Abstract
Mechanisms of HIV-mediated CD4+ T cell loss leading to immunodeficiency are amongst the most extensively studied yet unanswered questions in HIV biology. The level of CD4+ T cell depletion in HIV infected patients far exceeds the number of infected T cells suggesting an indirect mechanism of HIV pathogenesis termed bystander cell death. Evidence is accumulating that the HIV envelope glycoprotein (Env) is a major determinant of HIV pathogenesis and plays a critical role in bystander cell death. The complex structure and function of HIV Env makes the determination of the mechanism of Env mediated apoptosis more complex than previously thought. This review will examine the complex relationship between HIV Env phenotype, coreceptor expression and immune activation in determining HIV pathogenesis. We review data here corresponding to the role of HIV Env (hemi)fusion activity in HIV pathogenesis and how it interplays with other AIDS associated factors like chemokine receptor expression and immune activation.
Keywords: HIV-1, Env, pathogenesis, fusion, hemifusion, apoptosis
Envelope glycoprotein structure and function
The Envelope glycoprotein (Env) of HIV is arranged on the surface of the virus and virus-infected cells as a hetero-trimer. Each monomer is composed of a receptor-binding surface unit (gp120) and a fusogenic gp41 transmembrane unit [1]. The gp120 subunit binds to CD4 and a coreceptor either CXCR4 or CCR5 on T helper cells. Binding of HIV gp120 to CD4 triggers a complex sequence of events (Figure 1) involving several conformational changes in gp120 that result in exposure of coreceptor binding sites on gp120 and the N-terminal and C-terminal heptad repeat regions of gp41. Following engagement of gp120 with coreceptor, the gp41 heptad repeat domains interact with each other to form a six helix bundle catalyzing fusion of target and viral membranes [2]. Elucidation of mechanisms of fusion mediated by gp41 has been facilitated by high resolution determination of the gp41 core structure and by inhibition studies using peptides that mimic N or C-terminal heptad repeat sequences and interact with intermediate conformations of gp41 [3–7]. Although the function of HIV Env glycoprotein is to facilitate the entry of viral nucleocapsid into the target cell, its role in HIV pathogenesis is becoming increasingly evident [8].
Figure 1.
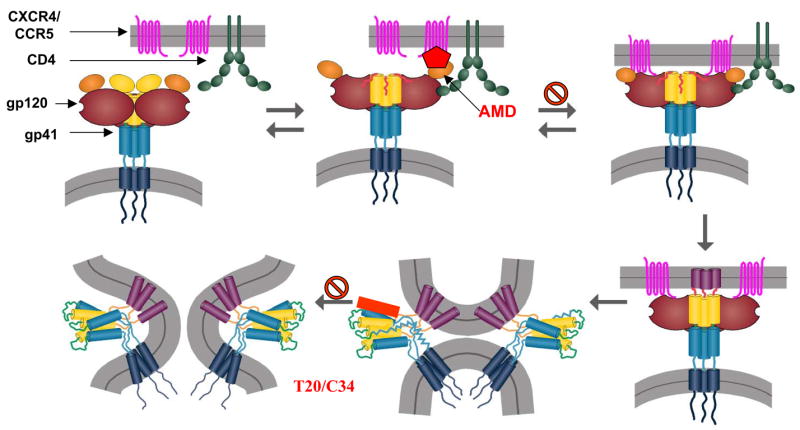
Schematic diagram of sequence of events involved in HIV Env mediated fusion. Binding of gp120 to receptor and coreceptor induces conformational changes that allow the exposure of gp41 Heptad Repeat (HR) regions. Interaction of HR1 and HR2 regions of gp41 results in six helix bundle formation resulting in fusion. The binding of CXCR4 antagonist AMD3100 and gp41 fusion inhibitors T20 and C34 are also shown.
Factors effecting HIV gp41 mediated fusion and hemifusion
Studies of membrane fusion mediated by viral Envs have revealed a number of intermediate steps in the fusion cascade that occur prior to the opening of the large fusion pore that enables the transfer of the nucleocapsid [9]. These steps include hemifusion and the opening of small fusion pores [10]. Hemifusion is defined as a membrane fusion event characterized by the mixing of the outer leaflets of the lipid bilayer without progression to fusion pore formation [11]. In the case of influenza hemagglutinin-expressing cells that fuse with red blood cells, stable hemifusion intermediates have been identified [12–16]. In the case if HIV-1 Env mediated cell fusion lipid mixing has been observed which is followed by separation of cells and the process does not progress to syncytia formation [17–19]. Although the latter process would represent “stunted” fusion rather than hemifusion from a fusion purist point of view, we will stick for the purpose of this review to the operational definition of hemifusion as lipid mixing without contents mixing. The rate of fusion depends not only on the fusogenic activity of the fusion protein like gp41 but also on the expression levels of both the fusion protein as well as the cognate receptor/coreceptor [20;21]. Hence depending on the conditions present in vitro or in vivo a fusion protein may mediate more hemifusion versus fusion or vice versa. There also seems to be clear disconnect between cell to cell fusion and virus cell fusion. A number of HIV Envs that are capable of inducing virus cell fusion and efficient viral replication do not induce cell to cell fusion or syncytia formation. These include a majority of CCR5 tropic Envs and some experimentally-designed CXCR4 tropic Envs [22;23]. This suggests that virus replication may be independent of cell to cell fusion and that Env may play a role in HIV pathogenesis that is related to the cell to cell fusion capacity of Env proteins. Another factor to keep in mind is the levels of coreceptor expression. The majority of CCR5 tropic viruses are Non Syncytia inducing (NSI) based on the low levels of CCR5 expression on cell lines and also in primary cells compared to CXCR4 expression [24;25]. Hence differences between the fusion capacity of CXCR4 and CCR5 viruses maybe directly related to the surface expression of coreceptors.
Gp41 structure and functional domains
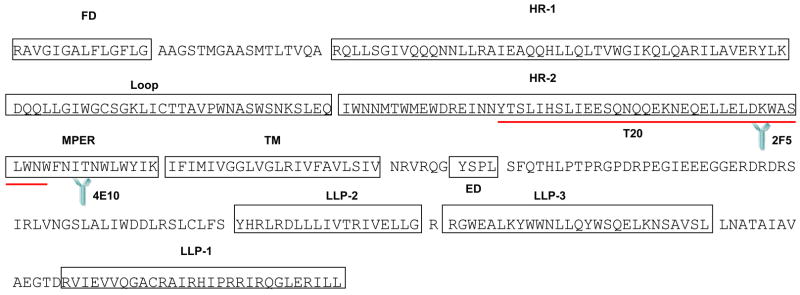
The gp41 is a complex transmembrane protein (Figure 2) that contains various well defined domains. The N terminal of the protein contains a hydrophobic fusion domain [26]. This is followed by N and C terminal heptad repeat (HR) regions that eventually form a six helix bundle which drives close membrane apposition and merging eventually leading to fusion pore formation [6;27]. The HR regions have been targets for development of numerous fusion inhibitors like T20 (Enfuvirtide) and C34 [7]. Although the HIV gp41 six helix bundle formation is the main driver of the fusion process, other gp41 domains may regulate fusion activity in numerous ways as indicated by studies on the effects of gp41 mutations on HIV Env mediated cell to cell or virus cell fusion [28]. The N-terminal and C-terminal heptad repeat regions are linked by an immunodominant loop region that plays a critical role in fusion. Mutations in the loop region of HIV have been shown to generate unique Envs that are restricted at the hemifusion step [19]. The tryptophan-rich membrane proximal ectodomain region (MPER) of HIV gp41 is also known to play a critical role in fusion. Mutation of more than one of the tryptophans in this region severely effects fusion activity [29;30]. Contrary to this replacement of tryptophans in this region with proline has been reported to enhance fusion activity [28]. This region is also highly investigated as a number of broadly neutralizing antibodies like 2F5 and 4E10 bind to this region and inhibit Env mediated fusion [31–33] making it an excellent target for vaccine development. The transmembrane region of gp41 is also important for Env activity as GPI anchored Env proteins fail to induce fusion [34] and a minimum length of transmembrane portion is critical for optimal Env function [35]. Interestingly the long cytoplasmic tail of gp41 not only regulates Env incorporation [36;37] but also regulates fusion [38]. The tail region of gp41 is known to contain 3 LLP (lentiviral lytic peptides) regions that interact with the viral membrane [39] and regulate fusion as evident from truncation mutants [38].
Figure 2.
Sequence of HIV-1 gp41 showing different domains. FD (Fusion Domain), HR (Heptad Repeat), MPER (Membrane Proximal Ectodomain Region), TM (Transmembrane Domain), ED (Endocytosis Domain), LLP (Lentiviral Lytic Peptide). Binding sites for neutralizing antibodies 2F5 and 4E10 are also shown along with the sequence of fusion inhibitor T20 (Enfuvirtide)
Env glycoprotein and bystander apoptosis
The amount of T cell depletion in HIV infected patients far exceeds the number of infected T cells suggesting an indirect mechanism of HIV pathogenesis termed bystander cell death [40;41]. Many studies have shown that bystander cell death induced by HIV shows characteristics of apoptosis [42;43]. In lymph node section of HIV-infected patients and SIV infected animals it appears that the majority of apoptotic cells are uninfected cells found in close proximity to infected cells [44]. Since HIV Env is expressed on the surface of infected cells that can interact with CD4+ bystander cells, Env is regarded as the major culprit in causing bystander cell death. This premise is supported by numerous in vitro studies that show that Env expressed on infected/transfected effector cells can interact with bystander target cells and induce apoptosis [45–48]. The binding of gp120 to its receptor (CD4) and a coreceptor (CXCR4/CCR5) is critical for this apoptosis induction [49;50]. This is evident from the fact that inhibition of this interaction with either anti CD4 antibodies or CXCR4 antagonists abolishes Env mediated bystander death [51–53]. Recently it was shown that HIV gp41 fusion inhibitors like T20 and C34 can also inhibit bystander cell death in models where HIV Env expressing cells are cocultured with receptor and coreceptor expressing bystander cells [17;54–57]. The inhibition of HIV Env mediated bystander apoptosis by T20 has also been shown in ex vivo Thymic cultures [56] further validating this phenomenon. This suggests that HIV gp41 plays a significant role in Env mediated bystander death. Hence the process of Env mediated apoptosis is quiet complex and is likely to be initiated via binding of gp120 to CD4 and CXCR4 and culminates in gp41 mediated membrane hemifusion/fusion. The nature of downstream signaling events that trigger the apoptotic cascade remains to be determined.
Signaling in HIV Env mediated apoptosis
Bystander cell death mediated via HIV Env shows classical signs of apoptosis. The apoptotic cascade initiated by HIV Env has been shown to involve caspase-3 activation [58], mitochondrial depolarization [53;59] as well as reactive oxygen species production [57]. The characteristics of apoptosis have also been reported in PBMCs from HIV infected individuals [60]. However the mechanism via which Env mediates these apoptotic features remains debated [8]. It is clear that this process is independent of Fas and TNF signaling [58;61]. As gp120 binds CD4 and CXCR4/CCR5 prior to fusion mediated by gp41, the role of signaling via either of these receptors becomes evident. Studies by Biard-Piechaczky et al using cells expressing cytoplasmic tail truncated form of CD4 showed that these cells still undergo Env mediated apoptosis [52] indicating that CD4 signaling may not be required for this phenomenon. However recent studies by Py et al suggest that the Siva protein interaction with CD4 may play a role in Env mediated apoptosis [62]. G protein dependent signaling via CXCR4 is also not likely to be involved in Env mediated apoptosis based on studies with pertussis toxin mediated inhibition of G protein [52;63]. However the role of G protein independent signaling via CXCR4 cannot be ruled out. The inhibition of HIV Env mediated bystander cell death by gp41 inhibitors like T20 and C34 suggest that the signaling event may in fact be initiated by gp41. In an attempt to understand this signaling mechanism we have shown that this process involves early caspase 3 activation that is propagated via a mitochondrial amplification loop [57]. Interestingly this apoptotic signaling via gp41 is inhibited by HIV protease inhibitor nelfinavir via its action on mitochondrial ANT transport system [64]. Other groups have also confirmed that nelfinavir inhibits HIV Env mediated apoptosis [65] establishing the critical role of mitochondria in HIV mediated apoptosis. This is further strengthened by findings that nelfinavir may have beneficial effects beyond virus suppression in HIV infected individuals by suppressing mitochondria mediated apoptosis [66].
Relationship between Env fusion and HIV pathogenesis
The fusogenic activity of HIV Env has long been associated with HIV pathogenesis both in vitro and in vivo. In clinical studies the finding of a highly fusogenic Syncytia inducing (SI) virus versus a Non Syncytia inducing (NSI) virus was associated with poor prognosis [67;68]. The classification of SI versus NSI is largely based on the syncytia formation by virus in MT2 cell lines [25]. This has often been correlated to coreceptor usage as CXCR4 utilizing viruses are most often SI while CCR5 viruses are NSI [69]. Furthermore, studies in chimeric SHIV containing HIV Env Rev Tat and Nef genes showed that passage of a non pathogenic chimera SHIV 89.6 resulted in a pathogenic SHIV89.6P which showed rapid CD4 loss in Rhesus Macaques [70;71]. The increased pathogenesis of SHIV89.6P could be mapped to the Env glycoprotein and correlated with fusion activity [72]. The role of SI phenotype in depletion of CD4+ T cells has also been demonstrated in various SCIDhu mouse models [73–75] as well as lymphnode [76;77] and thymus histocultures [56;78]. This suggests that a direct correlation between the fusogenic potential of HIV gp41 and CD4 T cell loss exists.
Gp41 mediated syncytial apoptosis
HIV Env expressing cells cocultured with CD4 and CXCR4/CCR5 expressing target cells undergo cell fusion. Cells undergoing fusion mediated by HIV gp41 form multinucleated syncytia that after a relatively short period 48–72h undergo apoptosis [79–83]. This method of apoptosis has been well characterized by Perfettini et al [84] and has been shown to involve p38 MAPK and mTOR mediated activation of p53. Under this model p53 dependent expression of bax and puma leads to mitochondrial depolarization and apoptosis [84;85]. In other studies with a slightly different model it has been shown that Env expressing cells on the verge of undergoing apoptosis can fuse with neighboring cells and transmit the lethal signal in a contagious fashion [86]. However the role of post-fusion hypotheses for HIV pathogenesis in vivo remains uncertain since little or no syncytia are observed in the lymph nodes of HIV infected individuals or SHIV infected Macaques [87].
Gp41 hemifusion mediated apoptosis
HIV gp41 mediates efficient fusion in a variety of cell lines expressing receptor and coreceptor. However, as mentioned above, in a number of situations the fusion process may not result in complete fusion of cells and maybe interrupted at the hemifusion step. This probably happens more often than thought especially in vivo where virus induced syncytia are rarely seen in infected patients. This process of hemifusion could be detrimental to the target cells forcing them to undergo apoptosis as observed by Blanco et al [17]. We and others have shown that in vitro the single cells dying after coculture of Env expressing cells with target cells have taken up a membrane dye from the effector cells [17;57]. These cells undergo classical apoptosis characterized by caspase 3 activation and mitochondrial dysfunction [57]. The inhibition of both membrane dye transfer and apoptosis by gp41 inhibitors like C34 suggests a direct role of gp41 in this process and forms the basis of the hemifusion hypothesis. To address this issue directly we have used a mutational approach [18] to show that mutations in the gp41 fusion domain that abolish fusion activity also inhibit apoptosis induction. Further support of the role of gp41 mediated hemifusion in bystander apoptosis comes form our demonstration that an Env glycoprotein mutant D589L that is restricted at the hemifusion step [19] mediates apoptosis in bystander cells in the absence of cell to cell fusion. The mechanism of apoptosis mediated by hemifusion restricted mutant is identical to wild type Env, based on the inhibition by nelfinavir and caspase 3 inhibitors, strengthening the hypothesis that gp41 mediated hemifusion is both required and sufficient for apoptosis induction. This hemfusion induced single cell death mediated by HIV Env has been aptly named the “Kiss of Death” [8] and its role in HIV pathogenesis is becoming increasingly evident.
Viral Synapse and bystander cell death
Recent evidence suggests that the transmission of HIV occurs more efficiently via cell to cell contact than free virus [88]. This cell contact dependent transmission of virus occurs across a viral synapse, which is similar to an immunological synapse, is formed between the infected and uninfected cell [89]. Upon contact of infected cell with uninfected cell there is a polarization of both HIV receptor and coreceptor on target cell as well as the budding of virus at the cell to cell interface [90;91]. Various cellular and viral proteins are known to facilitate the viral synapse formation including adhesion molecules like LFA-1, ICAM-1, ICAM-3 [92], tetraspanin [91], actin and tubulin cytoskeletal proteins [93], and Env glycoprotein interaction with CD4 and CXCR4 [94]. The formation of this cell to cell contact interface involving the Env glycoprotein further supports the importance of cell surface expressed HIV Env in mediating bystander cell death. It is important to note that this viral synapse formation can not only effect virus replication by enhancing virus transmission but may also mediate apoptosis via gp41 mediated events. In fact we have shown that replication of viruses that induce cell to cell fusion is slower than viruses that don’t [18]. Furthermore, inhibition of bystander apoptosis via caspase inhibitors results in faster replication of SI viruses in T cells as shown by us and others [18;95]. Hence while virus transmission across the viral synapse facilitates virus replication it may also come at the cost of bystander cell death in the case of pathogenic viruses.
Enfuvirtide resistant mutants and HIV pathogenesis
The hypothesis that HIV gp41 fusion/hemifusion activity correlates with pathogenesis suggests that drugs targeting gp41 function may alter HIV pathogenesis. In this context, it has been suggested that Enfuvirtide therapy may have beneficial effects by directly inhibiting gp41 mediated bystander cell death [96]. The effect of Enfuvirtide on bystander cell death is not restricted to direct inhibition of gp41 function. In a recent clinical study Aquaro et al [97] showed that certain resistant viruses emerging during Enfuvirtide therapy are associated with CD4 increase in patients even after virological failure. These mutations are specifically in the gp41 HR1 region and are known to effect gp41 fusion activity [98]. These findings were confirmed by Melby et al [99] and showing that a mutation specifically at position V38 (V549 based on Env numbering) are associated with increase in CD4 recovery in patients after virological failure. Similar findings have recently been reported by Svicher et al [100]. These findings not only support the hypothesis that gp41 mediated fusion/hemifusion are critical for HIV pathogenesis but also that targeting Env mediated fusion by inhibitors of gp41 should inhibit both virus replication and Env-mediated bystander cell death. Furthermore treatment strategies can be designed to select Enfuvirtide resistant mutants that seem to be less pathogenic via mutations in gp41 that effect fusion activity.
Coreceptor expression
The role of a specific type of coreceptor in HIV pathogenesis remains much debated. It is clear from various studies that switch of coreceptor usage by HIV from CCR5 to CXCR4 precedes the rapid decline in CD4 cells and AIDS development in numerous cases [67]. The differences in CXCR4 and CCR5 expression levels maybe related to the relative pathogenesis of these viruses [101]. Also the increased fusogenicity of CXCR4 viruses is suggested to be a factor in the pathogenesis of these viruses [68;76]. However in 50% of patients there is no switch in coreceptor and although the presence of CXCR4 tropic virus has been associated with poor prognosis in patients, it is evident that coreceptor switch may not be required for progression to AIDS [77]. Nevertheless, a selection of more pathogenic CCR5 utilizing HIV is possible and in recent studies by Oliveri et al [102] show that CCR5 tropic viruses isolated from patients early during disease are different from those isolated at the development of AIDS. More specifically, the Env fusion activity could be correlated with CD4 cell loss for CCR5 viruses as well. In vitro experiments have also suggested that the loss of CCR5 cells via R5 Tropic Env is mediated by gp41 [103]. The role of CCR5 in HIV infection and pathogenesis in vivo is supported by resistance to HIV infection in CCR5Δ32 homozygous populations [104;105]. On the other hand CCR5Δ32 heterozygous population has low levels of CCR5 expression making them susceptible to HIV infection but delayed progressing to AIDS [106;107]. In fact in a study by Scoggins et al in SCID-hu mice reconstituted with CCR5Δ32 heterozygous thymus grafts were resistant CCR5 virus mediated CD4 cell loss even in the presence of virus replication [108]. The role of CCR5 expression is further complicated by the polymorphism in CCR5 promoter region. Epidemiological studies suggest that the rate of disease progression in HIV infected individual correlates with CCR5 promoter polymorphism [109–112]. This promoter polymorphism in turn has been associated with surface expression levels of CCR5 [113]. The importance of CCR5 expression levels is further emphasized by the role of physiological levels of CCR5 expression on Env mediated fusion and virus replication [20;114]. Whether increased surface expression of CCR5 accounts for the Env hemifusion mediated apoptosis phenotype in certain R5 virus infected patients remains to be determined.
Immune activation and HIV pathogenesis
As HIV selectively targets CD4+ cells of the immune system, it is not surprising that it has complex immune manifestations. Whether HIV induced CD4 T cell loss is also immune mediated remains to be determined. Nevertheless, chronic immune activation remains a hallmark of pathogenic HIV infections [115;116]. The activation of CD4+ T cells as determined by surface expression of activations markers like Ki67, HLA-DR, CD25 and CD38 [117;118] have been associated with HIV disease progression. In fact immune activation is a better predictor of CD4 apoptosis than plasma viremia [119–122]. Although it is widely accepted that pathogenic HIV infections lead to chronic immune activation it is not clear what mediates this immune activation and whether it is a cause or consequence of CD4 T cell loss. HIV infection of resting T cells results in latent infection and immune activation drives the virus into productive replication. Also immune activation leads to an up regulation of coreceptors, both CXCR4 and CCR5, that not only facilitate virus infection [123] but may also enhance Env mediated apoptosis. Biancotto et al [124] have recently shown that infection of ex vivo human lymphoid tissue with HIV-1 leads to a unique pattern of T cell activation, characterized by CD25+/HLADR+ cells that facilitate virus replication. These studies underscore the fact that immune activation is virus mediated, although the mechanism is not clear. Recent studies by Rawson et al [125] demonstrate that cross presentation of caspase cleaved self antigens generated by T cells undergoing apoptosis may induce systemic immune activation providing a mechanistic understanding of immune activation. It is thus conceivable that during infection with pathogenic HIV-1, Env mediated apoptosis generates caspase cleaved antigens that in turn induce immune activation and perpetuates a vicious cycle that involves increased virus infection and replication at the cost of bystander cell death.
Conclusions and future directions
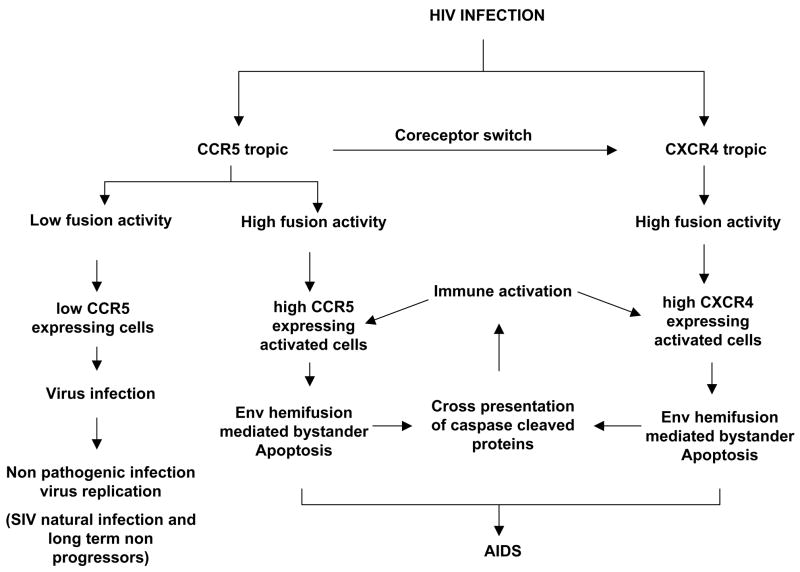
Although HIV Env glycoprotein structure and function have been studied extensively, the role it plays in HIV pathogenesis remains enigmatic. There seems to be a lack of consensus on whether HIV Env mediates bystander cell death and if so via what mechanism. In this review, we have provided evidence in favor of a role of the fusion activity of Env glycoprotein gp41 subunit in HIV pathogenesis. While the correlation between HIV Env fusogenicity and pathogenesis has long existed, it has been unclear as to the mechanism of this relation especially in terms of single cell death. Recent evidences suggest that gp41 mediated bystander cell death in single cells can be explained by the phenomenon of hemifusion. Application of the hemifusion hypothesis seems to put a consensus on the variety of observations over the years related to HIV pathogenesis. In this model, a complex interplay between HIV fusion activity, type and amount of coreceptor expression and immune activation play an intertwining role. Based on published studies, we can propose a model of HIV pathogenesis that tends to encompass all the above mentioned issues in a simple model (Figure 3). Based on recent evidence that pathogenesis mediated by Enfuviritde resistant Env gp41 subunit mutants is reduced; we are for the first time able to appreciate the role of Env mediated fusion in HIV pathogenesis in clinical settings. However future studies need to address the direct role of gp41 in HIV pathogenesis via in vivo and in vitro models. Some questions that remain unanswered include whether CCR5 polymorphism effects bystander apoptosis mediated by CCR5 Env, whether immune activation is mediated by apoptotic cells generated by Env mediated bystander cell death and whether the reduced pathogenesis of Enfuvirtide resistant mutants is due to lack of Env mediated apoptosis inducing capacity. Also further analysis of the cellular and biochemical changes occurring during this “kiss of death” phenomenon need to be examined in detail.
Figure 3.
Simplified model of HIV gp41 hemifusion mediated pathogenesis.
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 2.Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim Biophys Acta. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- 3.Jiang S, Lin K, Strick N, Neurath AR. Inhibition of HIV-1 infection by a fusion domain binding peptide from the HIV-1 envelope glycoprotein GP41. Biochem Biophys Res Commun. 1993;195:533–538. doi: 10.1006/bbrc.1993.2078. [DOI] [PubMed] [Google Scholar]
- 4.Jiang S, Lin K, Strick N, Neurath AR. HIV-1 inhibition by a peptide. Nature. 1993;365:113. doi: 10.1038/365113a0. [DOI] [PubMed] [Google Scholar]
- 5.Shugars DC, Wild CT, Greenwell TK, Matthews TJ. Biophysical characterization of recombinant proteins expressing the leucine zipper-like domain of the human immunodeficiency virus type 1 transmembrane protein gp41. J Virol. 1996;70:2982–2991. doi: 10.1128/jvi.70.5.2982-2991.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wild C, Dubay JW, Greenwell T, Baird T, Jr, Oas TG, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci U S A. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perfettini JL, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, Kroemer G. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005;12(Suppl 1):916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal R, Clague MJ, Durell SR, Epand RM. Membrane fusion. Chem Rev. 2003;103:53–69. doi: 10.1021/cr000036+. [DOI] [PubMed] [Google Scholar]
- 10.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 11.Chernomordik LV, Kozlov MM. Membrane hemifusion: crossing a chasm in two leaps. Cell. 2005;123:375–382. doi: 10.1016/j.cell.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Kemble GW, Danieli T, White JM. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell. 1994;76:383–391. doi: 10.1016/0092-8674(94)90344-1. [DOI] [PubMed] [Google Scholar]
- 13.Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiao H, Armstrong RT, Melikyan GB, Cohen FS, White JM. A specific point mutant at position 1 of the influenza hemagglutinin fusion peptide displays a hemifusion phenotype. Mol Biol Cell. 1999;10:2759–2769. doi: 10.1091/mbc.10.8.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melikyan GB, Markosyan RM, Roth MG, Cohen FS. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol Biol Cell. 2000;11:3765–3775. doi: 10.1091/mbc.11.11.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frolov VA, Cho MS, Bronk P, Reese TS, Zimmerberg J. Multiple local contact sites are induced by GPI-linked influenza hemagglutinin during hemifusion and flickering pore formation. Traffic. 2000;1:622–630. doi: 10.1034/j.1600-0854.2000.010806.x. [DOI] [PubMed] [Google Scholar]
- 17.Blanco J, Barretina J, Ferri KF, Jacotot E, Gutierrez A, rmand-Ugon M, Cabrera C, Kroemer G, Clotet B, Este JA. Cell-surface-expressed HIV-1 envelope induces the death of CD4 T cells during GP41-mediated hemifusion-like events. Virology. 2003;305:318–329. doi: 10.1006/viro.2002.1764. [DOI] [PubMed] [Google Scholar]
- 18.Garg H, Joshi A, Freed EO, Blumenthal R. Site-specific mutations in HIV-1 gp41 reveal a correlation between HIV-1-mediated bystander apoptosis and fusion/hemifusion. J Biol Chem. 2007;282:16899–16906. doi: 10.1074/jbc.M701701200. [DOI] [PubMed] [Google Scholar]
- 19.Bar S, Alizon M. Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J Virol. 2004;78:811–820. doi: 10.1128/JVI.78.2.811-820.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves JD, Gallo SA, Ahmad N, Miamidian JL, Harvey PE, Sharron M, Pohlmann S, Sfakianos JN, Derdeyn CA, Blumenthal R, Hunter E, Doms RW. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc Natl Acad Sci U S A. 2002;99:16249–16254. doi: 10.1073/pnas.252469399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao J, Vasir B, Sodroski JG. Changes in the cytopathic effects of human immunodeficiency virus type 1 associated with a single amino acid alteration in the ectodomain of the gp41 transmembrane glycoprotein. J Virol. 1994;68:4662–4668. doi: 10.1128/jvi.68.7.4662-4668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinomoto M, Yokoyama M, Sato H, Kojima A, Kurata T, Ikuta K, Sata T, Tokunaga K. Amino acid 36 in the human immunodeficiency virus type 1 gp41 ectodomain controls fusogenic activity: implications for the molecular mechanism of viral escape from a fusion inhibitor. J Virol. 2005;79:5996–6004. doi: 10.1128/JVI.79.10.5996-6004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Rij RP, Portegies P, Hallaby T, Lange JM, Visser J, de Roda Husman AM, van ‘t Wout AB, Schuitemaker H. Reduced prevalence of the CCR5 delta32 heterozygous genotype in human immunodeficiency virus-infected individuals with AIDS dementia complex. J Infect Dis. 1999;180:854–857. doi: 10.1086/314940. [DOI] [PubMed] [Google Scholar]
- 25.Schuitemaker H, Koot M, Kootstra NA, Dercksen MW, de Goede RE, van Steenwijk RP, Lange JM, Schattenkerk JK, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freed EO, Delwart EL, Buchschacher GL, Jr, Panganiban AT. A mutation in the human immunodeficiency virus type 1 transmembrane glycoprotein gp41 dominantly interferes with fusion and infectivity. Proc Natl Acad Sci U S A. 1992;89:70–74. doi: 10.1073/pnas.89.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melikyan GB, Markosyan RM, Hemmati H, Delmedico MK, Lambert DM, Cohen FS. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao J, Bergeron L, Helseth E, Thali M, Repke H, Sodroski J. Effects of amino acid changes in the extracellular domain of the human immunodeficiency virus type 1 gp41 envelope glycoprotein. J Virol. 1993;67:2747–2755. doi: 10.1128/jvi.67.5.2747-2755.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salzwedel K, West JT, Hunter E. A conserved tryptophan-rich motif in the membrane-proximal region of the human immunodeficiency virus type 1 gp41 ectodomain is important for Env-mediated fusion and virus infectivity. J Virol. 1999;73:2469–2480. doi: 10.1128/jvi.73.3.2469-2480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz-Barroso I, Salzwedel K, Hunter E, Blumenthal R. Role of the membrane-proximal domain in the initial stages of human immunodeficiency virus type 1 envelope glycoprotein-mediated membrane fusion. J Virol. 1999;73:6089–6092. doi: 10.1128/jvi.73.7.6089-6092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de RE, Vassell R, Jiang S, Kunert R, Weiss CD. Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes. J Virol. 2004;78:2627–2631. doi: 10.1128/JVI.78.5.2627-2631.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purtscher M, Trkola A, Grassauer A, Schulz PM, Klima A, Dopper S, Gruber G, Buchacher A, Muster T, Katinger H. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS. 1996;10:587–593. doi: 10.1097/00002030-199606000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Dimitrov AS, Jacobs A, Finnegan CM, Stiegler G, Katinger H, Blumenthal R. Exposure of the membrane-proximal external region of HIV-1 gp41 in the course of HIV-1 envelope glycoprotein-mediated fusion. Biochemistry. 2007;46:1398–1401. doi: 10.1021/bi062245f. [DOI] [PubMed] [Google Scholar]
- 34.Weiss CD, White JM. Characterization of stable Chinese hamster ovary cells expressing wild-type, secreted, and glycosylphosphatidylinositol-anchored human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1993;67:7060–7066. doi: 10.1128/jvi.67.12.7060-7066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owens RJ, Burke C, Rose JK. Mutations in the membrane-spanning domain of the human immunodeficiency virus envelope glycoprotein that affect fusion activity. J Virol. 1994;68:570–574. doi: 10.1128/jvi.68.1.570-574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami T, Freed EO. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci U S A. 2000;97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piller SC, Dubay JW, Derdeyn CA, Hunter E. Mutational analysis of conserved domains within the cytoplasmic tail of gp41 from human immunodeficiency virus type 1: effects on glycoprotein incorporation and infectivity. J Virol. 2000;74:11717–11723. doi: 10.1128/jvi.74.24.11717-11723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyss S, Dimitrov AS, Baribaud F, Edwards TG, Blumenthal R, Hoxie JA. Regulation of human immunodeficiency virus type 1 envelope glycoprotein fusion by a membrane-interactive domain in the gp41 cytoplasmic tail. J Virol. 2005;79:12231–12241. doi: 10.1128/JVI.79.19.12231-12241.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viard M, Ablan SD, Zhou M, Veenstra TD, Freed EO, Raviv Y, Blumenthal R. Photoinduced Reactivity of the HIV-1 Envelope Glycoprotein with a Membrane-Embedded Probe Reveals Insertion of Portions of the HIV-1 Gp41 Cytoplasmic Tail into the Viral Membrane. Biochemistry. 2008;47:1977–1983. doi: 10.1021/bi701920f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gougeon ML, Montagnier L. Apoptosis in AIDS. Science. 1993;260:1269–1270. doi: 10.1126/science.8098552. [DOI] [PubMed] [Google Scholar]
- 41.Gougeon ML, Colizzi V, Dalgleish A, Montagnier L. New concepts in AIDS pathogenesis. AIDS Res Hum Retroviruses. 1993;9:287–289. doi: 10.1089/aid.1993.9.287. [DOI] [PubMed] [Google Scholar]
- 42.Gougeon ML, Ledru E, Lecoeur H, Garcia S. T cell apoptosis in HIV infection: mechanisms and relevance for AIDS pathogenesis. Results Probl Cell Differ. 1998;24:233–248. doi: 10.1007/978-3-540-69185-3_11. [DOI] [PubMed] [Google Scholar]
- 43.Gougeon ML, Montagnier L. Programmed cell death as a mechanism of CD4 and CD8 T cell deletion in AIDS. Molecular control and effect of highly active anti-retroviral therapy. Ann N Y Acad Sci. 1999;887:199–212. doi: 10.1111/j.1749-6632.1999.tb07934.x. [DOI] [PubMed] [Google Scholar]
- 44.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 45.Laurent-Crawford AG, Krust B, Riviere Y, Desgranges C, Muller S, Kieny MP, Dauguet C, Hovanessian AG. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 46.Laurent-Crawford AG, Coccia E, Krust B, Hovanessian AG. Membrane-expressed HIV envelope glycoprotein heterodimer is a powerful inducer of cell death in uninfected CD4+ target cells. Res Virol. 1995;146:5–17. doi: 10.1016/0923-2516(96)80585-1. [DOI] [PubMed] [Google Scholar]
- 47.Ahr B, Robert-Hebmann V, Devaux C, Biard-Piechaczyk M. Apoptosis of uninfected cells induced by HIV envelope glycoproteins. Retrovirology. 2004;1:12. doi: 10.1186/1742-4690-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nardelli B, Gonzalez CJ, Schechter M, Valentine FT. CD4+ blood lymphocytes are rapidly killed in vitro by contact with autologous human immunodeficiency virus-infected cells. Proc Natl Acad Sci U S A. 1995;92:7312–7316. doi: 10.1073/pnas.92.16.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacotot E, Krust B, Callebaut C, Laurent-Crawford AG, Blanco J, Hovanessian AG. HIV-1 envelope glycoproteins-mediated apoptosis is regulated by CD4 dependent and independent mechanisms. Apoptosis. 1997;2:47–60. doi: 10.1023/a:1026435625144. [DOI] [PubMed] [Google Scholar]
- 50.Biard-Piechaczyk M, Robert-Hebmann V, Roland J, Coudronniere N, Devaux C. Role of CXCR4 in HIV-1-induced apoptosis of cells with a CD4+, CXCR4+ phenotype. Immunol Lett. 1999;70:1–3. doi: 10.1016/s0165-2478(99)00124-8. [DOI] [PubMed] [Google Scholar]
- 51.Blanco J, Barretina J, Henson G, Bridger G, De CE, Clotet B, Este JA. The CXCR4 antagonist AMD3100 efficiently inhibits cell-surface-expressed human immunodeficiency virus type 1 envelope-induced apoptosis. Antimicrob Agents Chemother. 2000;44:51–56. doi: 10.1128/aac.44.1.51-56.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Biard-Piechaczyk M, Robert-Hebmann V, Richard V, Roland J, Hipskind RA, Devaux C. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120) Virology. 2000;268:329–344. doi: 10.1006/viro.1999.0151. [DOI] [PubMed] [Google Scholar]
- 53.Roggero R, Robert-Hebmann V, Harrington S, Roland J, Vergne L, Jaleco S, Devaux C, Biard-Piechaczyk M. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J Virol. 2001;75:7637–7650. doi: 10.1128/JVI.75.16.7637-7650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stocker H, Scheller C, Jassoy C. Destruction of primary CD4(+) T cells by cell-cell interaction in human immunodeficiency virus type 1 infection in vitro. J Gen Virol. 2000;81:1907–1911. doi: 10.1099/0022-1317-81-8-1907. [DOI] [PubMed] [Google Scholar]
- 55.Scheller C, Jassoy C. Syncytium formation amplifies apoptotic signals: a new view on apoptosis in HIV infection in vitro. Virology. 2001;282:48–55. doi: 10.1006/viro.2000.0811. [DOI] [PubMed] [Google Scholar]
- 56.Meissner EG, Zhang L, Jiang S, Su L. Fusion-induced apoptosis contributes to thymocyte depletion by a pathogenic human immunodeficiency virus type 1 envelope in the human thymus. J Virol. 2006;80:11019–11030. doi: 10.1128/JVI.01382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garg H, Blumenthal R. HIV gp41-induced apoptosis is mediated by caspase-3-dependent mitochondrial depolarization, which is inhibited by HIV protease inhibitor nelfinavir. J Leukoc Biol. 2006;79:351–362. doi: 10.1189/jlb.0805430. [DOI] [PubMed] [Google Scholar]
- 58.Ohnimus H, Heinkelein M, Jassoy C. Apoptotic cell death upon contact of CD4+ T lymphocytes with HIV glycoprotein-expressing cells is mediated by caspases but bypasses CD95 (Fas/Apo-1) and TNF receptor 1. J Immunol. 1997;159:5246–5252. [PubMed] [Google Scholar]
- 59.Ferri KF, Jacotot E, Blanco J, Este JA, Kroemer G. Mitochondrial control of cell death induced by HIV-1-encoded proteins. Ann N Y Acad Sci. 2000;926:149–164. doi: 10.1111/j.1749-6632.2000.tb05609.x. [DOI] [PubMed] [Google Scholar]
- 60.Macho A, Castedo M, Marchetti P, Aguilar JJ, Decaudin D, Zamzami N, Girard PM, Uriel J, Kroemer G. Mitochondrial dysfunctions in circulating T lymphocytes from human immunodeficiency virus-1 carriers. Blood. 1995;86:2481–2487. [PubMed] [Google Scholar]
- 61.Gandhi RT, Chen BK, Straus SE, Dale JK, Lenardo MJ, Baltimore D. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J Exp Med. 1998;187:1113–1122. doi: 10.1084/jem.187.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Py B, Bouchet J, Jacquot G, Sol-Foulon N, Basmaciogullari S, Schwartz O, Biard-Piechaczyk M, Benichou S. The Siva protein is a novel intracellular ligand of the CD4 receptor that promotes HIV-1 envelope-induced apoptosis in T-lymphoid cells. Apoptosis. 2007;12:1879–1892. doi: 10.1007/s10495-007-0106-4. [DOI] [PubMed] [Google Scholar]
- 63.Blanco J, Jacotot E, Cabrera C, Cardona A, Clotet B, De CE, Este JA. The implication of the chemokine receptor CXCR4 in HIV-1 envelope protein-induced apoptosis is independent of the G protein-mediated signalling. AIDS. 1999;13:909–917. doi: 10.1097/00002030-199905280-00006. [DOI] [PubMed] [Google Scholar]
- 64.Weaver JG, Tarze A, Moffat TC, Lebras M, Deniaud A, Brenner C, Bren GD, Morin MY, Phenix BN, Dong L, Jiang SX, Sim VL, Zurakowski B, Lallier J, Hardin H, Wettstein P, van Heeswijk RP, Douen A, Kroemer RT, Hou ST, Bennett SA, Lynch DH, Kroemer G, Badley AD. Inhibition of adenine nucleotide translocator pore function and protection against apoptosis in vivo by an HIV protease inhibitor. J Clin Invest. 2005 doi: 10.1172/JCI22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vlahakis SR, Bren GD, geciras-Schimnich A, Trushin SA, Schnepple DJ, Badley AD. Flying in the face of resistance: antiviral-independent benefit of HIV protease inhibitors on T-cell survival. Clin Pharmacol Ther. 2007;82:294–299. doi: 10.1038/sj.clpt.6100140. [DOI] [PubMed] [Google Scholar]
- 66.Miro O, Villarroya J, Garrabou G, Lopez S, Rodriguez de la CM, Pedrol E, Martinez E, Giralt M, Gatell JM, Cardellach F, Casademont J, Villarroya F. In vivo effects of highly active antiretroviral therapies containing the protease inhibitor nelfinavir on mitochondrially driven apoptosis. Antivir Ther. 2005;10:945–951. [PubMed] [Google Scholar]
- 67.Koot M, van ‘t Wout AB, Kootstra NA, de Goede RE, Tersmette M, Schuitemaker H. Relation between changes in cellular load, evolution of viral phenotype, and the clonal composition of virus populations in the course of human immunodeficiency virus type 1 infection. J Infect Dis. 1996;173:349–354. doi: 10.1093/infdis/173.2.349. [DOI] [PubMed] [Google Scholar]
- 68.Spijkerman I, de WF, Langendam M, Schuitemaker H, Coutinho R. Emergence of syncytium-inducing human immunodeficiency virus type 1 variants coincides with a transient increase in viral RNA level and is an independent predictor for progression to AIDS. J Infect Dis. 1998;178:397–403. doi: 10.1086/515627. [DOI] [PubMed] [Google Scholar]
- 69.van Rij RP, Blaak H, Visser JA, Brouwer M, Rientsma R, Broersen S, de Roda Husman AM, Schuitemaker H. Differential coreceptor expression allows for independent evolution of non-syncytium-inducing and syncytium-inducing HIV-1. J Clin Invest. 2000;106:1039–1052. doi: 10.1172/jci7953c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Etemad-Moghadam B, Rhone D, Steenbeke T, Sun Y, Manola J, Gelman R, Fanton JW, Racz P, Tenner-Racz K, Axthelm MK, Letvin NL, Sodroski J. Membrane-fusing capacity of the human immunodeficiency virus envelope proteins determines the efficiency of CD+ T-cell depletion in macaques infected by a simian-human immunodeficiency virus. J Virol. 2001;75:5646–5655. doi: 10.1128/JVI.75.12.5646-5655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Etemad-Moghadam B, Sun Y, Nicholson EK, Fernandes M, Liou K, Gomila R, Lee J, Sodroski J. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J Virol. 2000;74:4433–4440. doi: 10.1128/jvi.74.9.4433-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LaBonte JA, Patel T, Hofmann W, Sodroski J. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J Virol. 2000;74:10690–10698. doi: 10.1128/jvi.74.22.10690-10698.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camerini D, Su HP, Gamez-Torre G, Johnson ML, Zack JA, Chen IS. Human immunodeficiency virus type 1 pathogenesis in SCID-hu mice correlates with syncytium-inducing phenotype and viral replication. J Virol. 2000;74:3196–3204. doi: 10.1128/jvi.74.7.3196-3204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Picchio GR, Gulizia RJ, Wehrly K, Chesebro B, Mosier DE. The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes. J Virol. 1998;72:2002–2009. doi: 10.1128/jvi.72.3.2002-2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaneshima H, Su L, Bonyhadi ML, Connor RI, Ho DD, McCune JM. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Penn ML, Grivel JC, Schramm B, Goldsmith MA, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci U S A. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Karlsson I, Grivel JC, Chen SS, Karlsson A, Albert J, Fenyo EM, Margolis LB. Differential pathogenesis of primary CCR5-using human immunodeficiency virus type 1 isolates in ex vivo human lymphoid tissue. J Virol. 2005;79:11151–11160. doi: 10.1128/JVI.79.17.11151-11160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meissner EG, Coffield VM, Su L. Thymic pathogenicity of an HIV-1 envelope is associated with increased CXCR4 binding efficiency and V5-gp41-dependent activity, but not V1/V2-associated CD4 binding efficiency and viral entry. Virology. 2005;336:184–197. doi: 10.1016/j.virol.2005.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferri KF, Jacotot E, Geuskens M, Kroemer G. Apoptosis and karyogamy in syncytia induced by the HIV-1-envelope glycoprotein complex. Cell Death Differ. 2000;7:1137–1139. doi: 10.1038/sj.cdd.4400748. [DOI] [PubMed] [Google Scholar]
- 80.Ferri KF, Jacotot E, Leduc P, Geuskens M, Ingber DE, Kroemer G. Apoptosis of syncytia induced by the HIV-1-envelope glycoprotein complex: influence of cell shape and size. Exp Cell Res. 2000;261:119–126. doi: 10.1006/excr.2000.5062. [DOI] [PubMed] [Google Scholar]
- 81.Ferri KF, Jacotot E, Blanco J, Este JA, Zamzami N, Susin SA, Xie Z, Brothers G, Reed JC, Penninger JM, Kroemer G. Apoptosis control in syncytia induced by the HIV type 1-envelope glycoprotein complex: role of mitochondria and caspases. J Exp Med. 2000;192:1081–1092. doi: 10.1084/jem.192.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castedo M, Kroemer G. The beauty of death. Trends Cell Biol. 2002;12:446. doi: 10.1016/s0962-8924(02)02346-2. [DOI] [PubMed] [Google Scholar]
- 83.Castedo M, Roumier T, Blanco J, Ferri KF, Barretina J, Tintignac LA, Andreau K, Perfettini JL, Amendola A, Nardacci R, Leduc P, Ingber DE, Druillennec S, Roques B, Leibovitch SA, Vilella-Bach M, Chen J, Este JA, Modjtahedi N, Piacentini M, Kroemer G. Sequential involvement of Cdk1, mTOR and p53 in apoptosis induced by the HIV-1 envelope. EMBO J. 2002;21:4070–4080. doi: 10.1093/emboj/cdf391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perfettini JL, Castedo M, Nardacci R, Ciccosanti F, Boya P, Roumier T, Larochette N, Piacentini M, Kroemer G. Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med. 2005;201:279–289. doi: 10.1084/jem.20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perfettini JL, Roumier T, Castedo M, Larochette N, Boya P, Raynal B, Lazar V, Ciccosanti F, Nardacci R, Penninger J, Piacentini M, Kroemer G. NF-kappaB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J Exp Med. 2004;199:629–640. doi: 10.1084/jem.20031216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andreau K, Perfettini JL, Castedo M, Metivier D, Scott V, Pierron G, Kroemer G. Contagious apoptosis facilitated by the HIV-1 envelope: fusion-induced cell-to-cell transmission of a lethal signal. J Cell Sci. 2004;117:5643–5653. doi: 10.1242/jcs.01486. [DOI] [PubMed] [Google Scholar]
- 87.Karlsson GB, Halloran M, Schenten D, Lee J, Racz P, Tenner-Racz K, Manola J, Gelman R, Etemad-Moghadam B, Desjardins E, Wyatt R, Gerard NP, Marcon L, Margolin D, Fanton J, Axthelm MK, Letvin NL, Sodroski J. The envelope glycoprotein ectodomains determine the efficiency of CD4+ T lymphocyte depletion in simian-human immunodeficiency virus-infected macaques. J Exp Med. 1998;188:1159–1171. doi: 10.1084/jem.188.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol. 2007;81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Piguet V, Sattentau Q. Dangerous liaisons at the virological synapse. J Clin Invest. 2004;114:605–610. doi: 10.1172/JCI22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fais S, Borghi P, Gherardi G, Logozzi M, Belardelli F, Gessani S. Human immunodeficiency virus type 1 induces cellular polarization, intercellular adhesion molecule-1 redistribution, and multinucleated giant cell generation in human primary monocytes but not in monocyte-derived macrophages. Lab Invest. 1996;75:783–790. [PubMed] [Google Scholar]
- 91.Jolly C, Sattentau QJ. Human immunodeficiency virus type 1 assembly, budding, and cell-cell spread in T cells take place in tetraspanin-enriched plasma membrane domains. J Virol. 2007;81:7873–7884. doi: 10.1128/JVI.01845-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jolly C, Mitar I, Sattentau QJ. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J Virol. 2007;81:13916–13921. doi: 10.1128/JVI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jolly C, Mitar I, Sattentau QJ. Requirement for an intact T-cell actin and tubulin cytoskeleton for efficient assembly and spread of human immunodeficiency virus type 1. J Virol. 2007;81:5547–5560. doi: 10.1128/JVI.01469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chinnaiyan AM, Woffendin C, Dixit VM, Nabel GJ. The inhibition of pro-apoptotic ICE-like proteases enhances HIV replication. Nat Med. 1997;3:333–337. doi: 10.1038/nm0397-333. [DOI] [PubMed] [Google Scholar]
- 96.Barretina J, Blanco J, Bonjoch A, Llano A, Clotet B, Este JA. Immunological and virological study of enfuvirtide-treated HIV-positive patients. AIDS. 2004;18:1673–1682. doi: 10.1097/01.aids.0000131350.22032.b5. [DOI] [PubMed] [Google Scholar]
- 97.Aquaro S, D’Arrigo R, Svicher V, Perri GD, Caputo SL, Visco-Comandini U, Santoro M, Bertoli A, Mazzotta F, Bonora S, Tozzi V, Bellagamba R, Zaccarelli M, Narciso P, Antinori A, Perno CF. Specific mutations in HIV-1 gp41 are associated with immunological success in HIV-1-infected patients receiving enfuvirtide treatment. J Antimicrob Chemother. 2006;58:714–722. doi: 10.1093/jac/dkl306. [DOI] [PubMed] [Google Scholar]
- 98.Reeves JD, Lee FH, Miamidian JL, Jabara CB, Juntilla MM, Doms RW. Enfuvirtide resistance mutations: impact on human immunodeficiency virus envelope function, entry inhibitor sensitivity, and virus neutralization. J Virol. 2005;79:4991–4999. doi: 10.1128/JVI.79.8.4991-4999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Melby TE, Despirito M, Demasi RA, Heilek G, Thommes JA, Greenberg ML, Graham N. Association between specific enfuvirtide resistance mutations and CD4 cell response during enfuvirtide-based therapy. AIDS. 2007;21:2537–2539. doi: 10.1097/QAD.0b013e3282f12362. [DOI] [PubMed] [Google Scholar]
- 100.Svicher V, Aquaro S, D’Arrigo R, Artese A, Dimonte S, Alcaro S, Santoro MM, Di PG, Caputo SL, Bellagamba R, Zaccarelli M, Visco-Comandini U, Antinori A, Narciso P, Ceccherini-Silberstein F, Perno CF. Specific Enfuvirtide-Associated Mutational Pathways In HIV-1 Gp41 Are Significantly Correlated With an Increase in CD4(+) Cell Count, Despite Virological Failure. J Infect Dis. 2008 doi: 10.1086/587693. [DOI] [PubMed] [Google Scholar]
- 101.Jekle A, Keppler OT, De CE, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846–5854. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olivieri K, Scoggins RM, Bor YC, Matthews A, Mark D, Taylor JR, Jr, Chernauskas D, Hammarskjold ML, Rekosh D, Camerini D. The envelope gene is a cytopathic determinant of CCR5 tropic HIV-1. Virology. 2007;358:23–38. doi: 10.1016/j.virol.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 103.Blanco J, Barretina J, Clotet B, Este JA. R5 HIV gp120-mediated cellular contacts induce the death of single CCR5-expressing CD4 T cells by a gp41-dependent mechanism. J Leukoc Biol. 2004;76:804–811. doi: 10.1189/jlb.0204100. [DOI] [PubMed] [Google Scholar]
- 104.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 105.Marmor M, Sheppard HW, Donnell D, Bozeman S, Celum C, Buchbinder S, Koblin B, Seage GR., III Homozygous and heterozygous CCR5-Delta32 genotypes are associated with resistance to HIV infection. J Acquir Immune Defic Syndr. 2001;27:472–481. doi: 10.1097/00126334-200108150-00009. [DOI] [PubMed] [Google Scholar]
- 106.Katzenstein TL, Eugen-Olsen J, Hofmann B, Benfield T, Pedersen C, Iversen AK, Sorensen AM, Garred P, Koppelhus U, Svejgaard A, Gerstoft J. HIV-infected individuals with the CCR delta32/CCR5 genotype have lower HIV RNA levels and higher CD4 cell counts in the early years of the infection than do patients with the wild type. Copenhagen AIDS Cohort Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:10–14. doi: 10.1097/00042560-199709010-00002. [DOI] [PubMed] [Google Scholar]
- 107.Mulherin SA, O’Brien TR, Ioannidis JP, Goedert JJ, Buchbinder SP, Coutinho RA, Jamieson BD, Meyer L, Michael NL, Pantaleo G, Rizzardi GP, Schuitemaker H, Sheppard HW, Theodorou ID, Vlahov D, Rosenberg PS. Effects of CCR5-Delta32 and CCR2-64I alleles on HIV-1 disease progression: the protection varies with duration of infection. AIDS. 2003;17:377–387. doi: 10.1097/01.aids.0000050783.28043.3e. [DOI] [PubMed] [Google Scholar]
- 108.Scoggins RM, Taylor JR, Jr, Patrie J, van’t Wout AB, Schuitemaker H, Camerini D. Pathogenesis of primary R5 human immunodeficiency virus type 1 clones in SCID-hu mice. J Virol. 2000;74:3205–3216. doi: 10.1128/jvi.74.7.3205-3216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McDermott DH, Zimmerman PA, Guignard F, Kleeberger CA, Leitman SF, Murphy PM. CCR5 promoter polymorphism and HIV-1 disease progression. Multicenter AIDS Cohort Study (MACS) Lancet. 1998;352:866–870. doi: 10.1016/s0140-6736(98)04158-0. [DOI] [PubMed] [Google Scholar]
- 110.Tang J, Rivers C, Karita E, Costello C, Allen S, Fultz PN, Schoenbaum EE, Kaslow RA. Allelic variants of human beta-chemokine receptor 5 (CCR5) promoter: evolutionary relationships and predictable associations with HIV-1 disease progression. Genes Immun. 1999;1:20–27. doi: 10.1038/sj.gene.6363640. [DOI] [PubMed] [Google Scholar]
- 111.An P, Martin MP, Nelson GW, Carrington M, Smith MW, Gong K, Vlahov D, O’Brien SJ, Winkler CA. Influence of CCR5 promoter haplotypes on AIDS progression in African-Americans. AIDS. 2000;14:2117–2122. doi: 10.1097/00002030-200009290-00007. [DOI] [PubMed] [Google Scholar]
- 112.Ometto L, Bertorelle R, Mainardi M, Zanchetta M, Tognazzo S, Rampon O, Ruga E, Chieco-Bianchi L, De RA. Polymorphisms in the CCR5 promoter region influence disease progression in perinatally human immunodeficiency virus type 1-infected children. J Infect Dis. 2001;183:814–818. doi: 10.1086/318828. [DOI] [PubMed] [Google Scholar]
- 113.Shieh B, Liau YE, Hsieh PS, Yan YP, Wang ST, Li C. Influence of nucleotide polymorphisms in the CCR2 gene and the CCR5 promoter on the expression of cell surface CCR5 and CXCR4. Int Immunol. 2000;12:1311–1318. doi: 10.1093/intimm/12.9.1311. [DOI] [PubMed] [Google Scholar]
- 114.Platt EJ, Durnin JP, Kabat D. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J Virol. 2005;79:4347–4356. doi: 10.1128/JVI.79.7.4347-4356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- 116.Grossman Z, Meier-Schellersheim M, Paul WE, Picker LJ. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat Med. 2006;12:289–295. doi: 10.1038/nm1380. [DOI] [PubMed] [Google Scholar]
- 117.Al-Harthi L, MaWhinney S, Connick E, Schooley RT, Forster JE, Benson C, Thompson M, Judson F, Palella F, Landay A. Immunophenotypic alterations in acute and early HIV infection. Clin Immunol. 2007;125:299–308. doi: 10.1016/j.clim.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Biancotto A, Grivel JC, Iglehart SJ, Vanpouille C, Lisco A, Sieg SF, Debernardo R, Garate K, Rodriguez B, Margolis LB, Lederman MM. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gougeon ML. T cell apoptosis as a consequence of chronic activation of the immune system in HIV infection. Adv Exp Med Biol. 1995;374:121–127. doi: 10.1007/978-1-4615-1995-9_11. [DOI] [PubMed] [Google Scholar]
- 120.Leng Q, Borkow G, Weisman Z, Stein M, Kalinkovich A, Bentwich Z. Immune activation correlates better than HIV plasma viral load with CD4 T-cell decline during HIV infection. J Acquir Immune Defic Syndr. 2001;27:389–397. doi: 10.1097/00126334-200108010-00010. [DOI] [PubMed] [Google Scholar]
- 121.Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RM. CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol. 2002;169:3400–3406. doi: 10.4049/jimmunol.169.6.3400. [DOI] [PubMed] [Google Scholar]
- 122.Resino S, Seoane E, Gutierrez MD, Leon JA, Munoz-Fernandez MA. CD4(+) T-cell immunodeficiency is more dependent on immune activation than viral load in HIV-infected children on highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:269–276. doi: 10.1097/01.qai.0000222287.90201.d7. [DOI] [PubMed] [Google Scholar]
- 123.Koning FA, Otto SA, Hazenberg MD, Dekker L, Prins M, Miedema F, Schuitemaker H. Low-level CD4+ T cell activation is associated with low susceptibility to HIV-1 infection. J Immunol. 2005;175:6117–6122. doi: 10.4049/jimmunol.175.9.6117. [DOI] [PubMed] [Google Scholar]
- 124.Biancotto A, Iglehart SJ, Vanpouille C, Condack CE, Lisco A, Ruecker E, Hirsch I, Margolis LB, Grivel JC. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rawson PM, Molette C, Videtta M, Altieri L, Franceschini D, Donato T, Finocchi L, Propato A, Paroli M, Meloni F, Mastroianni CM, d’Ettorre G, Sidney J, Sette A, Barnaba V. Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nat Med. 2007;13:1431–1439. doi: 10.1038/nm1679. [DOI] [PubMed] [Google Scholar]