Summary
Three pathogenic forms, or formae speciales, of Fusarium oxysporum infect the roots of Arabidopsis thaliana belowground, instigating symptoms of wilt disease in leaves aboveground. In prior reports, Arabidopsis mutants that are deficient in the biosynthesis of abscisic acid or salicylic acid or insensitive to ethylene or jasmonates exhibit more or less wilt disease than wild type, implicating the involvement of hormones in the normal host response to F. oxysporum. Our analysis of hormone-related mutants finds no evidence that endogenous hormones contribute to infection in roots. Mutants that are deficient in abscisic acid and insensitive to ethylene have no less infection than wild type, though they exhibit less disease. Whether a mutant that is insensitive to jasmonates affects infection depends on which forma specialis is infecting roots. Insensitivity to jasmonates suppresses infection by F. oxysporum forma specialis conglutinans and F. oxysporum forma specialis matthioli, which produce isoleucine- and leucine-conjugated jasmonate (JA-Ile/Leu) in culture filtrates; whereas, insensitivity to jasmonates has no effect on infection by F. oxysporum forma specialis raphani, which produces no detectable JA-Ile/Leu. Furthermore, insensitivity to jasmonates has no effect on wilt disease of tomato, and the tomato pathogen F. oxysporum forma specialis lycopersici produces no detectable jasmonates. Thus, some but not all F. oxysporum pathogens appear to utilize jasmonates as effectors, promoting infection in roots and/or development of symptoms in shoots. Only when infection of roots is promoted by jasmonates is wilt disease enhanced in a mutant deficient in salicylic acid biosynthesis.
Keywords: Arabidopsis thaliana, Fusarium oxysporum, Fusarium wilt, jasmonate, COI1
Introduction
Fusarium wilt of Arabidopsis thaliana is an experimental pathosystem for studying the genetics of host resistance to and pathogenesis of wilt fungi (Michielse and Rep; 2009). Wilt fungi are responsible for debilitating vascular infections in plant species that are important to agriculture, horticulture and silviculture (Mace et al., 1981). In A. thaliana, three pathogenic forms, or formae speciales, of the wilt fungus Fusarium oxysporum instigate disease (Diener and Ausubel, 2005). In the field, F. oxysporum forma specialis conglutinans (FOC), F. oxysporum forma specialis raphani (FOR) and F. oxysporum forma specialis mathioli (FOM) are isolated from diseased cabbage (Brassica species), radish (Raphanus sativus) and garden stock (Mathiola incana), respectively (Bosland and Williams, 1987). Foliar symptoms in field hosts, such as stunting, epinastic growth, perivascular yellowing and premature senescence of leaves are reproduced in infected A. thaliana (Diener and Ausubel, 2005).
Interactions between wilt fungi and their hosts, including F. oxysporum and A. thaliana, are described by three phases (Diener, 2012; Talboys, 1972): In the primary determinative (or prevascular) phase, soil-borne F. oxysporum enters roots usually at tips and colonizes extravascular tissue. In the secondary determinative (or vascular) phase, F. oxysporum invades the vascular cylinder and spreads via the water-conducting xylem vessels. In the expressive phase, symptoms appear in foliage aboveground in advance of the infection of roots belowground.
More or less wilt disease is a characteristic of genetically dissimilar cultivars or wild accessions of a host species (Beckman and Roberts, 1995). For instance, the standard laboratory accession Columbia-0 (Col-0) of A. thaliana expresses complete resistance to FOM, whereas accession Taynuilt-0 (Ty-0) is highly susceptible (Diener and Ausubel, 2005). This natural variation in resistance is specific to the infecting forma specialis as Col-0 and Ty-0 exhibit similar resistance when they are instead infected with FOC or FOR. Inheritance of RESISTANCE TO F. OXYSPORUM (RFO) genes explains the difference between FOM-infected Col-0 and Ty-0 and suggests that variation in resistance is the expression of diversity in innate immunity (Cole and Diener, 2013; Diener, 2012; Shen and Diener, 2013).
Arabidopsis mutants with defects in hormone biosynthesis or signaling exhibit more or less wilt disease when infected with FOC. On the one hand, genotypes that suppress pathogen-induced accumulation of salicylic acid (SA), such as enhanced disease susceptibility 5 (eds5), salicylic acid induction deficient 2 (sid2) and transgenic nahG, express more severe disease than wild type (Diener and Ausubel, 2005; Trusov et al., 2009). On the other hand, genotypes that are defective in the biosynthesis of abscisic acid (abscisic acid deficient 1, aba1 and aba2) or the perception of jasmonates (coronatine insensitive 1, coi1, and jasmonate insensitive 1, jin1) or ethylene (ethylene insensitive 2, ein2) or the signaling (auxin resistant 2) or transport of auxin (transport inhibitor resistant 3) express less disease or no disease altogether (Anderson et al., 2004; Kidd et al., 2011; Thatcher et al., 2009; Trusov et al., 2009).
The effect of hormones on disease may be interpreted in terms of host resistance (Glazebrook, 2005). In general, signaling by jasmonate and ethylene is associated with less disease from infection by necrotrophic pathogens, so jasmonate and ethylene are said to promote resistance to necrotrophs. SA accumulation often correlates with less disease from infection by biotrophic pathogens, so SA is said to promote resistance to biotrophs. Furthermore, hormones sometimes have synergistic effects or antagonistic effects that work at cross-purposes and negate the contribution of either or both (Kunkel and Brooks, 2002).
The effects of hormones may be the consequence of interactions between hormone signaling and virulence, in which case pathogenesis depends on how hormone signaling interacts with the strategy of pathogens (Grant and Jones, 2009; Seilaniantz et al., 2011). Indeed, the pathogen's lifestyle, biotrophic or necrotrophic, rather than taxonomic grouping anticipates the effects of hormones. Also, hormones usually have a more profound effect on infection by virulent pathogens than nonadapted pathogens. From this perspective, jasmonate signaling is antithetical to the virulence expressed by necrotrophs and conducive to the virulence of biotrophs. Attributing the roles of hormones to virulence rather than host resistance may better explain the inconsistent roles that hormones play during infection by the same or similar microbes expressing different strategies.
For example, jasmonate signaling in A. thaliana either promotes or suppresses resistance to F. oxysporum, depending on the disease syndrome being observed (Aboul-Soud et al., 2004; Berrocal-Lobo and Molina, 2004; Epple et al., 1997; McGrath et al., 2005; Thatcher et al., 2009; Trusov et al., 2009). When conditions favor rotting by F. oxysporum, there is more necrosis of leaves or whole seedlings in the jasmonate-insensitive mutant coi1 than wild type. Thus, jasmonate signaling is critical for resistance or susceptibility depending on the pathogenesis that F. oxysporum elaborates.
Here, we find that the host's perception of jasmonates is crucial for persistent root infection by F. oxysporum formae speciales that produce JA-Ile/Leu in vitro, that SA antagonizes the host's perception of jasmonates in roots and that endogenous plant hormones abscisic acid, ethylene and jasmonates have no critical role in immunity. Instead, endogenous abscisic acid and ethylene probably contribute to the expression of foliar wilt symptoms.
Results
COI1 promotes root infection by FOC
Prior studies show that the perception of jasmonates (by COI1) but not the synthesis of jasmonates (by ALLENE OXIDE SYNTHASE, AOS) is critical to the development of wilt disease in FOC-infected A. thaliana (Thatcher et al., 2009; Trusov et al., 2009). The effect and interaction of synthesis and perception of jasmonates were scrutinized in the nine genotypes generated by the selfed dihybrid AOS/aos COI1/coi1 (Figure 1). In particular, we measured the radius of rosettes, which quantified the stunted expansion of rosette leaves, a conspicuous symptom of Fusarium wilt. AOS corresponds to the first step in jasmonate biosynthesis, and aos fails to accumulate jasmonates in response to stimuli, including biotic stress (Chehab et al., 2008). At 20 days post infection (dpi), the rosette radii of FOC-infected plants that were capable (AOS/-) and incapable (aos) of accumulating jasmonates were similarly reduced (Figure 1). COI1 encodes the coreceptor of the biologically-active jasmonate (+)-7-iso-jasmonoyl-L-isoleucine (JA-Ile), and coi1 is insensitive to jasmonates, either produced by the plant or applied to the plant (Fonseca et al., 2009; Yan et al., 2009). In contrast, FOC-infected plants that were insensitive to jasmonates (coi1) developed no wilt symptoms, including no reduction in the rosette radius (Figure 1). Furthermore, the double mutant aos coi1 was unaffected, so absence of disease in coi1 was not an effect of endogenous jasmonates in the absence of COI1.
Figure 1. COI1 promotes wilt disease from FOC infection.
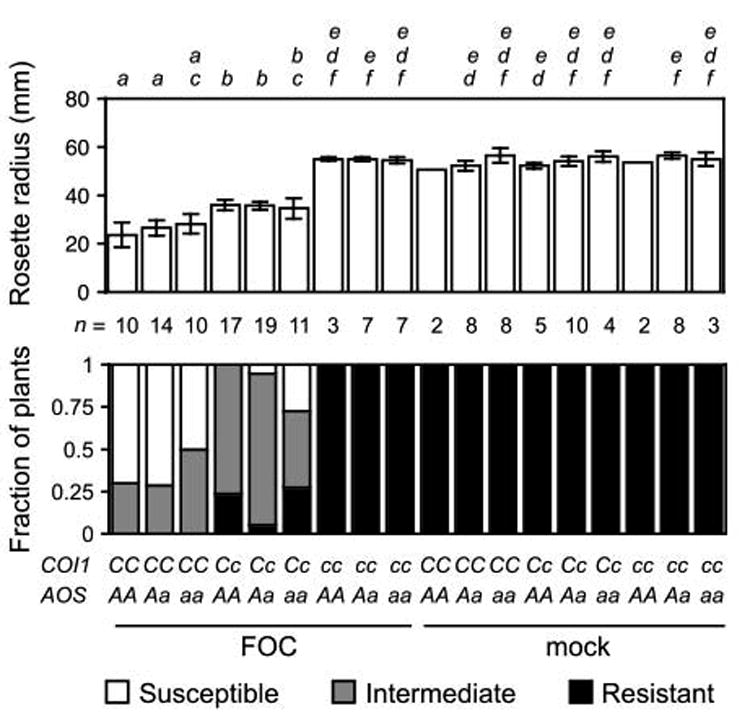
Genotypes of FOC-infected or mock-infected offspring of the dihybrid COI1/coi1 AOS/aos were COI1/COI1 (CC), COI1/coi1 (Cc) or coi1/coi1 (cc) and AOS/AOS (AA), AOS/aos (Aa) or aos/aos (aa). Wilt symptoms at 20 dpi: In upper graph, the mean radius of rosettes of n plants is in millimeters. Error bars are the confidence intervals of the means (α= 0.05). Means with the same italicized letter (above) are not significantly different, according to Student's t-test (p > 0.01). In lower graph, the fractions of n plants were resistant (health index, HI ≥ 4) or susceptible (HI < 3) or had intermediate resistance (HI ≥ 3 and HI < 4).
Interestingly, symptom severity was sensitive to gene dosage of COI1. The mean radius of rosettes of heterozygotes (COI1/coi1) was intermediate to that of coi1 and wild type (COI1). The intermediate size of COI1/coi1 rosettes was unperturbed by the presence or absence (aos) of AOS, which emphasized that COI1's role is unrelated to endogenously produced jasmonates.
To examine whether COI1 was critical for infection of roots in the primary and/or secondary determinative phases, infection in roots of coi1 and wild type was compared in the initial week after soil was infested with FOC. F. oxysporum infection in roots is stained blue when roots are treated with the indigogenic substrate of Fusarium-expressed arabinofuranosidase (ABF) 5-bromo-4-chloro-3-indoxyl-α-L-arabinofuranoside (X-Ara) (Diener, 2012). In the first few days after soil infection, both root tips and lateral root primordia (LRP) were stained blue by X-Ara in wild type and coi1, indicating that COI1 was not essential for FOC's initial invasion into roots. However, by four to five dpi, when wild-type rosettes still appeared asymptomatic, staining in roots of coi1 and wild type was noticeably different. In particular, fine staining in the central vascular cylinder that extended from colonized root apices of wild type (Figures 2a and 2b) rarely extended from those of coi1 (Figures 2c and 2d).
Figure 2. COI1promotes infection by FOC.
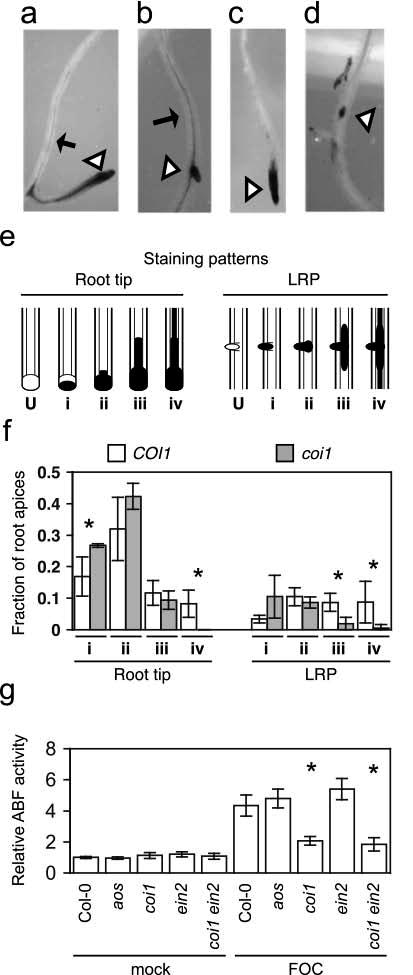
Representative FOC-infected apices (at triangles) of wild type, (a) and (b), or coi1, (c) and (d), with X-Ara staining pattern ii or iv, respectively. Arrows point to fine vascular staining. (e) Depictions of unstained root apices (U) common patterns of X-Ara-staining (filled) at root tips and lateral root primordia (LRP): (i) partial staining of undifferentiated apices, (ii) staining throughout undifferentiated apices, (iii) broad vascular staining extending for short distances from apices and (iv) fine, extensive vascular staining away from apices. (f) Fractions of patterns in whole root systems of three FOC-infected wild type (COI1) or coi1 at between 4 to 5 dpi. Error bars are confidence intervals of the means (α = 0.05). Asterisk indicates that the mean values of COI1 and coi1 are different, according to Student's t-test (α = 0.05). (g) Relative arabinofuranosidase (ABF) activity was quantified in FOC-infected wild type (Col-0, n = 5), aos (n = 5), coi1 (n = 4), ein2 (n = 5) and coi1 ein2 (n = 5) or mock-infected plants (n = 3) at 10 dpi, and values are relative to the mean of mock-infected Col-0 roots. Error bars are the confidence intervals of the means, and asterisks indicate that mean values of Col-0 and the mutant are different, according to Student's t-test (α = 0.05).
To quantify the difference at X-Ara-stained root apices, four patterns of staining were recognized (Figure 2e): (i) Undifferentiated tissue at apices was incompletely stained. (ii) Apices were stained throughout. (iii) Stain extended into the vascular cylinder a short distance from stained apices. (iv) Fine-staining in the vascular cylinder extended for some distance from stained apices.
At five dpi, X-Ara-stained roots were assigned to one of the four patterns, and patterns at root tips and LRP were tallied separately (Figure 2f). Comparable numbers of root tips from three whole root systems of coi1 (44, 48 and 71) and wild type (49, 55,and 63) were stained blue while significantly fewer LRP from root systems of coi1 (13, 15 and 15) than wild type (23, 24 and 29) were stained blue. However, because the total number of LRP was not determined, the discrepancy could be explained by fewer LRP emerging from coi1 rather than less infection in LRP of coi1. Showing that COI1 was crucial for infection of the differentiated vascular cylinder, pattern iv (as well as iii at LRP) was underrepresented among apices of coi1 (Figure 2f). Pattern iv was represented by only one LRP and no root tips of coi1 while 22 LRP and 20 roots tips of wild type were assigned to pattern iv. In addition to the deficit of pattern iv, coi1 had an excess of pattern i at root tips, and probably at LRP, in comparison to wild type, suggesting that COI1 also promoted colonization of undifferentiated cells at apices after invasion.
In the second week after infection, when wild type exhibited visible symptoms, ABF activity indicated that FOC infection was profoundly suppressed in coi1 but was no different in aos and wild type. X-Ara-staining in the vascular cylinder was extensive in wild type and sparse in coi1. This visible difference was quantified using the substrate nitrophenyl-α-L-arabinofuranoside (NP-Ara), and roots of wild type had 3.5-fold more Fusarium-derived ABF activity than coi1 (Figure 2g). In contrast, both visible staining and quantification of ABF activity in aos and wild type were similar and consistent with a similar appearance of foliar symptoms.
COI1 promotes symptoms in FOC-infected plants
Distinct roles for COI1 expression in roots and shoots were observed in grafted plants infected with FOC. Grafting was used to make the four possible combinations of scions and rootstocks from wild-type (COI1) and coi1 seedlings. As previously reported by Thatcher et al. (2009), all plants with coi1 rootstocks exhibited the same absence of wilt disease as ungrafted coi1 (Figures 3a and 3b). However, wilt disease in plants with COI1 rootstocks had an altered appearance depending on whether the scions were coi1 or COI1. In plants with coi1 scions, symptoms such as stunting of rosette leaves, accumulation of (anthocyanin) pigment in petioles and epinastic growth of leaf petioles were strongly suppressed while other symptoms such as perivascular yellowing and premature senescence were delayed in comparison to plants with COI1 scions (Figures 3a and 3b). At 21 dpi, X-Ara staining was largely restricted to root tips in coi1 rootstocks, so COI1 in roots alone was sufficient to promote infection of the vascular cylinder (Figure 3c). Interestingly, noticeably fewer roots in rootstocks of COI1 were stained by X-Ara when grafted to coi1 scions than COI1 scions (Figure 3c), suggesting that shoot-expressed COI1 might also influence the infection of roots.
Figure 3. COI1 promotes symptoms in FOC-infected plants.
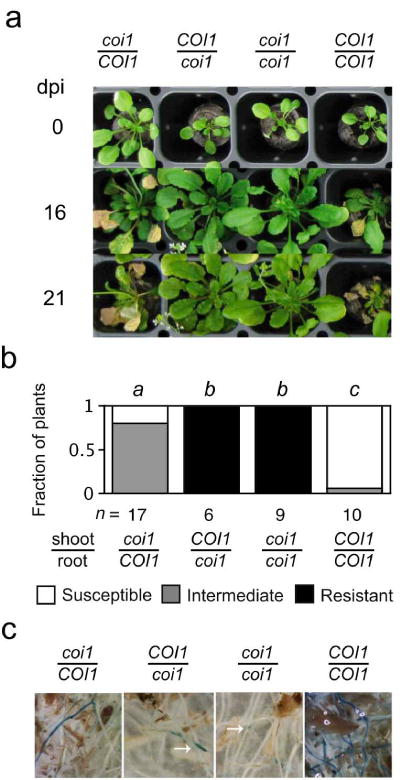
Grafted plants from scions (top) and rootstocks (bottom) that were wild type (COI1) or coi1 were infected with FOC. (a) Representative grafts are shown at 0, 16, and 21 dpi. (b) At 16 dpi, fractions of n grafted plants were susceptible (0 ≤ health index, HI < 2), had intermediate resistance (2 ≤ HI ≤ 3) or were resistant (3 < HI ≤ 5). Grafts with the same italicized letter (above) had similar HI scores, according to Mann-Whitney U test (p > 0.01). (c) At 21 days, X-Ara staining in roots of grafts is shown. Notice that staining of coi1 rootstocks is restricted to root tips (at arrows).
COI1 promotes wilt disease by FOM
In Diener and Ausubel (2005), Col-0 is resistant to wilt disease when soil is infested with FOM instead of FOC, so sensitivity to jasmonate is not sufficient to make A. thaliana susceptible to Fusarium wilt disease. To investigate whether COI1 nevertheless contributes to infection by FOM, wilt disease was evaluated in offspring of the selfed monohybrid rfo1 COI1/coi1 because rfo1 compromises immunity to F. oxysporum (Diener, 2012). FOM-infected offspring that were rfo1 COI1/- exhibited wilt symptoms, including a reduced rosette radius, while rfo1 coi1 offspring exhibited no disease (Figure 4a). A wider rosette radius in rfo1 COI1/coi1 heterozygotes and more severe symptoms in rfo1 COI1/COI1 homozygotes indicated that disease was sensitive to the gene dosage of COI1. Furthermore, X-Ara-staining of the vascular cylinder was extensive in roots of rfo1, whereas staining at root tips of rfo1 coi1 rarely extended into the differentiated vascular cylinder (Figure 4b).
Figure 4. COI1 promotes infection by FOM.
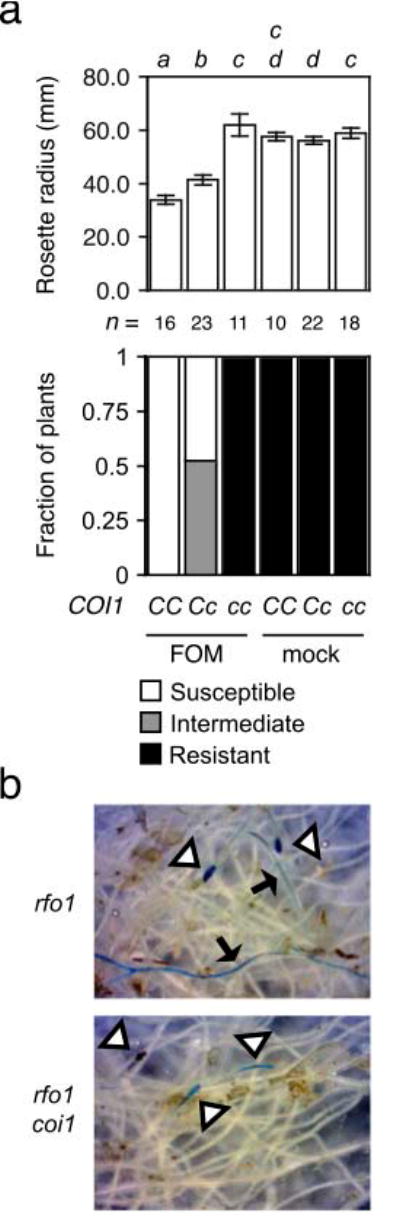
Genotypes of FOM- or mock-infected offspring of the monohybrid double mutant rfo1 COI1/coi were COI1/COI1 (CC), COI1/coi1 (Cc) or coi1/coi1 (cc). (a) Wilt symptoms at 20 dpi: In upper graph, the mean radius of rosettes of n plants is in millimeters. Error bars are the confidence intervals of the means (α = 0.05). Means with the same italicized letter (above) have no significant difference, according to Student's t-test (p > 0.01). In lower graph, fractions of n plants were resistant (HI = 5), susceptible (HI = 3) or had intermediate resistance (HI = 4). (b) At 20 dpi, X-Ara stains root apices (at triangles) of FOM-infected rfo1 and rfo1 coi1, and arrows point to fine vascular staining in roots of rfo1.
COI1 promotes symptoms in FOR-infected plants
Neither host synthesis nor perception of jasmonate was critical for infection by a third F. oxysporum pathogen, FOR. The root system and specifically the vascular cylinder in FOR-infected aos, coi1 and wild type was similarly stained by X-Ara, and similar amounts of Fusarium-derived ABF activity were measured in roots of aos, coi1 and wild type at 10 dpi (Figure 5a). Moreover, a dose of FOR that was lethal to wild type was also lethal to aos and coi1, though death of coi1 could be delayed by a few days in comparison to aos and wild type.
Figure 5. COI1 promotes symptoms in FOR-infected plants.
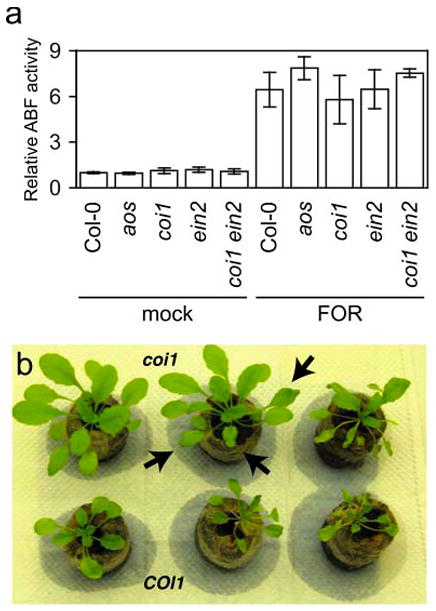
(a) Relative arabinofuranosidase (ABF) activity was quantified in FOR-infected wild type (Col-0), aos, coi1, ein2 and coi1 ein2 or mock-infected plants (n = 3) at 10 dpi. Error bars are the confidence intervals of the means (α = 0.05). (b) Foliar symptoms, such as stunting, epinasty and darkening (anthocyanin accumulation) of leaves, in FOR-infected wild type (bottom row) were suppressed in infected coi1 (top row). Notice wilted leaves (at arrows) on coi1 in watered soil.
Nevertheless, the appearances of FOR-infected coi1 and wild type were strikingly different (Figure 5b). Symptoms such as stunted growth of rosette leaves, epinastic growth of petioles and darker (anthocyanin) pigmentation, which were observed in wild type, were largely suppressed in coi1, even in plants on the verge of death (see coi1 plant in center in Figure 5b). Often only a wilting of leaves (at arrows in Figure 5b) preceded the sudden decline and death of coi1 plants (see coi1 on the right in Figure 5b). In contrast, wilt disease in FOR-infected aos and wild type was indistinguishable in all respects, so the promotion of wilt symptoms by COI1 could not be a response to endogenously produced jasmonates.
F. oxysporum produces jasmonates
Because host biosynthesis of jasmonates was unnecessary for the contributions of COI1, the production of jasmonate activity by pathogenic Fusarium strains, including the wheat head blight F. graminearum and five F. oxysporum formae speciales, FOC, FOM, FOR, F. oxysporum forma specialis lycopersici (FOL) and F. oxysporum forma specialis tulipae (FOT), was tested. Prior reports show that culture filtrate of FOM contains jasmonate activity that induces the expression of jasmonate-regulated gene THI2.1 and the reporter gene THI2.1p:uidA, in which the THI2.1 promoter sequence is fused to the Escherichia coli β-glucuronidase gene (Epple et al., 1998; Hilpert et al., 2001; Vignutelli et al., 1998). The previously described jasmonate-induced expression of β-glucuronidase (GUS) activity by THI2.1p:uidA was confirmed using the indigogenic substrate 5-bromo-4-chloro-3-indoxyl-β-D-glucuronic acid (X-Gluc) (Figure S1) (Bohlmann et al. 1998).
Culture filtrates of some but not all pathogenic Fusarium strains were capable of inducing THI2.1p:uidA expression. Transgenic seedlings that were wetted with filtrates from Fusarium cultures of FOC, FOM or FOT were stained blue by X-Gluc while no staining was evident after treatment with filtrates of FOR, FOL and F. graminearum (Figure 6). Because no GUS activity was evident in filtrate-treated wild type or aos without the transgene (Figure 6), expression of THI2.1p:uidA and not filtrates was the source of GUS activity. Because GUS activity was similarly induced in transgenic wild type and aos (Figure 6), filtrates and not endogenous biosynthesis were the source of the jasmonate hormone activity.
Figure 6. Fusariumculture filtrates induce THI2.1p:uidA.
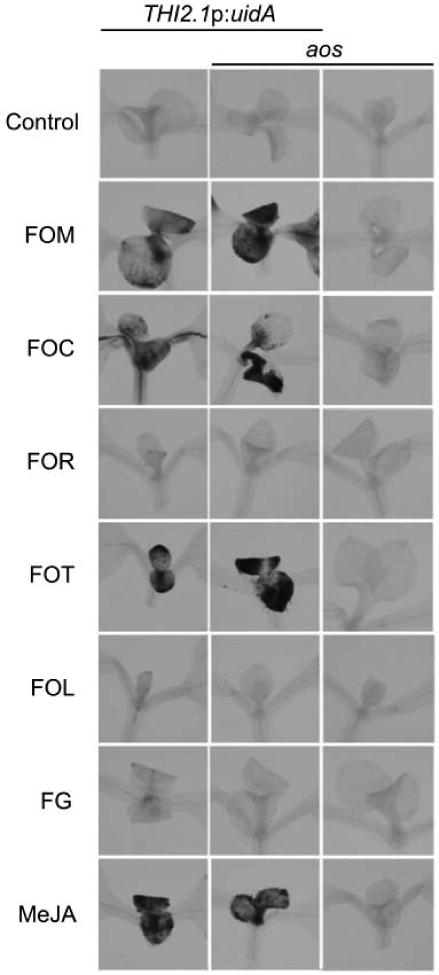
THI2.1p:uidA is induced by jasmonates in meristems and first true leaves of young seedlings and expresses β-glucuronidase, which hydrolyzes the indigogenic substrate X-Gluc (Vignutelli et al., 1998). β-glucuronidase activity in seedlings of THI2.1p:uidA (left), aos THI2.1p:uidA (middle) and aos (right) is stained blue by X-Gluc one day after treatment with culture filtrates of Fusarium strains, F. oxysporum f. sp. matthioli (FOM) race 2, F. oxysporum f. sp. conglutinans (FOC) race 1, F. oxysporum f. sp. raphani (FOR), F. oxysporum f. sp. tulipae (FOT), F. oxysporum f. sp. lycopersici race 2 (FOL), and F. graminearum (FG) or methyl jasmonate (MeJA). Results with FOL race 2 and race 3 (not shown) were similar.
Jasmonates in Fusarium culture filtrates
Accumulation of specific jasmonates, JA and JA-Ile/Leu, was measured in the culture filtrates of Fusarium strains. Jasmonates were extracted from filtrates of three-week old cultures, separated by HPLC and detected by electrospray ionization coupled to multiple reaction monitoring (MRM). A single peak for JA that coeluted with the JA standard and deuterated-JA was detected. JA was reproducibly present in cultures of FOC, FOM, FOR and FOT (in Table 1) but absent (< 4.8 nM) in cultures of FOL and F. graminearum. When amounts of JA were quantified, much less JA was measured in the cultures of FOC and FOR than FOM and FOT (in Table 1), and the most JA (200 nM) accumulated in cultures of FOT.
Table 1. JA, JA-Ile and JA-Leu in axenic cultures of pathogenic Fusarium.
| Fusarium pathogen | [JA]a | [JA-Ile] | [JA-Leu] |
|---|---|---|---|
| F. oxysporum f. sp. matthioli (FOM) | 120 ± 29b | 740 ± 160 | 410 ± 200 |
| F. oxysporum f. sp. conglutinans (FOC) | 10 ± 3.4 | 120 ± 27 | 74 ± 59 |
| F. oxysporum f. sp. raphani (FOR) | 8.7 ± 3.8 | nde | nd |
| F. oxysporum f. sp. tulipae (FOT) | 200 ± 77 | 6500 ± 1000 | 5800 ± 3400 |
| F. oxysporum f. sp. lycopersici (FOL)c | ndd | nd | nd |
| F. graminearum (FG) | nd | nd | nd |
nanomolar (nM)
the range (±) is the standard error of the mean (n = 3; α = 0.05)
Similar results were obtained with FOL race 2 and race 3
not detected, <4.8 nM
not detected, <6.2 nM
In the m/z 322→130 chromatograms, two closely-eluting peaks corresponding to the natural stereoisomers of JA-Ile and JA-Leu were present in the same samples. JA-Ile and JA-Leu standards and 13C-labeled JA-Ile internal control each eluted as two clearly separated peaks corresponding to alternative stereoisomers, one of which was the natural stereoisomer (Fonseca et al., 2009). The early peak (at 40.8 min) in Fusarium extracts coeluted with the later-eluting peak in the JA-Ile standard and internal control, while the later peak (at 41.5 min) in Fusarium extracts coeluted with the later-eluting peak in the JA-Leu standard. The order of reverse phase separation of stereoisomers of these compounds was the same as reported by Fonseca et al. (2009). Both JA-Ile and JA-Leu was detected and measured in all strains that produced JA with the exception of FOR, and neither amino acid conjugate was detected in FOR, FOL and F. graminearum. The molar amount of JA-Ile/Leu in extracts was consistently higher (from 3- to 32-fold more in Table 1) than JA, and the highest JA-Ile concentration was in FOT cultures (6.5 μM).
Infection of other hormone-related mutants
Other hormones may antagonize or reinforce the host's response to jasmonates. For example, wilt disease is more severe in sid2, which fails to accumulate SA (Diener and Ausubel, 2005; Nawrath and Métraux, 1999). The interaction of sid2 and coi1 in FOC-infected plants was scrutinized in the nine genotypes produced by the selfed dihybrid COI1/coi1 SID2/sid2 (Figure 7a). COI1 again exhibited incomplete dominance, and the rosette radius of heterozygotes was intermediate to the radii of wild type and mutant. Among progeny that perceived jasmonates (COI1/-), sid2 enhanced wilt disease, including a reduced radius. However, sid2 failed to suppress the resistance of the coi1 sid2 double mutant that was insensitive to jasmonates. In FOR-infected plants, in which COI1 has no effect on root infection, sid2 had no effect on wilt disease (Figure 7b); and, reduced expression of foliar symptoms in FOR-infected coi1 was independent of SID2 as symptoms in coi1 sid2 were less severe than those in COI1/- sid2 (Figure 7c).
Figure 7. COI1-dependent susceptibility is independent of SA.
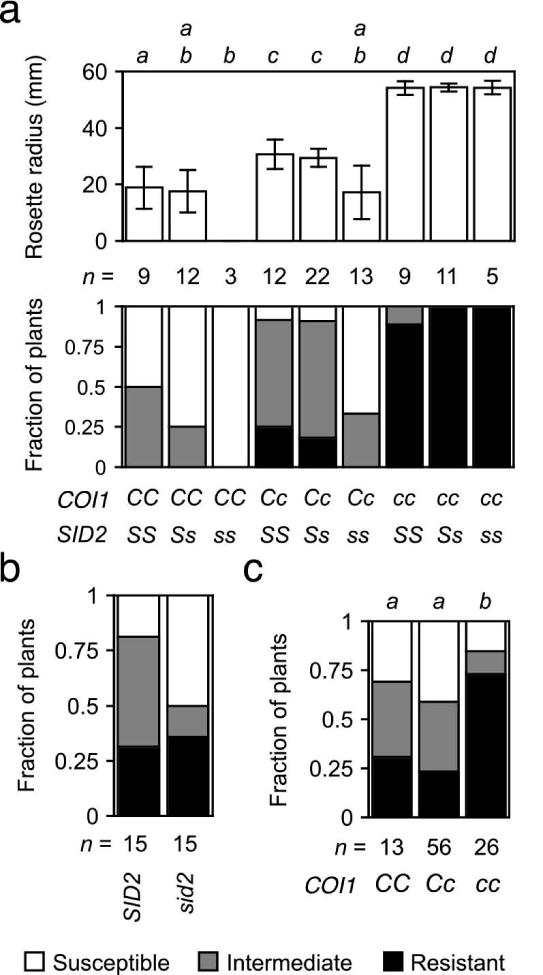
Wilt symptoms at 20 dpi: (a) FOC-infected offspring of the dihybrid COI1/coi1 SID2/sid2 were COI1/COI1 (CC), COI1/coi1 (Cc) or coi1/coi1 (cc) and SID2/SID2 (SS), SID2/sid2 (Ss) or sid2/sid2 (ss). In upper graph, the mean radius of rosettes of n plants is in millimeters. Error bars are the confidence intervals of the means (α = 0.05). Means with the same italicized letter (above) have no significant difference, according to Student's t-test (p > 0.01). In lower graph of (a) and (b) and (c), the fraction of n plants that were resistant (health index, HI > 3) or susceptible (HI < 2) or had intermediate resistance (2 ≤ HI ≤ 3). No symptoms were apparent in mock-infected plants (not shown). (b) Wild type (SID2) and sid2 were infected with FOR. (c) FOR-infected offspring of the monohybrid double mutant sid2 COI1/coi1 were COI1/COI1 (CC), COI1/coi1 (Cc) or coi1/coi1 (cc). Similar HI scores, according to Mann-Whitney U test (p > 0.01), have the same italicized letter above.
Some responses to jasmonates also depend on ethylene perception (Lorenzo et al., 2003), and Trusov et al. (2009) report that wilt disease is reduced in FOC-infected ein2, which is insensitive ethylene (Alonso et al., 1999). We observed less yellowing and premature senescence of rosette leaves in FOC- and FOR-infected ein2 in comparison to wild type; however, other symptoms and ultimately death were not appreciably affected by ein2. Infection by FOC and FOR produced similar X-Ara staining in roots of ein2 and wild type at 10 dpi. Furthermore, Fusarium-derived ABF activity was comparable in FOC- and FOR-infected roots of wild type and ein2, or coi1 and ein2 coi1 (Figures 2g and 5a, respectively).
Similar to coi1, the abscisic acid-deficient aba1 and aba2, which have abnormally small and dark green rosettes, exhibit less stunting, epinastic growth, yellowing and senescence of rosette leaves (Anderson et al., 2004; Trusov et al., 2009). However, X-Ara staining was not appreciably different in FOC-infected roots of aba2 and wild type (Figure 8a); in fact, the root system of aba2 had more Fusarium-derived ABF activity than that of wild type (Figure 8b). The smaller root mass of aba2 presumably accounted for this modest difference as F. oxysporum could colonize more of the smaller root system of aba2. In any case, aba2 did not enhance immunity to root infection.
Figure 8. FOC infection in aba2.
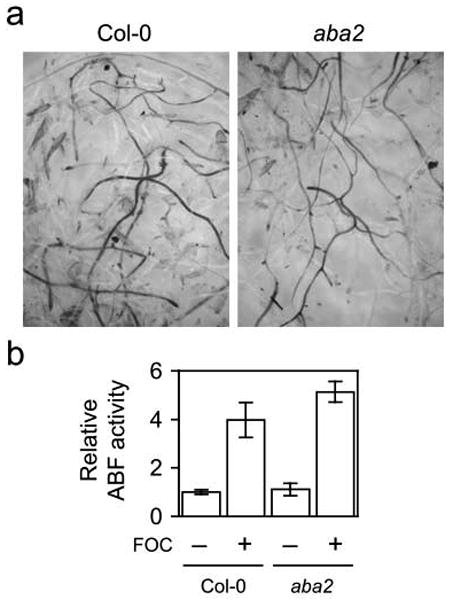
(a) At 7 dpi, X-Ara staining was similar in roots of FOC-infected wild type (Col-0) and aba2. (b) Relative arabinofuranosidase (ABF) activity in roots of FOC-infected (+; n = 5) or mock-infected (−; n = 3) plants was quantified using NP-Ara. Fresh weights (± standard deviations) of whole roots systems of wild type and aba2 were 28 (±8) and 15 (±3) milligrams, respectively. Comparison of ABF activity in infected wild type and aba2 using the Mann-Whitney U test gives two-tailed p = 0.026.
Jasmonate perception in Fusarium wilt of tomato
A role for the perception of jasmonates in Fusarium wilt of tomato was also tested. Jamonic Acid Insensitive 1 (JAI1) is the tomato ortholog of Arabidopsis COI1 (Li et al. 2004). The gross appearance of mock-infected jai1 and wild type was the same (Figure 9a). As well, FOL-infected JAI1 and jai1 plants expressed symptoms, such as epinastic growth of petioles and premature senescence of older leaves, with similar severity at 21 dpi (Figure 9b and 9c) and yielded similar distributions of disease index scores at 35 dpi (Figure 9d).
Figure 9. Fusarium wilt in tomato jai1.
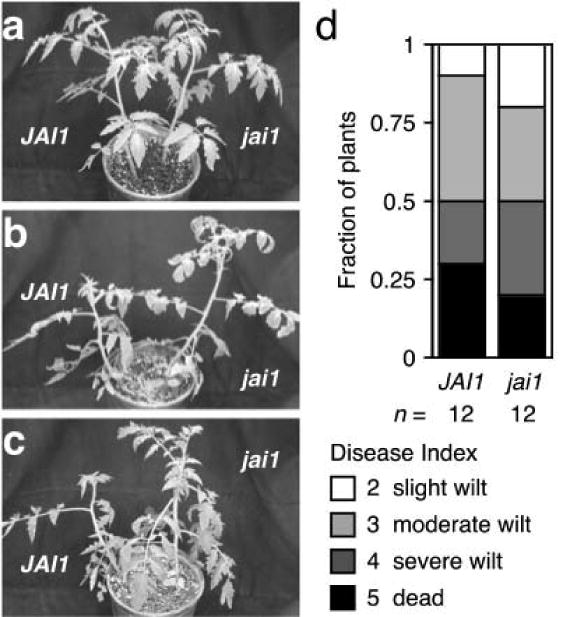
Representative wild type (JAI1) and jasmonate-insensitive jai1 plants that were mock-infected (a) or infected with FOL race 3 (b) and (c) are shown at 21 dpi. Lower leaves of FOL-infected plants exhibit epinasty and premature senescence when compared to uninfected plants. (d) At 35 dpi, symptoms of FOL-infected JAI1 (n = 12) and jai1 (n = 12) plants were scored using a disease index: healthy (0), slightly wilted (1), moderately wilted (2), severely wilted (4) to dead (5). Mock-infected JAI1 (n = 6) and jai1 (n = 6) exhibited no wilt symptoms.
Discussion
Our finding that COI1 primarily promoted infection by FOC contradicts Thatcher et al. (2009) who conclude that there is “no difference in the degree of fungal colonization of the wild-type and coi1 plants until later stages of infection, when host necrosis [is] well developed.” Instead, Thatcher et al. (2009) report that COI1 primarily promotes the expression of foliar symptoms and specifically senescence. Their conclusion rests on the detection of similar amounts of F. oxysporum DNA in coi1 and wild type early in the infection. However, Thatcher et al. (2009) quantify F. oxysporum DNA in shoots where little if any F. oxysporum would be present until late in the infection cycle. Indeed, when infected plants were stained with X-Ara, F. oxysporum infection was detected early in roots, late in stems and never in leaves (S.C., personal observation). The absence of F. oxysporum in shoots would explain why Thatcher et al. (2009) detected no early difference in fungal DNA in shoots of coi1 and wild type.
Our finding that endogenous jasmonates were inconsequential to host resistance is remarkable because the accumulation of jasmonates is a common response to wounding and infection in plants (Koo and Howe, 2009), and prior investigations clearly show the induction of jasmonate-regulated defense-related transcription in FOC-infected A. thaliana (Anderson et al., 2004; Kidd et al., 2011). Considering such precedent, Thatcher et al. (2009) posit that COI1 also mediates defense responses that restrict FOC growth. However, the absence of endogenous jasmonates (in aos) had no discernible effect on infection or wilt disease in general.
The effect of SA depended on COI1. When infected with FOR, COI1 was unnecessary for root infection, and a deficiency in SA had no effect on wilt disease. When infected with FOC, which depended on COI1 for root infection, sid2 exhibited more severe disease; however, this enhanced susceptibility depended on COI1 because the sid2 coi1 double mutant appeared as resistant as SID2/- coi1. Prior reports show that the enhanced susceptibility of SA-deficient genotypes nahG or eds5 is suppressed by coi1 as well (Thatcher et al. 2009; Trusov et al. 2009). Because JA signaling may be exaggerated in SA-deficient mutants (Kunkel and Brooks 2002), it is likely that enhanced susceptibility of sid2 is an abnormal response to Fusarium-derived jasmonates and that normal SA signaling is inconsequential to wilt disease in wild type.
Jasmonates, which Miersch et al. (1999a) detect in culture filtrates of FOM, are not previously identified products of other formae speciales. Miersch et al. (1999a) detect 22 jasmonate-related compounds, including JA and JA-Ile, in filtrates of an FOM isolate. However, in contrast to Miersch et al. (1999a), who report the accumulation of (20-fold) more JA than JA-Ile, we detected (6-fold) less JA than JA-Ile. Because more JA-Ile than JA accumulated in cultures of isolates from three formae speciales, the difference of major products in the two studies is probably a consequence of growth conditions rather than differences in the FOM strains used here and in Miersch et al. (1999a).
JA-Ile is the only reported amino acid conjugate of JA in fungal cultures, so the detection of comparable amounts of JA-Ile and JA-Leu was unanticipated (Cross and Webster, 1970; Miersch et al., 1999a). JA-Leu normally accumulates in plants too (Kramell et al., 1995); however, JA-Leu has equivalent or weaker hormone activity than JA-Ile when the two are compared in physiological tests, so the interactions of JA-Ile and JA-Leu with COI1 might yield distinct hormone responses (Katsir et al., 2008; Miersch et al., 1999b; Tamogami et al., 1997).
A role for COI1/JAI1 correlated with the in vitro production of JA and JA-Ile/Leu. Similarly, COI1 is critical to the pathogenesis of Pseudomonas syringae pathovars that are capable of producing the polyketide effector coronatine, which mimics JA-Ile (Bender et al, 1999; Feys et al., 1994); and, this suggests that jasmonates are effectors for some but not all F. oxysporum pathogens. For instance, FOL, which produced no detectable JA or JA-Ile/Leu, epitomized those formae speciales for which jasmonates are not critical effectors. On the other hand, formae speciales that produced JA, including FOR, FOC and FOM, relied on COI1 for the expression of foliar symptoms. Although accumulation of JA in filtrates of FOR was too low to induce THI2.1p:uidA, FOR-derived jasmonate in planta was crucial for the expression of foliar symptoms but inconsequential for root infection. Furthermore, formae speciales that produced substantial quantities of JA-Ile/Leu, namely FOC and FOM, were further dependent on COI1 for root infection and ultimately the death of the host. The separate associations of JA-Ile/Leu with root infection and JA with foliar symptoms are intriguing and suggest that different jasmonates might function as distinct effectors and target different host processes through the same COI1 receptor.
Fungal-derived jasmonates are also likely effectors in hosts other than A. thaliana and commonplace in fungal pathogenesis. The three jasmonate-producing formae speciales, FOC, FOM and FOR, represent three distinct phylogenetic lineages in the F. oxysporum species complex and are virulent to related dicotyledons in the mustard family (O'Donnell et al., 2009); whereas, the jasmonate-producing forma specialis FOT is virulent to tulip (Tulipa gesneriana), a monocotyledon. A characteristic of FOT-infected tulip bulbs is gum exudation, or gummosis, and MeJA-treatment of tulip bulbs phenocopies the gummosis associated with Fusarium bulb rot (Saniewski et al. 2004; Saniewski et al. 2006). JA was first identified in culture filtrates of Lasiodiplodia theobromae (Miersch et al. 1987), which is responsible for dieback diseases in peach and other fruit trees (Saniewski et al. 1998; Saniewski et al. 2006). A common symptom of Lasiodiplodia-instigated diseases is gummosis, which MeJA-treatment of peach stems phenocopies (Saniewski et al. 1998).
How Fusarium-derived jasmonates promote infection in roots remains unclear. Possibly, jasmonates promote gummosis (as cited above) and blockage of xylem vessels. Histological analysis has long associated infection by wilt fungi with pectin-like gum deposition in xylem vessels (Beckman 1987; Mace et al. 1981). Deposition of polysaccharide in nutrient-poor xylem sap may be beneficial to the growth of F. oxysporum. Alternatively, jasmonate signaling could be suppressing the host's defense response or the cell wall damage response (Denness et al., 2011; Millet et al., 2010).
Differences in the susceptibility of cultivated varieties usually correlate with the extent of infection in roots presumably because the differences reflect variation in innate immunity (Beckman and Roberts, 1995; Smith and Walker, 1930). However, when the relative susceptibility of hormone-related mutants are compared, the severity of symptoms may not correlate with the extent of infection because hormones may mediate the development of symptoms as well as the response to infection (Grant and Jones, 2009; Seilaniantz et al., 2011). Loss of abscisic acid biosynthesis (aba2) and insensitivity to ethylene (ein2) compromised the expression of foliar symptoms without curtailing infection, just as the auxin-related mutant tir3 suppresses the development of foliar symptoms without suppressing infection of roots (Diener, 2012; Kidd et al., 2011).
Experimental Procedures
Standards
(±) JA (Research Products International Corp., Mount Prospect, IL) and (±) 2H4 JA (C/D/N Isotopes Inc, Quebec, Canada) were purchased. JA-Leu, JA-Ile and 13C6-JA-Ile were generously provided by Paul Staswick (University of Nebraska, Lincoln).
Plant and Fusarium stocks
Published sources or the Arabidopsis Biological Resource Center (Ohio State University, Columbus) provided seeds: sid2-2 (Wildermuth et al., 2001), rfo1 (Diener and Ausubel, 2005), coi1-1 (Feys et al., 1994), aos (SALK_017756C), ein2-1 (CS3071), aba2-1 (CS156), THI2.1p:uidA (Epple et al., 1995) and JAI1/jai1 in the cultivar Castlemart (Li et al., 2004). Seeds were grown on Jiffy 7 peat pellets (Growers Solution, Cookeville, TN) under cool white fluorescent lighting with moderate intensity for a 12-hour daylength at 22°C before infection and 28°C after infection. Seeds were axenically grown on Plant Nutrient (PN) agar plates (Diener and Ausubel, 2005) with or without 0.5% sucrose. FOC race 1 (strain 777), FOR (strain 815) and FOM race 2 (strain 726) originate from P.H. Williams through H.C. Kistler (Bosland and Williams, 1987; Kistler et al., 1991). Kerry O'Donnell (USDA/ARS, Peoria, IL) provided FOL race 2 (strain 4287, Ma et al., 2010) and FOT (NRRL 26954), and H.C. Kistler provided FOL race 3 (MN-25) and F. graminearum (Gz3639).
Infection assays
Fusarium strains were grown on Czapek-Dox medium (Oxoid Ltd, Hampshire, UK), and conidial suspensions were harvested from 5-day old shaken cultures and washed 3 times with water. Two- to three-week old Arabidopsis plants were irrigated with 106 to 108 conidia/mL or mock-infected with water. Disease severity was scored using a health index (HI), described as a disease index in Diener and Ausubel (2005); briefly, HI scores ranged from 0 to 5 in increments of 0.5 and indicated the appearance of foliar symptoms: HI = 0 for dead plants, and HI = 5 for unaffected plants. Rosettes of plants with intermediate scores exhibited stunting (HI ≤ 4), perivascular yellowing (HI ≤ 3), senescence (HI ≤ 2) and necrosis (HI ≤ 1). ABF activity in infected roots was visualized and quantified as described in Diener (2012). The rosette radius was the mean length of the midrib of three leaves emerging from the stem at roughly 0°, 120° and 240°. Two-week old tomato seedlings in peat pellets were irrigated with 106 FOL conidia/mL (or water for mock-infection) and transplanted to soil pots. In each pot, jai1/jai1 and JAI1/JAI1 plants were paired: 12 pots were FOL-infected plants, and six were mock-infected plants. Tomato plants were scored using a disease index described in Figure 9.
Genotyping
Plants were genotyped as described in Diener and Ausubel (2005). Marker DNA of aos or AOS was PCR-amplified using primers, 5′-CTAACCGGAGGCTACCGTATC-3′ and either 5′-GCGTGGACCGCTTGCTGCAACT-3′ (LBb1) or 5′-AACAACAAAATCCTTACCGGC-3′, respectively. For coi1-1, a 612 bp product was PCR-amplified using primers 5′-tcgaccgggaagaaaggatta-3′ and 5′-acacagtttgtggaaacccca-3′, and XcmI digested wild-type but not coi1-1 DNA. Primer sequences for genotyping jai1, rfo1 and sid2-2 are described in Li et al. (2004), Diener and Ausubel (2005) and Wildermuth et al. (2001), respectively.
Quantification of jasmonates
Still cultures in 100 mL Czapek-Dox medium were kept in darkness at room temperature for three weeks, then centrifuged, filtered and stored at -80°C. Internal standards 2H4 JA (934 pmol) and 13C6 JA-Ile (978 pmol) were added to 1 ml aliquots of thawed culture filtrates, acidified to pH 2 with 0.1 N HCl, and extracted three times with 1 mL ethyl acetate. Pooled organic phases were dried under vacuum. Extracts were resuspended in 10 μL methanol/110 μL water, and 50 μl aliquots were separated by reverse phase HPLC (C18 Asentis Express, 15 cm × 2.1 mm × 2.7 μm, Supelco Analytics, Sigma-Aldrich, St. Louis, MO) equilibrated in 80% buffer A (water containing 10 mM HCO2NH4 and 0.1mM cetyl trimethylammonium bromide)/20% buffer B (CH3CN/aqueous 10mM HCO2NH4, 90/10, v/v) and eluted (200 ul/min) with an increasing concentration of buffer B (min/% B; 0/20, 2/20, 52/80, 55/20, 60/20). The effluent from the column was passed to a flow splitter and a proportion of the flow (about 20%) was passed to an Ionspray™ source connected to a triple quadrupole mass spectrometer (PE-Sciex ABI III+) operating in the negative ion tandem mass spectrometric multiple reaction monitoring (MRM) mode in which the intensity of specific parent → fragment ion transitions (JA m/z 209 → 59, 2H4 JA m/z 213 → 61, JA-Ile/JA-Leu m/z 322 → 130, 13C6 JA-Ile m/z 328 → 136) were monitored under previously optimized conditions (orifice -55 volts, argon collision gas at instrumental GCT setting of 180) using instrument-manufacturer supplied software for data acquisition (Tune version 2.5 and RAD version version 2.6) and analysis (MacSpec version 3.3 and BioMultiView version 1.3.1).
Two closely-eluting peaks in the sample chromatograms with the same transition as JA-Ile/Leu (m/z 322 → 130) were tentatively identified using JA-Ile and JA-Leu standards and co-chromatography experiments. Under the prescribed chromatographic conditions, the stereoisomers in the JA-Ile standard eluted as two equally intense peaks at 39.2 and 40.8 min, while the stereoisomers in the JA-Leu standard eluted as two equally intense peaks at 40.0 and 41.5 min. The four peaks of JA-Ile/Leu exhibited near-baseline separation when equal amounts of JA-Ile and JA-Leu standards were injected. Identity of JA-Ile/Leu peaks in sample extracts was confirmed by co-chromatography experiments by addition of authentic JA-Leu or JA-Ile to FOT fungal extract. In these experiments the peaks from the sample co-chromatographed with the early (40.8 min) or later (41.5 min) peak, respectively.
THI2.1p:uidA bioassay
Seedlings were grown for 7-day old seedlings on PN agar were vacuum infiltrated with culture filtrates, 100 μM MeJA in 0.02% acetone, 100 μM AgNO3 or mock-treated with corresponding solvent. Wounded seedlings were cut with a scalpel. Beta-glucuronidase activity was detected by staining overnight with 0.2 mg/ml 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-Gluc, Gold Biotechnologies, St. Louis, MO) in 0.1M sodium phosphate buffer, pH7.0 at 37°C. Seedlings were destained in ethanol/water, rehydrated and photographed.
Grafting
Grafts were essentially performed as described in Turnbull et al. (2002). MeJA-resistant coi1 progeny of COI1/coi1 were transferred to PN agar plates after selection on plates with 30μM MeJA. After joining scion and rootstocks, grafts were left on PN agar plates for ten days before transplanting to peat pellets. Four-week old grafted plants were infected two weeks after transplanting.
Supplementary Material
Figure S1. Induction of THI2.1p:uidA by methyl jasmonate, silver nitrate and wounding
Acknowledgments
An NIH graduate training program at UCLA supported S.C. The Dean of Life Sciences, UCLA and federal grants, NIH R37-GM48707 and NSF MCB-0519898, to F. Ausubel (MGH, Boston, MA) supported A.D. We thank P. Staswick for the provision of jasmonate standards.
References
- Aboul-Soud MA, Yun BW, Harrier LA, Loake GJ. Transformation of Fusarium oxysporum by particle bombardment and characterisation of the resulting transformants expressing a GFP transgene. Mycopathologia. 2004;158:475–482. doi: 10.1007/s11046-005-5370-7. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K. Antagonistic interaction between abscisic acid and jasmonate ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell. 2004;16:3460–3479. doi: 10.1105/tpc.104.025833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman CH. The nature of wilt diseases of plants. American Phytopathological Society; St. Paul: 1987. [Google Scholar]
- Beckman CH, Roberts EM. On the Nature and Genetic Basis for Resistance and Tolerance to Fungal Wilt Diseases of Plants. Adv Bot Res. 1995;21:35–77. [Google Scholar]
- Bender CL, Alarcón-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev. 1999;63:266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocal-Lobo M, Molina A. Ethylene response factor 1 mediates Arabidopsis resistance to the soilborne fungus Fusarium oxysporum. Mol Plant Microbe Interact. 2004;17:763–770. doi: 10.1094/MPMI.2004.17.7.763. [DOI] [PubMed] [Google Scholar]
- Bohlmann H, Vignutelli A, Hilpert B, Miersch O, Wasternack C, Apel K. Wounding and chemicals induce expression of the Arabidopsis thaliana gene Thi2.1, encoding a fungal defense thionin, via the octadecanoid pathway. FEBS Lett. 1998;437:281–286. doi: 10.1016/s0014-5793(98)01251-4. [DOI] [PubMed] [Google Scholar]
- Bosland PW, Williams PH. An evaluation of Fusarium oxysporum from crucifers based on pathogenicity isozyme polymorphism, vegetative compatibility and geographic origin. Can J Bot. 1987;65:2067–2073. [Google Scholar]
- Chehab EW, Kaspi R, Savchenko T, Rowe H, Negre-Zakharov F, Kliebenstein D, Dehesh K. Distinct roles of jasmonates and aldehydes in plant-defense responses. PLoS One. 2008;3:e1904. doi: 10.1371/journal.pone.0001904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SJ, Diener AC. Diversity in receptor-like kinase genes is a major determinant of quantitative resistance to Fusarium oxysporum f.sp. matthioli. New Phytol. 2013;200:172–184. doi: 10.1111/nph.12368. [DOI] [PubMed] [Google Scholar]
- Cross BE, Webster GRB. New Metabolites of Gibberella fujikuroi. Part XV. N-jasmonoyl-and n-dihydrojasmonoyl-isoleucine. J Chem Soc. 1970:1839–1842. doi: 10.1039/j39700001839. [DOI] [PubMed] [Google Scholar]
- Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M, Mansfield J, Zipfel C, Hamann T. Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol. 2011;156:1364–1374. doi: 10.1104/pp.111.175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener AC, Ausubel FM. RESISTANCE TO FUSARIUM OXYSPORUM 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics. 2005;171:305–321. doi: 10.1534/genetics.105.042218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener A. Visualizing and quantifying Fusarium oxysporum in the plant host. Mol Plant Microbe Interact. 2012;25:1531–1541. doi: 10.1094/MPMI-02-12-0042-TA. [DOI] [PubMed] [Google Scholar]
- Epple P, Apel K, Bohlmann H. Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell. 1997;9:509–520. doi: 10.1105/tpc.9.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple P, Vignutelli A, Apel K, Bohlmann H. Differential induction of the Arabidopsis thaliana Thi2.1 gene by Fusarium oxysporum f. sp. matthiolae. Mol Plant Microbe Interact. 1998;11:523–529. doi: 10.1094/MPMI.1998.11.6.523. [DOI] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R. (+)-7-iso-Jasmonoyl-L-isoleucine is the endogenous bioactive jasmonate. Nature Chem Biol. 2009;5:344–350. doi: 10.1038/nchembio.161. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Ann Rev Phytopathol. 2005;43:205–227. doi: 10.1146/annurev.phyto.43.040204.135923. [DOI] [PubMed] [Google Scholar]
- Grant MR, Jones JD. Hormone (dis)harmony moulds plant health and disease. Science. 2009;324:750–752. doi: 10.1126/science.1173771. [DOI] [PubMed] [Google Scholar]
- Hilpert B, Bohlmann H, op den Camp RO, Przybyla D, Miersch O, Buchala A, Apel K. Isolation and characterization of signal transduction mutants of Arabidopsis thaliana that constitutively activate the octadecanoid pathway and form necrotic microlesions. Plant J. 2001;26:435–446. doi: 10.1046/j.1365-313x.2001.2641036.x. [DOI] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci U S A. 2008;105:7100–7105. doi: 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd BN, Kadoo NY, Dombrecht B, Tekeoglu M, Gardiner DM, Thatcher LF, Aitken EA, Schenk PM, Manners JM, Kazan K. Auxin signaling and transport promote susceptibility to the root-infecting fungal pathogen Fusarium oxysporum in Arabidopsis. Mol Plant-Microbe Interact. 2011;24:733–748. doi: 10.1094/MPMI-08-10-0194. [DOI] [PubMed] [Google Scholar]
- Kistler HC, Momol EA, Benny U. Repetitive genomic sequences for determining relatedness among strains of Fusarium oxysporum. Phytopathology. 1991;81:331–336. [Google Scholar]
- Koo AJ, Howe GA. The wound hormone jasmonate. Phytochemistry. 2009;70:1571–80. doi: 10.1016/j.phytochem.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramell R, Atzorn R, Schneider G, Miersch O, Brückner C, Schmidt J, Sembdner G, Parthier B. Occurrence and identification of jasmonic acid and its amino acid conjugates induced by osmotic stress in barley leaf tissue. J Plant Growth Regul. 1995;14:29–36. [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol. 2002;5:325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Piqueras R, Sánchez-Serrano JJ, Solano R. ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell. 2003;15:165–178. doi: 10.1105/tpc.007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace ME, Bell AA, Beckman CH. Fungal wilt diseases of plants. Academic Press; New York: 1981. [Google Scholar]
- McGrath KC, Dombrecht B, Manners JM, Schenk PM, Edgar CI, Maclean DJ, Scheible WR, Udvardi MK, Kazan K. Repressor- and activator-type ethylene response factors functioning in jasmonate signaling and disease resistance identified via a genome-wide screen of Arabidopsis transcription factor gene expression. Plant Physiol. 2005;139:949–959. doi: 10.1104/pp.105.068544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielse CB, Rep M. Pathogen profile update: Fusarium oxysporum. Mol Plant Pathol. 2009;10:311–324. doi: 10.1111/j.1364-3703.2009.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miersch O, Preiss A, Sembdner G, Schreiber K. (+)-7-iso-jasmonic acid and related compounds from Botryodiplodia theobromae. Phytochemistry. 1987;26:1037–1039. [Google Scholar]
- Miersch O, Bohlmann H, Wasternack C. Jasmonates and related compounds from Fusarium oxysporum. Phytochemistry. 1999a;50:517–523. [Google Scholar]
- Miersch O, Kramell R, Parthier B, Wasternack C. Structure–activity relations of substituted, deleted or stereospecifically altered jasmonic acid in gene expression of barley leaves. Phytochemistry. 1999b;50:353–361. [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichhart D, Ausubel FM. Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell. 2010;22:973–990. doi: 10.1105/tpc.109.069658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K, Gueidan C, Sink S, Johnston PR, Crous PW, Glenn A, Riley R, Zitomer NC, Colyer P, Waalwijk C, Lee T, Moretti A, Kang S, Kim HS, Geiser DM, Juba JH, Baayen RP, Cromey MG, Bithell S, Sutton DA, Skovgaard K, Ploetz R, Kistler HC, Elliott M, Davis M, Sarver BA. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet Biol. 2009;46:936–948. doi: 10.1016/j.fgb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Saniewski M, Miyamoto K, Ueda J. Methyl jasmonate induces gums and stimulates anthocyanin accumulation in peach shoots. J Plant Growth Regul. 1998;17:121–124. [Google Scholar]
- Saniewski M, Ueda J, Miyamoto K, Okubo H, Puchalski J. Interaction of methyl jasmonate and ethephon in gum formation in tulip bulbs. J Fac Agric Kyushu Univ. 2004;49:207–215. [Google Scholar]
- Saniewski M, Ueda J, Miyamoto K, Horbowicz M, Puchalski J. Hormonal control of gummosis in Rosaceae. J Fruit Ornamental Plant Res. 2006;14:137–144. [Google Scholar]
- Seilaniantz RA, Grant M, Jones JD. Hormone crosstalk in plant disease and defense: more than just jasmonate salicylate antagonism. Ann Rev Phytopathol. 2011;49:317–343. doi: 10.1146/annurev-phyto-073009-114447. [DOI] [PubMed] [Google Scholar]
- Shen Y, Diener AC. Arabidopsis thaliana RESISTANCE TO FUSARIUM OXYSPORUM 2 implicates tyrosine-sulfated peptide signaling in susceptibility and resistance to root infection. PLoS Genet. 2013;9:e1003525. doi: 10.1371/journal.pgen.1003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Walker JC. A cytological study of cabbage plants in strains susceptible or resistant to yellows. J Agri Res. 1930;40:721–745. [Google Scholar]
- Talboys PW. Resistance to vascular wilt fungi. Proc R Soc London Ser B. 1972;181:319–332. [Google Scholar]
- Tamogami S, Rakwal R, Kodama O. Phytoalexin production by amino acid conjugates of jasmonic acid through induction of naringenin-7-O-methyltransferase, a key enzyme on phytoalexin biosynthesis in rice (Oryza sativa L.) FEBS Lett. 1997;401:239–242. doi: 10.1016/s0014-5793(96)01482-2. [DOI] [PubMed] [Google Scholar]
- Thatcher LF, Manners JM, Kazan K. Fusarium oxysporum hijacks COI1-mediated jasmonate signaling to promote disease development in Arabidopsis. Plant J. 2009;58:927–939. doi: 10.1111/j.1365-313X.2009.03831.x. [DOI] [PubMed] [Google Scholar]
- Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR. Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J. 2009;58:69–81. doi: 10.1111/j.1365-313X.2008.03755.x. [DOI] [PubMed] [Google Scholar]
- Vignutelli A, Wasternack C, Apel K, Bohlmann H. Systemic and local induction of an Arabidopsis thionin gene by wounding and pathogens. Plant J. 1998;14:285–295. doi: 10.1046/j.1365-313x.1998.00117.x. [DOI] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang C, Gu M, Bai Z, Zhang W, Qi T, Cheng Z, Peng W, Luo H, Nan F, Wang Z, Xie D. The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell. 2009;21:2220–2236. doi: 10.1105/tpc.109.065730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Induction of THI2.1p:uidA by methyl jasmonate, silver nitrate and wounding


