Abstract
Objectives
This study compares a statewide telephone health survey and EHR data from a large Wisconsin health system to estimate asthma prevalence in Wisconsin.
Methods
Frequency tables and logistic regression models were developed for children and adults using Wisconsin Behavioral Risk Factor Surveillance Survey (BRFSS) and University of Wisconsin primary care clinic data. Adjusted odds ratios (OR) from each model were compared.
Results
Between 2007 and 2009, the EHR database contained 376,000 patients (30,000 with asthma) compared to 23,000 (1,850 with asthma) responding to the BRFSS telephone survey. Adjusted ORs for asthma were similar in magnitude and direction for the majority of covariates, including gender, age, and race between survey and EHR models. The EHR data had greater statistical power to detect associations than survey data, especially in pediatric and ethnic populations, due to larger sample sizes.
Conclusions
EHRs can be used to estimate asthma prevalence in Wisconsin adults and children. EHR data may improve public health chronic disease surveillance using high quality data at the local level to better identify areas of disparity, risk factors, and guide education and healthcare interventions.
Asthma is a complex chronic disease with intermittent symptoms and varying degrees of severity. This often makes it difficult to determine its prevalence in a population. Nationally, asthma is estimated to affect approximately 10 percent of children ages 17 years and younger and 8 percent of adults1 and is associated with significant morbidity and substantial healthcare costs. The economic cost of asthma in the U.S. was estimated at $59.0 billion in 2007, including direct health care costs of $53.1 billion and indirect costs or lost productivity of $5.9 billion.2 These outcomes are largely preventable with targeted interventions.3 Ideally, asthma surveillance should allow identification of disproportionately affected populations and guide prevention and intervention efforts.
Surveillance data for chronic diseases are traditionally drawn from federally supported health surveys which provide estimates of asthma prevalence at the national and state levels but not at the local level where many policy decisions are made. The Behavioral Risk Factor Surveillance System (BFRSS) is the only source of data on health-related behaviors and outcomes for many states, and is the principal source of asthma prevalence data for Wisconsin.4 The Wisconsin telephone-based BRFSS survey contains self-reported disease and risk factor data for approximately 4,500 adults and 1,100 children annually. The BRFSS sample is dependent on available federal funding and may vary widely from year to year. Although data are provided at the county level, the sample size is often too small for direct estimation of disease prevalence at this geographical level.
Electronic Health Records (EHRs) are increasingly used in research to identify patients with chronic diseases for surveillance and epidemiological studies.5–7 This study aims to compare asthma prevalence estimates in the Wisconsin child and adult population from the traditional statewide BRFSS telephone survey and EHRs from a large Wisconsin health system. We hypothesized that a reliable estimate of asthma prevalence can be made from EHR data at a local level when compared with telephone survey data.
METHODS
Source of Health Survey Data
Cross-sectional data from the 2007–2009 Wisconsin BRFSS survey (http://www.cdc.gov/brfss) consisting of 22,945 adult and child residents were used to estimate asthma prevalence. The BRFSS is an ongoing, state-based telephone survey conducted by state health departments in collaboration with the Centers for Disease Control and Prevention to assess the health of the civilian non-institutionalized adult population aged 18 years and older in all 50 states. Data are collected annually from a random sample of adults via a telephone survey employing random-digit dialing. Information on children in the household is collected by proxy through the adult surveyed.
Source of Clinic Data
Our research group has developed the University of Wisconsin (UW) Electronic Health Record Public Health Information Exchange (eHealth-PHINEX), an EHR data exchange between UW Departments of Family Medicine, Pediatrics, and Internal Medicine clinics (UW clinics) and the Wisconsin Division of Public Health Information Network (PHIN) of all health care visits for patients seen in the UW clinics who had at least one encounter identified by a date of service in the clinical EHR between 1/1/2007 and 12/31/2009. During this three-year time period, we follow the health records of 376,054 patients with 5 million clinical encounters and 5.6 million associated diagnoses. The database contains extracted clinical care fields, geocoding to the census block group neighborhood level, and detailed socio-demographic data. The data exchange conforms to the HIPAA limited data set privacy rule (public health is blinded to patient/provider specific information) and has been approved by the UW Institutional Review Board (IRB) Protocol # M2009-1273 and by UW Health with data use agreements. The UW eHealth-PHINEX methodology was documented previously.8
UW clinics are located throughout the state, but the greatest patient density is seen in South Central Wisconsin (Dane and surrounding counties, including Sauk, Columbia, Dodge, Jefferson, Iowa, Rock, Green, and Marquette). These clinics provide care for Wisconsin residents of varied socioeconomic strata in both rural and urban settings.
Source of Community Level Data
The Esri Business Analyst Premium product9 contains over 6,000 variables at the census block group on demographics, socioeconomic segmentation, consumer behavior, business locations and type, street data, and market potential. For this study, we examined asthma risk by median household income at the Census Block Group, as calculated by the US Bureau of the Census.10
Measures
The primary outcome of interest was current asthma prevalence. From the BRFSS, current asthma was defined by an affirmative response to the following two questions: “Have you ever been told by a doctor, nurse or other health professional that you have asthma?” and the subsequent question, “Do you still have asthma?” Patients in the UW eHealth-PHINEX dataset were identified as having current asthma by presence of the ICD-9 code 493 in either a clinic encounter diagnosis or problem list fields of their EHR.
The following covariates (pre-established risk factors for asthma) were available from both the BRFSS survey and UW eHealth-PHINEX clinical record: gender, age group, race/ethnicity, adult body mass index (BMI) in kg/m2, and adult cigarette smoking. Child BMI from UW eHealth-PHINEX clinical data was categorized using BMI-for-age percentiles.11 Annual household income was available from BRFSS only. Because household income was not available for the UW eHealth-PHINEX patients, we used the 2010 median annual household income estimate by census block group from ESRI9 in analysis.12 A census block group is defined as a neighborhood area containing 600–3,000 people. Insurance status was available from the UW eHealth-PHINEX clinical record only. When a UW eHealth-PHINEX patient had more than one encounter in the three-year period, data were taken from the earliest encounter.
Analytical Methods
All analyses were conducted separately for children and adults. Descriptive analyses and prevalence by sociodemographic factors were calculated for both the WI BRFSS and UW eHealth-PHINEX datasets. We analyzed gender, age group, race/ethnicity, smoking status, BMI, household income and insurance status as covariates in the child and adult models when they were available. National and WI BRFSS data were analyzed with logistic regression models, adjusted for relevant covariates. UW eHealth-PHINEX data were analyzed using adjusted mixed-effects logistic regression, in which census block group was the random effect for median household income. Odds ratios (ORs) of covariates and 95% confidence intervals (CIs) were estimated from all multivariate logistic regression models. Analysis of UW eHealth-PHINEX data using a fixed effect logistic regression model resulted in estimates that were not significantly different in direction or magnitude from the mixed effects regression model (results not shown). Multivariate models were run two ways: including missing values as a separate category in analysis and excluding observations with missing values to key covariates. Final models were based on observations with complete covariate data; however, the results did not differ significantly when missing values were included in the analysis (results not shown).
BRFSS analyses incorporated sampling weights that adjusted for the multistage sampling frame and unequal probabilities of selection.4 In addition, BRFSS data were weighted proportionally to account for differences in sample size between the 3 years. Analyses were performed using SAS software Version 9.2 of the SAS System for Windows (Copyright © 2002–2008 SAS Institute Inc., Cary, NC, USA).
Data were graphically represented to illustrate asthma prevalence variation within each census tract in a map of Dane County, Wisconsin. Using a geographic information system (GIS), the patient address was geocoded and individual points were aggregated to the census tract (2500 and 8000 person county subdivision), providing a count of the overall total number of patients as well as those with asthma in order to determine the disease prevalence.
RESULTS
Demographics
The WI BRFSS sample consisted of 3,882 children under 18 years of age and 19,063 adults aged 18 years or older. The UW eHealth-PHINEX sample contained 93,791 children and 282,263 adults. A statewide comparison of census, BRFSS and UW eHealth-PHINEX demographics showed that the BRFSS and clinic samples were fairly representative of the Wisconsin statewide population (and were similar to one another), with the following exceptions (Table 1). UW eHealth-PHINEX data contained a significantly larger percentage of females (UW eHealth-PHINEX-53.09 (95% CI 52.86–53.32) versus Census-50.33 (95% CI 50.28–50.39) and BRFSS-50.54 (95% CI 49.48–51.59) percent) and children under 5 years of age (UW eHealth-PHINEX-8.88 (95% CI 8.78–8.98) versus Census-6.43 (95% CI 6.41–6.45) and BRFSS- 6.16 (95% CI 5.63–6.69) percent), compared to the Census and BRFSS data. Both UW eHealth-PHINEX and BRFSS samples contained significantly more non-Hispanic Whites than the general population (UW eHealth-PHINEX-87.99 (95% CI 87.68–88.30) and BRFSS-88.17 (95% CI 87.35–88.99) versus Census-85.46 (95% CI 85.38–85.53) percent) and fewer non-Hispanic Blacks and Hispanics.
Table 1.
Wisconsin Statewide Comparison of Census, BRFSS and UW eHealth-PHINEX Clinic Demographics (2007–2009)
| Wisconsin Census Data 2007–2009* | Wisconsin BRFSS Data 2007–2009 | Wisconsin UW eHealth-PHINEX Patients 2007–2009 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | Percent (95% CI) | N*† | Percent‡ (95% CI) | N** | Percent (95% CI) | |
| Overall | 5,627,985 | 22,945 | 376,054 | |||
|
| ||||||
| Sex | ||||||
| Male | 2,795,161 | 49.67 (49.61– 49.72) | 9,857 | 49.46 (48.41 – 50.52) | 176,416 | 46.91 (46.69 – 47.13) |
| Female | 2,832,824 | 50.33 (50.28 – 50.39) | 13,027 | 50.54 (49.48 – 51.59) | 199,631 | 53.09 (52.86 – 53.32) |
| Age Group | ||||||
| 0–4 | 361,847 | 6.43 (6.41 – 6.45) | 859 | 6.16 (5.63 – 6.69) | 33,408 | 8.88 (8.78 – 8.98) |
| 5–11 | 496,694 | 8.83 (8.80 – 8.85) | 1,230 | 8.57 (7.98 – 9.16) | 31,878 | 8.48 (8.39 – 8.57) |
| 12–17 | 458,426 | 8.15 (8.12 – 8.17) | 1,573 | 7.92 (7.43 – 8.41) | 28,505 | 7.58 (7.49 – 7.67) |
| 18–34 | 1,284,712 | 22.83 (22.79 – 22.87) | 2,529 | 23.05 (21.96 – 24.14) | 79,801 | 21.22 (21.07 – 21.37) |
| 35–64 | 2,277,326 | 40.46 (40.41 – 40.52) | 11,171 | 40.75 (39.83 – 41.67) | 155,120 | 41.25 (41.04 – 41.46) |
| 65+ | 748,981 | 13.31 (13.28 – 13.34) | 5,209 | 13.55 (12.98 – 14.11) | 47,342 | 12.59 (12.48 – 12.70) |
| Race/Ethnicity | ||||||
| Non-Hispanic | 4,809,406 | 85.46 (85.38 – 85.53) | 19,399 | 88.17 (87.35 – 88.99) | 315,730 | 87.99 (87.68 – 88.30) |
| White | ||||||
| Non-Hispanic | 352,101 | 6.26 (6.24 – 6.28) | 1,870 | 4.12 (3.67 – 4.57) | 15,652 | 4.36 (4.29 – 4.43) |
| Black | ||||||
| Non-Hispanic, | 178,549 | 3.17 (3.16 – 3.19) | 1,041 | 4.59 (4.04 – 5.15) | 13,878 | 3.87 (3.81 – 3.93) |
| Other | ||||||
| Hispanic | 287,930 | 5.12 (5.10 – 5.13) | 430 | 3.11 (2.61 – 3.62) | 13,553 | 3.78 (3.72 – 3.84) |
Average of 3 years of estimates (2007–2009), based on the 2000 census
Due to missing data within each variable, stratified counts may not sum to overall N
Unweighted N
Weighted percent
Prevalence
Child and adult asthma prevalence by select sociodemographic factors are shown in Table 2. Child asthma prevalence among UW eHealth-PHINEX patients compared to WI BRFSS respondents was not significantly different, both in terms of overall prevalence estimates (8.96 (95% CI 8.77–9.15) vs. 7.98 (95% CI 6.01–9.95) percent, respectively), as well as for the majority of the estimates by individual sociodemographic factors. However, due to the small sample size within strata, several of the WI BRFSS child asthma prevalence estimates had wide confidence intervals and relative standard error greater than 30%, which made the estimates less reliable. Smoking status, BMI and insurance status were not available for children from the WI BRFSS.
Table 2.
WI BRFSS and UW eHealth-PHINEX Current Asthma Prevalence by Select Sociodemographic Factors (2007–2009)
| WI BRFSS Data | UW eHealth-PHINEX | |||
|---|---|---|---|---|
|
| ||||
| N† | Percent‡ (95% CI) | N | Percent (95% CI) | |
| Child Asthma Prevalence | ||||
| Overall | 130 | 7.98 (6.01 – 9.95) | 8,403 | 8.96 (8.77 – 9.15) |
| Gender | ||||
| Male | 74 | 8.48 (5.67 – 11.29) | 4,913 | 10.20 (9.91 – 10.49) |
| Female | 56 | 7.48 (4.68 – 10.29) | 3,490 | 7.65 (7.40 – 7.90) |
| Age Group | ||||
| 0–4 | 24 | 6.75* (2.76 – 10.73) | 2,080 | 6.23 (5.96 – 6.50) |
| 5–11 | 46 | 8.20 (4.90 – 11.51) | 3,396 | 10.65 (10.29 – 11.01) |
| 12–17 | 59 | 8.42 (5.29 – 11.55) | 2,927 | 10.27 (9.90 – 10.64) |
| Race-Ethnicity | ||||
| Non-Hispanic, White | 79 | 7.32 (5.21 – 9.42) | 6,215 | 8.68 (8.46 – 8.90) |
| Non-Hispanic, Black | 38 | 18.02* (7.05 – 28.99) | 1,185 | 17.78 (16.77 – 18.79) |
| Non-Hispanic, Other | 8 | 11.70* (0.77 – 22.63) | 296 | 5.57 (4.94 – 6.20) |
| Hispanic | 5 | 4.38* (0.01 – 9.40) | 484 | 8.09 (7.37 – 8.81) |
| Smoking Status | ||||
| Never/Former | - | - | 6,176 | 9.35 (9.12 – 9.58) |
| Current | - | - | 256 | 15.48 (13.58 – 17.38) |
| Passive | - | - | 1,451 | 12.52 (11.88 – 13.16) |
| BMI | ||||
| Not Overweight/Obese | - | - | 4,639 | 10.50 (10.20 – 10.80) |
| (< 85th percentile) | ||||
| Overweight | - | - | 1,173 | 12.93 (12.19 – 13.67) |
| (≥ 85th and < 95th percentile) | ||||
| Obese (≥ 95th percentile) | - | - | 1,235 | 16.00 (15.11 – 16.89) |
| Household Income | ||||
| 75,000+ | 48 | 7.14 (4.44 – 9.84) | 2641 | 9.20 (8.85 – 9.55) |
| 50,000–<75,000 | 37 | 7.65 (4.42 – 10.88) | 3824 | 8.84 (8.56 – 9.12) |
| <50,000 | 37 | 13.44 (5.96 – 20.91) | 1443 | 9.24 (8.76 – 9.72) |
| Payer | ||||
| No Insurance | - | - | 66 | 2.32 (1.76 – 2.88) |
| Medicaid | - | - | 1,907 | 11.62 (11.10 – 12.14) |
| Commercial | - | - | 6,429 | 8.63 (8.42 – 8.84) |
|
| ||||
| Adult Asthma Prevalence | ||||
| Overall | 1,744 | 9.41 (8.70 – 10.13) | 21,390 | 7.58 (7.48 – 7.68) |
| Gender | ||||
| Male | 536 | 8.08 (6.98 – 9.17) | 7,180 | 5.60 (5.47 – 5.73) |
| Female | 1,208 | 10.71 (9.78 – 11.63) | 14,210 | 9.23 (9.08 – 9.38) |
| Age Group | ||||
| 18–34 | 300 | 11.11 (9.27 – 12.94) | 6,748 | 8.46 (8.26 – 8.66) |
| 35–64 | 997 | 8.81 (8.00 – 9.63) | 12,195 | 7.86 (7.72 – 8.00) |
| 65+ | 435 | 8.45 (7.33 – 9.57) | 2,447 | 5.17 (4.97 – 5.37) |
| Race-Ethnicity | ||||
| Non-Hispanic, White | 1,358 | 8.91 (8.18 – 9.64) | 18,611 | 7.62 (7.51 – 7.73) |
| Non-Hispanic, Black | 222 | 16.56 (12.39 – 20.74) | 1,142 | 12.71 (11.97 – 13.45) |
| Non-Hispanic, Other | 111 | 12.02 (7.91 – 16.13) | 455 | 5.31 (4.82 – 5.80) |
| Hispanic | 33 | 10.25* (3.55 – 16.94) | 472 | 6.23 (5.67 – 6.79) |
| Smoking Status | ||||
| Never | 799 | 8.65 (7.68 – 9.63) | 10,946 | 8.34 (8.18 – 8.50) |
| Former | 574 | 9.62 (8.44 – 10.79) | 5,881 | 8.74 (8.52 – 8.96) |
| Current | 365 | 11.14 (9.24 – 13.03) | 3,178 | 8.25 (7.96 – 8.54) |
| Passive | - | 225 | 10.19 (8.86 – 11.52) | |
| BMI | ||||
| Not Overweight/Obese | 480 | 8.75 (7.45 – 10.05) | 4,377 | 7.32 (7.10 – 7.54) |
| (<25.0) | ||||
| Overweight (25.0 – <30.0) | 514 | 7.90 (6.79 – 9.01) | 4,820 | 7.99 (7.76 – 8.22) |
| Obese (30.0 – <40.0) | 528 | 11.11 (9.70 – 12.52) | 5,133 | 10.19 (9.91 – 10.47) |
| Morbidly Obese (≥40.0) | 123 | 17.51 (12.11 – 22.90) | 1,834 | 15.89 (15.16 – 16.62) |
| Household Income | ||||
| 75,000+ | 473 | 8.83 (7.69 – 9.96) | 5,729 | 8.23 (8.02 – 8.44) |
| 50,000–<75,000 | 523 | 8.40 (7.30 – 9.49) | 9,916 | 7.76 (7.61 – 7.91) |
| <50,000 | 579 | 12.90 (10.84 – 14.95) | 4,097 | 7.35 (7.12 – 7.58) |
| Payer | ||||
| No Insurance | - | - | 441 | 2.44 (2.21 – 2.67) |
| Worker's Comp | - | - | 149 | 5.52 (4.63 – 6.41) |
| Medicaid | - | - | 1,791 | 12.31 (11.74 – 12.88) |
| Medicare | - | - | 2,917 | 6.11 (5.89 – 6.33) |
| Commercial | - | - | 16,092 | 8.08 (7.96 – 8.20) |
Unweighted N
Weighted percent
Relative Standard Error > 30% (unreliable estimate)
Adult asthma prevalence estimates differed significantly between UW eHealth-PHINEX and WI BRFSS data. Overall, adult asthma prevalence was lower among the UW eHealth-PHINEX population compared to WI BRFSS (7.58 (95% CI 7.48–7.68) vs. 9.41 (95% CI 8.70–10.13) percent, respectively). Males in the UW eHealth-PHINEX population had considerably lower asthma prevalence than male WI BRFSS respondents. Asthma prevalence was lower among the UW eHealth-PHINEX population's young adults (aged 18–34 years) and older adults (aged 65+years), compared to similarly aged WI BRFSS respondents. By race/ethnicity, other non-Hispanics in the UW eHealth-PHINEX population had lower asthma prevalence than WI BRFSS respondents, while asthma prevalence was similar among non-Hispanic Whites and non-Hispanic Blacks. Adult asthma prevalence within strata of household income differed only in the lowest income category. UW eHealth-PHINEX patients had substantially lower asthma prevalence in this category compared to WI BRFSS respondents (7.35 (95% CI 7.12–7.58) vs. 12.90 (95% CI 10.84–14.95) percent, respectively); however, median household income by census block group was utilized for UW eHealth-PHINEX patients, rather than individual patient household income. UW eHealth-PHINEX clinic patients covered by Medicaid had the highest asthma prevalence, compared to patients with commercial or no insurance. Insurance status was not available for adult WI BRFSS respondents.
Multivariate Analyses
Multivariable logistic regression models were created using BRFSS data for child and adult asthma prevalence. Estimates from these models were compared to mixed-effects logistic regression using UW eHealth-PHINEX data. Odds ratio (OR) estimates for asthma prevalence were similar between WI BRFSS and UW eHealth-PHINEX models, although small WI BRFSS sample size often resulted in non-significant estimates with wide confidence intervals. For this reason, estimates from a model based on U.S. BRFSS data are shown for comparison (Tables 3 and 4). The majority of the national BRFSS estimates were similar in direction and magnitude to the WI BRFSS estimates. Two exceptions were estimates for bon-Hispanic Blacks and those with a household income less than $50,000.
Table 3.
U.S. BRFSS, WI BRFSS and UW eHealth-PHINEX Multivariate Models for Child Current Asthma Prevalence (2007–2009)
| U.S. BRFSS * | WI BRFSS * | UW eHealth-PHINEX † | |
|---|---|---|---|
| ORadj (95% CI) | ORadj (95% CI) | ORadj (95% CI) | |
| Asthma Yes = 5,353 | Asthma Yes = 121 | Asthma Yes = 6,369 | |
| Asthma No = 53,914 | Asthma No = 1,196 | Asthma No = 47,230 | |
| Sex | |||
| Male | reference | reference | reference |
| Female | 0.76 (0.68 – 0.85) | 0.84 (0.48 – 1.45) | 0.73 (0.69 – 0.77) |
| Age Group | |||
| 0–4 | reference | reference | reference |
| 5–11 | 1.99 (1.68 – 2.34) | 1.38 (0.64 – 2.98) | 1.34 (1.25 – 1.44) |
| 12–17 | 1.77 (1.50 – 2.07) | 1.39 (0.67 – 2.89) | 1.30 (1.21 – 1.40) |
| Race/Ethnicity | |||
| Non-Hispanic, White | reference | reference | reference |
| Non-Hispanic, Black | 1.60 (1.36 – 1.88) | 2.74 (1.22 – 6.12) | 1.96 (1.79 – 2.15) |
| Non-Hispanic, Other | 0.99 (0.80 – 1.24) | 1.88 (0.64 – 5.50) | 0.76 (0.66 – 0.87) |
| Hispanic | 0.84 (0.72 – 0.98) | 0.63 (0.18 – 2.20) | 0.91 (0.81 – 1.03) |
| Smoking | |||
| Never/former | - | - | reference |
| Current | - | - | 1.44 (1.22 – 1.70) |
| Passive | - | - | 1.15 (1.07 – 1.24) |
| BMI | |||
| Not Overweight/Obese | - | - | reference |
| (< 85th percentile) | |||
| Overweight | 1.23 (1.14 – 1.32) | ||
| (≥ 85th and < 95th percentile) | |||
| Obese (≥ 95th percentile) | - | - | 1.45 (1.35 – 1.56) |
| Household Income | |||
| $75,000+ | reference | reference | reference |
| $50,000–<75,000 | 1.04 (0.90 – 1.22) | 2.21 (0.99 – 4.92) | 0.96 (0.89 – 1.03) |
| <$50,000 | 1.28 (1.13 – 1.45) | 1.75 (0.93 – 3.29) | 0.93 (0.85 – 1.03) |
| Insurance Status | |||
| Commercial | - | - | reference |
| Medicaid | - | - | 1.21 (1.12 – 1.30) |
| No Insurance | - | - | 0.29 (0.18 – 0.48) |
U.S. and WI BRFSS child asthma models adjusted for sex, age group, race/ethnicity and household income. Personal or passive smoking status, BMI and insurance status were not available for children in the BRFSS.
UW eHealth-PHINEX model adjusted for all variables in table, including sex, age group, race/ethnicity, smoking status, BMI, median household income for a patient's census block group and insurance status.
Table 4.
U.S. BRFSS, WI BRFSS and UW eHealth-PHINEX Multivariate Models for Adult Current Asthma Prevalence (2007–2009)
| U.S. BRFSS * | WI BRFSS * | UW eHealth-PHINEX † | |
|---|---|---|---|
| ORadj (95% CI) | ORadj (95% CI) | ORadj (95% CI) | |
| Asthma Yes = 92,828 | Asthma Yes = 1,492 | Asthma Yes = 14,373 | |
| Asthma No = 956,843 | Asthma No =14,795 | Asthma No = 142,005 | |
| Sex | |||
| Male | reference | reference | reference |
| Female | 1.76 (1.70 – 1.82) | 1.46 (1.19 – 1.79) | 1.70 (1.64 – 1.77) |
| Age Group | |||
| 18–34 | reference | reference | reference |
| 35–64 | 0.85 (0.82 – 0.88) | 0.71 (0.57 – 0.90) | 0.86 (0.82 – 0.89) |
| 65+ | 0.75 (0.72 – 0.78) | 0.73 (0.56 – 0.94) | 0.50 (0.46 – 0.55) |
| Race/Ethnicity | |||
| Non-Hispanic, White, | reference | reference | reference |
| Non-Hispanic, Black | 0.97 (0.92 – 1.02) | 1.79 (1.25 – 2.55) | 1.45 (1.33 – 1.58) |
| Non-Hispanic, Other | 1.08 (1.01 – 1.16) | 1.20 (0.78 – 1.86) | 0.74 (0.66 – 0.83) |
| Hispanic | 0.65 (0.61 – 0.70) | 0.81 (0.30 – 2.17) | 0.83 (0.74 – 0.93) |
| Smoking | |||
| Never | reference | reference | reference |
| Former | 1.21 (1.17 – 1.26) | 1.24 (1.01 – 1.52) | 1.11 (1.07 – 1.16) |
| Current | 1.31 (1.26 – 1.36) | 1.29 (1.01 – 1.66) | 0.99 (0.94 – 1.04) |
| Passive | - | - | 1.17 (0.98 – 1.40) |
| BMI | |||
| Not | reference | reference | reference |
| Overweight/Obese (<25.0) | |||
| Overweight (25.0 – <30.0) | 1.16 (1.11 – 1.20) | 1.00 (0.78 – 1.28) | 1.26 (1.20 – 1.32) |
| Obese (30.0 – <40.0) | 1.63 (1.57 – 1.70) | 1.41 (1.11 – 1.79) | 1.61 (1.54 – 1.69) |
| Morbidly Obese (≥40.0) | 2.79 (2.63 – 2.95) | 2.12 (1.38 – 3.25) | 2.38 (2.23 – 2.53) |
| Household Income | |||
| $75,000+ | reference | reference | reference |
| $50,000–<75,000 | 1.00 (0.95 – 1.05) | 1.07 (0.80 – 1.44) | 0.88 (0.83 – 0.94) |
| <$50,000 | 1.28 (1.24 – 1.33) | 1.03 (0.79 – 1.33) | 0.84 (0.78 – 0.91) |
| Insurance Status | |||
| Commercial | - | - | reference |
| Medicaid | - | - | 1.39 (1.30 – 1.49) |
| Medicare | - | - | 1.23 (1.13 – 1.33) |
| Worker's Comp | - | - | 0.89 (0.71 – 1.10) |
| No Insurance | - | - | 0.39 (0.34 – 0.46) |
U.S. and WI BRFSS adult asthma models adjusted for sex, age group, race/ethnicity, smoking status, BMI and household income. Passive smoking status and insurance status were not available for adults in the BRFSS.
UW eHealth-PHINEX model adjusted for all variables in table including sex, age group, race/ethnicity, smoking status, BMI, median household income for a patient's census block group and insurance status.
The child asthma prevalence model based on WI BRFSS data (Table 3) was adjusted for gender, age group, race and household income; however, the only significant covariate was race/ethnicity (p=0.0413). Due to the availability of additional sociodemographic variables, the UW eHealth-PHINEX model was more complete and also adjusted for smoking status, BMI and insurance coverage. Significant independent risk factors for asthma among children in the UW eHealth-PHINEX population included gender, age group, race, smoking status, BMI and health insurance status (all covariates p<0.0001). Specifically, male gender, older age, Black race, current or passive smoking, being overweight or obese, and having Medicaid health coverage were associated with higher asthma prevalence among children in the UW eHealth-PHINEX population. Median household income for the census block group was not significantly associated with asthma prevalence among children (p=0.332).
The adult asthma prevalence model based on WI BRFSS data (Table 4) was adjusted for gender, age group, race/ethnicity, smoking status, BMI and household income, with significant covariates including gender (p=0.0003), age group (p=0.0123), race/ethnicity (p=0.0114) and BMI (p<0.0001). Similarly, among adult UW eHealth-PHINEX patients, gender, age group, race/ethnicity and BMI were significant independent risk factors for asthma, as well as smoking and insurance status and median household income for the patient's census block group (all covariates p<0.0001). Household income was not a significant covariate in the WI BRFSS model.
Specifically, among adults in the UW eHealth-PHINEX population, females had significantly higher asthma prevalence than males after adjusting for other variables (OR 1.70, 95% CI 1.64–1.77). Compared to the youngest adults (18–34 years), older adults (35–64 and 65+ years) had lower asthma risk with ORs of 0.86 (95% CI 0.82–0.89) and 0.50 (95% CI 0.46–0.55), respectively. Race had a strong effect on asthma prevalence, non-Hispanic Blacks were almost 50% more likely to have asthma compared to Non-Hispanic Whites, after adjustment for other variables. Non-Hispanic other racial/ethnic groups and Hispanics both had reduced risk of asthma, compared to the reference group (non-Hispanic Whites). Both former and passive smoking were significant risk factors for asthma. Compared to adults who were not overweight or obese, a higher BMI was associated with an increased risk of asthma, with the greatest risk in the morbidly obese (OR 2.38, 95% CI 2.23–2.53). Insurance status was also a significant predictor of asthma prevalence; specifically, Medicaid and Medicare coverage were associated with a higher risk of asthma, compared to patients with commercial insurance. Lower household income was associated with reduced asthma risk.
DISCUSSION
We compared data from a traditional public health telephone survey and clinic EHRs to demonstrate that EHRs offer a promising source of health data to estimate asthma prevalence and associated risk factors in Wisconsin. Current surveillance systems have characterized chronic disease at the national and state level, but cannot meet the critical need for data at local levels within the state, where many public health policies and interventions ultimately are designed and implemented.13 There is also very little data on specific subpopulations such as children and racial and ethnic minorities. Data from the EHR can bridge these gaps in currently available public health information.
In a statewide comparison between UW eHealth-PHINEX demographics and census data, we found that the clinic samples were fairly representative of the Wisconsin statewide population.8 Furthermore, because the majority of the clinic patient population resided in seven counties surrounding Dane County, WI, we also made a demographic comparison to this area (data not shown). In these comparisons, UW eHEALTH-PHINEX demographics also resembled the seven-county population.
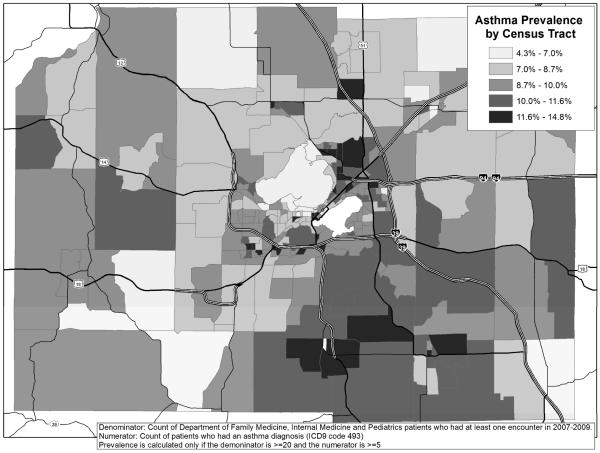
We determined asthma prevalence using EHR data from approximately 376,000 patients (30,000 with asthma), compared to 23,000 persons (1,850 with asthma) from the WI BRFSS. Adjusted ORs for asthma were similar in magnitude and direction for the majority of covariates, including gender, age and race, when comparing WI BRFSS and UW eHealth-PHINEX EHR models. Our EHR database was over sixteen-fold the sample size of the WI BRFSS, resulting in more precise estimates with tighter confidence intervals and greater power to detect associations with risk factors, especially in children. Furthermore, the EHR database provides the ability to estimate asthma prevalence at the neighborhood level (Figure 1).
Figure 1.
Asthma prevalence by census tract, Dane County, WI (2007–2009)
Overall prevalence estimates for children and adults differed slightly (non-significantly for children and significantly for adults) between the WI BRFSS and UW eHealth-PHINEX data. The direction of the UW eHealth-PHINEX estimates is more similar to what other studies have shown, specifically, that asthma prevalence is highest in childhood with a male predominance that reverses in adolescence to a higher prevalence of asthma among adult women.14–17 One surprising finding was that the UW eHealth-PHINEX asthma prevalence in males was much smaller that the WI BRFSS estimate and has a much narrower confidence interval. However, UW eHealth-PHINEX prevalence estimates were more similar in magnitude and direction to those obtained from the 2009 National Health Interview Survey (NHIS), an ongoing national household interview survey conducted by the Centers for Disease Control and Prevention (CDC) to assess the health of the civilian noninstitutionalized population. In the 2009 NHIS, child asthma prevalence was greater than adult asthma prevalence (9.6% (95% CI 8.9–10.3) and 7.7% (95% CI 7.3 – 8.1), respectively) and adult male asthma prevalence (5.5% (95% CI 5.0 – 6.0) was significantly lower than adult female asthma prevalence (9.7% (95% CI 9.1 – 10.3).18
While household income was not a significant risk factor for asthma among Wisconsin BRFSS respondents, having an annual household income less than $50,000 was associated with increased asthma prevalence in the national BRFSS data set. The association between low socioeconomic status (SES) and increased asthma risk has been observed in several studies.19–21 In contrast, the multivariate model based on UW eHealth-PHINEX data found a slightly protective association between household income less than $50,000 and asthma risk. We offer two explanations for the seemingly inconsistent result. The first is the narrow socioeconomic spectrum in the UW eHealth-PHINEX population. Compared to the national BRFSS data, this population and even the state BRFSS sample are fairly homogeneous with respect to household income, attenuating any association that may be detected. Second, it is important to highlight that we do indirectly detect poverty as a predictor of asthma through insurance status. In both the child and adult multivariate models, we see a strong increased risk differential between persons with Medicaid versus commercial insurance. Since the models control for insurance status, which is a measure of SES, any remaining effect of household income on asthma risk may be attenuated.
The adult WI BRFSS models showed a positive association between former and current smoking status and asthma risk, although only former smoking status was associated with asthma in the UW eHealth-PHINEX model. This result may be due to inconsistent or inaccurate smoking status documentation between the EHR and BRFSS. When compared to an anonymous telephone survey response, patients may be more likely to tell their physician during an in-person encounter that they have quit smoking or that they do not smoke when in fact they are smokers. Smoking status documentation will need to be further assessed as this is an important risk factor for many diseases.
Our data are limited to patients seen at UW clinics who reside primarily in an area of South Central Wisconsin that does not include Milwaukee, the largest city in the state with a large proportion of racial and ethnic minorities. Therefore, the magnitude of disparities in asthma prevalence is attenuated by racial and ethnic categories within our data. However, the data still do describe the relativity of the difference in asthma prevalence by racial categories, specifically that non-Hispanic Blacks are affected by increased asthma burden compared to other populations. In the national data set, the adjusted estimate for asthma associated with Black race was not significant, while both the WI BRFSS and UW eHealth-PHINEX asthma estimates were significantly elevated. Wisconsin may have more SES disparities in health outcomes by race than is seen on a national level. For example, the disparity between Milwaukee's black and white infant mortality rates is among the worst in the nation.22,23
EHR advantages
Public health data collection via telephone survey has several drawbacks in addition to low numbers and inability to assess diseases at the local level. The data is obtained by self-report which may exclude persons with undiagnosed asthma and no adjustment is made for variables related to geographic area such as race/ethnicity, which may improve disease estimates. Furthermore, low BRFSS response rates (~50%) might indicate response bias. The 2007–2009 BRFSS only sampled households with landline telephones, potentially resulting in the undersampling of certain populations due to the increasing use of cell phones. Wireless-only households tend to have younger occupants, have non-white racial backgrounds and lower incomes. Thus, the traditional public health telephone survey may not reflect the true prevalence of asthma nor highlight counties, neighborhoods (census block groups), or census tracts with the highest prevalence.
The EHR offers a rich source of high-quality population health data to study asthma or any other chronic disease. The objective diagnoses and measurements contained in clinical data can be linked with socio-demographic databases to describe risks in detail at the neighborhood level, allowing better insight to areas of interest such as where asthma is prevalent and uncontrolled. Local data can guide public health policy goals and targeting of health services delivery while providing a baseline for evaluation and quality improvement efforts.24,25 Using the EHR can greatly increase sample size, particularly among certain age, racial and ethnic subgroups critical to community health assessments and alleviate the inherent recall and response bias of traditional telephone surveys. EHR data can also provide additional disease risk factors not found in BRFSS such as BMI, tobacco smoke exposure and insurance coverage for children.
Electronic records are readily available for epidemiological analysis to study disease control as well as perform longitudinal surveillance in a timely manner. Costs are mainly limited to disease definition, identification of outcomes, and data extraction. With the recent widespread adoption of EHR by medical providers, EHR may offer a more sustainable data source as other systems may be less available given recent and anticipated government budget cuts. Thus, clinical EHR data exchange can be a robust method of partnering public health agencies with medical care organizations to inform mutual population health priorities.26–34 Indeed, the federal government awarded grants in 2010 to all of the states to facilitate electronic health information exchange among health care providers, hospitals, and public health.35 Public health departments can work with these organizations to assure that data exchange also supports public health surveillance priorities.35
EHR challenges and opportunities
There are challenges that arise in implementing a new method of disease surveillance. EHR data are limited to patients seen in participating clinics, and patients may not have a medical home within a single health system.27 The EHR may have missing values and inconsistent quality, which requires use of modeling techniques to account for missing data and attention to definitions of disease used to acquire data. There is also potential introduction of bias through misclassification of patients, even when disease identification has good sensitivity and specificity.36
In this study, a physician's diagnosis of asthma was the sole case definition criteria (presence of ICD-9 code 493 in encounter diagnosis or problem list fields of EHR). This may be problematic because there is no consensus on asthma diagnosis.37 For example, one study compared asthma status by ICD-9 code and criteria-based medical record review. It found that ICD code-based asthma ascertainment under-identified asthma cases when compared to a `gold standard' of manual record review. The authors concluded that “ICD codes may be useful for etiologic research but may not be suitable for surveillance of asthma epidemiology.”37 In our view, the problems of detection and subsequent documentation in the EHR would also likely affect self-report in the BRFSS telephone survey. In BRFSS, participants respond to the question “has a doctor ever told you that you have asthma.” But if a person is not diagnosed, it is unlikely that the physician will tell the patient that they have asthma. Thus EHR asthma cases that could found by chart review, but not ICD-9 codes, would also be cases that would be undetected by BRFSS. The BRFSS has been the mainstay for statewide surveillance of ambulatory chronic disease states. But as is the case with asthma, in many instances disease detection is dependent on self-report of physician recognition. In our study, the EHR-BRFSS prevalence estimate comparisons are for the most part remarkably similar, and the dependence upon physician recognition in both data systems may largely explain this finding. This then points to an additional advantage of the EHR and shortcoming of BRFSS. It is impossible to apply additional clinical criteria within BRFSS to find undetected cases. But along with diagnosis, other clinical indicators could be included in an EHR case definition. In this way, electronic health records may improve asthma case detection sensitivity in a way that is impossible with BRFSS. Indeed, we have a research study underway that will compare the asthma ICD-9 code only definition to one that includes additional clinical criteria present on the EHR.
Finally, EHR data are voluminous and very detailed and it is unclear how to best analyze and display these data for public health consumption.
Conclusions
Electronic health records can be used to estimate asthma prevalence in Wisconsin adults and children, and they provide estimates that are comparable to the traditional health telephone survey without many of its limitations. Development of EHR databases provides exciting opportunities to improve asthma as well as other chronic disease surveillance, prevention, and understanding of risk factors, highlight areas of disparity, and improve targeting of education and public health interventions.
Acknowledgments
The authors thank Brian G. Arndt, MD for his assistance in reviewing this manuscript and William R. Buckingham, PhD for generating the asthma prevalence map.
This research was funded in part with grants from The Robert Wood Johnson Foundation (Information Links; Common Ground), Centers for Disease Control and Prevention (Preparedness; Environmental Public Health Tracking; Infrastructure Improvement), and Centers for Medicare and Medicaid Services (Medicaid Transformation Grant).
Footnotes
Contributor Statement C. Tomasallo led the writing of the article and the data analysis. L.P. Hanrahan and T.W. Guilbert developed the conceptual framework for the analysis, participated in data interpretation and writing of the article. K.J. Cowan participated in writing the article. A. Tandias and T.S. Chang participated in data interpretation and refinement of analysis. All authors reviewed and helped revise the article.
Human Participant Protection This study was approved by the University of Wisconsin-Madison School of Medicine and Public Health Institutional Review Board Research Protocol M2009-1273, Family Medicine/Public Health Data Exchange.
REFERENCES
- 1.Moorman JE, Rudd RA, Johnson CA, et al. National surveillance for asthma--United States, 1980–2004. MMWR Surveill Summ. 2007 Oct 19;56(8):1–54. [PubMed] [Google Scholar]
- 2.Barnett SB, Nurmagambetov TA. Costs of Asthma in the United States: 2002–2007. Journal of Allergy and Clinical Immunology. 2011;127(1):145–52. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 3.National Asthma Education and Prevention Program . Expert Panel Report 3: guidelines for the diagnosis and management of asthma: clinical practice guidelines. 08. August. NIH/National Heart, Lung, and Blood Institute; Bethesda (MD): 2007. p. 4051. [Google Scholar]
- 4.CDCa . Behavioral risk factor surveillance system. U.S. Department of Health and Human Services, Center for Disease Control and Prevention; Atlanta, Georgia: [Accessed September 8, 2010]. [Google Scholar]
- 5.Himes BE, Kohane IS, Ramoni MF, Weiss ST. Characterization of patients who suffer asthma exacerbations using data extracted from electronic medical records. AMIA Annu Symp Proc. 2008:308–312. [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford AG, Cote C, Couto J, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity Electronic Medical Record database. Popul Health Manag. 2010 Jun;13(3):151–161. doi: 10.1089/pop.2009.0039. [DOI] [PubMed] [Google Scholar]
- 7.Esteban-Vasallo MD, Dominguez-Berjon MF, Astray-Mochales J, et al. Epidemiological usefulness of population-based electronic clinical records in primary care: estimation of the prevalence of chronic diseases. Family practice. 2009 Dec;26(6):445–454. doi: 10.1093/fampra/cmp062. [DOI] [PubMed] [Google Scholar]
- 8.Guilbert TW, Arndt B, Temte J, et al. The theory and application of UW eHealth-PHINEX, A Clinical Electronic Medical Record-Public Health Data Exchange. WMJ. 2012;111(3):124–133. [PubMed] [Google Scholar]
- 9.Esri Business Analyst Desktop Premium [computer program] Esri; Redlands, CA: [Accessed September 9, 2011]. http://www.esri.com/software/arcgis/extensions/buisnessanalyst/data-us-prem.html. [Google Scholar]
- 10.Esri The American Community Survey. [Accessed January 25, 2013];An Esri White Paper. 2011 Available at: http://www.esri.com/library/whitepapers/pdfs/the-american-community-survey.pdf.
- 11.About BMI for children and teens. Division of Nutrition, Physical Activity and Obesity. [Accessed September 9, 2011];Center for Disease Control and Prevention. http://www.cdc.gov/healthyweight/assessing/bmi/childrens_bmi/about_childrens_bmi.html.
- 12.ESRI Demographic Update Methodology [Accessed August 16, 2011];ESRI White Paper J-9800. 2009 http://www.esri.com/library/whitepapers/pdfs/demographic-update-methodology-2009 pdf.
- 13.Lurie N, Fremont A. Building bridges between medical care and public health. Jama. 2009 Jul 1;302(1):84–86. doi: 10.1001/jama.2009.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolai T, Pereszlenyiova-Bliznakova L, Illi S, Reinhardt D, von Mutius E. Longitudinal follow-up of the changing gender ratio in asthma from childhood to adulthood: role of delayed manifestation in girls. Pediatr Allergy Immunol. 2003 Aug;14(4):280–283. doi: 10.1034/j.1399-3038.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 15.Sears MR, Greene JM, Willan AR, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003 Oct 9;349(15):1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 16.Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis. 1980 Oct;122(4):567–575. doi: 10.1164/arrd.1980.122.4.567. [DOI] [PubMed] [Google Scholar]
- 17.Mandhane PJ, Greene JM, Cowan JO, Taylor DR, Sears MR. Sex differences in factors associated with childhood- and adolescent-onset wheeze. Am J Respir Crit Care Med. 2005 Jul 1;172(1):45–54. doi: 10.1164/rccm.200412-1738OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDCb . Table 4–1 Current Asthma Prevalence Percents by Age USNHIS, 2009. Centers for Disease Control and Prevention, National Center for Health Statistics; 2010. Interview Survey Data. [Google Scholar]
- 19.Almqvist C, Pershagen G, Wickman M. Low socioeconomic status as a risk factor for asthma, rhinitis and sensitization at 4 years in a birth cohort. Clin Exp Allergy. 2005 May;35(5):612–618. doi: 10.1111/j.1365-2222.2005.02243.x. [DOI] [PubMed] [Google Scholar]
- 20.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011 Jan 12;(32):1–14. [PubMed] [Google Scholar]
- 21.Bacon SL, Bouchard A, Loucks EB, Lavoie KL. Individual-level socioeconomic status is associated with worse asthma morbidity in patients with asthma. Respir Res. 2009;10:125. doi: 10.1186/1465-9921-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward TC, Mori N, Patrick TB, Madsen MK, Cisler RA. Influence of socioeconomic factors and race on birth outcomes in urban Milwaukee. WMJ. 2010 Oct;109(5):254–260. [PubMed] [Google Scholar]
- 23.Byrd DR, Katcher ML, Peppard P, Durkin M, Remington PL. Infant mortality: explaining black/white disparities in Wisconsin. Maternal and child health journal. 2007 Jul;11(4):319–326. doi: 10.1007/s10995-007-0183-6. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed August 1, 2011];President's Advisory Commission on Consumer Protection and Quality in the Health Care Industry. Archived Site: http://www.hcqualitycommission.gov/.
- 25.Honore PA, Wright D, Berwick DM, et al. Creating a framework for getting quality into the public health system. Health Aff. 2011;30(4):737–745. doi: 10.1377/hlthaff.2011.0129. [DOI] [PubMed] [Google Scholar]
- 26.Healthy People 2020 Objectives. 2011 http://www.healthypeople.gov/2020.
- 27.Hatahet MA, Bowhan J, Clough EA. Wisconsin Collaborative for Healthcare Quality (WCHQ): lessons learned. WMJ. 2004;103(3):45–48. [PubMed] [Google Scholar]
- 28.Touchpoints changes childhood asthma management. Pediatric nursing. 2000 Sep-Oct;26(5):538. [PubMed] [Google Scholar]
- 29.Committee for the Study of the Future of Public Health Division of Health Care Services Institute of Medicine National Academy Press. 1988 [Google Scholar]
- 30.Grossmann C, Powers B MJ. Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care: Workshop Series Summary. The National Academy of Sciences. 2011 [PubMed] [Google Scholar]
- 31.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343(15):1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 32. [Accessed August 1, 2011];Public Healht Systems and Services Research Overview. 2008 http://www.nlm.nih.gov/nichsr/phssr/phssrinto.html.
- 33. [Accessed August 1, 2011];Public Health Services and Systems Research: Setting the Research Agenda Update July 2011. http://www.publichealthsystems.org/media/file/PHSSR_ResearchAgenda_July2011.pdf.
- 34. [Accessed August 1, 2011];Digital Infrastructure for the Learning Health System: The Foundation for Continuous Improvement in Health and Health Care - Workshop Series Summary - Institute of Medicine. http://www.iom.edu/Reports/2011/Digital-Infrastructure-for-a-Learning-Health-System.aspx. [PubMed]
- 35. [Accessed January 25, 2013];State Health Information Exchange. Available at: http://www.healthit.gov/providers-professionals/state-health-information-exchange.
- 36.Manuel DG, Rosella LC, Stukel TA. Importance of accurately identifying disease in studies using electronic health records. Bmj. 2010;341:c4226. doi: 10.1136/bmj.c4226. [DOI] [PubMed] [Google Scholar]
- 37.Juhn Y, Kung A, Voigt R, Johnson S. Characterisation of children's asthma status by ICD-9 code and criteria-based medical record review. Prim Care Respir J. 2011;20(1):79–83. doi: 10.4104/pcrj.2010.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]