Abstract
Influenza is one of the most common infectious diseases endangering the health of humans, especially young children and the elderly. Although vaccination is the most effective means of protection against influenza, frequent mutations in viral surface antigens, low protective efficacy of the influenza vaccine in the elderly, slow production process and the potential of vaccine supply shortage during a pandemic are significant limitations of current vaccines. Adjuvants have been used to enhance the efficacy of a variety of vaccines; however, no adjuvant is included in current influenza vaccines approved in the United States. In this study, we found that a novel adjuvant, rOv-ASP-1, co-administrated with inactivated influenza vaccine using an aqueous formulation, substantially improved the influenza-specific antibody response and protection against lethal infection in a mouse model. rOv-ASP-1 enhanced the magnitude of the specific antibody response after immunization with low doses of influenza vaccine, allowing antigen-sparring by 10-fold. The rOv-ASP-1 formulated vaccine induced a more rapid response and a stronger Th1-associated antibody response compared to vaccine alone and to the vaccine formulated with the adjuvant alum. Importantly, rOv-ASP-1 significantly enhanced cross-reactive antibody responses and protection against challenge with an antigenically distinct strain. These results demonstrate that rOv-ASP-1 is an effective adjuvant that: 1) accelerates and enhances the specific antibody response induced by influenza vaccine; 2) allows for antigen sparing; and 3) augments a Th1-biased and cross-reactive antibody response that confers protection against an antigenically distinct strain.
Keywords: adjuvant, influenza, virus, vaccine
1. Introduction
Seasonal and pandemic influenza virus infections affect millions of people worldwide. Up to 20% of the world population may suffer from influenza any given year [1]. To prevent influenza, the trivalent inactivated influenza vaccine (TIV) has been widely used. While TIV can prevent influenza illness in up to 80% of healthy adults (<65 years of age) [2], their efficacy can be very low in years when influenza strains in the vaccine are not matched with circulating viruses due to antigenic drift and shift. Thus, generation of cross-reactive antibodies is important for developing a better influenza vaccine. In addition, TIV is not effective in the aged population; the protection rate in the elderly (≥ 65 years) can be as low as 30–40% even when the vaccine-induced antibodies are specific for the circulating influenza strain [2].
The use of adjuvants is a proven approach to enhancing the immunogenicity and protective efficacy of vaccines. Adjuvants have also been shown to enhance cross-reactive responses [3]. Furthermore, enhancement of immune responses by adjuvants can allow for antigen sparring, i.e. use of a lower dose of antigen that is still effective. Use of smaller amounts of antigen reduces vaccine cost, and more importantly allows more people to receive the vaccine when supply of vaccine antigen is limited [4, 5]. This is particularly relevant for influenza vaccines since its production in eggs is a long process (6 months) that cannot meet a sudden demand for large quantity of vaccine during an outbreak or pandemic.
At present, there are very few adjuvants commercially available for clinical use in humans [6]. Aluminum salt (Alum) is the first and only adjuvant approved in the United States for general use in vaccines [7, 8]. During the 1960s and 1970s, influenza vaccines commercially available in both the United States and Europe were alum-adsorbed. However, alum was removed from influenza vaccine formulations in the United States in the early 1980s because it only marginally enhances the antibody response while having increased adverse reactions, particularly in children [9–12].
One protein with adjuvant potential is the Onchocerca volvulus activation-associated secreted protein (Ov-ASP-1) [13]. Recent studies have demonstrated that recombinant Ov-ASP-1 (rOv-ASP-1) is a powerful immunostimulatory adjuvant using ovalbumin, HIV-1 polypeptide, SARS-CoV peptide and subunit antigens, and several commercially available vaccines [14–17]. It promotes a balanced Th1/Th2 antibody response and a Th1-biased cellular response to several vaccine antigens [14, 15]. In this study, we have demonstrated in a mouse model that rOv-ASP-1 not only substantially enhanced the magnitude of the specific antibody response after immunization with low doses of influenza vaccine, but also accelerated the antibody responses. Furthermore, rOv-ASP-1 significantly enhanced both cross-reactive antibody responses and protection against challenge with an antigenically distinct strain. Our results demonstrate that rOv-ASP-1 is a promising adjuvant that can be used with inactivated influenza vaccine, enabling both vaccine antigen sparing and enhanced protection against influenza infection.
2. Materials and Methods
2.1. Animals
6~8-week-old female C57BL/6 (B6) mice purchased from Jackson Laboratories (Bar Harbor, Maine) were used for all experiments. All mice were maintained in AAALAC-approved barrier facilities at Drexel University (Philadelphia, PA) and allowed to acclimate for at least one week in the animal facilities prior to use. All animal procedures performed in this study were conducted with the approval of the IACUC of Drexel University.
2.2. Immunization
The TIV used is FLUARIX (2010–2011 Formula) from GlaxoSmithKline and BEI Resources (http://www.beiresources.org) that contains hemagglutinin (HA) from each of the following 3 influenza viruses: A/California/7/2009 NYMC X-181(H1N1); A/Victoria/210/2009 NYMC X-187(H3N2); and B/Brisbane/60/2008. Mice were immunized intramuscularly (i.m.) with different doses of TIV, TIV mixed with purified rOv-ASP-1 [16] (20 μg/mouse), or TIV mixed with Imject Alum (Thermo Scientific, 1.2 mg/mouse).
2.3. Influenza virus and challenge
Influenza A/Puerto Rico/8/34 (PR8; H1N1) virus strain was propagated in specific pathogen-free fertile chicken eggs. Groups of 3–5 mice were challenged intranasally (i.n.) on day 21 post primary immunization or after a secondary immunization. Mice were anesthetized intraperitoneally with 2 mg ketamine and 0.15 mg xylazine and inoculated i.n. with 400xTCID50 of PR8 [18, 19]. Infected mice were weighed daily and those that lost > 25% of their initial body-weight were euthanized according to institutional IACUC guidelines.
2.4. ELISA for total IgG, IgG1, and IgG2c
The influenza-specific antibody response was measured by ELISA as previously described [15, 20]. Unlike BALB/c mice, B6 mice do not have an IgG2a gene, but express a different Th1 antibody IgG2c which cross-reacts with antibody to IgG2a [21–23], which was used to provide a relative measure of IgG2c levels as described previously [24, 25]. In some experiments, purified HA of PR8 used for coating was provided by BEI Resources. The end point titer of antibody was defined as the reciprocal of the highest dilution of plasma giving an optical density (OD) greater than 2 times that of a naïve mouse plasma sample.
2.5. Statistical analysis
The unpaired, two-tailed Student’s t-test was used to determine if the difference in immune responses between groups of mice was significant. Survival rates were analyzed by the log-rank (Mantel-Cox) test. Antibody titers are expressed as mean ± SEM. Results were considered statistically significant when the p value was less than 0.05.
3. Results
3.1. rOv-ASP-1 enhances antibody responses to low doses of influenza vaccine
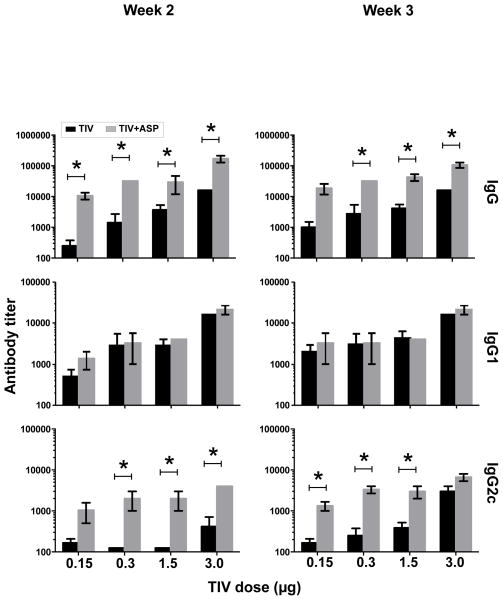
To determine if rOv-ASP-1 could enhance the influenza-specific antibody response, B6 mice were immunized with 0.15, 0.3, 1.5, or 3 μg of each HA of TIV with or without 20 μg rOv-ASP-1. As shown in Fig. 1, at both weeks 2 and 3 after immunization, rOv-ASP-1 significantly enhanced total IgG responses in plasma compared to immunization with TIV alone with each dose of vaccine (p < 0.05). Importantly, the level of IgG after immunization with 0.3 μg plus rOv-ASP-1 was comparable to that induced by 3 μg TIV alone at both week 2 and 3 (p > 0.05), demonstrating that with the addition of rOv-ASP-1 a 10-fold lower dose of vaccine could be utilized to generate a strong total IgG response.
Fig. 1.
Effect of rOv-ASP-1 on the primary antibody response after immunization with different doses of influenza vaccine. B6 mice were vaccinated with different doses (0.15, 0.3, 1.5, 3 μg) of TIV with and without 20 μg rOv-ASP-1. Influenza-specific antibodies (IgG, IgG1, and IgG2c) in the plasma at weeks 2 and 3 after immunization were determined by ELISA using TIV as antigen. Limitation of detection (LOD) is 250. The experiments were repeated 2 times (3–4 mice/group each time) with similar results. * p < 0.05, comparing TIV vs TIV+rOv-ASP-1 at each dose of vaccine.
To further characterize the antibody responses, we measured titers of IgG1 (Th2-type) and IgG2 (Th1-type) induced by immunization. IgG1 titers were not significantly changed with addition of rOv-ASP-1 at either week 2 or 3. In contrast to IgG1, the IgG2c responses were significantly enhanced by rOv-ASP-1 with 0.3, 1.5, and 3 μg of the vaccine at week 2, and with 0.15, 0.3, and 1.5 μg at week 3 (p < 0.05). These results show that addition of rOv-ASP-1 can significantly enhance influenza-specific total IgG and Th1-type IgG2c antibody responses compared to vaccine alone.
3.2. rOv-ASP-1 induces a stronger Th1-associated influenza-specific antibody response compared to the conventional adjuvant alum
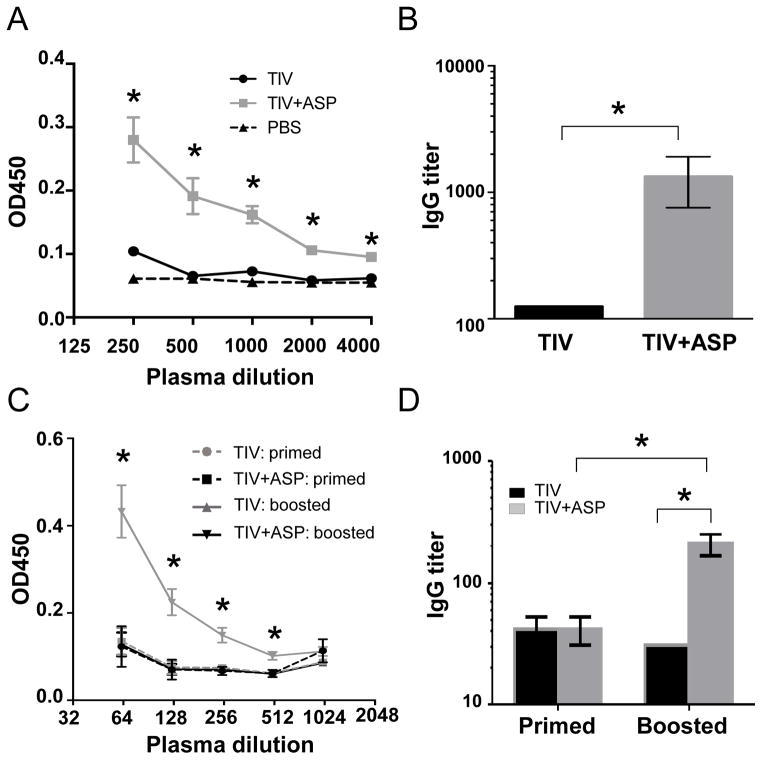
Alum is the only adjuvant licensed in the United States for general use in humans [8]. It strongly promotes a Th2-type, but not a Th1-type, associated antibody response [7, 26, 27]. Our results above show that rOv-ASP-1 enhances predominantly the Th1-type IgG2c response. Importantly, a recent study suggested that the Th1 response induced by influenza vaccine plays a more important role in protection against influenza [28]. Therefore, we compared the abilities of rOv-ASP-1 and alum to enhance the Th1 (IgG2c) and Th2 (IgG1) antibody responses after immunization with TIV. B6 mice were immunized with TIV+rOv-ASP-1 or TIV+Alum; two weeks after immunization titers of total IgG, IgG1, and IgG2c in plasma were determined by ELISA (Fig. 2). Both rOv-ASP-1 and alum significantly enhanced total IgG levels compared to TIV alone (p < 0.05) with no significant difference in total IgG between rOv-ASP-1-vaccinated and alum-vaccinated mice (p > 0.05). The level of IgG1 antibody was significantly higher in TIV+Alum group than both TIV alone and TIV+rOv-ASP-1 groups (p < 0.05). Notably, only TIV+rOv-ASP-1, but neither TIV+Alum nor TIV alone, induced a significant IgG2c response to influenza vaccine. These results demonstrate that rOv-ASP-1 enhances both total IgG and IgG1 responses similar to alum, but elicits a significant influenza-specific IgG2c that is not seen with either TIV alone or with alum in primary immunization.
Fig. 2.
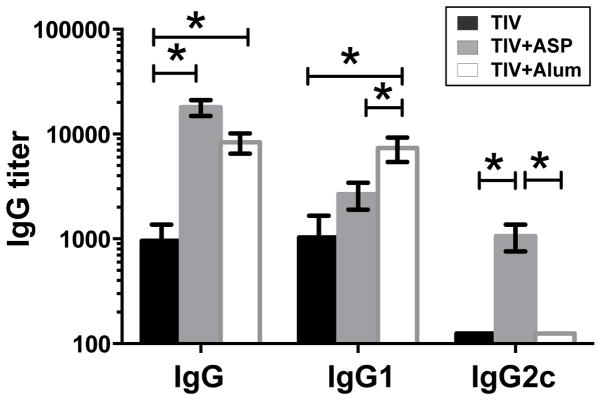
Comparison of rOv-ASP-1 and alum in inducing influenza-specific antibody responses after primary immunization with influenza vaccine. B6 mice were vaccinated with 0.3 μg of TIV alone or TIV combined with 20 μg rOv-ASP-1 or 1.2 mg alum. Titers of influenza-specific antibodies (IgG, IgG1, and IgG2c) in plasma at week 2 after immunization were determined by ELISA using TIV as antigen. Data is the combination of up to 6–12 mice/group from 2–3 independent experiments with similar results. * p < 0.05.
3.3. rOv-ASP-1 accelerates the influenza-specific antibody response after immunization
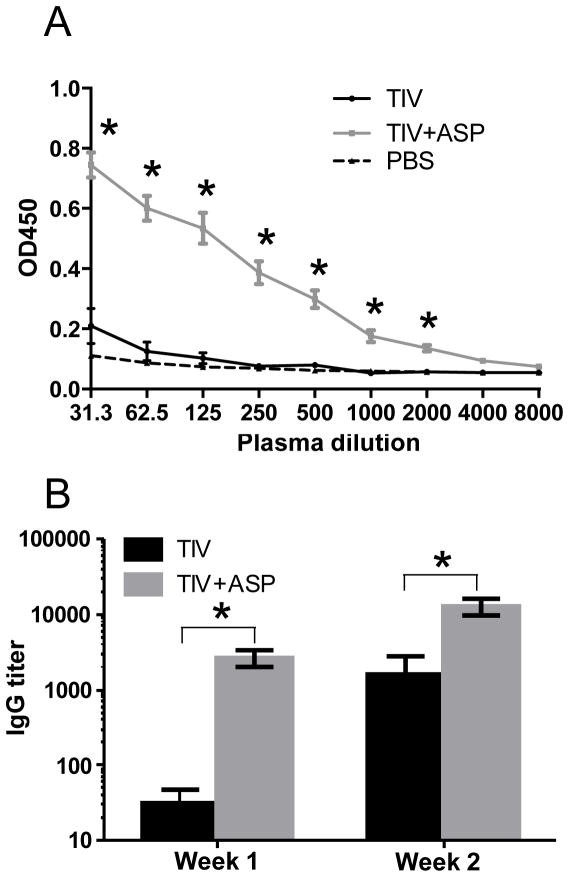
After immunization with inactivated influenza vaccine, it usually takes about two weeks to generate an antibody response. As such, the levels of influenza-specific total IgG in plasma were undetectable at week 1 after immunization with TIV alone. However, the production of influenza-specific IgG was significantly accelerated upon immunization with TIV+rOv-ASP-1 and was readily detectable at week 1 (Fig. 3A & B). Interestingly, the titer of total IgG in the TIV+rOv-ASP-1 group at week 1 was similar to that of the TIV alone group at week 2 (TIV+rOv-ASP-1 vs TIV: p > 0.05) (Fig. 3B). These results demonstrate that when co-administered with influenza vaccine, rOv-ASP-1 can significantly accelerate influenza-specific IgG production after a primary immunization.
Fig. 3.
rOv-ASP-1 accelerates the influenza-specific antibody response. B6 mice were vaccinated with 0.3 μg of TIV, or TIV combined with 20 μg rOv-ASP-1. Influenza-specific total IgG levels in the plasma at weeks 1 and 2 were determined by ELISA using TIV as antigen. (A) OD450 of plasma at week 1, and (B) titers of antibodies at weeks 1 and 2. (LOD is 31.25). The experiments were repeated 3 times (3–4 mice per group each time) with similar results. * p < 0.05, comparing OD450 (A) or IgG titer (B) of TIV+rOv-ASP-1 vs TIV.
3.4. rOv-ASP-1 enhances a cross-reactive antibody response
To test whether rOv-ASP-1 could enhance a cross-reactive antibody response, B6 mice were immunized with 0.3 μg TIV or TIV+rOv-ASP-1, then boosted with the same vaccine they originally received. The cross-reactive responses were determined by ELISA in which the plates were coated with inactivated PR8 virus. PR8 H1N1 virus is antigenically distinct compared to the 3 viral strains in the FLURIX 2010–2011 vaccine. Cross-reactive antibodies were not detected up to 3 weeks after primary immunization in any group (data not shown). However, cross-reactive IgG antibody was found at week 3 after secondary immunization with TIV+rOv-ASP-1 (Fig. 4A). The levels of IgG against PR8 H1N1 virus in the TIV+rOv-ASP-1 group were significantly higher (>10-folds, p < 0.05) than those of the TIV alone group (Fig. 4B).
Fig. 4.
rOv-ASP-1 enhances the influenza cross-reactive antibody response after boosting with influenza vaccine. B6 mice were vaccinated with 0.3 μg of TIV alone or TIV+rOv-ASP-1, and 3 weeks later were boosted with the same reagents in each group. PR8-specific total IgG in plasma was determined by ELISA using plates coated with inactivated PR8 virus (Fig. A & B, week 3 after boosted) or with purified HA of PR8 (Fig. C & D, week 2 after primed or week 3 after boosted). The experiments were repeated 3 times (3–4 mice/group each time) with similar results. * p < 0.05.
We next sought to determine if cross-reactive antibodies elicited by TIV+rOv-ASP-1 were specific for internal viral proteins or epitopes on HA that were conserved between PR8 H1N1 and the NYMC X-181 H1N1 vaccine strain. We performed additional ELISAs using plates coated with purified PR8 HA, instead of PR8 virions that share internal proteins, such as NP, with the vaccine strains. Similar to data from ELISAs coated with PR8 virions in Fig. 4A–B, no cross-reactive antibody was detected after primary immunization, while cross-reactive IgG to purified PR8 HA was found at week 2 after secondary immunization with TIV+rOv-ASP-1, but not with TIV alone (Fig. 4C). The titer of total IgG reactive to purified PR8 HA was significantly higher in the TIV+rOv-ASP-1 group than in the TIV alone group (p < 0.05, Fig. 4D). All plasma samples isolated after both primary and secondary immunization were hemagglutinination inhibition (HAI) assay negative by using PR8 H1N1 virus (data not shown), indicating no cross-reactive antibodies directed against the receptor binding domain of the HA globular head region. Together, these results show that rOv-ASP-1 enhances generation of cross-reactive antibody responses and these cross-reactive antibodies are targeted against HA epitopes conserved across influenza virus strains.
3.5. rOv-ASP-1 enhances the protective efficacy of influenza vaccine against challenge with an antigenically distinct strain
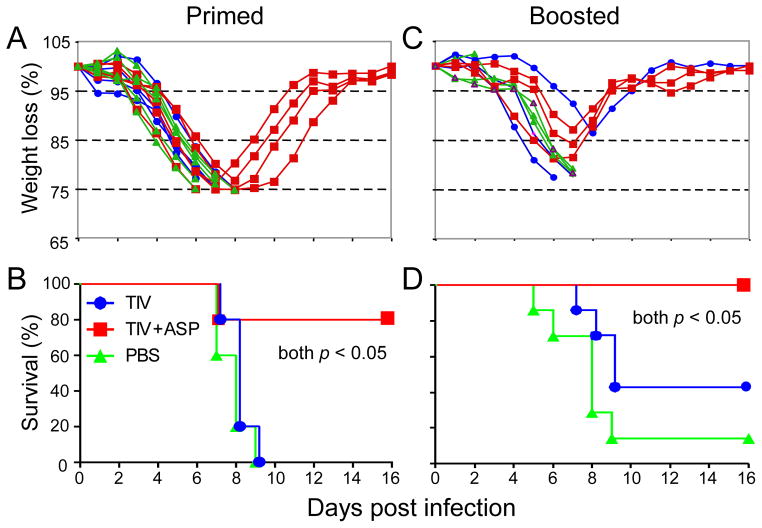
The ability of rOv-ASP-1 to enhance the cross-protective efficacy of influenza vaccine against influenza virus challenge was examined after single immunizations and prime-boost immunizations. B6 mice were immunized with TIV with or without rOv-ASP-1, and three weeks later were challenged with PR8 virus. Infected mice in all 3 groups (TIV, TIV+rOv-ASP-1, and PBS control) exhibited significant morbidity as indicated by loss of weight (Fig. 5A). However, only mice from the group that had received TIV with rOv-ASP-1 gradually recovered. Eighty percent of the mice in the TIV+ rOv-ASP-1 group survived, whereas all mice vaccinated with TIV alone and PBS control succumbed to infection by Day 9 (Fig. 5B). This recovery suggests that while the primary immune response was not sufficient to totally prevent influenza infection, a level of immunity was generated that limited the infection.
Fig. 5.
Morbidity and mortality of immunized mice following challenge with an antigenically distinct strain. B6 mice were vaccinated with 0.3 μg of TIV or TIV+rOv-ASP-1; some mice were boosted three weeks later. Mice were infected i.n. with a lethal dose (400TCID50) of influenza virus PR8, at week 3 after primary (A & B) or booster immunization (C & D). Survival was defined as ≤ 25% weight loss for PR8 infection. Three to five mice in each group. A and C are representative of two experiments with 3–5 mice with similar results. Ten mice/group for B, and 8~9 mice/group for D are the pooled data from the 2 experiments. * p < 0.05.
To test whether or not a protective response could be achieved, mice that received two immunizations (boosted three weeks after primary immunization) were challenged with PR8 three weeks after the second immunization. Mice boosted with TIV+ rOv-ASP-1 lost about 15% of their weight at the peak of infection around Day 7, and regained all lost weight by Day 10 after challenge (Fig. 5C). In addition, all mice boosted with TIV+rOv-ASP-1 survived challenge, compared to 80% survived with one immunization (Fig. 5D). The survival rates were significantly increased in TIV+rOv-ASP-1 group compared with either TIV alone (40%) or PBS group (10%). Together, these results clearly demonstrate that rOv-ASP-1 can significantly enhance the cross-protective efficacy with a low dose of influenza vaccine after both primary and booster immunizations.
4. Discussion and Conclusions
There are very few adjuvants approved by the FDA and commercially available for use in humans in the United States [6]. Recently, a protein with adjuvant properties, named rOv-ASP-1, has been discovered from the helminth parasite Onchocerca volvulus. It is a member of a family of proteins found in both free-living and parasitic nematodes [13]. The recombinant protein has a molecular weight of 24.9 kD and also has angiogenic activity in mice [13]. Our present study demonstrates that rOv-ASP-1 is a powerful adjuvant with immunogenic characteristics that may help overcome limitations of current influenza vaccines. rOv-ASP-1 significantly enhanced total IgG responses compared to immunization with TIV alone at each dose of influenza vaccine tested in our mouse model. Importantly, a low dose of vaccine administered with rOv-ASP-1 induced the total IgG response to a level as high as, if not higher than, immunization with ten times higher dose of vaccine alone. These results demonstrate the potency of rOv-ASP-1 as an adjuvant to enhance the IgG response to TIV and to allow for dose sparing of TIV antigen. Antigen sparing is crucial when there is a pandemic influenza outbreak because the slow process and limited capacity of current influenza vaccine production does not always meet the sudden need for a large amount of vaccine.
Like other adjuvants, the mechanisms of enhanced immune response induced by rOv-ASP-1 are not fully understood. Recently, it has been reported that rOv-ASP-1 primarily binds to APCs and induces Th1-dominated pro-inflammatory cytokines [16]. Consistent with this finding, we observed that addition of rOv-ASP-1 to TIV enhanced not only total IgG, but more significantly the Th1 IgG2c response. Neither vaccine alone nor vaccine with alum induced detectable amounts of IgG2c. This difference in antibody response may be responsible for the difference in protection we observed after challenge with a lethal dose of influenza virus: mice immunized with influenza vaccine plus rOv-ASP-1 provided better protection than immunization with vaccine alone, which contrasts to the report that mice immunized with TIV plus alum suffer from more severe weight loss and have significantly higher virus loads in lungs than those receiving vaccine alone [28]. A recent study indicates that the Th1 response induced by influenza vaccine plays a more important role in protection against influenza infection [28]. Our results are consistent with this finding and further suggest that the Th1 response induced by influenza vaccine plus rOv-ASP-1 may play an important role in cross-protection against influenza infection.
After immunization with TIV, it usually takes about 2 weeks to generate an antibody response. Our results indicate that rOv-ASP-1 can accelerate the generation of an IgG response after vaccination, resulting in high levels of antibodies one week after primary immunization. This is particularly advantageous during an unanticipated pandemic. In addition, the time window between the availability of appropriate vaccines and the start of a seasonal epidemic can be very short in some years due to delay in vaccine manufacture or early outbreaks. An accelerated response induced by influenza vaccine would offer a major advantage in these situations.
The ideal influenza vaccines should offer broad cross-reactive immunity. Cross-reactive antibody usually targets the HA stalk region since it is the conserved part of HA [29]. Our results have shown that co-administration of rOv-ASP-1 can enhance the production of cross-reactive antibodies and confer protection against infection caused by a different influenza virus strain. It is unknown if this effect is due to the enhanced antibody response to the stalk region of HA. However, the lack of HAI positive antibodies against PR8 virus in plasma of mice immunized with TIV+rOv-ASP-1, but protection of these mice from subsequent PR8 influenza infection, suggest that the PR8 protection afforded by the vaccine plus rOv-ASP-1 may be not related to anti-PR8 HA globular head antibodies, but due to enhancement of cross-reactive antibodies targeting the HA stalk or other conserved antigenic sites.
It is interesting that primary immunization with TIV plus rOv-ASP-1 does not induce detectable antibody to PR8 nor prevent weight loss after challenge with PR8 but does result in survival of most mice. This is actually similar to what has been observed in the elderly population receiving TIV: they have low levels of antibody responses to the circulating influenza virus and show influenza symptoms when infected with influenza virus, but demonstrate less severe disease as indicated by fewer hospitalizations than non-immunized elderly who are infected with influenza [30–34]. A secondary immunization of elderly with TIV annually is not a realistic public health strategy. However, if the cross-reactivity observed in our studies was demonstrated to be a broad based cross-reaction against multiple strains of influenza (e.g. all H1N1), influenza vaccination may not need to be annual if the duration of the cross-reactive protection afforded by two immunizations of TIV plus rOv-ASP-1 is shown to be long lasting. A booster immunization with TIV plus rOv-ASP-1 is possible since it could protect against multiple strains of influenza thus eliminating the need for annual immunization. Currently, we are testing if rOv-ASP-1 can enhance antibody response and protection against influenza in aged mice after TIV immunization and if the protection is long lasting.
In summary, we report that the protein adjuvant rOv-ASP-1 enhances and accelerates the influenza-specific antibody response during immunization and confers increased protection in a mouse model. The rOv-ASP-1 enhances a stronger Th1-associated antibody response to influenza vaccine compared to the conventional adjuvant alum, and it enhances cross-reactive antibody responses. Importantly, rOv-ASP-1 enhances the protection afforded by an inactivated influenza vaccine after challenge with an antigenically distinct strain. Our results clearly demonstrate that rOv-ASP-1 is an effective adjuvant in accelerating and enhancing the specific antibody response induced by influenza vaccine, and has the potential to allow antigen sparing and enhance heterologous protection.
Highlights.
rOv-ASP-1 enhances a stronger antibody response to influenza vaccine.
rOv-ASP-1 enhances cross-reactive antibody responses to influenza vaccine.
rOv-ASP-1 enhances the protection afforded by an inactivated influenza vaccine after challenge with a heterologous influenza virus.
Acknowledgments
This project was supported by research grants from the NIAID R43AI085783 and NIA R43AG042993 to J. J. We thank BEI Resources for kindly providing FLUARIX (2010–2011 Formula) and purified HA of PR8 strain.
Abbreviations used
- rOv-ASP-1
recombinant Onchocerca volvulus activation-associated secreted protein-1
- HAU
hemagglutination unit
Footnotes
Conflict of Interest Statement
J. J. is a part-time employee of DMX Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bridges CB, Fukuda K, Cox NJ, Singleton JA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2001;50(RR-4):1–44. [PubMed] [Google Scholar]
- 2.Palese P, Garcia-Sastre A. New directions in vaccine research. J Clin Invest. 2002;109(12):1517–1518. doi: 10.1172/JCI15876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamouda T, Sutcliffe JA, Ciotti S, Baker JR., Jr Intranasal immunization of ferrets with commercial trivalent influenza vaccines formulated in a nanoemulsion-based adjuvant. Clinical and vaccine immunology: CVI. 2011;18(7):1167–1175. doi: 10.1128/CVI.00035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhun AS, Akselsen PE, Sjursen H, Pedersen G, Svindland S, Nostbakken JK, Nilsen M, Mohn K, Jul-Larsen A, Smith I, et al. An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. Vaccine. 2010;29(2):266–273. doi: 10.1016/j.vaccine.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 5.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3(85):85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKee AS, Munks MW, Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Cox JC, Coulter AR. Adjuvants--a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 8.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, Vilaysane A, Mucsi AD, Fong Y, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17(4):479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 9.Davenport FM, Hennessy AV, Askin FB. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968;100(5):1139–1140. [PubMed] [Google Scholar]
- 10.Werner J, Kuwert EK, Stegmaier R, Simbock H. Local and systemic antibody response after vaccination with 3 different types of vaccines against influenza. II. Neuraminidase inhibiting antibodies (author’s transl) Zentralbl Bakteriol A. 1980;246(1):1–9. [PubMed] [Google Scholar]
- 11.D’Errico MM, Grasso GM, Romano F, Montanaro D. Comparison of anti-influenza vaccines: whole adsorbed trivalent, trivalent subunit and tetravalent subunit. Boll Ist Sieroter Milan. 1988;67(4):283–289. [PubMed] [Google Scholar]
- 12.Ionita E, Lupulescu E, Alexandrescu V, Matepiuc M, Constantinescu C, Cretescu L, Velea L. Comparative study of the immunogenicity of aqueous versus aluminium phosphate adsorbed split influenza vaccine C.I. Arch Roum Pathol Exp Microbiol. 1989;48(3):265–273. [PubMed] [Google Scholar]
- 13.Tawe W, Pearlman E, Unnasch TR, Lustigman S. Angiogenic activity of Onchocerca volvulus recombinant proteins similar to vespid venom antigen 5. Mol Biochem Parasitol. 2000;109(2):91–99. doi: 10.1016/s0166-6851(00)00231-0. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald AJ, Cao L, He Y, Zhao Q, Jiang S, Lustigman S. rOv-ASP-1, a recombinant secreted protein of the helminth Onchocercavolvulus, is a potent adjuvant for inducing antibodies to ovalbumin, HIV-1 polypeptide and SARS-CoV peptide antigens. Vaccine. 2005;23(26):3446–3452. doi: 10.1016/j.vaccine.2005.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao W, Du L, Liang C, Guan J, Jiang S, Lustigman S, He Y, Zhou Y. Evaluation of recombinant Onchocerca volvulus activation associated protein-1 (ASP-1) as a potent Th1-biased adjuvant with a panel of protein or peptide-based antigens and commercial inactivated vaccines. Vaccine. 2008;26(39):5022–5029. doi: 10.1016/j.vaccine.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He Y, Barker SJ, MacDonald AJ, Yu Y, Cao L, Li J, Parhar R, Heck S, Hartmann S, Golenbock DT, et al. Recombinant Ov-ASP-1, a Th1-biased protein adjuvant derived from the helminth Onchocerca volvulus, can directly bind and activate antigen-presenting cells. J Immunol. 2009;182(7):4005–4016. doi: 10.4049/jimmunol.0800531. [DOI] [PubMed] [Google Scholar]
- 17.Zhao G, Du L, Xiao W, Sun S, Lin Y, Chen M, Kou Z, He Y, Lustigman S, Jiang S, Zheng BJ, Zhou Y. Induction of protection against divergent H5N1 influenza viruses using a recombinant fusion protein linking influenza M2e to Onchocerca volvulus activation associated protein-1 (ASP-1) adjuvant. Vaccine. 2010;28(44):7233–7240. doi: 10.1016/j.vaccine.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 18.Po JL, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123(8):1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 19.Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marjuki H, Barman S, Webster RG, Webby RJ. Adaptation of pandemic H1N1 influenza viruses in mice. J Virol. 2010;84(17):8607–8616. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Tricoche N, Du L, Hunter M, Zhan B, Goud G, Didier ES, Liu J, Lu L, Marx PA, et al. The adjuvanticity of an O. volvulus-derived rOv-ASP-1 protein in mice using sequential vaccinations and in non-human primates. PloS one. 2012;7(5):e37019. doi: 10.1371/journal.pone.0037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouvin-Marche E, Morgado MG, Leguern C, Voegtle D, Bonhomme F, Cazenave PA. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29(2):92–97. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 22.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Method. 1998;212(2):187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 23.Petrushina I, Tran M, Sadzikava N, Ghochikyan A, Vasilevko V, Agadjanyan MG, Cribbs DH. Importance of IgG2c isotype in the immune response to beta-amyloid in amyloid precursor protein/transgenic mice. Neurosci Letter. 2003;338(1):5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C. TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol. 2007;179(11):7949–7958. doi: 10.4049/jimmunol.179.11.7949. [DOI] [PubMed] [Google Scholar]
- 25.Macleod MK, David A, Jin N, Noges L, Wang J, Kappler JW, Marrack P. Influenza nucleoprotein delivered with aluminium salts protects mice from an influenza A virus that expresses an altered nucleoprotein sequence. PloS one. 2013;8(4):e61775. doi: 10.1371/journal.pone.0061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32(3):155–172. doi: 10.1016/s0169-409x(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 27.Clements ML, Betts RF, Tierney EL, Murphy BR. Serum and nasal wash antibodies associated with resistance to experimental challenge with influenza A wild-type virus. J Clin Microbiol. 1986;24(1):157–160. doi: 10.1128/jcm.24.1.157-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bungener L, Geeraedts F, Ter Veer W, Medema J, Wilschut J, Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008;26(19):2350–2359. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 29.Doms RW. Immunology. Prime, boost, and broaden. Science. 2010;329(5995):1021–1022. doi: 10.1126/science.1195116. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17(1):82–94. doi: 10.1016/s0264-410x(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 31.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989;7(5):385–394. doi: 10.1016/0264-410x(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 32.Keren G, Segev S, Morag A, Zakay-Rones Z, Barzilai A, Rubinstein E. Failure of influenza vaccination in the aged. J Med Virol. 1988;25(1):85–89. doi: 10.1002/jmv.1890250112. [DOI] [PubMed] [Google Scholar]
- 33.Lang PO, Mendes A, Socquet J, Assir N, Govind S, Aspinall R. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence. Clin Interv Aging. 2012;7:55–64. doi: 10.2147/CIA.S25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(2–3):427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]