Abstract
In order to explore the potential association between the leptin receptor (LEPR) gene polymorphisms and essential hypertension (EH) risk in the Northern Han Chinese population, we recruited 823 hypertensive subjects and 491 healthy control subjects from the Northern Han Chinese. Genotyping was performed to identify the Lys109Arg, Gln223Arg and Lys656Asn polymorphisms of the LEPR gene. Significant associations were found in a dominant genetic model ([GG+AG] vs AA), P=0.007, odds ratio (OR)=3.697, 95% confidence interval (CI) 1.442–9.482), and in homozygote comparison (GG vs AA, P=0.005, OR=3.890, 95% CI 1.501–10.077) for the Gln223Arg polymorphism. No significant association could be found between Lys109Arg or Lys656Asn polymorphism and EH risk. Linkage disequilibrium was detected between the Lys109Arg and Gln223Arg polymorphisms, and haplotype analyses identified that the G-A haplotype was a protective haplotype for EH. Our studies demonstrated that the LEPR Gln223Arg polymorphism had an important role in a patient's susceptibility to EH in the Northern Han Chinese population.
Keywords: leptin receptor, polymorphism, essential hypertension, Chinese, linkage disequilibrium, haplotypes
INTRODUCTION
Essential hypertension (EH) is a worldwide escalating health problem, and 27.2% of the adult Chinese population from age 35 to 74 years old suffered from this disease.1 EH represents a major risk factor for stroke, myocardial infarction and renal disease, and often occurs in combination with other metabolic complications, such as hyperlipidemia, obesity and insulin resistance. The pathogenesis of EH is complex, characterized by the involvement of multiple genes and environmental factors. Leptin and leptin receptor (LEPR) genes have been suggested to be candidate genes for hypertension via their direct effects on regulation of blood pressure (BP) and adipose tissue metabolism, or indirect effect on obesity.2
Leptin is an adipocyte-derived hormone that suppresses food intake and increases energy expenditure by binding to and activating its specific receptor in the hypothalamus.3,4 In addition, it may also affect BP by stimulating sympathetic outflow.5,6 Leptin exerts its effects through the transmembrane LEPR, the gene that is located on the human chromosome 1P32. The LEPR, which has several isoforms, is a single-transmembrane protein belonging to the cytokine receptor family. A sequence variation in the LEPR gene will impair the efficacy of leptin binding to its receptor,7 attenuating the favorably regulatory effects on BP. Meanwhile, polymorphisms in the LEPR gene have been reported being associated with high-plasma leptin level, indicating leptin resistance and a lower whole-body plasma norepinephrine spillover, which is an index of blunted sympathetic nerve activity.8
Several previous studies had investigated the relationship between the polymorphisms of LEPR gene (Lys109Arg, Gln223Arg and Lys656Asn) and EH risk in different ethnic groups, but the results are controversial. Wiedemann et al.9 failed to identify any association in the German population, whereas the study by Rosmond et al.10 suggested that the variants of Lys109Arg and Gln223Arg seem to protect from hypertension development in the Swedish population. In order to clarify the roles of these three polymorphisms of the LEPR gene in the development of hypertension, we conducted a case-controlled study in the Northern Han Chinese population. Genomic locations and related mapping data were obtained from the National Center for Biotechnology Information (Figure 1).
Figure 1.
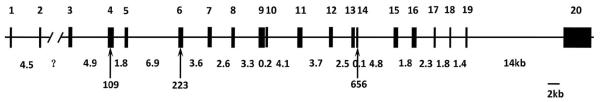
Polymorphism sites of the human LEPR gene. The human LEPR gene is composed of 20 exons and spans >70 kb of DNA. The exons are represented as vertical bars at double scale and are numbered above each exon. Estimated intron sizes are shown below except for intron no. 2 whose size is unknown. Polymorphisms are shown below the map and are identified by their amino-acid number.
MATERIALS AND METHODS
Study population
All normotensive participants and hypertensive patients were screened at the physical examination center and hypertension clinic at the Beijing Anzhen Hospital, Capital Medical University, Beijing, China. A total of 491 healthy, normotensive subjects (NT group) and 823 hypertensive patients (EH group) were screened. BP was accurately measured three times with a mercury sphygmomanometer by experienced internists at their offices. Measurements were recorded after the subjects had been seated in a chair with their feet on the floor and their arms supported at heart level for 10 min. The definition of normotension (systolic blood pressure (SBP)<120 mm Hg and diastolic blood pressure (DBP)<80 mm Hg) and hypertension (SBP≥140 mm Hg or DBP≥90 mm Hg) were based on the BP classification of the seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-VII).11 All hypertensive patients were diagnosed as being EH and without any treatment of antihypertensive medications. No EH patient is concurrently diagnosed with any other known disease, including secondary hypertension, primary renal disease, diabetic mellitus, hepatic disorders, cancer or other endocrine diseases, for example, hyper-thyroidism and so on. Smokers were defined as cigarette consumers who had smoked ≥100 cigarettes; and drinkers were defined as alcohol consumers who drank ≥12 times during the past year.12,13 Obese was defined as body mass index ≥25 kg m−2 according to the World Health Organization obesity guidelines on Asians.14 This study complied with the Declaration of Helsinki. All participants signed a consent form, and the study was approved by the Anzhen Hospital Ethics Committee of the Capital Medical University.15
Genotyping
Patient's peripheral blood sample was taken into EDTA-containing receptacles. Genomic DNA was extracted from the peripheral blood according to standard phenol–chloroform methods. We genotyped single-nucleotide polymorphisms (SNPs) using the TaqMan assay. The LEPR SNP TaqMan probes and primers were obtained from Applied Biosystems Assay-by-Design Service for SNP genotyping (Foster City, CA, USA). The sample DNA was amplified by PCR on a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems) following the manufacturer's recommendations. Genotypes were differentiated by analyzing the fluorescence levels of PCR products using an ABI PRISM 7900HT Sequence Detector (Applied Biosystems). Genotyping was performed blindly to all other data.
Statistics
In our experimental design, we used the published data in the literature to calculate the sample size, and the calculated power value was >0.8. We first used Gln223Arg data as a sample group to calculate the power value. We then used the statistical software `Power and Sample Size Calculations'16 to calculate the power value that was 0.882.
We used SPSS (Version 17.0; SPSS, Chicago, IL, USA) for database management and statistical analysis. All comparisons between specific groups for continuous variables were made using a two-sample t-test. Allelic and genotypic frequencies were compared between the hypertensive cases and the normotensive controls by using the χ2-test. To test for an association between each SNP and hypertension risk, we computed the overall genotypic test of association and genetic models (dominant, recessive, additive, allele and homozygote). A multinomial logistic regression was used to study the effect of LEPR Lys109Arg, Gln223Arg and Lys656Asn variants on hypertension status to allow incorporation of other variables into the model. All tests of association were adjusted for age, gender, body mass index, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum triglyceride and plasma glucose levels, as well as smoking and drinking habits. All analyses used the two-tailed estimation of significance. The statistical significance was defined by P<0.05. Multiple testing was adjusted using the Bonferroni correction.
The presence of Hardy–Weinberg equilibrium was tested by the χ2-test for goodness of fit based on a web program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Construction of the linkage disequilibrium map and haplotype blocks within polymorphisms of the LEPR gene was based on genotypes and utilized Haploview software (version 4.1) (http://www.broad.mit.edu/mpg/haploview/).17 The expectation maximization algorithm18 was performed to estimate haplotype frequencies and to obtain the best haplotype configuration for each multi-locus genotype. All haplotypes with frequency >1% in the combined case and control samples were examined. The χ2-test was conducted to compare the haplotype distributions between the hypertensives and the normotensives. Haplotype-specific testing was performed to compare a specific haplotype with the others. Assuming the highly prevalent haplotype as the baseline, each of the other haplotype was then compared with the baseline haplotype using a logistic regression model.14
RESULTS
Characteristics of the participants
A total of 1314 unrelated participating subjects comprising 823 hypertensive patients (531 men and 292 women; mean age 51.40±9.38) and 491 normotensive control subjects (293 men and 198 women; mean age 50.47±7.91) were recruited for the present study. The clinical and laboratory parameters of cases and controls were summarized in Table 1. In addition to BP changes, significant differences in body mass index, triglyceride level, glucose level and the ratio of drinkers were also observed between the EH and NT groups.
Table 1.
Clinical characteristics of normotensive and essential hypertensive participants
| Index | NT (491) | EH (823) | P-value |
|---|---|---|---|
| Gender (M/F) | 293/198 | 531/292 | 0.087 |
| Age (years) | 50.47 ± 7.91 | 51.40 ± 9.38 | 0.055 |
| SBP (mm Hg) | 107.43 ± 8.46 | 161.86 ± 18.31 | < 0.001 |
| DBP (mm Hg) | 69.22 ± 6.90 | 102.73 ± 14.10 | < 0.001 |
| BMI (kg m−2) | 69.22 ± 6.90 | 26.96 ± 3.50 | < 0.001 |
| ALT (UI−1) | 24.82 ± 14.67 | 26.30 ± 18.23 | 0.175 |
| TG (mmoll−1) | 1.63 ± 1.53 | 1.88 ± 1.53 | 0.006 |
| TC (mmoll−1) | 4.88 ± 0.99 | 4.90 ± 1.92 | 0.760 |
| HDL-C (mmoll−1) | 1.38 ± 0.95 | 1.31 ± 0.36 | 0.066 |
| LDL-C (mmoll−1) | 3.01 ± 0.80 | 2.95 ± 0.88 | 0.224 |
| Glu (mmoll−1) | 5.10 ± 0.72 | 5.34 ± 0.95 | < 0.001 |
| BUN (mmoll−1) | 5.70 ± 3.86 | 5.89 ± 2.88 | 0.439 |
| Cr (μmoll−1) | 77.71 ± 14.92 | 79.11 ± 19.21 | 0.221 |
| HR (b.p.m.) | 71.65 ± 9.09 | 71.66 ± 9.75 | 0.990 |
| Smokers (n) | 128 | 229 | 0.522 |
| Drinkers (n) | 75 | 248 | < 0.001 |
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; DBP, diastolic blood pressure; EH, essential hypertensive patients; F, female; Glu, blood glucose; HDL-C, high-density lipoprotein cholesterol; HR, heart rate; LDL-C, low-density lipoprotein cholesterol; M, male; NS, not significant; NT, normotensive subjects; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride. All the quantitative data were presented as mean ± s.d. P-values < 0.05 are provided in bold to emphasize their significance.
Association analyses
Among all the participants, 99.3% samples of Lys109Arg polymorphism, 98.8% samples of Gln223Arg polymorphism and 96% samples of Lys656Asn polymorphism were successfully detected in the laboratory. No deviation from the Hardy–Weinberg expectation was observed for the effect of LEPR Lys109Arg, Gln223Arg or Lys656Asn variants in either the EH group or the NT group (data not shown). The genotype frequencies for the three polymorphisms in the LEPR gene are shown in Table 2. Chi-square analyses indicated that the Gln223Arg polymorphism was significantly associated with EH. In contrast, no significant difference was found for the Lys109Arg or Lys656Asn polymorphisms. Our stratification analysis result did not show difference in LEPR polymorphisms (Lys109Arg, Gln223Arg and Lys656Asn) on triglyceride levels (data not shown) or obesity (Table 3).
Table 2.
The frequencies of the LEPR gene Lys109Arg, Gln223Arg and Lys656Asn polymorphisms genotypes
| Genotype (frequency, %) | P-value | Allele (frequency, %) | P-valuea | ||||
|---|---|---|---|---|---|---|---|
|
Lys109Arg
| |||||||
| GG | AG | AA | G | A | |||
|
| |||||||
| Case | 547 (67.1) | 340 (82.2) | 22 (2.7) | 0.601 | 1340 (82.2) | 290 (17.8) | 0.486 |
| Control | 323 (65.9) | 149 (30.4) | 18 (3.7) | 795 (81.1) | 185 (18.9) | ||
|
| |||||||
|
Gln223Arg
| |||||||
| Case | 608 (75.2) | 192 (23.8) | 8 (1.0) | 0.041 | 1408 (87.1) | 208 (12.9) | 0.189 |
| Control | 360 (73.5) | 116(23.7) | 14(2.9) | 836 (85.3) | 144 (14.7) | ||
|
| |||||||
|
Lys656Asn
| |||||||
| GG | GC | CC | G | C | |||
|
| |||||||
| Case | 684 (86.6) | 104 (13.2) | 2 (0.3) | 0.233 | 1472 (93.2) | 108 (6.8) | 0.098 |
| Control | 424 (94.8) | 47 (10.0) | 1 (0.2) | 895 (94.8) | 49 (5.2) | ||
P-value of the comparison of allelic frequencies. P-value <0.05 is provided in bold to emphasize its significance.
Table 3.
The genotype distributions and allele frequencies of the LEPR gene Lys109Arg, Gln223Arg and Lys656Asn polymorphisms in an obese and non-obese population
| Genotype (frequency, %) | P-valuea | Allele (frequency, %) | P-valueb | ||||
|---|---|---|---|---|---|---|---|
|
Lys109Arg
| |||||||
| GG | AG | AA | G | A | |||
|
| |||||||
| Total | 0.875 | 0.893 | |||||
| Obese | 545 (67.0) | 243 (29.9) | 26 (3.2) | 1333 (81.9) | 295 (18.1) | ||
| Non-obese | 325 (66.2) | 152 (31.0) | 14 (2.9) | 802 (81.7) | 180 (18.3) | ||
| Case | 0.415 | 0.908 | |||||
| Obese | 395 (67.6) | 171 (29.3) | 18 (3.1) | 961 (82.3) | 207 (17.7) | ||
| Non-obese | 152 (65.8) | 75 (32.5) | 4 (1.7) | 379 (82.0) | 83 (82.0) | ||
| Control | 0.909 | 0.849 | |||||
| Obese | 150 (65.2) | 72 (31.3) | 8 (3.5) | 372 (80.9) | 88 (19.1) | ||
| Non-obese | 173 (66.5) | 77 (29.6) | 10 (3.8) | 423 (81.3) | 97 (18.7) | ||
|
| |||||||
|
Gln223Arg
| |||||||
| Total | 0.895 | 0.728 | |||||
| Obese | 608 (75.0) | 189 (23.3) | 14 (1.7) | 1405 (86.6) | 217 (13.4) | ||
| Non-obese | 360 (73.9) | 119 (24.4) | 8 (1.6) | 839 (86.1) | 135 (13.9) | ||
| Case | 0.583 | 0.943 | |||||
| Obese | 438 (75.4) | 136 (23.4) | 7 (1.2) | 1012 (87.1) | 150 (12.9) | ||
| Non-obese | 170 (74.9) | 56 (24.7) | 1 (0.4) | 396 (87.2) | 58 (12.8) | ||
| Control | 0.934 | 0.915 | |||||
| Obese | 170 (73.9) | 53 (23.0) | 7 (23.0) | 393 (85.4) | 67 (14.6) | ||
| Non-obese | 190(73.1) | 43 (85.2) | 7 (2.7) | 443 (85.2) | 77 (14.8) | ||
|
| |||||||
|
Lys656Asn
| |||||||
| GG | GC | CC | G | C | |||
|
| |||||||
| Total | 0.407 | 0.658 | |||||
| Obese | 693 (87.6) | 95 (12.0) | 3 (0.4) | 1481 (93.6) | 101 (6.4) | ||
| Non-obese | 415 (88.1) | 56 (11.9) | 0 | 886 (94.1) | 56 (5.9) | ||
| Case | 0.440 | 0.515 | |||||
| Obese | 497 (87.2) | 71 (12.5) | 2 (0.4) | 1065 (93.4) | 75 (6.6) | ||
| Non-obese | 187 (85.0) | 33 (15.0) | 0 | 407 (92.5) | 33 (7.5) | ||
| Control | 0.464 | 0.369 | |||||
| Obese | 196(88.7) | 24 (10.9) | 1 (0.5) | 416 (94.1) | 26 (5.9) | ||
| Non-obese | 228 (90.8) | 23 (4.6) | 0 | 479 (95.4) | 23 (4.6) | ||
P-value of the comparison of the additive genetic model using the generalized linear model.
P-value of the comparison of allelic frequencies.
The data were then subjected to logistic regression analysis after adjusting for confounding risk variables. Significant association of Gln223Arg polymorphism with EH risk was found in the dominant genetic model ([GG+AG] vs AA), P=0.007, odds ratio (OR)=3.697, 95% confidence interval (CI) 1.442–9.482), and in homozygote comparison (GG vs AA, P=0.005, OR=3.890, 95% CI 1.501–10.077). For Lys656Asn polymorphism, a significantly higher prevalence of C allelic frequencies (P=0.044, OR 1.460, 95% CI 1.011–2.108) in the hypertensives than the normotensives was observed, suggesting that the C allele could be a risk factor for hypertension in the Northern Han Chinese. In addition, significant associations were found in the dominant genetic model (CC+GC) vs GG, P=0.034, OR=1.515, 95% CI 1.032–2.225 and additive genetic model (CC vs GC vs GG, P=0.041, OR=1.479, 95% CI 1.017–2.152). No significant association could be found between Lys109Arg polymorphism and EH risk (Table 4). To correct type I error, we applied the Bonferroni correction on the criteria of P=0.05/3 because three polymorphisms were tested. The statistical significance of the dominant genetic model and homozygote comparison between Gln223Arg polymorphism and EH risk remained, but the significance of allele comparison, dominant genetic model and additive genetic model between Lys656Asn polymorphism and EH risk disappeared.
Table 4.
Odds ratios for each single-nucleotide polymorphism genotype associated with EH in the Northern Han Chinese population
| SNP | Model | Contrast | OR (95% CI) | B | P-value | |
|---|---|---|---|---|---|---|
| Lys109Arg | Allele comparison | G vs A | 1.079 (0.867–1.342) | 0.076 | 0.497 | |
| Dominant genetic model | (GG + AG) vs AA | 1.643 (0.823–3.279) | 0.497 | 0.159 | ||
| Recessive genetic model | GG vs (AG + AA) | 1.037 (0.805–1.337) | 0.036 | 0.778 | ||
| Homozygote comparison | GG vs AA | 1.667 (0.826–3.364) | 0.511 | 0.154 | ||
| Additive genetic model | GG vs AG vs AA | 1.081 (0.866–1.350) | 0.078 | 0.490 | ||
| Gln223Arg | Allele comparison | G vs A | 1.165 (0.912–1.488) | 0.153 | 0.221 | |
| Dominant genetic model | (GG + AG) vs AA | 3.697 (1.442–9.482) | 1.308 | 0.007 | ||
| Recessive genetic model | GG vs (AG + AA) | 1.076 (0.818–1.415) | 0.073 | 0.599 | ||
| Homozygote comparison | GG vs AA | 3.890 (1.501–10.077) | 1.358 | 0.005 | ||
| Additive genetic model | GG vs AG vs AA | 1.168 (0.913–1.495) | 0.155 | 0.218 | ||
| Lys656Asn | Allele comparison | C vsG | 1.460 (1.011–2.108) | 0.378 | 0.044 | |
| Dominant genetic model | (CC + GC)vsGG | 1.515 (1.032–2.225) | 0.415 | 0.034 | ||
| Recessive genetic model | CC vs(GC + GG) | 0.920 (0.080–10.565) | −0.084 | 0.946 | ||
| Homozygote comparison | CC vs GG | 0.966 (0.084–11.128) | − 0.034 | 0.978 | ||
| Additive genetic model | CC vs GC vs GG | 1.479 (1.017–2.152) | 0.392 | 0.041 |
Abbreviations: B, coefficients; CI, confidence interval; OR, odds ratio; SNP, single-nucleotide polymorphism. ORs adjusted for age, gender, body mass index, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride level, glucose level, smoking habits and drinking habits. P-values <0.05 are provided in bold to emphasize their significance.
Haplotype analyses
The Lys109Arg and Gln223Arg variants were in linkage disequilibrium (D′ =0.83, r2=0.48). The Haploview program revealed that the Lys109Arg and Gln223Arg polymorphisms are in the same linkage disequilibrium block. The haplotype analyses of the two polymorphisms of the LEPR gene in hypertension and control subjects are shown in Table 5. The G-A haplotype was a protective haplotype (P=0.012, OR=0.482, 95% CI 0.273–0.850), whereas the G-G, A-A and A-G haplotypes were not associated with EH in the Northern Han Chinese (P=0.132, 0.647 and 0.660, respectively).
Table 5.
Haplotype analyses of the LEPR gene polymorphisms in hypertension and control subjects
|
Haplotype frequency
|
|||||||
|---|---|---|---|---|---|---|---|
| Lys109Arg | Gln223Arg | Cases | Controls | HS test P-valuea | OR (95% CI)a | P-valueb | OR (95% CI)b |
| G | G | 0.808 | 0.784 | 0.132 | 1.162 (0.956–1.413) | – | – |
| A | A | 0.114 | 0.120 | 0.647 | 0.944(0.738–1.207) | 0.519 | 1.085 (0.847–1.390) |
| A | G | 0.065 | 0.069 | 0.0690 | 0.931 (0.679–1.278) | 0.550 | 1.102 (0.802–1.514) |
| G | A | 0.014 | 0.028 | 0.012 | 0.482 (0.273-0.850) | 0.010 | 0.474 (0.269–0.837) |
Abbreviations: CI, confidence interval; HS test, haplotype-specific testing; OR, odds ratio. All haplotypes with frequency >1% detected in the haplotype analyses are shown in the table.
P-values and OR values derived from comparing of a specific haplotype with the other three.
P-values and OR values derived from comparing each haplotype with the baseline haplotype (G-G). P-values <0.05 are provided in bold to emphasize their significance.
The relative effects of these haplotypes were evaluated by the logistic regression. As the most highly prevalent haplotype, the G-G haplotype was defined as the baseline haplotype. Compared with the baseline haplotype, the G-A haplotype showed to be associated with a protective haplotype to EH (P=0.010, OR=0.474, 95% CI 0.269–0.837).
DISCUSSION
Both genetic and environmental factors have important roles in the pathogenesis and progression of EH.14,19 Genetic factor was considered to be of clinical importance by physicians and researchers in the pathogenesis, diagnosis, treatment and prevention of hypertension. With the advent of the human genome project and the international HapMap project, SNPs have become increasingly prominent in the studies of both multifactorial and multi-genomic diseases.15 The aim of the present study was to determine whether the polymorphisms in the LEPR gene were associated with EH development in a Northern Han Chinese population and whether there was a SNP–SNP interaction in the LEPR gene.
In our studies, no difference in genotype distribution of Lys109Arg between the EH patients and the controls was found, which is consistent with a previous report by Gu et al.20 However, our studies identified that the Gln223Arg polymorphism of the LEPR gene was associated with EH in the Northern Han Chinese, and G allele carriers of Gln223Arg (GG+AG) showed higher risks of hypertension than AA homozygotes (+P=0.007, OR=3.697, 95% CI 1.442–9.482). Interestingly, the study by Gu et al.20 showed a different result that Gln223Arg (AA+AG) had higher risk of hypertension than GG carriers (P=0.035, OR=1.549, 95% CI 1.031–2.036). It is likely that this discrepancy might be attributed to the following three reasons. First, in the report by Gu et al.,20 subjects were selected exclusively from the metropolitan areas of Shanghai and Nanjing, which are two cities in southern China with majority residential population of southern Han Chinese; whereas northern Chinese population was intentionally selected in our study in the northern areas of China. As China is the most populous country in the world, and the largest Chinese ethnic group Han Chinese (>90% of the total Chinese population) is further classified into two subgroups, northern and southern Han Chinese, with reportedly distinct genetic backgrounds.21,22 Second, two different inclusion criteria were used to determine the BP levels of the control group. The criteria for normotensive controls in our study were: SBP<120mmHg and DBP<80mmHg; whereas the inclusion criteria in the report by Gu et al.20 were: SBP<130mmHg and DBP<85mmHg. The different inclusion criteria would influence the results, as BP ranging from 120 to 139mmHg SBP and/or 80 to 89mmHg DBP is defined as prehypertension. People with prehypertension are considered to be at high risk of developing hypertension.11 Therefore, we believe that our inclusion criteria were stricter than the other report. Third, the adjustment variables, for example, smoking and drinking habits were considered in our analysis, but not in the other report by Gu et al.20 It has been suggested that smoking and alcohol intake are causative factors in the development of hypertension,23,24 thus they should be taken into account in our studies.
We did not find significant association between Lys656Asn polymorphism and EH risk. A previous report by de Luis Roman et al.25 showed that SBP decreased significantly in the Lys656 (GG) homozygotes, but not in the carriers of the Asn656 (C) allele. The ethnic background difference among these populations thus could be a main reason for such a variation. Furthermore, EH is a complex polygenic disease responsive to multiple environmental factors. As multiple genes and genetic interactions have been implicated in the regulation of BP, a single polymorphism of one gene likely has less impact on an individual's phenotype. Moreover, other causative factors for hypertension including environmental factors and lifestyle habits (for example, salt intake, smoking and alcohol consumption) ought to be considered in studies of EH.19 Currently, the relationship between Lys656Asn polymorphism and hypertension is still unclear and awaits further investigation.
To explore the interactive effects of obesity and the LEPR gene polymorphisms on hypertension, we analyzed the relation between polymorphisms (Lys109Arg, Gln223Arg and Lys656Asn) and hypertension by stratification analyses. We did not see a difference in the above LEPR polymorphisms on obesity. Masuo et al.26 reported that overweight obese subjects had significantly higher frequencies of the Arg223 (G) allele and the Arg223 (GG) homozygous allele of Gln223Arg and the Asn656 (C) allele of Lys656Asn compared with lean subjects in 129 young healthy normotensive men. However, a latest systematic review and analysis of primary data from the CoLaus study did not show an overall association between LEPR SNPs and overweight.27 In order to clearly define the relationships among LEPR polymorphism, obesity and hypertension, population stratification should be addressed in future genetic association studies.
Haplotype analysis is considered to be a powerful tool for studying the genetics of complex diseases. In our studies, two haplotypes with a frequency >1% were found, as previously described. There was linkage between Lys109Arg and Gln223Arg variations (D′ =0.83, r2=0.48). Haplotype analyses showed that the G-A haplotype was a protective haplotype (P=0.012, OR=0.482, 95% CI 0.273–0.850), which was consistent with the findings of the association analyses between the Gln223Arg polymorphism and EH risk. Currently, little is known about the haplotype of the LEPR gene in EH population in the literature. Gu et al.20 did not conduct haplotype analysis in their report either. Therefore, similar studies using haplotype analysis in EH patients are still needed.
The consequences of the LEPR polymorphism on LEPR function are currently unclear. The three polymorphisms of the LEPR gene in our studies are all located in the intracellular domain of the receptor, indicating that they could potentially be functionally important for leptin signaling.28 Recent studies showed that the Gln223Arg polymorphism affected leptin signaling via STAT3.29 The Gln223Arg polymorphism in LEPR may also be in linkage disequilibrium with another functional polymorphism impairing signaling capacity of the LEPR.30 The Gln223Arg mutation led to an attenuation of the antiapoptotic effect of leptin. In the Zucker fa/fa rat model, a missense mutation28 in a highly conserved extracellular domain of the LEPR led to an elevated leptin level (hyperleptinemia);31,32 whereas hyperleptinemia33,34 is known to have a pathophysiological role in the development of hypertension and other cardiovascular diseases including coronary artery diseases.35,36 In contrast, other reports suggested that the mutation of the LEPR gene may not directly influence the leptin level but could possibly advance the disease through inhibiting the biological effect of leptin.37
In summary, our studies clearly showed that the Gln223Arg polymorphisms in the LEPR gene are associated with EH risk in the Northern Han Chinese population. Interestingly, haplotype analyses suggested that the G-A haplotype was associated with the protection role of LEPR in EH development. Our studies suggested that the LEPR polymorphism had an important role in the susceptibility to EH in the Northern Han Chinese population. Meanwhile, experimental limitations are also present in our studies that could potentially affect our conclusions. For example, the age- and gender-matching between the case and control cohorts appear to be marginal as determined by their P-values, which could potentially influence our ability in detecting the associations between the SNPs and EH as well as other parameters. Our studies also did not cover the aspects of the functional research of LEPR gene polymorphisms, which might potentially overlook other gene variants that are strongly associated with EH. Future studies with larger sample size, more representative genetic backgrounds, multiple sample subgroups and functional research could potentially help further clarify the precise clinical roles of LEPR polymorphisms in the development of EH in different ethnic groups with distinct genetic backgrounds.
What is known about the topic
Animal studies indicate that leptin is involved in the regulation of BP through the LEPR.
The SNPs in our studies are the three most reported SNPs regarding LEPR polymorphisms and metabolism in literature.
Currently little is known about these three SNPs and hypertension in the literature, especially reports from the Chinese population.
What this study adds
In order to explore the potential association between the LEPR gene polymorphisms and EH risk in the Northern Han Chinese population, we designed a series of experiments.
Our studies demonstrated that the LEPR Gln223Arg polymorphism had an important role in the patient's susceptibility to EH in the Northern Han Chinese population.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health (NIH) (Grant No. 1R01-HL083218 and 3R01-HL083218-01A2S1) to LX, the National High Technology Research and Development Program of China (Grant No. 2008AA02Z441) and Beijing Natural Science Foundation (Grant No. 7133232, 7120001 and 7102045) to YL, S-JW and Z-GW.
Footnotes
CONFLICT OF INTEREST The authors declare no conflict of interest.
REFERENCES
- 1.Gu D, Reynolds K, Wu X, Chen J, Duan X, Muntner P, et al. Prevalence, awareness, treatment, and control of hypertension in china. Hypertension. 2002;40(6):920–927. doi: 10.1161/01.hyp.0000040263.94619.d5. [DOI] [PubMed] [Google Scholar]
- 2.Muy-Rivera M, Ning Y, Frederic IO, Vadachkoria S, Luthy DA, Williams MA. Leptin, soluble leptin receptor and leptin gene polymorphism in relation to preeclampsia risk. Physiol Res. 2005;54(2):167–174. [PubMed] [Google Scholar]
- 3.Masuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, Shigemoto M, et al. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes. 1995;44(7):855–858. doi: 10.2337/diab.44.7.855. [DOI] [PubMed] [Google Scholar]
- 4.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26(11):1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 5.Grassi G. Leptin, sympathetic nervous system, and baroreflex function. Curr Hypertens Rep. 2004;6(3):236–240. doi: 10.1007/s11906-004-0075-8. [DOI] [PubMed] [Google Scholar]
- 6.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45(1):9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 7.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 8.Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effect of exercise training on leptin levels in obese males. Am J Physiol. 1998;274(2 Pt 1):E280–E286. doi: 10.1152/ajpendo.1998.274.2.E280. [DOI] [PubMed] [Google Scholar]
- 9.Wiedemann A, Vocke F, Fitzgerald JS, Markert UR, Jeschke U, Lohse P, et al. Leptin gene (TTTC)(n) microsatellite polymorphism as well as leptin receptor R223Q and PPARgamma2 P12A substitutions are not associated with hypertensive disorders in pregnancy. Am J Reprod Immunol. 2010;63(4):310–317. doi: 10.1111/j.1600-0897.2009.00799.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, et al. Hypertension in obesity and the leptin receptor gene locus. J Clin Endocrinol Metab. 2000;85(9):3126–3131. doi: 10.1210/jcem.85.9.6781. [DOI] [PubMed] [Google Scholar]
- 11.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 12.Gu D, Su S, Ge D, Chen S, Huang J, Li B, et al. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47(6):1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 13.Ge D, Huang J, He J, Li B, Duan X, Chen R, et al. beta2-Adrenergic receptor gene variations associated with stage-2 hypertension in northern Han Chinese. Ann Hum Genet. 2005;69(Pt 1):36–44. doi: 10.1046/j.1529-8817.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 14.Lou Y, Liu J, Li Y, Liu Y, Wang Z, Liu K, et al. Association study of the beta2-adrenergic receptor gene polymorphisms and hypertension in the northern Han Chinese. PLoS One. 2011;6(4):e18590. doi: 10.1371/journal.pone.0018590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Liu Y, Liu J, Liu K, Lou Y, Wen J, et al. E-selectin gene polymorphisms are associated with essential hypertension: a case-control pilot study in a Chinese population. BMC Med Genet. 2010;11:127. doi: 10.1186/1471-2350-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11(2):116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 17.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 18.Qin ZS, Niu T, Liu JS. Partition-ligation-expectation-maximization algorithm for haplotype inference with single-nucleotide polymorphisms. Am J Hum Genet. 2002;71(5):1242–1247. doi: 10.1086/344207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu K, Liu J, Huang Y, Liu Y, Lou Y, Wang Z, et al. Alpha-adducin Gly460Trp polymorphism and hypertension risk: a meta-analysis of 22 studies including 14303 cases and 15961 controls. PLoS One. 2010;5(9):e13057. doi: 10.1371/journal.pone.0013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu P, Jiang W, Chen M, Lu B, Shao J, Du H, et al. Association of leptin receptor gene polymorphisms and essential hypertension in a Chinese population. J Endocrinol Invest. 2012;35(9):859–865. doi: 10.3275/8238. [DOI] [PubMed] [Google Scholar]
- 21.Wen B, Li H, Lu D, Song X, Zhang F, He Y, et al. Genetic evidence supports demic diffusion of Han culture. Nature. 2004;431(7006):302–305. doi: 10.1038/nature02878. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HG, Chen YF, Ding M, Jin L, Case DT, Jiao YP, et al. Dermatoglyphics from all Chinese ethnic groups reveal geographic patterning. PLoS One. 2010;5(1):e8783. doi: 10.1371/journal.pone.0008783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuruta M, Adachi H, Hirai Y, Fujiura Y, Imaizumi T. Association between alcohol intake and development of hypertension in Japanese normotensive men: 12-year follow-up study. Am J Hypertens. 2000;13(5 Pt 1):482–487. doi: 10.1016/s0895-7061(99)00238-1. [DOI] [PubMed] [Google Scholar]
- 24.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Zampi I, Battistelli M, et al. Cigarette smoking, ambulatory blood pressure and cardiac hypertrophy in essential hypertension. J Hypertens. 1995;13(10):1209–1215. doi: 10.1097/00004872-199510000-00016. [DOI] [PubMed] [Google Scholar]
- 25.de Luis Roman D, de la Fuente RA, Sagrado MG, Izaola O, Vicente RC. Leptin receptor Lys656Asn polymorphism is associated with decreased leptin response and weight loss secondary to a lifestyle modification in obese patients. Arch Med Res. 2006;37(7):854–859. doi: 10.1016/j.arcmed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Masuo K, Straznicky NE, Lambert GW, Katsuya T, Sugimoto K, Rakugi H, et al. Leptin-receptor polymorphisms relate to obesity through blunted leptin-mediated sympathetic nerve activation in a Caucasian male population. Hypertens Res. 2008;31(6):1093–1100. doi: 10.1291/hypres.31.1093. [DOI] [PubMed] [Google Scholar]
- 27.Bender N, Allemann N, Marek D, Vollenweider P, Waeber G, Mooser V, et al. Association between variants of the leptin receptor gene (LEPR) and overweight: a systematic review and an analysis of the CoLaus study. PLoS One. 2011;6(10):e26157. doi: 10.1371/journal.pone.0026157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qu Y, Yang Z, Jin F, Sun L, Feng J, Tang L, et al. The haplotype identified in LEPR gene is associated with type 2 diabetes mellitus in northern Chinese. Diabetes Res Clin Pract. 2008;81(1):33–37. doi: 10.1016/j.diabres.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Mackey-Lawrence NM, Guo X, Sturdevant DE, Virtaneva K, Hernandez MM, Houpt E, et al. Effect of the leptin receptor Q223R polymorphism on the host transcriptome following infection with Entamoeba histolytica. Infect Immun. 2013;81(5):1460–1470. doi: 10.1128/IAI.01383-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riestra P, Garcia-Anguita A, Torres-Cantero A, Bayonas MJ, de Oya M, Garces C. Association of the Q223R polymorphism with age at menarche in the leptin receptor gene in humans. Biol Reprod. 2011;84(4):752–755. doi: 10.1095/biolreprod.110.088500. [DOI] [PubMed] [Google Scholar]
- 31.Takaya K, Ogawa Y, Isse N, Okazaki T, Satoh N, Masuzaki H, et al. Molecular cloning of rat leptin receptor isoform complementary DNAs--identification of a missense mutation in Zucker fatty (fa/fa) rats. Biochem Biophys Res Commun. 1996;225(1):75–83. doi: 10.1006/bbrc.1996.1133. [DOI] [PubMed] [Google Scholar]
- 32.Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13(1):18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 33.Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, et al. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289(1):H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- 34.Knudson JD, Payne GA, Borbouse L, Tune JD. Leptin and mechanisms of endothelial dysfunction and cardiovascular disease. Curr Hypertens Rep. 2008;10(6):434–439. doi: 10.1007/s11906-008-0082-2. [DOI] [PubMed] [Google Scholar]
- 35.Soderberg S, Ahren B, Jansson JH, Johnson O, Hallmans G, Asplund K, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999;246(4):409–418. doi: 10.1046/j.1365-2796.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 36.Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS) Circulation. 2001;104(25):3052–3056. doi: 10.1161/hc5001.101061. [DOI] [PubMed] [Google Scholar]
- 37.Wang B, Fu E, Cao Y, Zhong Y, Fu G, Tian X, et al. Effect of leptin receptor mutation on the development of chronic bronchitis. Asia Pac J Public Health. 2013;25(Suppl 4):80S–87S. doi: 10.1177/1010539513497218. [DOI] [PubMed] [Google Scholar]


