Abstract
Objective
The FDA-approved trial, “A Phase 1, Open-label, First-in-human, Feasibility and Safety Study of Human Spinal Cord-derived Neural Stem Cell Transplantation for the Treatment of Amyotrophic Lateral Sclerosis, Protocol Number: NS2008-1,” is complete. Our overall objective was to assess the safety and feasibility of stem cell transplantation into lumbar and/or cervical spinal cord regions in ALS subjects.
Methods
Preliminary results have been reported on the initial trial cohort of 12 ALS subjects. Here, we describe the safety and functional outcome monitoring results for the final trial cohort, consisting of 6 ALS subjects receiving 5 unilateral cervical intraspinal neural stem cell injections. Three of these subjects previously received 10 total bilateral lumbar injections as part of the earlier trial cohort. All injections utilized a novel spinal-mounted stabilization and injection device to deliver 100,000 neural stem cells per injection, for a dosing range up to 1.5 million cells. Subject assessments included detailed pre- and post-surgical neurological outcome measures.
Results
The cervical injection procedure was well-tolerated and disease progression did not accelerate in any subject, verifying the safety and feasibility of cervical and dual-targeting approaches. Analyses on outcome data revealed preliminary insight into potential windows of stem cell biological activity and identified clinical assessment measures that closely correlate with ALSFRS-R scores, a standard assessment for ALS clinical trials.
Interpretation
This is the first report of cervical and dual-targeted intraspinal transplantation of neural stem cells in ALS subjects. This approach is feasible and well-tolerated, supporting future trial phases examining therapeutic dosing and efficacy.
INTRODUCTION
There is growing interest in the use of stem cells1–10 as a therapy in amyotrophic lateral sclerosis (ALS), a lethal neurological disorder characterized by the degeneration of motor neurons. Stem cells offer a means to replace lost cells, provide neurotrophic support, and improve the diseased microenvironment1,7–10. Preclinical in vitro and in vivo evidence support the therapeutic translation of stem cells9, and studies by our group and others demonstrate that human spinal stem cells (HSSCs) produce protective growth factor profiles, differentiate into neurons, form synapses with host motor neurons, and have beneficial effects after intraspinal transplantation in G93A-SOD1 rats, an established model of ALS2–6.
In 2009, the Food and Drug Administration (FDA) approved a Phase I clinical trial examining the safety and feasibility of HSSC injections into the spinal cords of 18 ALS subjects. HSSCs were delivered using a novel intraspinal stabilization and injection device developed by our group11–14. The first 12 trial subjects, representing cohorts A-C, received HSSC transplants into the L2-L4 lumbar segments of the spinal cord. Group A subjects were non-ambulatory and received 5 unilateral (A1, n=3) or 10 total bilateral (A2, n=3) lumbar injections. Subjects in Groups B and C were ambulatory and received 5 unilateral (n=3) or 10 total bilateral (n=3) lumbar injections, respectively. As previously described, interim results from these first 12 subjects demonstrated no serious adverse events associated with HSSC transplantation15, 16.
Those encouraging results along with the critical need to maintain respiratory function in ALS subjects enabled FDA approval to complete HSSC injections into C3-C5 cervical segments of the spinal cord, the region where motor neurons involved in diaphragmatic function reside. To support these injections, the lumbar stabilization and injection device was adapted and optimized for cervical intraspinal HSSC delivery12–14. Ambulatory subjects in Groups D (n=3) and E (n=3) received 5 unilateral cervical injections. Based on previous preclinical data demonstrating enhanced therapeutic efficacy of HSSC transplantation when injections were targeted to multiple spinal cord segments2, 4, subjects in Group E were the same subjects who previously received bilateral lumbar injections as part of Group C. This cohort represents the first examination of the feasibility of targeting both lumbar and cervical spinal cord segments in ALS subjects in separate surgeries.
The Phase I trial consisting of 18 intraspinal transplantation surgeries in 15 ALS subjects was completed in May 2013. Here, we present the functional outcome data from the 6 subjects undergoing cervical stem cell transplantation surgery, including 3 subjects receiving both bilateral lumbar and unilateral cervical HSSC transplants. Data are also presented from the continued follow-up of the first 12 subjects receiving lumbar intraspinal HSSC transplants. Overall, results demonstrate that HSSCs can be safely transplanted into both lumbar and/or cervical human spinal cord segments, warranting future trial phases focused on cellular dosing and therapeutic efficacy.
METHODS
Trial design and subject selection
The goals of this Phase I trial were to assess the safety and tolerability of the surgical procedure and the presence of neural stem cells in the spinal cord, and to examine the use of immunosuppression in ALS subjects, using a “risk escalation” study design consisting of 5 subject cohorts7, 15, 16. Subject selection criteria, demographics, and inclusion and enrollment criteria for Groups A-C have been previously described15, 16. For Groups D and E, inclusion criteria were the same as for Group B with the additional requirement of demonstrable arm weakness with an ALS Functional Rating Scale-Revised (ALSFRS-R) arm subscore between 1 and 3; all Group E subjects were recruited from Group C and had received prior lumbar intraspinal stem cell injections17. Detailed inclusion and exclusion criteria are available at: http://www.clinicaltrials.gov/ct2/show/NCT01348451.
Neural stem cell selection
The NSI-566RSC HSSC cell line used in the trial has been previously described6, 18, 19. The cells are stored under current Good Manufacturing Practice (cGMP) conditions and delivered to the surgery site at a concentration of 10,000 cells/μl15, 16. Cell viability was assessed prior to each surgery to ensure the required viability of at least 70% to proceed with transplantation15, 16.
Cervical stem cell transplantation approach
For cohorts D and E, adaptations were made to the lumbar stabilization and injection device and surgical procedure11–16 to accommodate cervical injections, including redesign of the mounting platform to adhere the device caudally to the C7 vertebrae and rostrally at the base of the skull12–14. Briefly, standard anesthetic and monitoring techniques were adhered to similar to those for lumbar injections15, 16, and the surgical procedure for Groups D and E involved a C3-C5 laminectomy. Subjects received 5 unilateral injections spaced 4 mm apart. Ten μl were delivered at a rate of 5 μl/min over 2 minutes, for a total of 500,000 cells in the 5 injections. Following completion of all injections, the dura and tissue incisions were closed and post-operative subject care was managed as previously described15, 16. A conservative lifelong, multi-agent immunosuppression approach was employed for the Phase I trial15, 16. For additional details of the cervical microinjection device, surgical procedure, and immunosuppression regimen for subjects in Groups D and E, refer to our technical approach and safety outcome report17.
Subject assessments
All subjects received an MRI during screening to calculate precise injection positioning and serve as a baseline for the assessment of post-operative MRI scans, which will be analyzed and reported separately. To determine progression of disease status, subjects regularly underwent standard clinical evaluations as well as regular functional assessments, including ALSFRS-R, seated forced vital capacity (FVC), grip strength assessments (GST), hand-held dynamometry (HHD), electrical impedance myography (EIM), and bladder ultrasounds14, 15, 19–21. Group A subjects were not ambulatory; these subjects were evaluated once pre-operatively and regularly following transplantation. All remaining subjects in Groups B, C and D were evaluated monthly for 3 months before surgery to establish a standard slope of disease progression and regularly following transplantation. Group E subjects previously received lumbar stem cell transplants as Group C; therefore, functional assessment schedules were already underway prior to surgery and were continued regularly following cervical transplantation. The schedule of all pre- and post-operative assessments is summarized in Supplemental Table 1.
Although this was a Phase I trial and functional outcome data were collected for the purpose of assessing safety, secondary analyses of these data were performed as a means to gain insight into how cellular transplantation affected disease progression rates and to inform outcome assessment approaches in future trial phases. Pre-surgical disease progression rates for the various functional outcome measures were first calculated using linear regression analyses for subjects with multiple available pre-surgical outcome assessment data points. These slopes were utilized to determine whether post-surgical assessment data points at 6, 9, 12, and 15 months were improved relative to predicted points extrapolated from the pre-surgical progression rates. In addition, Pearson correlation analyses were performed using available data points for the various functional measures to determine which outcome assessments most closely correlated with ALSFRS-R scores. Finally, we calculated progression rate slopes for ALSFRS-R scores and GST outcomes based on data points across 9-month sliding windows to determine if there were periods where progression rates were attenuated or improved relative to the pre-surgical progression rate. These analyses were performed for Group E subjects (individuals who received both lumbar and cervical transplantation), as they had the largest amount of available assessment data. Plotted values represent slopes generated from the available data points within each 9-month window. Best-fit curves were then generated for each subject using fourth-order polynomial analyses. All statistical analyses and curve fitting utilized R version 3.0.1 (http://cran.r-project.org/) and GraphPad Prism 6 for Windows (SanDiego, CA).
RESULTS
Subject selection and general surgical outcomes
Subject demographics for all cohorts are presented in Table 1. Enrolled subjects included 13 males and 2 females ranging in age from 35–66 years old. Disease duration ranged between 1.3–13 years at the time of surgery. All Group E subjects, one of two trial cohorts designated to receive cervical stem cell transplants, previously received lumbar stem cell transplants as Group C. In total, 15 ALS subjects underwent 18 surgeries15–17.
Table 1.
Subject demographics
| Group | Surgery details | Subject number |
Surgery number |
Subject age at surgery |
Disease duration at surgery (yrs) |
Gender | Death (mos post-surgery) |
|---|---|---|---|---|---|---|---|
| A1 | non-ambulatory unilateral lumbar |
1 | 1 | 61.8 | 5.2 | Male | 30 |
| 2 | 2 | 43.4 | 12.7 | Male | |||
| 3 | 3 | 51.1 | 2.1 | Male | 13 | ||
| A2 | non-ambulatory bilateral lumbar |
4 | 4 | 37.5 | 2 | Male | |
| 5 | 5 | 66.3 | 2.2 | Male | 19 | ||
| 6 | 6 | 55 | 2.2 | Male | 9 | ||
| B | ambulatory unilateral lumbar |
7 | 7 | 59 | 1.6 | Male | |
| 8 | 8 | 41.1 | 5.6 | Male | |||
| 9 | 9 | 54.6 | 1.3 | Male | 11 | ||
| C/E | ambulatory bilateral lumbar and unilateral cervical |
10 | 10 | 48.9 | 11.6 | Male | |
| 16 | 50.2 | 13 | |||||
| 11 | 11 | 39.3 | 1.6 | Male | |||
| 18 | 40.7 | 3 | |||||
| 12 | 12 | 65.1 | 3 | Male | |||
| 17 | 66.3 | 4.3 | |||||
| D | ambulatory unilateral cervical |
13 | 13 | 50.3 | 3.1* | Male | 20 |
| 14 | 14 | 54.3 | 1.8* | Female | 7 | ||
| 15 | 15 | 35.2 | 1.7 | Female |
Subject demonstrated features of bulbar onset ALS
Overall, the procedure was well-tolerated across all cohorts with minimal peri-operative or postoperative complications. Only a nominal number of serious adverse events were observed during the course of the Phase I trial17. For cervical injections in Groups D and E, detailed reports on the intraoperative and the immediate post-operative surgical outcomes and morbidity data are presented in our recent technical approach and safety outcome report17.
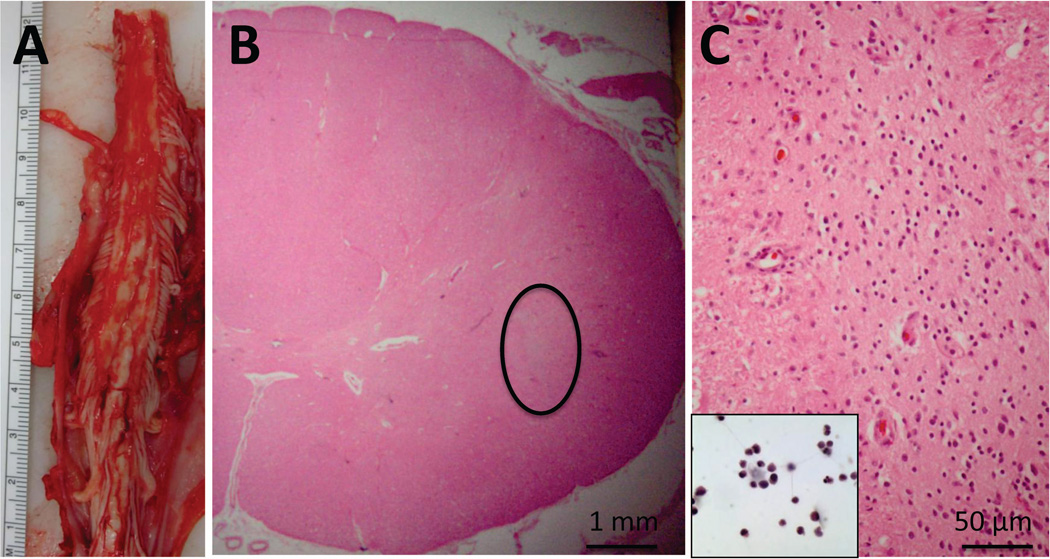
At this point, seven subject deaths have occurred (Table 1). As previously reported, subject 6 died suddenly and unexpectedly 8 months post-surgery due to a congenital cardiac defect, and subject 3 died of respiratory failure associated with disease progression 13 months post-surgery15, 16. Subjects 1, 5, 9, 13 and 14 also died of respiratory complications associated with ALS disease progression at 30, 19, 11, 20 and 7 months post-surgery, respectively. All patients underwent autopsy for analysis of tissue response to implantation and for the identification of the continued presence of the transplanted cells within the spinal cord. The detailed results of these analyses will be reported separately. Briefly, standard pathological analysis showed no evidence of hemorrhage, cyst formation, or inflammatory reaction within the sites of transplantation. A representative example of the initial post-mortem morphological findings is presented for subject 14 (Figure 1). This subject received cervical injections and died 7 months post-surgery. There were no morphological abnormalities within the sites of transplantation, however, a nest of cells likely composed of the transplanted cells was identified.
Figure 1. Neuropathological findings in patient 14.
(A) Gross image of cervical spinal cord at the time of autopsy. Serial sections through the region of transplantation did not demonstrate regions of cystic change, hemorrhage, or significant tissue disruption. (B) Representative cross section showing intact cord morphology using hematoxylin and eosin (H&E) staining. There is a “nest” of cells (circled) that are not intrinsic to the spinal cord, and do not stain with glial or neuronal markers (not shown). (C) Higher power of circled region in (B) showing the morphology of these cells, which is reminiscent of the morphology of the stem cells prior to transplantation (inset, H&E).
Functional outcome measures
Subjects regularly (see Supplemental Table 1) underwent clinical assessments. Interim results from subjects 1–12 demonstrated no obvious acceleration of disease progression, and subject 11 in Group C demonstrated modest improvements in post-operative ALSFRS-R, HHD and EIM measurements15. Continued functional outcome measure monitoring for Group A and B subjects are presented and discussed in Supplemental Figures 1 and 2, respectively. Overall, these subjects continued to demonstrate outcomes consistent with disease progression, but no acceleration of the disease course.
Group D – cervical injection
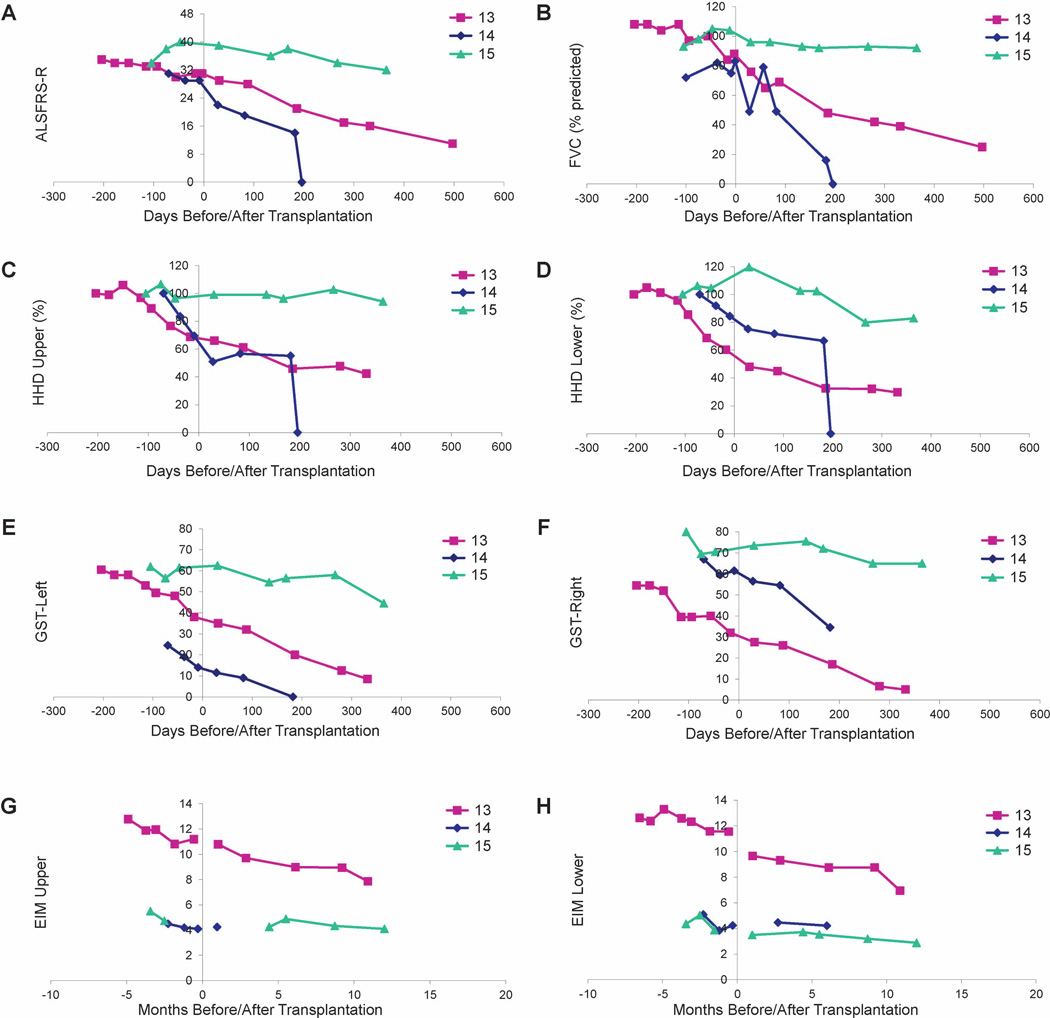
Functional outcomes are presented in Figure 2. Subjects 13 and 14 both had features of bulbar ALS. Subject 13 developed cervical kyphosis17 and died 20 months following transplantation, and subject 14 died 200 days following transplantation. While other clinical markers remained stable, subject 15 demonstrates a modest decline in ALSFRS-R and HHD following transplantation, reflecting a progression that appears slower than what is typically expected for ALS.
Figure 2. Evaluation of disease progression in Group D subjects.
Disease progression for subjects 13-15 as measured by ALSFRS-R (A), FVC (B), HHD (C-D), GST (E-F) and EIM (G-H). HHD is shown as a composite “megascore” for upper (C) or lower (D) extremities, normalized to the percent of the score at baseline. GST data are presented for left (E) and right (F) sides. EIM is shown as 50 kHz Phase all muscle average for upper (G) or lower (H) extremity muscles. X-axis is days pre- or post-surgery (day of surgery = day 0). Note that there were no precipitous declines in function after surgery for any subject. Note that a score of “0” for subject 14 indicates subject death on the day posttransplantation indicated on the x-axis.
Group E – dual-targeted injections
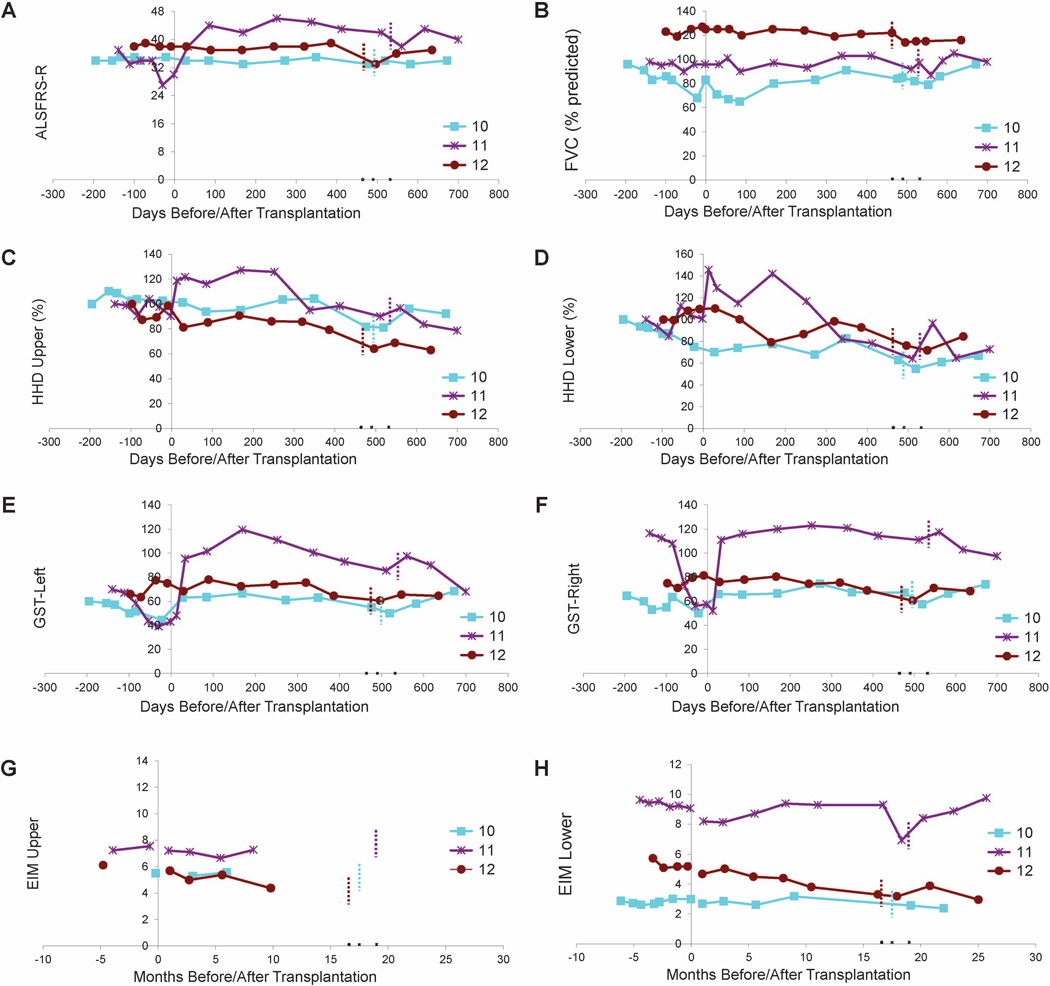
Functional outcome measures are presented in Figure 3. Subject 10 had a long disease duration and maintained a steady ALSFRS-R score accompanied by mild declines in other functional measures, suggestive of a very slowly progressive form of ALS. Subjects 11 and 12 had improved ALSFRS-R scores, steady FVC values, and modest declines in HHD megascores following HSSC transplantation, suggesting some progression of disease accompanied by multiple improved functional measures.
Figure 3. Evaluation of disease progression in Group E subjects.
Disease progression for subjects 10-12 as measured by ALSFRS-R (A), FVC (B), HHD (C-D), GST (E-F) and EIM (G-H). HHD is shown as a composite “megascore” for lower (C) or upper (D) extremities, normalized to the percent of the score at baseline. GST data are presented for left (E) and right (F) sides. EIM is shown as 50 kHz Phase all muscle average for upper (G) or lower (H) extremity muscles. X-axis is days pre- or post-lumbar surgery (day of surgery = day 0). Note that there were no precipitous declines in function after surgery for any subject. Note that Group E subjects are subjects initially enrolled in Group C and received lumbar stem cell injections, and the short dotted vertical bars indicate the number of days after the first surgery when the second stem cell transplantations (cervical injections) were administered. Note that subject 11 (purple line) showed apparent improvement in ALSFRS-R and upper and lower extremity HHD.
Advanced analyses of functional outcome measures
We performed additional analyses to gain insight into the effects of the intervention on disease progression and to identify appropriate functional outcome measures for future trial phases. Comparison of post-surgical outcome data to predicted outcome points extrapolated from pre-surgical disease progression slopes revealed improvements in a significant number of measures at 6, 9, 12, and 15 months post-surgery (Table 2). Of the 8 outcome assessments, at least 5 measures were improved in over 50% of subjects at each time point relative to the predicted outcome values extrapolated from pre-surgical progression rates. To identify which functional assessments coordinated most closely with ALSFRS-R scores, Pearson correlations were calculated between data points for the various functional outcome measures. Results indicate that GST measures most closely reflect ALSFRS-R values throughout the study period (Table 3), suggesting that ALSFRS-R and GST assessments will provide important outcome information in future trial phases.
Table 2.
Functional outcomes vs. predicted outcomes based on pre-surgical assessments
| Time post-surgery | 6 months | 9 months | 12 months | 15 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of subjects with pre- surgical slope data* |
Number of subjects with improved outcome |
Ratio | Number of subjects with pre- surgical slope data* |
Number of subjects with improved outcome |
Ratio | Number of subjects with pre- surgical slope data* |
Number of subjects with improved outcome |
Ratio | Number of subjects with pre- surgical slope data* |
Number of subjects with improved outcome |
Ratio | |
| ALSFRS | 9 | 3 | 33% | 9 | 4 | 44% | 9 | 3 | 33% | 9 | 3 | 33% |
| FVC-Seated | 9 | 3 | 33% | 9 | 3 | 33% | 9 | 3 | 33% | 9 | 3 | 33% |
| HHD-Upper | 9 | 7 | 78% | 9 | 8 | 89% | 9 | 8 | 89% | 9 | 7 | 78% |
| HHD-Lower | 9 | 5 | 56% | 9 | 4 | 44% | 9 | 4 | 44% | 9 | 5 | 56% |
| GST-Left | 8 | 7 | 88% | 8 | 8 | 100% | 8 | 7 | 88% | 8 | 7 | 88% |
| GST-Right | 8 | 6 | 75% | 8 | 6 | 75% | 8 | 6 | 75% | 8 | 6 | 75% |
| EIM-Upper | 4 | 3 | 75% | 4 | 3 | 75% | 4 | 3 | 75% | 4 | 3 | 75% |
| EIM-Lower | 10 | 6 | 60% | 10 | 6 | 60% | 10 | 6 | 60% | 10 | 7 | 70% |
Slopes were utilized to determine whether post-surgical assessment data points at 6, 9, 12, and 15 months were improved relative to predicted outcomes extrapolated from the pre-surgical progression rate slopes
Table 3.
Pearson correlations for outcome assessment measures
| ALSFRS | FVC.Seated | HHD.Upper | HHD.Lower | GST.Left | GST.Right | EIM.Upper | EIM.Lower | |
|---|---|---|---|---|---|---|---|---|
| ALSFRS | 1.00 | 0.79 | 0.63 | 0.15 | 0.86 | 0.79 | −0.19 | 0.49 |
| FVC.Seated | 0.79 | 1.00 | 0.53 | 0.34 | 0.68 | 0.57 | −0.04 | 0.12 |
| HHD.Upper | 0.63 | 0.53 | 1.00 | 0.54 | 0.64 | 0.59 | −0.19 | 0.16 |
| HHD.Lower | 0.15 | 0.34 | 0.54 | 1.00 | 0.40 | 0.45 | −0.31 | 0.08 |
| GST.Left | 0.86 | 0.68 | 0.64 | 0.40 | 1.00 | 0.89 | −0.06 | 0.19 |
| GST.Right | 0.79 | 0.57 | 0.59 | 0.45 | 0.89 | 1.00 | −0.44 | 0.17 |
| EIM.Upper | −0.19 | −0.04 | −0.19 | −0.31 | −0.06 | −0.44 | 1.00 | 0.93 |
| EIM.Lower | 0.49 | 0.12 | 0.16 | 0.08 | 0.19 | 0.17 | 0.93 | 1.00 |
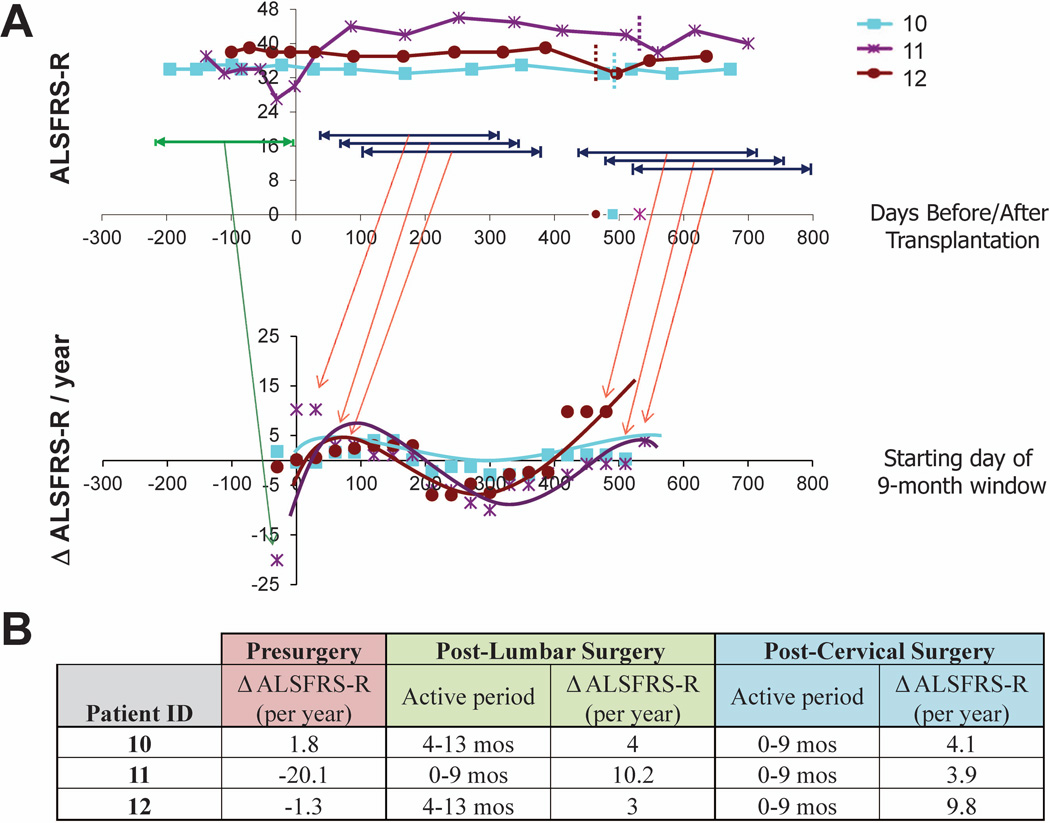
As shown in Figure 4, analysis of ALSFRS-R scores for Group E subjects exhibits improved outcomes (slope values higher than the pre-surgical slope at baseline reflect improved or attenuated progression rates during the designated window) beginning within the first month post-surgery,with slopes remaining positive for windows beginning up to 6 months post-surgery. While the rate of benefit then decreases over time, the overall progression rate generally remains attenuated relative to the pre-surgical slope through the time of the second surgery. Positive slopes are again observed across treatment windows beginning at approximately 13–14 months post-surgery for these subjects, reflecting the second HSSC transplant in subjects 10, 11, and 12 at 490, 532, and 464 days. This bimodal representation of HSSC benefit suggests that the biological activity of the cells shows the greatest benefits in the 6 months immediately following the surgeries (Figure 4B) while continuing to provide some benefit throughout the study evaluation period. Similar analyses on GST data for this cohort reflect comparable trends (data not shown).
Figure 4. Preliminary analysis of potential windows of HSSC biological activity in subjects 10-12.
To identify the most biologically active period of the injected HSSCs, post-surgery data points for Group E subjects were divided into a series of 9-month windows, beginning every month post-surgery, and slopes were calculated across each window. Slopes were also calculated using ALSFRS-R data points for the pre-surgical window. (A) The top panel demonstrates ALSFRS-R scores for Group E patients during the pre-surgical period (green) and representative ranges associated with the various sliding post-surgical 9-month windows (dark blue). The bottom panel demonstrates the slopes obtained for each sliding window, with the x-axis corresponding to the first month for each 9-month window (i.e., window 1 corresponds to months 1-10 post-surgery, window 2 corresponds to months 2-11 post-surgery, window 3 corresponds to months 3-12 post-surgery, etc.). The first plotted slope for each subject corresponds to their pre-surgical progression rate. Slope values higher than the pre-surgical slope at baseline represent improved or attenuated progression rates during the designated window. Note that the starting month of the final sliding window for each patient coincides with the dates of the second surgery, which occur at 17.5, 19, and 16.6 months after the initial cohort C surgery (time 0) for subjects 10, 11, and 12, respectively. (B) The pre-surgical slope and post-surgical slopes associated with the window correlating to the peak benefit windows for both the lumbar and cervical post-surgery time frames are summarized.
DISCUSSION
In this completed FDA-approved Phase I trial, 18 intraspinal transplantation surgeries in 15 ALS subjects were performed following a risk escalation paradigm, progressing from non-ambulatory to ambulatory subjects, lumbar to cervical spinal cord segments, and unilateral to bilateral injections across five cohorts. The encouraging interim results from Groups A-C15, 16, representing 12 subjects who received lumbar injections, supported the completion of the final trial cohorts D and E examining cervical injections in 6 ALS subjects. Notably, the final 3 subjects receiving cervical injections previously received bilateral lumbar injections. Our study represents the first report of successful intraspinal stem cell transplantation into the cervical spinal cord and of successful repeated intraspinal stem cell transplantation into lumbar and cervical spinal cord segments in ALS patients in an FDA-approved trial. Our ability to directly inject stem cells to target motor neurons in the region of the cervical spinal cord responsible for respiration represents a significant advance in the field of cellular therapy. In parallel, the dual-targeting approach, i.e. both cervical and lumbar transplantation, has the potential to preserve respiratory function and improve motor function in ALS patients4. What is now required are future studies to determine if these approaches provide sustained clinical improvement in ALS.
Of the 15 subjects in the Phase I trial, six subjects died of their disease and one subject died of a congenital heart defect unrelated to ALS between 7 and 30 months after surgery. Of the eight subjects who are still alive, three of them (subjects 2, 8, 10) had a long disease course prior to surgery, ranging 5.6, 11.6 and 12.7 years of known disease, likely representing atypical ALS, and have had little change in the trajectory of their disease. Subjects 7, 11, 12 and 15, who are alive with very slowly progressive or stabilized disease had two clinical characteristics in common: these individuals had no bulbar features of ALS and surgical transplantation occurred early within the course of their disease (average of 2 years and 1 month after symptom onset at the time of surgery). These preliminary results raise the possibility that intraspinal stem cell transplantation of ALS subjects with no bulbar symptoms early in the course of their disease could slow disease progression or even allow for functional improvement.
The majority of ALS trials utilize subject survival and ALSFRS-R scores for primary outcome measures20. Our data demonstrate that GST most closely correlates with the ALSFRS-R scores. Comparisons of pre-surgical slopes to post-transplant data revealed that over 50% of subjects demonstrated improvement across multiple clinical measures at 6, 9, 12 and 15 months post-surgery. Looking specifically at ALSFRS-R scores at the 9 month time point, the subjects who demonstrated improvements were part of Groups B, C, and E and exhibited an average disease duration under 2years prior to surgery, again suggesting that only subjects early in the disease course may experience clinical benefit. However, our experience did demonstrate a wide variation in pre-surgical progression rates for those individuals with multiple data points, emphasizing the importance of sufficient lead-in data to determine efficacy. Average declines of −1.1 ALSFRS-R score per month (−13.32 per year) have been reported 20; however, the varied slopes we observed and the heterogeneous presentation of ALS emphasize the need for subject-specific baseline data.
We acknowledge that this study was not powered to determine efficacy and there was no control arm. In addition, some subjects exhibited a significant disease burden prior to surgery and were unlikely to show benefit, sufficient preclinical data points were unavailable for some subjects, and best-fit pre-surgical slopes were not always significantly powered given the number of available data points. Despite these limitations, we were able to identify potential possible therapeutic windows in our advanced evaluation of Group C/E outcome data. Of note, the three subjects in this cohort received the highest number of injections and demonstrated the largest effects on progression rates, suggesting more injections are better, consistent with the neuroprotective mechanism of action hypothesized for this cell therapy7, 9. The ability to successfully administer 1.5 million HSSCs to ALS subjects over 15 total injections in Group E subjects into both lumbar and cervical spinal cord segments over the course of 2 surgeries is an important first step in evaluating the tolerance of the spinal cord for multiple HSSC transplantation procedures. The observed bimodal distribution in the 9-month sliding window slope analysis suggests there are maximal periods of benefit that correlate with the two surgical interventions. Furthermore, as the bell-shaped benefit curve associated with each intervention is likely due to disease progression, increasing the total cell dose and applying multiple applications of the stem cells may increase both the length and magnitude of potential benefit. These very preliminary observations on only 3 subjects provide the framework for future discussions of trial designs.
In conclusion, as we move forward, the continued assessment of data collected from subjects participating in Phase I of the trial, evaluation of post-surgical MRI data, and characterization of the cellular grafts in deceased subjects will provide further insight into the therapeutic mechanisms and potential efficacy of intraspinal stem cell transplantation in ALS. With improved definitions for subject selection criteria, careful evaluation of clinical history prior to surgery, and utilization of the most efficient neurological assessment measures, we are primed for continued progress in future trial phases. Phase II of this trial commenced in September 2013 (ClinicalTrials.gov identifier: NCT01730716).
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the study participants and their families for their trust and dedication to advancing the field of ALS therapeutics. Thank you to the trial Data Safety Monitoring Board, chaired by Dr. Zachary Simmons (Penn State). Thank you to Dr. Thomas G. Hazel for providing the cGMP stem cells, to Latoya Shaw for help with subject assessments, to Dr. Marla Gearing for assistance with neuropathology, to the staff of the Emory ALS Center, and to Jayna Duell, RN for assistance with table preparation. This Phase I study was funded by Neuralstem, Inc; the Phase II study is funded by the National Institutes of Health (NIH R01 NS077982 to JDG, ELF, NMB, SBR), the ALS Association (to ELF), and Neuralstem, Inc. Additional support for tissue and data analysis provided by the National Institutes of Health (NIA 5P50AG025688 to JDG) and theA. Alfred Taubman Medical Research Institute (to ELF and NMB). NMB is the inventor of devices to enable safe and accurate injection of the human spinal cord. Neuralstem, Inc. has purchased an exclusive license to this technology. NMB received an inventor’s share of this fee, and has the rights to royalty payments for distribution of this technology. KJ is an employee of Neuralstem, Inc.
Footnotes
AUTHORSHIP STATEMENT
ELF, NMB, and JDG designed the study. KJ provided critical study design input. NMB, TF, MP, JB, and JDG contributed to data acquisition. ELF, NMB, JH, SBR, SAS and JDG were responsible for data analysis and interpretation. ELF and SAS drafted the manuscript. All authors critically edited the content of the article and approved the final version.
REFERENCES
- 1.Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011 doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hefferan MP, Galik J, Kakinohana O, et al. Human neural stem cell replacement therapy for amyotrophic lateral sclerosis by spinal transplantation. PLoS One. 2012;7(8):e42614. doi: 10.1371/journal.pone.0042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009 Jun 1;514(4):297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Shen P, Hazel T, Johe K, Koliatsos VE. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett. 2011 May 2;494(3):222–226. doi: 10.1016/j.neulet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu L, Yan J, Chen D, et al. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006 Oct 15;82(7):865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 6.Yan J, Xu L, Welsh AM, et al. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007 Feb;4(2):e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulis NM, Federici T, Glass JD, Lunn JS, Sakowski SA, Feldman EL. Translational stem cell therapy for amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;8(3):172–176. doi: 10.1038/nrneurol.2011.191. [DOI] [PubMed] [Google Scholar]
- 8.Lunn JS, Hefferan MP, Marsala M, Feldman EL. Stem cells: comprehensive treatments for amyotrophic lateral sclerosis in conjunction with growth factor delivery. Growth Factors. 2009 Jun;27(3):133–140. doi: 10.1080/08977190902814855. [DOI] [PubMed] [Google Scholar]
- 9.Lunn JS, Sakowski SA, Federici T, Glass JD, Boulis NM, Feldman EL. Stem cell technology for the study and treatment of motor neuron diseases. Regen Med. 2011 Mar;6(2):201–213. doi: 10.2217/rme.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silani V, Calzarossa C, Cova L, Ticozzi N. Stem cells in amyotrophic lateral sclerosis: motor neuron protection or replacement? CNS Neurol Disord Drug Targets. 2010 Jul;9(3):314–324. doi: 10.2174/187152710791292666. [DOI] [PubMed] [Google Scholar]
- 11.Riley J, Butler J, Park J, et al. Targeted Spinal Cord Therapeutics Delivery: Stabilized Platform and MER Guidance Validation. Stereotactic and Functional Neurosurgery. 2007;86(2):67–74. doi: 10.1159/000112426. [DOI] [PubMed] [Google Scholar]
- 12.Riley JP, Raore B, Taub JS, Federici T, Boulis NM. Platform and Cannula Design Improvements for Spinal Cord Therapeutics Delivery. Neurosurgery. 2011 Apr 5; doi: 10.1227/NEU.0b013e3182195680. [DOI] [PubMed] [Google Scholar]
- 13.Raore B, Federici T, Taub J, et al. Cervical multilevel intraspinal stem cell therapy: assessment of surgical risks in Gottingen minipigs. Spine (Phila Pa 1976) 2011 Feb 1;36(3):E164–E171. doi: 10.1097/BRS.0b013e3181d77a47. [DOI] [PubMed] [Google Scholar]
- 14.Riley J, Federici T, Park J, et al. Cervical spinal cord therapeutics delivery: preclinical safety validation of a stabilized microinjection platform. Neurosurgery. 2009 Oct;65(4):754–761. doi: 10.1227/01.NEU.0000343524.45387.9E. discussion 61-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glass JD, Boulis NM, Johe K, et al. Lumbar Intraspinal Injection of Neural Stem Cells in Patients with Amyotrophic Lateral Sclerosis: Results of a Phase I Trial in 12 Patients. Stem cells (Dayton, Ohio) 2012 Mar 13;30(6):1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 16.Riley J, Federici T, Polak M, et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012 Aug;71(2):405–416. doi: 10.1227/NEU.0b013e31825ca05f. [DOI] [PubMed] [Google Scholar]
- 17.Riley J, Glass J, Feldman EL, et al. Intraspinal Stem Cell Transplantation in ALS: A Phase I Trial, Cervical Microinjection and Final Surgical Safety Outcomes. Neurosurgery. doi: 10.1227/NEU.0000000000000156. In Press, DOI:10.1097/NEU.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 18.Guo X, Johe K, Molnar P, Davis H, Hickman J. Characterization of a human fetal spinal cord stem cell line, NSI-566RSC, and its induction to functional motoneurons. J Tissue Eng Regen Med. 2010 Mar;4(3):181–193. doi: 10.1002/term.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes & development. 1996 Dec 15;10(24):3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 20.Healy BC, Schoenfeld D. Comparison of analysis approaches for phase III clinical trials in amyotrophic lateral sclerosis. Muscle & nerve. 2012 Oct;46(4):506–511. doi: 10.1002/mus.23392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.