if they developed an infection, or if the principal investigator felt it was in their best interest. As per protocol, subjects were followed for any type of adverse event, including irritation from wound dressing, infection, etc.
The data collected from these weekly visits, for example digital planimetry, were used to determine chronic wound healing rates (Gilman Formula) (19). Wound healing was calculated using two methods; digital planimetry and Image J. Digital planimetry was accomplished by tracing the wounds with Visitrak (a Smith and Nephew product). The wound tracings were then scanned onto a computer and the Image J program was used to determine the area and perimeter of the wound. The program, image J, is available from the National Institute of Health; website http://www.rsbweb.nih.gov/ij. This program analyzed planimetry data to facilitate calculation of the overall healing rates with the Gilman formula (19). In this formula, the healing rate= [(A1−A2)/{(P1+P2)/2}]/(T2−T1), where A is the area in square centimeters (cm2 ). P is the perimeter in centimeters and T the time in weeks. The formula is accepted and used as a practical way to quickly gauge healing with documented perimeter movement toward the center of the wound. When data was reviewed retrospectively, it became critical to account for healing rate instability and the Mean Adjusted Gilman Formula was derived (20). For data analysis in this study, the Mean Adjusted Gilman Formula was used to calculate the wound healing rate.
RESULTS
Overall, 7 subjects were enrolled in this study and six subjects completed the study. One subject was discontinued from the study due to noncompliance with the primary dressing. One subject missed the week 12 follow-up visit. There were no screen failures. The patient group was comprised of 4 men and 3 women, with a mean age at enrollment of 60 years (range: 47–78). Baseline wounds had a mean area of 13.28cm2 (range: 2.2cm2 to 30.7cm2). The wounds were present for a mean of 91.2 months (range: 8–240 months; see Table 1).
Table 1.
Patient demographics, wound area, wound healing rates, and ABI
| Subject | Age | Sex | Site | Duration of time (months) | Screening day area (cm2) | Day 1 area (cm2) | WK 2 area (cm2) | WK 8 area (cm2) | WK 12 area (cm2) | HR WK4 mm/wk | HR WK8 mm/wk | ABPI/ABI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 56 | M | LLML | 8 | 16.6 | 9.9 | 6.1 | 4.6 | 5.9 | 0.77 | 0.39 | 1.05 |
| 2 | 65 | M | LLLL | 192 | 4.2 | 2.2 | 0.8 | H | H | 0.64 | 0.68 | 1.04 |
| 3 | 61 | M | RLLL | 28 | 30.7 | 23.3 | 28.1 | 16.9 | 9.1 | 0.04 | 0.53 | 0.96 |
| 4 | 78 | F | LLLL | 14 | 6.2 | 4.7 | 3.4 | * | * | 0.99 | * | 1.07 |
| 5 | 54 | F | LLAL | 13 | 6.5 | 5.3 | 2.7 | H | 0.7 | 1.49 | 1.51 | 1.28 |
| 6 | 58 | M | RLLL | 144 | 20 | 16.4 | 12.5 | 9.3 | 6.1 | 0.53 | 0.59 | 1.05 |
| 7 | 47 | F | LLLL | 240 | 10.8 | 9.5 | 6.4 | 4.8 | NA | 0.51 | 0.49 | 0.84 |
This table depicts the patient demographics and features of their wounds such as location, duration of wound in months prior to enrollment in study, wound area, wound healing rate, and ABI at screening day.
Indicates that patient was discontinued from the study after week 4.
LLML: left lower medial leg; LLLL left lower lateral leg; LLPL: left lower posterior leg; LLAL: left lower anterior leg; RLML: right lower medial leg; RLLL: right lower lateral leg; RLPL: right lower posterior leg; RLAL: right lower anterior leg; HR: wound healing rate (Mean Adjusted Gilman); WK: week; ABPI/ABI: ankle-brachial pressure index/ankle-brachial index; H: healed; mm/wk; millimeter/week.
The primary endpoint for the study was increased wound healing rate (mm/week) with a secondary endpoint of compete wound closure. We have previously found a wound healing rate of 0.75mm/week to be 80% sensitive and specific for predicting ultimate wound closure by 4 weeks. A wound healing rate < 0.7mm/week indicates that the wound is not healing optimally and that a change in therapy should be considered (21).
The average wound area at the screening visit for the 6 subjects who completed all 8 week follow-up visits was 14.8 cm2. The percent decrease in wound area from the screening visit to week 8 was 71%±9.8%. From screening visit to week 12 there was a 79%±6.2 decrease in wound area (Figure 3). For example, subject three’s wound area at screening was 30.7 cm2, by week 12, the area of the wound measured 9.1cm2 (Table 1), which is approximately a 70% improvement from baseline (Figure 3).
Figure 3. Wound Area by Study Visit for Each Study Subject.
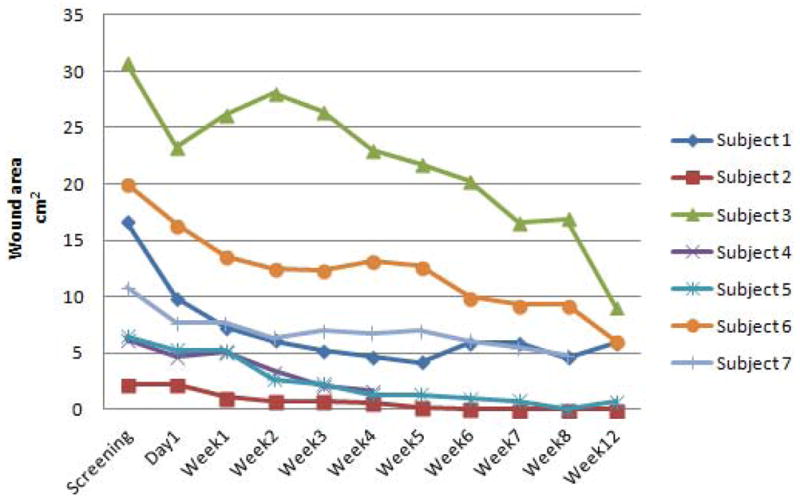
Wound area progression for each study patient per visit. Subject 4 was withdrawn from the study after week 4. Subject 7 did not return for the week 12 visit.
By using the Mean Adjusted Gilman’s equation, the wound size was measured and compared using the most previous visit (20). The average wound healing rate calculated at Week 4 and Week 8 for all subjects was 0.71±0.16 mm/wk and 0.69±0.17mm/wk, respectively. At week 4, three of the subjects had wound healing rates > than 0.75mm/week. All seven subjects had positive wound healing rates at week 4. All active subjects at week 8 had positive wound healing rates (Figure 4 and 5).
Figure 4. Wound Healing Rate at Week 4.
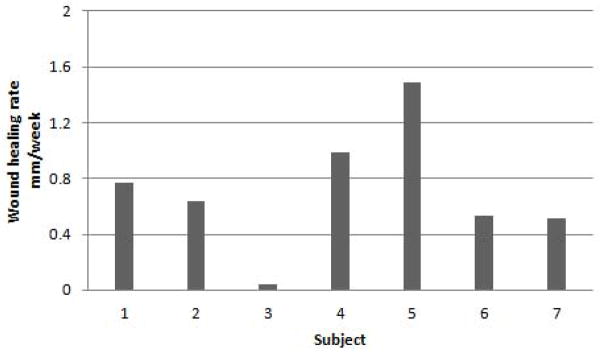
Calculated using the Mean Adjusted Gilman Formula. Three of the seven patients had a wound healing rate >0.75mm/week. All seven subjects had a positive wound healing rate. The average wound healing rate was 0.71mm/week±0.16.
Figure 5. Wound Healing Rate at Week 8.
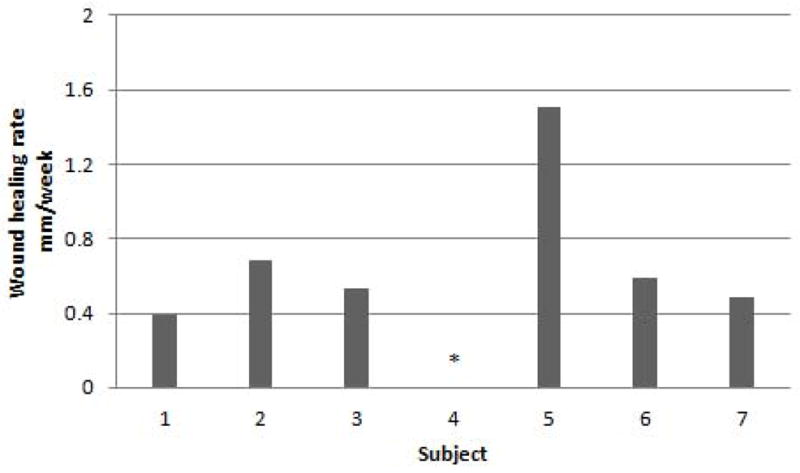
Calculated by the Mean Adjusted Gilman Formula. One study subject had a wound healing rate >0.75mm/week. All active subjects had positive wound healing rates. The average wound healing rate was 0.69mm/week±0.17.
In regards to our secondary study outcome, two of the 7 subjects achieved complete wound closure by week 8 (Figures 6 and 7). One of these subject’s ulcers had not previously been healed in 192 months (Figure 6). This study subject failed numerous other treatments including standard compression, and bioengineered skin. It is important to note that the average ulcer duration for patients in our trial was over 60 months, yet we achieved complete wound closure in two of the subjects in less than 10 weeks. One of the subjects that healed, the wound reopened slightly (0.7cm2) by the week 12 follow-up, and it is unclear why the wound reopened.
Figure 6. Representative Image From Subject 2.
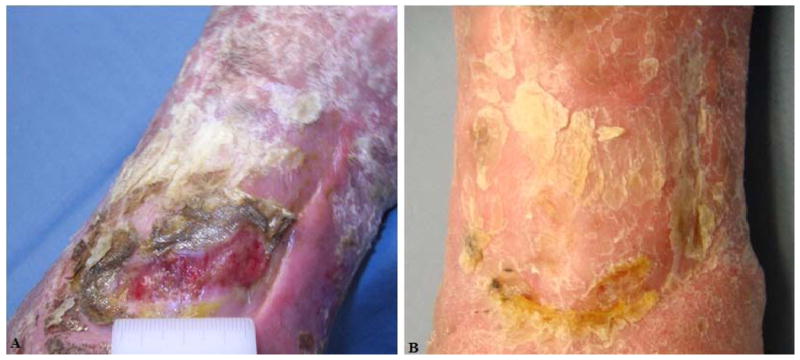
A) Screening visit photograph of VLU. B) Week 8 visit. The wound has healed. Their ulcer was present for 192 months prior to enrollment.
Figure 7. Representative image from Subject 5.
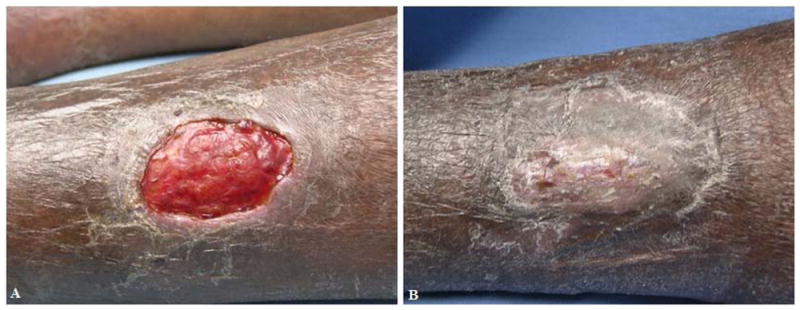
A) Screening visit photograph of VLU. B) Week 8 visit. The wound has healed. Their ulcer was present for 13 months prior to enrollment.
None of the subjects developed additional ulcers or clinically worsening inflammation while using the latex-free tubular elastic bandage. All of the subjects tolerated this form of compression well. In addition, no adverse effects such as contact dermatitis resulted from the primary hydrofiber dressing or secondary dressing used in the study.
DISCUSSION
The standard of care for venous ulcer treatment includes the use of compression therapy to reverse the effects of venous hypertension and the use of a dressing to maintain a moist wound-healing environment (22). Although all compression bandages are designed to improve venous return, there are many different types and many factors need to be considered when choosing the best option for patients. Compression bandaging systems vary by the following factors: construction (knitted, woven), components (elastic, non-elastic), performance (long-stretch, short stretch) and layers (single-layer, multiple layer) (23). They are classified into 3 groups based on the amount of pressure supplied at the ankle in mm Hg, Class I, II, and III. Class III stockings supply 40mm Hg or higher and are further subdivided based on the amount of pressure they produce (24). The selection of compression type depends on many factors including the ease of use and application, patient complaince and acceptability for both the doctor and patient, amount of pressure supplied, and cost (25). Four-layer compression bandage therapy provides a high compression level of 40mmHg and good healing rates (25). However, these bandages can be very uncomfortable to patients due to their bulky nature and lead to noncompliance (25). In addition, these types of bandages take a great deal of time and effort to apply, and must be applied by a trained professional. They are also much more costly compared to compression stockings. This is not only due to the cost of the dressing itself, but also the labor required including physician and nurse time (26). A Cochrane meta-analysis of 39 randomized controlled studies made the following conclusions: compression bandages with an elastic component are more effective than those of inelastic constituents, application of two-layer stockings appear to be more effective than a single layer, and two-component bandage systems appear to perform as well as four-layer bandage systems in terms of healing (27).
In our patient population we observed that patients were noncompliant with four layer compression bandages and non-elastic stockings such as the Unna boot. It has been previously reported that there are many advantages to the use of elastic stockings as opposed to multi-layer compression bandages. They are much easier for patients to apply at home, allow the use of ordinary shoes, bathing, and are much more comfortable. These factors should improve patient compliance and their quality of life (28). In addition, This simplified compression regimen is much cheaper and the patients can make far less office visits (28). This was a small, short term study to introduce the use of two layers of a latex-free tubular elastic bandage as a compromise to other forms of compression dressings that are not well tolerated by some patients. The results of this study were meant to be taken as “proof of principle”. Admittedly, the two layer tubular compression bandages reported here represent a compromise in terms of delivering an ideal compression to the leg. The advantage of applying double compression has also been previously reported in the literature. A study by Ham et al, showed that doubling compression on compression hosiery increased measured compression from 10mm Hg to 25–35mmHg. The patients in this study did not report increased discomfort with the addition of the second stocking (29). For this compression strategy to be fully implemented, a study would need to be implemented, requiring many more patients and arms to the study.
As previously reported, wound healing rate <0.7mm/week indicates that the wound is not healing optimally and that a change in therapy should be considered. Our results show that by week 4, the average wound healing rate was 0.71±0.16 mm/wk and by week 8 the average wound healing rate was 0.68±0.17 mm/wk. Recent review of the literature has shown that two-component bandage systems appear to perform as well as the four layer bandage system (27). Therefore, it is important to remember that some compression in patients with chronic VLUs is better than no compression. Based on our preliminary results, two layer latex-free tubular elastic compression stocking lead to acceptable wound healing rates, substantial decrease in wound area after 8 weeks of compression, and can lead to wound closure. Therefore, two layer latex tubular elastic compression is a good compromise in patients with chronic VLUs intolerant of high compression stockings.
Compression is only one piece in the complex process required to treat VLUs (30). Creating the optimal wound environment with a primary dressing is of great importance. A major breakthrough in the management of chronic wounds was the recognition that a moist environment is critical for wound healing (18). Moisture retentive wound dressings stimulate collagen synthesis, promote angiogenesis, accelerate re-epithelialization, and may decrease pain (18). Moist occlusive dressings actually decrease wound infection rates (18).
In the design of this trial we wanted to choose a form of compression that would be easy for our patients to change, at home, twice daily, and yet be capable of delivering enough pressure to help heal their wounds. There is a need for frequent dressing changes due to the exudative nature of many of these wounds. If chronic wound fluid is left to accumulate the surrounding area can become macerated which can lead to increase infection rates and further deterioration of the wound. Chronic wound fluid contains cytokines and matrix metalloproteinases (MMPs) production, which can adversely affect the function of resident cells (18). Thus, we decided on a moist hydrofiber as a primary dressing to be changed twice daily. They require moisture to function and are highly absorbent (can absorb approximately 20 times their weight) (22). The form of compression we choose was two layers of latex-free tubular elastic stocking because this type of compression allows for daily inspection of wounds, dressing changes at home, flexibility in the context of clinical trials, and is a compromise for patients that are intolerant to compression dressings. As our results indicate, the wound area decreased substantially in all subjects, wound healing rates were optimal, and two subjects obtained wound closure after 8 weeks of compression with two layers of compression bandage. In this pilot study, it appears that latex-free tubular elastic bandages can be safely used as a compromise in patients with venous leg ulcers that require frequent dressing changes. For this compression strategy to be fully implemented, a study would need to be implemented, requiring many more patients and arms to the study.
Key message.
Leg compression with a latex-free tubular elastic bandage can be safely used in patients with venous leg ulcers requiring frequent dressing changes
Acknowledgments
This work was supported by NIH grants P20GM103414 and AR060342
ABBREVIATION LIST
- VLU
Venous Leg Ulcer
- MMP
Matrix Metalloproteinase
- AVP
Ambulatory venous pressure
- ABI
Ankle-brachial index
- IRB
Institutional review board
- CHF
Congestive heart failure
- HR
Wound healing rate
Footnotes
- Marco Romanelli, MD, PhD, Department of Dermatology, University of Pisa, Via Roma, 67 56126 Pisa, Italy, m.romanelli@med.unipi.it
- William Marston, MD, Division of Vascular Surgery, CB #7212, UNC Department of Surgery, Chapel Hill, NC 27599-7212, Phone: 919-966-3391, Fax: 919-966-2898
- Herald Brem, MD, Chief, Division of Wound Healing and Regenerative Medicine, Winthrop University Hospital, 259 First Street, Mineola NY 11501, hbrem@winthrop.org
References
- 1.Rojas AI, Phillips TJ. In: Venous Ulcers and their management. Falanga V, editor. Martin Dunitz Publishers; 2001. p. 520. [Google Scholar]
- 2.Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. J Am Acad Dermatol. 2001;44(3):401–21. doi: 10.1067/mjd.2001.111633. [DOI] [PubMed] [Google Scholar]
- 3.Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7(4):201–7. doi: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 4.Falanga V. Venous Ulceration. J Dermatol Surg Oncol. 1993;19:764–71. doi: 10.1111/j.1524-4725.1993.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 5.Shull KC, Nicolaides AN, Fernandes é Fernandes J, Miles C, Horner J, Needham T, et al. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg. 1979;114(11):1304–6. doi: 10.1001/archsurg.1979.01370350106012. [DOI] [PubMed] [Google Scholar]
- 6.Browse NI, Burnard KG. The cause of venous ulceration. Lancet. 1982;2:243–5. doi: 10.1016/s0140-6736(82)90325-7. [DOI] [PubMed] [Google Scholar]
- 7.Burnand KG, Whimster IA, Browse NL. Pericapillary fibrin in the ulcer bearing skin of the leg: the cause of lipodermatosclerosis and venous ulceration. Br Med J. 1982;285:1071–2. doi: 10.1136/bmj.285.6348.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falanga V, Kirsner R, Katz MH, Gould E, Eaglstein WH, McFalls S. Pericapillary fibrin cuffs in venous ulceration: persistence with treatment and during ulcer healing. J Dermatol Surg Oncol. 1992;18:409–13. doi: 10.1111/j.1524-4725.1992.tb03694.x. [DOI] [PubMed] [Google Scholar]
- 9.Kobrin KL, Thompson PJ, van de Sheur M, Kwak T-H, Kim S, Falanga V. Evaluation of dermal pericapillary fibrin cuffs in venous ulceration using confocal microscopy. Wound repair and regeneration. 2008;16:503–6. doi: 10.1111/j.1524-475X.2008.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J. The role of the fibrin cuff in the development of venous leg ulcers. Journal of wound care. 2005;14(7):324–7. [PubMed] [Google Scholar]
- 11.Falanga V, Kruskal J, Franks JJ. Fibrin and fibrinogen related antigens in patients with venous disease and venous ulcerations. Arch Dermatol. 1991;127:75–8. [PubMed] [Google Scholar]
- 12.Falanga V, Moosa HH, Nemeth AJ, Alstadt SP, Eaglstein WH. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch Dermatol. 1987;123:620–3. [PubMed] [Google Scholar]
- 13.Kobrin KL, Thompson PJ, van de Scheur M, Kwak TH, Kim S, Falanga V. Evaluation of dermal pericapillary fibrin cuffs in venous ulceration using confocal microscopy. Wound Repair Regen. 2008 Jul-Aug;16(4):503–6. doi: 10.1111/j.1524-475X.2008.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falanga V, Eaglstein WH. The trap hypothesis of venous ulcerations. Lancet. 1993;341:1006–8. doi: 10.1016/0140-6736(93)91085-z. [DOI] [PubMed] [Google Scholar]
- 15.Coleridge Smith PD, Thomas P, Scurr JH, Dormandy JA. Causes of venous ulceration: a new hypothesis. British medical journal (Clinical research ed) 1988;296(6638):1726–7. doi: 10.1136/bmj.296.6638.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panuncialman J, Falanga V. The science of wound bed preperation. Clin Plast Surg. 2007;34(4):621–32. doi: 10.1016/j.cps.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Trent JT, Falabella A, Eaglstein WH, Kirsner RS. Venous Ulcers: Pathophysiology and Treatment Options. Ostomy Wound Management. 2005;51(5):38–54. [PubMed] [Google Scholar]
- 18.Hafner A, Sprecher E. In: Dermatology. 3. Bolognia JL, Jorizzo JL, Schaffer JV, editors. Philadelphia: Elsevier; 2012. p. 2572. [Google Scholar]
- 19.Margolis DJ, Gross EA, Wood CR, Lazarus GS. Planimetric rate of healing in venous ulcers of the leg treated with pressure bandage and hydrocolloid dressing. J Am Acad Dermatol. 1993 Mar;28(3):418–21. doi: 10.1016/0190-9622(93)70061-w. [DOI] [PubMed] [Google Scholar]
- 20.Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol. 1997 Oct;133(10):1231–4. [PubMed] [Google Scholar]
- 21.Donohue K, Falanga V. Healing rate as a prognostic indicator of complete healing: A Reappraisal. Wounds. 2003;15(3):71–6. [Google Scholar]
- 22.Sackheim K, De Arujo TSK, RS Compression modalities and dressings: their use in venous ulcers. Dermatol Ther. 2006;19:338–47. doi: 10.1111/j.1529-8019.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 23.Pham B, Harrison MB, Chen MH, Carley ME. Cost-effectiveness of compression technologies for evidence-informed leg ulcer care: results from the Canadian Bandaging Trial. BMC Health Service Research. 2012;12:1–8. doi: 10.1186/1472-6963-12-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palfreyman SJ, Lochiel R, Michaels JA. A systemic review of compression therapy for venous leg ulcers. Vascular Medicine. 1998;3:301–13. doi: 10.1177/1358836X9800300406. [DOI] [PubMed] [Google Scholar]
- 25.Hanna R, Bohbot S, Connolly N. A compression of inferface pressures of three compression bandage sytems. British Journ of Nursing. 2008;17(20):S16–S24. doi: 10.12968/bjon.2008.17.Sup9.31661. [DOI] [PubMed] [Google Scholar]
- 26.Kerstein MD. Economics of Quality Ulcer Care. Dermatology Nursing. 2003;15(1):59–61. [PubMed] [Google Scholar]
- 27.O’Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. Cochrane database of systematic reviews (Online) 2012;11:CD000265. doi: 10.1002/14651858.CD000265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geest AV, van Dooren-Greebe RJ, Go IH, Neumann H. An impressive therapeutic result of class III compression stockings in a patient with longstanding, extensive, combined leg ulcers. JEADV. 1999;14:15–7. doi: 10.1046/j.1468-3083.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 29.Ham S, Padmore HS. Two-layer compression hosiery for patients with venous leg ulceration. Nursing Standard. 2006;20(45):68–76. doi: 10.7748/ns2006.07.20.45.68.c4470. [DOI] [PubMed] [Google Scholar]
- 30.Castonguay G. Short-stretch or four-layer compression bandages: an overview of the literature. Ostomy Wound Management. 2008;54(3):50–5. [PubMed] [Google Scholar]