SUMMARY
There is comparatively little information about premorbid maturational brain abnormalities in schizophrenia. We investigated whether a history of childhood enuresis, a well-established marker of neurodevelopmental delay, is associated with schizophrenia and with measures of brain abnormalities also associated with schizophrenia. A DSM-IV based history of enuresis, volumetric brain MRI scans, and neuropsychological testing were obtained in patients with schizophrenia, their non-psychotic siblings, and non-psychiatric controls. The subjects were 211 patients (79.6% male), 234 of their siblings (43.2% male), and 355 controls (39.2% male). Frequency of enuresis was compared across groups and correlated with cognitive measures. Total and regional brain volumes were determined using VBM on matched subsets of probands (n=82) with or without enuresis (n=16, n=66, respectively) and controls (n=102) with or without enuresis (n=11, n=91, respectively). Patients with schizophrenia had higher rates of childhood enuresis (21%) compared with siblings (11%; χ2=6.42, p=0.01) or controls (7%; χ2=23.65, p<0.0001) and relative risk for enuresis was increased in siblings (λS=2.62). Patients with enuresis performed worse on two frontal lobe cognitive tests [Letter Fluency (t=1.97, p=0.05, df=200) and Category Fluency (t=2.15, p=0.03, df=200)] as compared with non-enuretic patients. VBM analysis revealed gray matter volume reductions in several frontal regions (right BA 9, right BA 10, and bilateral BA 45), and right superior parietal cortex (BA 7) in patients with a history of enuresis as compared with non-enuretic patients (all t>3.57, all p<0.001). The high frequency of childhood enuresis associated with schizophrenia, and abnormalities in prefrontal function and structure in patients with a childhood history of enuresis suggest that childhood enuresis may be a premorbid marker for neurodevelopmental abnormalities related to schizophrenia. These findings add to the evidence implicating prefrontal dysmaturation in this disorder, potentially related to genetic risk factors.
Keywords: Schizophrenia, Enuresis, Development, Neuroimaging, Frontal lobes
INTRODUCTION
Despite a prodromal period in late adolescence and early adulthood and a relatively fixed age of risk for onset, schizophrenia is generally conceptualized as a neurodevelopmental disorder. Pioneering prospective studies by Bender and later Fish described 'pandysmaturation' in motor, intellectual, and adaptive functioning of infants who subsequently developed schizophrenia (Bender 1947; Fish et al 1965). Developmental delays have been detected in infancy and early childhood in numerous studies both in offspring of patients with schizophrenia and 'pre-schizophrenics', including abnormalities in acquisition and refinement of language, gross motor delays in standing and walking, and delayed physical growth (Fish & Kendler 2005; Fish et al 1992; Isohanni et al 2001; Jones et al 1994).
The acquisition of volitional bladder control, particularly at night, is an important and well-established developmental neurological milestone. It usually occurs around 4 years of age (Johnson 1998). Rates of enuresis in otherwise healthy children vary from 10% among six year olds to 5% among ten year olds (Neveus et al 2000). In general, enuresis is more common in boys than girls (Johnson 1998; Neveus et al 2000). Persistent bedwetting has been associated with delayed achievement of language and motor milestones in otherwise healthy children (Touchette et al 2005). The DSM-IV characterizes enuresis as a disorder when there is a persistent loss of bladder control after age 5. In most cases, delays in the acquisition of volitional bladder control beyond age 5, in the absence of medical causes such as diabetes or spina bifida, represent a neurodevelopmental abnormality.
Few studies have explored the relationship between a history of childhood enuresis and schizophrenia, although Kraepelin, as early as 1919, anecdotally referred to “obstinate nocturnal enuresis” in childhood in cases of dementia praecox (Kraepelin 1919–1971). In a birth cohort study limited by the absence of statistical analysis, 40 ‘pre-schizophrenics’, assessed at regular intervals from 7 through 28 years of age, were said to be more likely enuretic by day at age 3 and by night at age 5 than controls (Crow et al 1995). In addition to a lack of statistical results, this study did not address whether and to what extent bedwetting persisted beyond age five, which DSM-IV criteria require for a diagnosis of enuresis. Another analysis of this cohort showed that at age 7, individuals who subsequently went on to develop schizophrenia were more likely to have incontinence problems than normal controls (p<0.10) (Jones 1997). A Finnish study of developmental progress at one year of age suggested that females with slower onset of urinary continence had a slightly increased risk of schizophrenia (Isohanni et al 2001). A study of child/adolescent-onset schizophrenia found late onset of urinary incontinence in 36% of subjects with schizophrenia (n=61), compared to 18% of patients with affective psychoses (n=48), and subjects with a preponderance of negative symptoms were more likely to have had incontinence (Hollis 2003). It is not clear from this study whether the emergence of incontinence in the setting of manifest illness was associated with enuresis prior to illness onset. This is an important point because the biologic meaning of incontinence during acute psychosis and enuresis during development may not be the same. Another study found increased rates of bedwetting at age 4 in offspring of schizophrenic women compared with controls (Henriksson & McNeil 2004). Finally, adult patients with schizophrenia have higher rates of urinary incontinence than patients with mood disorders (Bonney et al 1997). These various studies provide some evidence for delayed or impaired bladder control in individuals at risk for schizophrenia or in child/adolescent-onset schizophrenia. However, there has been no systematic study of the relationship between schizophrenia diagnosed in adulthood and antecedent childhood enuresis as defined by current criteria for persistent bedwetting beyond the age of five.
A limited number of studies have explored the relationship between childhood enuresis and other psychiatric disorders. Two studies found increased rates of childhood enuresis in children with ADHD (28–32%) (Biederman et al 1995; Neveus et al 2000); one study found increased rates of enuresis in childhood Tourette’s syndrome (22%) (Comings & Comings 1987); and one study found increased rates of enuresis in adult bipolar disorder (18%) (Henin et al 2007). Persistent enuresis has also been associated with an increased risk of adolescent suicidal behavior (Liu & Sun 2005). Persistent enuresis after the age of 10 years has been associated with a small but significantly increased risk of conduct problems, attention deficit behaviors, and anxiety/withdrawal in adolescence (Fergusson & Horwood 1994). Feehan and colleagues (1990) found that secondary enuresis (enuresis recurring after at least a year of continence), but not primary enuresis, was associated with an increased rate of behavioral problems (Feehan et al 1990). In Chinese children, persistent enuresis after 4 years of age is associated with increased behavior problems, as reported by both parents and teachers (Liu et al 2000). A similar finding was reported in US children with enuresis persisting after 5 years of age (Byrd et al 1996). These studies illustrate that enuresis as a putative marker of cerebral developmental delay is not an antecedent for a specific behavioral disorder any more that it is specifically associated with a single childhood neurological disorder.
To define forebrain structures involved in volitional bladder control, traditional clinical-neuropathological studies have relied upon the association between acquired brain lesions and the development of urinary incontinence. Nearly 75% of stroke patients with frontal lobe lesions developed urinary incontinence (Sakakibara et al 1999). Right superior frontal damage has been linked with transient urinary incontinence while bilateral damage has been associated with permanent incontinence (Mochizuki & Saito 1990). These studies suggest that the frontal lobes in general, and the superior frontal gyri in particular, are important in volitional bladder control.
Functional neuroimaging studies have more clearly defined the neural network involved in volitional bladder control. While peripheral muscles, nerves, spinal cord and brainstem structures play an important role in bladder control, forebrain structures are also important. In normal controls, the left superior frontal gyrus, supplementary motor area, cingulate gyrus, left orbitofrontal cortex, bilateral frontal opercula and insula were activated during a strong urge to void, using an fMRI paradigm (Kuhtz-Buschbeck et al 2005). Depending upon the physiological paradigm employed, PET studies have implicated the mid-cingulate and prefrontal cortices bilaterally, right inferior frontal gyrus, parts of the premotor cortex and cingulate gyri bilaterally, right anterior cingulate gyrus, left superior prefrontal, middle frontal, and anterior cingulate gyri, and left insular cortex in the neural regulation of volitional bladder control (Athwal et al 2001; Blok et al 1997; Blok et al 1998; Nour et al 2000). In summary, functional neuroimaging studies have implicated a complex neural network involving, among other regions, frontal, insular, and cingulate cortices in volitional bladder control.
Neuropathology, neuroimaging, and neuropsychological studies have consistently implicated the frontal lobes in the pathogenesis of schizophrenia (Weinberger et al 2001). We hypothesized that if these abnormalities in schizophrenia are related to frontal lobe development, then since maturation and functional competence of the frontal lobes also are important in the acquisition of volitional bladder control in children, schizophrenic patients would have a higher rate of childhood enuresis as a secondary manifestation of their frontal lobe developmental abnormalities. In a broad-based family study of schizophrenia, we assessed the rates of childhood enuresis in probands compared to their non-psychotic siblings and normal controls. Assessment of enuresis in siblings allowed us to address the potential heritability of this developmental condition. We also examined neuropsychological test performance and volumetric brain morphometry (voxel-based morphometry or VBM) of these groups, hypothesizing that subjects with a history of childhood enuresis would have evidence of persistent frontal lobe dysfunction and volumetric abnormalities continuing into adulthood. This is the first comprehensive study to examine the history of childhood enuresis in a large cohort of patients with adult-onset schizophrenia.
METHOD
Subject Selection
A total of 800 subjects participated, a subset of participants in the Clinical Brain Disorders Branch ‘Sibling Study’ protocol (NIMH #95-M-0150), an ongoing investigation of neurobiological abnormalities related to genetic risk for schizophrenia. Subjects were volunteers recruited nationally and through the local community, as well as from the NIMH Inpatient Schizophrenia Research Program. All of the subjects gave written informed consent according to the guidelines of the NIMH/ NIH Institutional Review Board and the regulations and ethical guidelines of the NIH Office of Human Subjects Research. Subjects were between the ages of 18–60. Participants included 211 patients with schizophrenia (herein “probands” or SCZ) (82.5% schizophrenia, 17.5% schizoaffective disorder, 79.6% male), 234 of their non-psychotic siblings (SIB) (43.2% male), and 355 non-psychiatric controls (NC) (39.2% male). All subjects were evaluated by a research psychiatrist for lifetime psychiatric illness and substance use according to DSM-IV, using the Structural Clinical Interview for DSM-IV Disorders (SCID) (First 1997). Subjects were excluded from any of the three diagnostic groups if they met DSM-IV criteria for substance use/dependence within the past year, and also if they had a lifetime history of abuse/dependence that exceeded five years. Furthermore, at the time of testing, all subjects received a blood toxicology screening to rule-out acute alcohol or drug intoxication. Subjects were also excluded if they had a history of significant medical or neurological disorders, such as epilepsy or traumatic brain injury.
In addition, detailed demographic information and clinical histories, including age of onset of prodromal symptoms and of illness, were compiled on each proband. Specifically, prodromal age and age of onset were determined by merging data from all past medical/psychiatric records, the SCID interview, and self/family completed application. In every case, a third party informant (i.e., either psychiatric records or a treating clinician) was used to verify symptoms and onset of illness. A second board-certified psychiatrist confirmed the diagnosis of schizophrenia for every proband. Additional information on subject recruitment, screening and examination are described elsewhere (Egan et al 2001). For demographic data on the subjects, see Table 1.
Table 1.
Demographic Data
| Variable | Diagnostic Groups | ||
|---|---|---|---|
| Probands (SCZ) (n=211) |
Siblings (n=234) |
Controls (n=355) |
|
| Age (yrs) | 33.7 (9.1) | 35.1 (9.0) | 31.6 (9.5) |
| Sex (M/F) | 168/43 | 68/84 | 139/216 |
| Race # (%) Caucasian | 172 (81.5) | 130 (85.5) | 297 (83.7) |
| Education (yrs) | 14.0 (2.1) | 15.9 (2.4) | 16.8 (2.4) |
| WAIS-FSIQ | 92.2 (11.4) | 106.0 (11.0) | 106.9 (9.7) |
| GAF | 46.4 (15.6) | 85.9 (6.7) | 86.5 (6.3) |
| Probands with and without Enuresis – Neuropsych subset | |||
| Variable | SCZ+Enuresis | SCZ-Enuresis | Group Comparison |
| (n=45) | (n=166) | ||
| Age (yrs) | 33.2 (9.2) | 33.8 (9.0) | t=0.43, p=0.67 |
| Sex (M/F) | 39/6 | 129/37 | χ2=1.75, p=0.19 |
| Race # (%) Caucasian | 38 (84.4) | 134 (80.7) | χ2=0.33, p=0.57 |
| Prodromal Age | 17.1 (6.3) | 19.2 (5.6) | t=2.06, p=0.04* |
| Age Onset Illness | 20.3 (4.8) | 21.6 (5.3) | t=1.39, p=0.17 |
| Education (yrs) | 13.7 (2.1) | 14.1 (2.0) | t=1.21, p=0.23 |
| GAF | 44.8 (13.9) | 46.8 (16.0) | t=0.76, p=0.45 |
| Age Offset Enuresis | 8.3 (3.3) | --- | --- |
| Probands with and without Enuresis – VBM imaging subset | |||
| Variable | SCZ+Enuresis | SCZ+Enuresis | Group Comparison |
| (n=16) | (n=16) | ||
| Age (yrs) | 34.7 (11.2) | 33.1 (9.5) | t=−0.60, p=0.55 |
| Sex (M/F) | 14/2 | 55/11 | Fisher exact, p=0.51 |
| Race # (%) Caucasian | 14 (87.5) | 53 (80.3) | Fisher exact, p=0.40 |
| Prodromal Age | 17.6 (6.8) | 19.7 (5.4) | t=1.31, p=0.20 |
| Age Onset Illness | 20.9 (6.2) | 21.7 (5.3) | t=0.53, p=0.60 |
| Education (yrs) | 13.6 (2.2) | 14.4 (2.2) | t=1.44, p=0.15 |
| GAF | 46.1 (11.8) | 47.7 (15.9) | t=0.39, p=0.70 |
| Age Offset Enuresis | 8.8 (2.6) | --- | |
Mean (SD) unless stated otherwise. SCZ=schizophrenia; WAIS-FSIQ=Wechsler Adult Intelligence Scale-revised, full-scale intelligence quotient; GAF=Global Assessment of Functioning Scale; Prodromal Age=age of onset of prodromal symptoms of schizophrenia
p≤0.05.
A history of enuresis was obtained from the mother of each participant by applying DSM-IV criteria (i.e., frequency of wetting >2 times per week for at least 3 consecutive months at age 5 or older, not due to a general medical condition or effect of a substance), as part of a birth and developmental history questionnaire. Neurological/medical co-morbidity contributing to childhood enuresis was evaluated through subject and maternal interviews. Subject cohorts were subdivided into those with or without a history of childhood enuresis. Rates of enuresis for each subject cohort were then compared. For the group comparisons with siblings, this particular analysis was limited to a subset of siblings (n=152) consisting of one randomly selected sibling per family.
Neuropsychological Tests
All subjects completed a battery of neuropsychological tests in order to assess academic skill (WRAT-R reading subtest) , general intellectual ability (a 4-subtest WAIS-R short form IQ) , psychomotor speed (Trail Making Tests A and B), working memory (1 and 2 back versions of the N-Back), problem-solving (Wisconsin Card Sorting Test), episodic memory (California Verbal Learning Test), and speeded verbal retrieval (Letter and Category Fluency) (Egan et al 2001).
Magnetic Resonance Imaging
Subjects
All structural imaging data free of visible artifact, distortion, incomplete acquisition, or evidence of neurological abnormality, were processed for whole-brain VBM analysis. Subjects had to have both available and usable structural imaging data meeting these quality control criteria and complete enuresis data to be included in the VBM sample. The resulting sample was 82 probands (16 SCZ+enuresis, 66 SCZ-enuresis) and 102 controls (11 NC+enuresis, 91 NC-enuresis). Thus, 20% of probands and 40.3% of controls from the original sample were excluded based on VBM quality control criteria. All probands were on antipsychotic medications at the timing of scan acquisition. Differences in demographic means between each diagnostic group were tested using an ANOVA in SPSS version 13.0 (www.spss.com). There were no significant differences between probands with and without a history of enuresis in age, sex, education, handedness, and WAIS full-scale IQ, nor were there significant differences between controls with and without a history of enuresis on these measures. Both the proband and control subgroups were matched for age, sex, WAIS full-scale IQ, handedness, and education.
Structural image processing and analyses
Image acquisition and data processing software have been described in a previous study from our group (Pezawas et al 2004), and most specifically in a recent study from this group involving a largely overlapping sample of subjects (Honea et al 2008). Customized template creation was based on a sample of 100 healthy volunteers, 100 non-psychotic siblings, and 100 probands taken from our study sample. These subjects were grouped to best represent the sample as a whole, and were matched for age (NC 35.6± 9.97 years; SCZ 36.18±9.54 years; p>.05). The iterative procedure used to make the customized template has been described previously (Pezawas et al 2004).
We used optimized VBM performed in SPM2, including the non-uniformity correction option (Ashburner & Friston 2000; Augood et al 1997). Images were modulated by multiplication with the determinant of the Jacobian of the spatial normalization function, and an integration of modulated gray matter voxel values resulted in estimates for total gray matter volume. Modulated images were smoothed 10mm for the whole brain VBM analysis.
The normalized, modulated and smoothed gray matter images were analyzed using the General Linear Model as implemented in SPM2. For this within-group analysis for NC and SCZ, we used an analysis of covariance model with enuresis history as factor, age and gender as covariates of interest, and total gray matter volume and second-order polynomial age expansions as covariates of no interest. Voxel-by-voxel t-tests were computed across the whole brain to determine global differences in gray matter volumes based on enuresis history, contrasting NC+enuresis vs. NC-enuresis, and SCZ+enuresis vs. SCZ-enuresis. There were an insufficient number of siblings to examine volumetric changes between SIB+enuresis and SIB-enuresis in our structural dataset. Significant voxels (thresholded at .001 uncorrected, cluster (k) > 5 contiguous voxels) are reported in Talairach space as in prior reports (Callicott et al 2005). A threshold was set at this level because of the lack of power, small numbers of those with a history of enuresis, and the fact that this was a within-group exploratory analysis which has never been performed previously.
Statistics
Statistical analyses of group differences in parameters other than MRI were computed with chi-square analyses or student t-tests using Statistica 7.1 (Statsoft, Inc., 2005; www.statsoft.com).
RESULTS
Probands had significantly higher rates of enuresis than their non-psychotic siblings (21.3% vs. 11.2%, χ2=6.42, p=0.01) and controls (21.3% vs. 7.3%, χ2=23.7, p<0.0001). Male probands also had significantly higher rates of enuresis than male siblings (23.2% vs. 10.3%, χ2=5.15, p=0.02) or male controls (23.2% vs. 11.5%, χ2=7.08, p=0.008). Female probands had significantly higher rates of enuresis than female controls (14% vs. 4.6%, χ2 = 5.38, p=0.02) but not female siblings (14% vs. 12%, χ2=0.11, p=0.72). No significant differences in rates of enuresis were found between siblings and controls in the overall sample or male subset, but were found in female siblings vs. female controls (12% vs. 4.6%, χ2=5.14, p=0.02). Siblings of probands with enuresis had significantly higher rates of enuresis than siblings of probands without enuresis (19.1% vs. 7.0%, χ2=6.56, p=0.01). Thus, familial relative risk for enuresis in probands with enuresis was calculated to be λS=2.62 (Risch 1990).
Probands with enuresis did not differ from probands without enuresis on any demographic variable, with the exception of being significantly younger at age of first prodromal symptoms (17.1 vs. 19.2, t=2.06, p=0.04). In analyses of cognitive data, we found that probands with enuresis performed significantly worse than probands without enuresis on two measures, both involving speed-dependent verbal retrieval [Letter Fluency (mean score 31.1 vs. 35.2, t=1.97, p=0.05, df=200) and Category Fluency (mean score 33.6 vs. 37.8, t=2.15, p=0.03, df=200)]. None of the other contrasts approached significance (all p>0.20, see Table 2). Given the number of tests performed without alpha correction, it is tempting to consider the verbal fluency findings as representing chance results. However, a nearly identical pattern of findings emerged in controls. Controls with enuresis performed significantly worse than controls without enuresis on Letter Fluency (mean score 40.7 vs. 45.3, t=1.96, df=335, p=0.05, two-tailed), and showed a trend towards worse performance on Category Fluency (mean score 50.0 vs. 53.8, t=1.71, df=335, p=0.09, two-tailed) in comparison to controls without enuresis. No other significant findings were found between the two control groups on neuropsychological measures. Thus, in both subject groups, enuresis was significantly and selectively associated with relatively poorer verbal fluency performance alone. No significant differences were found between siblings with or without enuresis on any neuropsychological measures.
Table 2.
Neuropsychological Assessment of Probands with and without Enuresis
| Variable | SCZ+Enuresis (n=45) |
SCZ-Enuresis (n=166) |
Group Comparison |
|---|---|---|---|
| General Intelligence | |||
| WAIS-FSIQ | 92.3 (9.3) | 92.2 (12.0) | t=−0.03, p=0.98 |
| Academic Skill | |||
| WRAT Reading | 99.6 (12.8) | 101.5 (11.9) | t=0.95, p=0.34 |
| Psychomotor Speed | |||
| Trails A | 41.8 (18.8) | 38.2 (15.3) | t=−1.29, p=0.20 |
| Trails B | 95.9 (50.2) | 96.1 (52.2) | t=0.02, p=0.98 |
| Working Memory | |||
| N-back (1-back) | 0.66 (0.25) | 0.68 (0.25) | t=0.77, p=0.77 |
| N-back (2-back) | 0.51 (0.23) | 0.50 (0.23) | t=−0.06, p=0.95 |
| Problem Solving | |||
| WCST %PE | 39.2 (11.7) | 39.4 (12.0) | t=0.09, p=0.93 |
| Episodic Memory | |||
| CVLT Standard | 23.1 (16.8) | 23.4 (15.6) | t=0.12, p=0.91 |
| CVLT Corrected | 39.3 (12.8) | 39.6 (11.5) | t=0.16, p=0.88 |
| Speeded Verbal Retrieval | |||
| Letter Fluency | 31.1 (10.1) | 35.2 (12.6) | t=1.97, p=0.05* |
| Category Fluency | 33.6 (9.4) | 37.7 (11.7) | t=2.15, p=0.03* |
SCZ=schizophrenia; WAIS-FSIQ=Wechsler Adult Intelligence Scale, full scale IQ; WRAT=Wide Range Achievement Test; WCST=Wisconsin Card Sorting Test; %PE=percentage of perseverative errors; CVLT=California Verbal Learning Test
p≤0.05.
Magnetic Resonance Imaging
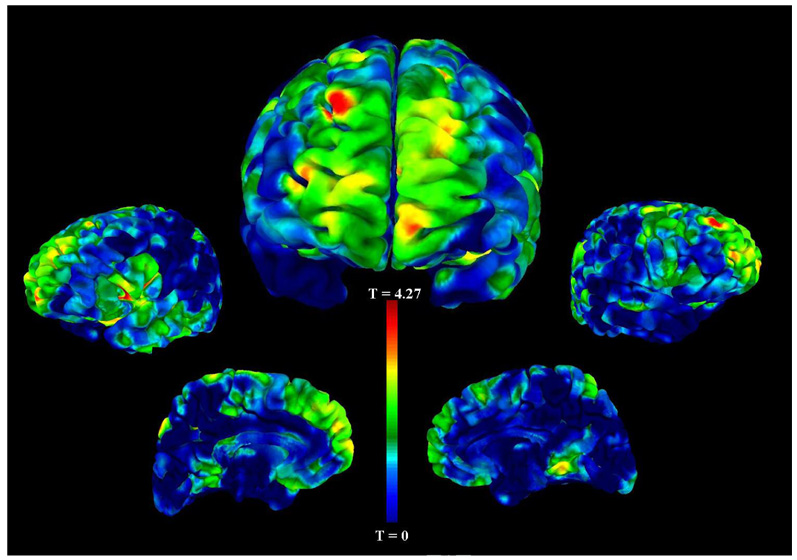
The VBM analysis of probands revealed frontal gray matter decreases in those with a history of enuresis (16 probands with enuresis and 66 probands without enuresis). Specifically, we found reductions of gray matter volume in the right superior frontal gyrus (BA 9, p<0.001, t=4.36), right middle frontal gyrus (BA 10, p<0.001, t=3.57), bilateral inferior frontal gyrus (BA 45, p<0.001, t=3.75), and right superior parietal cortex (BA 7, p<0.001, t=3.74) (see Table 3 and Figure 1).
Table 3.
Voxel-wise Statistical Analysis of GM Maps of Decreased Gray Matter in Probands with (n=16) vs. without Enuresis (n=66), and in Healthy Controls with (n=11) and without Enuresis (n=91)
| Probands | |||||||
|---|---|---|---|---|---|---|---|
| MNI Label | kE | T | Z | p | x | y | z |
| Right Middle Frontal Gyrus, BA 10 | 84 | 3.57 | 3.33 | 0.000* | 33 | 63 | 13 |
| Right Superior Frontal Gyrus, BA 9 | 404 | 4.36 | 4.10 | 0.000* | 22 | 51 | 39 |
| Left Inferior Frontal Gyrus, BA 45 | 248 | 3.75 | 3.58 | 0.000* | −44 | 27 | 7 |
| Right Inferior Frontal Gyrus, BA 45 | 117 | 3.75 | 3.58 | 0.000* | 47 | 18 | 13 |
| Left Superior Parietal Lobe, BA 7 | 51 | 3.74 | 3.42 | 0.000* | −12 | −79 | 57 |
| Controls | |||||||
| MNI Label | kE | T | Z | p | x | y | z |
| Right Medial Frontal Gyrus, BA 11 | 22112 | 3.42 | 3.31 | 0.000* | 0 | 20 | −12 |
| Right Middle Temporal Gyrus, BA 21 | 19033 | 3.42 | 3.31 | 0.000* | 62 | −25 | −9 |
| Left Middle Temporal Gyrus, BA 22 | 25372 | 4.08 | 3.90 | 0.000* | −53 | −43 | 3 |
| Left Cuneus | 1236 | 3.75 | 3.62 | 0.000* | −14 | −76 | 18 |
Peak coordinates listed rostral to caudal.
p value is p<0.001.
kE is the number of voxels in the significant cluster.
Figure 1. Gray Matter Volume Decreases in Probands with Enuresis Compared to Probands without Enuresis.
This figure depicts the statistical main effects of decreased regional gray matter volume comparing probands with a history of childhood enuresis to those without a history. The unthresholded VBM results are overlaid on an AFNI (Analysis of Functional Neuro-Images) pial surface and color-coded for the statistical t-score. The central figure is the frontal view, and the other figures, from left to right, are the left lateral, left medial, right medial, and right lateral hemispheric surface views. The most significant cluster of decreased gray matter volume in probands with enuresis is in the right superior frontal gyrus, BA 9 (coordinates x=22, y=51, z=39, p<0.001). Other clusters that were statistically significant are listed in Table 3.
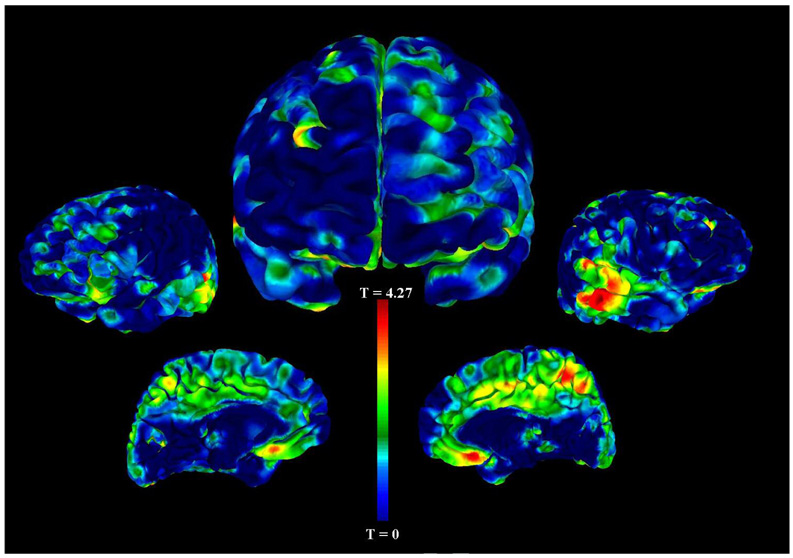
The VBM analysis on the subset of healthy controls also revealed areas of significantly decreased gray matter volume in those with a history of enuresis (11 healthy controls with enuresis and 91 healthy controls without enuresis). More specifically, we found reductions of gray matter volume in the right medial frontal gyrus (BA 11, p<0.001, t=3.42), right middle temporal gyrus (BA 21, p<0.001, t=3.42), left middle temporal gyrus (BA 22, p<0.001, t=4.08), and left cuneus (p<0.001, t=3.75) (see Table 3 and Figure 2).
Figure 2. Gray Matter Volume Decreases in Controls with Enuresis compared with Controls without Enuresis.
This figure depicts the statistical main effects of regional gray matter volume decreases in healthy controls with a history of childhood enuresis versus those without a history. The unthresholded VBM results are overlaid on an AFNI pial surface and color-coded for the statistical t-score. The central figure is a frontal view, and the other figures, from left to right, are the left lateral, left medial, right medial, and right lateral hemispheric surface views. The most significant cluster of reduced gray matter volume in healthy controls with enuresis is the left middle temporal gyrus, BA 22 (coordinates x=−53, y=−43, z=3, p<0.001). There was also a cluster of decreased gray from the medial frontal gyrus, BA 11 extending into the subgenual cingulate (coordinates x=0, y=20, z=−12, p<0.001). Other clusters that were statistically significant are listed in Table 3.
DISCUSSION
We have found that adult patients with schizophrenia have an increased rate of childhood enuresis (21%) compared with their healthy siblings (11%) or with normal controls (7%). This is the first and thus largest study to date of childhood enuresis in adult patients with schizophrenia, and the only study of adults with a history of enuresis examined with both psychological and structural brain imaging. Indeed, we did find evidence that enuresis is familial (and potentially heritable): the familial risk of enuresis was 2.6 times greater in siblings of enuretic probands than in siblings of non-enuretic probands. Moreover, we found that at least healthy female siblings of patients with schizophrenia have a significantly increased frequency of enuresis, suggesting that enuresis may be related to genetic risk factors for schizophrenia. In a preliminary report from our group, we had failed to find an increased rate of enuresis in probands compared to their non-psychotic siblings or controls (Egan et al 2001). However, that report contained a much smaller sample size, particularly for controls, than the current study and was relatively underpowered.
It is interesting to note that while the age of first diagnosis of schizophrenia was not significantly earlier for probands with a history of childhood enuresis, prodromal symptoms did appear two years earlier in these individuals. These findings suggest that childhood enuresis may be a marker of abnormal brain maturation that predates illness and indicates a predisposition towards the development of schizophrenia. This is not to say that childhood enuresis is a determinative and specific risk factor for schizophrenia, as most children with enuresis do not subsequently develop schizophrenia. The increased familial risk of childhood enuresis in siblings of probands with a history of childhood enuresis suggests that there may be a genetic component underlying this maturational defect. That is, the genetic risk factor may be one for alterations in prefrontal maturation, with childhood enuresis as one consequence, and schizophrenia as another. Since all enuretic subjects in this study eventually gained volitional bladder control, the underlying defect primarily is one of delayed or abnormal maturation, rather than a permanent loss of function.
Our study is not the first to implicate genetic risk in enuresis. In a large Finnish cohort (n=3206), if fathers were enuretic after age 4, the risk of offspring being enuretic was 7.1 times greater (Jarvelin et al 1988). Comparing monozygotic (n=1298 pairs) to dizygotic (n=2419 pairs) twin pairs, the concordance rate for childhood enuresis (at 4 years of age) was significantly higher in monozygotic twins. This was true in male pairs as well as female pairs (Hublin et al 1998). Twenty-three percent of probands with childhood enuresis have a positive family history of childhood enuresis (Wang et al 2007). In our study, the familial risk of enuresis was 2.6 times greater in siblings of enuretic probands than in siblings of non-enuretic probands. Linkage analysis in families with a high density of enuretic children have implicated a variety of chromosomal locations including 4p16.1, 12q, 13q, and 22q11 [see review (von Gontard et al 2001)]. Additional study is clearly needed to better define the genetic basis of childhood enuresis.
Environmental factors also have been implicated in the etiology of childhood enuresis. In the past, enuresis was largely attributed to a variety of factors often grouped as “psychosocial adversity” (Oppel et al 1968; Stein & Susser 1965). However, Fergusson and colleagues (Fergusson et al 1986) found no association between childhood enuresis and psychosocial factors including family, social, and economic background, family life events, or changes in family or residence. While adoption studies might help sort out the relative roles of genes and environment in the etiology of childhood enuresis, the linkage studies previously cited suggest a strong heritable component.
The VBM analysis on adult probands revealed that a childhood history of enuresis was associated with decreased gray matter in several frontal (right BA 9, right BA 10, bilateral BA 45) and one parietal region (right BA 7). In controls, a somewhat different pattern emerged, with decreased gray matter in frontal (right BA 11) and temporal (right BA 21, left BA 22) cortices, and the left cuneus. Lesion studies have associated acquired frontal lobe lesions in adulthood with the development of urinary incontinence (Sakakibara et al 1999). The findings from subjects with schizophrenia and controls are in agreement with the proposition that the frontal lobes are intimately involved in the development and maintenance of volitional bladder control. The fact that these structural differences are present in adults with no current incontinence but a childhood history of enuresis suggests that they represent vestigial developmental pathology that is functionally compensated.
The persistence of structural abnormalities in the adult brain related to early postnatal motor development has previously been reported with respect to infant motor development. Ridler and colleagues found that in normal controls, the earlier the age of learning to stand and walk, the greater the gray matter volumes in the premotor cortex, caudate nucleus, thalamus and cerebellum in adulthood (Ridler et al 2006). Similar to our findings, the study by Ridler and colleagues was notable for demonstrating continuity between behavioral markers of early postnatal development and adult brain structure in humans. Moreover, Ridler and colleagues found that patients with schizophrenia not only had significant delay in early motor development, but also had abnormal associations between early motor development scores and adult premotor cortical gray and fronto-parietal white matter volumes. Similarly, we found that subjects with delayed acquisition of urinary continence had smaller volumes of several cortical regions linked to micturition control. These abnormalities were found in both schizophrenics and normal controls with delayed acquisition of urinary continence, although the precise anatomical structures involved differed somewhat between groups.
Interestingly, right superior frontal damage has been associated with transient urinary incontinence in adulthood (Mochizuki & Saito 1990). In adult schizophrenic subjects, we found that decreased volume of the right superior frontal gyrus was associated with persistent yet ultimately transient urinary incontinence in childhood. This finding suggests that at least in schizophrenic subjects, delayed or abnormal development of the right superior frontal gyrus (BA9) may mediate childhood enuresis.
fMRI and blood-flow PET studies have implicated a wide cortical network in the volitional control of bladder function, with regional activation dependent upon the choice of physiological paradigm. Implicated regions include prefrontal, insular, cingulate, and parietal cortices (Athwal et al 2001; Blok et al 1997; Kuhtz-Buschbeck et al 2005; Matsuura et al 2002; Mochizuki & Saito 1990; Nour et al 2000). In both probands and controls with a history of childhood enuresis, there appears to be a developmental precursor within this neural network leading to abnormally small gray matter volumes in multiple prefrontal regions. The differences in the regions implicated by VBM analysis between probands and controls suggest that different genetic and/or environmental factors may be at work, altering the growth and maturation of different aspects of the neural network subserving the development of volitional bladder control in childhood.
Cognitive assessment with neuropsychological tests revealed that probands with a history of enuresis performed significantly worse than probands without enuresis on two measures of timed verbal retrieval (Letter Fluency and Category Fluency). One might have anticipated that a marker of neurodevelopmental compromise (i.e., childhood enuresis) would be associated with cognitive ability and academic skill in a broad sense. That is clearly not the case in this data set; schizophrenic subjects with and without a history of childhood enuresis had very similar scores on the WRAT-R reading and WAIS-R estimated Full Scale IQ. Instead, both probands and controls demonstrated specific decreases in both phonemic and semantic fluency. Such selective decreases in verbal fluency would primarily indicate left frontal and temporal lobe impairment based on the lesion and functional imaging literature (Baldo et al 2006; Baldo & Shimamura 1998; Baldo et al 2001; Costafreda et al 2006; Friston 1995; Gourovitch et al 2000; Mummery et al 1996). However, this interpretation is challenged by the fact that we did not observe any hint of an association between a history of childhood enuresis and poorer performance on other putative frontal lobe tests, such as the WCST and N-Back (Callicott et al 1998; Cohen et al 1997; Glahn et al 2005; Milner 1963; Weinberger et al 1986; Weiss et al 2004). One possible explanation might be that the WCST and N-Back typically activate a broader working memory network involving dorsal prefrontal areas bilaterally as well as superior parietal regions (Glahn et al 2005), whereas the fluency “network” involves frontal and temporal lobe structures, both of which were implicated in the VBM analysis. Thus, a dissociation of performance on these tasks may be supported by the functional neuroanatomy subserving performance.
Several studies have reported that language functions are less lateralized to the left hemisphere in schizophrenic subjects than controls (Sommer et al 2004; Weiss et al 2006). Accordingly, the right frontal VBM abnormalities noted in schizophrenic subjects with a history of enuresis might also mediate their poorer performance on tests of timed verbal retrieval. In contrast, the MRI data showed bilateral temporal lobe volumetric abnormalities in normal subjects with a history of enuresis. Controls with a history of childhood enuresis also performed more poorly on tests of timed verbal retrieval, possibly reflecting abnormal temporal lobe development. Given the relatively broad cortical network subserving volitional bladder control, it is not altogether surprising that different subregions within this network are differentially affected in probands and controls.
We did not observe the same effects on fluency in the sibling cohort. The sibling group is, in many respects, the most problematic of the three groups, presumably having both more risk and protective alleles for schizophrenia than the controls. This group was also disproportionately female, and contains the smallest number of enuretic subjects. Furthermore, it is also clinically heterogeneous. These factors may have obscured any effects on verbal fluency associated with enuresis.
While this report establishes as association between childhood enuresis and late adolescent/adult-onset schizophrenia, childhood enuresis has also been associated with a variety of childhood, adolescent, and adult-onset psychiatric disorders and many neurological disorders of childhood. These include ADHD (Biederman et al 1995; Neveus et al 2000), Tourette syndrome (Comings & Comings 1987), and bipolar disorder (Henin et al 2007), as well as less clearly characterized behavioral disorders of childhood and adolescence (Byrd et al 1996; Feehan et al 1990; Fergusson & Horwood 1994; Liu et al 2000). These reports are consistent with the view that childhood enuresis is a general reflection of maturational neurodevelopmental abnormalities that impede the acquisition of normal developmental milestones. This is not that surprising, given the complex and widely distributed neural network subserving volitional bladder control. What is novel here is the frequency with which childhood enuresis occurs in individuals who subsequently go on to develop schizophrenia, and the potential heritability of this dysmaturation in their non-schizophrenic siblings. This heritability, in conjunction with the MRI and neuropsychological testing results, suggests that some of the variations in genes that contribute liability towards childhood enuresis also may be involved in frontal lobe growth and development, and as such increase the risk of schizophrenia.
This study has several potential limitations. First, collection of enuresis data by retrospective maternal report may result in a selective recall bias and/or over-reporting of enuresis in subjects with schizophrenia. However, the fact that siblings as a group and normal control subjects had similar rates of enuresis mitigates against this possibility. The alternative strategy, i.e. prospective recruitment of enuretic subjects who might later develop schizophrenia would be impractical, and it is unlikely that attempts to gather pediatric records of childhood enuresis for adult subjects would yield much return, if any, given the age of the subjects in the study. Another possible limitation in this study is the discrepancy between male and female ratios for probands and controls. Since enuresis may be more predominant in males, it would be optimal to gather additional male control subjects in order to control for possible gender effects on our findings. However, when analysis was restricted to male subjects, the rates of enuresis were still significantly higher in schizophrenic subjects than their male siblings or male normal controls. Moreover, in the siblings, who were better sex matched with controls, there was a significant difference in frequency of childhood enuresis in the female sibling group.
In summary, childhood enuresis is much more common in schizophrenic subjects than in controls. There may be a heritable component to childhood enuresis in the families of patients with schizophrenia, as the non-schizophrenic siblings of schizophrenic subjects with enuresis also had a higher rate of enuresis. The genetic trait that underlies a familial predisposition towards childhood enuresis may be involved in prefrontal maturation and development. A history of childhood enuresis may be a marker of frontal cortical maldevelopment, as both adult schizophrenic subjects and controls with a history of childhood enuresis performed poorly on two tests of verbal fluency linked to frontal lobe function. Our anatomic data suggest that there is a persistent abnormality in regional cortical development, particularly involving portions of the frontal lobes. Taken together, the findings from this study advance the concept of schizophrenia being associated with early developmental difficulties, with particular focus on dysmaturation of the frontal lobes.
ACKNOWLEDGMENTS
We acknowledge all of the individuals and their families who participated in this study. We acknowledge Joann Berkson, RN, MEd and Mary Weirich, MSW, for assistance in data collection, and John Meyers, BS, for data management.
Funding/Support: This study was supported by the NIMH Intramural Research Program, NIH.
Abbreviations
- SCZ
schizophrenia
- SIB
sibling
- NC
non-psychiatric control
Footnotes
Disclosures or Conflicts of Interest: None reported.
Previous Presentation: This study was presented as a poster at the 62nd Society of Biological Psychiatry Scientific Convention and Meeting; May 17–19, 2007; San Diego, CA, USA.
REFERENCES
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, Frackowiak RS, Fowler CJ. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–377. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Westmore K, Emson PC. Phenotypic characterization of neurotensin messenger RNA-expressing cells in the neuroleptic-treated rat striatum: a detailed cellular co-expression study. Neuroscience. 1997;76:763–774. doi: 10.1016/s0306-4522(96)00449-6. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–267. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc. 2001;7:586–596. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Bender L. Childhood schizophrenia. American Journal of Orthopsychiatry. 1947;17:40–56. doi: 10.1111/j.1939-0025.1947.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Santangelo SL, Faraone SV, Kiely K, Guite J, Mick E, Reed ED, Kraus I, Jellinek M, Perrin J. Clinical correlates of enuresis in ADHD and non-ADHD children. J Child Psychol Psychiatry. 1995;36:865–877. doi: 10.1111/j.1469-7610.1995.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. A PET study on cortical and subcortical control of pelvic floor musculature in women. J Comp Neurol. 1997;389:535–544. [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121(Pt 11):2033–2042. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Bonney WW, Gupta S, Hunter DR, Arndt S. Bladder dysfunction in schizophrenia. Schizophr Res. 1997;25:243–249. doi: 10.1016/s0920-9964(97)00021-2. [DOI] [PubMed] [Google Scholar]
- Byrd RS, Weitzman M, Lanphear NE, Auinger P. Bed-wetting in US children: epidemiology and related behavior problems. Pediatrics. 1996;98:414–419. [PubMed] [Google Scholar]
- Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, van Gelderen P, Mattay VS, Frank JA, Moonen CT, Weinberger DR. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana B, Goldberg TE, Weinberger DR. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Comings DE, Comings BG. A controlled study of Tourette syndrome. VI. Early development, sleep problems, allergies, and handedness. Am J Hum Genet. 1987;41:822–838. [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Childhood precursors of psychosis as clues to its evolutionary origins. Eur Arch Psychiatry Clin Neurosci. 1995;245:61–69. doi: 10.1007/BF02190732. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, Bigelow L, Weinberger DR. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Feehan M, McGee R, Stanton W, Silva PA. A 6 year follow-up of childhood enuresis: prevalence in adolescence and consequences for mental health. J Paediatr Child Health. 1990;26:75–79. doi: 10.1111/j.1440-1754.1990.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Nocturnal enuresis and behavioral problems in adolescence: a 15-year longitudinal study. Pediatrics. 1994;94:662–668. [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Shannon FT. Factors related to the age of attainment of nocturnal bladder control: an 8-year longitudinal study. Pediatrics. 1986;78:884–890. [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders - Clinician Verson (SCID-CV) Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Fish B, Kendler KS. Abnormal infant neurodevelopment predicts schizophrenia spectrum disorders. J Child Adolesc Psychopharmacol. 2005;15:348–361. doi: 10.1089/cap.2005.15.348. [DOI] [PubMed] [Google Scholar]
- Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect. A review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–235. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- Fish B, Shapiro T, Halpern F, Wile R. The Prediction of Schizophrenia in Infancy: 3. a Ten-Year Follow-up Report of Neurological and Psychological Development. Am J Psychiatry. 1965;121:768–775. doi: 10.1176/ajp.121.8.768. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, Van Horn JD, Berman KF. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14:353–360. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Henin A, Biederman J, Mick E, Hirshfeld-Becker DR, Sachs GS, Wu Y, Yan L, Ogutha J, Nierenberg AA. Childhood antecedent disorders to bipolar disorder in adults: a controlled study. J Affect Disord. 2007;99:51–57. doi: 10.1016/j.jad.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Henriksson KM, McNeil TF. Health and development in the first 4 years of life in offspring of women with schizophrenia and affective psychoses: Well-Baby Clinic information. Schizophr Res. 2004;70:39–48. doi: 10.1016/j.schres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hollis C. Developmental precursors of child- and adolescent-onset schizophrenia and affective psychoses: diagnostic specificity and continuity with symptom dimensions. Br J Psychiatry. 2003;182:37–44. doi: 10.1192/bjp.182.1.37. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hublin C, Kaprio J, Partinen M, Koskenvuo M. Nocturnal enuresis in a nationwide twin cohort. Sleep. 1998;21:579–585. doi: 10.1093/sleep/21.6.579. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones PB, Moilanen K, Rantakallio P, Veijola J, Oja H, Koiranen M, Jokelainen J, Croudace T, Jarvelin M. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. 2001;52:1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- Jarvelin MR, Vikevainen-Tervonen L, Moilanen I, Huttunen NP. Enuresis in seven-year-old children. Acta Paediatr Scand. 1988;77:148–153. doi: 10.1111/j.1651-2227.1988.tb10614.x. [DOI] [PubMed] [Google Scholar]
- Johnson M. Nocturnal enuresis. Urol Nurs. 1998;18:259–273. quiz 274–5. [PubMed] [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Jones PD, D J. From birth to onset: a developmental perspective of schizophrenia in two national birth cohorts. In: Keshavan MSM, R M, editors. Neurodevelopment and Adult Psychopathology. Cambridge: Cambridge University Press; 1997. pp. 119–136. [Google Scholar]
- Kraepelin E. Dementia praecox. New York: Churchill Livingston, Inc; 1919–1971. [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, Junemann KP. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005;174:1477–1481. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun Z. Age of attaining nocturnal bladder control and adolescent suicidal behavior. J Affect Disord. 2005;87:281–289. doi: 10.1016/j.jad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun Z, Uchiyama M, Li Y, Okawa M. Attaining nocturnal urinary control, nocturnal enuresis, and behavioral problems in Chinese children aged 6 through 16 years. J Am Acad Child Adolesc Psychiatry. 2000;39:1557–1564. doi: 10.1097/00004583-200012000-00020. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002;168:2035–2039. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Archives of Neurology. 1963;9:100–110. [Google Scholar]
- Mochizuki H, Saito H. Mesial frontal lobe syndromes: correlations between neurological deficits and radiological localizations. Tohoku J Exp Med. 1990;161 Suppl:231–239. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating 'tiger' as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263:989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Neveus T, Lackgren G, Tuvemo T, Hetta J, Hjalmas K, Stenberg A. Enuresis--background and treatment. Scand J Urol Nephrol Suppl. 2000:1–44. [PubMed] [Google Scholar]
- Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain. 2000;123(Pt 4):781–789. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- Oppel WC, Harper PA, Rider RV. Social, psychological, and neurological factors associated with nocturnal enuresis. Pediatrics. 1968;42:627–641. [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, Murray GK, Haapea M, Jones PB, Isohanni MK, Bullmore ET. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:15651–15656. doi: 10.1073/pnas.0602639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. Am J Hum Genet. 1990;46:229–241. [PMC free article] [PubMed] [Google Scholar]
- Sakakibara R, Fowler CJ, Hattori T. Voiding and MRI analysis of the brain. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:192–199. doi: 10.1007/s001920050044. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, van Oel CJ, Kahn RS. Language activation in monozygotic twins discordant for schizophrenia. Br J Psychiatry. 2004;184:128–135. doi: 10.1192/bjp.184.2.128. [DOI] [PubMed] [Google Scholar]
- Stein ZA, Susser MW. Socio-medical study of enuresis among delinquent boys. Br J Prev Soc Med. 1965;19:174–181. doi: 10.1136/jech.19.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette E, Petit D, Paquet J, Tremblay RE, Boivin M, Montplaisir JY. Bed-wetting and its association with developmental milestones in early childhood. Arch Pediatr Adolesc Med. 2005;159:1129–1134. doi: 10.1001/archpedi.159.12.1129. [DOI] [PubMed] [Google Scholar]
- von Gontard A, Schaumburg H, Hollmann E, Eiberg H, Rittig S. The genetics of enuresis: a review. J Urol. 2001;166:2438–2443. doi: 10.1097/00005392-200112000-00117. [DOI] [PubMed] [Google Scholar]
- Wang QW, Wen JG, Zhang RL, Yang HY, Su J, Liu K, Zhu QH, Zhang P. Family and segregation studies: 411 Chinese children with primary nocturnal enuresis. Pediatr Int. 2007;49:618–622. doi: 10.1111/j.1442-200X.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Brinkhoff C, Kremser C, Felber S, Fleischhacker WW. Brain activation patterns during a verbal fluency test-a functional MRI study in healthy volunteers and patients with schizophrenia. Schizophr Res. 2004;70:287–291. doi: 10.1016/j.schres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Res. 2006;146:185–190. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]