Abstract
Neural precursor cells (NPCs) are the subject of intense investigation for their potential to treat neurodegenerative disorders, yet the consequences of neuroinvasive virus infection of NPCs remain unclear. This study demonstrates that NPCs support replication following infection by the neurotropic JHM strain of mouse hepatitis virus (JHMV). JHMV infection leads to increased cell death and dampens IFN-γ-induced MHC class II expression. Importantly, cytokines secreted by CD4+ T cells inhibit JHMV replication in NPCs, and CD8+ T cells specifically target viral peptide-pulsed NPCs for lysis. Furthermore, treatment with IFN-γ inhibits JHMV replication in a dose-dependent manner. Together, these findings suggest that T cells play a critical role in controlling replication of a neurotropic virus in NPCs, a finding which has important implications when considering immune modulation for NPC-based therapies for treatment of human neurologic diseases.
Keywords: Neural precursor cells, virus, T cells, host response
INTRODUCTION
Transplantation of multipotent neural precursor cells (NPCs) is emerging as a feasible therapeutic strategy for the treatment of a variety of neurological disorders. Recent studies have demonstrated both short and long-term clinical benefits following NPC engraftment within the context of rodent models of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and acute spinal cord injury (Blurton-Jones et al., 2009; McBride et al., 2004; van Gorp et al., 2013; Yasuhara et al., 2006). Furthermore, in murine and non-human primate models of the neuroinflammatory disease multiple sclerosis (MS) the ability of human NPCs to function as modulators of the immune system in addition to replacing lost or damaged neural cell populations has been suggested (Aharonowiz et al., 2008; Pluchino et al., 2009; Pluchino et al., 2003). However, despite the clinical and histological benefits of NPC transplantation in preclinical animal models of neurologic disease, there is limited evidence addressing the capacity of neural grafts to act as reservoirs for viral replication. Studies using the nonpolio enterovirus coxsackievirus B (CVB) demonstrate the ability of CVB to preferentially replicate in murine NPCs (Ruller et al., 2012). The ensuing carrier-state infection results in increased cell death and impaired differentiation potential in vitro, as well as inflammation, microgliosis, and a variety of CNS developmental defects in vivo (Ruller et al., 2012; Tsueng et al., 2011). Intracerebral infection of neonates with murine cytomegalovirus (MCMV) results in the loss of neural stem cells and their neuronal progeny, as well as a decrease in the production of neurotrophins imperative to normal brain development (Mutnal et al., 2011). Borna disease virus (BDV) infection of human fetal human NPCs results in cell death upon differentiation and impaired neurogenesis (Brnic et al., 2012). Thus, the role of neural stem and progenitors as targets for a variety of neuroinvasive viruses is evident, while the consequences of infection within the context of cellular therapy remain to be elucidated.
Complicating NPC-based therapies is the controversial issue of antigenicity of transplanted cells and immune-mediated recognition. A growing body of evidence suggests NPCs are not immunoprivileged, as has previously been reported (Hori et al., 2003). Indeed, we have shown that NPCs derived from post-natal C57BL/6 brains express the co-stimulatory molecules CD80 and CD86 and up-regulate major histocompatibility complex (MHC) molecules in response to the pro-inflammatory cytokine interferon gamma (IFN-γ) (Weinger et al., 2012). Furthermore, allogeneic NPCs are rapidly rejected via a T cell mediated mechanism following intraspinal transplantation into MHC-mismatched recipients (Weinger et al., 2012). Similarly, human NPCs have the capacity to express MHC I and II and induce T cell proliferation (Goya et al., 2011).The apparent antigenicity of NPCs suggests successful engraftment may require the use of immunomodulatory agents and lifelong suppression of the immune system, as with solid organ transplants. However, an unintended consequence of immune suppression is the potential for latent viruses to become activated, or for uncontrolled viral replication to occur following opportunistic infection (Crough et al., 2007; Jordan et al., 1977; Wynn et al., 2010; Young et al., 2012). Therefore, it is imperative to understand the consequences of neurotropic virus infection of NPCs as cell-replacement therapies continue to move into the clinic (Gupta et al., 2012; Riley et al., 2013).
In this study, we demonstrate that cultured murine NPCs are infected by the neurotropic JHM strain of mouse hepatitis virus (JHMV), which induces acute encephalomyelitis and chronic demyelination when injected intracranially into immunocompetent mice. JHMV-infected NPCs support replication that ultimately results in increased cell death over time. Importantly, CD8+ T cells kill NPCs pulsed with viral-peptides, and JHMV replication in NPCs was suppressed, in part, by IFN-γ secreted from virus-specific CD4+ T cells.
RESULTS
NPCs express the MHV receptor CEACAM1a and are infected by JHMV
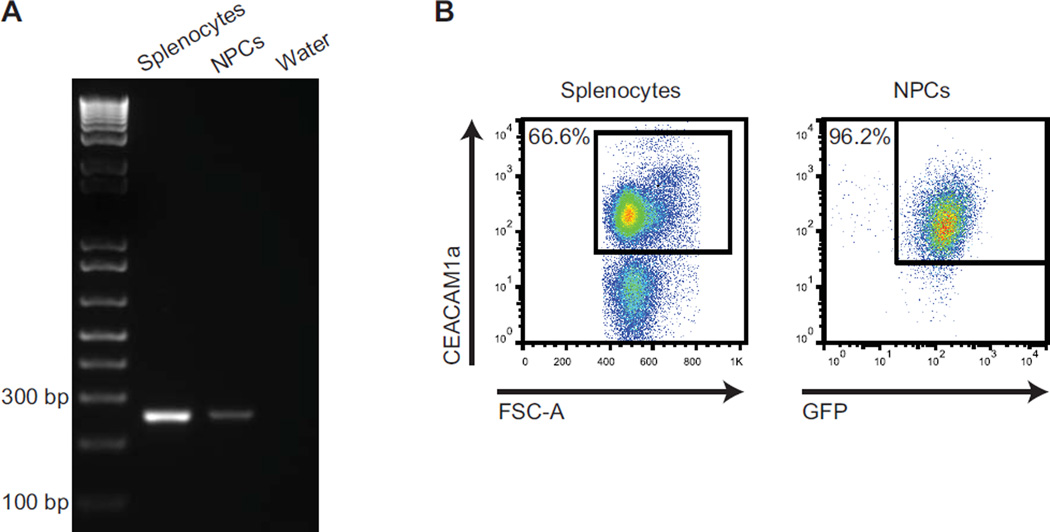
JHMV is a neurotropic coronavirus with relatively restricted tropism for glial cells through recognition and binding to the receptor carcinoembryonic antigen-cell adhesion molecule 1a (CEACAM1a) (Hirai et al., 2010; Thorp and Gallagher, 2004). CEACAM1a expression in mouse tissues is widespread and can be detected on the surface of a variety of epithelial cells in the gastrointestinal, respiratory, and reproductive tracts, as well as on small vascular endothelia and hematopoietic cells (Hemmila et al., 2004). However, CEACAM1a expression is not ubiquitous, and although it is known to be located at the surface of resident cells of the CNS including glia, expression by neural stem or progenitor cells has not been evaluated. To determine if NPCs derived from C57BL/6 transgenic mice engineered to express GFP (GFP-NPCs) express CEACAM1a, mRNA was isolated from cultured NPCs and receptor expression was evaluated by PCR. Using CEACAM1a-specific primers, PCR amplicons were detected in NPCs, as well as mixed splenocytes from C57BL/6 mice acting as controls (Figure 1A), and nucleotide sequencing confirmed homology with the specified region of the gene (data not shown). Furthermore, cell surface expression of CEACAM1a was confirmed with more than 90% of NPCs expressing the receptor as determined via flow cytometric analysis (Figure 1B).
Figure 1. Cultured NPCs express CEACAM1a.
(A) PCR amplification revealed that cultured C57BL/6 NPCs express transcripts specific for the JHMV receptor CEACAM1a. A 250 bp amplicon, specific for CEACAM1a, was amplified from cDNA generated from total RNA extracted from cultured NPCs; controls included water and splenocytes isolated from non-infected mice. (B) Representative dot plots showing CEACAM1a expression on the cell surface of C57BL/6 splenocytes (control) and cultured NPCs.
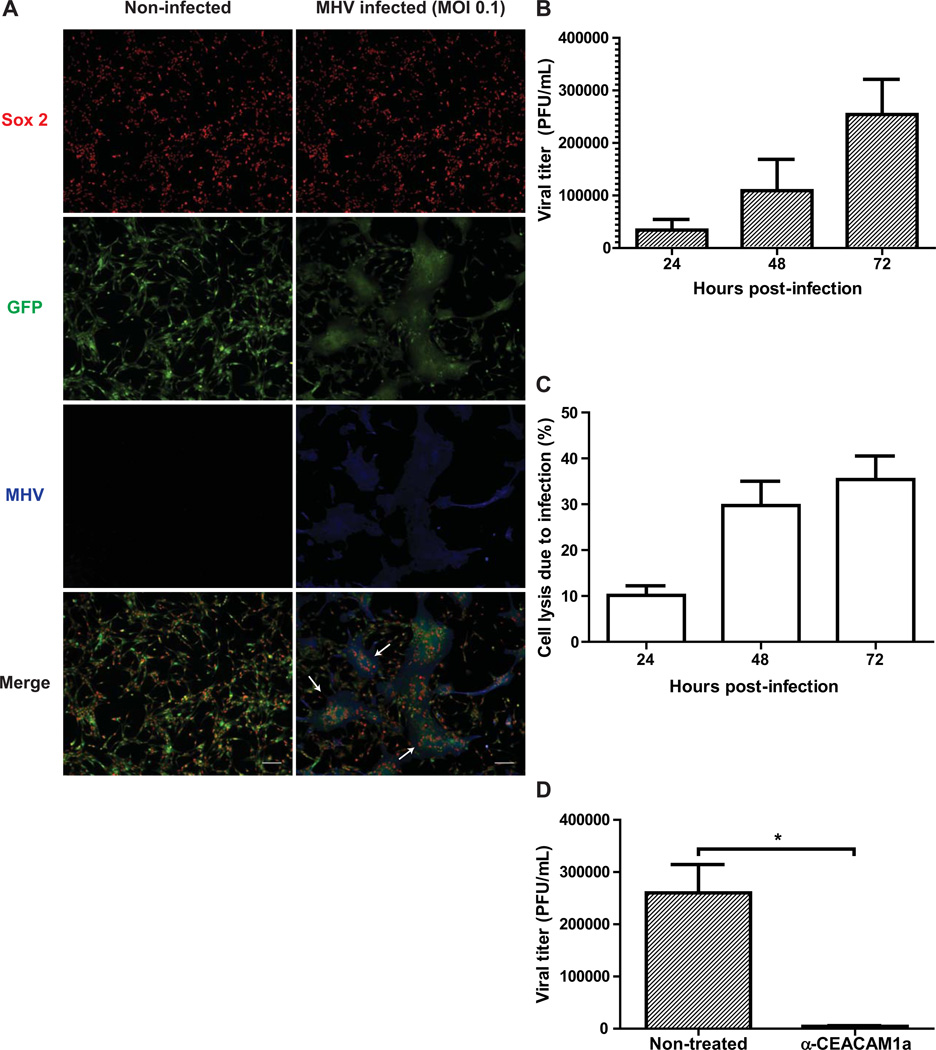
We next infected Sox2+ GFP-expressing NPCs with JHMV to assess susceptibility to infection. Infected NPC cultures were fixed 72 hours post-infection (p.i.) and stained with an antibody specific for the carboxyl terminus of the JHMV nucleocapsid (N) protein and subsequently imaged using fluorescence microscopy. Compared to non-infected NPCs that form a confluent monolayer when grown in tissue culture-treated, matrigel-coated vessels, Sox2+ NPCs infected at a multiplicity of infection (m.o.i.) of 0.1 displayed JHMV-specific syncytia formation by 72 hours post-infection (Figure 2A). Correspondingly, increasing viral titers were detected when plaque forming unit (PFU) assays were performed on supernatants harvested from JHMV-infected NPC cultures at 24, 48, and 72 hours p.i. (Figure 2B). Furthermore, determination of lactate dehydrogenase (LDH) released into the supernatants of infected cultures at defined times p.i. revealed increased NPC death over time, ranging from 10.1% ± 1.5% at 24 hours p.i., increasing to 29.7% ± 3.7% at 48 hours p.i., and peaking at 35.4% ± 3.6% by 72 hours (Figure 2C). As JHMV replication has been reported to occur via CEACAM1a-dependent and independent mechanisms (Nakagaki and Taguchi, 2005), we performed a monoclonal antibody blockade to determine the role of CEACAM1a in the spread of JHMV infection in cultured NPCs (Figure 2D). By 72 hours p.i., significant (p < 0.05) inhibition of viral replication was observed in anti-CEACAM1a-treated cells (4.4×103 ± 1.4×103 PFU/mL, n=3) when compared to non-treated, JHMV-infected NPCs (2.6×105 ± 5.4×104 PFU/mL).
Figure 2. JHMV infection of cultured NPCs.
(A) GFP-expressing NPCs were infected with JHMV (m.o.i. = 0.1) for 18 hours. After a 72 hour incubation, cells were fixed and stained with antibodies specific for Sox2 (red) and the JHMV nucleocapsid (N) protein (blue) and visualized by fluorescence microscopy (scale bar = 100 uM). Arrows indicate Sox2+, JHMV+ syncytia. (B) Supernatants were harvested from MHV-infected NPC cultures 24, 48, and 72 hours following infection and viral titers determined by plaque assay. (C) LDH released by infected NPCs was detected in viral supernatants and normalized to LDH levels from non-infected NPC cultures to evaluate cell death due to MHV infection. (D) Monoclonal CC1 antibody blockade of CEACAM1a following JHMV infection resulted in significantly (p < 0.05) reduced viral titers in cultured NPCs compared to non-treated controls by 72 hours p.i. For panels B–D, data is presented as average ± SEM and represents 3 independent experiments.
MHC class I and II expression by NPCs in response to JHMV infection
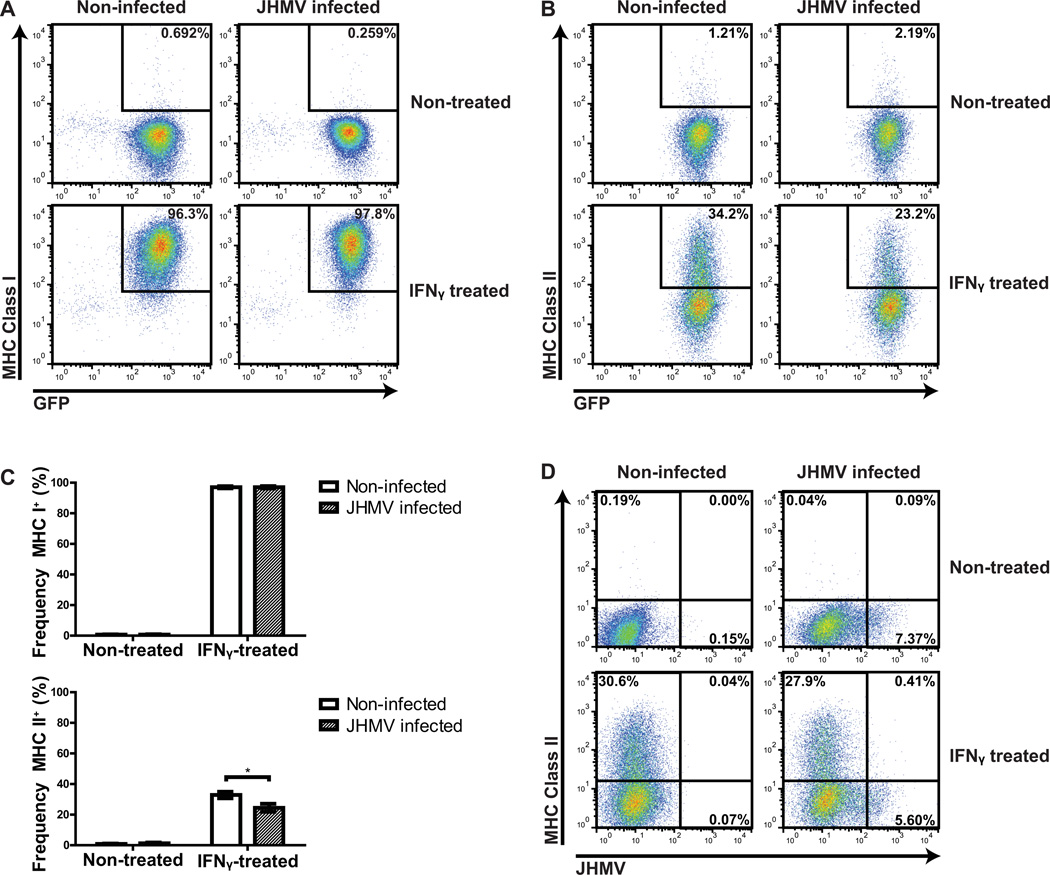
Under normal culture conditions, expression of MHC class I and II is undetectable on NPCs, yet MHC expression can be induced by treatment with IFN-γ (Chen et al., 2011; Weinger et al., 2012). To investigate if JHMV infection alters MHC class I and/or II expression on NPCs, we compared surface expression levels of these molecules on non-infected and infected cells in the absence or presence of 100 U/ml IFN-γ. Our findings indicated ≤1% of NPCs were found to be positive for MHC class I (Figures 3A and C) or II (Figures 3B and C) within non-infected, non-IFN-γ-treated groups. JHMV infection did not increase either MHC class I or II (0.7% ± 0.2% and 1.3% ± 0.5%, respectively) in medium-treated NPC cultures (Figures 3A and C). Treatment with IFN-γ (100 U/ml) for 24 hours dramatically increased levels of MHC class I (97% ± 0.7%) on non-infected NPCs, whereas only 32.9% ± 3.7% of non-infected, IFN-γ-treated NPCs expressed MHC class II (Figures 3A and C). IFN-γ treatment of JHMV-infected NPCs did not substantially increase MHC class I expression (97% ± 0.8%) in comparison to non-infected IFN-γ-treated cultures (97% ± 0.7%; Figures 3A–C). However, MHC class II was detected on a significantly (p < 0.05) lower fraction (24.5% ± 2.6%, n=3) of infected, IFN-γ-treated NPCs compared to non-infected, IFN-γ-treated NPCs (32.9% ± 2.1%, n=3) (Figure 3C). Furthermore, MHC class II could not be detected on the majority of JHMV-infected NPCs as determined by dual staining for viral antigen and MHC class II (Figure 3D).
Figure 3. MHC expression by NPCs in response to JHMV infection.
Cultured GFP NPCs were infected with JHMV (m.o.i.=0.1) for 18 hours and subsequently replenished with media alone or containing 100 U/ml IFN-γ. Cells were incubated for 24 h and MHC class I/II expression of non-infected and infected NPCs evaluated via flow cytometry. The frequency of NPCs expressing either MHC class I (A) or MHC class II (B) is shown in representative dot plots. (C) Quantification of the frequency of MHC I/II expression by infected or non-infected NPCs treated with media alone or in combination with IFN-γ. Data is presented as average ± SEM and represents 3 independent experiments (*p < 0.05).(D) Representative dot plots of MHC II-positive NPCs versus JHMV-positive NPCs.
Control of JHMV replication in infected NPCs
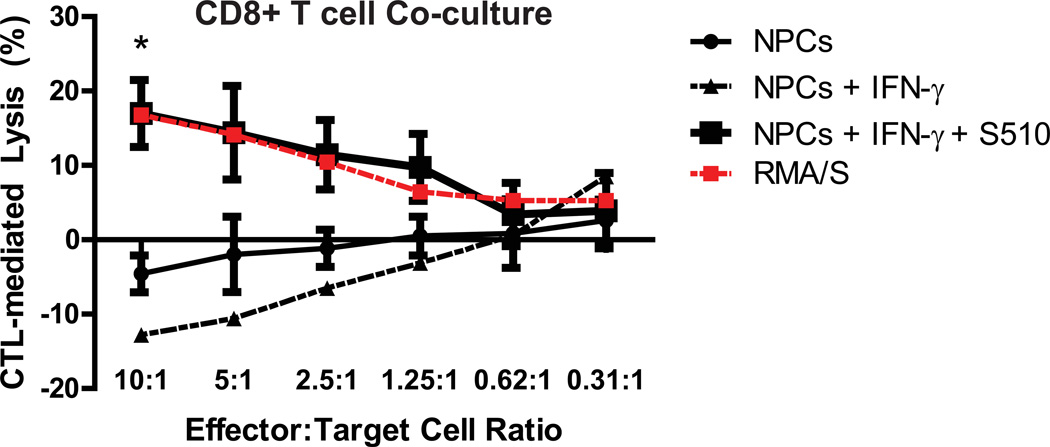
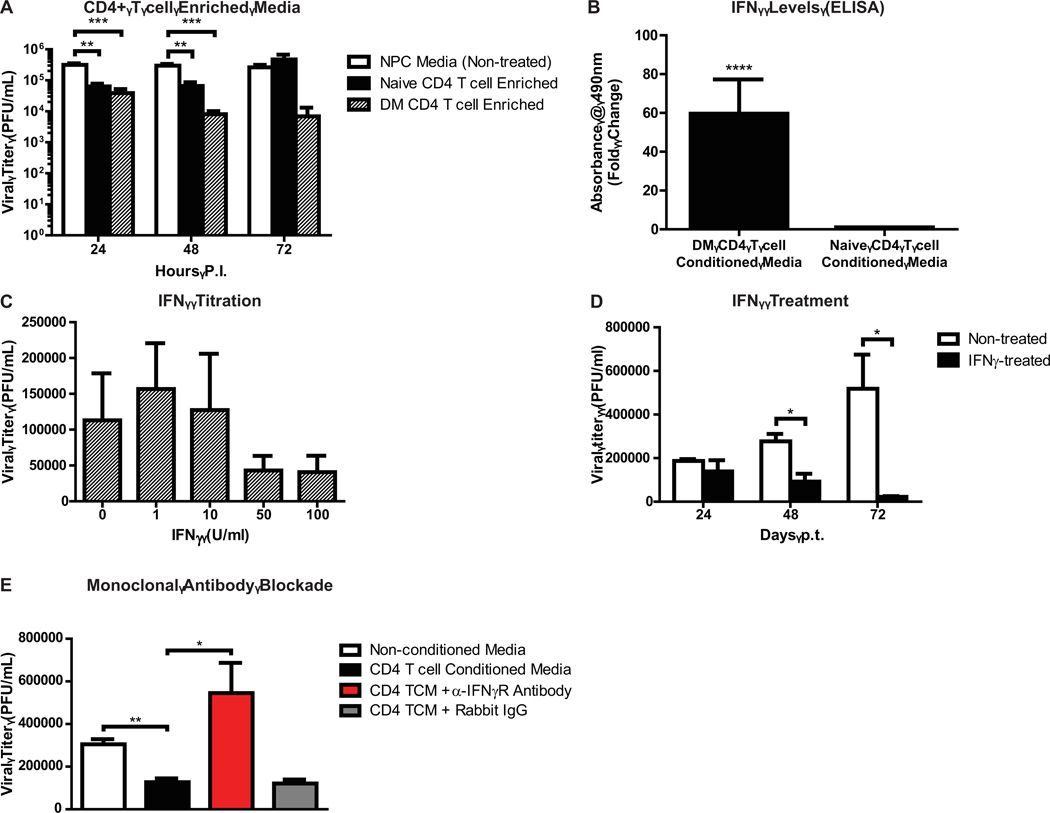
CD8+ and CD4+ T cells are pivotal in controlling JHMV replication within the infected CNS (Sussman et al., 1989; Williamson and Stohlman, 1990). Virus-specific effector CD8+ T cells help control replication in infected astrocytes and microglia through cytolytic activity (Bergmann et al., 2004). In addition to secreting IFN-γ that limits viral replication in oligodendrocytes, CD8+ T cells carry out perforin-dependent cytolysis of astrocytes and microglia (Bergmann et al., 2004; Williamson and Stohlman, 1990). We co-cultured virus-specific CTLs at diminishing effector-to-target (E:T) ratios with NPCs pulsed with the immunodominant CD8 peptide specific for JHMV spike (S) glycoprotein spanning amino acids 510–518 (S510–518), and treated with IFN-γ to induce MHC class I expression. Subsequently, LDH released in the supernatants was evaluated to quantify CTL-mediated NPC lysis; RMA-S cells, a murine lymphoma cell line that presents viral peptides to CTLs in an MHC class I dependent manner, were used as a positive control (Debruijn et al., 1991). NPCs pulsed with S510–518 peptide were specifically lysed by virus-specific CTLs at an E:T ratio of 10 to 1 (p < 0.05, n=3), indicating that virus-specific CD8+ T cells are capable of recognizing and directly killing JHMV-infected NPCs in vitro (Figure 4). Importantly, this cytolytic effect waned as the E:T to target ratio declined. CD4+ T cells have both indirect and direct antiviral roles during acute JHMV-induced encephalomyelitis, which include inducing the effector functions of virus-specific CTLs, along with IFN-γ secretion (Savarin et al., 2008; Stohlman et al., 2008). To evaluate if virus-sensitized CD4+ T cells can control replication of JHMV-infected NPCs, enriched populations of CD4+ T cells were isolated from C57BL/6 mice immunized against the DM variant of JHMV. Subsequently, splenocytes were isolated from JHMV-immunized and naïve mice, and magnetically-assisted negative selection was performed to purify the respective T cell populations. NPC media was conditioned with CD4+ T cell cytokines for 48 hours and then added to JHMV-infected NPCs. Supernatants from either naïve or virus-specific CD4+ T cells suppressed viral replication in NPCs at 24 and 48 hours post-infection, with the most significant inhibitory effects observed in groups treated with media enriched with virus-specific CD4+ T cell cytokines (Figure 5A). However, while the suppressive effects of naïve T cell media appeared to wane by 72 hours p.i. (4.7×105 ± 2×105 PFU/mL), supernatants from NPCs treated with virus-specific CD4+ T cell conditioned media maintained low viral titers (6.8×103 ± 6.3×103 PFU/mL) in comparison to non-treated controls (2.6×105 ± 5.4×104 PFU/mL; Figure 5A). T cell derived IFN-γ is critical in controlling JHMV replication in the CNS (Bergmann et al., 2004; Smith et al., 1991). Furthermore, treatment with IFN-γ specifically inhibits JHMV replication in oligodendrocyte progenitors (OPCs) derived from C57BL/6 NPCs, and inhibition of IFN-γ signaling in oligodendrocytes is associated with increased viral loads and mortality (Parra et al., 2010; Whitman et al., 2009). We evaluated levels of IFN-γ in naïve-versus-DM specific CD4+ T cell conditioned media by enzyme-linked immunosorbent assay (ELISA); absorbance values from media conditioned with DM-CD4+ T cells were increased ~60-fold when compared to naïve T cell conditioned media (p < 0.0001; Figure 5B). We subsequently treated JHMV-infected NPCs with varying amounts of mouse recombinant IFN-γ for 24 hours and determined its effects on viral titers. NPCs treated with 1 or 10 U/ml IFN-γ maintained high JHMV titers (1.6×105 ± 6.4×104 PFU/mL and 1.3×105 ± 7.8×104, respectively) in relation to non-treated groups (1.1×105 ± 6.6×104 PFU/mL; Figure 5C). However, JHMV replication in NPCs was reduced in cultures treated with 50 or 100 U/ml IFN-γ (4.3×104 ± 2×104 PFU/mL and 4.1×104 ± 2.3×104 PFU/mL, respectively; Figure 5C). We next performed a 72-hour time course to further probe the effects of IFN-γ (100U/mL) on JHMV-infected NPCs. A reduction from 2.8×105 ± 3.4×104 PFU/mL to 9.2×104 ± 4.9×104 PFU/mL was observed in IFN-γ-treated cultures by 48 hours post-treatment when compared to non-treated groups (p < 0.05, n=3), and JHMV levels were reduced to 2.3×104 ± 3.3×103 in IFN-γ treated cultures, versus 5.2×105 ± 1.6×105 in non-treated cultures, by 72 hours post-treatment (p < 0.05) (Figure 5D). We previously showed that multiple pro-inflammatory cytokines secreted by DM-specific T cells have synergistic effects with IFN-γ (Weinger et al., 2012). To confirm the role of IFN-γ as the major cytokine contributing to suppression of JHMV replication in infected NPC cultures, monoclonal antibody blockade against the IFN-γ receptor was performed on NPCs before and during treatment with virus-specific CD4+ T cell enriched media. As expected, by 48 hours p.t. JHMV levels were significantly (p < 0.01) reduced in conditioned media treated cultures compared to NPCs grown in non-conditioned media (1.3×105 ± 1.8×104 and 3.1×105 ± 2.4×104 PFU/mL, respectively; Figure 5E). However, treatment with anti-IFN-γ receptor resulted in higher (p < 0.05) viral titers (5.4×105 ± 1.4×105) compared to CD4+ T cell media treated cultures, thereby confirming the pivotal role of IFN-γ in CD4+ T cell mediated suppression of JHMV in NPCs. We have previously shown that IFN-γ treatment of JHMV-infected OPCs increases IFN-α/β secretion, and treatment with IFN-β suppresses JHMV replication (Whitman et al., 2009). Type I interferon (IFN-β) levels in JHMV-infected, IFN-γ treated NPC supernatants were assessed by ELISA and IFN-β was not detected above background levels (data not shown).
Figure 4. Virus-specific CD8+ T cells target S510–518 pulsed NPCs for lysis.
CTLs were harvested from mice immunized with the DM variant of JHMV and co-cultured at varying effector:target ratios with S510–518 pulsed, IFN-γ-treated NPCs for 4 hours, and lactate dehydrogenase released into the supernatant was subsequently measured. Non-IFN-γ-treated RMA/S cells pulsed with 50µM S510–518 were used as a positive lysis control.
Figure 5. Virus-specific CD4+ T cell enriched media reduces viral titers via IFN-γ.
(A) Media enriched with cytokines from naïve or CD4+ T cells significantly (p < 0.01 and p < 0.001, respectively) reduced viral titers in JHMV-infected NPCs at defined times p.i. compared to non-conditioned media. (B) Enzyme-linked immunosorbent assay for IFN-γ in naïve-versus-DM-specific CD4+ T cell supernatants. Absorbance values at 490nm were normalized to media alone and expressed as fold changes (DM absorbance / naïve absorbance). For statistical analysis, background-subtracted absorbance values were used (****p < 0.0001). (C) High doses of IFN-γ suppress JHMV replication in NPCs, and (D) treatment with 100 U/mL IFN-γ significantly inhibits virus replication at 72 hours p.t. (E) Monoclonal antibody blockade of IFN-γ receptor abrogated the virus-suppressive effects of DM CD4+ T cell conditioned media. For all panels, data is presented as average ± SEM and represents 3 independent experiments (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Effects of IFN-γ treatment on CEACAM1a and JHMV structural proteins
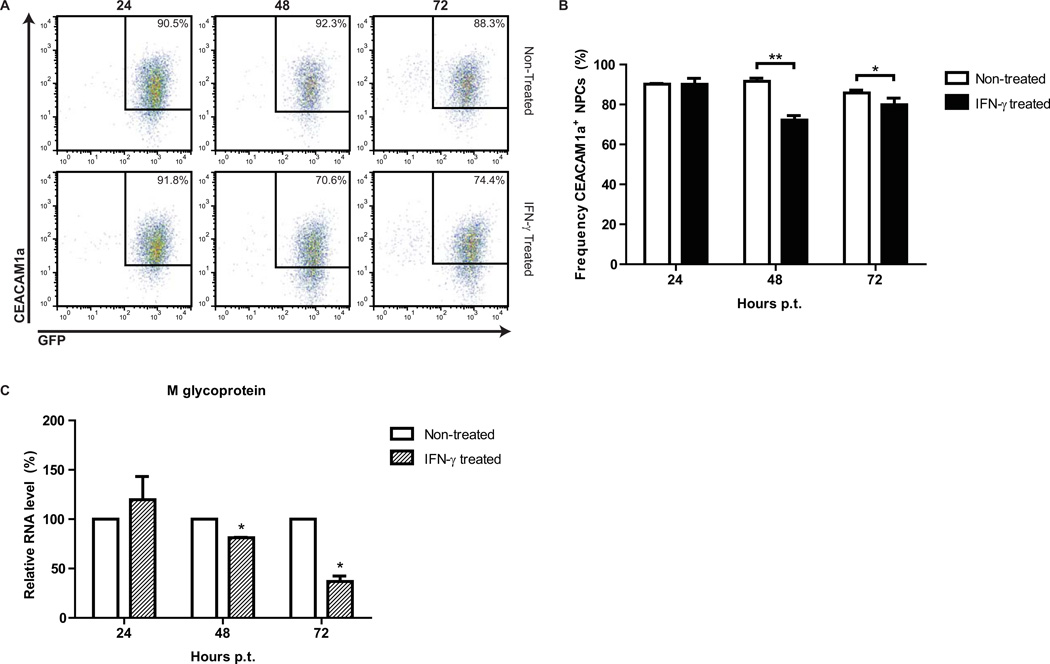
We evaluated the expression of the JHMV receptor CEACAM1a on NPCs following treatment with 100 U/mL IFN-γ and did not observe a change in the frequency of CECAM1a+ NPCs between treated and non-treated groups at 24 hours p.t. (Figures 6A and B). However, by 48 hours p.t. the frequency of CEACAM1a+ NPCs decreased from 86.1% ± 1.8% in non-treated NPCs to 62.4% ± 2.5% in IFN-γ treated cultures, and by 72 hours p.t. 61.9% ± 3.5% IFN-γ treated NPCs expressed CEACAM1a (compared to 73.3% ± 0.5%), indicating that IFN-γ dampens JHMV receptor expression by NPCs. The JHMV membrane (M) glycoprotein is critical in virus assembly (Matthews et al., 2002). To determine if M transcripts were decreased following IFN-γ treatment, gene-specific quantitative PCR (qPCR) was performed on total RNA extracts from JHMV-infected NPCs and M transcript levels were normalized to β-actin. M expression was significantly reduced in IFN-γ treated NPCs compared to non-treated NPCs at 48 and 72 hours p.t. (p < 0.05; Figure 6C). These findings suggest that the IFN-γ-induced inhibitory effect on JHMV replication within NPCs is related to both muted expression of CEACAM-1a as well as inhibiting viral RNA synthesis.
Figure 6. IFN-γ reduces JHMV receptor expression and JHMV transcripts in infected NPCs.
(A) Representative dot plots of CEACAM1a-expressing GFP NPCs at 24, 48, and 72 hours post-IFN-γ-treatment. (B) Quantification of the frequency of CEACAM1a+ NPCs with or without IFN-γ treatment. (C) Relative percent expression of JHMV membrane (M) protein transcripts from infected, IFN-γ-treated NPCs compared to non-infected, non-treated NPCs. For panels B and C, data is presented as average ± SEM and represents 3 independent experiments (*p ≤ 0.05). For panel C, ΔCt values were used for statistical analysis.
DISCUSSION
This study demonstrates that NPCs derived from the brains of post-natal C57BL/6-GFP mice express the JHMV receptor, CEACAM1a, and support viral replication following CEACAM1a-dependent infection. Additionally, JHMV infection of cultured NPCs induces cytopathic effects over time as evidenced by syncytia formation and elevated LDH levels. Within the context of JHMV infection of the CNS, these findings demonstrate that resident NPCs present within defined anatomical niches may be susceptible to viral infection. Moreover, we have previously shown that intraspinal transplantation of NPCs into mice persistently infected with JHMV results in clinical recovery associated with remyelination (Carbajal et al., 2010; Totoiu et al., 2004). Data presented within this report argues that transplanted NPCs may be susceptible to JHMV infection, a finding that highlights important clinical implications for emerging therapies utilizing NPCs to treat human neurologic disease as engrafted cells may be susceptible to infection by persistent neurotropic viruses.
JHMV infection has previously been shown to inhibit constitutive expression of MHC class I in mouse primary astrocyte cultures and to block IFN-γ-induced MHC class II expression on murine cerebral endothelial cells (Correale et al., 1995; Joseph et al., 1991). Here, we show that JHMV does not significantly affect MHC class I or II expression following infection of cultured NPCs in the absence of IFN-γ. However, IFN-γ-induced expression of MHC class II was reduced following JHMV infection. MHC expression plays an important role in immune surveillance during viral infection, and control of JHMV replication within the CNS requires antigen recognition by MHC class I and class II restricted CD8+ and CD4+ T cells (Bergmann et al., 2004; Sussman et al., 1989; Williamson and Stohlman, 1990). Impaired expression of MHC class II following IFN-γ-treatment of infected NPCs may be a mechanism employed to subvert detection by infiltrating virus-specific CD4+ T cells. Nonetheless, conditioned media from virus-specific CD4+ T cells was able to suppress JHMV replication within NPCs, likely due to the effects of IFN-γ. Supporting this notion, treatment of infected NPCs with recombinant mouse IFN-γ had a dose-dependent inhibitory effect on virus replication, and blocking IFN-γ receptor abrogated the observed suppressive effects. IFN-γ treatment resulted in fewer CEACAM1a-expressing NPCs with a concomitant decrease in JHMV membrane glycoprotein transcripts, suggestive of viral entry inhibition and reduced virion assembly. We also observed that NPCs pulsed with the CD8-specific viral peptide S510–518 were detected and killed by virus-specific CD8+ T cells, indicating that virally-infected NPCs may be targeted for lysis by CTLs infiltrating into the CNS in response to infection. Collectively, our findings argue that T cells are important for controlling viral replication within NPCs through both cytolytic activity and IFN-γ secretion.
Lineage fate mapping of neural stem/precursor cells residing within the subventricular zone of lateral ventricles and subgranular zone of the hippocampus demonstrate the ability of these cells to differentiate into neurons and glia throughout development (Doetsch, 2003; Gage, 2000). Furthermore, endogenous NPCs have been shown to proliferate, migrate, and differentiate in response to acute CNS inflammatory events, such as with spinal cord injury, stroke, and experimental models of chronic inflammatory demyelinating disorders (Picard-Riera et al., 2002; Yagita et al., 2001; Zhang et al., 2004). Though viewed as a glial tropic virus, this study highlights the potential for NPCs to serve as a reservoir for JHMV infection and replication. CTL-mediated lysis of JHMV-infected NPCs may be detrimental to NPC-mediated repair during CNS inflammation, and a loss of NPCs destined to become oligodendrocytes could contribute to limited remyelination observed in the JHMV-infected CNS. Additionally, our findings have clinical relevance, as NPCs are currently being employed in clinical trials for spinal cord injury as well as for treating Pelizaeus-Merzbacher disease, a genetic disorder that affects the growth of the myelin sheath (Gupta et al., 2012; Mayor, 2010). As NPCs used for clinical trials are unlikely to be “self-derived”, they would be subject to immune recognition and potential destruction by both innate and adaptive immune responses, necessitating long-term immune suppression to prevent graft rejection (Chen et al., 2011; Swijnenburg et al., 2008; Weinger et al., 2012). Several classes of immunosuppressive drugs used during transplantation, including calcineurin inhibitors i.e. cyclosporine and FK506, inhibit the activation and/or proliferation of T cells. Such immunosuppressive drugs would foster an environment whereby opportunistic infection or reactivation of latent virus might occur. This raises the possibility that transplanted NPCs may be subject to infection and, in the absence of adequate immune surveillance of the CNS, could lead to damage/death of engrafted cells. With this in mind, careful consideration should be given to potential viral infection when contemplating NPC grafting for treating neurological disease.
MATERIALS & METHODS
Virus
The JHM strain of mouse hepatitis virus (J2.2v-1) was added to NPC cultures at a multiplicity of infection (MOI) of 0.1 PFU/cell. Virus was allowed to absorb overnight (16–18 hours) before media was replaced. Supernatants of infected cultures were collected at defined times p.i. and viral titers were determined using the DBT astrocytoma cell line as previously described (Hirano et al., 1976).
Neural precursor cell culture and reagents
NPCs derived from the striatum of postnatal day 1 transgenic C57BL/6 mice expressing enhanced green fluorescent protein (GFP) were cultured as previously described (Carbajal et al., 2010). NPC media consisted of DMEM/F12 with Glutamax (Gibco), N2 supplement (1X, Gibco), ciprofloxacin hydrochloride (100 µg/mL, Cellgro), gentamicin (50 µg/mL, Sigma-Aldrich), Fungizone (2.5 µg/mL, Gibco), penicillin/streptomycin (1000 U/mL, Gibco), and human epidermal growth factor (20 ng/mL, Sigma-Aldrich). Recombinant mouse IFN-γ was purchased from Cell Sciences. For studies involving blockade of CEACAM-1a, NPCs were infected overnight and monoclonal antibody CC1 (eBiosciences) was subsequently added at a concentration of 1 µg/mL. Media was harvested 72 hours p.i. and plaque assay performed to determine viral titers. Experimental blockade of IFN-γ receptor was performed using JHMV-infected NPCs incubated with 250 nM (final) anti-mouse CD119 (IFN gamma receptor 1; eBiosciences) or 250 nM purified rabbit IgG (control; BD Pharmigen) for one hour before media was replaced with non-conditioned or CD4+ T cell conditioned media +/− anti mouse CD119 or rabbit IgG. Supernatants were harvested 48 hours post-treatment and viral titers determined.
Flow cytometric analysis
Cultured NPCs were dissociated using 0.05% trypsin-EDTA and suspended in PBS containing 0.5% BSA and 2 mM EDTA (Invitrogen). Cells were subsequently treated with blocking antibody (purified rat IgG2b anti-mouse CD16/CD32 monoclonal antibody, 1:200; BD Biosciences) for 20 minutes at 4°C before being incubated with antibodies specific for CEACAM1a (APC-conjugated, 0.06 µg/test, eBioscience), MHC class I (PE-conjugated, 1:200, eBioscience), or MHC class II (PE-conjugated, 1:200, BD Biosciences), for 20–30 minutes. In experiments where FACS analysis of JHMV was performed, NPCs were fixed with 4% paraformaldhyde for 15 minutes before being permeabilized using BD Perm/Wash buffer (BD Biosciences). The anti-JHMV mAb J.3.3 specific for the carboxyl terminus of the viral nucleocapsid (N) protein was conjugated to Alexa Fluor 647 using the APEX labeling system (Life Technologies) and used at a final concentration of 1.5 ng/mL. Detection of fluorescence was performed using a LSR II flow cytometer (BD Biosciences) and analysis of FACS data was performed with FlowJo software (Tree Star).
RNA Isolation, reverse transcription, and polymerase chain reaction
Total RNA was isolated from C57BL/6 splenocytes and NPCs using TRIzol reagent (Invitrogen) and purified by phenol-chloroform extraction. cDNA was reverse transcribed from RNA according to manufacturer’s instructions using the SuperScript III First-Strand Synthesis system (Invitrogen) and random hexamers. Standard PCR for CEACAM1a expression was performed with an Eppendorf Mastercycler using the Platinum Taq DNA polymerase kit (Invitrogen) and the following primers purchased from Integrated DNA Technologies: TTCCCTGGGGAGGACTACTG (forward primer) and TGTATGCTTGCC CCGTGAAAT (reverse primer). Gene products were run alongside a 1 Kb Plus DNA ladder (Invitrogen) on a 1% agarose gel containing ethidium bromide before being imaged using the Bio-Rad GelDoc system. For quantitative RT-PCR experiments, primers specific for the JHMV membrane protein (forward: CGAGCCGTAGCATGTTTATCTA; reverse: CGCATACACGCAATTGAACATA) were designed using PrimerQuest software (Integrated DNA Technologies, Inc.). SYBR Green Real-Time PCR Master Mix (Life Technologies) was used according to manufacturer’s specifications and RT-PCR was performed using the Applied Biosystem ViiA 7 Real-Time PCR System. Ct values of M protein transcripts were normalized to β-actin Ct values (forward: GGCCCAGAGCAAGAGAGGTATCC; reverse: ACGCACGATTTCCCTCTCAGC) and compared using the ΔΔCt method.
Immunofluorescence
To evaluate JHMV infection of cultured NPCs, cells were dissociated and plated on slides or cover slips coated with reduced growth factor Matrigel (BD Biosciences). NPCs were infected with JHMV overnight and fixed 72 hours p.i. with 4% paraformaldehyde for 10 minutes at room temperature. Immunofluorescence staining was performed as previously described (Whitman et al., 2009) using the anti-JHMV mAb J.3.3 (1:20 dilution) specific for the carboxyl terminus of the viral nucleocapsid (N) protein and the Alexa Fluor 405 goat anti-mouse IgG1 secondary antibody (Life Technologies), as well as rabbit monoclonal anti-Sox2 (Epitomics) and Alexa Fluor 568 goat anti-rabbit IgG1 secondary antibody (Life Technologies). Slides were imaged using a Nikon Eclipse Ti inverted microscope.
JHMV-induced cell death assay
NPC death due to JHMV infection was evaluated at 24, 48, and 72 hours p.i. by measuring lactate dehydrogenase released by lysed cells according to manufacturer’s recommendations using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). Briefly, spontaneous and virus-induced LDH levels were determined using the following formula: % Lysis = (Experimental LDH release) / (Maximum LDH release). LDH levels from JHMV-infected cultures were then normalized to spontaneously released LDH and expressed as cell death due to infection (%).
CD4+ T cell isolation for NPC media conditioning
C57BL/6 mice were infected with an i.p. injection of 2.5 × 105 PFU of a demyelinating (DM) variant of JHMV. On day 8 p.i., CD4+ T cells were isolated from spleens by negative selection according to manufacturer’s specifications using the EasySep Mouse CD4+ T cell Isolation kit (Stemcell Technologies). Briefly, red blood cell depleted splenocytes were suspended at a concentration of 1 × 108 cells/mL in PBS + 2% FBS with 1 mM EDTA. Normal rat serum was added at the appropriate concentration and cells were incubated with a cocktail containing a combination of biotinylated monoclonal antibodies directed against CD8a, CD11b, CD11c, CD19, CD45R/B220, CD49b, TCRγ/δ and TER119, for 10 minutes. Subsequently, a suspension of streptavidin-coated magnetic particles in PBS was added and incubated with the cells for 2.5 minutes; buffer was added to the appropriate volume, and cells were incubated in the EasySep Magnet for 2.5 minutes to foster binding of magnetically-labeled unwanted cells to the tube walls before CD4+ T cells were poured off. To generate CD4+ T cell conditioned NPC media, the magnetically-labeled fraction following depletion of total T cells was collected using the EasySep Mouse T Cell Isolation kit (Stemcell technologies). This enriched fraction was treated with 50 µg/ml mitomycin-C (AG Scientific), and 35 × 106 cells were co-cultured with 35 × 106 CD4+ T cells in 10 mL NPC media containing 5 µm CD4-specific membrane (M) glycoprotein spanning amino acid residues 133–147 (M133–147, Bio-Synthesis) for 48 hours. CD4+ T cell conditioned media was administered to JHMV-infected NPCs and supernatants were harvested 24, 48, and 72 hours p.i. for determination of viral titers. Levels of IFN-γ in CD4+ T cell conditioned media were determined by ELISA using the Mouse IFN-γ DuoSet according to manufacturer’s recommendations (R&D Systems). Interferon-beta levels in JHMV-infected NPC cultures were evaluated using the VeriKine Mouse Interferon Beta ELISA kit (PBL Assay Science). The animal protocols and procedures used for these studies were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
CD8+ T cell cytotoxicity assay
NPCs were seeded at a density of 20,000 cells/well in a flat-bottom 96-well format tissue culture plate (Corning Life Sciences) and pulsed overnight with 5 µM of the immunodominant CD8 peptide specific for MHV spike (S) glycoprotein spanning amino acids 510–518 (S510–518, Bio-Synthesis). NPCs were simultaneously treated overnight with 100 U/ml IFN-γ to induce MHC class I expression for the presentation of S510–518. CD8+ T cells isolated from DM-infected C57BL/6 mouse splenocytes (as mentioned for CD4+ T cells) using the EasySep Mouse CD8+ T cell Isolation kit (Stemcell Technologies) were then plated with NPCs at effector-to-target (E:T) ratios ranging from 10:1 to 0.31:1. Co-cultures were incubated for 4 hours at 37°C in 5% CO2 at a final volume of 200 µL/well. The amounts of lactate dehydrogenase released from lysed cells were determined using a CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega). The percentage of CTL-mediated lysis was determined as specified by the manufacturer's protocols. RMA-S cells pulsed overnight with 50 µM S510–518 were used as a positive control for cell lysis.
Statistical analysis
Statistical analysis was carried out using student's t test, one-way ANOVA, or repeated measures ANOVA and p ≤ 0.05 was considered significant.
HIGHLIGHTS.
Murine neural precursor cells are infected by JHMV in a CEACAM1a-dependent manner.
Peptide-pulsed NPCs are targeted for lysis by virus-specific CD8+ T cells.
JHMV replication in NPCs is suppressed by CD4+ T cells through IFN-γ secretion.
IFN-γ dampens CEACAM1a expression and JHMV protein production in NPCs.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (NIH) grant R01 NS074987 to T.E.L. C.M.W. is supported by California Institute for Regenerative Medicine (CIRM) grants RM1-01717 and TR3-05603, National Multiple Sclerosis Society (NMSS) Collaborative Center Research Award CA1058-A-8, and the Gleis Family Foundation. W.C.P. is supported by NIH pre-doctoral training grant 1T32NS082174-01 and J.G.W. is supported by NMSS postdoctoral fellowship FG 1960-A-1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aharonowiz M, Einstein O, Fainstein N, Lassmann H, Reubinoff B, Ben-Hur T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS One. 2008;3:e3145. doi: 10.1371/journal.pone.0003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CC, Parra B, Hinton DR, Ramakrishna C, Dowdell KC, Stohlman SA. Perforin and Gamma Interferon-Mediated Control of Coronavirus Central Nervous System Infection by CD8 T Cells in the Absence of CD4 T Cells. J Virol. 2004;78:1739–1750. doi: 10.1128/JVI.78.4.1739-1750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Muller FJ, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brnic D, Stevanovic V, Cochet M, Agier C, Richardson J, Montero-Menei CN, Milhavet O, Eloit M, Coulpier M. Borna disease virus infects human neural progenitor cells and impairs neurogenesis. J Virol. 2012;86:2512–2522. doi: 10.1128/JVI.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal KS, Schaumburg C, Strieter R, Kane J, Lane TE. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc Natl Acad Sci U S A. 2010;107:11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Phillips LK, Gould E, Campisi J, Lee SW, Ormerod BK, Zwierzchoniewska M, Martinez OM, Palmer TD. MHC mismatch inhibits neurogenesis and neuron maturation in stem cell allografts. PLoS One. 2011;6:e14787. doi: 10.1371/journal.pone.0014787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correale J, Li S, Weiner LP, Gilmore W. Effect of Persistent Mouse Hepatitis-Virus Infection on Mhc Class-I Expression in Murine Astrocytes. J Neurosci Res. 1995;40:10–21. doi: 10.1002/jnr.490400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crough T, Fazou C, Weiss J, Campbell S, Davenport MP, Bell SC, Galbraith A, McNeil K, Khanna R. Symptomatic and asymptomatic viral recrudescence in solid-organ transplant recipients and its relationship with the antigen-specific CD8(+) T-cell response. J Virol. 2007;81:11538–11542. doi: 10.1128/JVI.00581-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruijn MLH, Schumacher TNM, Nieland JD, Ploegh HL, Kast WM, Melief CJM. Peptide Loading of Empty Major Histocompatibility Complex-Molecules on Rma-S Cells Allows the Induction of Primary Cytotoxic Lymphocyte-T Responses. Eur J Immunol. 1991;21:2963–2970. doi: 10.1002/eji.1830211210. [DOI] [PubMed] [Google Scholar]
- Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Goya RL, Busch R, Mathur R, Coles AJ, Barker RA. Human fetal neural precursor cells can up-regulate MHC class I and class II expression and elicit CD4 and CD8 T cell proliferation. Neurobiol Dis. 2011;41:407–414. doi: 10.1016/j.nbd.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, Perry R, Farrell J, Jeremy RJ, Ulman M, Huhn SL, Barkovich AJ, Rowitch DH. Neural stem cell engraftment and myelination in the human brain. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3004373. 155ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmila E, Turbide C, Olson M, Jothy S, Holmes KV, Beauchemin N. Ceacam1a(−/−) Mice are completely resistant to infection by murine coronavirus mouse hepatitis virus A59. J Virol. 2004;78:10156–10165. doi: 10.1128/JVI.78.18.10156-10165.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai A, Ohtsuka N, Ikeda T, Taniguchi R, Blau D, Nakagaki K, Miura HS, Ami Y, Yamada YK, Itohara S, Holmes KV, Taguchi F. Role of Mouse Hepatitis Virus (MHV) Receptor Murine CEACAM1 in the Resistance of Mice to MHV Infection: Studies of Mice with Chimeric mCEACAM1a and mCEACAM1b. J Virol. 2010;84:6654–6666. doi: 10.1128/JVI.02680-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano N, Fujiwara K, Matumoto M. Mouse hepatitis virus (MHV-2). Plaque assay and propagation in mouse cell line DBT cells. Jpn J Microbiol. 1976;20:219–225. [PubMed] [Google Scholar]
- Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- Jordan MC, Shanley JD, Stevens JG. Immunosuppression Reactivates and Disseminates Latent Murine Cytomegalovirus. J Gen Virol. 1977;37:419–423. doi: 10.1099/0022-1317-37-2-419. [DOI] [PubMed] [Google Scholar]
- Joseph J, Knobler RL, Lublin FD, Hart MN. Mouse Hepatitis-Virus (Mhv-4, Jhm) Blocks Gamma-Interferon-Induced Major Histocompatibility Complex Class-Ii Antigen Expression on Murine Cerebral Endothelial-Cells. J Neuroimmunol. 1991;33:181–190. doi: 10.1016/0165-5728(91)90105-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews AE, Weiss SR, Paterson Y. Murine hepatitis virus--a model for virus-induced CNS demyelination. J Neurovirol. 2002;8:76–85. doi: 10.1080/13550280290049534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S. First patient enters trial to test safety of stem cells in spinal injury. Bmj. 2010;341:c5724. doi: 10.1136/bmj.c5724. [DOI] [PubMed] [Google Scholar]
- McBride JL, Behrstock SP, Chen EY, Jakel RJ, Siegel I, Svendsen CN, Kordower JH. Human neural stem cell transplants improve motor function in a rat model of Huntington's disease. J Comp Neurol. 2004;475:211–219. doi: 10.1002/cne.20176. [DOI] [PubMed] [Google Scholar]
- Mutnal MB, Cheeran MC, Hu S, Lokensgard JR. Murine cytomegalovirus infection of neural stem cells alters neurogenesis in the developing brain. PLoS One. 2011;6:e16211. doi: 10.1371/journal.pone.0016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagaki K, Taguchi F. Receptor-independent spread of a highly neurotropic murine coronavirus JHMV strain from initially infected microglial cells in mixed neural cultures. J Virol. 2005;79:6102–6110. doi: 10.1128/JVI.79.10.6102-6110.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra GI, Bergmann CC, Phares TW, Hinton DR, Atkinson R, Stohlman SA. Gamma interferon signaling in oligodendrocytes is critical for protection from neurotropic coronavirus infection. J Virol. 2010;84:3111–3115. doi: 10.1128/JVI.02373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Baron-Van Evercooren A. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S, Gritti A, Blezer E, Amadio S, Brambilla E, Borsellino G, Cossetti C, Del Carro U, Comi G, t Hart B, Vescovi A, Martino G. Human Neural Stem Cells Ameliorate Autoimmune Encephalomyelitis in Non-human Primates. Ann Neurol. 2009;66:343–354. doi: 10.1002/ana.21745. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Quattrini A, Brambilla E, Gritti A, Salani G, Dina G, Galli R, Del Carro U, Amadio S, Bergami A, Furlan R, Comi G, Vescovi AL, Martino G. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Riley J, Glass J, Feldman EL, Polak M, Bordeau J, Federici T, Johe K, Boulis NM. Intraspinal Stem Cell Transplantation in ALS: A Phase I Trial, Cervical Microinjection and Final Surgical Safety Outcomes. Neurosurgery. 2013 doi: 10.1227/NEU.0000000000000156. [DOI] [PubMed] [Google Scholar]
- Ruller CM, Tabor-Godwin JM, Van Deren DA, Jr., Robinson SM, Maciejewski S, Gluhm S, Gilbert PE, An N, Gude NA, Sussman MA, Whitton JL, Feuer R. Neural stem cell depletion and CNS developmental defects after enteroviral infection. The American Journal of Pathology. 2012;180:1107–1120. doi: 10.1016/j.ajpath.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C, Bergmann CC, Hinton DR, Ransohoff RM, Stohlman SA. Memory CD4+ T-cell-mediated protection from lethal coronavirus encephalomyelitis. J Virol. 2008;82:12432–12440. doi: 10.1128/JVI.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Barthold SW, Desouza MS, Bottomly K. The Role of Gamma Interferon in Infection of Susceptible Mice with Murine Coronavirus, Mhv-Jhm. Arch Virol. 1991;121:89–100. doi: 10.1007/BF01316746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohlman SA, Hinton DR, Parra B, Atkinson R, Bergmann CC. CD4 T cells contribute to virus control and pathology following central nervous system infection with neurotropic mouse hepatitis virus. J Virol. 2008;82:2130–2139. doi: 10.1128/JVI.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman MA, Shubin RA, Kyuwa S, Stohlman SA. T-Cell-Mediated Clearance of Mouse Hepatitis-Virus Strain Jhm from the Central Nervous-System. J Virol. 1989;63:3051–3056. doi: 10.1128/jvi.63.7.3051-3056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swijnenburg RJ, Schrepfer S, Govaert JA, Cao F, Ransohoff K, Sheikh AY, Haddad M, Connolly AJ, Davis MM, Robbins RC, Wu JC. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc Natl Acad Sci U S A. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp EB, Gallagher TM. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J Virol. 2004;78:2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totoiu MO, Nistor GI, Lane TE, Keirstead HS. Remyelination, axonal sparing, and locomotor recovery following transplantation of glial-committed progenitor cells into the MHV model of multiple sclerosis. Exp Neurol. 2004;187:254–265. doi: 10.1016/j.expneurol.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsueng G, Tabor-Godwin JM, Gopal A, Ruller CM, Deline S, An N, Frausto RF, Milner R, Crocker SJ, Whitton JL, Feuer R. Coxsackievirus preferentially replicates and induces cytopathic effects in undifferentiated neural progenitor cells. J Virol. 2011;85:5718–5732. doi: 10.1128/JVI.02261-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp S, Leerink M, Kakinohana O, Platoshyn O, Santucci C, Galik J, Joosten EA, Hruska-Plochan M, Goldberg D, Marsala S, Johe K, Ciacci JD, Marsala M. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res Ther. 2013;4:57. doi: 10.1186/scrt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger JG, Weist BM, Plaisted WC, Klaus SM, Walsh CM, Lane TE. MHC mismatch results in neural progenitor cell rejection following spinal cord transplantation in a model of viral-induced demyelination. Stem Cells. 2012;30:2584–2595. doi: 10.1002/stem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman L, Zhou H, Perlman S, Lane TE. IFN-gamma-mediated suppression of coronavirus replication in glial-committed progenitor cells. Virology. 2009;384:209–215. doi: 10.1016/j.virol.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JSP, Stohlman SA. Effective Clearance of Mouse Hepatitis-Virus from the Central-Nervous-System Requires Both Cd4+ and Cd8+ T-Cells. J Virol. 1990;64:4589–4592. doi: 10.1128/jvi.64.9.4589-4592.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn KK, Crough T, Campbell S, McNeil K, Galbraith A, Moss DJ, Silins SL, Bell S, Khanna R. Narrowing of T-cell receptor beta variable repertoire during symptomatic herpesvirus infection in transplant patients. Immunol Cell Biol. 2010;88:125–135. doi: 10.1038/icb.2009.74. [DOI] [PubMed] [Google Scholar]
- Yagita Y, Kitagawa K, Ohtsuki T, Takasawa K, Miyata T, Okano H, Hori M, Matsumoto M. Neurogenesis by progenitor cells in the ischemic adult rat hippocampus. Stroke. 2001;32:1890–1896. doi: 10.1161/01.str.32.8.1890. [DOI] [PubMed] [Google Scholar]
- Yasuhara T, Matsukawa N, Hara K, Yu G, Xu L, Maki M, Kim SU, Borlongan CV. Transplantation of human neural stem cells exerts neuroprotection in a rat model of Parkinson's disease. J Neurosci. 2006;26:12497–12511. doi: 10.1523/JNEUROSCI.3719-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GR, Eksmond U, Salcedo R, Alexopoulou L, Stoye JP, Kassiotis G. Resurrection of endogenous retroviruses in antibody-deficient mice. Nature. 2012;491:774–778. doi: 10.1038/nature11599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zhang Z, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J Neurosci. 2004;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]