Abstract
Lithium ephedrates and norcarane-derived lithium amino alkoxides used to effect highly enantioselective 1,2-additions on large scales have been characterized in toluene and tetrahydrofuran. The method of continuous variations in conjunction with 6Li NMR spectroscopy reveals that the lithium amino alkoxides are tetrameric. In each case, low-temperature 6Li NMR spectra show stereoisomerically pure homoaggregates displaying resonances consistent with an S4-symmetric cubic core rather than the alternative D2d core. These assignments are supported by density functional theory computations and conform to X–ray crystal structures. Slow aggregate exchanges are discussed in the context of amino alkoxides as chiral auxiliaries.
Introduction
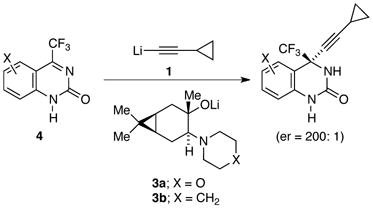
The idea of exploiting organolithium mixed aggregates to control organolithium reactivity and selectivity lurked for several decades,1 but it moved to center stage in the early 1980s largely owing to contributions of Seebach and coworkers.2 In a dramatic application of aggregate-based stereocontrol, the process group at Merck has shown that two equiv each of lithium cyclopropylacetylide 1 and lithium ephedrate 2b effect the 1,2-addition in eq 1 in 98% enantioselectivity.3 Synthesis of more than 50,000 kg of reverse transcriptase inhibitor efavirenz (Sustiva, Stocrin) using this protocol quashed any doubt about the practicality of stoichiometric amino alkoxide auxiliaries.4 Subsequently, DuPont Pharmaceuticals prepared more than 2000 kg of a second-generation reverse transcriptase inhibitor using a seemingly analogous 1,2-addition of lithium acetylide 1 to quinazolinone 4 with an extraordinary 99.5% enantioselectivity (eq 2).5 In this case, however, optimal selectivity was obtained using a 3:1 mixture of norcarane-derived amino alkoxide 3a and 1.
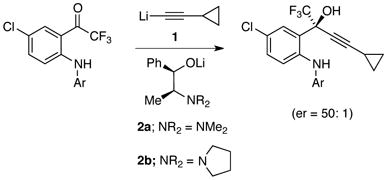 |
(1) |
 |
(2) |
A Cornell–Merck collaboration traced the high enantioselectivity in eq 1 to reaction of substrate with 2:2 (ROLi)2(R′Li)2 mixed tetramer 5.6 A subsequent Cornell–DuPont collaboration attributed the enantioselectivity in eq 2 to external attack of acetylide on 3:1 (ROLi)3(substrate)1 mixed tetramer 6.7 In both reactions, aging the reaction near ambient temperature before addition at low temperature was key to attaining high selectivity.
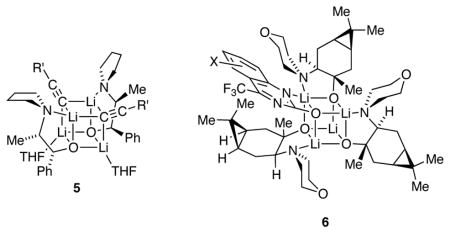
During these structural and mechanistic studies, the solution structures of the amino alkoxide homoaggregates proved elusive. The problem emanated from the difficulties associated with characterizing O-lithiated species in solution, wherein high symmetry and lack of O–Li coupling preclude direct NMR spectroscopic analysis.8 Arnett and coworkers have previously reported a crystal structure of ephedrate 2a displaying an S4-symmetric tetrameric core, but their efforts to determine the solution structure were less conclusive.9
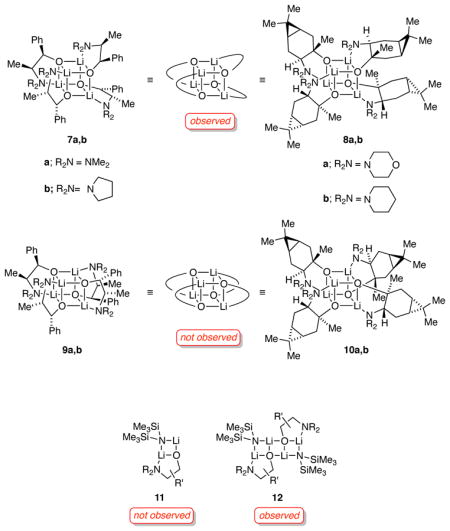
Considerable inroads have now been made toward characterizing the structures of O-lithiated species in solution.8,10,11,12 Using a combination of 6Li NMR spectroscopy, the method of continuous variations (MCV),8,12,13 and density functional theory computations, we show herein that amino alkoxides 2a,b and 3a,b form exclusively unsolvated homotetramers 7a,b and 8a,b.11,12,14 Several intraaggregate exchanges12,15 are shown to be remarkably slow. In conjunction with 6Li–15N double-labeling studies, the application of MCV is extended to distinguish lithium hexamethyldisilazide-lithium amino alkoxide dimers 11 and the corresponding ladders 1216—a structural ambiguity arising from opaque O–Li linkages that has dogged us for many years.12b,17 We also provide a more nuanced view of the benefits of catalytic lithium salts on the enantioselectivities observed by DuPont investigators.
Results
Homoaggregation
Lithium alkoxides 2a,b and 3a,b were generated in toluene by treating the corresponding alcohols3e,18 with 1.0 equiv of labeled lithium hexamethyldisilazide ([6Li]LiHMDS).19 To facilitate the narrative, we note at the outset that the data support cubic tetramers 7a,b and 8a,b bearing S4-symmetric cores.
Low-temperature 6Li NMR spectroscopy of all four alkoxides reveals two resonances (1:1) that coalesce above −15 °C to afford a single sharp resonance above 0 °C consistent with a single aggregate containing two magnetically inequivalent lithium nuclei. Note that S4-symmetric cubic tetramers 7a,b and 8a,b would show such a pair, whereas tetramers 9a,b and 10a,b with D2d-symmetric cores would each display a single 6Li resonance. The coalescence temperature in toluene (approximately −20 °C for all four alkoxides) is higher than that in neat THF (Tcoalesc ≈ −35 °C), suggesting that THF assists the exchange. We attribute these behaviors to a degenerate rearrangement of the chelates about the cubic tetramer frameworks of 7a,b and 8a,b (eq 3).
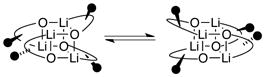 |
(3) |
Despite the THF dependence on the rate of chelate exchange, we conclude that THF is coordinated only transiently based on a simple and powerful diagnostic probe as follows.12 Pyridine strongly coordinates lithium nuclei and shifts 6Li resonances markedly (0.5 to >1.0 ppm) downfield even in neat THF solutions.12c,20 Amino alkoxides 2a,b and 3a,b showed no measurable change in chemical shift in solutions of 1.2 M pyridine/toluene compared with that in THF/toluene or toluene solutions, demonstrating that the chelates occupy all available coordination sites.
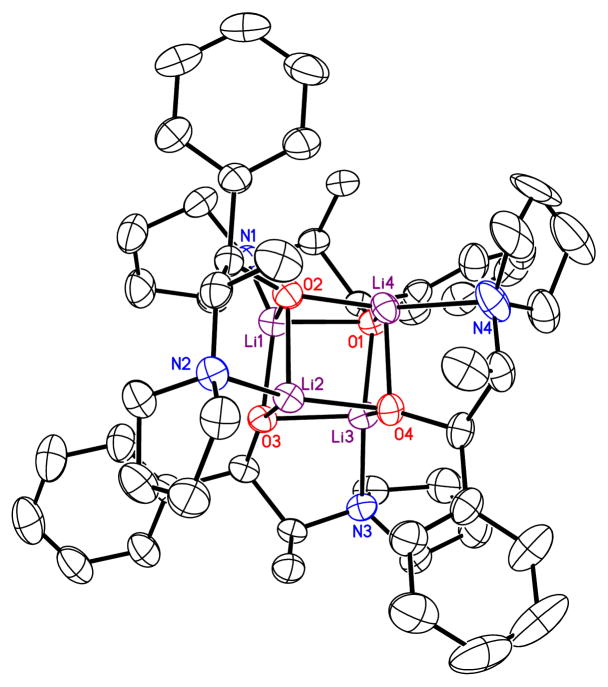
The assignment of 7a is consistent with a crystal structure by Arnett and coworkers.9 The assignment of 7b was corroborated by an X–ray crystal structure of 5a showing the S4-symmetric cubic tetramer core (Figure 1; supporting information).
Figure 1.
ORTEP of 5a as fully chelated tetramer bearing an S4-symmetric cubic core.
Heteroaggregation and MCV
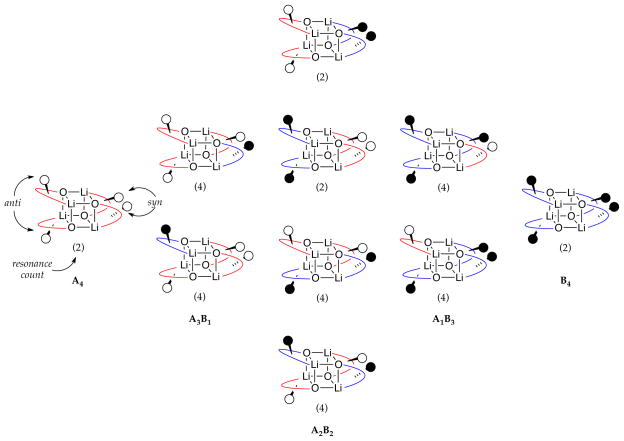
Assignment of 2a,b and 3a,b as tetramers 7a,b and 8a,b relied critically on MCV.8,12,13 In this experiment, the high symmetries of the lithium alkoxides are disrupted by forming ensembles of homo- and heteroaggregates (eq 4).21,22 The number and symmetries of the heteroaggregates and the dependence of the distribution on the mole fraction (XA or XB) attest to the structures of the homoaggregates, An and Bn. In most previous applications of MCV, cubic tetramers appear as a series of five homo- and heteroaggregates with the characteristic resonance counts illustrated in Chart 1.8,12
Chart 1.
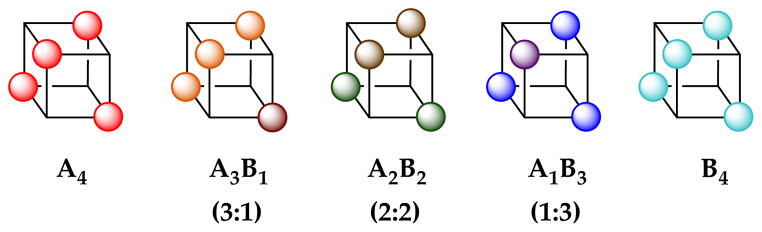
| (4) |
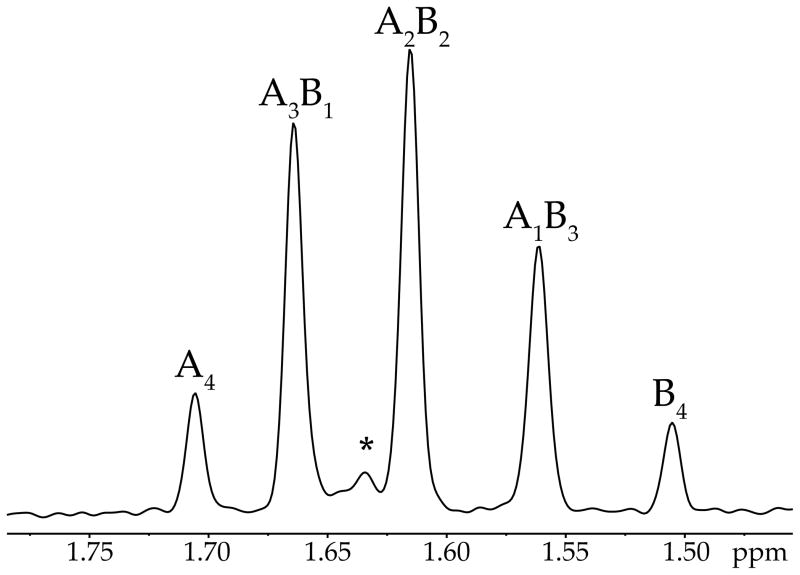
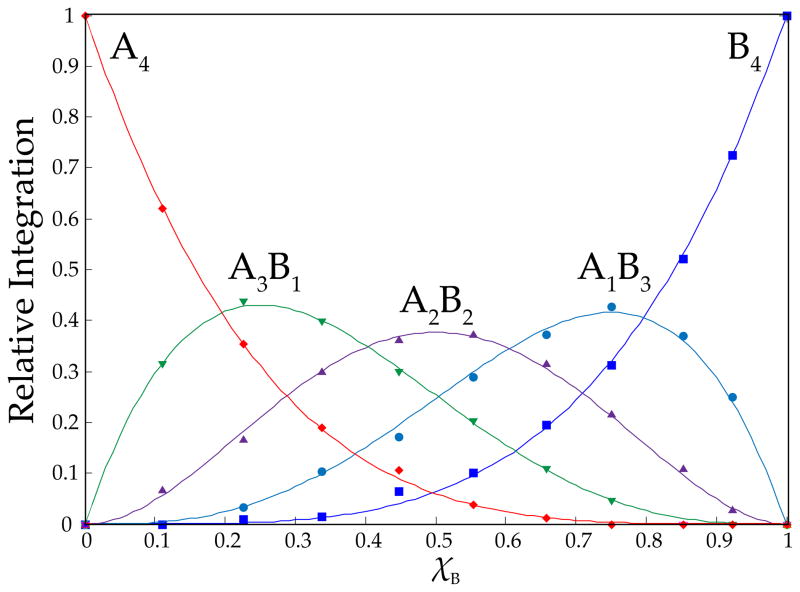
Characterization of the alkoxides as tetramers using MCV is illustrated with 2a and 2b emblematically. Mixtures of 2a and 2b in a 1:1 ratio in toluene or THF give intractable NMR spectra at low temperature. The complexity inherent to ensembles is exacerbated by the stereochemistry of chelation (discussed in detail below). On warming, however, the resonances coalesce to afford a sharp 5-peak ensemble at +60 °C consistent with a tetramer ensemble—A4, A3B1, A2B2, A1B3, and B4—with each stoichiometry appearing as a single resonance (Figure 2). The apparent intraaggregate Li–Li exchange8,12,15,20a is well-precedented and has been useful in characterizing O-lithiated species, but it is usually significantly more facile. The exchange shows minor acceleration by THF relative to toluene. The aggregates were monitored in the high temperature limit with varying proportions of 2a and 2b and fixed total alkoxide concentration. The relative integrations of the five distinct aggregates are plotted versus measured mole fractions23 (XA or XB) of the two components in Figure 3. The curves result from a parametric fit as described previously.8,12 The number of aggregates and quality of the fit confirm the tetramer assignment. In conjunction with the symmetry of the homoaggregates at low temperature and solvent-independent chemical shift, MCV completes the assignment of alkoxides 2a and 2b as solvent-free tetramers 8a and 8b.
Figure 2.
6Li NMR spectrum of a 1:1 mixture of lithium ephedrates 2a and 2b in toluene recorded at +60 °C. The labels indicate the relative AmBn stoichiometries. The asterisk denotes an unknown impurity.
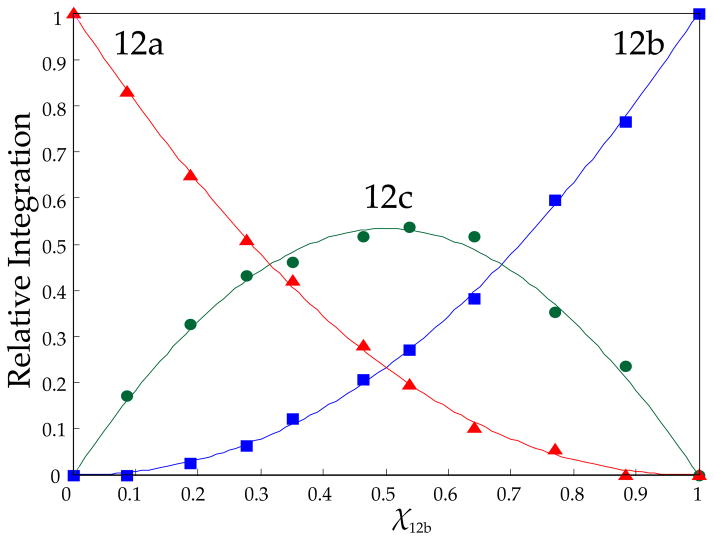
Figure 3.
Job plot showing the relative integrations of tetrameric homo- and heteroaggregates versus measured mole fractions23 of 2a (XA) for 0.10 M mixtures of lithium ephedrates [6Li]2a (A) and [6Li]2b (B) in toluene at +60 °C.
Studies of norcarane-derived alkoxides 3a and 3b afforded results fully analogous to those of 2a and 2b in every respect, supporting unsolvated cubic tetramers 8a and 8b. Relatively minor quantitative differences include slightly faster chelate exchanges and slightly slower intraaggregate Li–Li site exchanges.
The stereochemical preference for S4 rather than D2d cubic cores was examined using density functional theory computations at the B3LYP level of theory with the 6–31G(d) Pople basis set.24 Free energies were calculated from an MP2-derived single-point energy [6–31G(d) basis set] and a B3LYP-derived thermal correction [6–31G(d)] at 195 K and 1 atm. The 21 kcal/mol preference for the S4 form in 7b (eq 5) is fully consistent with the experimental data. Although we often use computations only qualitatively, this difference is very large for isodesmic25 stereoisomers. Computations of a sterically less congested variant in which the phenyl and methyl moieties along the backbone of ephedrate 2a were omitted show a reduced but still sizeable 7 kcal/mol preference for the S4 core (eq 6).
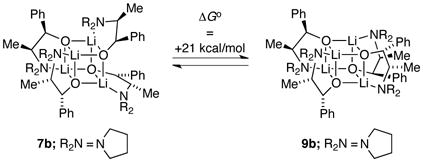 |
(5) |
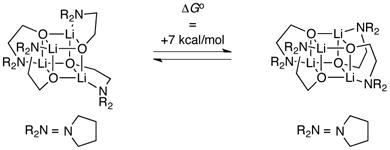 |
(6) |
Lithium alkoxide–LiHMDS mixed aggregates
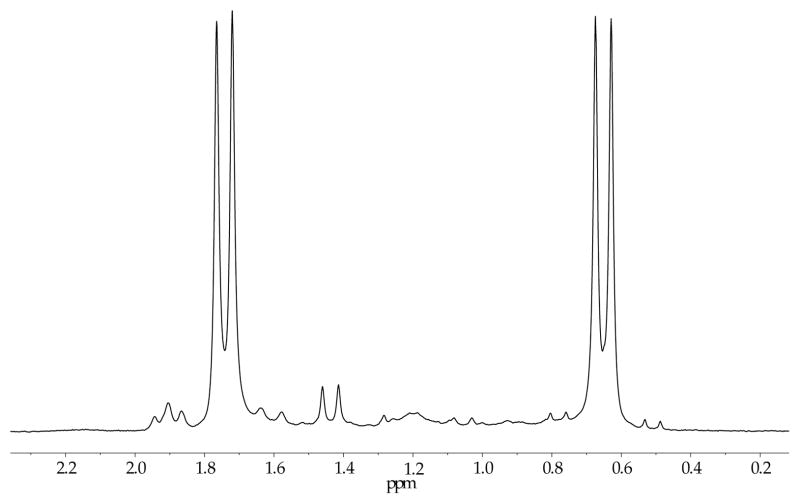
During the studies described above, we detected lithium alkoxide–LiHMDS mixed aggregates that formed quantitatively with 1.0 equiv of excess LiHMDS.22 For example, lithium ephedrate 2a with 1.0 equiv excess [6Li,15N]LiHMDS displays two 6Li doublets in a 1:1 ratio (JLi–N = 1.0 Hz) and a single resonance appearing as a quintet in the 15N NMR spectrum (Figure 4). The data are consistent with the basic mixed dimer subunits 11a,b or the corresponding ladder 12a. Once again, the spectroscopically opaque Li–O linkages posed a problem, and MCV offered the solution.
Figure 4.
6Li NMR spectrum recorded on a 1:1 mixture of [6Li,15N]LiHMDS (0.10 M) and lithium amino alkoxides 2a (0.10 M total concentration) in toluene cosolvent at −30 °C.
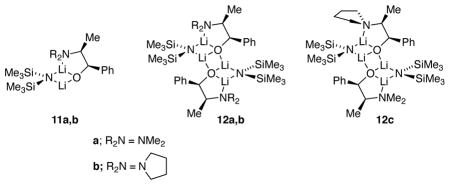
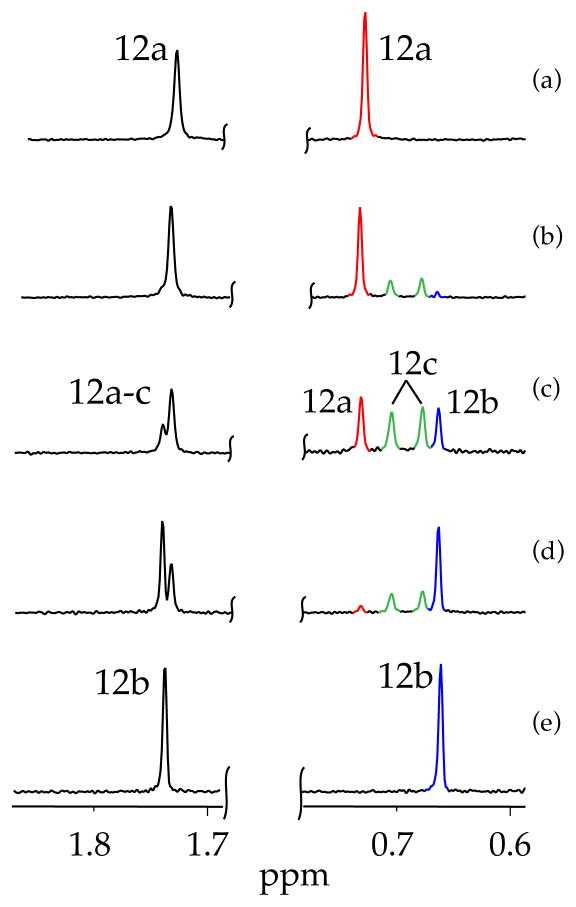
Mixtures of lithium ephedrates 2a and 2b in toluene at varying proportions but constant lithium alkoxide titer in the presence of 1.0 equiv of LiHMDS afford 6Li spectra showing the two original resonance pairs along with additional resonances consistent with mixed ladder 12c. The downfield ensemble is not well resolved, yet the upfield resonances clearly show 12a and 12b along with two resonances (1:1) attributed to mixed ladder 12c. We suspect that the well-resolved upfield resonances correspond to those bearing the dialkylamino chelates. Maintaining the total concentration of excess LiHMDS at 0.10 M and the total alkoxide titer at 0.10 M while varying proportions (mole fractions) of the two alkoxides afforded a mole fraction-dependent distribution consistent with ladders 12a, 12b, and 12c. The resulting Job plot is illustrated in Figure 6. The resonance counts and quality of the fit confirm the 1:1 association of two mixed dimeric subunits and the overall ladder motif.
Figure 6.
Job plot showing the relative integrations of mixed ladders 12a (A2), 12b (B2), and 12c (AB) versus measured mole fractions of 2b–LiHMDS (XB) in mixtures containing 0.10 total amino alkoxide and 0.10 M LiHMDS at −30 °C.
Discussion
Synthetically important lithium amino alkoxides pose an interesting challenge for structural organolithium chemists. Arnett and coworkers have shown that crystalline 2a is cubic tetramer 9a, but their efforts to determine the solution structure were less conclusive. Messy 6Li NMR spectra cast doubt on the colligative measurements, which are notoriously sensitive to impurities.8 We previously studied amino alkoxides 2a and 3a using NMR spectroscopy and gleaned no useful information.6,7 The current paper describes how a combination of 6Li NMR spectroscopy and MCV allowed us to characterize 2a,b and 3a,b as stereochemically pure cubic tetramers 9a,b and 10a,b. Computational studies suggest that the S4-symmetric cubic core is inherently more stable than the D2d core, a preference that is amplified by the substituents along the chelate backbone (eqs 4 and 5). During these studies, we made a number of observations and achieved some tactical developments in MCV that call for further elaboration.
In the low temperature limit, all four homoaggregates display two distinct resonances that, with warming, coalesce into a single resonance owing to facile degenerate isomerizations of the chelates (eq 3). Although this observation proved critical to complete the structural assignments, it foreshadowed severe technical problems with the use of MCV. In typical applications of MCV to characterize tetramer ensembles (eq 4), we would observe three heteroaggregates of stoichiometries—3:1, 2:2, and 1:3—displaying resonance counts and integrations reflecting the symmetries (Chart 1).12 The amino alkoxides, by contrast, show a markedly increased resonance count arising from stereochemical complexity (Chart 2). The homoaggregates each show two rather than the usual one resonance. The 3:1 and 1:3 heterotetramers exist as two distinct diastereomers each displaying eight resonances total. There are potentially four diastereomeric 2:2 heterotetramers—two C2-symmetric diastereomers displaying two resonances each and two C1-symmetric diastereomers containing four discrete lithium resonances each. Thus, the tetramer ensemble in the slow exchange limit would include 32 resonances in total. It is not shocking, therefore, that ensembles generated from 2a/2b or 3a/3b pairings are intractable in the low temperature limit.
Chart 2.
Two general classes of intraaggregate exchanges would, in principle, simplify the spectra. Chelate–chelate exchange (eq 3) without further deepseated adjustments within the cubic core would reduce the complexity of Chart 2 to the simpler distribution depicted in Chart 1 and lower the 6Li resonance count from 32 to eight. Intraaggregate exchange of all 6Li nuclei12,15 within each aggregate would further symmetrization, causing the five-aggregate ensemble to appear as five discrete 6Li singlets. In practice, warming the samples appeared to elicit rapid chelate exchange, but we could not readily observe all eight resonances at a single temperature owing to differential exchange rates of the different aggregates. Warming of the samples to 60–70 °C, however, elicited the hoped-for rapid intraaggregate Li–Li site exchanges. We have examined structures in the limit of rapid intraaggregate exchange before,12,20a but the temperatures required for vicinal amino alkoxides are remarkably high.
We previously noted the maxim “like aggregates with like.”12 Ensembles generated from lithium alkoxides and related O-lithiated species of differing aggregation states resist heteroaggregation, affording no heteroaggregates whatsoever or an ensemble of homo- and heteroaggregates that deviates significantly from statistical.12 The most compelling assignments stem from structurally related ROLi/R′OLi pairs. At the outset, however, we thought that pairing structurally very different alkoxides would be required to obtain sufficient resolution in the 6Li NMR spectra. Nonetheless, the 2a/2b and 3a/3b pairs differing marginally at the dialkylamino appendages provide convincing results.26 More heterogeneous pairing of lithium ephedrate and norcarane-derived lithium alkoxides—2/3 pairs—also appeared to provide tetramer ensembles, but rapid intraaggregate demanded very high (>80 °C) temperatures.
Mounting evidence suggests that cubic tetramers of enolates and related O-lithiated species are far more robust (less dynamic) than we ever suspected.27,28 Effects of aging (warming–cooling cycles) and catalytically active lithium salts on aggregate equilibrations may profoundly influence stereo- and regiochemical outcomes. Both chelate–chelate and Li–Li site exchanges are observed at lower temperatures in THF than in toluene, indicating a role of THF.
During the studies of homoaggregates we detected lithium alkoxide–LiHMDS mixed aggregates in toluene. (LiHMDS does not form mixed aggregates in THF.29) The connectivities obtained from 6Li–15N double-labeling studies do not distinguish cyclic dimer 11 from ladder 12, a distinction that has eluded us previously.17 We used MCV to reveal that mixtures of LiHMDS and alkoxides afford mixed ladders (12a–c). The chirality of these mixed aggregates may also pique curiosity among those interested in enantioselective reactions of lithium amides.
Conclusion
We have shown that cubic tetramers are a dominant form of several lithium amino alkoxides. This study and others28 suggest that such tetramers composed of O-lithiated species are very robust. It is not difficult to imagine, therefore, that practitioners using lithium enolates to achieve stereocontrolled carbon–carbon bond formation have been thwarted by undetected aging and salt effects.
The importance of lithium amino alkoxides as auxiliaries in organolithium chemistry has grown markedly in the absence of any structural insights whatsoever.3,4,5 Notably, structural studies of aggregates underlying the Merck chemistry (eq 1)6 have played a direct role in the development of the protocols subsequently used at DuPont (eq 2).5,7 In this context, we note a curious observation that may prove important. Inserting lithium salts into the cubic tetramers of 7a and 8a to form the mixed tetramers 5 and 6 central to Merck’s and DuPont’s enantioselective additions requires disruption of the chelate orientations of the S4 core structure of homoaggregates 7a and 8a. We wonder: would mixed aggregates that allow three of the four chelates in the S4 core to remain intact (eq 7) offer a more generalized control of stereochemistry? Studies are, of course, ongoing.
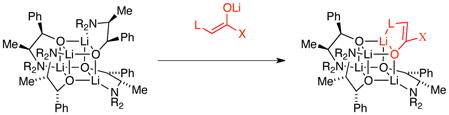 |
(7) |
Experimental Section
Reagents and Solvents
Toluene, THF, and pyridine were distilled from blue solutions containing sodium benzophenone ketyl. The toluene contained approximately 1% tetraglyme to dissolve the ketyl. [6Li]LiHMDS and [6Li,15N]LiHMDS were prepared and recrystallized using modified literature protocols.19 Air- and moisture-sensitive materials were manipulated under argon using standard glove box, vacuum line, Schlenk, and syringe techniques. NMR samples were prepared using protocols described previously.12c 6Li NMR spectra were typically recorded on a 500 or 600 MHz spectrometer with the delay between scans set to >5 x T1 to ensure accurate integrations. Chemical shifts are reported relative to a 0.30 M 6LiCl/MeOH standard at −80 °C.
Supplementary Material
Figure 5.
6Li NMR spectra recorded on mixtures of [6Li]LiHMDS (0.10 M) and lithium amino alkoxides 2a and 2b (0.10 M total concentration) in toluene cosolvent at −30 °C: (a) 0.10 M [6Li]2b; (b) 0.080 M [6Li]2b and 0.020 M [6Li]2a; (c) 0.050 M [6Li]2b and 0.050 M [6Li]2a; (d) 0.020 M [6Li]2b and 0.080 M [6Li]2a; and (e) 0.10 M [6Li]2a.
Acknowledgments
We thank the National Institutes of Health (GM077167) for support and Merck and Bristol–Myers Squibb (formerly DuPont) for a number of amino alcohols.
Footnotes
Supporting Information: Spectra, additional Job plots, and authors for reference 24 (25 pages). This material is available free of charge via the Internet at http://pubs.acs.org.
References and Footnotes
- 1.(a) Szwarc M, editor. Ions and Ion Pairs in Organic Reactions. 1 and 2 Wiley; New York: 1972. [Google Scholar]; (b) Wardell JL. In: In Comprehensive Organometallic Chemistry. Wilkinson G, Stone FGA, Abels FW, editors. Vol. 1. Pergamon; New York: 1982. Chapter 2. [Google Scholar]; (c) Wakefield BJ. The Chemistry of Organolithium Compounds. Pergamon Press; New York: 1974. [Google Scholar]; (d) Brown TL. Pure Appl Chem. 1970;23:447. [Google Scholar]
- 2.Seebach D. Angew Chem, Int Ed Engl. 1988;27:1624. [Google Scholar]; Seebach D. Welch Foundation Conferences on Chemistry and Biochemistry. Wiley; New York: 1984. In Proceedings of the Robert A. [Google Scholar]
- 3.(a) Grabowski EJJ. Reflections on Process Research. In: Abdel-Magid AF, Ragan JA, editors. In Chemical Process Research: The Art of Practical Organic Synthesis. American Chemical Society; Washington D.C.: USA: 2004. pp. 1–21. [Google Scholar]; (b) Thompson AS, Corley EG, Huntington MF, Grabowski EJJ. Tetrahedron Lett. 1995;36:8937. [Google Scholar]; (c) Huffman MA, Yasuda N, DeCamp AE, Grabowski EJJ. J Org Chem. 1995;60:1590. [Google Scholar]; (d) Tan L, Chen CY, Tillyer RD, Grabowski EJJ, Reider PJ. Angew Chem, Int Ed Engl. 1999;38:711. doi: 10.1002/(SICI)1521-3773(19990301)38:5<711::AID-ANIE711>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]; (e) Pierce ME, Parsons RL, Jr, Radesca LA, Lo YS, Silverman S, Moore JR, Islam Q, Choudhury A, Fortunak JMD, Nguyen D, Luo C, Morgan SJ, Davis WP, Confalone PN, Chen CY, Tillyer RD, Frey L, Tan LS, Xu F, Zhao D, Thompson AS, Corley EG, Grabowski EJJ, Reamer R, Reider PJ. J Org Chem. 1998;63:8536. [Google Scholar]
- 4.(a) Ye M, Logaraj S, Jackman LM, Hillegass K, Hirsh K, Bollinger AM, Grosz AL, Mani V. Tetrahedron. 1994;50:6109. [Google Scholar]; (b) Rochet P, Vatele JM, Gore J. Synlett. 1993:105. [Google Scholar]; (c) Vijn RJ, Speckamp WN, De Jong BS, Hiemstra H. Angew Chem. 1984;96:165. [Google Scholar]; (d) Dieter RK, Datar R. Can J Chem. 1993;71:814. [Google Scholar]; (e) Boireau G, Abenhaim D, Henry-Basch E. Tetrahedron. 1979;35:1457. [Google Scholar]; (f) Boireau G, Abenhaim D, Bourdais J, Henry-Basch E. Tetrahedron Lett. 1976;17:4781. [Google Scholar]; (g) Zweig JS, Luche JL, Barreiro E, Crabbe P. Tetrahedron Lett. 1975;16:2355. [Google Scholar]; (h) Vadecard J, Plaquevent JC, Duhamel L, Duhamel P. J Org Chem. 1994;59:2285. [Google Scholar]; (i) Malmvik AC, Obenius U, Henriksson U. J Chem Soc, Perkin Trans 2. 1986:1899. [Google Scholar]; (j) Gerlach U, Haubenreich T, Huenig S. Chem Ber. 1994;127:1969. [Google Scholar]; (k) Kumamoto T, Aoki S, Nakajima M, Koga K. Tetrahedron: Asymmetry. 1994;5:1431. [Google Scholar]; (l) Fehr C, Stempf I, Galindo J. Angew Chem, Int Ed Engl. 1993;32:1042. [Google Scholar]; (m) Milne D, Murphy PJ. J Chem Soc, Chem Commun. 1993:884. [Google Scholar]; (n) Mukaiyama T, Soai K, Sato T, Shimizu H, Suzuki K. J Am Chem Soc. 1979;101:1455. [Google Scholar]; (o) Schön M, Naef R. Tetrahedron: Asymmetry. 1999;10:169. [Google Scholar]; (p) Gärtner P, Letschnig M, Knollmüller M. Monatsh Chem. 2000;131:867. [Google Scholar]; (q) Knollmüller M, Ferencic M, Gärtner P. Tetrahedron: Asymmetry. 1999;10:3969. [Google Scholar]; (r) Scott MS, Lucas AC, Luckhurst CA, Prodger JC, Dixon D. J Org Biomol Chem. 2006;4:1313. doi: 10.1039/b515356e. [DOI] [PubMed] [Google Scholar]; (s) Riant O, Hannedouche J Org Biomol Chem. 2007;5:873. doi: 10.1039/b617746h. [DOI] [PubMed] [Google Scholar]; (t) Khartabil HK, Gros PC, Fort Y, Ruiz-Lopez MF. J Am Chem Soc. 2010;132:2410. doi: 10.1021/ja910350q. [DOI] [PubMed] [Google Scholar]; (u) Coldham I, Raimbault S, Whittaker DTE, Chovatia PT, Leonori D, Patel JJ, Sheikh NS. Chem Eur J. 2010;16:4082. doi: 10.1002/chem.200903059. [DOI] [PubMed] [Google Scholar]; (v) Coldham I, Dufour S, Haxell TFN, Howard S, Vennall GP. Angew Chem Int Ed. 2002;41:3887. doi: 10.1002/1521-3773(20021018)41:20<3887::AID-ANIE3887>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]; (w) Gros P, Fort Y. Eur J Org Chem. 2002:3375. doi: 10.1021/jo016064p. [DOI] [PubMed] [Google Scholar]; (x) Gros P, Fort Y, Caubère PJ. Chem Soc, Perkin Trans. 1997;1:3071. [Google Scholar]
- 5.Parsons RL., Jr Curr Opin Drug Discovery Dev. 2000;3:783. [PubMed] [Google Scholar]; Kauffman GS, Harris GD, Dorow RL, Stone BRP, Parsons RL, Pesti JA, Magnus NA, Fortunak JM, Confalone PN, Nugent WA. Org Lett. 2000;2:3119. doi: 10.1021/ol006321x. [DOI] [PubMed] [Google Scholar]
- 6.Thompson A, Corley EG, Huntington MF, Grabowski EJJ, Remenar JF, Collum DB. J Am Chem Soc. 1998;120:2028. [Google Scholar]; Xu F, Reamer RA, Tillyer R, Cummins JM, Grabowski EJJ, Reider PJ, Collum DB, Huffman JC. J Am Chem Soc. 2000;122:11212. [Google Scholar]
- 7.Parsons RL, Jr, Fortunak JM, Dorow RL, Harris GD, Kauffman GS, Nugent WA, Winemiller MD, Briggs TF, Xiang B, Collum DB. J Am Chem Soc. 2001;123:9135. doi: 10.1021/ja0105616. [DOI] [PubMed] [Google Scholar]
- 8.For extensive leading references to solution structural studies of enolates and related O-lithiated species see: McNeil AJ, Toombes GES, Gruner SM, Lobkovsky E, Collum DB, Chandramouli SV, Vanasse BJ, Ayers TA. J Am Chem Soc. 2004;126:16559. doi: 10.1021/ja045144i.
- 9.(a) Arnett EM, Nichols MA, McPhail AT. J Am Chem Soc. 1990;112:7059. [Google Scholar]; (b) Nichols MA, McPhail AT, Arnett EM. J Am Chem Soc. 1991;113:6222. [Google Scholar]
- 10.Streitwieser A. J Org Chem. 2009;74:4433. doi: 10.1021/jo900497s. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diffusion-ordered NMR spectroscopy (DOSY) shows considerable potential to examine the structures of LiX salts: Li D, Keresztes I, Hopson R, Williard PG. Acc Chem Res. 2009;42:270. doi: 10.1021/ar800127e.
- 12.(a) Liou LR, McNeil AJ, Ramírez A, Toombes GES, Gruver JM, Collum DB. J Am Chem Soc. 2008;130:4859. doi: 10.1021/ja7100642. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) De Vries TS, Goswami A, Liou LR, Gruver JM, Jayne E, Collum DB. J Am Chem Soc. 2009;131:13142. doi: 10.1021/ja9047784. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Tomasevich LL, Collum DB. J Org Chem. 2013;78:7498. doi: 10.1021/jo401080n. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Renny JS, Tomasevich LL, Tallmadge EH, Collum DB. Angew Chem, Int Ed. 2013;52:11998. doi: 10.1002/anie.201304157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Herberich GE, Spaniol TP, Fischer A. Chem Ber. 1994;127:1619. [Google Scholar]; (b) Armstrong DR, Davies RP, Raithby PR, Snaith R, Wheatley AEH. New J Chem. 1999;23:499. [PubMed] [Google Scholar]; (c) Strohmann C, Seibel T, Schildbach D. J Am Chem Soc. 2004;126:9876. doi: 10.1021/ja048216e. [DOI] [PubMed] [Google Scholar]; (d) Iwasaki M, Narita T, Umino Y. J Organomet Chem. 2011;696:2763. [Google Scholar]; (e) Al-Masri HT, Sieler J, Hey-Hawkins E. Appl Organometal Chem. 2003;17:63. [Google Scholar]; (f) Morris JJ, MacDougall DJ, Noll BC, Henderson KW. Dalton Trans. 2008:3429. doi: 10.1039/b719565f. [DOI] [PubMed] [Google Scholar]; (g) van der Schaaf PA, Jastrezebski JTBH, Hogerheide MP, Smeets WJJ, Spek AL, Boersma J, van Koten G. Inorg Chem. 1993;32:4111. [Google Scholar]; (h) Khartabil HK, Gros PC, Fort Y, Ruiz-Lopez MF. J Org Chem. 2008;73:9393. doi: 10.1021/jo8019434. [DOI] [PubMed] [Google Scholar]; (i) Khartabil HK, Martins-Costa MTC, Gros PC, Fort Y, Ruiz-Lopez MF. J Phys Chem B. 2009;113:6459. doi: 10.1021/jp809211y. [DOI] [PubMed] [Google Scholar]
- 15.(a) Arvidsson PI, Ahlberg P, Hilmersson G. Chem Eur J. 1999;5:1348. [Google Scholar]; (b) Bauer W. J Am Chem Soc. 1996;118:5450. [Google Scholar]; (c) Bauer W, Griesinger C. J Am Chem Soc. 1993;115:10871. [Google Scholar]; (d) DeLong GT, Pannell DK, Clarke MT, Thomas RD. J Am Chem Soc. 1993;115:7013. [Google Scholar]; (e) Thomas RD, Clarke MT, Jensen RM, Young TC. Organometallics. 1986;5:1851. [Google Scholar]; (f) Bates TF, Clarke MT, Thomas RD. J Am Chem Soc. 1988;110:5109. [Google Scholar]; (g) Fraenkel G, Hsu H, Su BM. In: In Lithium: Current Applications in Science, Medicine, and Technology. Bach RO, editor. Wiley; New York: 1985. pp. 273–289. [Google Scholar]; (h) Heinzer J, Oth JFM, Seebach D. Helv Chim Acta. 1985;68:1848. [Google Scholar]; (i) Fraenkel G, Henrichs M, Hewitt JM, Su BM, Geckle MJ. J Am Chem Soc. 1980;102:3345. [Google Scholar]; (j) Lucht BL, Collum DB. J Am Chem Soc. 1996;118:3529. [Google Scholar]; (k) Knorr R, Menke T, Ferchland K, Mahlstäubl J, Stephenson DS. J Am Chem Soc. 2008;130:14179. doi: 10.1021/ja8026828. [DOI] [PubMed] [Google Scholar]
- 16.Mulvey RE. Chem Soc Rev. 1991;20:167. [Google Scholar]
- 17.Hall PL, Gilchrist JH, Harrison AT, Fuller DJ, Collum DB. J Am Chem Soc. 1991;113:9575. [Google Scholar]
- 18.Fedyunina IV, Plemenkov VV, Bikbulatova S, Nikitina LE, Litvinov IA, Kataeva ON. Chem Nat Compds. 1992;28:173. [Google Scholar]
- 19.Romesberg FE, Bernstein MP, Gilchrist JH, Harrison AT, Fuller DJ, Collum DB. J Am Chem Soc. 1993;115:3475. An improvement based on a dissolving metal protocol will be reported. [Google Scholar]
- 20.(a) Jackman LM, DeBrosse CW. J Am Chem Soc. 1983;105:4177. [Google Scholar]; (b) Reich HJ, Kulicke KJ. J Am Chem Soc. 1996;118:273. [Google Scholar]; (c) Deana RK, Recklinga AM, Chena H, Daweab LN, Schneiderac CM, Kozak CM. Dalton Trans. 2013;42:3504. doi: 10.1039/c2dt32682e. [DOI] [PubMed] [Google Scholar]; (d) Reed D, Barr D, Mulvey RE, Snaith R. J Chem Soc, Dalton Trans. 1986:557. [Google Scholar]; (e) MacDougall DJ, Noll BC, Kennedy AR, Henderson KW. J Chem Soc Dalton Trans. 2006;15:1875. doi: 10.1039/b515011f. [DOI] [PubMed] [Google Scholar]; (f) Boyle TJ, Pedrotty DM, AlamTMVick SC, Rodriguez MA. Inorg Chem. 2000;39:5133. doi: 10.1021/ic000432a. [DOI] [PubMed] [Google Scholar]
- 21.(a) Weingarten H, Van Wazer JR. J Am Chem Soc. 1965;87:724. [Google Scholar]; (b) Goralski P, Legoff D, Chabanel M. J Organomet Chem. 1993;456:1. [Google Scholar]; (c) Desjardins S, Flinois K, Oulyadi H, Davoust D, Giessner-Prettre C, Parisel O, Maddaluno J. Organometallics. 2003;22:4090. [Google Scholar]; (d) Günther H. J Braz Chem Soc. 1999;10:241. [Google Scholar]; (e) Kissling RM, Gagne MR. J Org Chem. 2001;66:9005. doi: 10.1021/jo016105h. [DOI] [PubMed] [Google Scholar]
- 22.After surveying a subset of the community, we have chosen to refer to (LiX)n and (LiX)m(LiX′)n (such that X and X′ contain the same heteratom) as a “homoaggregate” and “heteroaggregate”, respectively, and reserve the term “mixed aggregate” for (LiX)m(LiY)n (such that X and Y are different heteroatoms).
- 23.Measuring the mole fraction within only the ensemble of interest rather than the overall mole fraction of lithium alkoxides added to the samples eliminates the distorting effects of impurities.
- 24.Frisch MJ, et al. GaussianVersion 3.09. Gaussian, Inc; Wallingford, CT: 2009. revision A.1. [Google Scholar]
- 25.When the chemical bonds broken in the reactant are the same as the type of bonds formed the reaction is isodesmic.
- 26.Although homo- and heterosolvated dimeric enolates coordinated by N,N,N′,N′-tetraalkyldiamines fail resolve (unpublished), the corresponding mixed diamine solvates of n-BuLi and PhLi resolve well: Hoffmann D, Collum DB. J Am Chem Soc. 1998;120:5810.Rutherford JL, Hoffmann D, Collum DB. J Am Chem Soc. 2002;124:264. doi: 10.1021/ja002979u.
- 27.Casy BM, Flowers RA. J Am Chem Soc. 2011;133:11492. doi: 10.1021/ja205017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.For examples of reactions that are fast relative to the rates of aggregate-aggregate exchanges see: McGarrity JF, Ogle CA. J Am Chem Soc. 1985;107:1810.Jones AC, Sanders AW, Bevan MJ, Reich HJ. J Am Chem Soc. 2007;129:3492. doi: 10.1021/ja0689334.Thompson A, Corley EG, Huntington MF, Grabowski EJJ, Remenar JF, Collum DB. J Am Chem Soc. 1998;120:2028.Jones AC, Sanders AW, Sikorski WH, Jansen KL, Reich HJ. J Am Chem Soc. 2008;130:6060. doi: 10.1021/ja8003528.Reich HJ. J Org Chem. 2012;77:5471. doi: 10.1021/jo3005155.
- 29.Lucht BL, Collum DB. Acc Chem Res. 1999;32:1035. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.