Abstract
Attention-deficit/hyperactivity disorder (ADHD) is characterized by inattention, hyperactivity and impulsivity, but there is no consensus regarding whether ADHD exists on the extreme end of a continuum of normal behavior or represents a discrete disorder. In this study, we sought to characterize both the categorical and dimensional variations in network functional connectivity in order to identify neural connectivity mechanisms of ADHD. Functional connectivity analyses of resting-state fMRI data from 155 children with ADHD and 145 typically-developing children (TDC) defined the dorsal attention network (DA), default mode network (DM), salience processing network (SAL) and executive control network (CON). Regional alterations in connectivity associated with categorical diagnoses and dimensional symptom measures (inattention and hyperactivity/impulsivity) as well as their interaction were systematically characterized. Dimensional relationships between symptom severity measures and functional connectivity that did not differ between TDC and children with ADHD were observed for each network, supporting a dimensional characterization of ADHD. However, categorical differences in functional connectivity magnitude between TDC and children with ADHD were detected after accounting for dimensional relationships, indicating the existence of categorical mechanisms independent of dimensional effects. Additionally, differential dimensional relationships for TDC versus ADHD children demonstrated categorical differences in brain-behavior relationships. The patterns of network functional organization associated with categorical versus dimensional measures of ADHD accentuate the complexity of this disorder and support a dual characterization of ADHD etiology featuring both dimensional and categorical mechanisms.
Keywords: attention-deficit/hyperactivity disorder, resting state functional magnetic resonance imaging, dimensional disorders, attention, impulsivity, functional neural networks
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is characterized by developmentally inappropriate levels of inattention, impulsivity and hyperactivity(Kuntsi, et al., 2006). A clinical diagnosis of ADHD is assigned when symptoms surpass a particular threshold of severity, implying an underlying categorical mechanism(Sonuga-Barke, 1998). However, an alternative perspective considers symptoms as lying at the extreme end of normal behavior(Chabernaud, et al., 2012), a conceptualization supported by genetic association studies(Bidwell, et al., 2011; Larsson, et al., 2012; Thapar, et al., 2006), the graded relationship of subthreshold symptoms of ADHD and psychiatric comorbidities(Fergusson and Horwood, 1995; Malmberg, et al., 2011), and taxometric analyses of ADHD-related behavioral measures(Haslam, 2007; Marcus and Barry, 2011). The integration of dimensional measures of psychopathology into standard diagnostic criteria has thus gained support in recent years(Helzer, 2006; Hudziak, et al., 2007; Marcus and Barry, 2011; Swanson, et al., 2011). In fact, the latest edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-V) adopts an approach that employs both categorical diagnosis and symptom severity assessments. Whether ADHD represents a discrete disorder or lies on an extreme of a continuum of normal behaviors has implications for how this disorder is diagnosed, treated and studied. However, the precise nature of the neural mechanisms that underlie ADHD has not been systematically characterized.
The brain mediates the impact of genetic and environmental etiological factors on the outward expression of symptoms, making it a prime target for investigating the mechanisms of ADHD. Particularly, the identification of large-scale functional neural networks enabled by the recently emerged resting state functional fMRI (rsfMRI) technique(Biswal, et al., 1995; Lowe, et al., 1998; Paloyelis, et al., 2007) provides a compelling means of exploring the neural mechanisms underlying different brain disorders(Chabernaud, et al., 2012; Greicius, 2008). Regarding ADHD, several candidate functional neural networks may be of particular importance. Specifically, the default mode network (DM)( Raichle, et al., 2001) of posterior cingulate cortex, medial prefrontal cortex, bilateral angular gyrus and temporal cortex demonstrates hypo-connectivity among children with ADHD(Fair, et al., 2010), and a failure to down-regulate the DM during external goal-directed tasks is thought to contribute to attentional lapses(Gao, et al., 2013; Mason, et al., 2007; Sonuga-Barke and Castellanos, 2007; Weissman, et al., 2006). Additionally, the dorsal attention network (DA)( Corbetta and Shulman, 2002), covering bilateral intraparietal sulcus (IPS), frontal eye fields (FEF) and middle temporal visual regions (MT), is engaged during tasks requiring attention to external goals and has similarly been implicated in the etiology of ADHD(Castellanos and Proal, 2012; Cortese, et al., 2012; Vance, et al., 2007). A frontal-parietal control system(Dosenbach, et al., 2008; Seeley, et al., 2007) consisting of a cingulo-opercular “salience network” (SAL) and a frontal-parietal “executive control network” (CON) may likewise contribute to ADHD: the CON network of dorsolateral, ventrolateral, and dorsomedial prefrontal and bilateral parietal connectivity is implicated in control of attention(de Fockert, et al., 2001; Gao and Lin, 2012; Rossi, et al., 2009; Turatto, et al., 2004) and exhibits decreased activity in children with ADHD during attention tasks(Cortese, et al., 2012; Hart, et al., 2012). The SAL network of anterior insula and anterior cingulate connectivity is involved in salience detection(Menon and Uddin, 2010), including behavioral monitoring and error detection(Ullsperger, et al., 2010), processes impaired in individuals with ADHD(Liotti, et al., 2005; O'Connell, et al., 2009). Therefore, a closer examination of these large-scale neural networks associated with ADHD and its behavioral symptoms may expose the underlying categorical and dimensional characteristics of ADHD.
Adopting a network functional connectivity perspective, we aimed to unveil the neural mechanisms of ADHD characterized by a large sample of both typically developing children (TDC, n=145) and children with ADHD (n=155). Given evidence supporting both categorical and dimensional properties of ADHD(Chabernaud, et al., 2012; Lubar, 2001; Thapar, et al., 2006), we tested a hybrid model based on a systematic rsfMRI investigation of four networks implicated in cognitive processes impaired in ADHD (i.e., DA, DM, SAL, and CON). To characterize the mechanisms underpinning ADHD-related brain abnormalities, we investigated the contributions of dimensional symptom measures, categorical diagnosis, and their interaction to functional connectivity of these networks. Three types of effects were explored: 1) Functional connectivity-behavior relationships that do not differ between TDC and ADHD children and are independent of categorical diagnosis, which we term “congruent dimensional relationships,” would suggest that ADHD lies on a continuum; 2) On the other hand, categorical differences in functional connectivity after controlling for dimensional relationships would suggest the existence of categorical mechanisms of ADHD; 3) Furthermore categorical differences between TDC and ADHD children in the relationship of functional connectivity to behavior would suggest that symptoms of ADHD qualitatively differ from the spectrum of normal behavior, providing further evidence for categorical mechanisms of ADHD. We present results supporting each of these characterizations and subsequently discuss the implications.
METHODS
Subjects
Data from 145 TDC and 155 ADHD children were obtained from the ADHD-200 Sample database(Milham, et al.) (http://fcon_1000.projects.nitrc.org/indi/adhd200/). The ADHD sample included 88 children diagnosed with the combined type, 64 with the predominantly inattentive type and 3 with the predominantly hyperactive-impulsive type. Given known sex differences in brain structure and function(Cahill, 2006), we focused this study on males, as this sex constitutes the majority of childhood ADHD cases(Cuffe, et al., 2005). Only data from research sites contributing resting-state fMRI scans from both TDC and ADHD children in addition to dimensional measures of ADHD were included (i.e., Kennedy Krieger Institute, Johns Hopkins University (KKI), New York University Child Study Center (NYU), and Peking University (PU)). Study procedures were approved by the Johns Hopkins Medical and New York University Institutional Review Boards and Research Ethics Review Board of the Institute of Mental Health at Peking University. Parental written informed consent and child assent were obtained following explanation of study procedures.
Inclusion/Exclusion Criteria by Site
KKI
ADHD children met criteria for ADHD based on the Diagnostic Interview for Children and Adolescents, Fourth Edition (DICA-IV (Reich, et al., 1997)) and had a T-score of at least 65 on the Conners Parent Rating Scale-Revised, Long Form (CPRS (Conners, et al., 1998)) for either DSM-IV Inattentive and/or DSM-IV Hyperactive/Impulsive subscales or met ADHD criteria on the ADHD Rating Scale IV (DuPaul, 1998). TDC children had T-scores lower than 60 or lower on both the DSM-IV Inattentive and DSM-IV Hyperactive/Impulsive subscales. Subjects were excluded for an estimated IQ below 80, language disorder or reading disability, visual or hearing impairment, psychoactive medication use other than stimulants for ADHD children, neurological disorders, or psychiatric disorders other than specific phobias or oppositional defiant disorder (ADHD subjects only).
NYU
ADHD diagnosis required a diagnosis based on parent and child responses to the Schedule of Affective Disorders and Schizophrenia for Children-Present and Lifetime Version (K-SADS (Kaufman, et al., 1997)) and a T-score of at least 65 on either the DSM-IV Inattentive and/or DSM-IV Hyperactive/Impulsive subscales of the CPRS. TDC children had a T-score below 60 on any CPRS ADHD subscale. Exclusion criteria were an IQ below 80, left-handedness, and chronic medical conditions. TDC children had no Axis-I psychiatric disorders.
PU
ADHD was assessed with the Computerized Diagnostic Interview Schedule IV (Bacon, 1997) and verified with parent responses on the K-SADS. Subjects were excluded for an IQ below 80, left-handedness, loss of consciousness due to head trauma, neurological illness, schizophrenia, affective disorder, pervasive development disorder, or substance abuse.
Parent ratings from the CPRS (KKI, NYU) or ADHD Rating Scale IV (PU) provided dimensional measures of symptoms of inattention and hyperactivity/impulsivity related to DSM-IV diagnostic criteria. Corresponding subscale scores for these two instruments demonstrate good convergent validity(Zhang, et al., 2005). To control for differences in ranges of potential scores obtained from different instruments and to enable comparison across sites, symptom subscales were rescaled to have a range of 0.0–1.0 for each site by normalizing all scores to their corresponding maximum. Intelligence (IQ) was assessed with the Wechsler Intelligence Scale for Children, Fourth Edition (Kaufman, et al., 2006) (KKI), Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) (NYU), or the Wechsler Intelligence Scale for Chinese Children-Revised(Dan, et al., 1990) (PU). For the 116 children with ADHD for which medication status was available, 69 (59%) were medication naive. The inattention and impulsivity scores used in the current analysis were obtained as part of the study procedures and therefore reflect symptoms exhibited after medication use for treated subjects. Subjects from all imaging sites were free of stimulant medication for at least 24 hours prior to the scan.
Image Acquisition
Magnetic resonance imaging time series were collected in resting conditions using Siemens Magnetom Allegra and Trio (Siemens Medical Solutions, Erlangen, Germany) for NYU and PU and Philips Gyroscan (Philips Medical Systems, Amsterdam, Netherlands) 3 Tesla MRI scanners for KKI. Detailed imaging parameters are presented in Table S1.
Preprocessing
Functional images were preprocessed using Statistical Parametric Mapping 8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm/) and Analysis of Functional NeuroImages (AFNI(Cox, 1996)) software. The first five volumes were removed to allow magnetization to reach equilibrium. Images were corrected for slice timing and realigned to the second available scan in each functional series. Next, the subject’s T1 image was registered to an MNI template in SPM8 and the functional images were warped using the same transformation field and then re-sliced to 3mm cubic voxels. Functional images were spatially smoothed with an 8 mm FWHM Gaussian kernel and band-pass filtered between 0.008~0.08Hz in AFNI. Regression analysis was performed to remove nuisance signals from white matter, cerebral spinal fluid, global signal, and six motion parameters. To further remove motion artifacts, a new data scrubbing method was implemented(Power, et al., 2012). Specifically, thresholds for global signal change at each volume and displacement between acquired volumes were set at less than 0.5% BOLD signal and 0.5 mm, respectively. Briefly, if both measures of any volume reached their respective thresholds, that volume, the one previous and the two after were removed. Total displacement between consecutive volumes was measured by taking the sum of the distance moved across six directions (Power, et al., 2012), including three translations (x, y, z) and three rotations (roll, pitch, yaw, converted into distance). An analysis of the mean volume-to-volume displacement across all volumes indicated no significant difference in motion between ADHD children (0.19 ± 0.20 mm) and TDC (0.14 ± 0.22 mm). An average of 7.3 ± 14.3 (4.2 ± 8.1%) and 6.8 ± 14.3 (4.4 ± 9.2%) volumes were removed for ADHD children and TDC, respectively, indicating no significant difference between groups in the total number (t=0.30, p>0.05) or percent (t=−0.12, p>0.05) of volumes removed.
Network Functional Connectivity
Network-level functional connectivity was defined as the voxel-wise Pearson correlation with a reference time series using AFNI’s 3dfim+. Reference time series were extracted as the simple average time series of all voxels within a 6 mm spherical seed. In accordance with Seeley and colleagues(Seeley, et al., 2007), the CON was defined by connectivity with a right dorsolateral prefrontal cortex seed (Montreal Neurologic Institute coordinates (MNI): 44, 36, 20) and the SAL by a seed in the right anterior insula (MNI: 38, 26, −10). Similarly, DM and DA were defined by seeds in the posterior cingulate cortex (MNI: 1, −55, 17) and bilateral intraparietal sulcus (MNI: −27, −52, 57; 24, −56, 55), respectively, as in (Gao and Lin, 2012) and (Vincent, et al., 2008). Pearson correlation maps were subsequently normalized using a Fisher-z transform.
Statistical Analyses
Linear regression models included categorical diagnosis and either inattention or hyperactivity/impulsivity as predictors of functional connectivity, covarying for age and imaging sites. Inattention and hyperactivity/impulsivity were tested in separate models to avoid multicollinearity as these variables share a large portion of variance (R2=0.56). These models enabled the detection of dimensional effects that demonstrate consistent linear relationships across both groups (i.e. not explained by categorical differences). We describe these effects as “congruent dimensional relationships” in subsequent sections. Furthermore, these analyses also identified those regions for which categorical differences in functional connectivity magnitude were evident after controlling for effects of dimensional variables. Such effects are referred to as “categorical effects on functional connectivity” in subsequent sections. To account for the full effects of both inattention and hyperactivity/impulsivity, categorical effects were defined using a conjunction analysis of significant effects of ADHD diagnosis that were present in both models. Significant categorical and dimensional effects (p<0.05) were cluster-level-corrected to α <0.05 with a minimum cluster size of 154 voxels based on 10,000 Monte Carlo simulations conducted with 3dClustSim in AFNI.
Furthermore, to determine if ADHD is associated with categorical effects on dimensional relationships, we tested the interaction of diagnosis and dimensional variables as predictors of voxel-wise functional connectivity in linear regression models, with age and imaging sites included as covariates. Again, inattention and hyperactivity/impulsivity symptoms were modeled separately. These models identified those regions for which the linear relationship between symptom measures and functional connectivity was categorically different between TDC and ADHD. Subsequent sections refer to these effects as “categorical effects on brain-behavior relationships.” The threshold for a significant interaction effect was set at p<0.05 with a minimum cluster size of 154 voxels based on 3dClustSim in AFNI providing a corrected false positive rate of 0.05.
Finally, the intersection of the maps of significant (cluster-level corrected) regions showing categorical effects (in functional connectivity magnitude or in brain-behavior relationships) with a map of significant regions showing congruent dimensional relationships was calculated in order to identify regions that demonstrate effects of both mechanisms.
RESULTS
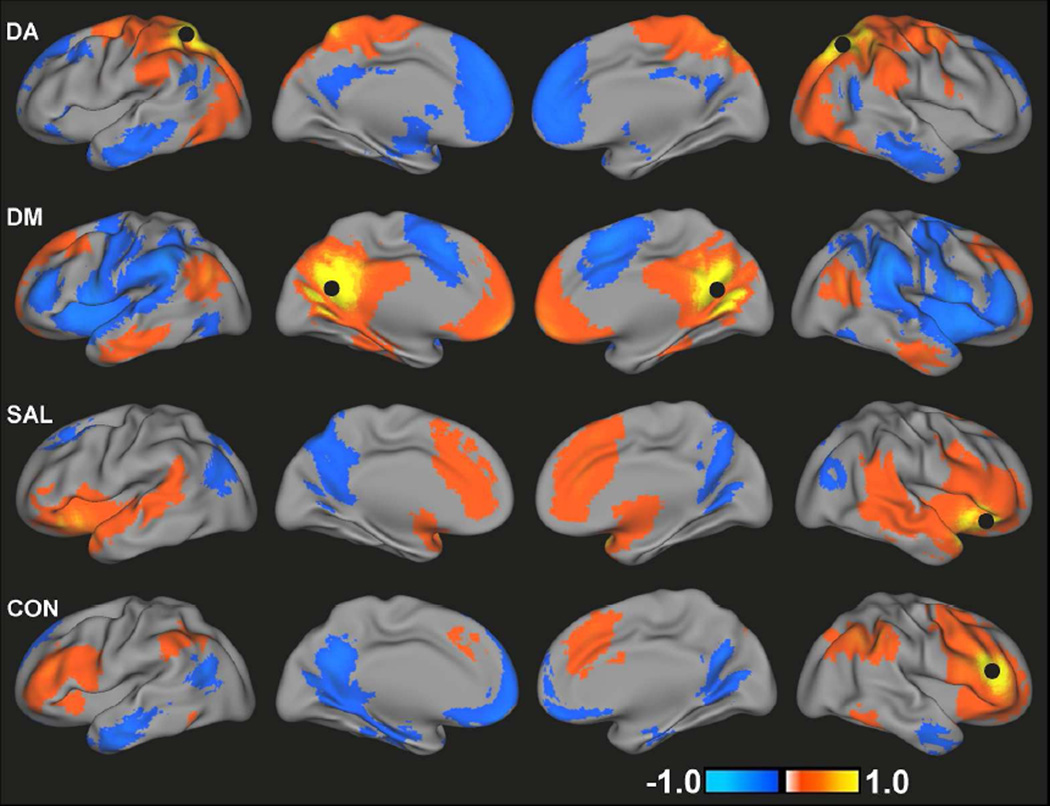
Demographic and clinical variables for TDC and ADHD subjects are summarized in Table 1. Figure 1 presents the spatial maps of all four networks for the TDC group. Consistent with previous reports(Fox, et al., 2005), the DA consisted of positive correlations between the superior parietal lobules/IPS, FEF, inferior and middle frontal gyri, MT, and cerebellum. DM connectivity was present in posterior cingulate cortex, inferior parietal lobules, medial prefrontal cortex, middle temporal cortex and parahippocampal gyrus(Buckner, et al., 2008; Greicius, et al., 2003). Consistent with the network topology of healthy adults(Seeley, et al., 2007), the SAL included bilateral inferior frontal cortex/insula, anterior cingulate cortex/medial prefrontal cortex, and inferior parietal lobules and the CON included bilateral middle and inferior frontal gyri, dorsomedial prefrontal cortex and bilateral parietal cortex. The spatial topologies of all four functional connectivity maps for children with ADHD were qualitatively similar to those for TDC (Figure S1).
Table 1.
Demographic and clinical variables for typically-developing children, ADHD patients and the pooled sample.
| All Sites | KKI | NYU | PU | |||||
|---|---|---|---|---|---|---|---|---|
| N | 300 | 46 | 132 | 122 | ||||
| TDC | 145 | 34 | 45 | 66 | ||||
| ADHD | 155 | 12 | 87 | 56 | ||||
| Age | 11.7 | 2.4 | 10.4 | 1.4 | 11.7 | 2.9 | 12.3 | 1.8 |
| TDC | 11.8 | 2.3 | 10.3 | 1.4 | 12.3 | 3.2 | 12.1 | 1.7 |
| ADHD | 11.7 | 2.5 | 10.4 | 1.5 | 11.4 | 2.7 | 12.4 | 2.0 |
| IQ | 112.7 | 15.2 | 113.0 | 13.8 | 109.3 | 14.6 | 116.1 | 15.6 |
| TDC | 117.4 | 13.6 | 115.3 | 13.0 | 113.5 | 13.6 | 121.1 | 13.2 |
| ADHD | 108.4 | 15.2 | 106.5 | 14.5 | 107.3 | 14.6 | 110.4 | 16.3 |
| Inattention Score | 43.9 | 21.4 | 51.2 | 11.0 | 60.8 | 13.6 | 21.7 | 7.4 |
| TDC | 33.5 | 15.7 | 45.4 | 5.3 | 45.0 | 7.2 | 15.4 | 3.6 |
| ADHD | 57.3 | 21.5 | 66.7 | 6.0 | 68.7 | 8.0 | 28.3 | 3.6 |
| Impulsivity Score | 42.2 | 23.0 | 52.8 | 11.4 | 60.1 | 14.2 | 17.7 | 7.0 |
| TDC | 31.7 | 16.9 | 47.0 | 4.6 | 45.3 | 4.6 | 13.1 | 3.5 |
| ADHD | 51.2 | 23.8 | 68.2 | 9.9 | 67.4 | 11.3 | 22.4 | 6.6 |
Mean and standard deviation (italics) are provided for each continuous measure. N, number; IQ, intelligence quotient; ADHD, attention-deficit/hyperactivity disorder; TDC, typically-developing children; KKI, Kennedy Krieger Institute; NYU, New York University; PU, Peking University.
Figure 1.
Group mean functional connectivity maps for DA, DM, SAL and CON for TDC. Black circles indicate the location of seed regions used to generate each map. Images are displayed using a threshold of an absolute value of r>0.1.
Congruent dimensional relationships across TDC and ADHD
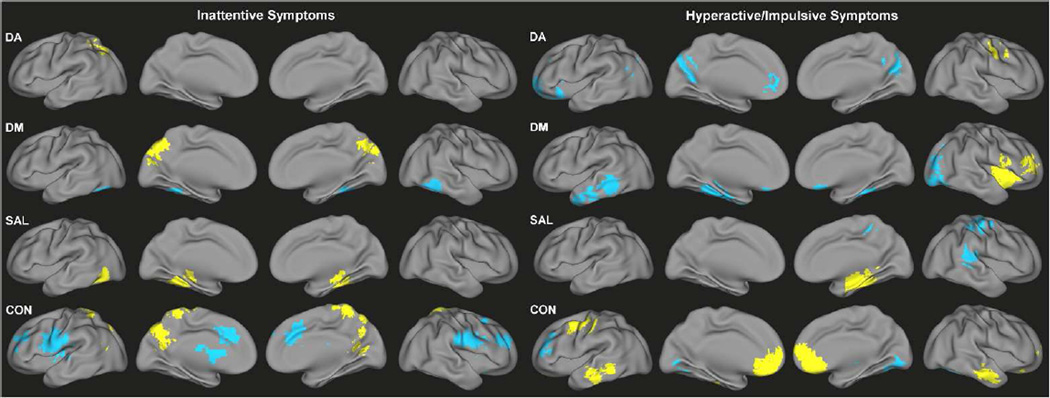
Significant dimensional effects on functional connectivity across both children with ADHD and TDC were observed for all networks tested (Figure 2, Tables S2–S3). Higher inattention scores were associated with connectivity of bilateral fusiform gyrus for DM (decreased) and right fusiform gyrus for SAL (increased), as well as increased connectivity of the precuneus for DM and CON. There was also a notable association of greater inattention with lesser connectivity of anterior cingulate cortex and bilateral middle frontal gyrus regions within CON. Greater severity of hyperactive/impulsive symptoms was associated with connectivity of the fusiform and parahippocampal gyri for DM (decreased) and SAL (increased), medial prefrontal cortex for DA (decreased) and CON (increased), left inferior/middle temporal gyrus and right lateral occipital cortex for DM (decreased), and bilateral inferior/middle temporal gyrus for CON (increased).
Figure 2.
Congruent functional connectivity-behavior relationships across TDC and ADHD for inattention scores (left) and hyperactivity/impulsivity scores (right) for DA, DM, SAL, and CON. Yellow indicates positive associations with symptoms; blue indicates negative associations with symptoms.
Categorical effects of ADHD on functional connectivity
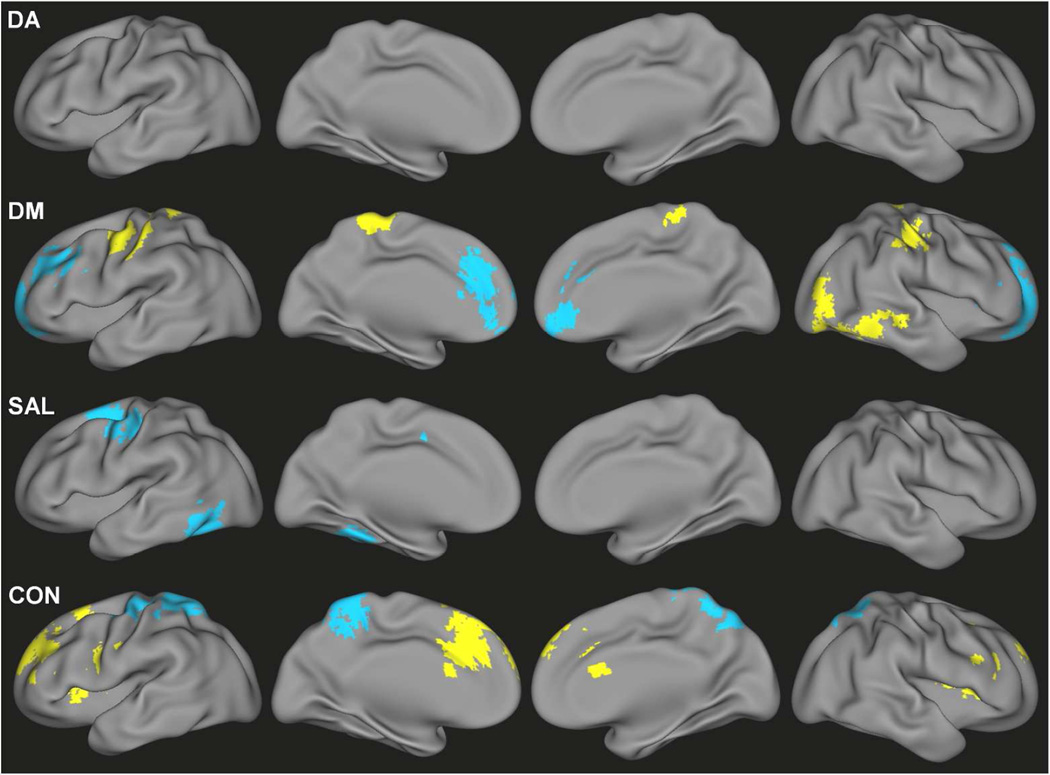
After accounting for congruent dimensional relationships, there were regions that demonstrated categorical effects of ADHD diagnosis on functional connectivity of DM, SAL and CON (Figure 3, Table S4). Hyper-connectivity in children with ADHD – after adjusting for dimensional relationships with symptom severity – was observed for DM connectivity with sensorimotor and visual association regions and CON connectivity with anterior cingulate cortex, superior frontal gyrus, insula, and cerebellum. Hypo-connectivity associated with categorical effects of ADHD was observed for DM connectivity with the medial prefrontal cortex and superior frontal gyrus, SAL connectivity with the left fusiform gyrus and left frontal eye field, and CON connectivity with the precuneus and sensorimotor cortex.
Figure 3.
Categorical differences in functional connectivity values associated with an ADHD diagnosis and unaccounted for by either inattentive or hyperactive/impulsive scores for DA, DM, SAL and CON. Yellow indicates ADHD>TDC; blue indicates TDC>ADHD.
Categorical effects of ADHD on brain-behavior relationships
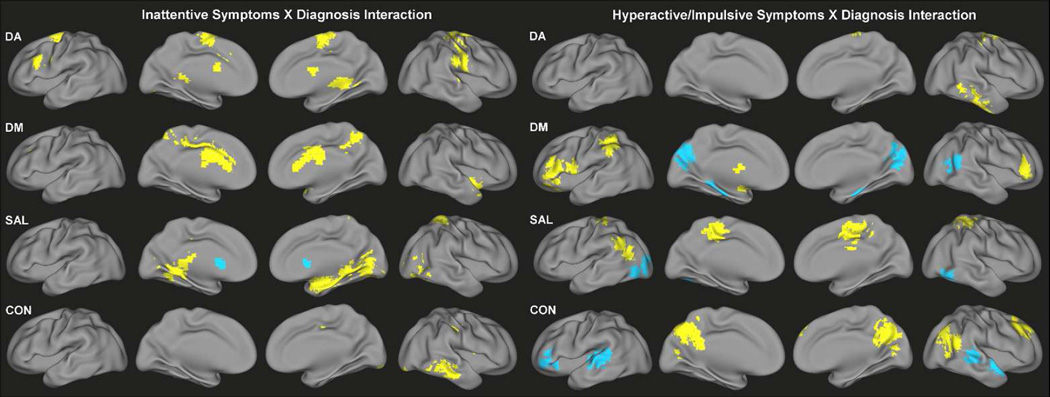
Categorical differences in the slope of the relationship of ADHD-related behaviors to functional connectivity were also identified across all four networks (Figure 4, Tables S5–S6). Significant interaction effects between ADHD diagnosis and inattentive symptoms on functional connectivity were identified for DA primarily along the precentral and postcentral gyri, supplementary motor area and cerebellum, showing a greater positive relationship in children with ADHD. Similar effects were observed in the precuneus and anterior cingulate cortex for DM, in the right amygdala and parahippocampal gyrus for SAL, and in the middle temporal gyrus for CON. Conversely, the relationship between inattention and functional connectivity of the bilateral caudate with SAL was weaker in ADHD children than TDC.
Figure 4.
Significant interaction effects of ADHD diagnosis and symptoms of inattention (left) and significant interaction effects of ADHD diagnosis and symptoms of hyperactivity/impulsivity (right) on functional connectivity of DA, DM, SAL and CON. Yellow indicates more positive association with symptoms for children with ADHD; blue indicates more positive association with symptoms for TDC
For the interaction of ADHD diagnosis and hyperactive/impulsive symptoms, children with ADHD demonstrated a greater relationship of symptoms to DA connectivity with the right middle and inferior temporal gyrus, DM connectivity with bilateral inferior frontal gyrus and inferior parietal lobule, SAL connectivity with bilateral paracentral lobule and left angular gyrus, and CON connectivity with the posterior cingulate cortex, precuneus, right superior frontal gyrus and right angular gyrus. There was also a decreased relationship among children with ADHD of hyperactive/impulsive symptoms to functional connectivity of the cuneus/precuneus and right superior temporal gyrus with DM, cerebellum and visual associations regions with SAL, and bilateral superior temporal gyrus and left inferior frontal gyrus with CON.
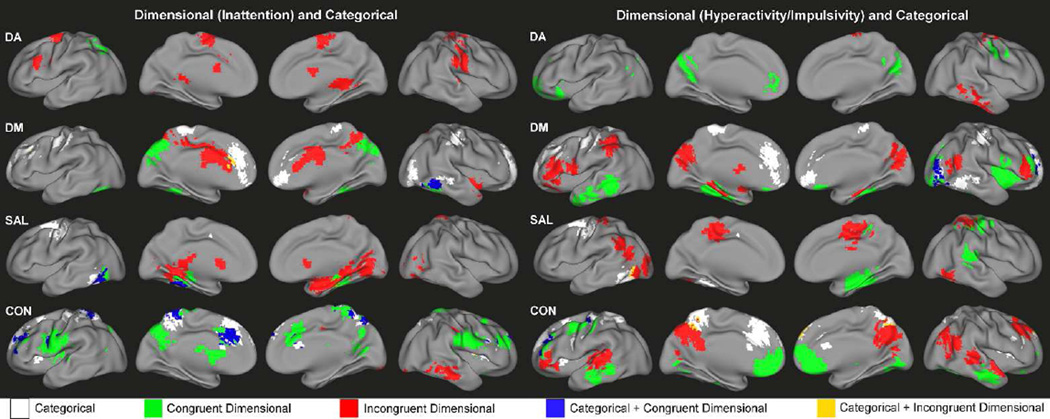
Overlap of categorical and dimensional mechanisms
Overall, the patterns of dimensional effects and categorical effects on the functional connectivity of DA, DM, SAL and CON suggest these mechanisms largely impact separate regions. However, there were also several regions affected by both mechanisms, showing consistent brain-behavior relationships across TDC and ADHD children in addition to categorical effects on functional connectivity that exist independent of dimensional relationships (blue colors, Figure 5). Such regions included the precuneus, anterior cingulate cortex, and bilateral middle frontal gyrus for CON, the fusiform gyrus for DM and SAL, as well as lateral visual areas for DM. Minimal overlap was observed between brain regions showing categorical effects on functional connectivity and those associated with categorical effects on brain-behavior relationships (yellow colors, Figure 5).
Figure 5.
Composite maps showing the regional connectivity alterations associated with categorical effects of ADHD diagnosis (categorical, white), dimensional effects of inattention (left) or hyperactivity/impulsivity (right) (congruent dimensional, green), the interaction of categorical and dimensional effects (incongruent dimensional, red), the overlap of categorical and congruent dimensional effects (blue) and the overlap of categorical and incongruent dimensional effects (yellow) for DA, DM, SAL and CON.
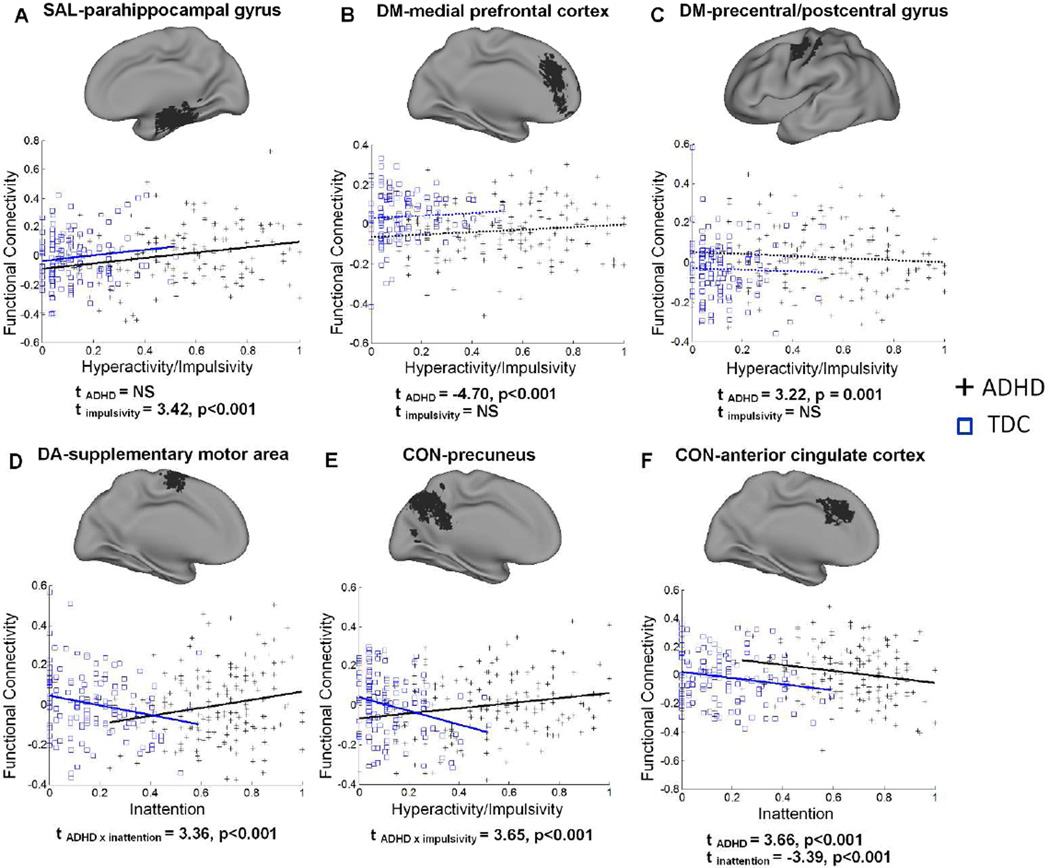
Exemplar network connectivity effects showing various dimensional and/or categorical relationships are provided in Figure 6. An example of only congruent dimensional effects on SAL connectivity is presented in Figure 6A; two examples of DM connectivity showing only categorical effects on functional connectivity are presented in Figure 6B, C; two examples of DA and CON connectivity depicting an interaction between categorical diagnosis and dimensional brain-behavior relationships are shown in Figure 6D, E; finally, an example of CON connectivity demonstrating both congruent dimensional and categorical effects is presented in Figure 6F.
Figure 6.
Scatter plots of the relationship between behavior scores and functional connectivity for TDC and children with ADHD for selected regions. Least-squares regression lines demonstrate statistically significant relationships (solid lines) or non-significant relationships (dashed lines). T-statistics for the effects of categorical and dimensional variables on regional connectivity are reported below each plot demonstrating dimensional effects only (A), categorical effects only (B, C), an interaction of categorical and dimensional effects (D, E), and both dimensional and categorical effects (F). Functional connectivity values (y-axis) represent residuals after removing effects of age and site.
DISCUSSION
In this study, both categorical differences associated with ADHD diagnosis and significant dimensional effects of two symptom measures were observed for functional connectivity across four theoretically derived neural networks (i.e., DA, DM, SAL, and CON). The detection of a number of regions associated with consistent brain-behavior relationships across both TDC and ADHD endorses the dimensional characteristics of this disorder. Additionally, categorical differences in functional connectivity magnitude that were not driven by dimensional effects, as well as the presence of brain-behavior relationships that were moderated by ADHD diagnosis, indicate the existence of categorical mechanisms of ADHD. Therefore, our results support a dual characterization of ADHD etiology and highlight the importance of considering influences from both categorical and dimensional factors.
The consistent, linear relationship of dimensional variables to functional connectivity across both ADHD children and TDC (congruent dimensional relationships) provides strong evidence for a dimensional characterization of the functional connectivity etiology of ADHD (Chabernaud, et al., 2012). The subscales for the CPRS and ADHD Rating Scale, which provided the dimensional variables for this study, measure symptoms that correspond with the DSM-IV inattention and hyperactive/impulsive symptom criteria for a diagnosis of ADHD. However, these scales are modeled on the recognition that the behaviors they assess are present to a varying degree in all children. The correspondence of these dimensional subscales to functional connectivity indicates that the degree of expression of ADHD-related behaviors is driven by greater or lesser connectivity in particular brain regions, exemplifying the dimensional aspect of ADHD etiology. One example of such a dimensional mechanism is shown in Figure 6A; regardless of the presence of an ADHD diagnosis, greater expression of hyperactive/impulsive behaviors was related to greater connectivity of the SAL seed with the right hippocampus/parahippocampal gyrus. Intuitively, aberrant connectivity in such regions would promote the increased expression of ADHD symptomology, resulting in a positive diagnosis of ADHD. This relationship is thus consistent with the perspective of ADHD as existing along a continuum that includes typical brain function.
In addition, categorical differences in functional connectivity magnitude that were independent of symptom measures (Figure 3; Figure 6B, C) as well as categorically different functional connectivity-behavior relationships for TDC versus ADHD (Figure 4; Figure 6D, E), were also observed across multiple networks and regions. A series of factors could contribute to the existence of such categorical effects. First, clinically-defined ADHD may encompass impairments in constructs not fully accounted for by the two studied behavioral domains. For example, functional alterations in sensorimotor cortical regions(Mostofsky, et al., 2006; Tian, et al., 2008) may contribute to sensory processing impairments in ADHD(Cheung and Siu, 2009; Yochman, et al., 2004). Other processes associated with a categorical diagnosis of ADHD, including temporal discounting behavior(Paloyelis, et al., 2010), error processing(O'Connell, et al., 2009; Senderecka, et al., 2012) and reward processing(Paloyelis, et al., 2012) could also have differential underlying neurobiology and contribute to the observed categorical effects. However, it is unlikely that these secondary behavioral deficits could account for the presence of wide-spread categorical effects on both functional connectivity values (Figure 3) and brain-behavior relationships (Figure 4) that are independent of the examined dimensional relationships as shown in this study. Secondly, part of the observed categorical effects may represent effects of comorbidities, as childhood ADHD frequently presents alongside anxiety disorders, conduct disorder and oppositional-defiant disorder(Costello, et al., 2003). However, many comorbid psychiatric and neurological disorders were considered exclusion criteria across the three imaging sites. Therefore, it is again unlikely that secondary or subthreshold disorders would be sufficient to account for the observed wide-spread categorical effects.
A remaining possibility, which we tentatively support, is that ADHD etiology comprises categorical mechanisms in addition to dimensional characteristics. This explanation largely corroborates factor analytic studies indicating that a separate “general” ADHD mechanism together with “specific” inattention and hyperactive/impulsive factors best account for variation in ADHD symptoms(Martel, et al., 2010; Toplak, et al., 2009). Such mechanisms are supported by the finding that a number of regions showed categorical differences in their functional connectivity after controlling for dimensional effects. For example, sensorimotor regions exhibited categorical hypo-connectivity with CON but hyper-connectivity with DM. The CON plays a role in initiating task sets for adaptive control of behavior by preparing secondary sensory and motor processes (Dosenbach, et al., 2008; Pochon, et al., 2001). The observed disruption of connectivity between CON and sensorimotor regions may represent a deficit in coordination between these regions that contributes, in a qualitative manner, to heightened symptoms of ADHD. On the other hand, the DM and sensorimotor network are negatively correlated during rest and finger tapping in healthy adults(Gao and Lin, 2012), a relationship which may reduce interference from internally-directed processes of the DM during externally-directed motor behaviors. Thus an increased association between DM and sensorimotor regions could again contribute, in a qualitative manner, to impaired motor control(Tseng, et al., 2004) and heightened responsiveness to sensory stimuli(Dunn and Bennett, 2002) in children with ADHD. The diminished medial prefrontal cortical connectivity within the DM, which is a replicated deficit in ADHD(Castellanos, et al., 2008; Fair, et al., 2010; Qiu, et al., 2010), persisted after controlling for behavioral measures (Figure 3, Figure 6B). It could be speculated that hypo-connectivity of the medial prefrontal cortex within the DM hinders uniform DM suppression during externally-directed attention, contributing, in a categorical manner, to difficulties with maintaining attention(Sonuga-Barke and Castellanos, 2007). Finally, children with ADHD also exhibited hyper-connectivity of CON in medial and inferior frontal/insula cortical regions consistent with core SAL regions. The CON and SAL undergo functional segregation throughout typical development(Fair, et al., 2007), becoming functionally distinct in adulthood(Dosenbach, et al., 2008; Elton and Gao, In Press; Seeley, et al., 2007). These networks appear to be less functionally segregated in children with ADHD, potentially contributing to cognitive deficits in this disorder(Fair, et al., 2007).
Evidence that the presence of psychopathology alters the relationship of behaviors to functional connectivity in particular regions across all four networks relationships also supports the postulation of categorical mechanisms. The implications for such a discrepancy in brain-behavior relationships between TDC and ADHD children is that the expression of ADHD symptomology does not lie exclusively on the continuum of normal behavioral expression. For example, greater CON connectivity with a cluster extending from the precuneus to posterior cingulate cortex was associated with greater severity of hyperactive/impulsive symptoms in children with ADHD; however, this same region showed a negative brain-behavior relationship in TDC (Figure 6E). In healthy individuals, the posterior cingulate and precuneus, core regions of the DM, functionally interact with the CON to support goal-directed planning (Gerlach, et al., 2011; Spreng, et al., 2010). However, in children with ADHD, this same functional interaction is apparently associated with increased impulsive behavior, suggesting poorer planning ability (Marzocchi, et al., 2008). Thus, at least for certain regions, ADHD symptoms exhibit a qualitatively different profile of functional connectivity from those behaviors that fall in the non-clinical range. Such findings not only provide evidence for a categorical nature of ADHD but also highlight the importance of considering both categorical and dimensional measures when characterizing this disorder (Chabernaud, et al., 2012). Thus, overall, our results indicate that both dimensional factors and categorical mechanisms likely contribute to ADHD.
Finally, there were several regions including the fusiform gyrus in DM and SAL and the anterior cingulate cortex in CON that showed effects of both dimensional and categorical variables. As shown in Figure 6F, for the connectivity of the anterior cingulate cortex to the CON seed, there were remaining categorical differences after controlling for the consistent negative relationship between functional connectivity and inattention symptom across both TDC and ADHD. The existence of such regions highlights the fact that dimensional and categorical mechanisms of ADHD not only express separately in different brain areas but could also function independently in the same brain region. Such convergence of categorical and dimensional effects on common neural targets may indicate a functionally relevant etiological mechanism and deserves further investigation.
Limitations
Several methodological limitations should be considered. First, we only considered males in this study, but future work should investigate potential sex differences in the neural network alterations underlying ADHD. Second, although imaging site was a covariate in all statistical analyses, we further tested the interaction of site and ADHD diagnosis on regions of each network showing a significant effect of ADHD in the pooled sample to confirm our results were not driven by a single site. There were no significant effects of site on ADHD-related connectivity alterations for any network. Third, due to the extent of missing data regarding psychostimulant medication use, our analyses did not control for this variable. However, we explored the potential contribution of medications(Konrad, et al., 2007) in the subsample of ADHD children for which medication status was available by conducting a two-sample t-test on the mean functional connectivity within regions showing an effect of ADHD in primary analyses. We found no significant differences between medication naive and non-medication naive children in ADHD-affected regional connectivity. Furthermore, we opted not to adjust for intelligence (IQ) since cognitive-behavior deficits in ADHD tend to produce lower IQ scores and controlling for this variable can provide counterintuitive estimates of effects of interest(Dennis, et al., 2009). Nonetheless, post-hoc tests of the effects of IQ on regional connectivity related to categorical and dimensional measures of ADHD confirmed that intelligence did not account for our findings. We also investigated potential differences between ADHD subtypes in regions identified in primary analyses and detected no significant effects of the combined type (n=88) versus inattentive type (n=64) on either categorically-defined regions or dimensional brain-behavior relationships. Because hyperactivity/impulsivity and inattention are correlated, including hyperactivity/impulsivity and inattention variables in separate models reduced the interpretability of observed effects as being specific to the symptom being tested. However, since these variables share a substantial portion of variance (56%), on top of the multicollinearity concern in linear regression, including both of these variables in the same model would have minimized the effects of either and potentially resulted in an overestimation of categorical effects. Also, the contention that observed correlations between brain regions are revealed rather than “introduced” by removal of the global signal time series is debated (Fox, et al., 2009; Murphy, et al., 2009), and thus it is possible that such processing contributed to our results. Additionally, although the study sample consisted of children, the data was registered to an adult template (MNI), potentially resulting in a larger degree of registration error than would be observed for adult studies. Finally, the use of full Pearson correlation for estimating network connectivity limits inferences regarding “direct” versus “indirect” connections between regions and may be less sensitive to detecting true connections than other methods (Smith, et al., 2011). Future work utilizing partial correlation or other connectivity measures may provide a more informed picture of ADHD-related functional connectivity abnormalities.
Conclusions
In this study, we characterized the effects of dimensional behavioral measures of ADHD on functional connectivity of four large-scale neural networks. We also documented categorical differences, in both brain-behavior relationships and functional connectivity magnitude, that were distinct from effects of dimensional relationships, potentially reflecting certain categorical mechanisms underlying the etiology of ADHD. This study contributes novel insight to the ongoing debate regarding diagnostic and investigative models of ADHD and provides support for a characterization that includes both categorical diagnosis and symptom severity indices.
Supplementary Material
ACKNOWLEDGMENTS
Funding was provided to contributing research institutions by The Autism Speaks Foundation, Johns Hopkins General Clinical Research Center, National Center for Resource, Intellectual and Developmental Disabilities Research Center, NIMH, The Stavros Niarchos Foundation, The Leon Levy Foundation, an endowment from Phyllis Green and Randolph Cōwen, The Commonwealth Sciences Foundation (Ministry of Health, China), The National Foundation (Ministry of Science and Technology, China), The National Natural Sciences Foundation (China), The Funds for International Cooperation of the National Natural Science Foundation of China, and Open Research Fund of the State Key Laboratory of Cognitive Neuroscience and Learning.
We would also like to acknowledge the UNC start-up fund to WG.
Footnotes
FINANCIAL DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Bacon W. NIMH-computerized diagnostic interview schedule for children-version IV (C-DISC-4) New York: Columbia University; 1997. [Google Scholar]
- Bidwell LC, Willcutt E, McQueen M, DeFries J, Olson R, Smith S, Pennington B. A Family Based Association Study of DRD4, DAT1, and 5HTT and Continuous Traits of Attention-Deficit Hyperactivity Disorder. Behavior Genetics. 2011;41:165–174. doi: 10.1007/s10519-010-9437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabernaud C, Mennes M, Kelly C, Nooner K, Di Martino A, Castellanos FX, Milham MP. Dimensional Brain-Behavior Relationships in Children with Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2012;71:434–442. doi: 10.1016/j.biopsych.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung PP, Siu AM. A comparison of patterns of sensory processing in children with and without developmental disabilities. Research in developmental disabilities. 2009;30:1468–1480. doi: 10.1016/j.ridd.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JDA, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of abnormal child psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP, Castellanos FX. Toward Systems Neuroscience of ADHD: A Meta-Analysis of 55 fMRI Studies. American Journal of Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cuffe SP, Moore CG, McKeown RE. Prevalence and correlates of ADHD symptoms in the national health interview survey. J Atten Disord. 2005;9:392–401. doi: 10.1177/1087054705280413. [DOI] [PubMed] [Google Scholar]
- Dan L, Jin Y, Steven GV, Yuemei Z, Caihong T. Report on Shanghai norms for the Chinese translation of the Wechsler Intelligence Scale for Children-Revised. Psychological reports. 1990;67:531–541. doi: 10.2466/pr0.1990.67.2.531. [DOI] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The Role of Working Memory in Visual Selective Attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W, Bennett D. Patterns of sensory processing in children with attention deficit hyperactivity disorder. OTJR: Occupation, Participation and Health. 2002;22:4–15. [Google Scholar]
- DuPaul GJ. ADHD Rating Scale IV: checklists, norms, and clinical interpretation. New York: Guilford; 1998. [Google Scholar]
- Elton A, Gao W. Divergent Task-Dependent Functional Connectivity of Executive Control and Salience Networks. Cortex. doi: 10.1016/j.cortex.2013.10.012. (In Press) [DOI] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Predictive Validity of Categorically and Dimensionally Scored Measures of Disruptive Childhood Behaviors. Journal of the American Academy of Child & Adolescent Psychiatry. 1995;34:477–487. [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The Global Signal and Observed Anticorrelated Resting State Brain Networks. Journal of Neurophysiology. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Gilmore JH, Alcauter S, Lin W. The dynamic reorganization of the default-mode network during a visual classification task. Front Syst Neurosci. 2013;7:34. doi: 10.3389/fnsys.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Hum Brain Mapp. 2012;33:192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL. Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage. 2011;55:1816–1824. doi: 10.1016/j.neuroimage.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current opinion in neurology. 2008;21:424. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of Functional Magnetic Resonance Imaging Studies of Inhibition and Attention in Attention-deficit/Hyperactivity Disorder: Exploring Task-Specific, Stimulant Medication, and Age Effects. Arch Gen Psychiatry. 2012;17:1–14. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Haslam N. The latent structure of mental disorders: A taxometric update on the categorical vs dimensional debate. Current Psychiatry Reviews. 2007;3:172–177. [Google Scholar]
- Helzer JE. The feasibility and need for dimensional psychiatric diagnoses. Psychological Medicine. 2006;36:1671. doi: 10.1017/S003329170600821X. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16:S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Flanagan DP, Alfonso VC, Mascolo JT. Review of Wechsler Intelligence Scale for Children, (WISC-IV) 2006. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997 doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Fink GR, Herpertz-Dahlmann B. Long-Term Effects of Methylphenidate on Neural Networks Associated With Executive Attention in Children With ADHD: Results From a Longitudinal Functional MRI Study. Journal of the American Academy of Child & Adolescent Psychiatry. 2007;46:1633–1641. doi: 10.1097/chi.0b013e318157cb3b. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, McLoughlin G, Asherson P. Attention deficit hyperactivity disorder. Neuromolecular medicine. 2006;8:461–484. doi: 10.1385/NMM:8:4:461. [DOI] [PubMed] [Google Scholar]
- Larsson H, Anckarsater H, Råstam M, Chang Z, Lichtenstein P. Childhood attention-deficit hyperactivity disorder as an extreme of a continuous trait: a quantitative genetic study of 8,500 twin pairs. Journal of Child Psychology and Psychiatry. 2012;53:73–80. doi: 10.1111/j.1469-7610.2011.02467.x. [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Perez R, Kothmann D, Woldorff MG. Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex. 2005;41:377–388. doi: 10.1016/s0010-9452(08)70274-0. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- Lubar JF. The development of a quantitative electroencephalographic scanning process for attention deficit-hyperactivity disorder: reliability and validity studies. Neuropsychology. 2001;15:136–144. doi: 10.1037//0894-4105.15.1.136. [DOI] [PubMed] [Google Scholar]
- Malmberg K, Edbom T, Wargelius H-L, Larsson J-O. Psychiatric problems associated with subthreshold ADHD and disruptive behaviour diagnoses in teenagers. Acta Paediatrica. 2011;100:1468–1475. doi: 10.1111/j.1651-2227.2011.02363.x. [DOI] [PubMed] [Google Scholar]
- Marcus DK, Barry TD. Does attention-deficit/hyperactivity disorder have a dimensional latent structure? a taxometric analysis. Journal of Abnormal Psychology. 2011;120:427. doi: 10.1037/a0021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Von Eye A, Nigg JT. Revisiting the latent structure of ADHD: is there a ‘g’factor? Journal of Child Psychology and Psychiatry. 2010;51:905–914. doi: 10.1111/j.1469-7610.2010.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzocchi GM, Oosterlaan J, Zuddas A, Cavolina P, Geurts H, Redigolo D, Vio C, Sergeant JA. Contrasting deficits on executive functions between ADHD and reading disabled children. Journal of Child Psychology and Psychiatry. 2008;49:543–552. doi: 10.1111/j.1469-7610.2007.01859.x. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milham MP, Fair D, Mennes M, Mostofsky SH. Frontiers: The adhd-200 consortium: a model to advance the translational potential of neuroimaging in clinical neuroscience. Frontiers in Systems Neuroscience. 2012;6 doi: 10.3389/fnsys.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Rimrodt SL, Schafer JG, Boyce A, Goldberg MC, Pekar JJ, Denckla MB. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, Fitzgerald M, Foxe JJ, Robertson IH. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD) Neuropsychologia. 2009;47:1149–1159. doi: 10.1016/j.neuropsychologia.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:722–732. doi: 10.1016/j.jaac.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert review of neurotherapeutics. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon J-B, Levy R, Poline J-B, Crozier S, Lehéricy S, Pillon B, Deweer B, Le Bihan D, Dubois B. The Role of Dorsolateral Prefrontal Cortex in the Preparation of Forthcoming Actions: an fMRI Study. Cerebral Cortex. 2001;11:260–266. doi: 10.1093/cercor/11.3.260. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu MG, Ye Z, Li QY, Liu GJ, Xie B, Wang J. Changes of Brain Structure and Function in ADHD Children. Brain Topogr. 2010 doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z, Herjanic B. Diagnostic interview for children and adolescents-IV (DICA-IV) Multi-Health Systems. Toronto, Canada. 1997 [Google Scholar]
- Rossi A, Pessoa L, Desimone R, Ungerleider L. The prefrontal cortex and the executive control of attention. Experimental Brain Research. 2009;192:489–497. doi: 10.1007/s00221-008-1642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderecka M, Grabowska A, Szewczyk J, Gerc K, Chmylak R. Response inhibition of children with ADHD in the stop-signal task: an event-related potential study. Int J Psychophysiol. 2012;85:93–105. doi: 10.1016/j.ijpsycho.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF, Nichols TE, Ramsey JD, Woolrich MW. Network modelling methods for FMRI. NeuroImage. 2011;54:875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. Categorical models of childhood disorder: A conceptual and empirical analysis. Journal of Child Psychology and Psychiatry. 1998;39:115–133. [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J, Wigal T, Lakes K, Volkow N. Attention deficit hyperactivity disorder: Defining a spectrum disorder and considering neuroethical implications. Oxford handbook of neuroethics. 2011:309–342. [Google Scholar]
- Thapar A, Langley K, O'donovan M, Owen M. Refining the attention deficit hyperactivity disorder phenotype for molecular genetic studies. Molecular psychiatry. 2006;11:714–720. doi: 10.1038/sj.mp.4001831. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y. Enhanced resting-state brain activities in ADHD patients: a fMRI study. Brain and Development. 2008;30:342–348. doi: 10.1016/j.braindev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Pitch A, Flora DB, Iwenofu L, Ghelani K, Jain U, Tannock R. The unity and diversity of inattention and hyperactivity/impulsivity in ADHD: evidence for a general factor with separable dimensions. Journal of Abnormal Child Psychology. 2009;37:1137–1150. doi: 10.1007/s10802-009-9336-y. [DOI] [PubMed] [Google Scholar]
- Tseng MH, Henderson A, Chow SMK, Yao G. Relationship between motor proficiency, attention, impulse, and activity in children with ADHD. Developmental Medicine & Child Neurology. 2004;46:381–388. doi: 10.1017/s0012162204000623. [DOI] [PubMed] [Google Scholar]
- Turatto M, Sandrini M, Miniussi C. The role of the right dorsolateral prefrontal cortex in visual change awareness. NeuroReport. 2004;15:2549–2552. doi: 10.1097/00001756-200411150-00024. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance A, Silk T, Casey M, Rinehart N, Bradshaw J, Bellgrove M, Cunnington R. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Molecular psychiatry. 2007;12:826–832. doi: 10.1038/sj.mp.4001999. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Yochman A, Parush S, Ornoy A. Responses of preschool children with and without ADHD to sensory events in daily life. The American journal of occupational therapy. 2004;58:294–302. doi: 10.5014/ajot.58.3.294. [DOI] [PubMed] [Google Scholar]
- Zhang S, Faries D, Vowles M, Michelson D. ADHD rating scale IV: psychometric properties from a multinational study as clinician-administered instrument. International Journal of Methods in Psychiatric Research. 2005;14:186–201. doi: 10.1002/mpr.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.