Abstract
Adoptive immunotherapy—in particulary T cell therapy—has recently emerged as a useful strategy with the potential to overcome many of the limitations of antiviral drugs for the treatment of viral complications after hematopietic stem cell transplantation (HSCT). In this review we briefly summarize the current methods for virus- specific T cell isolation or selection and we report results from clinical trials employing these techniques, focusing specifically on the strategies aimed to broaden the application of this technology.
Keywords: Stem Cell Transplantation, Virus, T cell, Immunotherapy
INTRODUCTION
Allogenic Hematopoietic stem cell transplantation (HSCT) has emerged as one of the best therapeutic options available for many patients with malignant and non-malignant diseases involving the hematopoietic system. The use of donors other than HLA-matched siblings requires the depletion of host-attacking donor T cells in order to prevent graft versus host disease (GVHD). The broader use of alternative stem cell donor sources, such as unrelated donors, haploidentical related donors and umbilical cord blood (CB) have, however, resulted in an increased incidence of viral infections due to the T cell depletion strategies required to prevent GVHD. As a result, infection is one of the main causes of transplant-related mortality and morbidity in this setting.(1)
Cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (Adv) infections are particularly frequent among HSCT recipients and are often described as important risk factors affecting prognosis post-HSCT.(2–4) Although the introduction of sensitive viral screening techniques and pre-emptive treatment strategies have reduced mortality related to these complications, current antiviral drugs have some important limitations. First, depending on the drug, antiviral pharmacotherapy can result in bone marrow suppression and substantial toxicities(5, 6) that are difficult to manage in patients who have undergone intense chemotherapy and radiation. Secondly, effective antiviral drugs do exist for CMV and EBV and can be beneficial, but the effectiveness of these agents in patients with Adv infection has only been suggested by non-randomized and uncontrolled clinical trials, and in our experience they are often not effective.(7) Antiviral drugs – especially those used for CMV – can lead to late-onset CMV disease. Once the antiviral pharmacotherapy is removed, the late-onset CMV may be worse than the original reactivation since the use of these agents can delay virus-specific immune recovery.(2) As a result, patients developing viral complications may require multiple treatment courses, which is not only expensive, but drug resistance may also occur. In the case of CMV, 94% of strains resistant to the common antiviral drug ganciclovir are due to mutations in the UL97 gene. Further, Nichols et al reported that despite the use of antiviral drugs, about one third of transplant recipients had increasing viral loads after initiating antiviral therapy (8) Hence, one of the most attractive and innovative approaches to overcome the limits of current antiviral pharmacotherapies is adoptive immunotherapy using virus specific T cells.
CURRENT METHODOLOGIES FOR VIRUS-SPECIFIC T CELL GENERATION OR SELECTION
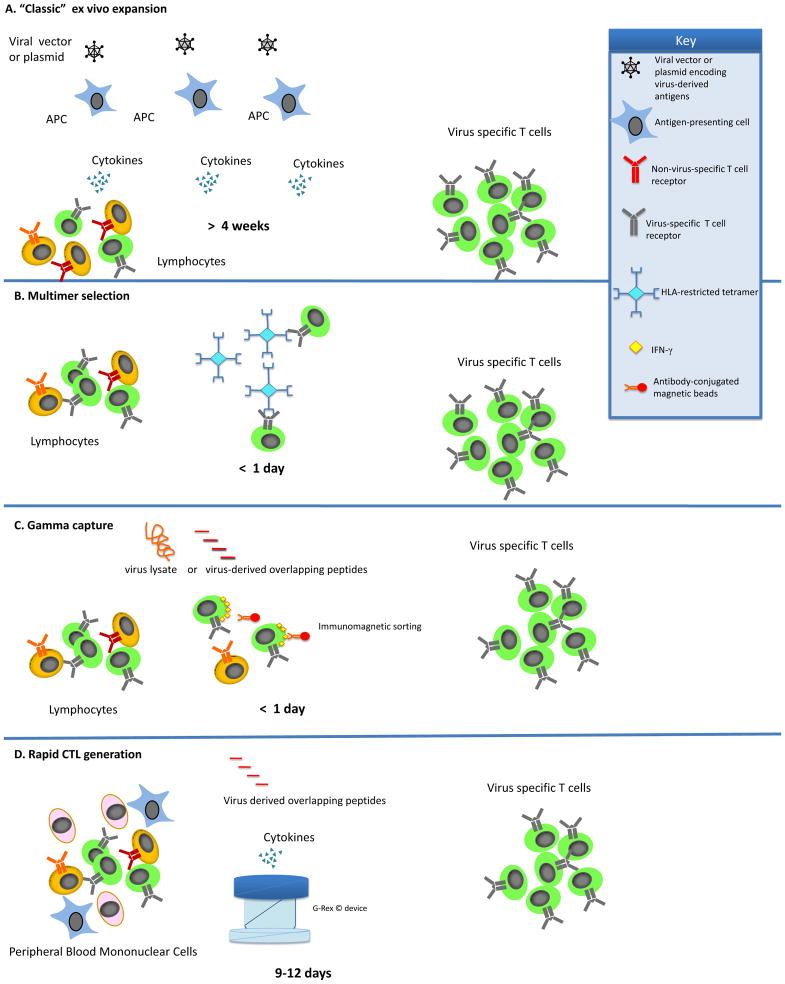
Over the past 20 years, there has been an increasing use of virus specific adoptive T cell therapies where donor-derived virus-specific T lymphocytes are administered to patients with the primary goal of counteracting the effects of uncontrolled viral replication in immunosuppressed patients after HSCT. In order to optimize this approach, numerous in vitro studies have been conducted by various groups in an attempt to identify the best methodology for the expansion or selection of virus-specific T lymphocytes for clinical use (Figure 1 and Table 1).(9–15)
Figure 1. GMP-Applicable Approaches for the Generation of Virus-Specific T cells.
a) In the “classic” ex vivo-expansion, T cells are combined with antigen presenting cells (APC) that have been transduced with either a viral vector or plasmids encoding the antigens of interest. The antigen presenting cells are used to stimulate the T cells until cells of sufficient specificity and number have been expanded. b) To prepare virus-specific T cells using multimers, T cells are incubated with multimers that mimic the peptide:MHC binding of an APC. The T cells that bind the multimer are then isolated using magnetic beads or fluorescence-activated cell sorting (FACS). c) In the gamma-capture technique, T cells are activated using the peptide of interesting to stimulate the T cells. Once the T cells are stimulated, antibodies bind IFN-γ and the T cell allowing the T cells to be isolated by magnetic selection. d)To improve upon the “classic” ex vivo expansion system, the rapid system utilizes the APCs present in the peripheral blood mononuclear cells (PBMC). The PBMCs are pulsed with overlapping peptides representing the viral antigens(s) of interest. APCs pulsed with the peptides then stimulate the T cells to grow. When coupled with a G-rex gas permeable culture device, these CTL are ready 9–12 days after initiation.
Table 1.
Advantages and disadvantages of various methods of virus-specific T cell generation
| Reference | Method | Advantages | Disadvantages |
|---|---|---|---|
| Einsele 2002(18). Peggs 2009(19) |
Stimulation of PBMC with virus lysate |
|
|
| Cobbold, 2005(9) | Tetramer selection |
|
|
| Leen, 2006(11) | GMP-grade adenoviral-transduction of APCs to stimulate CTL |
|
|
| Gerdemann, 2009(12) | Nucleofection of APCs used to stimulate CTL |
|
|
| Hanley, 2009(14) | GMP-grade adenoviral-transduction of APCs to stimulate CTL |
|
|
| Peggs, 2011(10) | Selection of IFNγ-secreting T cells |
|
|
| Gerdemann, 2012(13) | Direct stimulation of PBMC with peptides |
|
|
CTL: cytotoxic T lymphocytes, APC: antigen-presenting cell, CMV: cytomegalovirus, EBV: Epstein-Barr virus, AdV: adenovirus
The first experiences using antiviral adoptive immunotherapy used T cells expanded with CMV-infected fibroblasts.(16, 17) or CMV lysate(18, 19). Although effective, this made them difficult to export because of the regulatory hurdles required for such a production. Indeed, the expansion of virus-specific T cells often requires clean rooms, quality control, quality assurance, release testing, and documentation to meet current good manfuacturing practices (cGMP) compliance. One of the first cGMP-compliant strategies reported for the manufacture of virus specific T cells was the selection of virus-specific T cells from bulk donor’s T lymphocytes by tetramer selection.(9) The advantages of this method were the rapid availability of the T cells and the ease of the selection process, which does not require antigen-presenting cells, exogenous cytokines, or extended ex vivo manipulation and can be performed using closed-system devices outside of a dedicated clean room or GMP facility. However, tetramer-mediated selection only selects T cells specific for a single HLA restricted epitope of a single virus (in this case CMV) and is generally only available for donors with the most common of Human Leukocyte Antigen (HLA) types. While sometimes effective, focusing the antiviral response to one epitope leaves the patient vulnerable to antigenic escape as has been observed clinically for EBV.(20, 21)
Another method to isolate virus-specific T cells is by immunomagnetically selecting T cells that secrete IFN-γ in response to virus-derived overlapping peptides.(10, 22) This technique is advantageous because the cells are rapidly available and do not require extensive manipulation while still targeting entire antigens or viruses, depending on the stimuli. However, the selection of unexpanded T cells has been associated with GvHD and as with the tetramer technology, this option is currently only available for donors who are seropositive for the virus being targeted.
Another GMP-applicable method to generate virus-specific T cells involves the stimulation of peripheral blood mononuclear cells (PBMC) with antigen-presenting cells (APCs). This approach was investigated in the 1990’s to generate Epstein-Barr Virus (EBV)-specific Cytotoxic T cells (CTL) by stimulating PBMC with EBV-transformed lymphoblastoid cell lines (LCL)(23) and was later modified to include a first stimulation with dendritic cells transduced with clinical grade adenoviral vectors expressing viral antigens for EBV or CMV thus expanding the antiviral specificity of the CTL. (11)
Furthermore, CTL expanded in this way enable T cells to recognize three viruses (EBV (from the LCL), CMV (from the engineered adenoviral vector), and adenovirus (from the adenoviral vector)) in a single culture with a very high specificity starting from a relative low blood volume (50–60 mL). The limitation of this approach is that it is time consuming (up to 3 months), requires the use of a clinical grade viral vector which is expensive, and can be a major regulatory hurdle.
To remove the need for viral vectors, more recent approaches have used dendritic cells which were either nucleofected with plasmid DNA encoding different viral antigens or pulsed with overlapping peptides for viral antigens to stimulate and expand multi-virus specific T cells. After only a single stimulation (a total of about 10–17 days) the CTL were frozen and ready for use pending the release testing.(12);(13)
Despite the manufacturing advances made for the generation of virus-specific T cells, none of the approaches described above are able to expand virus-specific T cells from donors that are virus-seronegative. This is a limitation since one of the biggest risks for viral infection (excluding immune suppression) is when the graft doesn’t contain a specific T memory compartment (such as in cord blood or seronegative adult donors) and the recipient is latently infected by these pathogens (24–26) To address this unmet need, several groups have evaluated strategies to stimulate the naïve T cells present in cord blood.(27–31) Further, using the G-Rex gas permeable device,(32) it was possible to expand cord blood-derived T cells to numbers sufficient for clinical use, demonstrating for the first time that it is possible to generate multivirus-specific T cells in a virus-inexperienced setting in a cGMP-applicable manner.(14, 33)
CLINICAL EXPERIENCES WITH VIRUS-SPECIFIC CTL
Treating cytomegalovirus
The first clinical protocols whereby CMV specific T cells were cultured from hematopoietic stem cells donors and then transferred to the recipients were successfully performed in the early 1990’s: Seattle group treated 14 HSCT recipients with CMV specific clones generated from stem cell donors observing neither CMV viremia increase or CMV disease in any of them.(16, 17)
In an attempt to more rapidly generate CMV specific T cells several groups have explored strategies which limit the ex vivo expansion time. Peggs and colleagues from the University College of London generated CMV-specific T cells by selecting Interferon-γ secreting T cells after exposure to viral antigens. They treated 18 matched related donor (MRD) and matched unrelated donor (MUD) HSCT recipients both as prophylaxis and as pre-emptive therapy. The results of this trial were promising for patients treated as prophylaxis (6/7 patients didn’t experience CMV re-activation after CMV-specific T cell infusion), but this approach seemed to be less efficient in clearing ongoing infections, as 9 out of 11 patients treated pre-emptively still required additional antiviral drugs. Moreover, in this trial GvHD was observed in multiple patients, probably as a consequence of selecting highly activated T cells.(10) (Tables 3 and 4)
Table 3.
T cell therapy for cytomegalovirus infection/reactivation after stem cell transplant
| Reference | n | HSCT type | Strategy | End points | Results |
|---|---|---|---|---|---|
| Cobbold, 2005(9) | 9 | MRD, MUD | Pre-emptive therapy | Safety Efficacy CTL persistence |
|
| Leen, 2006(11) and Hanley 2013(15) | 34 | MRD, MUD, haplo | Prophylaxis | Safety Efficacy CTL expansion |
|
| Peggs, 2011(10) | 18 | MRD, MUD | Prophylaxis Pre- emptive therapy | Safety Efficacy CTL expansion |
|
| Hanley, 2012(51) | 7 | cord blood | Prophylaxis Treatment | Safety Efficacy CTL expansion |
|
| Blyth, 2013(49) | 50 | MRD, MUD | Prophylaxis | Safety Efficacy |
|
MRD: matched related donor, MUD: matched unrelated donor, haplo: haploidentical HSCT, CTL: cytotoxic T lymphocytes, aGvHD: acute graft-versus-host disease, cGvHD: chronic graft-versus-host disease.
Table 4.
T cell therapy for adenovirus infection after stem cell transplant
| Reference | n | HSCT type | Strategy | End points | Results |
|---|---|---|---|---|---|
| Feuchtinger, 2006(38) | 9 | MRD, MUD, MMUD, haplo | Treatment (refractory/unresponsive to antiviral drugs) | Safety Efficacy CTL expansion |
|
| Leen, 2006(11) and Hanley 2013(15) | 34 | MRD, MUD, haplo | Prophylaxis | Safety Efficacy CTL expansion |
|
| Leen, 2009(46) | 13 | MUD, haplo | Prophylaxis (12) Treatment (1) |
Safety Efficacy CTL expansion |
|
| Hanley, 2012(51) | 7 | cord blood | Prophylaxis Treatment | Safety Efficacy CTL expansion |
|
MRD: matched related donor, MUD: matched unrelated donor, haplo: haploidentical HSCT, CTL: cytotoxic T lymphocytes, GvHD: graft-versus-host disease, AdV: adenovirus.
The use of tetramer-selected T cells was first reported by Cobbold and colleagues. They treated nine patients who had undergone matched-related donor (MRD) and matched unrelated donor (MUD) HSCTs and who had CMV re-activation after HSCT. After the administration of tetramer-selected CMV specific T cells, eight of 9 patients cleared the virus but 2 cases of GvHD were observed.(9) These data are confirmed by several other clinical trials showing that this therapy is safe and often able to overcome some of the limitations of antiviral drugs.(34, 35) In one recent study, Qasim et al treated pediatric patients by selecting IFN-γ-secreting T cells after exposure to Hexon. However, two of five patients did not have detectable hexon-specific T cells and third party donors were needed. (36)
Treating adenovirus
Feuchtinger and colleagues reported the first experience where AdV specific T cells were used to treat AdV infection in patients undergoing HSCT.(37, 38) In their clinical trial 9 patients underwent MRD, MUD, mismatched unrelated donor (MMUD) and haplo HSCT and developed either refractory AdV infection or were unresponsive to antiviral drugs; these patients were treated with AdV specific T cells: 5 cleared viremia spontaneously and only 1 case of already established GvHD re-aggravation was reported.
Treating Epstein-Barr virus
The opportunity to readily activate EBV specific T cells from healthy EBV-seropositive HSCT donors using LCL as APCs and the seriousness of this infection in HSCT recipients (Post Transplant Lymphoproliferative Disease, PTLD) also made EBV one of the first targets suitable for adoptive immunotherapy.(39, 40) (Table 2) In a multi-institutional study enrolling 114 patients undergoing MRD, MUD and haplo HSCT, none of the 101 patients who received donor-derived EBV-specific CTLs both as prophylaxis and as pre-emptive therapy developed PTLD and no cases of de novo GvHD occurred after CTL infusion.(41) Historically, in patients who received T cell depleted grafts with similar conditioning regimens, the rate of PLTD was approximately 11%. Further, of the 13 patients who were treated for overt PTLD, 11 achieved complete remissions that were sustained without recurrence. The most significant adverse effects seen were localized, but reversible, and included swelling at sites of disease during the therapeutic response in four patients with bulky disease at the time of T cell therapy. In addition, the group at MSKCC reported the results of another large study using EBV specific CTLs for the treatment of PTLD: 47 HSCT patients were treated for PTLD with donor or third party derived EBV CTLs with an overall response rate of 68% without evidence of de novo or recurrent GvHD.(42) Other smaller studies reported similar results in terms of both efficacy and safety of EBV-specific CTLs obtained by LCL stimulation(43, 44). Hence the adoptive transfer of EBV-specific T cells is a safe and effective strategy both in the prophylaxis or therapeutic setting.(45)
Table 2.
T cell therapy studies for EBV reactivation and post-transplant lymphproliferative disorder occurring after HSCT.
| Reference | n | HSCT type | Strategy | End points | Results |
|---|---|---|---|---|---|
| Gustafsson, 2000(43) | 9 | MRD, MUD, MMUD | Pre-emptive therapy | Antiviral effect |
|
| Leen, 2006(11) and Hanley 2013(15) | 34 | MRD, MUD, haplo | Prophylaxis Pre-emptive therapy PTLD treatment |
Safety Efficacy CTL expansion |
|
| Comoli, 2007(44) | 4 | haplo | Prophylaxis | Safety Efficacy |
|
| Leen, 2009(46) | 12 | haplo, MUD | Prophylaxis | Safety Efficacy |
|
| Heslop, 2010(45) | 114 | MRD, MUD, haplo | Prophylaxis (101 patients) PTLD treatment (13 patients) |
Safety Efficacy |
|
| Hanley, 2012(51) | 7 | cord blood | Prophylaxis Treatment | Safety Efficacy CTL Expansion |
|
MRD: Matched related donor, MUD: matched unrelated donor, MMUD: mismatched unrelated donor, haplo: haploidentical stem cell transplant, PTLD: post-transplant lymphoproliferative disease, CTL: cytotoxic T lymphocytes, GvHD, graft-versus-host disease.
Treating multiple viruses
To further broaden the specificity of CTL several groups have explored the use of multi-virus specific T cells including T cells targeting EBV and Adv(46) or CMV and Adv(47) or CMV, Ad and EBV.(11, 48) One large study from Australia demonstrated the efficacy and the safety of infusing bi-virus specific T cells to 50 MRD and MUD HSCT recipients. These patients received T cells specific for CMV (+/− Adenovirus) as prophylaxis and observed the same incidence of GvHD and a statistically significant reduced incidence of CMV re-activation compared to a cohort of patients who received the same HSCT protocol but did not receive CMV specific T cells.(49) To further broaden the specificity of CTL, the group at Baylor College of Medicine expanded T cells targeting CMV, EBV and adenovirus in a single culture. Among the 33 MRD, MUD and haploidentical (Haplo) HSCT patients who received CMV, EBV and AdV specific T cells they described 8 cases of CMV re-activation, 11 cases of Adv infection and 10 cases of EBV reactivation/PTLD.
All viral infections resolved post CTL therapy. EBV-and CMV-specific T cells were detected in the peripheral blood post infusion in almost all patients treated. However, AdV specific T cells were found only in patients who developed AdV infection. Importantly however, no cases of GvHD were observed despite the fact that the majority of the patients were recipients of alternate donor grafts.(11, 41)
In addition to CMV, EBV and Adv, HSCT recipients are also susceptible to other viral infections such as BK virus, JC, HHV6, HHV7, influenza, parainfluenza, coronavirus, and RSV, all of which may cause severe morbidity and mortality.(50) In order to extend this approach to others viruses, the group at Baylor College of Medicine described a method by which it is possible to rapidly generate a single preparation of polyclonal (CD4+ and CD8+) T cells which are specific for seven viruses (EBV, CMV, Adv, BK, HHV6, RSV, and Influenza) frequently described as important risk factors affecting prognosis post HSCT.(13) These broadly virus specific T cells are now been evaluated clinically (ClinicalTrials.gov Identifier NCT01570283).
In all the above-mentioned clinical trials, one of the inclusion criteria was the availability of a seropositive donor. The clinical experience of T cell adoptive immunotherapy from seronegative donors is still limited, but cord blood-derived CTLs are currently being tested in a Phase I clinical trial. Of nine patients receiving multivirus-(CMV, EBV and AdV) specific CTL, only 3 patients developed viral reactivations. One patient had both CMV reactivation and adenovirus infection. After T cell infusion, there was an increase in CMV-specific T cells detected in the peripheral blood that coincided with a decrease in CMV viral load. The patient also successfully cleared the adenovirus infection without additional antiviral therapy. Two patients had EBV reactivation or infection either before or soon after CTL infusion that was controlled without additional therapy coinciding with the detection of EBV-specific T cells in the peripheral blood.(51)
Strategies to broaden the application of antiviral adoptive immunotherapy
The widespread use of T cells to prevent or treat viral complications occurring after HSCT is mainly limited by their relatively long production time and by the complexity of the production process itself that limits the therapy to a relatively small number of Centers. Several groups have investigated different approaches to overcome these limitations and several strategies have emerged as potentially suitable for clinical investigation or are already being investigated in ongoing studies. Tetramer selection and IFNγ-secreting T cell isolation are by themselves two encouraging approaches in terms of handiness and reproducibility, but as mentioned above they still have some pitfalls that emerged when these approaches were tested clinically.(9, 10, 52–54)
Another potential strategy to broaden the application of virus-specific T cell is the use of third party T cells. The generation and the banking of HLA-typed virus-specific T cell lines ready to be infused could represent an option for patients suitable for antiviral T cell based immunotherapy but for whom the clinical condition requires a prompt intervention (Table 5). This “off-the-shelf” approach was initially evaluated by Haque and colleagues who treated 33 patients (HSCT recipients and solid organ transplantation recipients) with third party EBV specific CTL for PTLD. They observed overall response rates of 64% at 5 weeks, and 52% at 6 months with no cases of GvHD or graft rejection. The best results were observed in patients who received donor T cells that were best-HLA matched with the recipient.(55) The Memorial Sloan Kettering Group also confirmed the safety of this strategy describing their results comparing the use of third party EBV specific CTLs with the use of Donor Lymphocytes Infusion (DLI) in patients who developed PTLD after CB HSCT. While the response rate was similar in both groups, GvHD occurred more frequently in patients treated with DLI.(56) In 2011 Quasim and colleagues also described the results of a trial enrolling patients developing AdV infection after MUD HSCT treated with third party AdV specific T cells highlighting a high risk of GvHD development, but in this case T cells were generated using the IFNγ capture technology which may increase the risk for the presence of alloreactive T cells in the infused product.(57)
Table 5.
T cell therapy using third-party CTL for viral infections post-stem cell transplant
| Reference | n | Target | Type of HSCT | Serious Adverse Events | Results |
|---|---|---|---|---|---|
| Barker, 2010(56) and Doubrovina, 2012(42) | 5 | EBV | Cord blood | None |
|
| Uhlin, 2010(61) | 1 | EBV | Cord blood | None |
|
| Leen, 2010(62) and Leen, 2012(63) | 44 | EBV, CMV, AdV | MRD, MUD, Cord blood |
|
|
| Qasim, 2011(57) | 1 | Adv | MMUD | Grade II–IV GvHD (skin, liver) |
|
EBV: Epstein-Barr virus, CMV: cytomegalovirus, AdV: adenovirus, MRD: Matched related donor, MUD: matched unrelated donor, MMUD: mismatched unrelated donor, CTL: cytotoxic T lymphocytes, GvHD, graft-versus-host disease.
Finally, a recent multi-center study used banked third-party multi-virus specific T cells and showed that this is a feasible and safe approach to rapidly treat multiple viral infections occurring after HSCT. This study showed that a clinical effect is possible even when the CTL donor and transplant recipient only share one single HLA locus. The caveat is that the shared allele must be known to present a known and effective epitope of the virus meaning that extensive in vitro characterization of the CTL lines must be done by laboratories with extensive expertise.(58) Nevertheless, this approach resulted in a 74% complete or partial response rate. Importantly, 50 patients with an array of HLA types were successfully treated using only 18 cell lines. Further, this approach demonstrated that an in-depth characterization of the CTL product not only serves as a measure of potency, but can also be used to customize a treatment for each patient using the banked T cells.
MOVING BEYOND PHASE 1
The age of antiviral immunotherapy being a “boutique” therapy is, hopefully, coming to an end. Since the first clinical trial testing the efficacy of these cells, advances have been trending towards making the technology faster, more stream-lined, standardized, and more affordable. To move beyound phase I/II, later phase clinical trials are typically sponsored by large pharmaceutical companies interested in marketing their product and this type of partnership is essential for cell therapy to continue to move beyond early phase clinical trials. From an academic perspective, laboratories need to continue to innnovate and make laborious procceses more efficient, cost effective, and more broadly applicable. Beyond processing, alternative transduction methods such as transposons and overlapping peptides will need to be optimized to limit the use of viral vectors.(59)
Finally, although third-party CTL appear to be the most appealing way to manufacture CTL and administer them to patients, these CTL will only become the standard of care if manufacturing technologies continue to improve. The use of the gas permeable culture device technology represents a signficiant advance for the rapid expansion of T cells, but these devices still require multiple manipulations that are exposed to the environment (increasing the risk of contamination) and need to be performed in biosafety cabinets. Large-scale cultures bags and devices are now being produced,(60) but whether they can support complex expansions like those required for antigen-specific T cells remain to be tested.
CONCLUSION
With improved detection techniques and the introduction of pre-emptive strategies the prognosis for patients developing viral complications after HSCT has improved but pharmacotherapeutic agents still have some important pitfalls. One of most investigated and promising approaches to overcome some of the limitations of antiviral drugs is adoptive immunotherapy using virus-specific T cells. At the moment different virus-specific T cell isolation/generation techniques are available: each of them has its own advantages and limitations and none of them has been shown to be superior in pre-clinical studies. In order to move virus specific T cell therapy to the “mainstream,” larger studies are necessary to simplify the manufacturing process and to extend the availability to all HSCT recipients irrespective of their donor status. Rapid T cell generation and third party derived T cell infusion are being currently investigated as potential solution to overcome some of the limitations regarding availability, but in the meantime, other solutions (i.e. CTL generation from virus naïve sources) should also continue to be tested in order to further broaden the application of this approach.
Acknowledgments
This work was supported by Solidarity Banks Onlus awarded to FS, a postdoctoral fellowship, PF-13-046-01-LIB, from the American Cancer Society awarded to PJH, and CPRIT RO1 RP100469, and NCI PO1 CA148600-02 awards to CMB.
ABBREVIATIONS
- HSCT
Hematopoietic stem cell transplantation
- GVHD
graft versus host disease
- CMV
Cytomegalovirus
- EBV
Epstein-Barr virus
- Adv
Adenovirus
- HLA
Human Leukocyte Antigen
- cGMP
Good Manfuacturing Practices
- PBMC
peripheral blood mononuclear cells
- APCs
antigen-presenting cells
- CTL
cytotoxic T cells
- LCL
EBV-transformed lymphoblastoid cell lines
- MRD
matched related donor
- MUD
matched unrelated donor
- MMUD
mismatched unrelated donor
- PTLD
post transplant lymphoproliferative disease
- Haplo
haploidentical hematopoietic stem cell transplantation
- DLI
donor lymphocytes infusion
Footnotes
CONFLICT OF INTEREST STATEMENTS
The authors declare no conflict of interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kennedy-Nasser AA, Bollard CM, Myers GD, Leung KS, Gottschalk S, Zhang Y, et al. Comparable outcome of alternative donor and matched sibling donor hematopoietic stem cell transplant for children with acute lymphoblastic leukemia in first or second remission using alemtuzumab in a myeloablative conditioning regimen. Biol Blood Marrow Transplant. 2008 Nov;14(11):1245–1252. doi: 10.1016/j.bbmt.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003 Jan 15;101(2):407–414. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006 Oct 15;108(8):2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myers GD, Krance RA, Weiss H, Kuehnle I, Demmler G, Heslop HE, et al. Adenovirus infection rates in pediatric recipients of alternate donor allogeneic bone marrow transplants receiving either antithymocyte globulin (ATG) or alemtuzumab (Campath) Bone Marrow Transplant. 2005 Dec;36(11):1001–1008. doi: 10.1038/sj.bmt.1705164. [DOI] [PubMed] [Google Scholar]
- 5.Salzberger B, Bowden RA, Hackman RC, Davis C, Boeckh M. Neutropenia in allogeneic marrow transplant recipients receiving ganciclovir for prevention of cytomegalovirus disease: risk factors and outcome. Blood. 1997 Sep 15;90(6):2502–2508. [PubMed] [Google Scholar]
- 6.Kumar D, Humar A. Cytomegalovirus prophylaxis: how long is enough? Nat Rev Nephrol. 2010 Jan;6(1):13–14. doi: 10.1038/nrneph.2009.207. [DOI] [PubMed] [Google Scholar]
- 7.Matthes-Martin S, Feuchtinger T, Shaw PJ, Engelhard D, Hirsch HH, Cordonnier C, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011) Transpl Infect Dis. 2012 Dec;14(6):555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 8.Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M, et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood. 2001 Feb 15;97(4):867–874. doi: 10.1182/blood.v97.4.867. [DOI] [PubMed] [Google Scholar]
- 9.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005 Aug 1;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peggs KS, Thomson K, Samuel E, Dyer G, Armoogum J, Chakraverty R, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011 Jan 1;52(1):49–57. doi: 10.1093/cid/ciq042. [DOI] [PubMed] [Google Scholar]
- 11.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Oct;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 12.Gerdemann U, Christin AS, Vera JF, Ramos CA, Fujita Y, Liu H, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009 Sep;17(9):1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdemann U, Keirnan JM, Katari UL, Yanagisawa R, Christin AS, Huye LE, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012 Aug;20(8):1622–1632. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009 Aug 27;114(9):1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanley PJ, Leen AM, Gee AP, Leung K, Martinez C, Krance R, et al. MULTI-VIRUS-SPECIFIC T-CELL THERAPY FOR PATIENTS AFTER HEMATOPOIETIC STEM CELL AND CORD BLOOD TRANSPLANTATION. ASH Annual Meeting Abstracts 2013 [Google Scholar]
- 16.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992 Jul 10;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 17.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995 Oct 19;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 18.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002 Jun 1;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 19.Peggs KS, Verfuerth S, Pizzey A, Chow SL, Thomson K, Mackinnon S. Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin Infect Dis. 2009 Dec 15;49(12):1851–1860. doi: 10.1086/648422. [DOI] [PubMed] [Google Scholar]
- 20.Hanley PJ, Shaffer DR, Cruz CR, Ku S, Tzou B, Liu H, et al. Expansion of T cells targeting multiple antigens of cytomegalovirus, Epstein-Barr virus and adenovirus to provide broad antiviral specificity after stem cell transplantation. Cytotherapy. 2011 Sep;13(8):976–986. doi: 10.3109/14653249.2011.575356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottschalk S, Ng CY, Perez M, Smith CA, Sample C, Brenner MK, et al. An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood. 2001 Feb 15;97( 4):835–843. doi: 10.1182/blood.v97.4.835. [DOI] [PubMed] [Google Scholar]
- 22.Moosmann A, Bigalke I, Tischer J, Schirrmann L, Kasten J, Tippmer S, et al. Effective and long-term control of EBV PTLD after transfer of peptide-selected T cells. Blood. 2010 Apr 8;115(14):2960–2970. doi: 10.1182/blood-2009-08-236356. [DOI] [PubMed] [Google Scholar]
- 23.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995 Jan 7;345(8941):9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 24.Ugarte-Torres A, Hoegh-Petersen M, Liu Y, Zhou F, Williamson TS, Quinlan D, et al. Donor serostatus has an impact on cytomegalovirus-specific immunity, cytomegaloviral disease incidence, and survival in seropositive hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2011 Apr;17(4):574–585. doi: 10.1016/j.bbmt.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Jaskula E, Bochenska J, Kocwin E, Tarnowska A, Lange A. CMV Serostatus of Donor-Recipient Pairs Influences the Risk of CMV Infection/Reactivation in HSCT Patients. Bone Marrow Res. 2012;2012:375075. doi: 10.1155/2012/375075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merindol N, Salem Fourati I, Brito RM, Grenier AJ, Charrier E, Cordeiro P, et al. Reconstitution of protective immune responses against cytomegalovirus and varicella zoster virus does not require disease development in pediatric recipients of umbilical cord blood transplantation. J Immunol. 2012 Nov 15;189(10):5016–5028. doi: 10.4049/jimmunol.1201759. [DOI] [PubMed] [Google Scholar]
- 27.Park KD, Marti L, Kurtzberg J, Szabolcs P. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood. 2006 Sep 1;108(5):1770–1773. doi: 10.1182/blood-2005-10-006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safdar A, Decker WK, Li S, Xing D, Robinson SN, Yang H, et al. De novo T-lymphocyte responses against baculovirus-derived recombinant influenzavirus hemagglutinin generated by a naive umbilical cord blood model of dendritic cell vaccination. Vaccine. 2009 Mar 4;27(10):1479–1484. doi: 10.1016/j.vaccine.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Pedron B, Guerin V, Jacquemard F, Munier A, Daffos F, Thulliez P, et al. Comparison of CD8+ T Cell responses to cytomegalovirus between human fetuses and their transmitter mothers. J Infect Dis. 2007 Oct 1;196(7):1033–1043. doi: 10.1086/521196. [DOI] [PubMed] [Google Scholar]
- 30.Sun Q, Burton R, Reddy V, Lucas KG. Safety of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for patients with refractory EBV-related lymphoma. Br J Haematol. 2002 Sep;118(3):799–808. doi: 10.1046/j.1365-2141.2002.03683.x. [DOI] [PubMed] [Google Scholar]
- 31.Savoldo B, Cubbage ML, Durett AG, Goss J, Huls MH, Liu Z, et al. Generation of EBV-specific CD4+ cytotoxic T cells from virus naive individuals. J Immunol. 2002 Jan 15;168(2):909–918. doi: 10.4049/jimmunol.168.2.909. [DOI] [PubMed] [Google Scholar]
- 32.Vera JF, Brenner LJ, Gerdemann U, Ngo MC, Sili U, Liu H, et al. Accelerated production of antigen-specific T cells for preclinical and clinical applications using gas-permeable rapid expansion cultureware (G-Rex) J Immunother. 2010 Apr;33( 3):305–315. doi: 10.1097/CJI.0b013e3181c0c3cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanley PJ, Lam S, Shpall EJ, Bollard CM. Expanding cytotoxic T lymphocytes from umbilical cord blood that target cytomegalovirus, Epstein-Barr virus, and adenovirus. J Vis Exp. 2012;(63):e3627. doi: 10.3791/3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008 Jan-Feb;40(1):63–67. doi: 10.1016/j.bcmd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Luo XH, Huang XJ, Liu KY, Xu LP, Liu DH. Protective immunity transferred by infusion of cytomegalovirus-specific CD8(+) T cells within donor grafts: its associations with cytomegalovirus reactivation following unmanipulated allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010 Jul;16(7):994–1004. doi: 10.1016/j.bbmt.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Qasim W, Gilmour K, Zhan H, Derniame S, McNicol AM, Ip W, et al. Interferon-gamma capture T cell therapy for persistent Adenoviraemia following allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2013 May;161(3):449–452. doi: 10.1111/bjh.12251. [DOI] [PubMed] [Google Scholar]
- 37.Feuchtinger T, Lang P, Hamprecht K, Schumm M, Greil J, Jahn G, et al. Isolation and expansion of human adenovirus-specific CD4+ and CD8+ T cells according to IFN-gamma secretion for adjuvant immunotherapy. Exp Hematol. 2004 Mar;32(3):282–289. doi: 10.1016/j.exphem.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006 Jul;134(1):64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 39.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996 May;2(5):551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 40.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998 Sep 1;92(5):1549–1555. [PubMed] [Google Scholar]
- 41.Melenhorst JJ, Leen AM, Bollard CM, Quigley MF, Price DA, Rooney CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010 Nov 25;116(22):4700–4702. doi: 10.1182/blood-2010-06-289991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012 Mar 15;119(11):2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustafsson A, Levitsky V, Zou JZ, Frisan T, Dalianis T, Ljungman P, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000 Feb 1;95(3):807–814. [PubMed] [Google Scholar]
- 44.Comoli P, Basso S, Zecca M, Pagliara D, Baldanti F, Bernardo ME, et al. Preemptive therapy of EBV-related lymphoproliferative disease after pediatric haploidentical stem cell transplantation. Am J Transplant. 2007 Jun;7(6):1648–1655. doi: 10.1111/j.1600-6143.2007.01823.x. [DOI] [PubMed] [Google Scholar]
- 45.Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010 Feb 4;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009 Nov 5;114(19):4283–4292. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008 Nov 15;112(10):3974–3981. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 48.Gerdemann U, Katari UL, Papadopoulou A, Keirnan JM, Craddock JA, Liu H, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013 Jun 20; doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blyth E, Clancy L, Simms R, Ma CK, Burgess J, Deo S, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013 May 2;121(18):3745–3758. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 50.Schonberger S, Meisel R, Adams O, Pufal Y, Laws HJ, Enczmann J, et al. Prospective, comprehensive, and effective viral monitoring in children undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010 Oct;16(10):1428–1435. doi: 10.1016/j.bbmt.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Hanley PJ, Martinez C, Leung K, Savoldo B, Dotti G, Brenner MK, et al. Improving Immune Reconstitution After Cord Blood Transplantation Using Ex Vivo Expanded Virus-Specific T Cells: A Phase I Clinical Study. ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):224. 2012. [Google Scholar]
- 52.Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013 Jan 1;31(1):39–48. doi: 10.1200/JCO.2011.39.8495. [DOI] [PubMed] [Google Scholar]
- 53.Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010 Nov 18;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 54.Meij P, Jedema I, Zandvliet ML, van der Heiden PL, van de Meent M, van Egmond HM, et al. Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8+ T-cell lines. J Immunother. 2012 Oct;35(8):621–628. doi: 10.1097/CJI.0b013e31826e35f6. [DOI] [PubMed] [Google Scholar]
- 55.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007 Aug 15;110(4):1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 56.Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010 Dec 2;116(23):5045–5049. doi: 10.1182/blood-2010-04-281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qasim W, Derniame S, Gilmour K, Chiesa R, Weber M, Adams S, et al. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. Br J Haematol. 2011 Jul;154(1):150–153. doi: 10.1111/j.1365-2141.2011.08579.x. [DOI] [PubMed] [Google Scholar]
- 58.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013 Jun 27;121(26):5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakazawa Y, Huye LE, Salsman VS, Leen AM, Ahmed N, Rollins L, et al. PiggyBac-mediated cancer immunotherapy using EBV-specific cytotoxic T-cells expressing HER2-specific chimeric antigen receptor. Mol Ther. 2011 Dec;19(12):2133–2143. doi: 10.1038/mt.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh V. Disposable bioreactor for cell culture using wave-induced agitation. Cytotechnology. 1999 Jul;30(1–3):149–158. doi: 10.1023/A:1008025016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol Immunother. 2010 Mar;59(3):473–477. doi: 10.1007/s00262-009-0789-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Most Closely HLA-Matched Allogeneic Virus Specific Cytotoxic T-Lymphocytes (CTL) to Treat Persistent Reactivation or Infection with Adenovirus, CMV and EBV After Hemopoietic Stem Cell Transplantation (HSCT) ASH Annual Meeting Abstracts. 2010 Nov 19;116(21):829. 2010. [Google Scholar]
- 63.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter Study of “off-the-Shelf” Third Party Virus-Specific T Cells (VSTs) to Treat Adenovirus (Adv), Cytomegalovirus (CMV) or Epstein Barr Virus (EBV) Infection After Hemopoietic Stem Cell Transplantation (HSCT) ASH Annual Meeting Abstracts. 2012 Nov 16;120(21):457. 2012. [Google Scholar]