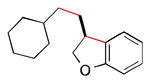
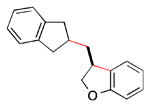
Abstract
As part of our ongoing effort to expand the scope of cross-coupling reactions of alkyl electrophiles, we have pursued a strategy wherein the nucleophilic coupling partner includes a pendant olefin; after transmetalation by such a substrate, if β-migratory insertion proceeds faster than direct cross-coupling, an additional carbon–carbon bond and stereocenter can be formed. With the aid of a nickel/diamine catalyst (both components are commercially available), we have established the viability of this approach for the catalytic asymmetric synthesis of 2,3-dihydrobenzofurans and indanes. Furthermore, we have applied this new method to the construction of the dihydrobenzofuran core of fasiglifam, as well as to a cross-coupling with a racemic alkyl electrophile; in the latter process, the chiral catalyst controls two stereocenters, one that is newly generated in a β-migratory insertion and one that begins as a mixture of enantiomers.
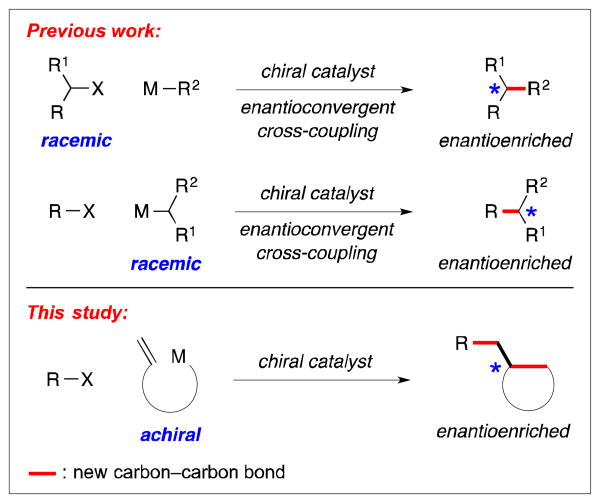
In recent years, significant progress has been reported on the development of methods for the transition metal-catalyzed cross-coupling of alkyl electrophiles to generate carbon–carbon bonds, including enantioselective processes.1 To date, most investigations of asymmetric catalysis have focused on stereoconvergent reactions of racemic secondary electrophiles,2 although an advance has also been described with a racemic secondary nucleophile (top of Figure 1).3
Figure 1.
Asymmetric cross-couplings of alkyl electrophiles.
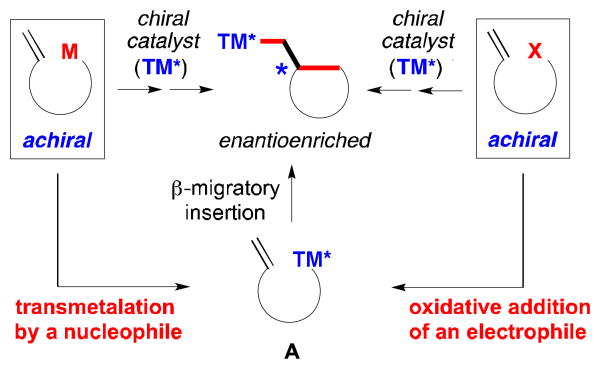
As part of our ongoing effort to expand the scope of enantioselective cross-couplings of alkyl electrophiles, we are pursuing an approach wherein an organometallic reagent that bears a pendant olefin is employed as the nucleophilic coupling partner (bottom of Figure 1).4,5,6 In the presence of a chiral catalyst, transmetalation and then β-migratory insertion (left side of Figure 2), followed by alkyl–alkyl coupling, could lead to the formation of two carbon–carbon bonds and a new stereocenter (bottom of Figure 1). This strategy complements asymmetric coupling processes wherein an intermediate of type A is generated through oxidative addition of an electrophile (right side of Figure 2).7
Figure 2.
Complementary approaches to generating a precursor (A) for catalytic enantioselective cyclizations.
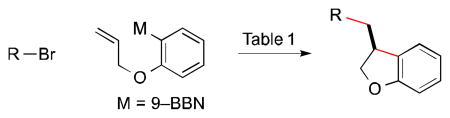
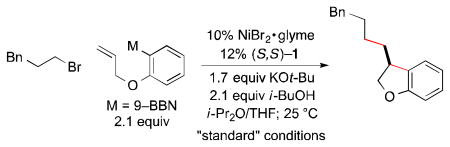
In this report, we establish that a transmetalation–insertion sequence can indeed be used to generate two, rather than one, carbon–carbon bonds in a cross-coupling with an alkyl electrophile and that this process can be achieved with good enantioselectivity. Specifically, we describe couplings of arylboron reagents that bear a pendant olefin with unactivated alkyl halides, thereby furnishing 2,3-dihydrobenzofurans8,9 and indanes10,11 in high ee (eq 1).
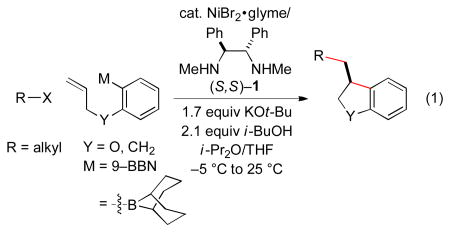
In order to enhance the likelihood of cyclization (β-migratory insertion) prior to coupling with the electrophile, we chose to focus on an organometallic coupling partner that could form a five-membered ring upon insertion, since such cyclizations are often facile. At the outset, it was unclear what catalyst would enable the desired sequence of bond-forming processes, much less achieve high enantioselectivity.
Interestingly, we have determined that a nickel/1,2-diaminebased catalyst, which we have found to be useful for enantioconvergent alkyl–alkyl couplings,3,12 is also effective for the desired cyclization/cross-coupling sequence (Table 1, entry 1). Thus, in the presence of NiBr2•glyme and ligand 1, both of which are commercially available, the target 2,3-dihydrobenzofuran is generated in good ee and yield. Under these conditions, essentially none of the product of direct cross-coupling (without cyclization of the nucleophile) or of endo cyclization is observed (<5%).
Table 1. Catalytic Enantioselective Cyclization/Cross-Coupling with an Alkyl Electrophile: Influence of Reaction Parametersa.
| |||
|---|---|---|---|
| entry | variation from the “standard” conditions | ee (%) | yield (%)b |
| 1 | none | 96 | 82 |
| 2 | no NiBr2 • glyme | – | <5 |
| 3 | no (S,S)–1 | – | <5 |
| 4 | no i-BuOH | – | <5 |
| 5 | 1.5 equiv of arylboron reagent | 81 | 67 |
| 6 | (S,S)–2, instead of (S,S)–1 | 39 | 64 |
| 7 | (S,S)–3, instead of (S,S)–1 | – | <5 |
| 8 | (S,S)–4, instead of (S,S)–1 | 61 | 33 |
| 9 | BnCH2CH2Cl, instead of BnCH2CH2Br | – | <5 |
|
| |||
All data are the average of two experiments.
The yield was determined by GC analysis with the aid of a calibrated internal standard.
In the absence of NiBr2•glyme, ligand 1, or i-BuOH the desired cyclization/cross-coupling product did not form in appreciable yield (Table 1, entries 2–4),13 and the use of a smaller excess of the arylboron reagent led to somewhat lower ee and yield (entry 5).14 Other ligands that we have found to be useful for enantioconvergent couplings of alkyl electrophiles were not effective for this new asymmetric cross-coupling with an alkyl halide (entries 6–8).15 If the alkyl bromide was replaced with the corresponding alkyl chloride, essentially no 2,3-dihydrobenzofuran was observed (entry 9).16
We next examined the scope of this method for asymmetric cyclization/cross-coupling with alkyl bromides (Table 2).17 A range of functionalized electrophiles serve as suitable reaction partners, furnishing the desired 2,3-dihydrobenzofuran in very good enantiomeric excess. A silane, an acetal, and an imide are compatible with the reaction conditions. The method is not limited to unhindered primary alkyl bromides–a β-branched primary and a secondary bromide also undergo cyclization/cross-coupling (entries 6 and 7).
Table 2. Catalytic Enantioselective Cyclization/Cross-Coupling with Alkyl Electrophilesa.
All data are the average of two experiments.
Yield of purified product.
15% NiBr2•glyme and 17% ligand 1 were used.
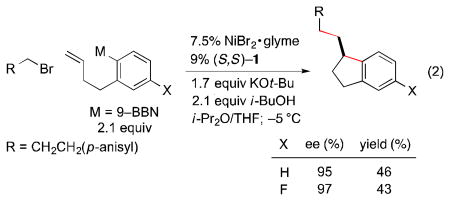
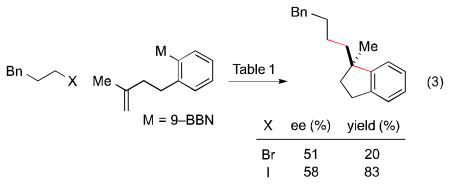
Under similar conditions, indane derivatives can also be produced in high ee, although modest yield (eq 2).17c An attempt to generate a quaternary stereocenter furnished a promising initial result (eq 3).
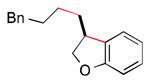
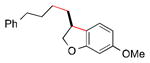
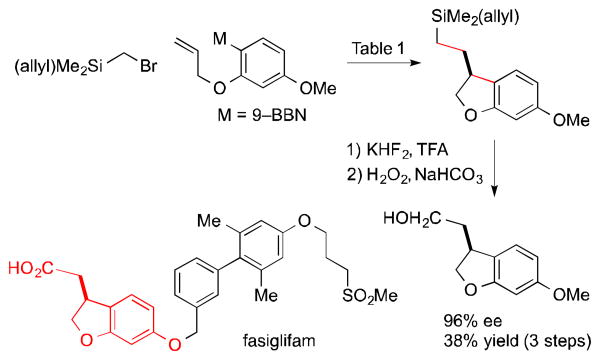
A number of optically active 2,3-dihydrobenzofurans exhibit interesting biological activity,8,9 including fasiglifam (Takeda Pharmaceuticals: TAK–875), which progressed to Phase 3 clinical trials for type 2 diabetes until being withdrawn due to concerns about liver safety.18 We have applied our method to a catalytic asymmetric synthesis of the dihydrobenzofuran core of fasiglifam (Scheme 1).
Scheme 1. Catalytic Asymmetric Synthesis of the 2,3-Dihydrobenzofuran Core of Fasiglifam.
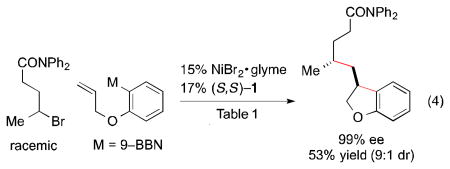
In view of the similarity of the optimized conditions for this new asymmetric cyclization/cross-coupling process to those for our stereoconvergent cross-coupling of racemic γ-haloamides,12d we investigated the possibility that a single chiral catalyst could accomplish two distinct enantioselective transformations: create a new stereocenter through the cyclization of an achiral nucleophile, as well as control the absolute stereochemistry of a second stereocenter through an enantioconvergent coupling of a racemic electrophile. As illustrated in eq 4, this objective can indeed be achieved (minor diastereomer: 86% ee).
In summary, we have expanded the scope of cross-coupling reactions of alkyl electrophiles by incorporating an olefin in the nucleophilic partner, which leads to the formation of an additional carbon–carbon bond and stereocenter, when compared with a simple cross-coupling. With the aid of a nickel/diamine catalyst (both components are commercially available), we have established that this strategy enables the synthesis of highly enantioenriched 2,3-dihydrobenzofurans and indanes through couplings with a range of alkyl halides. We have applied this new method to the generation of the dihydrobenzofuran core of fasiglifam, as well as to a transformation wherein the chiral catalyst controls the stereochemistry of two rather different processes: a β-migratory insertion and an enantioconvergent coupling of a racemic alkyl halide. Ongoing studies are directed at further enlarging the scope of cross-coupling reactions of alkyl electrophiles, as well as elucidating the mechanisms of these transformations.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01–GM62871). We thank Yufan Liang, Dr. Allen G. Oliver (University of Notre Dame), Dr. Nathan D. Schley, and Dr. Scott C. Virgil (Caltech Center for Catalysis and Chemical Synthesis, supported by the Gordon and Betty Moore Foundation) for assistance.
Footnotes
Notes: The authors declare no competing financial interest.
Supporting Information: Experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For reviews and leading references, see: Glasspoole BW, Crudden CM. Nature Chem. 2011;3:912–913. doi: 10.1038/nchem.1210.. Rudolph A, Lautens M. Angew Chem Int Ed. 2009;48:2656–2670. doi: 10.1002/anie.200803611.. Glorius F. Angew Chem Int Ed. 2008;47:8347–8349. doi: 10.1002/anie.200803509.. Swift EC, Jarvo ER. Tetrahedron. 2013;69:5799–5817. doi: 10.1016/j.tet.2013.05.001.
- 2.For a few examples and leading references, see: Do HQ, Chandrashekar ERR, Fu GC. J Am Chem Soc. 2013;135:16288–16291. doi: 10.1021/ja408561b.. Schmidt T, Kirschning A. Angew Chem Int Ed. 2012;51:1063–1066. doi: 10.1002/anie.201106762.. Tsuji T, Yorimitsu H, Oshima K. Angew Chem Int Ed. 2002;41:4137–4139. doi: 10.1002/1521-3773(20021104)41:21<4137::AID-ANIE4137>3.0.CO;2-0.. Shields JD, Ahneman DT, Graham TJA, Doyle AG. Org Lett. 2014;16:142–145. doi: 10.1021/ol4031364.. Caeiro J, Sestelo JP, Sarandeses LA. Chem Eur J. 2008;14:741–746. doi: 10.1002/chem.200701035.
- 3.Cordier CJ, Lundgren RJ, Fu GC. J Am Chem Soc. 2013;135:10946–10949. doi: 10.1021/ja4054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.For reviews on asymmetric carbometalation, see: Ojima I, Kaloko JJ, Chaterpaul SJ, Teng YHG, Lin CF. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley; Hoboken: 2010. pp. 643–681.. Negishi Ei, Tan Z. Top Organomet Chem. 2005;8:139–176.. Hoveyda AH, Heron NM. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Springer–Verlag; Berlin: 1999. pp. 431–454.
- 5.McDonald RI, Liu G, Stahl SS. Chem Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For recent examples of related catalytic asymmetric couplings wherein the nucleophilic site is a nitrogen, rather than a carbon, and the electrophile is not an alkyl halide, see: Mai DN, Wolfe JP. J Am Chem Soc. 2010;132:12157–12159. doi: 10.1021/ja106989h.. Ingalls EL, Sibbald PA, Kaminsky W, Michael FE. J Am Chem Soc. 2013;135:8854–8856. doi: 10.1021/ja4043406.
- 7.For example, asymmetric Heck reactions: McCartney D, Guiry PJ. Chem Soc Rev. 2011;40:5122–5150. doi: 10.1039/c1cs15101k.. Dounay AB, Overman LE. In: Mizoroki–Heck Reaction. Oestreich M, editor. Wiley; Chichester, UK: 2009. pp. 533–568.. Shibasaki M, Vogl EM, Ohshima T. Adv Synth Catal. 2004;346:1533–1552.
- 8.For reviews of methods for the synthesis of 2,3-dihydrobenzofurans, as well as descriptions of their significance, see: Sheppard TD. J Chem Res. 2011;35:377–385.. Bertolini F, Pineschi M. Org Prep Proced Int. 2009;41:385–418.. Lachia M, Moody CJ. Nat Prod Rep. 2008;25:227–253. doi: 10.1039/b705663j.
- 9.Codeine is an example of a bioactive compound that includes a 2,3-dihydrobenzofuran.
- 10.For a review of methods for the synthesis of indanes, see: Hong Bc, Sarshar S. Org Prep Proced Int. 1999;31:1–86.
- 11.Rasagiline, which is used for the treatment of Parkinson's disease, is an example of a simple bioactive compound that includes an indane: Hoy SM, Keating GM. Drugs. 2012;72:643–669. doi: 10.2165/11207560-000000000-00000.
- 12.(a) Saito B, Fu GC. J Am Chem Soc. 2008;130:6694–6695. doi: 10.1021/ja8013677. [DOI] [PubMed] [Google Scholar]; (b) Owston NA, Fu GC. J Am Chem Soc. 2010;132:11908–11909. doi: 10.1021/ja105924f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lu Z, Wilsily A, Fu GC. J Am Chem Soc. 2011;133:8154–8157. doi: 10.1021/ja203560q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zultanski SL, Fu GC. J Am Chem Soc. 2011;133:15362–15364. doi: 10.1021/ja2079515. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wilsily A, Tramutola F, Owston NA, Fu GC. J Am Chem Soc. 2012;134:5794–5797. doi: 10.1021/ja301612y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The failure to observe a significant amount of the cross-coupling product in the absence of i-BuOH (entry 4 of Table 1) could be due to less effective transmetalation in the absence of a less bulky alkoxide.
- 14.Some of the nucleophile is consumed in the reduction of the Ni(II) pre-catalyst to the active catalyst. A small amount also undergoes protodeborylation under the reaction conditions.
- 15.For example, see: Pybox ligand. Fischer C, Fu GC. J Am Chem Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509.. Bis(oxazoline) ligand. Lou S, Fu GC. J Am Chem Soc. 2010;132:1264–1266. doi: 10.1021/ja909689t.
- 16.The alkyl chloride is largely intact at the end of the reaction (>95%).
- 17.Notes: Under our standard conditions (Table 1): (a) The coupling illustrated in Table 2, entry 1 proceeded in 96% ee and 67% yield on a gram-scale (1.07 g of product). (b) An initial attempt to form a six-membered ring through cyclization/cross-coupling of a homologated arylboron reagent was not successful. (c) In general, the primary undesired side reactions are reduction (hydrodehalogenation) and electrophile homocoupling. (d) PhBr is not a suitable electrophile. (e) An indoline can be generated with promising enantioselectivity and yield (54% ee, 40% yield).
- 18.For a discussion and leading references, see: Takeda web page. [accessed January 18, 2014]; http://www.takeda.com/news/2013/20131227_6117.html.. Negoro N, Sasaki S, Mikami S, Ito M, Suzuki M, Tsujihata Y, Ito R, Harada A, Takeuchi K, Suzuki N, Miyazaki J, Santou T, Odani T, Kanzaki N, Funami M, Tanaka T, Kogame A, Matsunaga S, Yasuma T, Momose Y. ACS Med Chem Lett. 2010;1:290–294. doi: 10.1021/ml1000855.. Kaku K. Expert Opinion on Pharmacotherapy. 2013;14:2591–2600. doi: 10.1517/14656566.2013.851668.. de Lartigue J. Drugs of the Future. 2011;36:813–818.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.