Abstract
With advances in genetic and imaging techniques, investigating axon regeneration after spinal cord injury in vivo is becoming more common in the literature. However, there are many issues to consider when using animal models of axon regeneration, including species, strains and injury models. No single particular model suits all types of experiments and each hypothesis being tested requires careful selection of the appropriate animal model. In this review, we describe several commonly-used animal models of axon regeneration in the spinal cord and discuss their advantages and disadvantages.
Keywords: pyramidotomy, strain differences, contusion, injury models
Introduction
There is a fundamental difference between studying axon regeneration and other research areas. Unlike development, cancer, or many neurodegenerative diseases, axon regeneration is a process that does not typically occur in the adult mammalian central nervous system (CNS). Therefore, in addition to investigating the basic cellular and molecular processes that promote axon regeneration, the investigator must also have a keen understanding of how to detect regenerating axons since this is a major endpoint of the study. First, an operational definition of what constitutes an axon that has regenerated is needed. Does regeneration refer to any type or a specific type of axonal growth? This topic has been discussed many times[1-3], and is not the focus of this review. Rather, we introduce some of the popular in vivo models of axon regeneration that are used to help investigators gain a better understanding of the pros and cons of each model.
Rats versus Mice
Most of the earlier studies on spinal cord injury (SCI) and axon regeneration were performed in rats. However, with advances in genetic mouse models and their increased availability to the research community, these models have gained much popularity recently. Using mice with knockout of a target molecule has become the gold-standard for functional testing, and Cre-Lox technology along with increasing numbers of transgenic mice have provided greater temporo-spatial control of the knockout strategy that has proven invaluable for providing mechanistic insights into the cellular and molecular processes of axon regeneration. Therefore, many scientists have been drawn to using mice to study axon regeneration, but certain limitations must be considered.
One important difference between rat and mouse models of SCI is that while rats develop large fluid-filled cystic cavities at the injury site (thereby mimicking the human pathology), mice do not[4]. Instead, the injury site in mice is densely packed with cells and actually decreases in size over time[4]. The exact reason for such a significant pathological difference between such closely-related species is not known. Since axons do not regenerate regardless of the presence or the absence of a cavity, the lack of a cavity in mice may not matter in most instances. However, if the focus is on targeting cells present at the injury site, such as the cellular composition of the scar, then the findings of the study must be interpreted with this difference in mind. In addition, transplantation strategies (and findings) may be significantly different between rats (that have a cyst) and mice (that do not have a cyst). Therefore, rats are preferable for studies where mimicking the human pathology is important. These include preclinical studies that focus on the efficacy of novel cellular and/or pharmacological therapies. However, to gain mechanistic insights into the basic cellular and molecular biology of SCI, mouse models may have more to offer.
Another important factor to consider when using mouse models is strain differences. That different strains respond differently to SCI is now well-described in the literature. After contusive SCI, different strains display different rates of locomotor recovery[5] and histopathology[6-8]. Interestingly, the C57BL/6 mouse, which is perhaps the most commonly used strain in axon regeneration studies, has been reported to have a worse locomotor outcome[5], the greatest immunological response[6], and the least axonal growth[7] compared to other inbred mouse strains. In fact, adult dorsal root ganglia cultures from 129X/SvJ mice show much more axon growth than those from C57BL/6 mice[9]. In addition, the same genetic deletion of the myelin-associated inhibitor, Nogo-A, shows much more axonal growth in a 129X1/SvJ background than a C57BL/6 background[9]. While there is no clear consensus about which genetic background is ideal for axon regeneration studies, it is clear that the backgrounds of experimental and control groups should be well-matched.
Matching genetic background is possible with inbred mice after many generations of backcrossing. Since this can significantly delay research progress, some studies have used mixed backgrounds. However, when the targeted allele is present in a mixed background, the genetic differences between mutant and wild-type animals is not as clear as a pure background, leading to a spectrum of phenotypes which may increase the variability in the outcome measures[10]. With a single mutation, this issue can be addressed by using littermate controls; a homozygous mutant mouse is bred to a heterozygous mouse to produce offspring that are either mutants or heterozygotes, which can be used as littermate controls that should have the same genetic background. However, this strategy becomes impractical when the experiment involves compound mutants (i.e. two or more genetic deletions). An alternative strategy is to establish a new founder line(s) using compound heterozygotes to generate the wild-type, single and/or compound mutants necessary for the study. Then these mutant (and wild-type) mice can be used to establish a breeding colony that will generate the animals to be used in the study. As long as the breeding scheme is isolated from other genetic backgrounds and genetic drift is avoided, this strategy should provide a similar (but not identical) background between the different groups.
Large Animal Models
While the focus of this review is on rodent models of SCI, we briefly discuss the utility of large animal models such as cats, pigs and non-human primates. These models are not as commonly used as rodents for several reasons including size, cost, availability, housing facilities, medical care and ethics. However, they have provided valuable information on our understanding of SCI pathophysiology and have served as important preclinical models for testing new therapies. Cats have been a popular model for spinal cord electrophysiologists and have been used for decades to decipher the physiology of the normal and injured spinal cords[11, 12]. Due to their large size and greater similarity to human physiology, pig models are becoming more important as a preclinical model that is intermediate in size between rodents and humans[13, 14]. There is obvious utility in using non-human primates as a preclinical model (e.g. bipedal locomotion), but ethical reasons typically limit their use in the SCI field. Even with this limited use, non-human primate models have provided important information on anatomical plasticity[15] and behavior[16, 17], and have been instrumental in advances in brain-machine-interface devices[18]. Therefore, while the rodent is still the popular model among basic scientists, large animal models remain a necessity for finding effective therapies for SCI.
Penetrating Lesions
Dorsal Hemisection
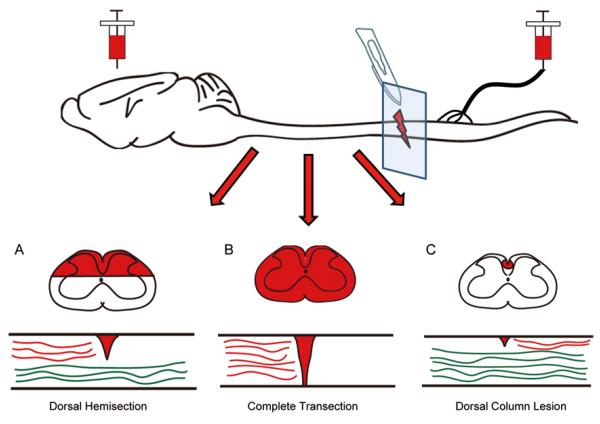
The dorsal hemisection model (Fig. 1A) has become the model-of-choice for investigating regeneration of corticospinal tract (CST) axons. In this model, the top one-half to two-thirds of the spinal cord is cut usually at the thoracic level, which injures the descending CST and rubrospinal tract (RST) axons as well as the ascending sensory axons in the dorsal columns[19]. Thus, in a typical experiment, the spinal cord lesion is followed by injections of anterograde tracers (e.g. biotinylated dextran amine) into the motor cortex (for the CST) or the red nucleus (for the RST). Ascending sensory axons are typically studied using a dorsal column lesion as described below. If performed correctly in mice, there should be virtually no detectable traced CST/RST axons caudal to the injury site under normal circumstances. This can be confirmed by observing cross-sections of the caudal spinal cord. The presence of significant numbers of traced axons caudal to the injury site in control animals confounds the interpretation of increased numbers of axons in the experimental animals; the increased number could be due to frank regeneration of severed axons that have extended down the spinal cord and/or more spared axons that display increased growth.
Fig. 1.
Diagram of different mid-thoracic penetrating SCI lesions. Coronal and sagittal views of the injury site after dorsal hemisection (A), complete transection (B) and a dorsal column lesion (C). After tracers (red) are injected into the cortex (for dorsal hemisection and complete transection) or into the peripheral nerve (for dorsal column lesion), the traced axons typically fail to regenerate across the lesion and are absent from the other side of the spinal cord. Green denotes spared axons that are not labeled by the tracer and intentionally left intact.
Another important difference between rats and mice is the detection of the ventral CST using conventional tracing methods. Injection of anterograde tracers into the motor cortex in rats labels the dorsal CST axons (the main tract in the dorsal column and the minor tract in the dorsolateral white matter) as well as the ventral CST axons located in the ventromedial white matter[20, 21]. However, for unknown reasons, the ventral CST is rarely labeled in mice[22] even though genetic labeling studies clearly demonstrate their presence[23]. Therefore, a dorsal hemisection in mice results in the caudal spinal cord being virtually devoid of any traced CST axons, whereas a similar experimental approach in rats displays many CST axons in the caudal spinal cord. Under these circumstances, a therapeutic treatment in mice that leads to increased CST axons caudal to the lesion may be interpreted as enhanced axon regeneration, whereas similar results in rats could be due to any kind of axonal growth.
Complete Transection
A complete transection of the spinal cord (Fig. 1B) is the most severe type of injury and can be considered as the most rigorous test of axon regeneration since the two stumps of the spinal cord are completely separated and no axons are spared. However, under such harsh conditions, the scar tissue may be too much of an inhibitory barrier for axons that may otherwise be able to regenerate across healthier tissue that is left after partial injuries. This is an advantage of the dorsal hemisection model described above where a bridge of non-lesioned tissue remains in the ventral white matter that may serve as a more preferable substrate for axon growth than the glial scar. Complete transections are necessary for studying axons that are distributed throughout the spinal cord, such as serotonin axons.
Dorsal Column Lesion
Dorsal column lesions (Fig. 1C) typically involve either a cut or a crush of the ascending sensory axons located in the dorsal part of the dorsal columns. Since this tract is located at the dorsal surface of the cord, it is easily accessible and can be lesioned without significantly affecting other axonal pathways. These axons can be labeled by injecting retrograde transganglionic tracers (such as cholera toxin beta) into the peripheral nerve or into the associated muscle. Alternatively, tracer can be injected directly into the dorsal root ganglia, although this may cause some damage and likely leads to a conditioning lesion response as described below.
The dorsal column sensory axons offer a unique model system in that their cell bodies, the dorsal root ganglia, have a peripheral branch as well as a central branch stemming from the same soma. Interestingly, the peripheral branch can regenerate, while the intraspinal central branch of the same neuron cannot. This supports the theory that the failure of regeneration in the CNS is due, at least in part, to the inhibitory (non-growth-permissive) nature of the CNS. Moreover, a lesion of the peripheral nerve prior to a lesion of the corresponding central axons can promote a limited degree of regeneration of the central axons[24], indicating that the peripheral lesion enhances the intrinsic state of growth that enables the central branch to regenerate. This “conditioning lesion” paradigm has served as a model system to study regeneration-associated mechanisms such as the expression of growth-associated genes[25-28]. However, the exact mechanism behind conditioning lesions remains poorly understood.
Contusion
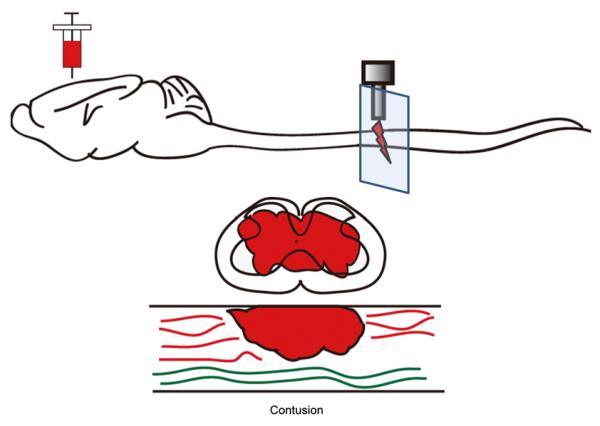
The contusion model of SCI (Fig. 2) is the preferred model for mimicking the pathophysiology that occurs most commonly in humans. Although there are various contusion models for rats and mice[29-34], the basic principle is to use an injury device to deliver a force that can be adjusted to control the contusion severity. The mechanical force that generates this type of injury leads to a lesion morphology that is very different from penetrating injuries such as those described above. In addition to the dura remaining intact, contusion results in a lesion that is typically larger than a penetrating injury and leaves a peripheral rim of spared white matter[35]. By design, lesions in penetrating injuries are typically limited to the target areas without significant damage to adjacent areas as occurs in contusive injuries. Because of their clinical relevance, most behavioral assays are based on contusive models that have become the gold-standard for pre-clinical trials of therapeutic agents after SCI.
Fig. 2.
Diagram of a mid-thoracic contusion injury. A contusion injury is typically induced by a device that impacts the surface of the spinal cord with a predetermined force. This results in a pathology where a peripheral rim of white matter is spared. In many instances, corticospinal axons are partially spared as denoted by the red axons caudal to the injury site.
While contusion injuries are useful for understanding the pathophysiology of SCI, they have some disadvantages in the study of axon regeneration. Since the biomechanical force of the injury can vary and is more difficult to predict than manual lesions, it is difficult to control which axons are severed and which are spared. For example, when performed correctly, a dorsal hemisection in mice eliminates virtually all CST axons in the caudal spinal cord. However, contusion injuries usually spare the dorsolateral CST, which could be misinterpreted as regeneration. This is also true of serotonin axons that are distributed throughout the entire spinal cord; while a complete transection is guaranteed to lesion all descending serotonin axons, even severe contusive injuries typically display serotonin axons in the caudal cord (personal observations).
One case where contusion injuries can be used to study axon regeneration is in attempts to observe axons in the actual lesion site (a region devoid of astrocytes sometimes called the GFAP-negative region) or in tissue that has been transplanted into the lesion site[36-39]. Since most axons fail to grow into the lesion, experimental manipulations that lead to axons being detected in the lesion can be interpreted as having caused regeneration. This is possible in mice since the lesion is filled with cells that can serve as a substrate, but not the case in rats where a fluid-filled cyst develops. However, in rats, transplants that promote the growth of axons into the transplant can be interpreted as axon regeneration since the original transplant was devoid of axons. Of course, this criterion also applies to any injury model involving transplants.
Pyramidotomy
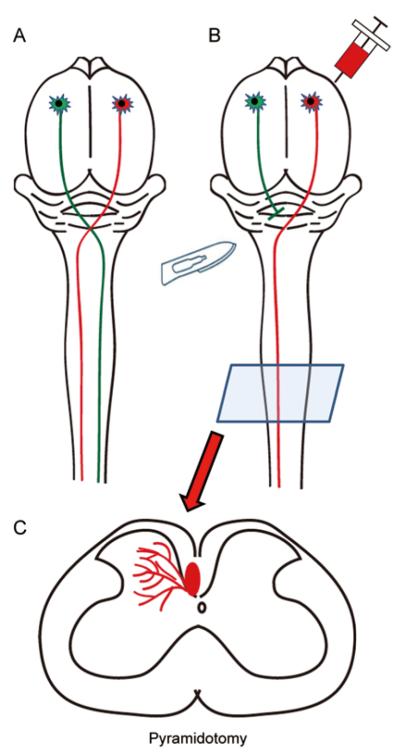
Pyramidotomy (Fig. 3) involves lesion of the CST (typically unilaterally) at the level of the pyramidal tract located at the ventral surface of the brainstem. Unlike the SCI models described above, a pyramidotomy does not lesion the spinal cord and therefore has limited utility for understanding SCI pathophysiology. However, as a CST-selective lesion, this model has been used extensively for understanding the contribution of CST axons to forelimb movements[40, 41] and as a model for promoting CST axonal growth[42-45]. After a unilateral pyramidotomy, one side of the spinal cord (contralateral to the lesion since the lesion is made before the pyramidal decussation) is virtually devoid of CST innervation while the other side is not significantly affected since CST axons in rodents typically do not innervate the spinal cord bilaterally to a significant degree. The intact CST is labeled with a tracer and the growth of these axons across the midline is observed at a specified time after injury. Since only the intact axons are labeled, this is a model to study purely axonal sprouting rather than bona fide regeneration. The completeness of the lesion can be verified by PKCγ immunohistochemistry of spinal cross-sections, which show much more robust immunostaining in the intact main CST[41, 42, 44, 45].
Fig. 3.
Diagram of a pyramidotomy model. Corticospinal axons originate from layer 5 of the motor cortex, decussate at the medullary pyramids and descend the length of the spinal cord without significant bilateral innervation in rodents (A). A pyramidotomy lesions the corticospinal axons at the level of the brainstem before the decussation so that the contralateral spinal cord is virtually devoid of corticospinal innervation (B). Injection of a tracer into the intact tract can be observed at the level of the spinal cord (C) and assessed for axonal growth into the contralateral side.
One unique feature of the pyramidotomy model is the absence of a significant glial scar, which is typically observed after SCI. While there is reported evidence of some microglial activation[46], pyramidotomy does not lead to the significant glial scar that typically forms after CNS injury. This can be advantageous because the glial scar does not serve as an inhibitory barrier to axonal growth in the pyramidotomy model, increasing the likelihood of observing a positive effect by the experimental treatment. On the contrary, lack of axonal growth in a spinal cord injury model can be attributed to either the presence of the inhibitory glial scar or the lack of an intrinsic growth mechanism. Therefore, the pyramidotomy model offers a simpler experimental model to specifically manipulate CST axons while reducing the likelihood of producing false-negatives.
In vivo Imaging
The most definitive way of verifying whether an axon has regenerated or not is to document the process while it is happening. Live images of an axon re-growing from its injured tip are irrefutable evidence of axon regeneration. But this is technically very difficult and only recently have there been the tools necessary to make significant advances in this field. In vivo imaging of single axons in the spinal cord requires animal models with appropriate fluorescent labeling. A popular choice has been to use transgenic mouse lines developed by Feng et al.[47] in which different fluorescent markers are expressed under the Thy1 promoter in specific subsets of neurons. Using the GFP-S line of these transgenic mice, Kerschensteiner et al.[48] were the first to describe the response of lesioned ascending dorsal sensory axons through repetitive imaging in vivo. Using a thin needle to sever the axons, they discovered that after a period of acute degeneration of both the proximal and distal tips, the proximal axons often grew in the wrong direction. Therefore, it seems that ascending sensory axons fail to regenerate, at least in part, due to the absence of proper navigational cues. In addition, through live imaging of axons at the dorsal root entry zone, Di Maio et al.[49] demonstrated that axons may regenerate across this border into CNS territory but stall after exhibiting presynaptic features[50].
More recently, there have been improvements in in vivo imaging of the spinal cord including spinal clamps to reduce movement artifacts from respiration, the use of two-photon microscopy, and the use of chronically-implanted glass chambers[51-54]. The use of two-photon microscopy has many advantages including deep penetration into the tissue and low phototoxicity. In the context of axon regeneration, an additional advantage is being able to use the laser to lesion axons. This provides unparalleled control of the lesion and produces minimal scarring, which may be useful in distinguishing between neuron-intrinsic and -extrinsic mechanisms of axon regeneration. This laser ablation technique has also been used in the C. elegans model of axon regeneration[55, 56]. Such rapid improvements in in vivo imaging techniques will provide unprecedented temporo-spatial resolution of axon regeneration.
So Which Model Should I Choose?
Animal models of SCI require skilled small-animal surgeons with proper knowledge of neuroanatomy as well as the ability to administer proper postoperative care such as manual bladder expression. If behavioral assessment is planned, then additional training in these techniques is required. In addition, the necessary equipment, such as a stereotaxic frame and contusion device, must be available. If any of these resources are not available, then the choice of animal models is limited. For example, if twice-daily manual bladder expression for the duration of the experiment is not logistically possible, then models such as dorsal hemisection or mid-thoracic contusion are not feasible. Or if stereotaxic injections of anterograde axonal tracers are not feasible, then the study may be limited to axons that can be detected immunohistochemically, such as serotonin axons.
The particular goals of the study are also important factors in the selection of the animal model. If the main focus is on the intrinsic mechanisms of CST growth, then the pyramidotomy model offers several advantages such as ease of animal care and absence of a glial scar. However, if the goal is to overcome the glial scar, then an injury model with a robust glial response, such as a dorsal hemisection model, is suitable. These advantages and disadvantages are summarized in Table 1.
Table 1.
Summary of the advantages and disadvantages of different injury models to study axon regeneration
| Injury model | Advantages | Disadvantages |
|---|---|---|
| Dorsal hemisection | Most common model to study CST regeneration; many hindlimb behavioral assays can be adapted |
Large variability between different surgeons |
| Complete transection | Most rigorous model to show regeneration | Injury may be too severe to observe growth; minimal behavioral recovery limits choice of assays; dedicated postoperative care required |
| Dorsal column lesion | Can be combined with conditioning lesion paradigm; distinct anatomical tract that is easily located; bladder expression not required |
Limited choice of behavioral assays |
| Pyramidotomy | Well-established CST sprouting model; does not pro- duce major glial scar; bladder expression not required |
Not clinically relevant; behavioral assays limited to fine forelimb tests |
| Contusion | Use of same device among labs; well-established behavioral assays; most clinically relevant |
Requires an injury device; difficult to distinguish between spared and regenerated axons |
| Laser ablation with two-photon imaging |
Most convincing way to show regeneration; allows study of axons in real-time; bladder expression not required |
No behavioral assays; not clinically relevant; requires advanced microscopy; technically difficult |
ACKNOWLEDGEMENTS
This review was supported by NINDS 1R01NS081040-01, 1R21NS082835-01, US Army W81XWH1010737, The Miami Project to Cure Paralysis, and Buoniconti Fund.
REFERENCES
- [1].Cafferty WB, McGee AW, Strittmatter SM. Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci. 2008;31:215–220. doi: 10.1016/j.tins.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steward O, Zheng B, Tessier-Lavigne M. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- [3].Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Inman D, Guth L, Steward O. Genetic influences on secondary degeneration and wound healing following spinal cord injury in various strains of mice. J Comp Neurol. 2002;451:225–235. doi: 10.1002/cne.10340. [DOI] [PubMed] [Google Scholar]
- [5].Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- [6].Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ma M, Wei P, Wei T, Ransohoff RM, Jakeman LB. Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J Comp Neurol. 2004;474:469–486. doi: 10.1002/cne.20149. [DOI] [PubMed] [Google Scholar]
- [8].Kostyk SK, Popovich PG, Stokes BT, Wei P, Jakeman LB. Robust axonal growth and a blunted macrophage response are associated with impaired functional recovery after spinal cord injury in the MRL/MpJ mouse. Neuroscience. 2008;156:498–514. doi: 10.1016/j.neuroscience.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dimou L, Schnell L, Montani L, Duncan C, Simonen M, Schneider R, et al. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Doetschman T. Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol. 2009;530:423–433. doi: 10.1007/978-1-59745-471-1_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rossignol S, Frigon A. Recovery of locomotion after spinal cord injury: some facts and mechanisms. Annu Rev Neurosci. 2011;34:413–440. doi: 10.1146/annurev-neuro-061010-113746. [DOI] [PubMed] [Google Scholar]
- [12].Rossignol S, Bouyer L, Langlet C, Barthelemy D, Chau C, Giroux N, et al. Determinants of locomotor recovery after spinal injury in the cat. Prog Brain Res. 2004;143:163–172. doi: 10.1016/S0079-6123(03)43016-1. [DOI] [PubMed] [Google Scholar]
- [13].Lee JH, Jones CF, Okon EB, Anderson L, Tigchelaar S, Kooner P, et al. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013;30:142–159. doi: 10.1089/neu.2012.2386. [DOI] [PubMed] [Google Scholar]
- [14].Kuluz J, Samdani A, Benglis D, Gonzalez-Brito M, Solano JP, Ramirez MA, et al. Pediatric spinal cord injury in infant piglets: description of a new large animal model and review of the literature. J Spinal Cord Med. 2010;33:43–57. doi: 10.1080/10790268.2010.11689673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rosenzweig ES, Courtine G, Jindrich DL, Brock JH, Ferguson AR, Strand SC, et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat Neurosci. 2010;13:1505–1510. doi: 10.1038/nn.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bortoff GA, Strick PL. Corticospinal terminations in two new-world primates: further evidence that corticomotoneuronal connections provide part of the neural substrate for manual dexterity. J Neurosci. 1993;13:5105–5118. doi: 10.1523/JNEUROSCI.13-12-05105.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Courtine G, Bunge MB, Fawcett JW, Grossman RG, Kaas JH, Lemon R, et al. Can experiments in nonhuman primates expedite the translation of treatments for spinal cord injury in humans? Nat Med. 2007;13:561–566. doi: 10.1038/nm1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ethier C, Oby ER, Bauman MJ, Miller LE. Restoration of grasp following paralysis through brain-controlled stimulation of muscles. Nature. 2012;485:368–371. doi: 10.1038/nature10987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]
- [20].Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- [21].Brosamle C, Schwab ME. Ipsilateral, ventral corticospinal tract of the adult rat: ultrastructure, myelination and synaptic connections. J Neurocytol. 2000;29:499–507. doi: 10.1023/a:1007297712821. [DOI] [PubMed] [Google Scholar]
- [22].Steward O, Zheng B, Ho C, Anderson K, Tessier-Lavigne M. The dorsolateral corticospinal tract in mice: an alternative route for corticospinal input to caudal segments following dorsal column lesions. J Comp Neurol. 2004;472:463–477. doi: 10.1002/cne.20090. [DOI] [PubMed] [Google Scholar]
- [23].Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11:1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- [24].Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- [25].Hoffman PN. A conditioning lesion induces changes in gene expression and axonal transport that enhance regeneration by increasing the intrinsic growth state of axons. Exp Neurol. 2010;223:11–18. doi: 10.1016/j.expneurol.2009.09.006. [DOI] [PubMed] [Google Scholar]
- [26].Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, et al. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–5573. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Blesch A, Lu P, Tsukada S, Alto LT, Roet K, Coppola G, et al. Conditioning lesions before or after spinal cord injury recruit broad genetic mechanisms that sustain axonal regeneration: superiority to camp-mediated effects. Exp Neurol. 2012;235:162–173. doi: 10.1016/j.expneurol.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Stam FJ, MacGillavry HD, Armstrong NJ, de Gunst MC, Zhang Y, van Kesteren RE, et al. Identification of candidate transcriptional modulators involved in successful regeneration after nerve injury. Eur J Neurosci. 2007;25:3629–3637. doi: 10.1111/j.1460-9568.2007.05597.x. [DOI] [PubMed] [Google Scholar]
- [29].Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–126. doi: 10.1089/neu.1992.9.123. discussion 126–128. [DOI] [PubMed] [Google Scholar]
- [30].Wrathall JR, Pettegrew RK, Harvey F. Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol. 1985;88:108–122. doi: 10.1016/0014-4886(85)90117-7. [DOI] [PubMed] [Google Scholar]
- [31].Stokes BT. Experimental spinal cord injury: a dynamic and verifiable injury device. J Neurotrauma. 1992;9:129–131. doi: 10.1089/neu.1992.9.129. discussion 131–124. [DOI] [PubMed] [Google Scholar]
- [32].Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG, et al. Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma. 2000;17:299–319. doi: 10.1089/neu.2000.17.299. [DOI] [PubMed] [Google Scholar]
- [33].Scheff SW, Rabchevsky AG, Fugaccia I, Main JA, Lumpp JE., Jr Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma. 2003;20:179–193. doi: 10.1089/08977150360547099. [DOI] [PubMed] [Google Scholar]
- [34].Nishi RA, Liu H, Chu Y, Hamamura M, Su MY, Nalcioglu O, et al. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J Neurotrauma. 2007;24:674–689. doi: 10.1089/neu.2006.0204. [DOI] [PubMed] [Google Scholar]
- [35].Noble LJ, Wrathall JR. Spinal cord contusion in the rat: morphometric analyses of alterations in the spinal cord. Exp Neurol. 1985;88:135–149. doi: 10.1016/0014-4886(85)90119-0. [DOI] [PubMed] [Google Scholar]
- [36].Li W, Cai WQ, Li CR. Repair of spinal cord injury by neural stem cells modified with BDNF gene in rats. Neurosci Bull. 2006;22:34–40. [PubMed] [Google Scholar]
- [37].Barakat DJ, Gaglani SM, Neravetla SR, Sanchez AR, Andrade CM, Pressman Y, et al. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- [38].Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, et al. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- [39].Gao M, Lu P, Bednark B, Lynam D, Conner JM, Sakamoto J, et al. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials. 2013;34:1529–1536. doi: 10.1016/j.biomaterials.2012.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Whishaw IQ, Pellis SM, Gorny B, Kolb B, Tetzlaff W. Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav Brain Res. 1993;56:59–76. doi: 10.1016/0166-4328(93)90022-i. [DOI] [PubMed] [Google Scholar]
- [41].Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, et al. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Expl Neurol. 2005;195:524–539. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- [42].Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thallmair M, Metz GA, Z’Graggen WJ, Raineteau O, Kartje GL, Schwab ME. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- [44].Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Leong SK, Ling EA, Fan DP. Glial reaction after pyramidotomy in mice and rats. Neurodegeneration. 1995;4:403–413. doi: 10.1006/neur.1995.0049. [DOI] [PubMed] [Google Scholar]
- [47].Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- [48].Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- [49].Di Maio A, Skuba A, Himes BT, Bhagat SL, Hyun JK, Tessler A, et al. In vivo imaging of dorsal root regeneration: rapid immobilization and presynaptic differentiation at the CNS/PNS border. J Neurosci. 2011;31:4569–4582. doi: 10.1523/JNEUROSCI.4638-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Han SB, Kim H, Skuba A, Tessler A, Ferguson T, Son YJ. Sensory Axon Regeneration: A Review from an in vivo imaging Perspective. Exp Neurobiol. 2012;21:83–93. doi: 10.5607/en.2012.21.3.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Farrar MJ, Bernstein IM, Schlafer DH, Cleland TA, Fetcho JR, Schaffer CB. Chronic in vivo imaging in the mouse spinal cord using an implanted chamber. Nat Methods. 2012;9:297–302. doi: 10.1038/nmeth.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Davalos D, Lee JK, Smith WB, Brinkman B, Ellisman MH, Zheng B, et al. Stable in vivo imaging of densely populated glia, axons and blood vessels in the mouse spinal cord using two-photon microscopy. J Neurosci Methods. 2008;169:1–7. doi: 10.1016/j.jneumeth.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Fenrich KK, Weber P, Hocine M, Zalc M, Rougon G, Debarbieux F. Long-term in vivo imaging of normal and pathological mouse spinal cord with subcellular resolution using implanted glass windows. J Physiol. 2012;590:3665–3675. doi: 10.1113/jphysiol.2012.230532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Davalos D, Akassoglou K. In vivo imaging of the mouse spinal cord using two-photon microscopy. J Vis Exp. 2012:e2760. doi: 10.3791/2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- [56].Chen L, Wang Z, Ghosh-Roy A, Hubert T, Yan D, O’Rourke S, et al. Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron. 2011;71:1043–1057. doi: 10.1016/j.neuron.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]