Abstract
Lack of an appropriate small animal model remains a major hurdle for studying the immunotolerance and immunopathogenesis induced by hepatitis B virus (HBV) infection. In this study, we report a mouse model with sustained HBV viremia after infection with a recombinant adeno-associated virus (AAV) carrying a replicable HBV genome (AAV/HBV). Similar to the clinical HBV carriers, the mice infected with AAV/HBV were sero-negative for antibodies against HBV surface antigen (HBsAg). Immunization with the conventional HBV vaccine in the presence of aluminum adjuvant failed to elicit an immune response against HBV in these mice. To identify a vaccine that can potentially circumvent this tolerance, the TLR9 agonist CpG was added to HBsAg as an adjuvant. Vaccination of mice with HBsAg/CpG induced not only clearance of viremia, but also strong antibody production and T-cell responses. Furthermore, both the DNA replication and protein expression of HBV were significantly reduced in the livers of AAV/HBV-infected mice. Accordingly, AAV/HBV-infected mice may be used as a robust model for investigating the underlying mechanism(s) of HBV immunotolerance and for developing novel immunotherapies to eradicate HBV infections.
Keywords: AAV vector, HBV, immunotherapy, immunotolerance, mouse model
INTRODUCTION
Over 350 million people worldwide are affected by chronic hepatitis B (CHB), which remains the leading cause of liver cancer in developing countries.1,2 Current hepatitis B virus (HBV) treatments, including antiviral nucleos(t)ides and alpha interferons, are administered to suppress viral replication but cannot induce a protective immune response or viral clearance.2,3 Interruptions of these treatments would result in unavoidable viral rebound. Moreover, long-term treatment may be hampered by escape mutants, drug resistance, side effects and heavy economic burdens. Novel treatments, including therapeutic vaccines, are urgently needed to effectively control the HBV epidemic and eventually eradicate chronic HBV infection. However, the lack of a robust animal model that closely mimics chronic HBV infection in patients2,4 has hindered the development of novel treatments for CHB.
Transgenic mice containing the HBV genome either persistently express distinct HBV antigens or produce infectious virions.5-7 Although such mouse models have been used to test numerous drugs for HBV infection, the central tolerance induced by the transgenic gene products, starting from as early as the embryonic stage, makes the study of therapeutic HBV vaccines in this model system difficult. Furthermore, these mice are not suitable for monitoring viral clearance because the integrated HBV genome persists in every mouse cell.5,7,8
Alternative models were developed by hydrodynamic injection (HDI) of the HBV genome into the tail vein.9 A later study showed that HDI of the HBV genome, which was cloned into an adeno-associated virus (AAV) vector, would lead to a stable HBV replication and long-lasting viremia due to AAV-mediated immune suppression.10 Unfortunately, neonatal and young mouse models of CHB could not be successfully established using this procedure. In addition, high levels of HBV, which are commonly detected in patients with CHB, could not be produced in this model due to the induction of immune clearance. This phenomenon has also been observed in another mouse model infected by an adenovirus vector harboring the HBV genome.11
It has been reported that mice infected with a recombinant AAV carrying the HBV genome (AAV/HBV) exhibited continuous viremia for more than 30 weeks.12-14 In the present study, we further characterized the anti-HBV immune response in AAV/HBV-infected mice and demonstrated that these mice can be used as an appropriate model for examining the mechanisms of HBV tolerance and developing novel therapies for CHB.
MATERIALS AND METHODS
Mice and virus
C57BL/6 mice were purchased from Vital River Laboratories (Beijing, China). Six- to eight-week-old male mice were used in all experiments unless otherwise specified. All mice were housed under controlled temperature and light conditions following the Institutional Animal Care guidelines. AAV/HBV virus was provided by Beijing FivePlus Molecular Medicine Institute (Beijing, China). This recombinant virus carries 1.3 copies of the HBV genome (genotype D, serotype ayw) and is packaged in AAV serotype 8 (AAV8) capsids.
AAV/HBV infection
Adult C57BL/6 mice were injected with the indicated amounts of recombinant virus (diluted to 200 μl with phosphate-buffered saline) through tail vein injection. Neonatal mice (3 days after birth) were infected with AAV/HBV through intraliver injection. The mice were bled retro-orbitally at the indicated time points to monitor HBV surface antigen (HBsAg), HBV e antigen (HBeAg), HBs antibody (HBsAb) and HBV genomic DNA in serum.
HBsAg vaccination
HBsAg with an aluminum-containing adjuvant (EngerixB; GlaxoSmithKline Biological, Middlesex, UK) was used as a control vaccine in some experiments. CpG (1826) was synthesized by Life Technologies Corporation (Carlsbad, USA) (TCCATGACGTTCCTGACGTT) and mixed with 2 μg of HBsAg. All vaccines were injected subcutaneously.
HDI
HDI of the plasmid pBlue-HBV1.3 was performed according to previous reports.9,10 In brief, various doses of endo-free plasmid DNA (extracted using a Qiagen kit) were diluted in a volume of saline equivalent to 8% of the body weight of the mouse. The total injection volume was administered within 5–8 s.
Serological and biochemical analysis
Serum was harvested by retro-orbital bleeding. Serum alanine aminotransferase activity (ALT kit; BioSino Bio-technology and Science Inc, Beijing, China) was determined using a SpectraMax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) following the manufacturer’s instructions. HBV antigens and antibodies were monitored by enzyme-linked immunosorbent assay (ELISA).
ELISA assay
Serum HBsAg was measured by ELISA (Shanghai Kehua Bioengineering Co., Ltd, Shanghai, China) according to the manufacturer’s instructions. The lower limit of detection for HBsAg was 0.5 ng/ml. Serum dilutions of 2.5- to 100-fold were used to obtain values within the linear range of the standard curve. HBsAb was analyzed on a precoated HBsAg plate and developed by HRP-labeled anti-mouse IgG (Zhong ShanGolden Bridge Biological Technology Co., Ltd, Beijing, China). Regarding the liver specimens, 50 mg of tissue was homogenized in RIPA lysis buffer and centrifuged at 12 000 r.p.m. for 5 min. The supernatant was collected for HBsAg ELISA according to the manufacturer’s instructions.
Real-time PCR
Serum HBV DNA was extracted from 100 μl of serum and measured following the manufacturer’s instructions (Qiagen, Hilden, Germany). Liver HBV DNA was extracted from 50 mg of liver tissues using a genomic DNA kit (TIANGEN Biotech, Beijing, China). The DNA samples were analyzed by real-time quantitative PCR (qPCR) using HBV-specific primers (5′-CACATCAGGATTCCTAGGACC-3′; 5′-GGTGAGTGATTGGAGGTTG-3′).
Mouse GAPDH was used as the control for quantification (forward 5′-GGTGAAGGTCGGTGTGAACG-3′; reverse 5′CTCGCTCCTGGAAGATGGTG-3′).
Immunohistochemistry
Liver tissues were fixed with 10% neutral buffered formalin and embedded in paraffin. After deparaffinization, the sections were incubated with primary antibodies (polyclonal rabbit anti-HBcAg; DAKO, Glostrup, Denmark) and the DAKO Envision System. The sections were also counter stained with hematoxylin.
Synthetic peptides and ELISPOT assay
ENV190 (VWLSVIWM) peptides and OVA257 (SIINFEKL) peptides were synthesized by Invitrogen. All peptides were disusolved in dimethyl sulfoxide (DMSO) before use. The organs were harvested at the indicated time points after immunization with HBs plus CpG1826 vaccines. An enzymatic digestion method was utilized for the isolation of intrahepatic lymphocytes. In brief, liver tissues were digested by collagenase IV (Roche, Basel, Switzerland) at 37 °C for 15 min. The suspension was centrifuged at 30g for 1 min to remove hepatocytes. Lymphocytes were then pelleted by centrifugation at 400g for 10 min and further purified with 40% and 70% Percoll solutions by centrifuging at 800g for 20 min at room temperature. Cells were collected from the interface, and red blood cells were removed with ACK buffer to make a single-cell suspension. For detecting an antigen-specific immune response, liver lymphocytes (1.5×105) were incubated for 48 h at 37 °C in complete medium containing 10 μg/ml ENV190 peptide or OVA257 peptide in an IFNγ ELISPOT plate (Merck Millipore, Billerica, Massachusetts, USA). After incubation, IFN-γ secretion was analyzed using a biotinylated anti-IFN-γ antibody and streptavidin-HRP (BD Biosciences, Franklin Lakes, New Jersey, USA). Finally, spots were visualized with AEC substrate and quantified with the auto-analyzing system.
Statistical analysis
Statistical analyses were performed using a two-tailed unpaired t-test and Prism software (GraphPad Software, San Diego, California, USA). P<0.05 was considered statistically significant.
RESULTS
AAV/HBV induces sustained viremia in neonatal and adult mice
To determine whether injection of mice using the AAV/HBV system could establish a persistent HBV infection with viremia in immunocompetent mice, 5×1010 viral genome equivalents (vg) of recombinant virus was intravenously injected into adult C57BL/6 mice. All the mice (n=12) became serum HBsAg-positive by 14 days post-infection (Figure 1a). Importantly, we observed that male hosts had higher HBsAg than female hosts. This phenomenon is in accordance with clinical observations.15,16 We also found that neonatal mice became HBsAg-positive after intrahepatic injection of 1×1010 or 2×1010 vg AAV/HBV (n=30, Figure 1b). This is the first mouse model of persistent HBV viremia in an immunocompetent host after neonatal infection.
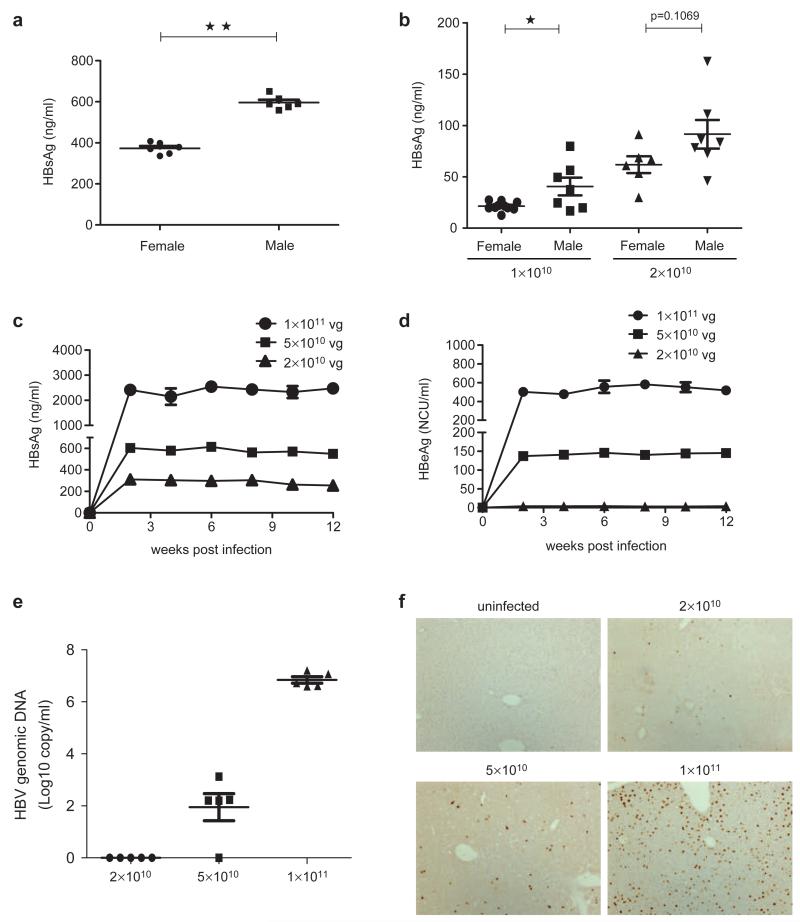
Figure 1.
AAV/HBV inoculation resulted in persistent HBV viremia in immunocompetent mice. (a) Female or male C57BL/6 mice (n=6, 6–8 weeks old) were infected with AAV/HBV at 5×1010 viral genome equivalents (vg) through tail vein injection. At 14 days post-infection, blood samples were collected and serum HBsAg was measured by ELISA. (b) Intraliver injection of 3-day-old neonatal C57BL/6 mice (n=6–7) was performed with 1×1010 or 2×1010 vg AAV/HBV, and serum HBsAg titers were examined at 48 days post-infection. (c) C57BL/6 mice (n=6, 6–8 weeks old) were infected with AAV/HBV at 2×1010, 5×1010 or 1×1011 vg. The mice were bled biweekly after infection. Serum HBsAg was measured by ELISA. The lower limit of detection was 0.5 ng/ml. (d) Adult C57BL/6 mice were intravenously injected with 2×1010, 5×1010 or 1×1011 vg virus and were bled biweekly to monitor serum HBeAg titers. (e) On day 40 post-AAV/HBV infection, HBV genomic DNA in the serum was determined by real-time PCR. (f) Immunohistochemical staining of HBcAg in the liver of AAV/HBV infected mice (12 weeks post-infection). AAV, adeno-associated virus; HBcAg, HBV core antigen; HBsAg, HBV surface antigen; HBV, hepatitis B virus.
To test the dose-dependent response of the AAV/HBV infection, we delivered three doses of AAV/HBV virus ranging from 231010 to 131011 vg per mouse and monitored HBV viremia for an extended period of 3 months. We found that serum HBsAg levels ranged from 319612 ng/ml to 24146171 ng/ml (Figure 1c), correlating with the inoculation dosages. In addition, nearly 100% (263/265) of the injected mice became HBV-positive during the 3-month time period (data not shown). The viremia lasted for more than 1 year in high-dose AAV/HBV-infected mice (Supplementary Figure 1a and b).
Additionally, HBeAg was also detected in the blood of infected mice (Figure 1d), indicating active HBV replication in this CHB model.17 HBV genomic DNA in the sera of infected mice also exhibited a dose-dependent correlation. The HBV DNA levels, ranging from 6.61×106 to 7.20×107 copies/ml in the mice infected with 1×1011 vg AAV/HBV (Figure 1e), are comparable to those of CHB patients in the immunotolerant stage.18-20 Furthermore, the immunohistochemical staining for HBV core antigen (HBcAg) in liver cells from infected mice revealed that the frequency of HBcAg-positive cells was proportional to infection dose of AAV/HBV (Figure 1f). HBV core proteins were not detected in any other organs (heart, kidney or lung) by immunohistochemical staining (data not shown). In addition, viral transcripts and replicative intermediates were detected in the mouse liver, but not in the heart, kidney or lung. This experimental evidence confirmed that chronic HBV replication was restricted to the liver (data not shown).
Immune response against HBV in AAV/HBV-infected mice
Given the lasting expression of HBV proteins, we then examined whether this chronicity could provoke host immune responses against viral antigens. We used an HBV plasmid hydrodynamic injection model as the positive control for an anti-HBV response.9 The pBlue-HBV1.3 plasmid contains an HBV sequence identical to that in AAV/HBV. These control mice developed high-titer anti-HBs antibody (HBsAb) (Figure 2a). However, HBsAb could not be detected in the mice receiving AAV/HBV. This suggests that AAV/HBV infection is more physiologically accurate than plasmid hydrodynamic injection for modeling persistent HBV infection and immunotolerance with a wider range of viremia.
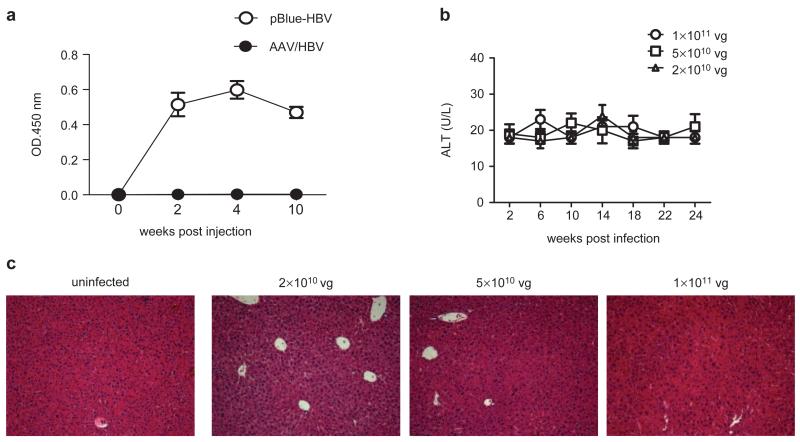
Figure 2.
The immune response was undetectable in AAV/HBV-infected mice. (a) Serum HBsAb levels in C57BL/6 mice receiving 15 μg of pBlue-HBV1.3 DNA via hydrodynamic injection or infected with 1×1011 vg of AAV/HBV (n=3). (b) ALT levels of HBV carrier mice were measured over time (n=3). (c) H&E staining of liver sections from mice infected with different doses of AAV/HBV. One of two independent experiments is presented. AAV, adeno-associated virus; ALT, alanine aminotransferase; HBsAg, HBV surface antigen; HBV, hepatitis B virus; H&E, hematoxylin and eosin.
To address whether AAV/HBV chronic infection causes liver damage, serum samples were collected from mice infected with different doses of AAV/HBV and measured for alanine aminotransferase (ALT) activity, a key indicator of hepatic injury. No significant increase in ALT was observed in infected adult mice over a 6-month period (Figure 2b and Figure 1c). No obvious infiltrating lymphocytes or other abnormalities were observed in the infected liver tissue by hematoxylin and eosin (H&E) staining (Figure 2c). These results indicate that AAV/HBV infection did not induce detectable liver injury.
Induction of HBV immune tolerance in AAV/HBV-infected mice
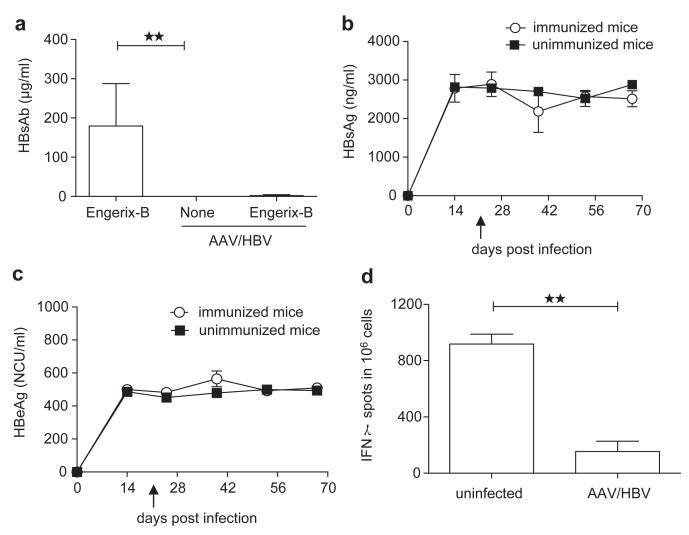
In CHB patients, seroconversion to HBsAb is considered to be a marker of disease resolution. However, the induction of HBsAb is a challenging clinical goal to achieve due to HBV-induced tolerance. We tested whether mice infected with AAV/HBV virus could resist the challenge of a conventional HBsAg vaccine, as observed in HBV infections. We used a clinical HBV vaccine with aluminum adjuvant (Engerix-B). Whereas naive mice developed considerable antibodies against HBsAg at day 28 post-vaccination, AAV/HBV-infected mice failed to develop anti-HBsAg antibody responses, which indicated immune tolerance toward HBsAg (Figure 3a). By 42 days post-vaccination, none of the AAV/HBV-infected mice that received Engerix-B treatment showed significant reduction of serum HBsAg or HBeAg when compared with the unimmunized mice (Figure 3b and c). To detect the HBsAg-specific T-cell tolerance in this model, we challenged the AAV/HBV (1×1011 vg)-infected mice with HBsAg plus CpG and analyzed HBsAg-specific CD8 T cells in the liver using IFN-γ ELISPOT. AAV/HBV-infected mice generated very low levels of IFN-γ-producing T cells compared with naive mice after vaccination (Figure 3d).
Figure 3.
AAV/HBV infection induces immune tolerance. (a) Adult mice were infected with 1×1011 vg AAV/HBV followed by Engerix-B immunization at 3 weeks post-infection. Serum HBsAb levels were monitored by ELISA at 4 weeks post-vaccination. Uninfected mice immunized with Engerix-B and non-immunized AAV/HBV-infected mice were chosen as controls (n=5). (b) The serum HBsAg titer was monitored by ELISA at different time points pre- and post-Engerix-B immunization (n=3). The arrow indicates vaccine immunization. (c) Following the same immunization protocol, the HBeAg titer was measured by ELISA (n=3). The arrow indicates vaccine immunization. (d) Adult mice infected with AAV/HBV (1×1011 vg) were treated with HBsAg (2 μg/mouse) plus CpG (50 μg per mouse) at 3 weeks post-infection and euthanized on day 10 postvaccination. ENV190-specific CD8 T cells of liver leukocytes were detected by IFN-γ ELISPOT (n=3). AAV, adeno-associated virus; HBeAg, HBV e antigen; HBsAg, HBV surface antigen; HBV, hepatitis B virus.
Therapeutic vaccination with CpG adjuvant in AAV/HBV-infected mice
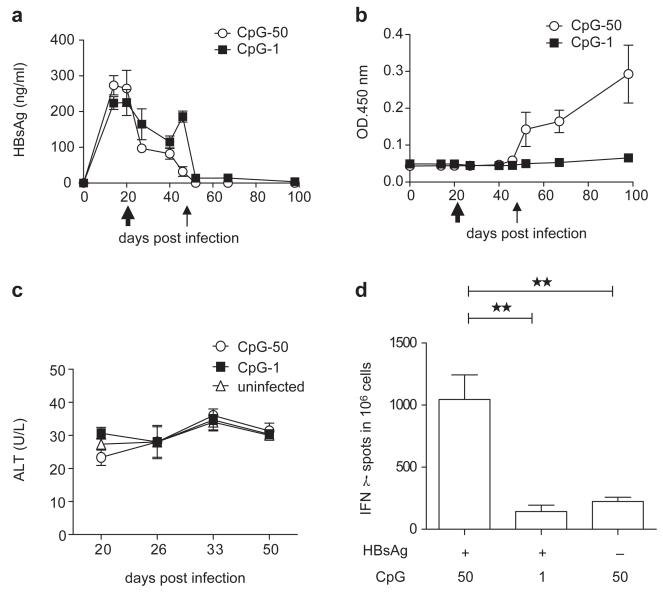
To explore an effective vaccine that is capable of breaking immune tolerance, we reconstituted the conventional HBsAg vaccine with a strong TLR9 agonist, CpG, as an adjuvant. HBV carrier mice that received 2×1010 vg AAV/HBV were vaccinated with an equal amount of HBsAg in the presence of either 1 or 50 μg of CpG1826 and subsequently boosted with Engerix-B. HBsAg levels rapidly declined after vaccination and remained undetectable without rebound (Figure 4a). HBsAb induction is a clinical indication of immune protection. More impressively, mice receiving a vaccine with a high dose of CpG developed protective anti-HBsAbs in a dose-dependent manner, as vaccination with a low dose of CpG as an adjuvant failed to induce protective anti-HBsAbs (Figure 4b). There was no significant upregulation of ALT in either of the vaccinated groups (Figure 4c). Because CD8+ T-cell responses are an important indicator of anti-viral immune responses and immune clearance, we examined whether this CpG-adjuvant vaccine could induce an HBsAg-specific CD81 T-cell response. Indeed, vaccination with HBsAg plus a high dose of CpG (50 μg) provoked a stronger ENV190-specific CD8+ T-cell response in the liver (Figure 4d). These data indicate that an HBsAg vaccine containing a proper dose of the CpG adjuvant may have a potent therapeutic effect in carriers with low-level viremia and could restore the host immune response to HBV.
Figure 4.
AAV/HBV-infected mice respond to HBsAg vaccination with CpG. (a) C57BL/6 mice were infected with AAV/HBV (2×1010 vg) and were subsequently immunized with HBsAg plus CpG (50 or 1 μg per mouse) at 3 weeks post-infection (wpi) followed by a boost with Engerix-B (2 μg/mouse) at 7 wpi. Serum HBsAg levels in the different groups were monitored by ELISA (n=3). (b) HBsAb titers were measured by ELISA. (c) The ALT in immunized mice was monitored at different time points post-vaccination. (d) AAV/HBV (2×1010 vg)-infected mice were treated with HBsAg plus two respective doses of CpG (50 or 1 μg per mouse) or high-dose CpG alone at 3 wpi. ENV190-specific CD8+ T cells in the liver were detected by IFN-γ ELISPOT at 10 days post-vaccination (n=5). AAV, adeno-associated virus; ALT, alanine aminotransferase; HBsAg, HBV surface antigen; HBV, hepatitis B virus.
However, carriers with high-level viremia (mice infected with 1×1011 vg) failed to respond to HBsAg plus CpG vaccination, as the level of HBsAg in the blood and liver tissue was not reduced (Supplementary Figure 2). The means by which a proper antibody response can be induced in mice with such severe viremia remains to be determined.
Vaccination with a CpG adjuvant leads to a reduction of HBV in the liver
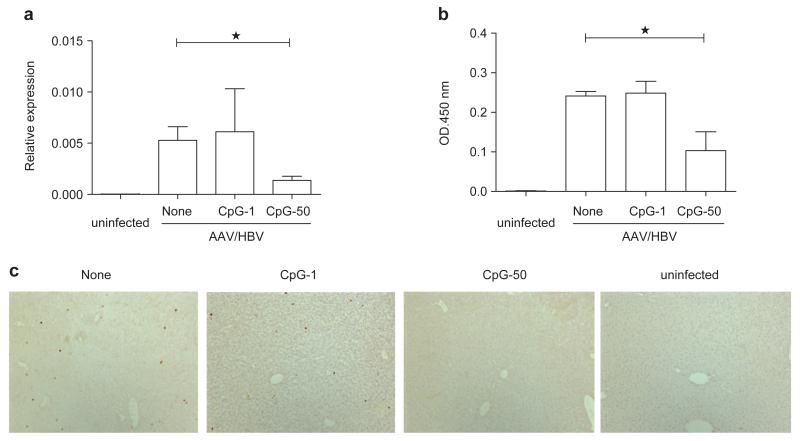
Although HBsAg serological conversion is the currently accepted marker for a cured HBV infection, viral DNA may still be present in the liver, which could result in viral rebound when the immune system is suppressed.21-23 Thus, clearance of HBV DNA in the liver is considered as the ultimate goal of anti-HBV therapy. To evaluate the therapeutic effect of vaccination with CpG adjuvant, we tested the HBV DNA level from liver extracts by real-time PCR at 11 weeks post-vaccination. Interestingly, we found that HBV DNA replication in the liver decreased after vaccination with highdose CpG (50 μg) but not low-dose CpG (1 μg) (Figure 5a). Meanwhile, HBsAg in the liver was significantly reduced in mice that received high-dose CpG compared with those that received low-dose CpG vaccination (Figure 5b). Furthermore, HBcAg, an important marker for HBV replication, was also remarkably decreased in the high-dose CpG vaccination group (Figure 5c).
Figure 5.
Vaccination with CpG adjuvant reduces HBV DNA in the liver. (a) AAV/HBV (2×1010 vg)-infected mice were treated with the indicated vaccine combinations. HBV DNA levels in the liver were determined by real-time PCR at 11 weeks post-vaccination. (b) HBsAg levels in the liver were measured by ELISA at 11 weeks post-vaccination with homogenized liver tissues. (c) Immunohistochemical staining of HBcAg in the liver sections of different groups (n=3). AAV, adeno-associated virus; HBcAg, HBV core antigen; HBsAg, HBV surface antigen; HBV, hepatitis B virus.
DISCUSSION
It was recently reported that AAV/HBV infection in mice established persistent viremia for more than 30 weeks.12,13 Here, we further reveal that male mice exhibit a higher plasma viral load than females. The AAV/HBV-infected mice lacked an anti-HBV immune response regardless of the levels of HBV viremia present. Furthermore, these mice were resistant to immunization with the conventional vaccine with aluminum adjuvant, which is an indication of immune tolerance. These findings are similar to clinical observations in HBV-infected patients and demonstrate that these AAV/HBV-infected mice can be utilized as a robust model to study immunotolerance induced by HBV infection.
With the same AAV/HBV infection model, Dion et al.24 reported that HBV-specific CD8+ T cells could be detected in both peripheral lymphoid and liver tissues upon immunization with a DNA vaccine expressing HBsAg. However, despite the presence of HBV-specific T-cell responses, all infection markers remained unchanged at 2 weeks post-immunization,24 including the levels of plasma HBeAg and HBsAg, intrahepatic expression of HBcAg and HBV DNA replication intermediates. This piece of experimental data is similar to that obtained from the clinical trial of a DNA vaccine,25 which highlights the complexity of HBV-induced dysfunction of the immune system.
Moreover, significant liver inflammation and liver damage in AAV/HBV-infected mice, manifested by increased ALT levels, fibrosis and steatosis, were reported by another group. In addition, 100% of these mice (12 out of 12) developed visible tumor nodules in the liver within 16 weeks.26 We did not observe any tumor formation in 33 HBV-infected mice that had been monitored for more than 1 year, although we used the same HBV strain (a genotype D virus). In our study, one AAV construct containing the entire HBV genome was used, whereas Tao et al.27 used two AAVs each containing one half of the HBV genome. However, whether this difference in AAV constructs contributes to liver cancer formation remains to be determined in the future.
Using our new HBV infection model, we demonstrated the therapeutic potential of vaccines containing CpG as an adjuvant. CpG is an oligodeoxynucleotide containing unmethylated CpG motifs. It serves as a ‘danger signal’ to improve dendritic and B cell functions through Toll-like receptor 9 signaling.28-30 A clinical trial of an HBV vaccine with CpG adjuvant showed significant improvement over a conventional vaccine with aluminum adjuvant.31-34 Immunization with CpG adjuvant promotes Th1 immune cell differentiation, which inhibits the replication of intracellular pathogens.35 The data presented here provide further support that CpG may serve as a potential adjuvant for an HBV therapeutic vaccine.
In summary, we have demonstrated that AAV/HBV-infected mice are a new and clinically relevant model that may be used for delineating the mechanism of HBV-induced immunotolerance and for developing therapeutic vaccines or other novel immunotherapies to eradicate HBV infections.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Dr Xiaoyan Dong for providing AAV/HBV and for helpful discussions. This work was supported by grants from the National Basic Research Program 973 of China (Nos. 2009CB522502, 2012CB910203 and 2012CB519000) and the National Science and Technology Major Project of China (No. 2012ZX10002006) to YXF; grants from the Ministry of Science and Technology (2009CB522507, 2010-Biols-CAS-0201 and KSCX20YW-R-150) to LZ; grants from the Ministry of Health (2011ZX10004503-007) to LZ and LS; and funding from the National Institutes of Health (NIH) (R01AI095097) to LS. We would like to give special thanks to Mendy Miller for editing and to the NIH for partial funding (DK095962) provided to Y-XF and LS.
Footnotes
AUTHOR CONTRIBUTIONS
The work presented here was a collaboration among all the authors. DY, LL and DZ performed the experiments. LZ and Y-XF provided conceptual and technical guidance. LZ, Y-XF and DY wrote the paper. LL, DZ, HP and LS provided reagents, suggestions and editing.
COMPETING FINANCIAL INTERESTS
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Cellular & Molecular Immunology website.
References
- 1.World Health Organization . Hepatitis B. Fact sheet. SWHO; Geneva: [Accessed on May 10th, 2013]. 2012. Available from: http://www.who.int/mediacentre/factsheets/fs204/en. [Google Scholar]
- 2.Scaglione SJ, Lok AS. Effectiveness of hepatitis B treatment in clinical practice. Gastroenterology. 2012;142:1360–1368.e1. doi: 10.1053/j.gastro.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Chen DS. Toward elimination and eradication of hepatitis B. J Gastroenterol Hepatol. 2010;25:19–25. doi: 10.1111/j.1440-1746.2009.06165.x. [DOI] [PubMed] [Google Scholar]
- 4.Pol S, Michel ML. Therapeutic vaccination in chronic hepatitis B virus carriers. Expert Rev Vaccines. 2006;5:707–716. doi: 10.1586/14760584.5.5.707. [DOI] [PubMed] [Google Scholar]
- 5.Schirmbeck R, Wild J, Stober D, Blum HE, Chisari FV, Geissler M, et al. Ongoing murine T1 or T2 immune responses to the hepatitis B surface antigen are excluded from the liver that expresses transgene-encoded hepatitis B surface antigen. J Immunol. 2000;164:4235–4243. doi: 10.4049/jimmunol.164.8.4235. [DOI] [PubMed] [Google Scholar]
- 6.Chisari FV, Filippi P, Buras J, McLachlan A, Popper H, Pinkert CA, et al. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc Natl Acad Sci USA. 1987;84:6909–6913. doi: 10.1073/pnas.84.19.6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wirth S, Guidotti LG, Ando K, Schlicht HJ, Chisari FV. Breaking tolerance leads to autoantibody production but not autoimmune liver disease in hepatitis B virus envelope transgenic mice. J Immunol. 1995;154:2504–2515. [PubMed] [Google Scholar]
- 8.Loirat D, Mancini-Bourgine M, Abastado JP, Michel ML. HBsAg/HLA-A2 transgenic mice: a model for T cell tolerance to hepatitis B surface antigen in chronic hepatitis B virus infection. Int Immunol. 2003;15:1125–1136. doi: 10.1093/intimm/dxg117. [DOI] [PubMed] [Google Scholar]
- 9.Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci USA. 2002;99:13825–13830. doi: 10.1073/pnas.202398599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang LR, Wu HL, Chen PJ, Chen DS. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103:17862–17867. doi: 10.1073/pnas.0608578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LR, Gäbel YA, Graf S, Arzberger S, Kurts C, Heikenwalder M, et al. Transfer of HBV genomes using low doses of adenovirus vectors leads to persistent infection in immune competent mice. Gastroenterology. 2012;142:1447–1450.e3. doi: 10.1053/j.gastro.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Dong X, Yu CJ, Wang G, Tian WH, Lu Y, Zhang FW, et al. Establishment of hepatitis B virus(HBV) chronic infection mouse model by in vivo transduction with a recombinant adeno-associated virus 8 carrying 1.3 copies of HBV genome(rAAV8-1.3HBV) Chin J Virol. 2010;26:425–431. [PubMed] [Google Scholar]
- 13.Wang G, Dong XY, Tian WH, Yu CJ, Zheng G, Gao J, et al. Study on the differences of two mouse models of hepatitis B virus infection by transduction with rAAV8-1.3HBV. Chin J Virol. 2012;28:541–547. [PubMed] [Google Scholar]
- 14.Wang G, Wang G, Dong X, Tian W, Yuchi J, Wei G, et al. Anti-HBV effect of nucleotide analogues on mouse model of chronic HBV infection mediated by recombinant adeno-associated virus 8. Chin J Biotechnol. 2013;29:95–106. Chinese. [PubMed] [Google Scholar]
- 15.Wang SH, Yeh SH, Lin WH, Yeh KH, Yuan Q, Xia NS, et al. Estrogen receptor alpha represses transcription of HBV genes via interaction with hepatocyte nuclear factor 4alpha. Gastroenterology. 2012;142:989–998.e4. doi: 10.1053/j.gastro.2011.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Tian Y, Kuo CF, Chen WL, Ou JH. Enhancement of hepatitis B virus replication by androgen and its receptor in mice. J Virol. 2012;86:1904–1910. doi: 10.1128/JVI.06707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andres LL, Sawhney VK, Scullard GH, Smith JL, Merigan TC, Robinson WS, et al. Dane particle DNA polymerase and HBeAg: impact on clinical, laboratory, and histologic findings in hepatitis B-associated chronic liver disease. Hepatology. 1981;1:583–585. doi: 10.1002/hep.1840010604. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen T, Sawhney VK, Scullard GH, Smith JL, Merigan TC, Robinson WS, et al. Hepatitis B surface antigen levels during the natural history of chronic hepatitis B: a perspective on Asia. J Hepatol. 2010;52:508–513. doi: 10.1016/j.jhep.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Jaroszewicz J, Calle Serrano B, Wursthorn K, Deterding K, Schlue J, Raupach R, et al. Hepatitis B surface antigen (HBsAg) levels in the natural history of hepatitis B virus (HBV)-infection: a European perspective. J Hepatol. 2010;52:514–522. doi: 10.1016/j.jhep.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- 21.Wijaya I, Hasan I. Reactivation of hepatitis B virus associated with chemotherapy and immunosuppressive agent. Acta Med Indones. 2013;45:61–66. [PubMed] [Google Scholar]
- 22.Xunrong L, Yan AW, Liang R, Lau GK. Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy—pathogenesis and management. Rev Med Virol. 2001;11:287–299. doi: 10.1002/rmv.322. [DOI] [PubMed] [Google Scholar]
- 23.Manzano-Alonso ML, Castellano-Tortajada G. Reactivation of hepatitis B virus infection after cytotoxic chemotherapy or immunosuppressive therapy. World J Gastroenterol. 2011;17:1531–1537. doi: 10.3748/wjg.v17.i12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dion S, Bourgine M, Godon O, Levillayer F, Michel ML. Adeno-associated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLA-DR1 molecules. J Virol. 2013;87:5554–5563. doi: 10.1128/JVI.03134-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michel ML, Deng Q, Mancini-Bourgine M. Therapeutic vaccines and immune-based therapies for the treatment of chronic hepatitis B: perspectives and challenges. J Hepatol. 2011;54:1286–1296. doi: 10.1016/j.jhep.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 26.Huang YH, Fang CC, Tsuneyama K, Chou HY, Pan WY, Shih YM, et al. A murine model of hepatitis B-associated hepatocellular carcinoma generated by adeno-associated virus-mediated gene delivery. Int J Oncol. 2011;39:1511–1519. doi: 10.3892/ijo.2011.1145. [DOI] [PubMed] [Google Scholar]
- 27.Huang YH, Fang CC, Tsuneyama K, Chou HY, Pan WY, Shih YM, et al. A murine model of hepatitis B-associated hepatocellular carcinoma generated by adeno-associated virus-mediated gene delivery. Int J Oncol. 2011;39:1511–1519. doi: 10.3892/ijo.2011.1145. [DOI] [PubMed] [Google Scholar]
- 28.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 29.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 30.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–368. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 31.Cooper CL, Davis HL, Morris ML, Efler SM, Adhami MA, Krieg AM, et al. CPG 7909, an immunostimulatory TLR9 agonist oligodeoxynucleotide, as adjuvant to Engerix-B HBV vaccine in healthy adults: a double-blind phase I/II study. J Clin Immunol. 2004;24:693–701. doi: 10.1007/s10875-004-6244-3. [DOI] [PubMed] [Google Scholar]
- 32.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 33.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Cooper C, Mackie D. Hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine: a review of HEPLISAV safety and efficacy. Expert Rev Vaccines. 2011;10:417–427. doi: 10.1586/erv.10.162. [DOI] [PubMed] [Google Scholar]
- 35.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.