Abstract
When used effectively, cognitive reappraisal of distressing events is a highly adaptive cognitive emotion regulation (CER) strategy, with impairments in cognitive reappraisal associated with greater risk for psychopathology. Despite extensive literature examining the neural correlates of cognitive reappraisal in healthy and psychiatrically ill adults, there is a dearth of data to inform the neural bases of CER in children, a key gap in the literature necessary to map the developmental trajectory of cognitive reappraisal. In this fMRI study, psychiatrically healthy schoolchildren were instructed to use cognitive reappraisal to modulate their emotional reactions and responses of negative affect after viewing sad photos. Consistent with the adult literature, when actively engaged in reappraisal compared to passively viewing sad photos, children showed increased activation in the vlPFC, dlPFC, and dmPFC as well as in parietal and temporal lobe regions. When children used cognitive reappraisal to minimize their experience of negative affect after viewing sad stimuli they exhibited dampened amygdala responses. Results are discussed in relation to the importance of identifying and characterizing neural processes underlying adaptive CER strategies in typically developing children in order to understand how these systems go awry and relate to the risk and occurrence of affective disorders.
Keywords: Emotion, Emotion Regulation, Cognitive Reappraisal, fMRI, Sadness, Late Childhood
1. Introduction
The development and effective implementation of adaptive CER strategies (e.g., reappraisal and problem solving) are core components of mental and physical health throughout the lifespan. Failure to develop adaptive ER strategies, the inability to use them effectively, or chronic reliance on maladaptive CER strategies (e.g., rumination, suppression, catastrophizing) has been associated with negative biological, psychological, and social outcomes (Gross, 1998; Gross and John, 2003; Zlomke and Hahn, 2010). The use of maladaptive/ineffective emotion regulation strategies has been linked to impulsivity, poor social functioning, and is a core symptom in more than 50% of all DSM-IV axis I psychiatric disorders (Gross and Munoz, 1995). Thus, identifying and characterizing the neurobehavioral basis of adaptive CER strategies in typical developing children may inform whether, when and how atypical aberrations in these processes relate to the development of childhood psychopathology.
During middle childhood, children’s ER skills progress from rudimentary behavioral strategies (e.g., covering ears) to more sophisticated cognitive-based ER strategies, such as reappraisal, the cognitive re-interpretation of emotion eliciting events (Davis and Levine, 2013; Dennis and Hajcak, 2009; Garnefski et al., 2007; McRae et al., 2008). Historically, the developmental ER literature has emphasized the examination of behavioral forms of ER in children up to age 6 and cognitive forms of ER from early adolescence to adulthood. This has left a critical gap in the ER literature with fewer studies examining CER during middle childhood (ages 7-to-12), a critical developmental period for children’s transition from behavioral to cognitive strategies for regulating emotions (Legerstee et al., 2010). The developmental period immediately preceding adolescence is especially important in relation to the study of CER for several reasons. First, the CER strategies that are developed and used with increasing frequency during adolescence are predictive of the strategies used throughout adulthood (Garnefski and Kraaij, 2006). Thus, establishing good strategies during the earliest possible developmental period may have important protective effects and could reduce the risk of adult onset psychopathology (Luby and Belden, 2006). Second, it is well established that starting in adolescence (and in relation to the commencement of puberty) the risk for the onset of psychopathology increases significantly for all children, with the risk of affective disorders rising exponentially for adolescent girls (Costello et al., 2011). Third, brain regions that support children’s affective interpretation/appraisals are thought to complete structural maturation around puberty. Interestingly, puberty also marks the start of substantial structural and functional changes in brain regions known to support social cognition, emotion, and cognitive control (Luna et al., 2010). Taken together these factors suggest that understanding the neural bases for CER immediately preceding children’s entry into puberty, the “storm and stress” period of development, may provide invaluable insight into how typical trajectories of ER may go awry and increase risk for mental illness (Siener and Kerns, 2012).
One CER strategy that has received the bulk of empirical focus is cognitive reappraisal (Buhle, 2013; Ochsner et al., 2012). By reinterpreting the affective meaning of emotion-eliciting situations, one may regulate and modify their emotional responses to a distressing event. Reappraisal has been consistently found to minimize the mental and/or physical toll associated with the experience of negative emotions and stressful life events (Gross, 1999; Gross, 2002; Gross and John, 2003; Gross and Munoz, 1995; Gross and Thompson, 2007). Cognitive reappraisal is an ideal starting point for examining the neural bases of emotion regulation in children. That is, cognitive reappraisal has been studied extensively in healthy and psychiatrically ill adults, has clinical value (e.g., often taught in Cognitive Behavioral Therapy), and is feasible to teach and implement in neuroimaging settings with younger samples.
While the literature suggests that children as young as 5 show the ability for cognitive ER (DeCicco et al., 2012), there are very few studies that have used fMRI to examine the neural substrates associated with the cognitive reappraisal of sadness in children prior to age 13 (Levesque et al., 2003). This is an especially important area of study given that once children have the cognitive and neurobiological processes in place to support cognitive reappraisal (with varying degrees of effectiveness), it is likely that they will rely on this ER strategy with increasing frequency as they age (Garnefski et al., 2002b). Furthermore, fMRI studies of reappraisal in children and adults have emphasized the down-regulation of highly arousing negative emotions such as disgust and fear. Although sadness is low in arousal compared to disgust or fear it was the focus of the current study for one primary reason. That is, sadness has a prevailing role in the occurrence and course of childhood onset depression. Our overarching future goal is to compare neural activation in healthy compared to depressed children using this same reappraisal of sadness task. Based on this, we focused our study on the CER of sadness.
1.1. Neural Substrates of Cognitive Reappraisal in Adulthood
Cognitive reappraisal is well characterized and commonly used in adults (Ochsner and Gross, 2007; Ochsner et al., 2012) where it has been linked to neural activation in two distinct, but anatomically connected, brain systems (Gross and Thompson, 2007; Ochsner et al., 2001; Phillips et al., 2008). One system of particular interest for the current study includes emotional reaction/processing/generation regions including the amygdala, ventral striatum, ventromedial PFC, the insula, and others. Although an oversimplification of the complexity of the neural circuitry of emotion processing, these regions are often referred to as a “ventral” system implicated in “bottom-up” generation of emotion (Ochsner et al., 2001; Ochsner and Gross, 2003, 2005; Ochsner and Gross, 2007, 2008; Ochsner and Phelps, 2007). The amygdala is implicated in the perception, labeling, and encoding of stimuli with a particular sensitivity to threat and fear (Cunningham et al., 2008; Kim et al., 2010b; Neta and Whalen, 2011). The ventral striatum is thought to be involved in determining which cues from the environment predict rewarding or reinforcing outcomes (Van Leijenhorst et al., 2010). Affective appraisals of stimuli processed in the amygdala and ventral striatum, along with input from other regions (e.g., medial temporal lobules), are integrated within the ventromedial prefrontal cortex (vmPFC). The vmPFC tracks the positive or negative appraisal of stimuli in a goal-dependent manner (Ochsner et al., 2012). Similar to the amygdala and ventral striatum, the insula is another region implicated in emotion reactivity/processing of emotionally distressing experiences (Eugene et al., 2003; Johnstone et al., 2007).
The second neural system utilized for cognitive reappraisal involves PFC and cingulate regions and is thought to support “top-down” control processes, which modulate activity in posterior and subcortical systems that generate emotional responses (Ochsner et al., 2002). The dorsolateral prefrontal cortex (dlPFC) and posterior portions of the PFC, in conjunction with inferior parietal regions, are implicated in attending to reappraisal-relevant stimulus features and maintaining awareness of reappraisal goals (Wager and Smith, 2003). Dorsal regions of the anterior cingulate cortex (ACC) are implicated in performance monitoring and are thought to help organize the extent to which reappraisals change emotional responses in the intended way (Botvinick et al., 2004). The ventrolateral prefrontal cortex (vlPFC) is also implicated in cognitive reappraisal, selecting goal-appropriate responses, and inhibiting goal-inappropriate responses (Thompson-Schill et al., 2005). Furthermore, the vlPFC is implicated in the deliberate selection of new stimulus-appropriate reappraisal (Badre and Wagner, 2007).
1.2. Neural Substrates of Cognitive Reappraisal in Childhood
Behavioral studies demonstrate that around age 5 children begin to demonstrate the capacity to reappraise, and by age 12, strategies for reappraisal of negative events are increasingly stable and consistent with those used throughout adulthood (Garnefski et al., 2002a). Examining event related potentials, Dennis and Hajcak have provided evidence for neural changes associated with cognitive ER in children between the ages of 5-10-years-old (Dennis and Hajcak, 2009). However to date, fMRI has been mainly used to study reappraisal in adolescents and adults, though some studies have included children as young as 7-years-old (Pitskel et al., 2011b). In the only other fMRI study of school-age children’s reappraisal of sadness, Levesque and colleagues reported that girls’ reappraisal of sad film clips was associated with activation in bilateral lateral PFC, OFC, and medial PFC as well as right-sided activation in ACC and vlPFC (Levesque et al., 2003). Pitskel and colleagues examined children’s emotional responses to pictures designed to elicit feelings of disgust and found that in boys and girls, 7-17-years-old, reappraising feelings of disgust was associated with decreased activation in the occipital cortex, insula, and thalamus, as well as in the right dlPFC, postcentral gyrus, left precentral gyrus, and the ACC (Pitskel et al., 2011b). In the same study, region-of-interest (ROI) analyses examining correlations between limbic and prefrontal ER-related regions demonstrated that greater activation in the vmPFC during reappraisal was associated with decreased activation in the amygdala and insula. These studies show many similar regional patterns of activation associated with reappraisal in children as in studies of adults. However, developmental differences in amygdala activation between children and adults have also been suggested, with children showing a less specific amygdala response to different facial expressions of emotion compared to neutral faces (Burghy et al., 2012; Pagliaccio et al., in press; Pagliaccio et al., 2013). Thus, one aim of the current study was to further investigate differences in schoolchildren’s neural activation within specific amygdala subregions after being instructed to reappraise versus passively view sad and neutral images.
In general, fMRI studies examining emotion processing and regulation as it relates to the amygdala have treated it as single unit. Thus, there continues to be a dearth of empirical literature examining specific amygdala nuclei and their contribution to distinct functions that result from varying interactions with cortical and subcortical regions. Two major amygdala sub regions are the basolateral amygdala (BLA) and the centromedial amygdala (CMA). In adult and animal studies both the BLA and CMA play a pivotal role in numerous emotion-related functions. Specifically, the CMA is thought to be critical for controlling the expression of fear (Qin et al., 2012) whereas the BLA is thought to play a critical role in perception, appraisal, and regulation of emotionally salient stimuli (Kim et al., 2010a). Thus, we hypothesized that activity in both the BLA and CMA would be modulated by CER.
Current Study
The objectives of the current study were to investigate the neural correlates of cognitive reappraisal of sadness in sample of healthy school-age children. We tested for specific differences in the amygdala and its subregions as well as differences at the whole-brain level. To do this, we adapted a cognitive reappraisal paradigm commonly used in adults for children (8-to-12-years-old), where participants are instructed to passively view neutral and sad photos and to actively down-regulate their emotional responses to sad photos (Ochsner et al., 2004). We aimed to address the following questions: Do healthy school-age children: 1) exhibit greater neural activation in the emotion processing regions (amygdala, ventral striatum, vmPFC, and insula) thought to support the appraisal of emotionally evocative stimuli when passively viewing sad compared to neutral photos? 2) Show greater activation in ER regions (dlPFC, vlPFC, dmPFC, inferior parietal regions, dorsal regions of the anterior cingulate cortex) when attempting to regulate their responses to sad photos as compared to viewing sad photos? 3) Show reduced activation in emotion processing related regions such as amygdala, ventral striatum, ventromedial PFC, and insula when attempting to regulate their responses to sad photos? 4) Show activation differences in centromedial and or laterobasal subregions of the amygdala over time and between conditions when instructed to passively view vs. reappraise their response to sad photos?
2. Materials and Methods
2.1. Participants
Nineteen healthy children (n=11 male) between 8-to-12-years-old (mean=10.05 years; SD=1.17) participated in the current study after providing consent according to the guidelines of the Washington University School of Medicine Institutional Review Board. Child participants and their primary caregivers were recruited as part of a larger parent study examining the longitudinal course of preschool onset depression. The current sample was limited to psychiatrically healthy children.
2.2. Materials
Children’s self-reported use of cognitive reappraisal ER strategies was assessed using the 4-item positive refocusing subscale of CERQ-k (Garnefski et al., 2007). This is a well-validated subscale (alpha = .80 in our sample) that assess the degree to which children report trying to reframe their experience of negative events in a more positive light. We focused on this subscale because it was the aspect of reappraisal most similar to that used in the fMRI task. The positive refocusing subscale of the CERQ-k uses a 5-point Likert scale. Children are asked: what do you think when you experience negative/unpleasant events. After given this instruction children rate themselves on the following items: 1. I think of nicer things than what I have experienced, 2. I think of pleasant things that have nothing to do with it, 3. I think of something nice instead of what has happened, and 4. I think about pleasant experiences. The positive refocusing subscale score is the average of children’s ratings on these four items.
2.3. Task Design
Children were instructed to decrease their experience of negative emotions in response to viewing sad images using positive cognitive reappraisal strategies. The trial structure was similar to investigations of cognitive reappraisal conducted with older children and adults (Ochsner et al., 2004; Perlman et al., 2012; Perlman and Pelphrey, 2011; Pitskel et al., 2011a; Wager et al., 2008), modified for use with school-age children. As shown in Figure 1, at the start of each trial, a photo (i.e., neutral or sad) was presented for a 4-second interval. Next an instruction appeared below the photo, ‘VIEW’ appeared to indicate non-regulation trials or ‘MAKE-POSITIVE’ appeared to indicate regulation trials (the instruction and photo remained on the screen together for another 4 seconds). Then, the photo disappeared but the instruction remained on screen for an additional 4-seconds. Following each picture, children were prompted to answer the question ‘How do you feel?’. Children had 4 seconds to rate their negative affect, on a scale from 1 to 4. Responses were made on a 4-button box. After the affect rating period, the word ‘RELAX’ appeared on the screen for 4 to 8 seconds (pseudo-randomly determined). The combinations of neutral and sad photos with non-regulate versus regulate instructions resulted in 3 conditions: view neutral (non-emotional), view sad (sadness inducing but no reappraisal), and reappraise sad (instructed to engage in reappraisal while viewing sad photo).
Figure 1.
Example of a Single Trial for the Reappraise Sad Condition.
Stimuli were taken from the International Affective Picture Series (Bradley et al., 2001; Bradley et al., 2008) and were supplemented for from an in-house set of images selected to be appropriate for viewing by children (e.g., photos of other children crying). IAPS stimuli have been rated for valence (1 to 9; extremely negative to extremely positive) and arousal (1 to 9; no arousal to extreme arousal). The images we used had valence scores less than 4 and arousal scores greater than 4. We used 20 total neutral and 40 total sad pictures during the fMRI task.
Each run presented 12 trials divided equally among view neutral photos (4 per run), view sad photos (4 per run), and reappraise sad photos (4 per run). Trial orders were pseudo-randomized to allow for estimates of BOLD responses to each trial type. Stimuli used for the “VIEW” versus “MAKE-POSITIVE” conditions were counterbalanced so that stimuli were not confounded with condition. In its entirety, the ER task included 5 runs of 12 trials each (60 trials total, 20 in each condition). Each trial lasted 16 seconds (followed by a 4-8 second jitter) and each run lasted approximately 4 minutes and 40 seconds.
2.4. Procedure
A comprehensive pre-scan training procedure was used to assure that children understood the fMRI reappraisal task. Reappraisal training details are provided in the Supplementary Material.
2.5. fMRI Data Acquisition and Data Analyses
All fMRI data were acquired on Siemens 3T Tim TRIO MRI scanner. Acquisition and data processing details are given in Supplementary Material.
2.5.1. Behavioral Data
Pearson correlations, t-tests and chi-square tests were conducted to test for significant differences and/or associations between demographic characteristics (e.g., age, sex, SES, and ethnicity) of children in the current sample and their affective ratings during the reappraisal condition of the fMRI task and children’s scores on the positive refocusing subscale of the CERQ-K. Specifically, we tested the hypotheses that the children who are more likely to report engaging in positive refocusing will be the most able to decrease amygdala activation during the reappraisal condition.
2.5.2. fMRI Statistical Image Analysis
For each participant, we computed a General Linear Model (GLM). We did not assume a hemodynamic response shape because of concerns about potential developmental differences in the shape or timing of this response. Instead, a finite impulse response approach was used where 16 timepoints (taken at 2-second TRs) were estimated. This included 8 timepoints to cover the 16 seconds of the trial (average trial length) plus an additional 16 seconds for the evolution of the hemodynamic response), with a separate estimate for each of the task conditions. The results of these fixed effects analyses for each individual subject were entered into second level analyses, treating subjects as a random factor. Frames 5-to-8 (10-to-18-seconds into each trial) were used for the current analyses, reflecting BOLD responses hypothesized to be related to picture viewing and reappraisal of sad emotion, when taking into account the lag in the hemodynamic response. The first set of analyses focused on the effect of stimulus type using repeated-measures ANOVA, which included condition (view sad versus view neutral) and timepoint (5-8) as within subject factors. The second set of analyses focused on the effect of reappraisal and included condition (reappraise sad versus view sad) and timepoints (5-8) as within subject factors. In the results section we focused on regions that showed main effects of condition as well as regions showing interactions between condition and timepoint.
Two approaches to the analysis of the fMRI data were used. First, we conducted whole-brain voxel-by-voxel analyses, using the ANOVA models described above, with a corrected whole brain false positive rate of p<.05 (using p-value+cluster size thresholding of Z>3.00 and a minimum cluster size of 13. Montecarlo simulations have indicated that this cluster size and significance level of thresholding provides a whole-brain corrected false positive rate of 0.05 (McAvoy et al., 2001). We also used a ROI analysis to examine these effects on amygdala subregion activity. Specifically, we used ROIs for centromedial, laterobasal, and superficial subdivions of the amygdala in both right and left hemispheres. The six amygdala subdivision ROIs were based on prior findings from Roy and colleagues and our ROI mask was provided by these authors (Roy et al., 2009).
We also conducted a separate correlational ROI analysis to examine amygdala activation during the fMRI reappraisal condition in relation to children’s self-reported scores on the positive refocusing subscale of the CERQ-K.
2.6. Comparison to Meta-analysis in adults
Given that the current study only included children, we wished to compare our results to those of a recent meta-analyses of findings with cognitive emotion regulation in adults. We focused on the, (Diekhof et al., 2011) meta-analysis, since it included studies focused on cognitive down-regulation of negative emotion (albeit primarily fear stimuli), similar to the current study. We created ROIs from the coordinates provided in the Diekhof meta-analysis and conducted analyses in our sample using these ROIs. In the Diekhof manuscript, there were coordinates for 23 peaks showing consistent effects of emotion regulation of negative stimuli (see Table 3 in that manuscript and Table 3 in the current manuscript). We used these coordinates to create spherical ROIs 18 mm in diameter, and computed the same analyses as described above, using the average across voxels within each of the Diekhof ROIs.
Table 3.
Results using regions from Diekhof meta-analysis of cognitive emotion regulation in adults
| Regions | x | y | z | Effect of Condition |
|---|---|---|---|---|
| L/R dorsomedial PFC/ACC | −6 | 16 | 58 | 0.007 |
| L/R dorsomedial PFC/ACC | 2 | 32 | 44 | 0.046 |
| L middle frontal gyrus/inferior frontal sulcus/IFJ | −42 | 18 | 44 | 0.007 |
| L middle frontal gyrus/inferior frontal sulcus/IFJ | −42 | 4 | 48 | 0.01 |
| R middle frontal gyrus/inferior frontal sulcus | 40 | 22 | 44 | 0.26 |
| L inferior frontal gyrus/anterior insula | −50 | 30 | −10 | 0.0002 |
| L inferior frontal gyrus/anterior insula | −54 | 22 | −2 | 0.0001 |
| L inferior frontal gyrus/anterior insula | −52 | 42 | −6 | 0.51 |
| R inferior frontal gyrus | 50 | 30 | −10 | 0.06 |
| L intraparietal cortex | −46 | −66 | 36 | 0.08 |
| L intraparietal cortex | −42 | −56 | 38 | 0.07 |
| L intraparietal cortex | −38 | −60 | 30 | 0.08 |
| R intraparietal cortex | 50 | −58 | 42 | 0.122 |
| L inferior temporal sulcus | −60 | −36 | −2 | 0.001 |
| L anterior insula/frontal operculum | −38 | 20 | −4 | 0.04 |
| R anterior insula/frontal operculum | 46 | 14 | 0 | 0.2 |
| L/R VMPFC | 6 | 40 | −22 | 0.53 |
| L/R VMPFC | 0 | 38 | −18 | 0.43 |
| L middle temporal gyrus | −64 | −4 | −22 | 0.01 |
| R frontomarginal sulcus | 34 | 60 | 8 | 0.33 |
| R inferior frontal gyrus | 60 | 26 | 6 | 0.49 |
| L ACC | −8 | 28 | 28 | 0.03 |
| R superior frontal gyrus | 18 | 24 | 58 | 0.23 |
Note: Regions were from Table 3 of Diekhof meta-analysis. Regions in dark gray were significant at p<.05, while regions in light grey were trend level (p<.10).
3. Results
3.1. Behavioral
As expected, children reported significantly greater negative affect for sad compared to neutral images in the view condition, t(17) =6.85, p<.001. As hypothesized, children reported significantly less negative affect in the reappraise sad (M=7.46; SD=3.53) versus view sad condition (M=5.83; SD=2.72), t(16)=2.09, p<.05. Children’s sex (t = −1.37, p = .20), IQ (r = .21, p = .42), pubertal status (r = .13, p = .62), and age (r = .18, p = .50), as well as their familial income (r = .04, p = .86) did not differ significantly in relation to children’s affective ratings during the fMRI reappraise sad condition. Similar results emerged when examining sex (t = −.30, p = .98), income (r = .09, p = .73), age(r = .22, p = .38), pubertal status (r = .07, p = .78), and IQ scores (r = −.24, p = .31) in relation to positive refocus scores on the CERQ. Children’s affective rating scores during the reappraise sad trials were not associated with the positive refocus scores on the CERQ, r = −.09, p = .89.
3.2. fMRI Whole Brain Analyses: Passive Viewing of Sad Versus Neutral Photos
3.2.1 Effects of Condition and Condition x Timepoint
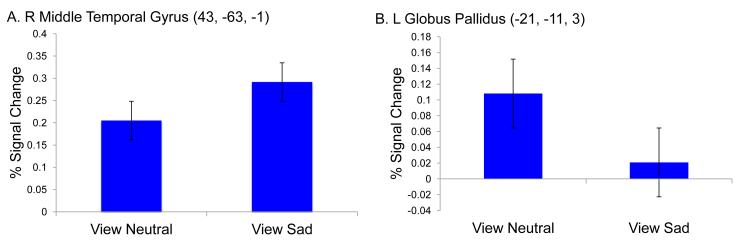
Results from the 2 (View Sad versus View Neutral) x 4 (frames 5-8) repeated measures ANOVA demonstrated a significant main effect of condition on neural activity in 2 regions (see Table 1). Overall, children showed significantly greater activity in the middle temporal gyrus while passively viewing sad compared to neutral photos (see Figure 2A). In contrast, children showed significantly greater activation in the lateral globus pallidus during the view neutral compared to view sad condition (see Figure 2B). There was one region that showed a condition X timepoint interaction in the culmen, with children showing increased activation when viewing sad compared to neutral photos (see Table 1).
Table 1.
Significant Main Effects of Condition (Sad vs Neutral) & Condition × Timepoint Interaction Effect
| Region of Activation | Direction of Effect | BA | x | y | z | Z-score | Cluster (3mm3) |
|---|---|---|---|---|---|---|---|
| Whole Brain Analysis | |||||||
| Main Effect of Condition (View Sad vs. View Neutral) | |||||||
| R Middle Temporal Gyrus (see Figure 2a) | Sad>Neutral | 37 | 43 | −63 | −1 | 3.77 | 58 |
| L Lateral Globus Pallidus (see Figure 2b) | Neutral>Sad | * | −21 | −11 | 3 | 4.28 | 31 |
| Condition (View Sad vs. View Neutral) × Timepoint Interaction | |||||||
| L Culmen | Sad>Neutral | * | −2 | −56 | −18 | 3.65 | 16 |
Sad = View Sad; Neutral = View Neutral
BA = Brodmann Area, Z-score for peak voxel in each ROI, Cluster = number of 3mm isotropic voxels in each ROI
Figure 2.
Neutral vs. Sad: Main Effect of Condition. A: Effect of condition in the Right Middle Temporal Gyrus; B: Effect of condition in the Left Globus Pallidus
3.3. Amygdala ROI Analyses
Despite children’s behavioral ratings of negative affect being significantly greater for the view sad versus view neutral condition there was no significant difference in amygdala subregion activation between conditions and there was no significant interaction effect between condition and timepoint on subregion specific amygdala activation.
3.4. fMRI Whole Brain Analyses: Reappraise Sad VS View Sad
3.4.1. Effects of Condition and Condition x Timepoint
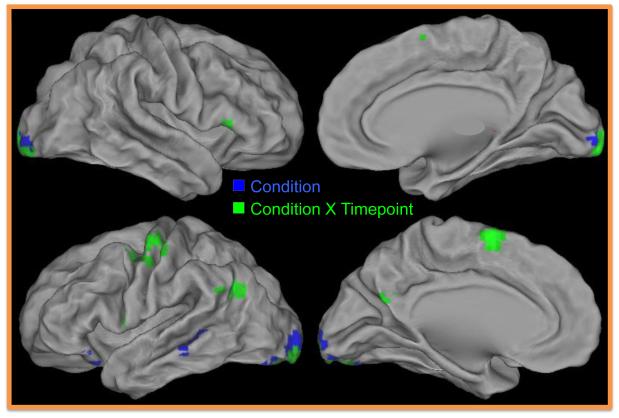
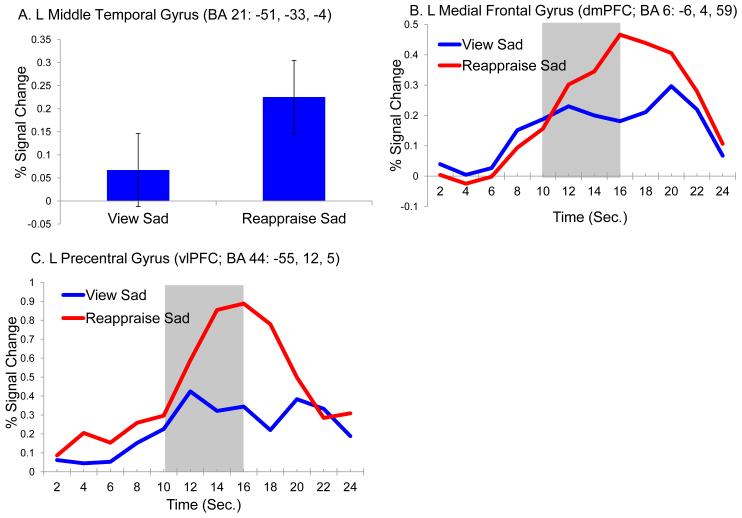
Results from the repeated measures ANOVA demonstrated a significant main effect of condition on five brain regions (see Table 2 and Figure 3). Two regions in the ventrolateral prefrontal cortex (BA47) as well as the middle temporal (BA21) the inferior occipital (BA17) gyrus and the cuneus (BA17) all showed significantly greater activation while children reappraised compared to passively viewed sad photos (see Figure 4A for main effect of condition). In addition, a number of other regions showed an interaction between condition and timepoint (see Table 2 and Figure 3). In all of these regions, including four regions of the left prefrontal cortex including vlPFC (BA44, 45), dlPFC (BA6), as well as dmPFC (BA6), activity was greater overtime while reappraising compared to passively viewing sad images. Although there are too many regions to provide timecourse data for each, we provided the most representative timecourse patterns found across all regions using two specific prefrontal regions from Table 2 (e.g., see Table 2 and Figures 4B; 4C).
Table 2.
Significant Main Effects of Condition (Reappraise Sad Vs. View Sad) & Condition × Timepoint Interaction Effects
| Region of Activation | Direction of Effects | BA | x | y | z | Z- score |
Cluster (3mm3) |
|---|---|---|---|---|---|---|---|
| Whole Brain Analysis | |||||||
| Main Effect of Condition (Reappraise vs. View Sad) | |||||||
| L Inferior Frontal Gyrus | Reappraise Sad>View Sad | 47 | −35 | 25 | −17 | 3.87 | 17 |
| L Inferior Frontal Gyrus | Reappraise Sad>View Sad | 47 | −45 | 25 | −10 | 3.73 | 14 |
| L Middle Temporal Gyrusa | Reappraise Sad>View Sad | 21 | −51 | −33 | −4 | 3.73 | 53 |
| L Inferior Occipital Gyrus | Reappraise Sad>View Sad | 17 | −16 | −92 | −5 | 4.27 | 175 |
| R Cuneus | Reappraise Sad>View Sad | 17 | 20 | −93 | −1 | 4.38 | 88 |
| Condition (Reappraise vs. View Sad) × Timepoint (Frames 5 to 8) Interaction Effects | |||||||
| L Inferior Frontal Gyrus | Reappraise Sad>View Sad | 45 | 46 | 24 | 10 | 4.02 | 18 |
| L Precentral Gyrusc | Reappraise Sad>View Sad | 44 | −55 | 12 | 5 | 4.19 | 32 |
| L Precentral Gyrus | Reappraise Sad>View Sad | 6 | −42 | −8 | 50 | 4.44 | 156 |
| L Medial Frontal Gyrusb | Reappraise Sad>View Sad | 6 | −6 | 4 | 59 | 4.55 | 114 |
| L Middle Temporal Gyrus | Reappraise Sad>View Sad | 39 | −46 | −60 | 25 | 3.81 | 51 |
| L Supramarginal Gyrus | Reappraise Sad>View Sad | 40 | −58 | −51 | 26 | 4.22 | 38 |
| L Precuneus | Reappraise Sad>View Sad | 31 | −5 | −60 | 23 | 3.51 | 13 |
| L Lingual Gyrus | Reappraise Sad>View Sad | 18 | −3 | −94 | −6 | 4.57 | 357 |
| R Declive | Reappraise Sad>View Sad | * | 34 | −71 | −21 | 3.94 | 24 |
| R Culmen | Reappraise Sad>View Sad | * | 38 | −57 | −24 | 4.21 | 23 |
|
| |||||||
| Amygdala ROI Analysis: | |||||||
| Main Effect of Condition (Reappraise vs. View Sad) | |||||||
| R Centromedial Amygdala | View Sad>Reappraise Sad | * | 25 | −11 | −4 | 3.22 | 9 |
BA = Brodmann Area, Z-score for peak voxel in each ROI, Cluster = number of 3mm isotropic voxels in each ROI
Superscript a, b, & c indicate that these effects are plotted in Figure 4
Figure 3.
Brain Regions Showing Main Effect of Condition and Condition X Time Interaction Effects in the Whole Brain Analysis of Reappraise Vs. View Sad. Regions in blue showed a main effect of condition. Regions in green showed a condition X timepoint interaction.
Figure 4.
Reappraise vs. View Sad: Significant Condition and Condition x Time Effects A: Effect of condition in the Left Middle Temporal Gyrus; B: condition X timepoint interaction in the Left Medial Frontal Gyrus. C: condition X timepoint interaction in the Left Precentral Gyrus. Shaded areas indicate the timepoints included in the analysis.
3.5. Amygdala ROIs
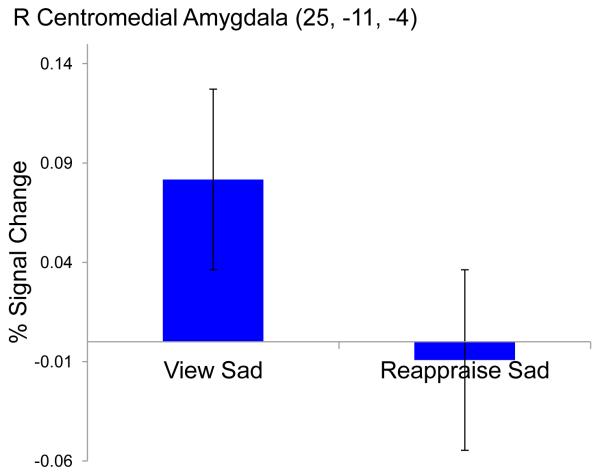
Results from the ROI analyses of amygdala subdivision activation during the reappraise compared to view sad condition indicated a significant main effect of condition in the right centromedial subdivision of the amygdala, see Table 2, Figure 5. Results from the follow up paired t-tests indicated children showed significantly, t=-3.06, p=.007, reduced activation in the centromedial subdivision of the amygdala when instructed to reappraise compared to naturally view sad photos.
Figure 5.
Reappraise vs. View Sad Condition Effects from Amygdala ROI Analysis. Effect of condition effect in Right Centromedial Amygdala
An amygdala ROI analysis was also conducted to test whether children’s greater self-reported use of adaptive cognitive regulation strategies (i.e., the positive refocusing subscale of the CERQ-k) was associated with decreased amygdala activation while engaged in reappraisal during the fMRI task. Results indicated that children with higher scores on the positive refocusing scale had significantly less amygdala activation within the right superficial subdivision during the reappraisal condition of the fMRI task, r(df 17) = −.59, z = −2.66, p < .001. No significant correlations were found between activation and CER scores in the 5 other amygdalae ROI. Given the especially small sample size and the associated reduction in power the null results should be interpreted with caution. However, the positive finding between decreased amygdala activation and children’s greater self-reported use of reappraisal is intriguing and highly consistent with established literature.
3.6 Regional Activation During Reappraisal in Children vs Adults
Our final set of analyses were focused on results using the ROIs created from the coordinates reported in the meta-analysis of studies on the reappraisal of negative affect in adults (Diekhof et al., 2011). As shown in Table 3, we found that 10 out these 23 regions showed a significant effect of passive viewing versus reappraising sad stimuli at a p<.05. An additional four regions showed trend level effects (p<.10). The pattern of activation in all of the significant or trend level regions was in the same direction as reported in the Diekhof meta-analysis. Further, the pattern in all of the remaining regions except for one (VMPFC, 6,40,-22) was also in the same direction as Diekhof. These results suggest an interesting amount of consistency between adults and children in the regions engaged in emotion regulation, with the strongest effects in dorsomedial PFC, left middle and inferior frontal cortex, and left anterior insula.
4. Discussion
Delineating the neural patterns that underlie children’s utilization of cognitive reappraisal prior to entering puberty, the “storm and stress” period of adolescence, may advance our understanding of the neural basis of affective psychopathology. Although significant strides have been made in the past decade in our understanding of brain processes underlying CER in adults, especially engagement in cognitive reappraisal, there remains a dearth of research to inform the neurodevelopmental basis of these strategies during middle and late childhood. The main aim of the current study was to begin identifying and characterizing neural activation associated with cognitive reappraisal of sad photos among healthy school-age children.
To date, fMRI studies examining children’s use of cognitive reappraisal have largely focused on highly aversive emotions such as disgust or fear. This is first study to our knowledge to examine the cognitive reappraisal of sadness in a sample of both boys and girls 8 to 12-years-old. Children’s capacity to reappraise feelings of sadness has great functional and adaptive importance, as it is a frequently experienced emotion in daily life and the ability to regulate sadness is thought to be central to the experience of and risk for depression (Zaki and Ochsner, 2009). In addition to focusing on a discrete basic emotion (i.e., sadness) the current study also examined a narrower age range than the few prior studies of reappraisal in children. Although the limited age range limits our capacity to infer developmental change or differences, this is also a strength of the current study. That is, studies examining neural correlates of childhood reappraisal have included small subsamples of school-age children within larger total samples with much broader age ranges (McRae et al., 2012; Pitskel et al., 2011b). Therefore these studies have been limited in their ability to examine the neural characteristics of reappraisal specific to school-age children given small subsample sizes. The current study provides data to inform the neural characteristics specific to CER in children ages 8 to 12. Future studies will benefit from examining trajectories of neural activation during reappraisal using longitudinal design with multiple age ranges.
Consistent with our hypotheses, school-age children showed patterns of activation in many of the regions thought to sub-serve emotion processing and regulation similar to those found in reappraisal studies of healthy adults, as well as in the small body of available data in children. Consistent with several studies of adults, children showed significantly greater activation in the inferior temporal gyrus when viewing sad versus neutral photos (Goldin et al., 2005; Goldin et al., 2008; Walter et al., 2009). As hypothesized, children’s active reappraisal of sad images was associated with significantly greater activation in several regions thought to support cognitive ER in adults, including dorsal portions of the right ACC (BA32), left vlPFC, left dlPFC, as well as left dmPFC. Consistent with several prior studies in adults (Kanske et al., 2011; McRae et al., 2010), we also found greater activation during the reappraise sad versus view sad conditions in the temporal cortex (i.e., left middle temporal gyrus [BA21/39]) and in the supramarginal gyrus a region near the temporal-parietal junction.
In addition, it was hypothesized that children would show greater activity in the amygdala when viewing sad versus neutral photos, but that engaging in reappraisal versus passively viewing sad images would be associated with significantly less activation in the amygdala as well as in the insula. Interestingly, we did not find greater activity in the amygdala when children viewed sad versus neutral photos. This result is consistent with a growing body of fMRI literature that suggests typically developing school age children (prior to adolescence) show undifferentiated amygdala responses to emotionally evocative versus neutral stimuli and that differential responses to emotional stimuli arise later in development (Pagliaccio et al., in press; Tottenham et al., 2011). Nonetheless, we did find that children showed significantly less right centromedial amygdala activity when they were instructed to reappraise versus naturally view sad photos, suggesting that the ability to modulate amygdala responses may be subdivision specific at least to some extent, prior to adolescence. Prior results of modulated amygdala responses during reappraisal of aversive stimuli have been mixed in studies of children and adults, with previous studies frequently using aversive stimuli designed to elicit fear or disgust (McRae et al., 2012; Pitskel et al., 2011a). However, the current findings suggest that children were able to modulate amygdala responses associated with the reappraisal of a potentially less intense “everyday” basic emotion such as sadness.
The finding that neural responses similar to those seen in older children and adults are already evident during the school age period is important to begin to address the developmental psychopathology of disorders like depression. Elucidation of these neural pathways can help to inform treatment targets as well as provide neurobiological markers of risk as well as response to treatment. The mapping of these neural pathways will allow investigations of neural as well as behavioral markers of risk, resilience and treatment response. Future work that investigates whether these neurobehavioral patterns associated with the reappraisal of sadness differentiate typically developing children versus children with or at risk for psychopathology is an important next scientific step.
Several limitations should be considered when interpreting results of the present study. The first is the relatively small sample size, making replication in a larger sample a key next step. The use of subjective ratings of emotional experiences is another limitation, as self-reports are known to have issues with validity. As such, it will be important for researchers to bolster self-report data with physiological measures (e.g. vagal tone; (Ohira et al., 2006) in future work. However, subjective ratings continue to be the most commonly used measure of emotional experience in the reappraisal literature. Another limitation is that the current study examined a single emotion (i.e., sadness) and a single cognitive ER strategy (i.e., reappraisal). Future studies are needed to examine whether the same neural processes operate when children reappraise other discrete negative as well as positive emotions, or use other types of cognitive ER strategies. Future studies should also examine neural function associated with other adaptive as well as maladaptive cognitive ER strategies (e.g., suppression). Finally given that we did not include a sample of adults in the current study, our ability to make direct inferences about developmental differences in the neural bases of reappraisal related to sadness is limited. The findings illustrating the overlap in our results and those from the meta-analysis of cognitive emotion regulation in adults (i.e., Diekhof et al., 2011) suggest a great deal of consistency between regional brain activation during reappraisal in children and adults. However, it is difficult to know whether the findings would remain consistent had an adult sample completed our exact imaging protocol. Last the relatively small sample size and particularly findings related to correlational analyses examining self-report reappraisal on the CERQ and children’s amygdala activation should be interpreted with caution and treated as preliminary and as an impetus for additional work with larger samples.
Despite these limitations, the present study provides valuable new data that advances our understanding of the neural correlates of children’s reactions to and reappraisals of sad feelings. We found that school age children showed evidence of the ability to down regulate amygdala activity using cognitive reappraisal, as well as evidence for increased activity in inferior frontal and temporal regions associated with ER. Difficulties in reappraising negative emotions and distressing life events has been associated with alterations in neural functioning in adults and has been proposed as a potential marker in the neuropathogenesis of depressive disorders in adulthood (Drevets, 2001; Phillips et al., 2008). Thus, future studies that investigate the dynamic interplay between the neural and behavioral factors associated with the use of reappraisal in healthy children and those at risk for depression during their transition into adolescence are imperative for mapping trajectories of emotion centered neurodevelopmental disorders such as major depressive disorder.
Supplementary Material
Highlights.
! ! Children’s use of reappraisal was associated with increased prefrontal activation.
! ! Children showed deactivation in the amygdala while reappraising sad photos.
! ! Children’s PFC activity during reappraisal was consistent with adult findings.
Acknowledgments
This work was supported by the National Institutes of Health [MH090515 to A.C.B., 2R01MH064769 to J.L.L., and R01MH090786 to Co-PIs J.L.L. and D.M.B]; and the McDonnell Center for Systems Neuroscience [NRG to A.C.B]. The sponsors had no role in designing the study, collecting or analyzing data, preparing the manuscript, or deciding to publish the article.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved either experimental animals or human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
Funding This work was supported by the National Institutes of Health [MH090515 to A.C.B., 2R01MH064769 to J.L.L., and R01MH090786 to Co-PIs J.L.L. and D.M.B]; and the McDonnell Center for Systems Neuroscience [NRG to A.C.B]. The sponsors had no role in designing the study, collecting or analyzing data, preparing the manuscript, or deciding to publish the article.
Footnotes
Conflict of Interest Drs. Belden, Luby, Barch and Mr. Pagliaccio wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral cortex (New York, N.Y. 1991) 2013 doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E, Copeland W, Angold A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? Journal of Child Psychology and Psychiatry. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham WA, Van Bavel JJ, Johnsen IR. Affective flexibility: evaluative processing goals shape amygdala activity. Psychol Sci. 2008;19:152–160. doi: 10.1111/j.1467-9280.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Davis EL, Levine LJ. Emotion regulation strategies that promote learning: reappraisal enhances children’s memory for educational information. Child Dev. 2013;84:361–374. doi: 10.1111/j.1467-8624.2012.01836.x. [DOI] [PubMed] [Google Scholar]
- DeCicco JM, Solomon B, Dennis TA. Neural correlates of cognitive reappraisal in children: an ERP study. Dev Cogn Neurosci. 2012;2:70–80. doi: 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. J Child Psychol Psychiatry. 2009;50:1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Eugene F, Levesque J, Mensour B, Leroux JM, Beaudoin G, Bourgouin P, Beauregard M. The impact of individual differences on the neural circuitry underlying sadness. Neuroimage. 2003;19:354–364. doi: 10.1016/s1053-8119(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences. 2006;40:1659–1669. [Google Scholar]
- Garnefski N, Legerstee J, Kraaij V, van den Kommer T, Teerds J. Cognitive coping strategies and symptoms of depression and anxiety: A comparison between adolescents and adults. Journal of Adolescence. 2002a;25:603–611. doi: 10.1006/jado.2002.0507. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Legerstee J, Kraaij VV, Van Den Kommer T, Teerds J. Cognitive coping strategies and symptoms of depression and anxiety: a comparison between adolescents and adults. J Adolesc. 2002b;25:603–611. doi: 10.1006/jado.2002.0507. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Rieffe C, Jellesma F, Meerum Terwogt M, Kraaij V. Cognitive emotion regulation strategies and emotional problems in 9-11-year-old children: The development of an instrument. European Child & Adolescent Psychiatry. 2007;16:1–9. doi: 10.1007/s00787-006-0562-3. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JD, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. Neuroimage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion Regulation: Past, Present, Future. Cognition & Emotion. 1999;13:551–573. [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Munoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- Gross JJ, Thompson RA. Handbook of emotion regulation. Guilford Press; New York, NY US: 2007. Emotion Regulation: Conceptual Foundations; pp. 3–24. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanske P, Heissler J, Schönfelder S, Bongers A, Wessa M. How to regulate emotion? Neural networks for reappraisal and distraction. Cereb Cortex. 2011;21:1379–1388. doi: 10.1093/cercor/bhq216. [DOI] [PubMed] [Google Scholar]
- Kim J, Lyoo I, Estes A, Renshaw P, Shaw D, Friedman S, Kim D, Yoon S, Hwang J, Dager S. Laterobasal Amygdalar Enlargement in 6-to 7-Year-Old Children With Autism Spectrum Disorder. Archives of General Psychiatry. 2010a;67:1187–1197. doi: 10.1001/archgenpsychiatry.2010.148. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Neta M, Davis FC, Oler JA, Mazzulla EC, Whalen PJ. Behind the mask: the influence of mask-type on amygdala response to fearful faces. Soc Cogn Affect Neurosci. 2010b;5:363–368. doi: 10.1093/scan/nsq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerstee JS, Garnefski N, Jellesma FC, Verhulst FC, Utens EM. Cognitive coping and childhood anxiety disorders. Eur Child Adolesc Psychiatry. 2010;19:143–150. doi: 10.1007/s00787-009-0051-6. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, Bourgouin P, Beauregard M. Neural correlates of sad feelings in healthy girls. Neuroscience. 2003;121:545–551. doi: 10.1016/s0306-4522(03)00528-1. [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden AC. Mood disorders: phenomenology and a developmental emotion reactivity model. In: Luby JL, editor. Handbook of Preschool Mental Health: Development, Disorders, and Treatment. Guilford Press; New York: 2006. pp. 209–230. [Google Scholar]
- Luna B, Padmanabhan A, O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment of significant activation in fMRI. Neuroimage. 2001;13:198–198. [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JD, Gross JJ, Ochsner KN. The neural bases of distraction and reappraisal. J Cogn Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ. Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes & Intergroup Relations. 2008;11:143–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Whalen PJ. Individual differences in neural activity during a facial expression vs. identity working memory task. Neuroimage. 2011;56:1685–1692. doi: 10.1016/j.neuroimage.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. The neural bases of reappraisal. Cognitive Neuroscience Society, New York; New York: 2001. [Google Scholar]
- Ochsner KN, Gross JJ. Thinking Makes It So: A Social Cognitive Approach to Emotion Regulation. In: Vohs K, Baumeister R, editors. The Handbook of Self-Regulation. Erlbaum, NJ: 2003. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Handbook of emotion regulation. Guilford Press; New York, NY US: 2007. The Neural Architecture of Emotion Regulation; pp. 87–109. [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Phelps E. Emerging perspectives on emotion-cognition interactions. Trends Cogn Sci. 2007;11:317–318. doi: 10.1016/j.tics.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, Isowa T, Iidaka T, Sato A, Fukuyama S, Nakajima T, Yamada J. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Pagliaccio D, Luby J, Gaffrey M, Belden A, Botteron K, Harms M, Barch D. Amygdala Activation to Emotional and non-Emotional Faces in Healthy Children: Evidence for Developmentally Undifferentiated Amygdala Function During the School Age Period. Cognitive, Affective, & Behavioral Neuroscience. doi: 10.3758/s13415-013-0167-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Gaffrey MS, Belden AC, Botteron KN, Harms MP, Barch DM. Functional brain activation to emotional and nonemotional faces in healthy children: Evidence for developmentally undifferentiated amygdala function during the school-age period. Cogn Affect Behav Neurosci. 2013 doi: 10.3758/s13415-013-0167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman G, Simmons A, Wu J, Hahn K, Tapert S, Max J, Paulus M, Brown G, Frank G, Campbell-Sills L, Yang T. Amygdala response and functional connectivity during emotion regulation: A study of 14 depressed adolescents. Journal of Affective Disorders. 2012;139:75–84. doi: 10.1016/j.jad.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S, Pelphrey K. Developing connections for affective regulation: Age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology. 2011;108:607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel N, Bolling D, Kaiser M, Crowley M, Pelphrey K. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Developmental Cognitive Neuroscience. 2011a;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011b;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Young C, Supekar K, Uddin L, Menon V. Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7941–7946. doi: 10.1073/pnas.1120408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siener S, Kerns KA. Emotion Regulation and Depressive Symptoms in Preadolescence. Child Psychiatry & Human Development. 2012;43:414–430. doi: 10.1007/s10578-011-0274-x. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Bedny M, Goldberg RF. The frontal lobes and the regulation of mental activity. Curr Opin Neurobiol. 2005;15:219–224. doi: 10.1016/j.conb.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Walter H, von Kalckreuth A, Schardt D, Stephan A, Goschke T, Erk S. The Temporal Dynamics of Voluntary Emotion Regulation. Plos One. 2009:4. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The need for a cognitive neuroscience of naturalistic social cognition. Ann N Y Acad Sci. 2009;1167:16–30. doi: 10.1111/j.1749-6632.2009.04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlomke K, Hahn K. Cognitive emotion regulation strategies: Gender differences and associations to worry. Personality and Individual Differences. 2010;48:408–413. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.