Abstract
Background
Health information reaching the public today is often characterized by what decision theorists have termed ‘ambiguity’ – i.e. uncertainty regarding the information’s reliability, credibility or adequacy. This is a critical problem, as growing research suggests that ambiguity has important effects–promoting pessimistic judgments about risks and potential outcomes of risk-reducing behaviours, and lowering adoption of these behaviours. However, little is known about the public’s perceptions of ambiguity in the health information domain, the effects of these perceptions, and the factors that influence these effects.
Objective
To examine associations between perceived ambiguity regarding cancer prevention recommendations and prevention-related perceptions and behaviours, and to explore how these associations differ by cancer type.
Study design and participants
Cross-sectional analysis of data on 4070 adults participating in the 2005 US Health Information National Trends Survey.
Main variables and outcome measures
We examined associations between perceived ambiguity about colon, skin and lung cancer prevention recommendations and two main outcome variables: (i) risk-related cognitions (perceived cancer risk and preventability, cancer-related worry) and (ii) risk-modifying behaviours (colon cancer screening, sunscreen use and smoking abstinence).
Results
Perceived ambiguity was inversely associated with perceptions of the preventability of all three cancers, and with cancer-specific risk-modifying behaviours including sigmoidoscopy–colonoscopy testing, sunscreen use and smoking abstinence. Relationships with cancer risk perceptions and worry varied across different cancer types.
Conclusions
Perceived ambiguity about cancer prevention recommendations has significant and predictable associations with cancer prevention-related cognitions and behaviours, and some associations differ by cancer type. These findings have implications for future research and communication efforts.
Keywords: ambiguity, cancer prevention, health behaviours, perceptions, recommendations
Introduction
Health information reaching the public today through various channels is increasing in both magnitude and complexity. Popular media coverage of both breakthroughs and controversies in health care has exploded in recent years,1–3 while promotional activities of pharmaceutical companies and health advocacy groups have grown in number, diversity and influence.4,5 At the same time, the medical profession has devoted increasing attention to disseminating complex health information to the lay public. A growing emphasis on informing and involving patients in health-care decisions–goals articulated through the ideals of informed and shared decision making6–11–has fuelled concerted efforts to educate the public about the benefits, risks and uncertainties surrounding various medical interventions.12
What are the effects of these trends? Ideally, greater exposure to health information should enable people to be more educated, competent consumers of health care, and better equipped to make reasoned choices. This potential outcome is a central justification for health communication efforts as well as patient education and counselling interventions such as health decision aids.11,13–17 To what extent this outcome is actually attained, however, is not known. Although the provision of health information through formal educational interventions has been shown to improve patient knowledge,13 it may have mixed psychological effects.
For example, research in other decision-making domains suggest that exposure to complex information may overwhelm, frighten or confuse people, rather than empowering them. Multiple options have been shown to impose a heavy cognitive burden that may hinder optimal decision making.18–21 Furthermore, the presence of what decision theorists have termed ‘ambiguity’ – i.e. uncertainty regarding the reliability, credibility or adequacy of information about risks and the potential outcomes of decisions22– has been shown to have distinct psychological and behavioural effects. Specifically, ambiguity causes people to judge risks and choice outcomes pessimistically, and to avoid decision making.22–26
This response to ambiguity, known as ‘ambiguity aversion’, pertains to information concerning various types of risks; however, it has particular relevance for understanding the outcomes of exposure to complex health information. The health interventions that often receive the greatest attention in informed decision-making efforts are those that are the most ambiguous – for which existing evidence on the balance of benefits and harms is unclear and more than one reasonable course of action exists – e.g. hormone replacement therapy and prostate-specific antigen screening.7,9,10,27,28 At the same time, it is ambiguity that defines the complex nature of the information presented to patients. Informed decision making entails the provision of comprehensive information regarding not only the projected risks and benefits of a health service, but the uncertainties surrounding these projections and the range of clinical options available.6,7,9,12 An instrumental goal of informed decision making, in other words, is to increase patients’ awareness of ambiguity.
For this reason, it is important to understand the effects of ambiguity in health decisions. Relatively little research has been devoted to this problem, although past work suggests that ambiguity aversion pertains to at least some health decision-making domains. In a previous study, we focused on the domain of cancer prevention and explored how perceptions of ambiguity regarding cancer prevention recommendations relate to other risk-related cognitions known to influence health-related decisions and behaviours.29 In that study, we analysed cross-sectional data from a large nationally representative survey of the US public, the National Cancer Institute’s 2003 Health Information National Trends Survey (HINTS 2003).
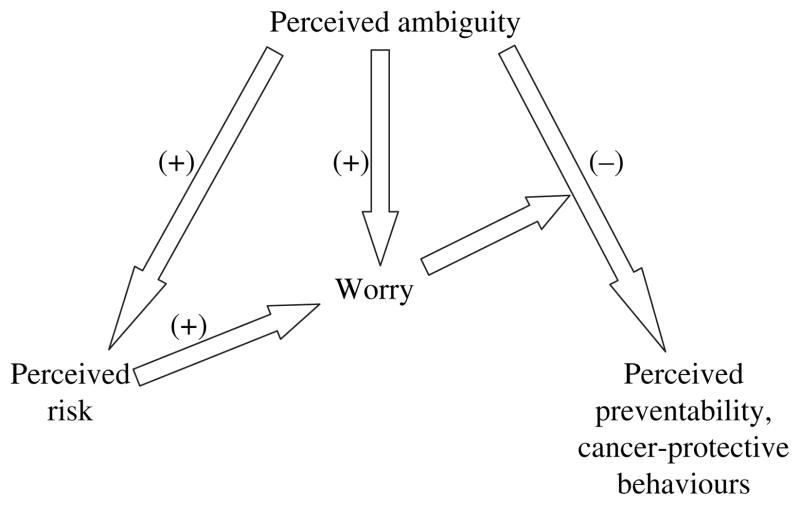
We found that perceptions of ambiguity about cancer prevention recommendations were related to other important psychological variables in ways consistent with the phenomenon of ambiguity aversion (Fig. 1)29. Higher perceptions of ambiguity were associated with lower perceptions of the preventability of cancer, higher perceived cancer risk, and higher cancer-related worry. Also consistent with predictions, cancer-related worry moderated the degree of ambiguity aversion–higher worry being associated with a more negative relationship between perceptions of ambiguity and cancer preventability. Finally, the association between perceived ambiguity and worry appeared to be partially mediated by perceived risk – i.e. perceived ambiguity influenced worry not only directly, but indirectly through its influence upon perceived risk. Although the cross-sectional nature of this study restricted inferences about causality, the results supported theory-based predictions and the findings of related research, and raised the possibility that exposure to ambiguous health information may indeed have negative psychological consequences.
Figure 1.
Predicted relationships between perceived ambiguity, perceived cancer preventability, perceived cancer risk, cancer worry and cancer-protective behaviours.
To what extent these findings can be replicated in other samples and health information domains, and with respect to actual health risk-modifying behaviours – i.e. behaviours aimed at either primary or secondary prevention – remains to be seen. Limited evidence from studies utilizing hypothetical scenarios has suggested that ambiguity of information concerning environmental health risks and the outcomes of primary prevention measures such as immunizations lead people to heightened perceptions of these health risks and lowered interest in these measures.30–33 Similarly, in the domain of secondary prevention, intervention studies have demonstrated that educating people about uncertainties surrounding particular controversial cancer screening measures decreases their interest in screening, further implying that people are ambiguity averse.34–37
In the current study, we attempted to build upon this work and to replicate and expand upon our earlier findings regarding the potential outcomes of perceived ambiguity about cancer prevention recommendations. Applying methods similar to those used in our previous study,29 we analysed data on the new sample population surveyed by the most recent HINTS (2005). Furthermore, because the 2005 HINTS – unlike the earlier survey – measured cognitions pertaining to the prevention of specific malignancies (colon, skin and lung cancer) rather than to cancer in general, we were able to expand our analysis to how ambiguity perceptions relate to specific cancer risk-modifying behaviours (e.g. colon cancer screening, sunscreen use and smoking abstinence), as well as cognitions, and to determine how cancer type influences these relationships.
Methods
Data source and study population
The HINTS is a biennial telephone-based nationwide survey conducted by the National Cancer Institute and aimed at tracking trends in the public’s cancer-related knowledge, attitudes and behaviours. The HINTS surveys a nationally representative sample of US adults aged 18 and older, utilizing a complex stratified sampling design with oversampling of African-American and Hispanic households.38 For the HINTS 2005, interviews were completed with a total of 5586 adults aged 18 and older; the overall response rate was 21%. Further details about the study methods, survey design and variables in HINTS are published elsewhere.38 We limited our analysis to the subpopulation of individuals aged 40 and older (N = 4070), with the rationale that it is this group for which cancer prevention and screening recommendations are most salient and potentially controversial, and with the objective of comparing findings to those of our earlier study which employed this age cutoff in a different population sample.29
Data collection
The HINTS assesses several cancer-related cognitions known to be important determinants of cancer-protective behaviour: perceived preventability of cancer, perceived cancer risk, cancer- related worry and perceived ambiguity about cancer prevention recommendations. These cognitions represented the primary outcome and predictor variables for the current analyses. Unlike the 2003 survey, however, HINTS 2005 measured these variables not in terms of cancer in general, but in terms of three specific cancer types: colon, skin and lung cancer. Individual respondents were randomly assigned, using a computer-generated number for each sampled household, to answer cancer cognition items about only one of these three malignancies. The final study sample included a total of 1447, 1231 and 1392 respondents, respectively, for the colon, skin and lung cancer items. Unequal totals for these groups were due to missing data and non-response. HINTS 2005 also included questions about several cancer-specific prevention and screening behaviours, which were asked of all survey respondents in our sample population, although the survey design was such that questions on colon cancer screening behaviours were asked only of respondents aged 45 and older.
Cancer risk-related cognitions
The primary outcome variables for our analyses were measured by three survey items coded on Likert scales, with each item made specific to one of the three cancer types (colon, skin or lung). Perceived preventability of cancer was assessed by the question, ‘There is not much you can do to lower your chances of getting (colon / skin / lung) cancer’. Response options were ‘agree’ and ‘disagree’. Perceived cancer risk was assessed by the question, ‘How likely do you think it is that you will develop (colon / skin / lung) cancer in the future?’ Response options were ‘very low’, ‘somewhat low’, ‘moderate’, ‘somewhat high’ and ‘very high’. Cancer-related worry was assessed by the question, ‘How often do you worry about getting (colon / skin / lung) cancer?’ Response options were ‘rarely or never’, ‘sometimes’, ‘often’ and ‘all the time’.
Cancer risk-modifying behaviours
Several risk-modifying behaviours specific to the three cancers were measured and treated as additional outcome variables in our analyses. Colon cancer risk-modifying behaviours consisted of the secondary preventive interventions of past colon cancer testing by colonoscopy or flexible sigmoidoscopy and by faecal occult blood testing (FOBT), which were ascertained by separate questions asking respondents whether or not they had ever had these tests. Skin cancer risk-modifying behaviours consisted of the primary preventive intervention of sunscreen use, which was measured by a single item, ‘When you go outside for more than 1 h on a warm, sunny day, how often do you wear sunscreen?’ Response options were: ‘always’, ‘often’, ‘sometimes’, ‘rarely’ and ‘never’. Lung cancer risk-modifying behaviours consisted of lifetime history of cigarette smoking at least 100 cigarettes, which was measured by a single item with ‘yes’ and ‘no’ response options, and current cigarette smoking in respondents with a past smoking history, which was measured by a single item, ‘Do you now smoke cigarettes…’ Response options were ‘every day’, ‘some days’, ‘not at all’, ‘do not know’ and ‘refused’.
Perceived ambiguity
The primary independent variable for all analyses was perceived ambiguity about cancer prevention recommendations. This construct was assessed by the question, ‘There are so many different recommendations about preventing (colon / skin / lung) cancer, it is hard to know which one to follow’. Response options were ‘agree’ and ‘disagree’.
Sociodemographic variables
Various sociodemographic factors were treated as covariates in all analyses. Age was coded using four response categories (40–49, 50–59, 60–69 and 70 and older). Race was coded using three response categories (White, Black and Other) while education level used four response categories (less than high school, high school graduate, some college and college graduate). Gender was also treated as a covariate. These factors have been shown to be associated with both health cognitions – e.g. perceived risk and preventability beliefs – as well as health-protective behaviours such as cancer screening; the potential for confounding effects justified controlling for these variables in our analysis.35,39–44 Income was previously shown to be highly correlated with education level, and was not included in subsequent analyses to avoid potential problems with multicollinearity.
Data analysis
To adjust for the complex sampling design of the HINTS,38 the statistical program SUDAAN (version 9.0.2, Research Triangle Institute, Research Triangle Park, NC, USA) was used in all analyses,45 utilizing sample weights provided with the HINTS public use data file. These weights were post-stratified to the US census distributions by age, sex and race / ethnicity for the year 2005 to provide estimates representative of the general US population. Variances of parameter estimators were calculated using a jackknife method.
We excluded individuals with ‘not ascertained’, ‘no opinion’, ‘do not know’ or ‘refused’ responses to any of the main survey items examined. The proportion of excluded data in the study sample exceeded 5% for the following individual variables: perceived colon cancer risk (10.4%), perceived colon cancer preventability (5.7%), and perceived ambiguity about colon cancer recommendations (7.1%).
Univariate and multivariate analyses were performed. Binary and ordinal logistic regression analyses were used to examine the relationships between perceived ambiguity and each of the primary outcome variables, adjusting for sociodemographic variables. We also explored predicted interactions and mediating relationships between perceived ambiguity and the primary outcome variables.
Predictions
Based on our previously derived theoretical model and the findings from our earlier study,29 we predicted that perceived ambiguity about cancer prevention recommendations would demonstrate several associations with other cognitive and behavioural variables (Fig. 1), in directions consistent with the theoretical concept of ‘ambiguity aversion’.
Lower perceived preventability of cancer.
Higher perceived cancer risk.
Higher cancer-related worry.
Lower adherence to cancer-specific risk-modifying behaviours (colon cancer testing, sunblock use and cigarette smoking abstinence).
Based on past research, we also predicted an interaction between baseline perceived ambiguity and cancer-related worry (perceived ambiguity × cancer-related worry), such that higher levels of worry would be associated with a stronger inverse relationship between perceived ambiguity and perceived cancer preventability, compared with the associations present at lower levels of worry. In addition, we predicted that perceived ambiguity would influence worry as well as cancer risk-modifying behaviours not only directly but indirectly, through its effects upon risk and preventability perceptions, respectively, which would act as mediating variables. We had no a priori reasons to expect cancer-specific differences in any of these predicted relationships.
Results
Distributions and US population-weighted percentages for the sociodemographic and cognitive variables examined in this study are shown in Tables 1 and 2. Most respondents were less than age 60 and white, and reported high school or greater education. The proportion of respondents who agreed with the perceived ambiguity item, ‘There are so many different recommendations about preventing cancer, it is hard to know which ones to follow’, ranged from 44% for lung cancer recommendations to 54% for colon cancer recommendations. Nevertheless, with respect to each of the three cancer types, most respondents still reported high preventability beliefs and low levels of perceived risk and cancer-related worry. With respect to cancer-protective behaviours, at least 40% of the entire study population reported past sigmoidoscopy– colonoscopy and FOBT, as well as at least some sunscreen use and past smoking history of >100 cigarettes. Of the 52% of respondents with a prior smoking history, 37% reported that they currently smoked.
Table 1.
Distribution and weighted percentages of sociodemographic characteristics of Health Information National Trends Survey (HINTS) respondents aged 40 and older (2005 HINTS)*
| Sociodemographic variables | N* | Percentage† |
|---|---|---|
| Age | ||
| 40–49 | 1029 | 34.6 |
| 50–59 | 1057 | 27.2 |
| 60–69 | 872 | 19.9 |
| 70+ | 1112 | 18.3 |
| Education level | ||
| Less than high school | 500 | 14.1 |
| High school graduate | 1094 | 35.3 |
| Some college | 1113 | 25.4 |
| College graduate | 1226 | 25.2 |
| Race | ||
| White | 3351 | 83.2 |
| Black | 337 | 10.0 |
| Others | 156 | 6.8 |
| Gender | ||
| Female | 2708 | 47.1 |
| Male | 1380 | 52.9 |
Total sample N = 4070; decreased and unequal ‘n’ for individual variables because of excluded and missing data.
Percentages weighted to the US population using 2005 census data.
Table 2.
Distribution and weighted percentages of cognitive and behavioural variables by cancer type, for Health Information National Trends Survey (HINTS) respondents aged 40 and older with no history of cancer (HINTS 2005)*
| Colon cancer | N† | Percentage‡ | Skin cancer | N§ | Percentage‡ | Lung cancer | N¶ | Percentage‡ |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Cognitive variables | ||||||||
| Perceived ambiguity | ||||||||
| Low | 610 | 46.0 | Low | 623 | 55.3 | Low | 761 | 55.7 |
| High | 701 | 54.0 | High | 493 | 44.7 | High | 560 | 44.3 |
| Perceived preventability | ||||||||
| Low | 296 | 23.3 | Low | 180 | 15.3 | Low | 247 | 18.6 |
| High | 1036 | 76.7 | High | 950 | 84.7 | High | 1084 | 81.4 |
| Perceived cancer risk | ||||||||
| Very low | 497 | 40.8 | Very low | 325 | 27.5 | Very low | 672 | 47.8 |
| Somewhat low | 380 | 27.5 | Somewhat low | 275 | 24.7 | Somewhat low | 264 | 20.3 |
| Moderate | 322 | 26.3 | Moderate | 351 | 33.6 | Moderate | 253 | 21.1 |
| Somewhat high | 57 | 4.6 | Somewhat high | 109 | 10.3 | Somewhat high | 82 | 6.8 |
| Very high | 11 | 0.8 | Very high | 44 | 3.8 | Very high | 33 | 4.0 |
| Cancer-related worry | ||||||||
| Rarely / never | 1099 | 77.1 | Rarely / never | 762 | 64.6 | Rarely / never | 1092 | 78.1 |
| Sometimes | 270 | 19.0 | Sometimes | 300 | 28.4 | Sometimes | 198 | 15.2 |
| Often | 29 | 2.1 | Often | 49 | 4.2 | Often | 44 | 3.3 |
| All the time | 12 | 1.9 | All the time | 35 | 2.8 | All the time | 33 | 3.4 |
| Behavioural variables | ||||||||
| Past colonoscopy | Sunscreen use | Lifetime cigarette smoking | ||||||
| Yes | 1890 | 49.2 | Always | 717 | 15.7 | Yes | 2017 | 51.7 |
| No | 1564 | 50.8 | Often | 610 | 14.0 | No | 1997 | 48.3 |
| Past faecal occult blood testing | Sometimes | 769 | 19.8 | Current smoking | ||||
| Yes | 1608 | 40.0 | Rarely | 643 | 17.3 | Every day | 521 | 30.1 |
| No | 1863 | 60.0 | Never | 1126 | 33.2 | Some days | 124 | 6.5 |
| Not at all | 1371 | 63.4 | ||||||
Total sample N = 4070; decreased and unequal ‘n’ for individual variables because of missing data.
n = 1447 for cognitive variables, n = 3487 for behavioural variables.
Percentages weighted to the US population using 2005 census data.
n = 1231 for cognitive variables, n = 4041 for behavioural variables.
n = 1392 for cognitive variables, n = 4025 for behavioural variables.
Relationships between perceived ambiguity and perceived preventability of cancer
Across all three cancer types, a strong inverse relationship was found between perceived ambiguity and perceived preventability of cancer, controlling for sociodemographic variables (Table 3). As predicted and consistent with the phenomenon of ambiguity aversion, perceived ambiguity was associated with lower preventability beliefs. Sociodemographic covariates demonstrating significant associations with perceived cancer preventability included age (for colon and lung cancer) and education level (for skin and lung cancer), with older age and lower education levels predicting lower perceived preventability.
Table 3.
Multivariate logistic regression models of the relationship between perceived ambiguity and perceived cancer preventability, by cancer type (2005 Health Information National Trends Survey)*
| Variables | Colon cancer preventability (n = 1199)
|
Skin cancer preventability (n = 1031)
|
Lung cancer preventability (n = 1243)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value† | OR | 95% CI | P-value† | OR | 95% CI | P-value† | |
| Age | |||||||||
| 40–49 | 1.00 | 0.040 | 1.00 | 0.264 | 1.00 | 0.019 | |||
| 50–59 | 0.52 | 0.24–1.11 | 0.97 | 0.45–2.09 | 0.94 | 0.45–1.97 | |||
| 60–69 | 0.76 | 0.38–1.52 | 0.95 | 0.48–1.85 | 0.54 | 0.30–0.97 | |||
| 70+ | 0.43 | 0.23–0.81 | 0.55 | 0.28–1.08 | 0.74 | 0.39–1.41 | |||
| Education level | |||||||||
| Less than high school | 1.00 | 0.059 | 1.00 | 0.008 | 1.00 | 0.007 | |||
| High school graduate | 1.19 | 0.68–2.10 | 1.70 | 0.87–3.34 | 1.97 | 1.13–3.45 | |||
| Some college | 2.54 | 1.18–5.47 | 2.83 | 1.29–6.22 | 2.29 | 1.20–4.36 | |||
| College graduate | 1.99 | 0.88–4.47 | 6.25 | 1.83–21.32 | 2.89 | 1.51–5.54 | |||
| Race | |||||||||
| White | 1.00 | 0.303 | 1.00 | 0.324 | 1.00 | 0.234 | |||
| Black | 0.60 | 0.23–1.53 | 0.72 | 0.34–1.55 | 0.78 | 0.36–1.70 | |||
| Others | 0.49 | 0.16–1.55 | 0.56 | 0.24–1.28 | 0.43 | 0.18–1.02 | |||
| Gender | |||||||||
| Female | 1.00 | 0.376 | 1.00 | 0.273 | 1.00 | 0.720 | |||
| Male | 0.81 | 0.50–1.31 | 1.33 | 0.79–2.24 | 0.92 | 0.58–1.46 | |||
| Perceived ambiguity | |||||||||
| Low | 1.00 | <0.001 | 1.00 | <0.001 | 1.00 | <0.001 | |||
| High | 0.27 | 0.15–0.46 | 0.20 | 0.10–0.41 | 0.23 | 0.14–0.37 | |||
Total sample N = 1414; separate models fitted for each cancer type; decreased and unequal ‘n’ for individual models (indicated in parentheses) because of excluded and missing data.
For Wald chi-squared test of association.
Contrary to predictions, perceived ambiguity did not interact with cancer-related worry (perceived ambiguity × cancer-related worry) in its relationship with perceived cancer preventability, for any of the three cancer types.
Relationships between perceived ambiguity and perceived cancer risk
For lung cancer only, a positive relationship [odds ratio (OR) 1.85, 95% CI: 1.27–2.69 and P = 0.001] was found between perceived ambiguity and perceived cancer risk, controlling for sociodemographic variables (Table 4). Consistent with predictions, high perceived ambiguity was associated with higher levels of perceived lung cancer risk. However, this relationship was not observed with perceived colon and skin cancer risk. Sociodemographic covariates demonstrating significant associations with perceived lung cancer risk included age, education level, and race, with older age and higher education being associated with lower perceived lung cancer risk, and non-white, non-black race being associated with higher perceived lung cancer risk.
Table 4.
Multivariate ordinal logistic regression models of the relationship between perceived ambiguity and perceived cancer risk, by cancer type (2005 Health Information National Trends Survey)*
| Variables | Colon cancer perceived risk (n = 1141)
|
Skin cancer perceived risk (n = 1010)
|
Lung cancer preventability risk (n = 1214)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value† | OR | 95% CI | P-value† | OR | 95% CI | P-value† | |
| Age | |||||||||
| 40–49 | 1.00 | 0.022 | 1.00 | <0.001 | 1.00 | <0.001 | |||
| 50–59 | 1.00 | 0.67–1.48 | 0.78 | 0.54–1.11 | 1.01 | 0.70–1.47 | |||
| 60–69 | 0.98 | 0.66–1.43 | 0.70 | 0.44–1.13 | 0.92 | 0.58–1.48 | |||
| 70+ | 0.54 | 0.36–0.82 | 0.26 | 0.16–0.43 | 0.48 | 0.32–0.72 | |||
| Education level | |||||||||
| Less than high school | 1.00 | 0.646 | 1.00 | 0.067 | 1.00 | 0.052 | |||
| High school graduate | 1.36 | 0.83–2.24 | 1.92 | 1.12–3.28 | 1.16 | 0.70–1.92 | |||
| Some college | 1.34 | 0.75–2.41 | 1.78 | 1.05–3.01 | 0.79 | 0.47–1.33 | |||
| College graduate | 1.25 | 0.70–2.25 | 1.94 | 1.08–3.50 | 0.63 | 0.36–1.09 | |||
| Race | |||||||||
| White | 1.00 | 0.033 | 1.00 | <0.001 | 1.00 | 0.039 | |||
| Black | 0.47 | 0.21–1.08 | 0.23 | 0.12–0.45 | 0.86 | 0.48–1.53 | |||
| Others | 0.42 | 0.17–1.00 | 0.29 | 0.16–0.54 | 2.58 | 1.18–5.64 | |||
| Gender | |||||||||
| Female | 1.00 | 0.116 | 1.00 | 0.868 | 1.00 | 0.751 | |||
| Male | 1.34 | 0.92–1.94 | 0.97 | 0.68–1.39 | 1.06 | 0.74–1.50 | |||
| Perceived ambiguity | |||||||||
| Low | 1.00 | 0.209 | 1.00 | 0.921 | 1.00 | 0.001 | |||
| High | 1.24 | 0.88–1.77 | 0.98 | 0.67–1.43 | 1.85 | 1.27–2.69 | |||
Total sample N = 1414; separate models fitted for each cancer type; decreased and unequal ‘n’ for individual models (indicated in parentheses) because of excluded and missing data.
For Wald chi-squared test of association.
Relationships between perceived ambiguity and cancer-related worry
For lung cancer only, a positive relationship (OR 1.48, 95% CI: 1.00–2.19 and P = 0.046) was found between perceived ambiguity and lung cancer-related worry, controlling for sociodemographic variables (Table 5). As predicted, higher levels of perceived ambiguity were associated with higher levels of lung cancer-related worry. This relationship was not observed with colon and skin cancer-related worry, although the association with colon cancer approached statistical significance (OR 1.41, 95% CI: 0.95– 2.11 and P = 0.081). Sociodemographic covariates demonstrating significant associations with lung cancer-related worry included age (P < 0.001) and education level (P = 0.018), with older age and higher education being associated with lower lung cancer-related worry.
Table 5.
Multivariate ordinal logistic regression models of the relationship between perceived ambiguity and cancer-related worry, by cancer type (2005 Health Information National Trends Survey)*
| Variables | Colon cancer worry (n = 1242)
|
Skin cancer worry (n = 1041)
|
Lung cancer worry (n = 1262)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value† | OR | 95% CI | P-value† | OR | 95% CI | P-value† | |
| Age | |||||||||
| 40–49 | 1.00 | 0.755 | 1.00 | <0.001 | 1.00 | <0.001 | |||
| 50–59 | 0.80 | 0.48–1.34 | 0.69 | 0.42–1.13 | 0.77 | 0.51–1.16 | |||
| 60–69 | 0.84 | 0.49–1.44 | 0.72 | 0.43–1.21 | 0.82 | 0.47–1.44 | |||
| 70+ | 0.82 | 0.54–1.26 | 0.29 | 0.18–0.47 | 0.32 | 0.18–0.56 | |||
| Education level | |||||||||
| Less than high school | 1.00 | 0.237 | 1.00 | 0.057 | 1.00 | 0.018 | |||
| High school graduate | 0.68 | 0.35–1.31 | 2.07 | 1.00–4.33 | 0.81 | 0.42–1.55 | |||
| Some college | 0.52 | 0.27–1.02 | 1.90 | 0.93–3.88 | 0.46 | 0.21–1.00 | |||
| College graduate | 0.51 | 0.25–1.05 | 2.72 | 1.28–5.77 | 0.47 | 0.24–0.89 | |||
| Race | |||||||||
| White | 1.00 | 0.086 | 1.00 | 0.108 | 1.00 | 0.122 | |||
| Black | 2.27 | 0.88–5.83 | 0.35 | 0.12–0.96 | 1.10 | 0.62–1.96 | |||
| Others | 1.68 | 0.78–3.61 | 0.84 | 0.41–1.75 | 1.86 | 1.01–3.44 | |||
| Gender | |||||||||
| Female | 1.00 | 0.091 | 1.00 | 0.062 | 1.00 | 0.774 | |||
| Male | 1.40 | 0.94–2.08 | 0.74 | 0.53–1.02 | 0.93 | 0.58–1.51 | |||
| Perceived ambiguity | |||||||||
| Low | 1.00 | 0.081 | 1.00 | 0.837 | 1.00 | 0.046 | |||
| High | 1.41 | 0.95–2.11 | 1.04 | 0.70–1.55 | 1.48 | 1.00–2.19 | |||
Total sample N = 1414; separate models fitted for each cancer type; decreased and unequal ‘n’ for individual models (indicated in parentheses) because of excluded and missing data.
For Wald chi-squared test of association.
Mediational effects
To follow up on the significant main effects found for lung cancer cognitions, we conducted mediational analyses to test the prediction that perceived ambiguity would appear to influence worry not only directly but indirectly, through its effects upon risk perceptions. Using Baron and Kenny’s procedure,46 we first confirmed significant associations between perceived ambiguity and worry (P = 0.046), perceived ambiguity and perceived risk (P = 0.001), and perceived risk and worry (P < 0.001), in separate logistic regression models. Next, we regressed worry on perceived ambiguity while controlling for perceived risk; this reduced the previously significant association between perceived ambiguity and worry to non-significance (P = 0.370), consistent with a mediational effect of perceived risk, and the OR for the association decreased from 1.48 (95% CI: 1.00–2.19) to 1.17 (95% CI: 0.82–1.68). Supporting the hypothesized causal direction of this effect, we also found no evidence that worry mediated the relationship between perceived ambiguity and perceived risk. The association between perceived ambiguity and perceived risk remained significant (P < 0.001) even when controlling for worry.
Relationships between perceived ambiguity and cancer-specific risk-modifying behaviours
Colon cancer screening
Perceived ambiguity about colon cancer prevention recommendations was inversely associated with self-reported past flexible sigmoidoscopy or colonoscopy (OR 0.59, 95% CI: 0.38–0.92 and P = 0.018) (Table 6). Consistent with the phenomenon of ambiguity aversion, perceived ambiguity was associated with less past sigmoidoscopy–colonoscopy. A similar trend was seen with past FOBT, although this association did not reach statistical significance (OR 0.80, 95% CI: 0.57–1.13 and P = 0.066). Further mediational analyses 46 showed only a borderline significant mediating effect of perceived colon cancer preventability in the relationship between perceived ambiguity and sigmoidoscopy–colonoscopy screening. Inclusion of the potential mediating variable in the regression model reduced the significant association between perceived ambiguity and sigmoidoscopy–colonoscopy to non-significance, although the change in P-value was relatively small (from 0.018 to 0.054).
Table 6.
Multivariate logistic regression models of the relationship between perceived ambiguity and cancer-specific risk-related behaviours, by cancer type (2005 Health Information National Trends Survey)*
| Variables | Colon cancer | Skin cancer | Lung cancer | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonoscopy / sigmoidoscopy (n = 1085)
|
Faecal occult blood testing (n = 1090)
|
Sunscreen use (n =998)
|
Lifetime history of smoking 100 cigarettes‡(n= 1263)
|
Current smoking in ever smokers‡(n = 655)
|
|||||||||||
| OR | 95% CI | P-value† | OR | 95% CI | P-value† | OR | 95% CI | P-value† | OR | 95% CI | P-value† | OR | 95% CI | P-value† | |
| Age | |||||||||||||||
| 40–49 | 1.00 | <0.001 | 1.00 | <0.001 | 1.00 | 0.944 | 1.00 | 0.013 | 1.00 | <0.001 | |||||
| 50–59 | 5.26 | 3.07–9.02 | 1.68 | 0.83–3.37 | 1.03 | 0.71–1.50 | 1.41 | 0.87–2.28 | 0.41 | 0.24–0.69 | |||||
| 60–69 | 12.69 | 6.57–24.53 | 4.56 | 2.12–9.81 | 0.89 | 0.55–1.42 | 2.04 | 1.28–3.27 | 0.42 | 0.22–0.81 | |||||
| 70+ | 14.16 | 7.36–27.23 | 6.15 | 3.03–12.48 | 0.94 | 0.58–1.52 | 1.27 | 0.80–2.02 | 0.14 | 0.06–0.30 | |||||
| Education level | |||||||||||||||
| Less than high school | 1.00 | 0.002 | 1.00 | 0.015 | 1.00 | <0.001 | 1.00 | 0.093 | 1.00 | <0.001 | |||||
| High school graduate | 1.80 | 1.01–3.22 | 1.45 | 0.81–2.59 | 3.37 | 1.79–6.35 | 0.71 | 0.38–1.34 | 0.97 | 0.46–2.06 | |||||
| Some college | 1.93 | 1.07–3.49 | 1.76 | 1.05–2.94 | 3.63 | 1.81–7.29 | 0.62 | 0.32–1.21 | 0.42 | 0.18–0.98 | |||||
| College graduate | 3.31 | 1.76–6.23 | 2.03 | 1.29–3.20 | 6.49 | 3.47–12.15 | 0.55 | 0.31–0.95 | 0.27 | 0.12–0.62 | |||||
| Race | |||||||||||||||
| White | 1.00 | 0.040 | 1.00 | 0.141 | 1.00 | <.001 | 1.00 | 0.937 | 1.00 | 0.408 | |||||
| Black | 0.45 | 0.24–0.87 | 0.93 | 0.40–2.13 | 0.20 | 0.10–0.40 | 0.96 | 0.51–1.80 | 0.82 | 0.39–1.72 | |||||
| Others | 1.00 | 0.44–2.25 | 0.42 | 0.16–1.06 | 0.61 | 0.25–1.50 | 1.10 | 0.48–2.54 | 1.60 | 0.68–3.78 | |||||
| Gender | |||||||||||||||
| Female | 1.00 | 0.387 | 1.00 | 0.613 | 1.00 | <0.001 | 1.00 | <0.001 | 1.00 | 0.254 | |||||
| Male | 0.85 | 0.59–1.23 | 1.09 | 0.78–1.52 | 0.38 | 0.27–0.53 | 2.08 | 1.40–3.10 | 0.76 | 0.46–1.23 | |||||
| Perceived ambiguity | |||||||||||||||
| Low | 1.00 | 0.018 | 1.00 | 0.066 | 1.00 | 0.044 | 1.00 | 0.164 | 1.00 | 0.015 | |||||
| High | 0.59 | 0.38–0.92 | 0.80 | 0.57–1.13 | 0.68 | 0.47–1.00 | 1.22 | 0.92–1.61 | 1.85 | 1.11–3.07 | |||||
Total sample N = 1414; separate models fitted for each cancer type and behaviour; decreased and unequal ‘n’ for individual models (indicated in parentheses) because of excluded and missing data.
For Wald chi-squared test of association.
Analysis using ordinal regression.
Sunscreen use
Perceived ambiguity about skin cancer prevention recommendations was inversely associated with sunscreen use (OR 0.68, 95% CI: 0.47–1.00 and P = 0.044) (Table 6). Consistent with the phenomenon of ambiguity aversion, perceived ambiguity was associated with lower levels of sunscreen use. Mediational analysis46 showed no significant mediating effect of perceived skin cancer preventability in the relationship between perceived ambiguity and sunscreen use.
Cigarette smoking
Perceived ambiguity about lung cancer prevention recommendations was not significantly associated with lifetime history of smoking at least 100 cigarettes (OR 1.22, 95% CI: 0.92–1.61 and P = 0.164), although it was positively associated (OR 1.85, 95% CI: 1.11–3.07 and P = 0.015) with current cigarette smoking in respondents with a past smoking history (n = 655) (Table 6). No mediators in this relationship were identified.
Discussion
In this nationally representative cross-sectional survey, we found significant relationships between perceived ambiguity about cancer prevention recommendations and other important cognitions pertaining to specific cancers. Consistent with the phenomenon of ‘ambiguity aversion’, perceived ambiguity had a strong negative relationship with perceptions of the preventability of all three types of cancer – colon, skin and lung examined in the study. These results support both theory-based predictions and the findings of our earlier study which was conducted in a different sample population29 and which focused solely on the relationship between perceived ambiguity and cognitions pertaining to cancer in general.
Furthermore, in addition to these predicted relationships with cancer-related cognitions, perceived ambiguity about the prevention of specific cancers was inversely related to several corresponding risk-modifying behaviours, including sigmoidoscopy–colonoscopy testing, sunscreen use and smoking abstinence (among respondents with a past smoking history). This finding of an association between perceived ambiguity about cancer prevention recommendations and actual preventive behaviours expands upon our previous work and adds to growing evidence that ambiguity perceptions in this domain may have behavioural as well as cognitive manifestations,47–49 potentially influencing both primary (sunscreen use and smoking abstinence) and secondary (sigmoidoscopy– colonoscopy testing) prevention behaviours.
The current study, however, did not support all of our predictions regarding the potential effects of perceived ambiguity. Across all cancer types, perceived ambiguity did not demonstrate consistent associations with cancer risk perceptions or worry. Although perceived ambiguity was positively related to both perceived risk and worry pertaining to lung cancer, these relationships were not seen for colon and skin cancer. This finding was contrary to study predictions and the results of our earlier analysis of non-cancer-specific cognitions, suggesting that cancer type moderates the potential cognitive effects of ambiguity perceptions. Because smokers are the individuals most at risk for lung cancer, this finding may reflect tighter coherence among cognitions about cancers for which there is one primary risk factor. This hypothesis needs to be tested.
These cancer-specific differences highlight the need to understand the potential influence of ambiguity perceptions within the larger context of key cognitions that constitute people’s mental representations of illness. Important cognitions that might be affected by ambiguity – e.g. perceptions regarding the risk and controllability of a disease, and the risks of both undertaking or foregoing a disease-protective intervention – are components of specific mental models that individuals develop over time as they process health information from different sources.50,51 People’s responses to ambiguity may be altered by these mental models in various ways. The extent to which ambiguity about preventing a particular cancer heightens risk perceptions or cancer-related worry may be moderated by preexisting beliefs about the cancer’s causes, consequences or natural history. Furthermore, the coherence – as well as the content – of these mental models may influence people’s responses to ambiguity. Pertinent illness representations including perceptions of both the preventability and risk of cancer may be well formed and strongly held, because of several factors – e.g. the influence of mass media and disease advocacy groups,5,52 cultural values regarding the good of cancer prevention and screening.53–55 This may make people more or less resistant to ambiguous information about cancer prevention.
At the same time, the observed relationships between perceived ambiguity and various cancer risk-modifying behaviours suggest that the potential causal pathways from ambiguity perceptions to behaviours are complex. Cancer risk perceptions and worry may play little role in these pathways, at least for the colon and skin cancer protective behaviours examined. For sigmoidoscopy–colonoscopy screening, perceived ambiguity may influence behaviour through its effect on cancer preventability beliefs, while for other behaviours such as sunscreen use and smoking abstinence, preventability beliefs may not be important mediating factors. Factors related to the protective behaviour itself may also moderate the influence of ambiguity perceptions; this may explain why, for example, perceived ambiguity was associated with sigmoidoscopy–colonoscopy testing, but not with FOBT. Other variables may also act as moderators in these pathways, although contrary to predictions, no significant interactions were found between cancer-specific worry and perceived ambiguity in relation to any of the other outcome variables. These findings remain tentative, however, given that the study may have had insufficient power to detect all potentially significant interactions.
Much more work is needed to elucidate the mechanisms underlying ambiguity aversion. Causal directions need to be firmly established, and this represents the main limitation of the current study. Although our hypothesized causal model and the study’s specific findings are consistent with a large body of past research on the outcomes of ambiguity perceptions, longitudinal and experimental studies are necessary to confirm our findings and validate the model.
Furthermore, a large number of associations were examined, and although most of our findings were consistent with theoretical predictions and past empirical work, some observed associations could have resulted from chance alone. We also excluded subjects with ‘do not know’ responses to several items including those used to measure perceived ambiguity. This could have biased our findings, if such responses indicated high ambiguity perceptions – a possibility supported by additional analyses in which similar associations with outcome variables, such as perceived colon cancer preventability, were found for the ‘do not know’ (OR 0.19, 95% CI: 0.09–0.42) and ‘high’ (OR 0.27, 95% CI: 0.15–0.46) response categories of the perceived ambiguity variable. This suggests that any bias introduced by excluding ‘do not know’ respondents was likely conservative (biased towards the null), and an acceptable trade-off to avoid analytic problems posed by small cell sizes or imputed responses.
Other study limitations point to additional research needs. HINTS 2005 did not ascertain the specific targets of perceived ambiguity; thus we do not know, for example, whether respondents’ ambiguity perceptions related to random exposure to health messages from mass media, or to deliberate communication efforts by health professionals. Nor do we know whether respondents’ exposure to ambiguous information was passive or the result of active information seeking. These unmeasured variables, however, might be critical determinants of people’s responses to ambiguity. Another methodological concern is that perceived ambiguity and the constructs of perceived cancer preventability, risk and worry were each measured by single items with unknown reliability and validity. This may limit the strength of our conclusions, although cognitions such as risk perceptions have been shown to be highly reliable over time,56 and single-item measures of constructs such as cancer risk and cancer worry have been found in other studies to predict behavioural endpoints such as cancer screening.57–59
The behavioural variables analysed in our study may also have had limited validity as indicators of the effects of ambiguity perceptions. HINTS 2005 did not ascertain whether sigmoidoscopy–colonoscopy testing, for example, was performed for the purpose of cancer screening as opposed to diagnostic evaluation. In the strictest sense, furthermore, screening interventions such as sigmoidoscopy, colonoscopy and FOBT do not prevent cancer; therefore, ambiguity about colon cancer prevention recommendations should not influence screening-related cognitions and behaviours. Likewise, because both sunscreen use and smoking abstinence serve purposes other than cancer prevention, perceived ambiguity about skin and lung cancer prevention might have limited effect on these behaviours. The lack of ascertainment of behaviours more explicitly aimed at the primary prevention of all three cancers likely reduced our ability to determine the full impact of ambiguity perceptions related to cancer prevention recommendations. The fact that perceived ambiguity nevertheless demonstrated significant associations with all these variables suggests that ambiguity perceptions are powerful determinants of behaviour, sometimes being overgeneralized to circumstances in which they do not strictly apply.
Finally, aspects of the study population may limit the generalizability of our findings. The relatively low-response rate for the HINTS 2005 reflects an unfortunate trend in survey research,60,61 and may have biased our findings. The exclusion from analyses of participants with ‘do not know’ and ‘refused’ responses may have also introduced bias, as non-response and indecision might reflect high perceived ambiguity. Furthermore, because the study sample was predominantly white and well educated, we are less able to generalize our findings to members of other population groups.
In spite of these limitations, the current study provides convergent evidence that ambiguity surrounding cancer prevention recommendations may have predictable effects on important cognitions, emotions and behaviours. It may diminish beliefs in the preventability of cancer, lower adherence to cancer-protective interventions and in some cases heighten perceptions of vulnerability to cancer. The communication of ambiguity to the public thus poses potential ethical trade-offs for clinicians, communication experts, and policy makers who are interested in promoting informed and shared decision making. In the cancer prevention domain at least, these approaches to making health care more patient-centred62 could end-up diminishing patient well-being. A critical task, then, is to determine when heightened ambiguity perceptions are truly appropriate and warranted, and how ambiguity should be communicated in these circumstances.
Yet, the current study also suggests that the potential outcomes of communicating ambiguity are not straightforward; ambiguity aversion may manifest in different ways and degrees depending on the type of cancer involved, and other factors. Not all ambiguous situations are aversive, and not everyone confronting ambiguity will react in the same way. But this means that we need to understand much more about the factors that mitigate people’s responses to ambiguity, to anticipate these responses and to design effective, supportive, and ethical interventions for communicating ambiguity in cancer control and in health care generally. We view the current study as a preliminary step towards that goal.
References
- 1.Viswanath K. Science and society: the communications revolution and cancer control. Nature Reviews Cancer. 2005;5:828–835. doi: 10.1038/nrc1718. [DOI] [PubMed] [Google Scholar]
- 2.Copper CP, Yukimura D. Science writers’ reactions to a medical “breakthrough” story. Social Science and Medicine. 2002;54:1887–1896. doi: 10.1016/s0277-9536(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 3.Friedman SM, Dunwoody S, Rogers CL, editors. Communicating Uncertainty: Media Coverage of New and Controversial Science. Mahway, NJ: Lawrence Erlbaum Associates; 1999. (LEA’s Communication Series) [Google Scholar]
- 4.Moynihan R, Heath I, Henry D. Selling sickness: the pharmaceutical industry and disease mongering. British Medical Journal. 2002;324:886–891. doi: 10.1136/bmj.324.7342.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamey G, Wilkes M. The PSA storm. British Medical Journal. 2002;324:431. [Google Scholar]
- 6.Bekker H, Thornton JG, Airey CM, et al. Informed decision making: an annotated bibliography and systematic review. Health Technology Assessment. 1999;3:1–156. [PubMed] [Google Scholar]
- 7.Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango) Social Science and Medicine. 1997;44:681–692. doi: 10.1016/s0277-9536(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 8.Emanuel EJ, Emanuel LL. Four models of the physician-patient relationship. The Journal of the American Medical Association. 1992;267:2221–2226. [PubMed] [Google Scholar]
- 9.Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Annals of Internal Medicine. 2004;140:54–59. doi: 10.7326/0003-4819-140-1-200401060-00012. [DOI] [PubMed] [Google Scholar]
- 10.Whitney SN. A new model of medical decisions: exploring the limits of shared decision making. Medical Decision Making. 2003;23:275–280. doi: 10.1177/0272989X03256006. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Crossing the Quality Chasm: a New Health System for the Twenty-first Century. Washington: National Academies Press; 2001. [PubMed] [Google Scholar]
- 12.Woolf SH, Chan EC, Harris R, et al. Promoting informed choice: transforming health care to dispense knowledge for decision making. Annals of Internal Medicine. 2005;143:293–300. doi: 10.7326/0003-4819-143-4-200508160-00010. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor AM, Stacey D, Entwistle V, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systemic Reviews. 2003;2:CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor AM, Rostom A, Fiset V, et al. Decision aids for patients facing health treatment or screening decisions: systematic review. British Medical Journal. 1999;319:731–734. doi: 10.1136/bmj.319.7212.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Affairs. 2004 Nov-Dec;23(6):VAR63–VAR72. doi: 10.1377/hlthaff.var.63. [DOI] [PubMed] [Google Scholar]
- 16.Barry MJ. Health decision aids to facilitate shared decision making in office practice. Annals of Internal Medicine. 2002;136:127–135. doi: 10.7326/0003-4819-136-2-200201150-00010. [DOI] [PubMed] [Google Scholar]
- 17.Molenaar S, Sprangers MA, Postma-Schuit FC, et al. Feasibility and effects of decision aids. Medical Decision Making. 2000;20:112–127. doi: 10.1177/0272989X0002000114. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar SS, Lepper MR. When choice is demotivating: can one desire too much of a good thing? Journal of Personality and Social Psychology. 2000;79:995–1006. doi: 10.1037//0022-3514.79.6.995. [DOI] [PubMed] [Google Scholar]
- 19.Redelmeier DA, Shafir E. Medical decision making in situations that offer multiple alternatives. The Journal of the American Medical Association. 1995;273:302–305. doi: 10.1001/jama.1995.03520280048038. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz B. The tyranny of choice. Scientific American. 2004;290:70–75. doi: 10.1038/scientificamerican0404-70. [DOI] [PubMed] [Google Scholar]
- 21.Ubel PA. Is information always a good thing? Helping patients make “good” decisions. Medical Care. 2002;9 (Suppl):V39–V44. doi: 10.1097/01.MLR.0000023954.85887.69. [DOI] [PubMed] [Google Scholar]
- 22.Ellsberg D. Risk, ambiguity, and the savage axioms. Quarterly Journal of Economics. 1961;75:643–669. [Google Scholar]
- 23.Camerer C, Weber M. Recent developments in modeling preferences: uncertainty and ambiguity. Journal of Risk and Uncertainty. 1992;5:325–370. [Google Scholar]
- 24.Curley SP, Yates JF. An empirical evaluation of descriptive models of ambiguity reactions in choice situations. Journal of Mathematical Psychology. 1989;33:397–427. [Google Scholar]
- 25.Einhorn HJ, Hogarth RM. Decision making under ambiguity. Journal of Business. 1986;59:S225–S250. [Google Scholar]
- 26.Einhorn HJ, Hogarth RM. Ambiguity and uncertainty in probabilistic inference. Psychological Review. 1985;92:433–461. [Google Scholar]
- 27.Rimer BK, Briss PA, Zeller PK, Chan EC, Woolf SH. Informed decision making: what is its role in cancer screening? Cancer. 2004;101 (Suppl 5):1214–1228. doi: 10.1002/cncr.20512. [DOI] [PubMed] [Google Scholar]
- 28.Briss P, Rimer B, Reilley B, et al. Promoting informed decisions about cancer screening in communities and healthcare systems. American Journal of Preventive Medicine. 2004;26:67–80. doi: 10.1016/j.amepre.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Han PK, Moser RP, Klein WM. Perceived ambiguity about cancer prevention recommendations: relationship to perceptions of cancer preventability, risk, and worry. Journal of Health Communication. 2006;11 (Suppl 1):51–69. doi: 10.1080/10810730600637541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritov I, Baron J. Reluctance to vaccinate: omission bias and ambiguity. Journal of Behavioral Decision Making. 1990;3:263–277. [Google Scholar]
- 31.Meszaros JR, Asch DA, Baron J, Hershey JC, Kunreuther H, Schwartz-Buzaglo J. Cognitive processes and the decisions of some parents to forego pertussis vaccination for their children. Journal of Clinical Epidemiology. 1996;49:697–703. doi: 10.1016/0895-4356(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 32.Viscusi WK, Magat WA, Huber J. Communication of ambiguous risk information. Theory and Decision. 1991;31:159–173. [Google Scholar]
- 33.Viscusi WK, Magat WA, Huber J. Smoking status and public responses to ambiguous scientific risk evidence. Southern Economic Journal. 1999;66:250–270. [Google Scholar]
- 34.Jepson RG, Forbes CA, Sowden AJ, Lewis RA. Increasing informed uptake and non-uptake of screening: evidence from a systematic review. Health Expectations. 2001;4:116–126. doi: 10.1046/j.1369-6513.2001.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolf AM, Nasser JF, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Archives of Internal Medicine. 1996;156:1333–1336. [PubMed] [Google Scholar]
- 36.Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Annals of Family Medicine. 2003;1:22–28. doi: 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frosch DL, Kaplan RM, Felitti V. The evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. Journal of General Internal Medicine. 2001;16:391–398. doi: 10.1046/j.1525-1497.2001.016006391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson DE, Kreps GL, Hesse BW, et al. The Health Information National Trends Survey (HINTS): development, design, and dissemination. Journal of Health Community. 2004;9:443–460. doi: 10.1080/10810730490504233. (discussion 481–444) [DOI] [PubMed] [Google Scholar]
- 39.Nijs HG, Essink-Bot ML, DeKoning HJ, Kirkels WJ, Schroder FH. Why do men refuse or attend population-based screening for prostate cancer? Journal of Public Health Medicine. 2000;22:312–316. doi: 10.1093/pubmed/22.3.312. [DOI] [PubMed] [Google Scholar]
- 40.Myers RE, Wolf TA, McKee L, et al. Factors associated with intention to undergo annual prostate cancer screening among African American men in Philadelphia. Cancer. 1996;78:471–479. doi: 10.1002/(SICI)1097-0142(19960801)78:3<471::AID-CNCR14>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 41.Wolf AM, Philbrick JT, Schorling JB. Predictors of interest in prostate-specific antigen screening and the impact of informed consent: what should we tell our patients? The American Journal of Medicine. 1997;103:308–314. doi: 10.1016/s0002-9343(97)00155-1. [DOI] [PubMed] [Google Scholar]
- 42.Myers RE, Hyslop T, Jennings-Dozier K, et al. Intention to be tested for prostate cancer risk among African-American men. Cancer Epidemiology, Biomarkers and Prevention. 2000;9:1323–1328. [PubMed] [Google Scholar]
- 43.Myers RE. African American men, prostate cancer early detection examination use, and informed decision-making. Seminars in Oncology. 1999;26:375–381. [PubMed] [Google Scholar]
- 44.Demark-Wahnefried W, Strigo T, Catoe K, et al. Knowledge, beliefs, and prior screening behavior among blacks and whites reporting for prostate cancer screening. Urology. 1995;46:346–351. doi: 10.1016/S0090-4295(99)80218-0. [DOI] [PubMed] [Google Scholar]
- 45.Shah B, Barnwell B, Bieler G. SUDAAN. Research Triangle Park, NC: Research Triangle Institute; 1997. [Google Scholar]
- 46.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 47.Meissner HI, Rimer BK, Davis WW, Eisner EJ, Siegler IC. Another round in the mammography controversy. Journal of Women’s Health (Larchmt) 2003;12:261–276. doi: 10.1089/154099903321667609. [DOI] [PubMed] [Google Scholar]
- 48.Han PKJ, Kobrin SC, Klein WMP, Davis WW, Stefanek ME, Taplin SH. Perceived ambiguity about screening mammography recommendations: association with future mammography uptake and perceptions. Cancer Epidemiology, Biomarkers and Prevention. 2007;16:458–466. doi: 10.1158/1055-9965.EPI-06-0533. [DOI] [PubMed] [Google Scholar]
- 49.Rimer BK, Halabi S, Strigo TS, Crawford Y, Lipkus IM. Confusion about mammography: prevalence and consequences. Journal of Women’s Health and Gender-Based Medicine. 1999;8:509–520. doi: 10.1089/jwh.1.1999.8.509. [DOI] [PubMed] [Google Scholar]
- 50.Leventhal H, Brissette I, Leventhal EA. The common-sense model of self-regulation of health and illness. In: Cameron LD, Leventhal H, editors. The Self-regulation of Health and Illness Behaviour. London: Routledge; 2003. pp. 42–65. [Google Scholar]
- 51.Cameron LD. Conceptualizing and assessing risk perceptions: a self-regulatory perspective. Paper Presented at the National Cancer Institute Workshop on Conceptualizing and Measuring Risk Perceptions; Washington, DC. 13–14 February 2003. [Google Scholar]
- 52.Katz ML, Sheridan S, Pignone M, et al. Prostate and colon cancer screening messages in popular magazines. Journal of General Internal Medicine. 2004;19:843–848. doi: 10.1111/j.1525-1497.2004.30504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrell MH, Murphy MA, Schneider CE. How underlying patient beliefs can affect physician–patient communication about prostate-specific antigen testing. Effective Clinical Practice. 2002;5:120–129. [PubMed] [Google Scholar]
- 54.Schwartz LM, Woloshin S. News media coverage of screening mammography for women in their 40s and tamoxifen for primary prevention of breast cancer. The Journal of the American Medical Association. 2002;287:3136–3142. doi: 10.1001/jama.287.23.3136. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. The Journal of the American Medical Association. 2004;291:71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 56.Shepperd JA, Helweg-Larsen M, Ortega L. Are comparative risk judgments stable across time and events? Personality and Social Psychology Bulletin. 2003;29:1169–1180. doi: 10.1177/0146167203254598. [DOI] [PubMed] [Google Scholar]
- 57.Stefanek ME, Wilcox P. First degree relatives of breast cancer patients: screening practices and provision of risk information. Cancer Detection and Prevention. 1991;15:379–384. [PubMed] [Google Scholar]
- 58.Lipkus IM, Iden D, Terrenoire J, Feaganes JR. Relationships among breast cancer concern, risk perceptions, and interest in genetic testing for breast cancer susceptibility among African-American women with and without a family history of breast cancer. Cancer Epidemiology, Biomarkers and Prevention. 1999;8:533–539. [PubMed] [Google Scholar]
- 59.Diefenbach MA, Miller SM, Daly MB. Specific worry about breast cancer predicts mammography use in women at risk for breast and ovarian cancer. Health Psychology. 1999;18:532–536. doi: 10.1037//0278-6133.18.5.532. [DOI] [PubMed] [Google Scholar]
- 60.Goyder J, Warriner K, Miller S. Evaluating socioeconomic status (SES) bias in survey nonresponse. Journal of Official Statistics. 2002;18:1–12. [Google Scholar]
- 61.de Leeuw E, de Heer W. Trends in household survey nonresponse: a longitudinal and international comparison. In: Groves DADRM, Eltinge JL, Little RJA, editors. Survey Nonresponse. New York, NY: John Wiley; 2002. pp. 121–134. [Google Scholar]
- 62.Sepucha KR, Fowler FJ, Mulley AG. Policy support for patient-centered care: the need for measurable improvements in decision quality. Health Affairs. 2004 Nov-Dec;23(6):VAR54–VAR62. doi: 10.1377/hlthaff.var.54. [DOI] [PubMed] [Google Scholar]