Abstract
Objective
The neuropathogenesis of postoperative delirium remains unknown. Low cerebrospinal fluid (CSF) βamyloid protein (Aβ) and high CSF Tau levels are associated with Alzheimer’s disease. We therefore assessed whether lower preoperative CSF Aβ/Tau ratio was associated with higher incidence and greater severity of postoperative delirium.
Methods
One hundred and fifty three participants (71±5 years, 53% males) who had total hip/knee replacement under spinal anesthesia were enrolled. CSF was obtained during initiation of spinal anesthesia. The incidence and severity of postoperative delirium were determined by Confusion Assessment Method (CAM) and Memorial Delirium Assessment Scale (MDAS) on postoperative day 1 and 2. Aβ40, Aβ42, and Tau levels in the CSF were measured by enzyme-linked immunosorbent assay. The relationships among these variables were determined, adjusting for age and gender.
Results
Participants in the lowest quartile of preoperative CSF Aβ40/Tau and Aβ42/Tau ratio had higher incidence (32% versus 17%, P=0. 0482) and greater symptom severity of postoperative delirium (Aβ40/Tau ratio: 4 versus 3, P=0. 034; Aβ42/Tau ratio: 4 versus 3, P=0. 062, the median of the highest Memorial Delirium Assessment Scale score) as compared to the combination of the rest of the quartiles. The preoperative CSF Aβ40/Tau or Aβ42/Tau ratio was inversely associated with Memorial Delirium Assessment Scale score (Aβ40/Tau ratio: −0.12±0.05, P=0.014, adj. −0.12±0.05, P=0.018; Aβ42/Tau ratio: −0.65±0.26, P=0.013, adj. −0.62±0.27, P=0.022).
Interpretation
Lower CSF Aβ/Tau ratio could be associated with postoperative delirium, pending confirmation of our preliminary results in further studies. These findings suggest potential roles of Aβ and/or Tau in postoperative delirium neuropathogenesis.
Keywords: Cerebrospinal fluid, Aβ/Tau ratio, delirium, surgery
Introduction
Postoperative delirium1 is one of the most common postoperative complications in elderly patients2. It has been shown that postoperative delirium has independent adverse effects on short and long-term mortality and morbidity, including poor functional recovery, postoperative cognitive dysfunction, deterioration in quality of life, and increased costs [3–6, reviewed in7, 8]. However, at the present time, postoperative delirium is a clinical phenomenon, and its neuropathogenesis remains unknown. This gap in knowledge has become a barrier that limits further studies, including development of potential interventions for postoperative delirium.
βAmyloid protein (Aβ), including Aβ40 and Aβ42, is the key component of senile plaques in Alzheimer’s disease (AD) patients. Tau is the major protein component of intraneuronal neurofibrillary tangles. Both Aβ and Tau are hallmark features of AD neuropathogenesis [reviewed in9].
Lower levels of CSF Aβ42 have been found to be associated with higher brain amyloid amounts10–13, and appear in AD patients as compared to normal controls14, 15. Higher levels of CSF Tau are associated with elevated brain Tau levels13, 16, and with the progression of AD17–19. Therefore, higher CSF Tau to Aβ42 ratio20 or lower CSF Aβ42 to Tau ratio21 can distinguish AD patients from healthy controls and predict the development of AD [reviewed in22]. In addition to Aβ42, Aβ40 has been shown to induce neurotoxicity23–25 and is associated with cognitive dysfunction and dementia26–28. Moreover, we recently published a study that reported both CSF Aβ40/Tau ratio and Aβ42/Tau ratio were associated with postoperative cognitive changes, although each ratio was associated with changes in different cognitive domains29. We therefore used the CSF Aβ/Tau ratio, not the mathematically different but scientifically equivalent CSF Tau/Aβ ratio, in the studies, and assessed whether human CSF Aβ40/Tau or Aβ42/Tau ratio would be associated with postoperative delirium.
Therefore, we performed a prospective investigation in patients who had elective total hip/knee replacement under spinal anesthesia to assess whether there were associations between preoperative human CSF Aβ40/Tau or Aβ42/Tau ratio and the incidence and severity of postoperative delirium. The current proof of concept study was performed in one hundred and fifty three participants. The primary hypothesis in our study was that lower preoperative CSF Aβ42/Tau ratio would be associated with greater severity of postoperative delirium. Our secondary hypotheses were that preoperative CSF Aβ40/Tau ratio would be associated with postoperative delirium severity, and that both CSF Aβ42/Tau ratio and Aβ40/Tau ratio would be associated with the incidence of postoperative delirium. We chose the severity of postoperative delirium in the current research because there have been no studies to determine the association between the human CSF biomarkers and the severity of postoperative delirium. Moreover, given our anticipated relatively small sample size, using the continuous outcome of delirium severity maximizes statistical power, which is appropriate for this hypothesis generating study. The outcomes from this study mainly served to establish a system and to generate a concept that Aβ and/or Tau might contribute to the neuropathogenesis of postoperative delirium, which would promote more studies to further investigate the neuropathogenesis of postoperative delirium.
Methods
Study enrollment
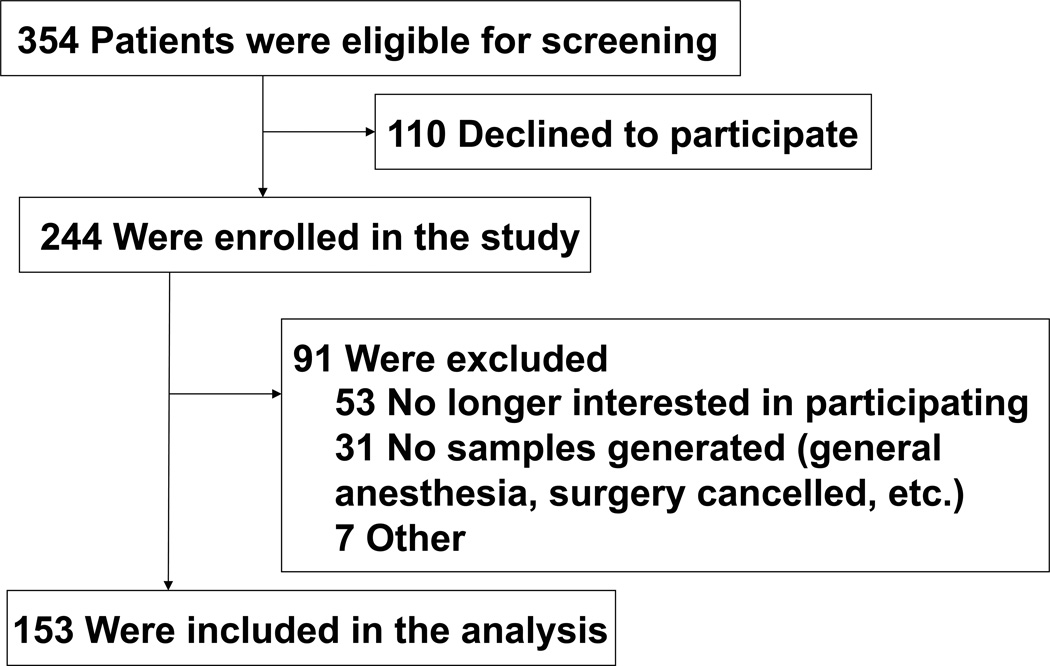
The protocol was approved by the Institutional Review Board of Partners Human Research Committee, Boston, Massachusetts, United States of America. A total of 354 adults who were scheduled to have elective total hip or knee replacement surgery at the Massachusetts General Hospital were asked to participate in this study (see Figure 1, the Flow diagram). The inclusion criteria included: (1) 63 years old or older; (2) proficient in English, and (3) candidates for spinal anesthesia. Individuals who met these criteria were further screened in an initial interview. After reviewing participant medical records, individuals excluded from participation were those identified as having: (1) past medical history of neurological and psychiatric diseases including AD, other forms of dementia, stroke, or psychosis; (2) severe visual or hearing impairment; and (3) unwillingness to comply with the protocol or procedures. Consent was obtained by study coordinators in the Pre-Admissions Testing Area at Massachusetts General Hospital when the participants came to the hospital for preoperative evaluation. A total of 244 participants were enrolled in the study from September 2011 to May 2013, though 91 were excluded due to dropping out preoperatively or changing their mind about spinal anesthesia, bringing the total number of participants to 153. There have been no major changes in the surgery or anesthesia practice since the start of our studies in 2011. We calculated that a sample size of 150 participants would be sufficient to determine a correlation > 0.20 between Aβ/Tau ratio and Memorial Delirium Assessment Scale (MDAS) score (which measures the severity of delirium30) with 80% power and 5% type I error. The power calculation was performed in the design phase of the study and was based on our primary hypothesis that lower preoperative Aβ42/Tau ratio was associated with greater severity of postoperative delirium. We powered our study to be able to detect a correlation of greater than 0.20 based on the best estimation from our previous studies examining the association between the preoperative CSF Aβ40/Tau ratio with the postoperative Brief Visuospatial Memory Test Total Recall score, and between the preoperative CSF Aβ42/Tau ratio with the postoperative Hopkins Verbal Learning Test Retention score29. Our power calculation determined that a sample size of 150 participants would be sufficient.
Figure 1. Flow diagram.
The flow diagram shows that 354 participants were initially screened for the studies and finally 153 participants were included in the data analysis.
Anesthesia, CSF sample collection, and measurement of β-Amyloid and Tau
All of the participants had spinal anesthesia for the scheduled surgery. One ml of CSF was collected from a spinal needle by anesthesiologists during the spinal anesthesia before the administration of the local anesthetic. The CSF was collected in an eppendorf tube and was immediately placed in ice. The CSF was then stored in a −80 °C degree freezer until the time of measurement when the samples were thawed. Levels of Aβ (including Aβ40 and Aβ42) and total Tau in the CSF were measured by using Enzyme-linked immunosorbent assay (ELISA) kits (Aβ40: Cat. # 292-62301; Aβ42: Cat. # 296-64401, Wako, Richmond, VA; Tau: Cat. # KHB0041, Invitrogen, San Francisco, CA) as described in our previous studies29. The assay to measure the CSF Aβ level was performed in triplicate. The assay to measure the levels of CSF Tau was performed in duplicate because we did not have a sufficient volume of CSF. The average level of CSF Aβ40, Aβ42, and Tau was obtained and used for the data analysis. All of the CSF samples in the current study were analyzed using the same methods by one person (Y.D.); therefore the relative differences in the values between the participants were consistent.
Surgery
All of the participants had total hip or total knee replacement under spinal anesthesia by one surgeon to avoid potential confounding factors owing to varying surgery skills or different surgical practices. All of the participants had standardized perioperative care, including spinal anesthesia, sedation and postoperative pain control. The spinal anesthesia included the administration of 0.5% bupivacaine into spinal space (mean dose: 3.24 ± 0. 63 ml). Most of patients received versed (midazolam, intravenous administration, mean dose: 2.38 ± 0.88 mg) before the surgery and propofol (intravenous administration, mean dose: 279.89 ± 162.1 mg) during the surgery for sedation. We did not measure the depth of sedation in the current studies. The postoperative pain control included a standard postoperative pain management, e.g., morphine patient controlled analgesia (1 mg morphine per injection, interval time of injection was 6 minutes with total of 10 mg morphine per hour). There were no major complications among the participants during the immediate postoperative period.
Postoperative interviews
Trained clinical research assistants (C.S. and S.W.) interviewed the patients on the first and second day post-surgery. The assessment of delirium was performed once per day between 8:00 am to 10:00 am. Patient notes were not reviewed for episodes of delirium which could occur outside the time of assessment. The clinical research assistants who performed the delirium assessments in the current study had good training and went through quality control procedures. We used state-of-the-art delirium detection methods, which tend to report a higher incidence of delirium. The interview included the Confusion Assessment Method (CAM) and Memorial Delirium Assessment Scale (MDAS). CAM is a diagnostic algorithm used to determine the presence or absence of delirium30, 31. The CAM algorithm consists of four clinical criteria: (1) acute onset and fluctuating course, (2) inattention, (3) disorganized thinking, and (4) altered level of consciousness. For delirium to be defined, both the first and the second criteria have to be present, plus either the third and/or the fourth criterion. MDAS was used to determine the severity of delirium30, 32 by quantifying the symptoms related to delirium based on 10 features, including reduced level of consciousness/awareness, disorientation, short-term memory impairment, impaired digit span, reduced ability to maintain and shift attention, disorganized thinking, perceptual disturbance, delusions, decreased or increased psychomotor activity, and sleep-wake cycle disturbance. Each of the features is scored from 0 (best) to 3 (worst symptom) with a maximal score of 30 for all of the features. Given that MDAS can evaluate the features of delirium described in CAM and can be administrated by trained nonclinical interviewers, we chose to use the MDAS rather than the Delirium Rating Scale (DRS)33. The highest MDAS score from the postoperative day one and day two was presented in the studies. MDAS scores were evaluated for all patients, regardless of whether they met CAM criteria on that day.
Statistical analysis
The data were presented as median and interquartile range (25% percentile – 75% percentile) for Aβ40/Tau or Aβ42/Tau ratio, and mean ± standard deviation (SD) for other measurements. Postoperative delirium incidence was presented as a percentage. We used Mann-Whitney test to determine the difference in Aβ40/Tau or Aβ42/Tau ratio between the participants with and without postoperative delirium. Chi-square test was used to compare the postoperative delirium incidence between the participants who had first (lowest) quartile of Aβ40/Tau or Aβ42/Tau ratio and the combination of the participants who had 2nd (lowest), 3rd] (lowest) and 4th (highest) quartile of Aβ40/Tau or Aβ42/Tau ratio. Finally, we applied simple linear regression to determine the association between the Aβ40/Tau or Aβ42/Tau ratio and MDAS scores, and multiple linear regression to determine the association after the adjustment of age and gender. The regression coefficient ± standard error (SE) was used to illustrate the association between Aβ40/Tau or Aβ42/Tau ratio and MDAS score. P-values less than 0.05 were considered statistically significant. We used SAS (SAS institute Inc., Cary, NC) software (version 9.2) and Prism 6 software (La Jolla, CA) to analyze the data.
Results
Characteristics of participants
Three hundred and fifty-four eligible participants were screened, among them 244 participants provided informed consent for the study. Ninety-one participants were subsequently excluded from the study owing to various reasons (see Figure 1, the Flow diagram), yielding 153 participants who were included in the final data analysis. The age, gender, and education of the 91 patients who were excluded were comparable to those of the 153 participants who were finally included in the data analysis. The demographic and clinical data of the participants were presented in Table 1. Thirty-one participants (20%) were defined as having postoperative delirium by using CAM. The median of the highest MDAS score of all participants (regardless of delirium status) over the first two postoperative days was 3 (2 – 5) (median and 25% – 75% percentile). The median of the highest MDAS score of participants with postoperative delirium was 7 (5 – 10), which was higher than that of the participants without postoperative delirium [3 (2 – 4) (P<0.0001)]. Because we have previously demonstrated that MDAS scores have prognostic significance even in patients without delirium30, we analyzed the MDAS scores in the entire population, not just in those with delirium.
Table 1.
Characteristics of the participants
| (N = 153) | ||
|---|---|---|
| Age (years) | ||
| Mean ± SD | 71 ± 5 | |
| 64–69 | 73 (48%) | |
| 70–75 | 43 (28%) | |
| 76–80 | 37 (24%) | |
| Male sex - no. (%) | 80 (52%) | |
| Race or ethnic group, No. (%) | ||
| White | 151 (98.7%) | |
| Black | 1 (0.65%) | |
| Hispanic | 0 | |
| Asian Indian | 0 | |
| Others or unknown | 1 (0.65%) | |
| Education, No. (%) | ||
| Graduate of College or Postgraduate School | 110 (72%) | |
| Some College/Vocational/Technical Program | 17 (11%) | |
| High School Graduate/GED | 19 (12%) | |
| Less than high school graduation | 4 (3%) | |
| Unknown | 3 (2%) | |
| Height (cm) Mean ± SD | 170 ± 10 | |
| Body Weight (kg) Mean ± SD | 85 ± 17 | |
| BMI (kg/m2) Mean ± SD | 29 ± 5 | |
| ASA Class | ||
| I | 1 | |
| II | 119 | |
| III | 27 | |
| Unknown | 6 | |
| Length of Anesthesia (minutes) | 125 ± 18 | |
| Length of Surgery (minutes) | 81 ± 16 | |
| Total Hip Arthroplasty/Replacement | 72 (46%) | |
| Total Knee Arthroplasty/Replacement | 83 (54%) | |
| Estimated Blood Loss (ml) Mean ± SD | 162 ± 93 | |
| Aβ40 (Median and 25% – 75% percentile) | 4820 (3800 – 6411) pg/mL | |
| Aβ42 (Median and 25% – 75% percentile) | 570 (370 – 768) pg/mL | |
| Tau (Median and 25% – 75% percentile) | 380 (303 – 519) pg/mL | |
| Aβ40/Tau ratio (Median and 25% – 75% percentile) | 12.6 (9.2 – 16.1) | |
| Aβ42/Tau ratio (Median and 25% – 75% percentile) | 1.4 (0.9 – 2.1) | |
The length of Anesthesia was defined from the time anesthesiologists started the spinal anesthesia in the participants to the time when the participants were sent to the post-anesthesia care unit. The length of surgery was defined from the time of initial incision to the time of the closure of the skin. The values of Aβ40, Aβ42, Tau, Aβ40/Tau ratio and Aβ42/Tau ratio in the CSF of all 153 participants were obtained using ELISA methods (see text for details). ASA, American Society of Anesthesiologists; GED, general educational development; cm, centimeter; min, minute; kg, kilogram; ml, milliliter, SD, standard deviation; Aβ, β-amyloid; CSF, cerebrospinal fluid.
We did not include patients with dementia, stroke, or psychosis in the studies because we believed we would not be able to recruit enough participants with dementia, stroke, or psychosis in the studies to determine the contribution of these variables to postoperative cognitive delirium. The primary goal of the current studies was to assess a concept that Aβ and/or Tau may contribute to the neuropathogenesis of delirium, rather than validating Aβ and Tau as a biomarker for delirium. Therefore, pre-operative cognitive function (e.g., MMSE) was not determined in the current studies.
The distribution of postoperative delirium incidence in quartiles of preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio
The intra-assay coefficient of variations of CSF Aβ40, Aβ42, and Tau were 21.8%, 18.7% and 6.8%, respectively. The inter-participant coefficient variations of CSF Aβ40, Aβ42, and Tau were 35.5%, 45.7% and 50.4%, respectively. There was no significant difference among the values of CSF Aβ40 (F = 0.028, P = 0.963, N.S., One-Way ANOVA), Aβ42 (F = 1.600, P = 0.215, N.S., One-Way ANOVA), and Tau (P = 0.789, N.S., student t-test) obtained in different measurements.
The average ratio of preoperative CSF Aβ40/Tau and Aβ42/Tau from the participants were 12.6 (9.2 – 16.1) and 1.4 (0.9 – 2.1) (median and 25% – 75% percentile), respectively. We then compared these ratios in the participants with postoperative delirium and those without it. Mann-Whitney test showed that the preoperative CSF Aβ40/Tau [12.2 (8.1 – 14.8) or Aβ42/Tau ratio [1.3 (0.7 – 1.9)] in the participants who developed postoperative delirium was not significantly different from those who did not develop postoperative delirium [Aβ40/Tau: 12.6 (9.6 – 16.1), P = 0.241; or Aβ42/Tau ratio: 1.4 (1.0 – 2.1), P = 0.192].
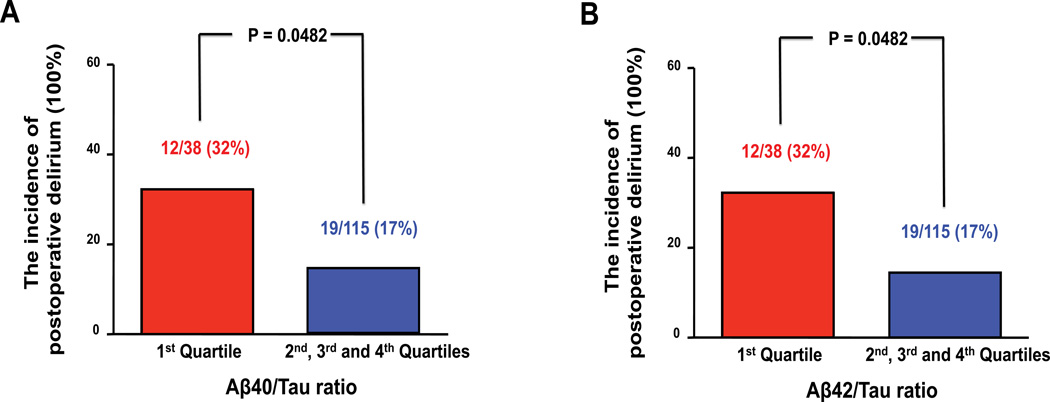
However, the relationship between preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio and postoperative delirium incidence may not be linear (e.g. a threshold effect), we therefore divided the participants into quartiles according to the levels of preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio. We compared the incidence of postoperative delirium among these quartiles and found that more postoperative delirium occurred in the lowest quartile of CSF Aβ40/Tau (32%) or CSF Aβ42/Tau ratio (32%) than in the rest of three quartiles of the Aβ40/Tau ratio (2nd: 11%, 3rd: 21% and 4th: 18%) or the Aβ42/Tau ratio (2nd: 8%, 3rd: 24% and 4th: 18%). Therefore, we dichotomized the preoperative Aβ/Tau ratio and compared the postoperative delirium incidence between the participants in the 1st quartile and the participants in the combination of the 2nd, 3rd, and 4th quartiles. We found a significantly higher incidence of delirium in participants with CSF Aβ40/Tau ratio in the lowest quartile versus all others (32% versus 17%, P = 0.0482) and in participants with CSF Aβ42/Tau ratio in the lowest quartile versus all others (32% versus 17%, P = 0.0482) (Figure 2).
Figure 2. The postoperative delirium incidence in the 1st quartile and the combination of the other three quartiles of CSF Aβ40/Tau or Aβ42/Tau ratio.
Chi-square test shows that there is a significant difference in the postoperative delirium incidence between the 1st quartile (red bar) and the combination of the 2nd, 3rd, and 4th quartiles (blue bar) of CSF Aβ40/Tau (A) or Aβ42/Tau ratio (B).
The distribution of postoperative delirium severity in quartiles of preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio
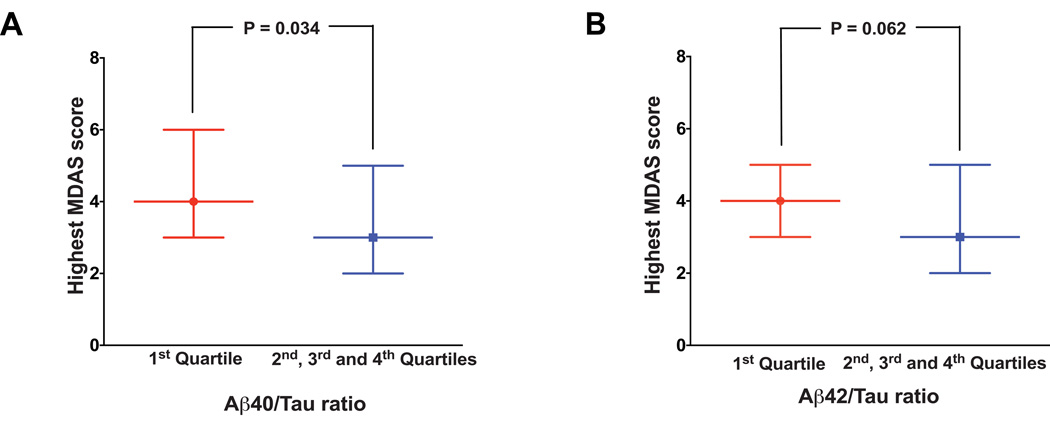
Next, we asked whether the preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio could also be associated with the postoperative delirium severity. We therefore compared the MDAS score, the measurement of delirium severity, between the participants in the 1st quartile and the participants in the combination of the 2nd, 3rd, and 4th quartiles. We found that the median of the highest MDAS score (4, 2 – 5) of the participants in the 1st quartile of CSF Aβ40/Tau ratio was significantly higher than that of the participants in the combination of the 2nd, 3rd, and 4th quartiles of CSF Aβ40/Tau ratio (3, 2 – 5, P = 0.034, Figure 3A). The median of the highest MDAS score (4, 2 – 6) of the participants in the 1st quartile of CSF Aβ42/Tau ratio were borderline higher than that of the participants in the combination of the 2nd, 3rd, and 4th quartiles of CSF Aβ42/Tau ratio (3, 2 – 5, P = 0.062, Figure 3B).
Figure 3. The postoperative delirium severity in the 1st quartile and the combination of the other three quartiles of CSF Aβ40/Tau or Aβ42/Tau ratio.
A. Mann-Whitney test suggests that there is a significant difference in the highest MDAS score between the 1st quartile (red bar) and the combination of the 2nd, 3rd, and 4th quartiles (blue bar) of CSF Aβ40/Tau. B. Mann-Whitney test suggests that there is a borderline difference in the highest MDAS score between the 1stquartile (red bar) and the combination of the 2nd, 3rd, and 4th quartiles (blue bar) of CSF Aβ42/Tau ratio.
Preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio and postoperative delirium severity
Finally, we determined the linear association between the preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio and the MDAS score post-surgery. Using an unadjusted simple linear regression, we found that the preoperative CSF Aβ40/Tau or Aβ42/Tau ratio was significantly correlated (negatively) with the highest MDAS score (Aβ40/Tau −0.12±0.05, P = 0.014; Aβ42/Tau: −0.65±0.26, P = 0.013) (Table 2). Thus, as CSF Aβ/Tau goes down, the MDAS scores go up, as would be expected given the previous association of low CSF Aβ40/Tau ratio with the postoperative delirium incidence. Multiple linear regression, after adjusting for age and gender, showed that the preoperative CSF Aβ40/Tau (−0.12±0.05, P = 0.018) or Aβ42/Tau (−0.62±0.27, P = 0.022) ratio remained significantly correlated (negatively) with the highest MDAS score (Table 2). Age did not contribute to the association between Aβ40/Tau ratio (P = 0.631) or Aβ42/Tau ratio (P = 0.757) and the highest MDAS score; gender also did not contribute to the association between Aβ40/Tau ratio (P = 0.439) or Aβ42/Tau ratio (P = 0. 679) and the highest MDAS score. We did not include evaluation of other peri-operative variables (e.g., postoperative pain medication) in the analysis. As opposed to age and gender, these postoperative variables are highly complicated and require sophisticated adjustment. For instance, nearly all patients are exposed to postoperative opioids, therefore, meaningful adjustment for postoperative pain medication requires consideration of exposures to individual opioid agents, the dose of exposure, and the time and duration of exposure. Our current study was not sufficiently large to adjust for such complex variables. We will take these factors into account in future larger scale studies.
Table 2.
Correlation between MDAS score and the CSFAβ40/Tau orAβ42/Tau ratio
| Highest MDAS score | ||||
|---|---|---|---|---|
| Un-adjusted | Adjusted by age and gender | |||
| Regression coefficient ± SE | P | Regression coefficient ± SE | P | |
| Aβ40/Tau ratio | −0.12 ± 0.05 | 0.014 | −0.12 ± 0.05 | 0.018 |
| Aβ42/Tau ratio | −0.65 ± 0.26 | 0.013 | −0.62 ± 0.27 | 0.022 |
The left panel of the table illustrates the results of the regression coefficients for CSF Aβ40/Tau or Aβ42/Tauratio with highest MDAS score in a simple linear regression. The right panel of the table shows the regression coefficients in multiple linear regressions including adjustment of age and gender.
MDAS, Memorial Delirium Assessment Scale; CSF, cerebrospinal fluid; SE, standard error.
Discussion
We assessed the association between preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio and the incidence and severity of postoperative delirium in this prospective study of 153 older adults who had total hip and knee replacement under spinal anesthesia. Aβ40, Aβ42, and Tau have been reported to contribute to neurotoxicity, cognitive dysfunction, dementia, and postoperative cognitive changes [14, 15, 17–19, 23–29, reviewed in9], and dementia is a risk factor of delirium4, 34. Therefore, we chose Aβ40, Aβ42 and Tau in the studies to determine whether these proteins may also contribute to postoperative delirium.
We found that the patients in the lowest quartile of CSF Aβ40/Tau and CSF Aβ42/Tau ratios (consistent with the AD biomarker) had the highest incidence of delirium (Figure 2) and more severe symptoms of delirium represented by higher MDAS scores (Figure 3). We also found that lower CSF Aβ40/Tau and CSF Aβ42/Tau ratios were significantly associated with greater postoperative delirium severity represented by a higher MDAS score (Table 2). Collectively, these findings suggest that Aβ and Tau may contribute to the neuropathogenesis of postoperative delirium, pending further studies.
Delirium incidence is a dichotomous outcome, therefore we did not use linear models to analyze the incidence of postoperative delirium. Preoperative CSF Aβ/Tau ratio was not significantly associated with the postoperative delirium incidence, but was associated with the severity of postoperative delirium. We believe this difference is explained primarily by issues of statistical power. It is notable that lower preoperative CSF Aβ/Tau ratio is associated with both higher incidence and greater symptom severity of postoperative delirium, which was demonstrated in Figures 2 and 3, respectively.
The postoperative delirium incidence depends on type of surgery, varying from 12% (otolaryngological surgery) up to 50% (major abdominal surgery) [reviewed in7]. The postoperative delirium incidence in the patients who have total joint replacement has been reported to be from 3.6 to 41% [35–39, reviewed in40]. The incidence of postoperative delirium after total joint replacement in all ages of patients is 9 – 15% [reviewed in40], and this incidence of postoperative delirium will be higher in older adults. The variation in the postoperative delirium incidence could be due to the influence of perioperative factors, including sedation levels41, cognitive status, history of central nervous system disease, and postoperative pain levels42. Therefore, the postoperative delirium incidence (20%) in the current study was consistent with the incidence reported in other studies of total knee and hip replacement, demonstrating validity to our delirium assessment methods.
In a prospective cohort study in older adults with hip fracture, Witlox et al. found that cerebrospinal fluid Aβ42, Tau, and phosphorylated Tau were not associated with postoperative delirium43. However, the studies by Witlox et al. did not assess the association of preoperative CSF Aβ/Tau ratio with the postoperative delirium43. In the current studies, we did not find the association between preoperative CSF Aβ40, Aβ42, or Tau with the incidence or severity of postoperative delirium either (data not shown). However, we investigated the association between the preoperative CSF Aβ40/Tau or Aβ42/Tau ratio and the incidence and severity of postoperative delirium, and we found an inverse association. Also of note, the subject population in the studies by Witlox et al. (patients who had surgical repair for acute hip fracture) was different from the participants in the current studies (patients who had elective total hip or total knee replacement). Taken together, the cohort and the variables measured were different between the studies by Witlox et al. and our current studies, which might explain the different findings and conclusions.
Lower levels of CSF Aβ42 and higher levels of CSF Tau are associated with the progression of AD14, 15, 17–19. Therefore, CSF Tau/Aβ42 ratio has been used for the diagnosis of AD dementia and prediction of its progress. The higher the CSF Tau/Aβ42 ratio20 or the lower CSF Aβ42 to Tau ratio21, the worse the cognitive function in AD patients [reviewed in22]. Consistently, the current findings showed that the lower preoperative CSF Aβ40/Tau or Aβ42/Tau ratio might also predict postoperative delirium and suggested that more severe postoperative delirium symptoms may occur. Dementia is known as the most consistent risk factor of delirium4, 34. Taken together, these findings suggest that some AD neuropathogenesis, e.g., accumulation of Aβ in the brain, could also be part of the neuropathogenesis of postoperative delirium. The future studies to further test this hypothesis are warranted.
Moreover, low CSF A level represents high brain A amounts, owing to the sequestration of Aβ into brain amyloid plaques44, 45, and CSF Tau levels represent brain Tau levels44, 45. Therefore, the findings that a lower CSF Aβ/Tau ratio is associated with higher incidence and greater severity of postoperative delirium also suggest that elevated brain Aβ levels may be associated with postoperative delirium. This hypothesis is supported by the fact that age is a risk factor of delirium46, and aging is associated with elevated levels of Aβ47 in the brain. Further studies are needed to test this hypothesis by determining whether the amount of amyloid in the brain is associated with the incidence and severity of postoperative delirium, e.g., by using positron emission tomographic imaging (PET) for amyloid48.
We previously demonstrated that preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio was associated with certain domains of postoperative cognitive change29, and our current study showed that the preoperative CSF Aβ40/Tau or CSF Aβ42/Tau ratio was associated with the incidence and severity of postoperative delirium symptoms. These results suggest that changes in the levels of Aβ and Tau in CSF and brain may be a common neuropathogenesis underlying both postoperative delirium and postoperative cognitive dysfunction. This hypothesis is further supported by the findings that postoperative delirium is associated with a significant decline in cognitive ability during the first year after cardiac surgery4. Collectively, these results would promote further studies to determine the potential association, neuropathologically and behaviorally, among postoperative delirium, postoperative cognitive dysfunction, and dementia.
Postoperative delirium has been suggested to relate to neuroinflammation49. However, although all patients may have a surgery-induced increase in pro-inflammatory cytokines in the blood that can enter the brain through the blood brain barrier50, 51 to induce neuroinflammation, not every patient develops postoperative delirium. Thus, it is plausible that patients who develop postoperative delirium have other changes in the brain that facilitate neuroinflammation. The findings from the current studies that lower CSF Aβ/Tau ratio is associated with higher incidence and greater severity of postoperative delirium symptoms suggest that Aβ or Tau could be one of these changes.
It has been reported that there are large variations in the levels of CSF Aβ and Tau between the different studies52, which could be caused by the differences in analytical procedures and the analytical kits. The values of Aβ and Tau in our current studies might also be different from those of the other studies. However, the CSF Aβ and Tau levels in the current studies were measured using the same methods by the same person, who had ample experience with the assays (Y.D). Therefore, although the absolute values of CSF Aβ and Tau may differ from those reported in other studies, the relative differences in the CSF Aβ and Tau levels between the subjects are consistent. Moreover, the intra-assay coefficient of variations of CSF Aβ40 (21.8%), Aβ42 (18.7%), and Tau (6.8%) were still in the acceptable to good range, and within the range reported elsewhere in the literature (13%36%)52. Finally, there was no significant difference among the repeated values of CSF Aβ40, Aβ42, and Tau obtained in different measurements of our current studies. Taken together, we believe the results provide strong evidence that the associations between the CSF Aβ/Tau ratio and the postoperative delirium incidence and severity in the current studies are valid.
Our study has several limitations. First, the majority of the participants were white and had education beyond high school. It is unknown whether the association between CSF Aβ/Tau ratio and postoperative delirium would still exist in non-white participants with lower education levels. Second, we did not determine the preoperative cognitive function (e.g., MMSE) in current studies. Therefore, it is unknown whether the relationship between preoperative CSF Aβ/Tau ratio and postoperative delirium remains significant after adjusting for measured preoperative cognitive function. However, the primary goal of the current studies was not to validate the CSF Tau/Aβ ratio as an independent predictive biomarker for delirium, but rather to assess a concept that Aβ and Tau might contribute to the neuropathogenesis of delirium. Therefore, we did not want to adjust for MMSE because low preoperative cognitive function (measured by MMSE) would be on the causal pathway between Aβ and Tau effects and delirium. However, given we did not perform baseline cognitive function, we could have missed some (milder) cases of dementia. Third, we did not assess whether patients had preoperative delirium. However, the patients all came to hospital by themselves for elective surgery, and they were assessed, including mental function examination, by both nurses and anesthesiologists before surgery. Therefore, the likelihood of participants having preoperative delirium is low. Finally, the spectrum of delirium was mild in the current study as the MDAS score was relatively low. The observation could be due to the fact that the population of the current study was relatively healthy and, it is possible that MDAS scores might represent mild reversible cognitive impairment. Nevertheless, our findings with delirium severity complement and extend the findings with CAM-defined postoperative delirium.
In conclusion, we have found that the patients who have lower preoperative CSF Aβ40/Tau or Aβ42/Tau ratio, particularly those in the lowest quartile, are more likely to develop postoperative delirium and have more severe symptoms. These findings have established a system and generated a concept for the future studies. If confirmed and extended in future studies, these findings may shed light on the currently undefined neuropathogenesis of postoperative delirium. These studies will hopefully promote the development of more targeted interventions to prevent and treat postoperative delirium, ultimately leading to safer surgery care and better postoperative outcomes for patients.
Acknowledgements
This study was supported by National Institutes of Health grants (Bethesda, Maryland) (R21 AG029856, R01 GM088801, R21AG038994, and R01 AG041274); Investigator-Initiated Research Grant from Alzheimer’s Association (Chicago, IL); and Cure Alzheimer’s Fund (Wellesley, MA) (to Dr. Zhongcong Xie). Dr. Marcantonio was funded in part by grants R01AG030618, P01AG031720, and a Mid-Career Investigator Award (K24AG035075) from the National Institute on Aging.
Abbreviations
- Aβ
β-Amyloid protein
- CSF
cerebrospinal fluid
- CAM
Confusion Assessment Method
- MDAS
Memorial Delirium Assessment Scale
Footnotes
Disclosure Statement:
The authors have no conflict of interest to disclose.
Author Contribution:
Study concept and design: Marcantonio, Sunder and Burke, and Xie.
Acquisition of data: Swain, Ward, and Dong.
Analysis and interpretation of data: Marcantonio, Zhang, Escobar, Zheng and Xie
Drafting of the manuscript: Marcantonio and Xie.
Critical revision of the manuscript for important intellectual content: Marcantonio and Xie.
Obtained funding: Xie.
Administrative, technical, and material support: Sunder, Burke, Zhang and Xie.
Study supervision: Xie.
Zhongcong Xie had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994 Jan 12;271(2):134–139. [PubMed] [Google Scholar]
- 2.Liu LL, Leung JM. Predicting adverse postoperative outcomes in patients aged 80 years or older. Journal of the American Geriatrics Society. 2000 Apr;48(4):405–412. doi: 10.1111/j.1532-5415.2000.tb04698.x. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK. Delirium in older persons. N Engl J Med. 2006 Mar 16;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 4.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012 Jul 5;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010 Feb;97(2):273–280. doi: 10.1002/bjs.6843. [DOI] [PubMed] [Google Scholar]
- 6.Jankowski CJ, Trenerry MR, Cook DJ, et al. Cognitive and functional predictors and sequelae of postoperative delirium in elderly patients undergoing elective joint arthroplasty. Anesthesia and analgesia. 2011 May;112(5):1186–1193. doi: 10.1213/ANE.0b013e318211501b. [DOI] [PubMed] [Google Scholar]
- 7.Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best practice & research Clinical anaesthesiology. 2012 Sep;26(3):277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. British journal of anaesthesia. 2009 Dec;103(Suppl 1):i41–i46. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010 Jan 28;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 10.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of Neurology. 2006 Mar;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 11.Forsberg A, Engler H, Almkvist O, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiology of aging. 2008 Oct;29(10):1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Grimmer T, Riemenschneider M, Forstl H, et al. Beta amyloid in Alzheimer's disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biological psychiatry. 2009 Jun 1;65(11):927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolboom N, van der Flier WM, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009 Sep;50(9):1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 14.Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA : the journal of the American Medical Association. 2003 Apr 23–30;289(16):2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 15.Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2004 Apr;1(2):213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Archives of Neurology. 2009 Mar;66(3):382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 17.Blom ES, Giedraitis V, Zetterberg H, et al. Rapid progression from mild cognitive impairment to Alzheimer's disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE epsilon4/epsilon4 genotype. Dementia and geriatric cognitive disorders. 2009;27(5):458–464. doi: 10.1159/000216841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samgard K, Zetterberg H, Blennow K, Hansson O, Minthon L, Londos E. Cerebrospinal fluid total tau as a marker of Alzheimer's disease intensity. International journal of geriatric psychiatry. 2010 Apr;25(4):403–410. doi: 10.1002/gps.2353. [DOI] [PubMed] [Google Scholar]
- 19.Wallin AK, Hansson O, Blennow K, Londos E, Minthon L. Can CSF biomarkers or pre-treatment progression rate predict response to cholinesterase inhibitor treatment in Alzheimer's disease? International journal of geriatric psychiatry. 2009 Jun;24(6):638–647. doi: 10.1002/gps.2195. [DOI] [PubMed] [Google Scholar]
- 20.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009 Apr;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet neurology. 2009 Jul;8(7):619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 22.Holtzman DM. CSF biomarkers for Alzheimer's disease: current utility and potential future use. Neurobiol Aging. 2011 Dec;32(Suppl 1):S4–S9. doi: 10.1016/j.neurobiolaging.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomonov I, Korkotian E, Born B, et al. Zn2+-Abeta40 complexes form metastable quasi-spherical oligomers that are cytotoxic to cultured hippocampal neurons. The Journal of biological chemistry. 2012 Jun 8;287(24):20555–20564. doi: 10.1074/jbc.M112.344036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatip FF, Hatip-Al-Khatib I, Matsunaga Y, Suenaga M, Sen N. Effects of 8-residue beta sheet breaker peptides on aged Abeta40-induced memory impairment and Abeta40 expression in rat brain and serum following intraamygdaloid injection. Current Alzheimer research. 2010 Nov;7(7):602–614. doi: 10.2174/156720510793499048. [DOI] [PubMed] [Google Scholar]
- 25.Shiwany NA, Xie J, Guo Q. Cortical neurons transgenic for human Abeta40 or Abeta42 have similar vulnerability to apoptosis despite their different amyloidogenic properties. Int J Clin Exp Pathol. 2009;2(4):339–352. [PMC free article] [PubMed] [Google Scholar]
- 26.Gabelle A, Roche S, Geny C, et al. Decreased sAbetaPPbeta, Abeta38, and Abeta40 cerebrospinal fluid levels in frontotemporal dementia. Journal of Alzheimer's disease : JAD. 2011;26(3):553–563. doi: 10.3233/JAD-2011-110515. [DOI] [PubMed] [Google Scholar]
- 27.Gao CM, Yam AY, Wang X, et al. Abeta40 oligomers identified as a potential biomarker for the diagnosis of Alzheimer's disease. PLoS One. 2010;5(12):e15725. doi: 10.1371/journal.pone.0015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spies PE, Slats D, Sjogren JM, et al. The cerebrospinal fluid amyloid beta42/40 ratio in the differentiation of Alzheimer's disease from non-Alzheimer's dementia. Current Alzheimer research. 2010 Aug;7(5):470–476. doi: 10.2174/156720510791383796. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z, McAuliffe S, Swain CA, et al. Cerebrospinal Fluid Abeta to Tau Ratio and Postoperative Cognitive Change. Annals of surgery. 2013 Aug;258(2):364–369. doi: 10.1097/SLA.0b013e318298b077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcantonio E, Ta T, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002 May;50(5):850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 31.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990 Dec 15;113(12):941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 32.Breitbart W, Rosenfeld B, Roth A, Smith MJ, Cohen K, Passik S. The Memorial Delirium Assessment Scale. J Pain Symptom Manage. 1997 Mar;13(3):128–137. doi: 10.1016/s0885-3924(96)00316-8. [DOI] [PubMed] [Google Scholar]
- 33.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988 Jan;23(1):89–97. doi: 10.1016/0165-1781(88)90037-6. [DOI] [PubMed] [Google Scholar]
- 34.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Annals of internal medicine. 1993 Sep 15;119(6):474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 35.Bruce AJ, Ritchie CW, Blizard R, Lai R, Raven P. The incidence of delirium associated with orthopedic surgery: a meta-analytic review. Int Psychogeriatr. 2007 Apr;19(2):197–214. doi: 10.1017/S104161020600425X. [DOI] [PubMed] [Google Scholar]
- 36.Lynch EP, Lazor MA, Gellis JE, Orav J, Goldman L, Marcantonio ER. The impact of postoperative pain on the development of postoperative delirium. Anesthesia and analgesia. 1998 Apr;86(4):781–785. doi: 10.1097/00000539-199804000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Rade MC, Yadeau JT, Ford C, Reid MC. Postoperative delirium in elderly patients after elective hip or knee arthroplasty performed under regional anesthesia. Hss J. 2011 Jul;7(2):151–156. doi: 10.1007/s11420-011-9195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams-Russo P, Urquhart BL, Sharrock NE, Charlson ME. Post-operative delirium: predictors and prognosis in elderly orthopedic patients. Journal of the American Geriatrics Society. 1992 Aug;40(8):759–767. doi: 10.1111/j.1532-5415.1992.tb01846.x. [DOI] [PubMed] [Google Scholar]
- 39.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA : the journal of the American Medical Association. 2012 Jul 4;308(1):73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011 May;112(5):1202–1211. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010 Jan;85(1):18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leung JM, Sands LP, Lim E, Tsai TL, Kinjo S. Does Preoperative Risk for Delirium Moderate the Effects of Postoperative Pain and Opiate Use on Postoperative Delirium? The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013 May 6; doi: 10.1016/j.jagp.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witlox J, Kalisvaart KJ, de Jonghe JF, et al. Cerebrospinal fluid beta-amyloid and tau are not associated with risk of delirium: a prospective cohort study in older adults with hip fracture. Journal of the American Geriatrics Society. 2011 Jul;59(7):1260–1267. doi: 10.1111/j.1532-5415.2011.03482.x. [DOI] [PubMed] [Google Scholar]
- 44.Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol. 2009 Mar;66(3):382–389. doi: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 45.Seppala TT, Nerg O, Koivisto AM, et al. CSF biomarkers for Alzheimer disease correlate with cortical brain biopsy findings. Neurology. 2012 May 15;78(20):1568–1575. doi: 10.1212/WNL.0b013e3182563bd0. [DOI] [PubMed] [Google Scholar]
- 46.Inouye SK. Delirium in older persons. The New England journal of medicine. 2006 Mar 16;354(11):1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 47.Fukumoto H, Rosene DL, Moss MB, Raju S, Hyman BT, Irizarry MC. Beta-secretase activity increases with aging in human, monkey, and mouse brain. The American journal of pathology. 2004 Feb;164(2):719–725. doi: 10.1016/s0002-9440(10)63159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of Neurology. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 49.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010 Feb 27;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 50.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993 Sep;47(2):169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 51.Gaykema RP, Goehler LE, Tilders FJ, et al. Bacterial endotoxin induces fos immunoreactivity in primary afferent neurons of the vagus nerve. Neuroimmunomodulation. 1998 Sep-Oct;5(5):234–240. doi: 10.1159/000026343. [DOI] [PubMed] [Google Scholar]
- 52.Mattsson N, Andreasson U, Persson S, et al. The Alzheimer's Association external quality control program for cerebrospinal fluid biomarkers. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 Jul;7(4):386–395. e6. doi: 10.1016/j.jalz.2011.05.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]