Abstract
Introduction
Limited information is available about the clinical features of Paget’s disease of bone among unselected patients in the community. We examined morbidity and mortality associated with this condition in a large inception cohort of Olmsted County, MN, residents with a new diagnosis of Paget’s disease from 1950 through 1994.
Materials and Methods
Survival was estimated using the Kaplan-Meier method. Cox proportional hazards models were used to assess the impact of various covariates on death.
Results
Paget’s disease of bone was diagnosed in 236 Olmsted County residents (mean age at diagnosis, 69.6 yr; 55% men). The majority were symptomatic at diagnosis (58%), and the proportion with symptoms did not change from the prescreening era (1950 to June 1974) to the postscreening era (July 1974–1994). Most patients had polyostotic disease (72%), and the pelvis (67%), vertebra (41%), and femur (31%) were the most common sites of involvement. Skeletal complications attributable to Paget’s disease included bowing deformities (7.6%), fracture of pagetic bone (9.7%), and osteosarcoma (0.4%). Osteoarthritis was observed in 73% of patients, and 11% had a hip or knee replacement. Nonskeletal complications related to Paget’s disease included cranial nerve (0.4%), peripheral nerve (1.7%), and nerve root (3.8%) compression, basilar invagination (2.1%), hypercalcemia (5.2%), and congestive heart failure (3.0%). Hearing loss, noted in 61%, was significantly higher than previously reported.
Conclusions
Compared with white Minnesota residents, overall survival was slightly better than expected (p = 0.010). No clinical risk factors were identified that were associated with an increased risk of death.
Keywords: epidemiology, morbidity, mortality, Paget’s disease, alkaline phosphatase
INTRODUCTION
Paget’s disease of bone is a localized bone remodeling disorder of uncertain etiology.(1) The onset of Paget’s disease is insidious and generally occurs later in life. Both men and women are affected, with a slight male preponderance.(1–5) Bone that is remodeled by this pathologic process becomes enlarged and mechanically weakened. Pain, skeletal deformity, and fracture may develop because of structurally inferior bone, but most patients with Paget’s disease are discovered because of an increased alkaline phosphatase level or an incidental X-ray finding and are asymptomatic. Additional clinical consequences of Paget’s disease can include secondary arthritis, neurologic compression syndromes, hearing loss, high-output cardiac failure, and sarcomatous transformation.(1,6–10) To date, there has been only a single study in North America estimating the complications associated with Paget’s disease, but it did not directly assess patients’ records, relying instead on diagnostic codes from claims records.(10)
Little information is available on the risk of death in patients with Paget’s disease of bone. Death related to sarcomatous transformation in Paget’s disease has been declining, corresponding to a similar decline in the prevalence and severity of the disease.(11) In Britain, data from the General Practice Research Database suggest that, compared with control subjects, patients with Paget’s disease had reduced survival.(9,12) There is no comparable information on mortality associated with Paget’s disease of the bone from countries outside of Britain. To fill these gaps, we examined the morbidity and mortality associated with Paget’s disease among residents of Olmsted County, MN, where the Rochester Epidemiology Project provides access to essentially all documented medical history in the community.(13)
MATERIALS AND METHODS
Population-based epidemiologic research can be conducted in Olmsted County because medical care is virtually self-contained within the community, and there are relatively few providers. Most endocrinologic and orthopedic care, for example, is provided by the Mayo Clinic, which has maintained a common medical record system with its two affiliated hospitals (St Marys and Rochester Methodist) for 100 yr.(13) The Mayo Clinic dossier-type record thus contains both inpatient and outpatient data, and the diagnoses and surgical procedures recorded in these records are indexed. The index includes the diagnoses for outpatients seen in office or clinic consultations, emergency room visits, or nursing home care, as well as the diagnoses recorded for hospital inpatients, at autopsy examination, or on death certificates. Medical records of the other providers who serve the local population, most notably the Olmsted Medical Center with its affiliated hospital, are also indexed and retrievable. Thus, details of almost all of the medical care provided to residents of the County are available for review in approved studies.(13)
After approval by Mayo’s Institutional Review Board, we used this unique medical records linkage system (the Rochester Epidemiology Project) to identify all Olmsted County residents with a new diagnosis of Paget’s disease of bone who first came to clinical attention during the period 1950–1994. To accomplish this, we reviewed the complete (inpatient and outpatient) medical records of every resident with any of the following diagnoses: Paget’s disease of bone, osteitis deformans (various skeletal sites), osteoporosis circumscripta, Schuller’s disease, and osteodystrophy. However, all qualifying cases were listed under Paget’s disease of bone or osteitis deformans. Paget’s disease is predominantly a radiographic diagnosis, and characteristic radiographic and laboratory findings are diagnostic in virtually all instances. To confirm the diagnosis of Paget’s disease in the patients listed, a nurse abstractor reviewed the reports of radiographic procedures and laboratory results. If a radiologist diagnosed Paget’s disease based on the radiographic findings, the diagnosis of Paget’s disease was accepted. Otherwise, the radiographic findings, clinical features, and laboratory results were reviewed by an expert (RDT) to determine eligibility. Because of complete community coverage and the redundancy of the data system, we believe that all Olmsted County residents with Paget’s disease initially diagnosed during the study period were identified. However, the histories of two potential cases could not be examined because they had not provided an authorization for review of their medical records for research.(14)
Two patient groups were defined based on introduction of the automated serum chemistry panel at Mayo in June 1974, which included the measurement of serum alkaline phosphatase. The “prescreening” era was defined as those patients recognized from 1950 to June 1974, whereas “post-screening” patients were those identified in July 1974–1994. Symptoms at baseline were defined as pain, deformity, warmth, pathologic fracture, or other symptom attributable to Paget’s disease. Baseline complications were defined as any that occurred before or within 6 mo of the initial diagnosis of Paget’s disease. Skeletal complications directly attributed to Paget’s disease were defined as osteosarcoma, bowing, and pathologic fractures. Nonskeletal complications directly related to Paget’s disease were defined as cranial nerve compression, basilar invagination, peripheral nerve compression, nerve root compression, and congestive heart failure (CHF) attributed to Paget’s disease by the attending physicians. Osteoarthritis and auditory manifestations were considered nonspecific and therefore were analyzed independently. Hip or knee replacement came from an internal Joint Registry, which incorporates data from 1970 to the present.
Categorical variables were summarized using frequencies and percentages. Characteristics in subjects diagnosed in the prescreening time period (1950 to June 1974) were compared with those diagnosed in the postscreening era (July 1974–1994) using χ2 and Fisher’s exact tests as appropriate.
Survival was estimated using the Kaplan-Meier method with expected survival rates based on Minnesota white decennial life tables. Observed and expected survival curves were compared using the one-sample log-rank test statistic. Standardized mortality ratios (SMRs) were used to compare the number of cause-specific deaths in our cohort to the number expected for the Minnesota white population generally.(15) 95% CIs for the SMRs were calculated assuming that the expected rates are fixed, and the observed events follow a Poisson distribution. Expected numbers were derived by applying age-, sex-, and calendar year–specific mortality rates from the general population to the age-, sex-, and calendar year–specific person-years of follow-up in this cohort, which was updated in 2007.
Cox proportional hazards models, which do not incorporate the population expected rates, were used to assess the impact of various covariates on death. Stepwise methods with forward selection and backward elimination were used to choose independent variables for the final models. The dependent variable was time until death, and the independent variables were the clinical characteristics. The pagetic involvement of sites and fracture of pagetic bone were handled as time-dependent variables. Hip or knee replacement was also handled as a time-dependent variable, but it only included follow-up from 1970 onward. Relative risk regression models, using the Minnesota population expected death rates, were used to assess the impact of various covariates on death adjusting for the impact of the expected survival for each subject. These analyses used follow-up data obtained with the abstraction of the clinical variables in 1998.
RESULTS
Clinical features
Over the 45-yr period, 1950–1994, 236 Olmsted County residents were first diagnosed with Paget’s disease of bone. All but two were white, consistent with the racial composition of the community (98% white in 1980). The mean age at diagnosis was 69.6 yr (median, 70.6 yr; range, 23–95 yr), and there were more men (55%) than women (45%). Monostotic disease was noted initially in 155 patients (66%), of whom 90 were subsequently found to be polyostotic. Thus, polyostotic disease was initially present in only 80 patients (34%) but later documented in 170 (72%). The status of one patient was unknown. The pelvis was the most common site of involvement (67%), followed by vertebrae (41%), femur (31%), skull/face (12%), and tibia/fibula (7.2%), with rare involvement of other skeletal sites. The majority of patients were symptomatic at diagnosis (58%). The proportion of symptomatic patients and the distribution of symptoms were similar in the prescreening and post-screening periods despite more patients having an alkaline phosphatase measured within 2 yr of diagnosis in the post-screening era (95%) compared with the prescreening era (50%) (Table 1).
Table 1.
Symptoms and Complications at Baseline Among Olmsted County, MN, Residents With Paget’s Disease of Bone First Diagnosed in 1950–1994, by Prescreening (1950 to June 1974) and Postscreening (July 1974–1994) Time Periods
| 1950 to June 1974 (N = 109) | July 1974–1994 (N = 127) | p | |
|---|---|---|---|
| Age at diagnosis (mean ± SD) | 66.2 ± 11.1 | 72.6 ± 12.3 | <0.001 |
| Symptomatic presentation, any [N (%)] | 63 (58.9) | 71 (57.7) | 0.859 |
| Pain [N (%)] | 47 (43.9) | 48 (40.0) | 0.550 |
| Deformity [N (%)] | 13 (12.0) | 18 (14.6) | 0.563 |
| Warmth [N (%)] | 4 (3.7) | 1 (0.8) | 0.186 |
| Fracture [N (%)] | 4 (3.7) | 2 (1.6) | 0.421 |
| Other [N (%)] | 17 (15.7) | 28 (22.4) | 0.199 |
| Skeletal complications [N (%)] | 5 (4.6) | 5 (3.9) | 0.999 |
| Bowing deformities [N (%)] | 4 (3.7) | 2 (1.6) | 0.419 |
| Osteosarcoma [N (%)] | 0 (0) | 0 (0) | — |
| Fracture of pagetic bone [N (%)] | 1 (0.9) | 3 (2.4) | 0.626 |
| Nonskeletal complications [N (%)] | 6 (5.5) | 2 (1.6) | 0.149 |
| Cranial nerve compression [N (%)] | 0 (0) | 0 (0) | — |
| Basilar invagination [N (%)] | 1 (0.9) | 0 (0) | 0.462 |
| Peripheral nerve compression [N (%)] | 0 (0) | 0 (0) | — |
| Nerve root compression [N (%)] | 4 (3.7) | 0 (0) | 0.044 |
| CHF attributed to Paget’s disease [N (%)] | 1 (0.9) | 2 (1.6) | 0.999 |
| Nonspecific conditions [N (%)]* | 63 (64.9) | 89 (74.2) | 0.140 |
| Osteoarthritis [N (%)] | 45 (41.7) | 59 (46.8) | 0.428 |
| Hip or knee replacement [N (%)] | 1 (0.9) | 5 (3.9) | 0.221 |
| Hearing loss [N (%)]* | 10 (11.1) | 42 (41.6) | <0.001 |
| Tinnitus [N (%)] | 6 (5.6) | 11 (8.8) | 0.342 |
| Vertigo [N (%)] | 8 (7.4) | 25 (20.0) | 0.006 |
| Hypercalcemia [N (%)] | 1 (0.9) | 4 (3.2) | 0.376 |
| Gout [N (%)] | 2 (1.9) | 6 (4.8) | 0.292 |
| Kidney stones [N (%)] | 4 (3.7) | 11 (8.9) | 0.114 |
Forty-five patients with unknown date of diagnosis.
Specific complications related to Paget’s disease were noted in 46 patients (19%). Over the entire period of observation, skeletal complications occurred in 34 patients (14%) and included osteosarcoma (1 patient, 0.4%), bowing deformities (18 patients, 7.6%), and 33 pathologic fractures (multiple fractures in some individuals) attributed to Paget’s disease (23 patients, 9.7%; Table 2). Nonskeletal complications directly related to Paget’s disease were observed in 22 patients (9.3%) and included cranial nerve compression (1 patient, 0.4%), basilar invagination (5 patients, 2.1%), peripheral nerve compression (4 patients, 1.7%), nerve root compression (9 patients, 3.8%), and CHF attributed to Paget’s disease (7 patients, 3.0%); 69 other individuals had CHF that was not attributed to Paget’s disease.
Table 2.
Symptoms at Presentation and Complications at Any Time Among Olmsted County, MN, Residents With Paget’s Disease of Bone First Diagnosed in 1950–1994
| Men (N = 129) | Women (N = 107) | Both sexes (N = 236) | |
|---|---|---|---|
| Age at diagnosis (mean ± SD) | 69.5 ± 11.9 | 69.8 ± 12.6 | 69.6 ± 12.2 |
| Symptomatic presentation [N (%)] | 67 (53.2) | 67 (64.4) | 134 (58.3) |
| Pain [N (%)] | 48 (38.7) | 47 (45.6) | 95 (41.9) |
| Deformity [N (%)] | 12 (9.4) | 19 (18.3) | 31 (13.4) |
| Warmth [N (%)] | 3 (2.4) | 2 (1.9) | 5 (2.2) |
| Fracture [N (%)] | 3 (2.3) | 3 (2.9) | 6 (2.6) |
| Other [N (%)] | 23 (18.0) | 22 (21.0) | 45 (19.3) |
| Skeletal complications [N (%)] | 14 (10.9) | 20 (18.7) | 34 (14.4) |
| Bowing deformities [N (%)] | 8 (6.2) | 10 (9.3) | 18 (7.6) |
| Osteosarcoma [N (%)] | 1 (0.8) | 0 (0.0) | 1 (0.4) |
| Fracture of pagetic bone [N (%)] | 9 (7.0) | 14 (13.1) | 23 (9.7) |
| Nonskeletal complications [N (%)] | 10 (7.8) | 12 (11.2) | 22 (9.3) |
| Cranial nerve compression [N (%)] | 0 (0.0) | 1 (0.9) | 1 (0.4) |
| Basilar invagination [N (%)] | 1 (0.8) | 4 (3.7) | 5 (2.1) |
| Peripheral nerve compression [N (%)] | 1 (0.8) | 3 (2.8) | 4 (1.7) |
| Nerve root compression [N (%)] | 5 (3.9) | 4 (3.7) | 9 (3.8) |
| CHF attributed to Paget’s disease [N (%)] | 4 (3.1) | 3 (2.8) | 7 (3.0) |
| Nonspecific conditions [N (%)] | 113 (88.3) | 97 (91.5) | 210 (89.7) |
| Osteoarthritis [N (%)] | 89 (69.5) | 81 (76.4) | 170 (72.6) |
| Hip or knee replacement [N (%)] | 15 (11.6) | 10 (9.3) | 25 (10.6) |
| Hearing loss [N (%)] | 79 (61.2) | 66 (61.7) | 145 (61.4) |
| Tinnitus [N (%)] | 15 (11.8) | 27 (25.5) | 42 (18.0) |
| Vertigo [N (%)] | 25 (19.7) | 32 (30.2) | 57 (24.5) |
| Hypercalcemia [N (%)] | 3 (2.4) | 9 (8.5) | 12 (5.2) |
| Gout [N (%)] | 13 (10.3) | 6 (5.7) | 19 (8.2) |
| Kidney stones [N (%)] | 18 (14.4) | 9 (8.5) | 27 (11.7) |
Several nonspecific conditions that have been associated with Paget’s disease were also observed. Osteoarthritis was documented in 170 patients (73%), and either hip or knee replacement was performed in 25 (10.6%). The auditory manifestations observed included hearing loss (145 patients, 61%), tinnitus (42 patients, 18%), and vertigo (57 patients, 24%). Other clinical conditions observed in this cohort included hypercalcemia (12 patients, 5.2%), gout (19 patients, 8.2%), and kidney stones (27 patients, 12%).
Survival
After the diagnosis of Paget’s disease, patients were followed for a total of 3408 person-years through 2007 (median, 13.4 yr). Compared with white Minnesota residents of similar age and sex distribution, survival was slightly better than expected (p = 0.010): by 10 yr after the diagnosis of Paget’s disease, 62% were still alive compared with an expected 57% (Fig. 1). The men had significantly better survival, with 59% alive compared with an expected 52% at 10 yr (p = 0.044), whereas the women had a trend toward better survival with 66% alive compared with an expected 63% at 10 yr (p = 0.111). The most frequent causes of death in this cohort were diseases of the circulatory system (45%), neoplasms (19%), and diseases of the respiratory system (12%), but mortality caused by these conditions was not increased compared with the general population (Table 3).
FIG. 1.
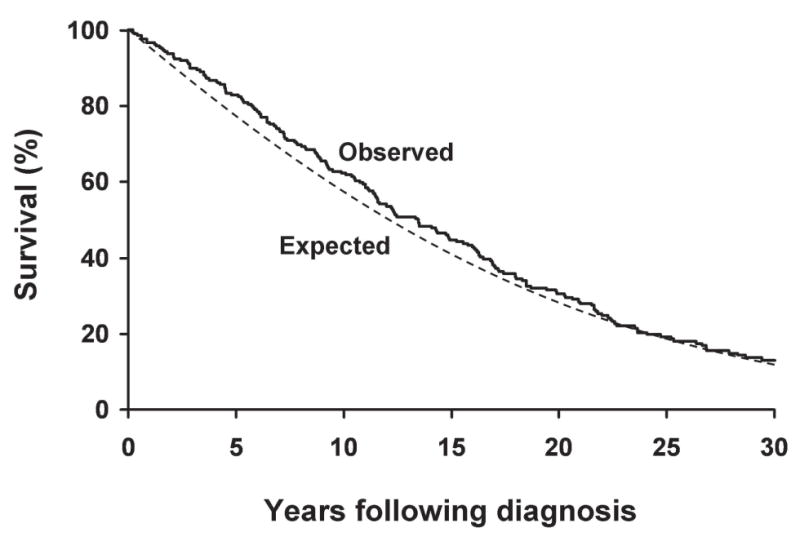
Overall age- and sex-adjusted survival among Olmsted County, MN, residents first diagnosed with Paget’s disease of bone in 1950–1994 compared with that expected for Minnesota white residents of similar age and sex (p = 0.01 for difference between observed and expected survival).
Table 3.
SMRS, With 95% CIs, by Underlying Cause of Death,* Among Olmsted County, MN, Residents With Paget’s Disease of Bone First Diagnosed in 1950–1994 Compared With Expected Deaths in White Minnesota Residents
| Cause of death | Observed | Expected | SMR (95% CI) |
|---|---|---|---|
| Cardiovascular disease | 91 | 110.5 | 0.82 (0.66–1.01) |
| Cancer | 39 | 38.1 | 1.02 (0.73–1.40) |
| Respiratory disease | 25 | 20.9 | 1.20 (0.77–1.77) |
| Gastrointestinal disease | 5 | 6.5 | 0.77 (0.25–1.80) |
| All other causes | 36 | 34.8 | 1.03 (0.73–1.43) |
| All causes | 204 | 210.9 | 0.97 (0.84–1.11) |
Eight people had an unknown cause of death.
Using the original follow-up through 1998 to match data abstraction of clinical features, the clinical features of Paget’s disease that were significantly associated with an increased risk of death in a univariate model included age at diagnosis (hazard ratio [HR] per 10-yr increase, 2.4; 95% CI, 2.0–2.8), year of diagnosis per 10-yr increase (HR, 1.3; 95% CI, 1.1–1.5), time period of diagnosis July 1974 or after (HR, 1.5; 95% CI 1.1–2.1), and male sex (HR, 1.4; 95% CI, 1.1–2.0; Table 4). However, after adjusting for the expected survival among those of similar age, sex, and calendar year, there was no overall impact of these factors on survival. Symptoms at presentation and hip or knee replacement were also associated with a lower and higher risk of death, respectively, in the univariate analysis; once again, after adjusting for population survival differences, the impact of symptoms at presentation and joint replacement disappeared. Specific skeletal sites of pagetic involvement including the spine, skull, face, pelvis, and femur were not associated with reduced survival. Likewise, there was no difference in survival between monostotic and polyostotic disease, as well as those with normal alkaline phosphatase levels compared with patients with elevated alkaline phosphatase values. Fracture of pagetic bone also did not influence survival. Many complications, such as nerve root compression, CHF, osteosarcoma, and bowing deformities were too infrequent to allow assessment of their impact on survival.
Table 4.
Predictors of All-Cause Mortality Among Olmsted County, MN, Residents First Diagnosed With Paget’s Disease of Bone in 1950–1994
| Variable | Hazard ratio (95% CI) | p |
|---|---|---|
| Univariate model | ||
| Age at diagnosis per 10-yr increase | 2.36 (1.98–2.82) | <0.001 |
| Year of diagnosis per 10-yr increase | 1.28 (1.08–1.51) | 0.004 |
| Year of diagnosis, July 1974or after | 1.48 (1.06–2.06) | 0.022 |
| Male | 1.44 (1.06–1.97) | 0.020 |
| AlkPhos high, within 2 years of diagnosis | 1.12 (0.77–1.64) | 0.538 |
| Symptoms at presentation | 0.69 (0.50–0.94) | 0.021 |
| Hearing loss before or within 6 mo after dx | 1.44 (0.97–2.12) | 0.068 |
| Pagetic involvement of spine | 1.33 (0.97–1.81) | 0.074 |
| Pagetic involvement of skull | 1.31 (0.84–2.06) | 0.238 |
| Pagetic involvement of pelvis | 1.24 (0.89–1.72) | 0.198 |
| Pagetic involvement of femur | 1.24 (0.89–1.72) | 0.208 |
| Monostotic vs. polyostotic | 1.40 (0.98–1.99) | 0.063 |
| Hip or knee replacement | 1.95 (1.27–3.00) | 0.002 |
| Fracture of pagetic bone | 1.22 (0.70–2.12) | 0.478 |
| Multivariate model | ||
| Age at diagnosis per 10-yr increase | 2.50 (2.08–3.02) | <0.001 |
| Male | 1.68 (1.22–2.30) | 0.001 |
DISCUSSION
Paget’s disease of bone is associated with significant morbidity related to skeletal deformity and fracture.(1,7) Only one study, however, has specifically addressed mortality associated with Paget’s disease. In that study, the British General Practice Research Database identified 2465 patients diagnosed with Paget’s disease of bone from 1988 to 1999; retrospective review indicated that 5-yr survival was 67% in patients with Paget’s disease compared with 72% in control patients.(9) In contrast to the findings from van Staa et al.,(9) this first North American study to assess the impact of Paget’s disease on mortality found that survival was not decreased among unselected patients in the community, and in fact, was better than expected, especially among men with Paget’s disease of bone. We also did not identify an increased risk of mortality caused by cancer, cardiovascular disease, pulmonary disease, or gastrointestinal disease in this cohort.
This discrepant finding might be explained if disease in our population was less severe than that observed in Britain. Surveys from Britain and New Zealand have suggested milder disease in more recent decades,(9,16) and over one half of our cohort presented from July 1974–1994. However, 58% of our patients were symptomatic, and unexpectedly, the introduction of automated serum chemistry panels in June 1974, which included the measurement of serum alkaline phosphatase, did not impact the proportion of patients presenting with symptoms. In addition, if more severe disease were associated with decreased survival, higher levels of alkaline phosphatase, symptomatic disease, and more extensive skeletal involvement would be expected to be associated with an increased risk of death. We did not observe reduced survival in those with higher alkaline phosphatase levels, symptomatic disease at presentation, or polyostotic disease. The site of skeletal involvement and fracture of pagetic bone also did not increase the risk of death. These findings are consistent with data suggesting that the relationship between total alkaline phosphatase levels and quality of life caused by complications is poor.(17,18)
As part of this comprehensive assessment, we were able to evaluate the complications associated with Paget’s disease. The extensive medical record system of the Rochester Epidemiology Project makes Olmsted County one of the few places in the world where long-term studies of disease natural history can be performed.(13) Several important skeletal complications were observed, including fractures, bone deformity, and osteosarcoma. Fractures directly attributed to Paget’s disease occurred in 21 patients, and 14% of all observed fractures (33/240 fractures) were attributed to Pagetic bone involvement. We previously noted an increased risk of vertebral and rib fractures of nonpagetic bone, but after excluding fractures through pagetic bone, there was no increase in the overall subsequent fracture risk in this cohort.(6) The most serious complication associated with Paget’s disease of bone, osteosarcoma, was seen only once (0.4%). This is consistent with prior estimates of osteosarcoma in <1% of patients with Paget’s disease of bone.(1) Osteoarthritis associated with bowing deformities caused by Paget’s disease has important implications for physical functioning and quality of life(17) in addition to significant economic consequences.(10) Skeletal bowing was observed in 8% of these patients and most frequently involved the femur (6/18) or tibia (10/18), the typical sites previously described.(7) Bowing deformity at these weight-bearing sites can result in gait alteration, which can accelerate degeneration of the hip and knee, culminating in hip or knee arthroplasty.(19,20) Osteoarthritis was noted in 170 patients, and 25 (11%) had a hip or knee replacement, which is higher than previously reported.(9,10)
Several notable nonskeletal complications were also observed including hypercalcemia, gout, neurologic compression syndromes, and CHF attributed to Paget’s disease. Hypercalcemia was noted in 5.2% of the patients in our cohort. Hypercalcemia has been described in Paget’s disease in two specific circumstances: concomitant primary hyperparathyroidism(21) and, rarely, in immobilized patients with extensive Paget’s disease.(1,22) Of the 12 patients with hypercalcemia in our cohort, 10 patients (4.2%; 8 women and 2 men) likely had underlying primary hyperparathyroidism. One of these patients did have an increase in serum calcium to 15.6 mg/dl when immobilized for orthopedic surgery related to her Paget’s disease. Of the two remaining subjects with hypercalcemia, one had tertiary hyperparathyroidism caused by longstanding renal disease and the other only had a single calcium elevation. Gout,(23,24) which has also been associated with Paget’s disease caused by hyperuricemia,(25) was observed in 8.2% of the cohort.
Reports from referral centers indicate that neurological complications occur in one third of patients with Paget’s disease, with cranial nerve lesions (21%) and spinal cord/nerve root (10%) being the most common forms of involvement.(7) However, in this cohort, neurological complications were much lower than previously described, with only 0.4% of patients developing a cranial nerve compression and 5.5% with nerve root or peripheral nerve impingement. All of the patients with nerve root compression presented in the prescreening time period. Hearing loss caused by pagetic involvement of the cochlea is another well-described feature of the disease.(26) Hearing loss was observed in nearly two thirds of our cohort, significantly higher than previous prevalence estimates of 2.4–13.5%.(9,10) The number of patients identified with auditory disease at presentation increased in the most recent time period; however, 45 people were missing from this summary because of an unknown date of hearing loss. The majority of patients identified with hearing loss did not have skull imaging (bone scan or plain skull film). Because of this limitation, we could not confidently attribute hearing loss directly to Paget’s disease, because hearing loss in this setting can be caused by presbycusis, Paget’s disease itself, or a combination of both.(7) In part, this higher prevalence of hearing loss relative to other studies may reflect more complete evaluation of cases through direct chart review, which includes all audiology testing. Hearing loss was observed in 21 of the 29 subjects (72%) with radiographically documented skull involvement. High-output CHF has also been associated with Paget’s disease when there is extensive skeletal involvement. In our cohort, CHF attributed to Paget’s disease was noted in 3% of patients, which is similar to previous estimates.(9)
There are several limitations of this study. Our study population is relatively small and located in a midwestern community that is disproportionately white and slightly younger than the U.S. population. However, the sociodemographic characteristics of Olmsted County residents are similar to those of U.S. whites in general.(13) In addition, Paget’s disease has a heterogenous disease expression, and some cases will be missed even though there is widespread application of radiographs and biochemical testing in Olmsted County. Thus, an estimated 11% of this entire population had at least one serum alkaline phosphatase test performed at Mayo in 1990–1994.(2) However, if Paget’s disease was ever found, it was likely to have been documented in our medical records linkage system, which records the diagnoses made through both inpatient and out-patient care by essentially all of the providers of medical care to local residents.(13) We were unable to address the contribution of a family history of Paget’s disease because this specific information was often not documented in the medical record. We also did not evaluate the impact of treatment of Paget’s disease of bone because few patients in this cohort were treated with antiresorptive agents. Most potent bisphosphonates were introduced near the end of our study, and only 25 patients used them (etidronate disodium in 22 patients and alendronate in 3 patients) at any time. Similarly, we did not directly assess the relationship of alkaline phosphatase to disease complications because only one third of our patients had a documented elevation in alkaline phosphatase within 30 days of diagnosis. This may be because of discovery of Paget’s disease radiologically in patients with quiescent disease, as well as less frequent measurement of serum alkaline phosphatase before introduction into our automated serum chemistry panel in July 1974.
In summary, in contrast to other studies, Paget’s disease of bone was not associated with a reduction in overall survival in an unselected community population, and no clinical factors were identified that were associated with a reduced survival. Specifically, features seen in more severe disease, such as symptoms at presentation, polyostotic disease, and higher alkaline phosphatase levels, did not increase the risk of death. The introduction of an automated chemistry panel increased serum alkaline phosphatase testing but did not significantly alter the clinical presentation of Paget’s disease. Complications that were observed more frequently than previously described included hearing loss, osteoarthritis, and joint replacement, whereas neurological complications were less frequent than prior estimates. Although Paget’s disease does not seem to compromise survival, it does have significant clinical sequela that impact quality of life and health care cost. If these data are confirmed, it will be important to identify specific treatment strategies that may prevent these complications in the future.
Acknowledgments
The authors thank Margaret L Bitzer-Abramowitz for help with data collection and Mary G Roberts for help in preparing the manuscript. This work was supported by a contract from Sanofi-Synthelabo and National Institutes of Health Grant AR-30582.
References
- 1.Siris ES, Roodman GD. Paget’s disease of bone. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6. American Society for Bone and Mineral Research; Washington, DC, USA: 2006. pp. 320–330. [Google Scholar]
- 2.Tiegs RD, Lohse CM, Wollan PC, Melton LJ. Long-term trends in the incidence of Paget’s disease of bone. Bone. 2000;27:423–427. doi: 10.1016/s8756-3282(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ. The epidemiology of Paget’s disease of bone. Br Med Bull. 1984;40:396–400. doi: 10.1093/oxfordjournals.bmb.a072011. [DOI] [PubMed] [Google Scholar]
- 4.Kanis JA. Epidemiology, Pathophysiology and Treatment of Paget’s Disease of Bone. 2. Martin Dunitz; London, UK: 1998. pp. 1–11. [Google Scholar]
- 5.Sissons HA. Epidemiology of Paget’s disease. Clin Orthop. 1966;45:73–79. [PubMed] [Google Scholar]
- 6.Melton LJ, III, Tiegs RD, Atkinson EJ, O’Fallon WM. Fracture risk among patients with Paget’s disease: A population-based cohort study. J Bone Miner Res. 2000;15:2123–2128. doi: 10.1359/jbmr.2000.15.11.2123. [DOI] [PubMed] [Google Scholar]
- 7.Bone HG. Nonmalignant complications of Paget’s disease. J Bone Miner Res. 2006;21(S2):64–68. doi: 10.1359/jbmr.06s212. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA. Clinical features and complications. In: Kanis JA, editor. Pathophysiology and Treatment of Paget’s Disease of Bone. 2. Martin Dunitz; London, UK: 1991. pp. 110–138. [Google Scholar]
- 9.van Staa TP, Selby P, Leufkens HG, Lyles K, Sprafka JM, Cooper C. Incidence and natural history of Paget’s disease of bone in England and Wales. J Bone Miner Res. 2002;17:465–471. doi: 10.1359/jbmr.2002.17.3.465. [DOI] [PubMed] [Google Scholar]
- 10.Briesacher BA, Orwig D, Seton M, Omar M, Kahler KH. Medical care costs of Paget’s disease of bone in a privately insured population. Bone. 2006;38:731–737. doi: 10.1016/j.bone.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Cooper C, Dennison E, Schafheutle K, Kellingray S, Guyer P, Barker D. Epidemiology of Paget’s disease of bone. Bone. 1999;24:3S–5S. doi: 10.1016/s8756-3282(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 12.Cooper C, Harvey NC, Dennison EM, van Staa TP. Update on the epidemiology of Paget’s disease of bone. J Bone Miner Res. 2006;21(S2):3–8. doi: 10.1359/jbmr.06s201. [DOI] [PubMed] [Google Scholar]
- 13.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 14.Melton LJ., III The threat to medical-records research. N Engl J Med. 1997;337:1466–1470. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Wide-ranging On Line Data for Epidemiologic Research The Compressed Mortality File Data Set. [Accessed December 3, 2007]; Available online at http://wonder.cdc.gov/mortSQL.html.
- 16.Cundy HR, Gamble G, Wattie D, Rutland M, Cundy T. Paget’s disease of bone in New Zealand: Continued decline in disease severity. Calcif Tissue Int. 2004;75:358–364. doi: 10.1007/s00223-004-0281-z. [DOI] [PubMed] [Google Scholar]
- 17.Langston AL, Campbell MK, Fraser WD, Maclennan G, Selby P, Ralston SH. Clinical determinants of quality of life in Paget’s disease of bone. Calcif Tissue Int. 2007;80:1–9. doi: 10.1007/s00223-006-0184-2. [DOI] [PubMed] [Google Scholar]
- 18.Ralston SH, Langston AL, Campbell MK, MacLennan G, Selby PL, Fraser WD. Initial results from the PRISM Study: A large randomized comparative trial of intensive therapy to normalized alkaline phosphatase levels versus symptomatic treatment for Paget’s disease. Presented at the 28th Annual Meeting of the American Society for Bone and Mineral Research; September 15–19, 2006; Philadelphia, PA, USA. 2006. [Google Scholar]
- 19.Lewallen DG. Hip arthroplasty in patients with Paget’s disease. Clin Orthop. 1999;369:243–250. doi: 10.1097/00003086-199912000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Cameron HU. Total knee replacement in Paget’s disease. Orthop Rev. 1989;18:206–208. [PubMed] [Google Scholar]
- 21.Brandi ML, Falchetti A. What is the relationship between Paget’s disease of bone and hyperparathyroidism? J Bone Miner Res. 2006;21(S2):69–74. doi: 10.1359/jbmr.06s213. [DOI] [PubMed] [Google Scholar]
- 22.Reifenstein E, Albright F. Paget’s disease: Its pathologic physiology and the importance of this in the complications arising from fracture and immobilization. N Engl J Med. 1944;231:343–355. [Google Scholar]
- 23.Kuo JS, Fallon MD, Gannon FH, Goldmann D, Schumacher HR, Haddad JG, Kaplan FS. The articular manifestations of Paget’s disease of bone. A case report. Clin Orthop. 1992;285:250–254. [PubMed] [Google Scholar]
- 24.Lluberas-Acosta G, Hansell JR, Schumacher HR., Jr Paget’s disease of bone in patients with gout. Arch Intern Med. 1986;146:2389–2392. [PubMed] [Google Scholar]
- 25.Cawley MI. Complications of Paget’s disease of bone. Gerontology. 1983;29:276–287. doi: 10.1159/000213127. [DOI] [PubMed] [Google Scholar]
- 26.Monsell EM. The mechanism of hearing loss in Paget’s disease of bone. Laryngoscope. 2004;114:598–606. doi: 10.1097/00005537-200404000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]


