Summary
Most multicellular organisms show a physiological decline in immune function with age. However, little is known about the mechanisms underlying these changes. We examined Drosophila melanogaster, an important model for identifying genes affecting innate immunity and senescence, to explore the role of phagocytosis in age-related immune dysfunction. We characterized the localized response of immune cells at the dorsal vessel to bacterial infection in one-week and five-week old flies. We developed a quantitative phagocytosis assay for adult Drosophila and utilized this to characterize the effect of age on phagocytosis in transgenic and natural variant lines. We showed that genes necessary for bacterial engulfment in other contexts are also required in adult flies. We found that blood cells from young and old flies initially engulf bacteria equally well, while cells from older flies accumulate phagocytic vesicles and thus are less capable of destroying pathogens. Our results have broad implications for understanding how the breakdown in cellular processes influences immune function with age.
Keywords: immunosenescence, phagocytosis, hemocytes, Drosophila, immunity, senescence
Introduction
Age-related decline in immune function, or immunosenescence, appears to be a general hallmark of aging in multicellular organisms. For humans this decline poses a serious health risk and commonly leads to hospitalization in the elderly (High 2004; Ongradi & Kovesdi 2010). Although changes in adaptive immunity were once thought to be the primary cause of immunosenescence, recent evidence demonstrates that age-related decline in functional components of the innate immune system also plays a significant role (Plowden et al. 2004; Panda et al. 2009).
To examine the key factors in age-related functional decline of innate immunity, we employed a genetic model organism, the fruit fly, Drosophila melanogaster. Drosophila and humans share a similar response to bodily infections, particularly in the molecular pathways of the innate immune response (Lemaitre & Hoffmann 2007). With only an innate immune system, studies in Drosophila can focus on the consequences of age-related changes in innate immunity without the complicating interactions arising from adaptive immune components.
Adult Drosophila respond to infection in two ways: clearance of pathogens by phagocytic hemocytes (also called blood cells or plasmatocytes) and production of antimicrobial proteins (AMPs) (reviewed in (Lemaitre & Hoffmann 2007)). Phagocytosis begins in minutes in response to bacteria that have breached the cuticle or digestive tract, and is required for survival of an infection (Nehme et al. 2007; Stuart & Ezekowitz 2008; Charroux & Royet 2009). This cellular response has been best characterized in embryos, larvae, and in cell culture assays (reviewed in (Stuart & Ezekowitz 2008; Fauvarque & Williams 2011; Ulvila et al. 2011)), but a few qualitative studies have been carried out in adult flies (Elrod-Erickson et al. 2000; Kocks et al. 2005; Garver et al. 2006; Mackenzie et al. 2011; Nehme et al. 2011). Bacteria that are not immediately engulfed can be destroyed by AMPs, which are released by various cells (Lemaitre & Hoffmann 2007; Stuart & Ezekowitz 2008). However, transcriptionally-regulated AMP production is delayed compared to phagocytosis (Ramet et al. 2002; Lemaitre & Hoffmann 2007; Haine et al. 2008). While decline in either or both of these components could contribute to immunosenescence, their individual roles have not been characterized in most studies because common assays, like the ability to survive and/or clear bacterial infection, combine the effects of both.
Research in a variety of organisms suggests a model for how phagocytic cells destroy bacteria (reviewed in (Nordenfelt & Tapper 2011)). Contact with a pathogen activates cell-surface receptors on hemocytes, triggering cytoskeletal changes and the formation of a phagocytic cup that develops into a membrane-bound phagosome containing the invading pathogen(s). Phagosomes fuse with lysosomes to create phagolysosomes, and the low pH in these compartments destroys the bacteria. Only some of the molecules required for this cellular process are known.
Mutant analyses in Drosophila have identified several genes required for efficient bacterial clearance by hemocytes (Stuart & Ezekowitz 2008; Fauvarque & Williams 2011), including the PDGF/VEGF receptor ortholog Pvr, (Wood et al. 2006), the pattern recognition receptor PGRP-SC1/picky (Garver et al. 2006), cell-surface proteins encoded by nimrod (Kurucz et al. 2007) and eater (Kocks et al. 2005), and vesicular transport and fusion regulators, such as psidin (Brennan et al. 2007) and full-of-bacteria (fob) (Akbar et al. 2011). Gene expression and tissue-specific knockdown studies implicate many other molecular players (for examples, see (Ramet et al. 2002; Zettervall et al. 2004; Cronin et al. 2009; Zanet et al. 2009; Kadandale et al. 2010)).
Phagocytosis has been characterized primarily in cell culture or early developmental stages with little work focused on hemocytes in aging adult flies. It has been reported that new phagocytic cells are produced only during the early phases of the life cycle, and not in adult Drosophila (Lemaitre & Hoffmann 2007), suggesting that the effects of age on these cells can be estimated with precision. Hemocyte activity in young adult flies has been tested using fluorescent bacteria, providing gross estimates of phagocytic abilities (Elrod-Erickson et al. 2000; Kocks et al. 2005; Garver et al. 2006). However, this whole-fly measurement is unlikely to be sensitive enough to detect small changes that might occur with age or within natural populations. Furthermore, this type of assay cannot distinguish the efficiency of phagocytic function from individual variation in hemocyte size, number, or location. An alternative method examined the phagocytic character of cells from adults, but only included non-adherent cells at one time point post-infection (Kocks et al. 2005; Mackenzie et al. 2011). Such work suggested that the number and proportion of phagocytically active cells declines with age (Mackenzie et al. 2011). Interestingly, studies on human neutrophils and macrophages reveal declines in phagocytic ability with age, although responses are variable depending on the cell types and assays (Plowden et al. 2004; Kovacs et al. 2009). Mechanisms underlying age-related changes in immune function remain unclear.
As phagocytosis acts as a first line of defense against pathogens after infection, we examined age-related changes in this process. Here we evaluate the effect of age on heart-associated hemocytes and explore the aspects through which the detrimental effects of age could act on phagocytosis. We focused on heart-associated cells because many hemocytes are concentrated at this tissue (called the dorsal vessel) in adult flies (Elrod-Erickson et al. 2000), and recent work in mosquitoes showed preferential adhesion of hemocytes near the heart after pathogen injection (King & Hillyer 2013).
To evaluate the age-specific immunological function of hemocytes in vivo, we developed and employed a quantitative phagocytosis assay. Use of Hemese-Gal4-driven Green Fluorescent Protein (GFP), which specifically marks hemocytes (Kurucz et al. 2003; Zettervall et al. 2004), demonstrated that these cells are the predominant phagocytic cells in young and old flies. Knockdown of nimrod C1 or eater in heart-associated adult blood cells reduced phagocytosis to a similar extent as that in larvae (Kocks et al. 2005; Kurucz et al. 2007). We also found that Rab5 promotes phagocytosis after engulfment in adults, similar to observations in cell culture (Agaisse et al. 2005; Cheng et al. 2005; Philips et al. 2005; Peltan et al. 2012).
We found a consistent decline in phagocytic efficiency with age. At older ages, we observed fewer heart-associated hemocytes. The rate of phagocytic uptake and the proportion of active hemocytes were similar in young and old flies. However, clearance of bacteria from the cell once engulfed decreased dramatically with age. This suggests that a decline in phagocytic efficiency contributes to overall immunosenescence. These observations, and a new quantitative assay for measuring phagocytic ability in adults, show that adult Drosophila can provide a powerful system for study of the genetic basis of age-related changes in immune response.
Results
A whole-fly assay did not reveal changes in immune function with age
Previous work using inbred Drosophila lines derived from a natural population showed significant variation in the ability of different genotypes to clear a bacterial infection at different ages (Felix et al. 2012). To test if these disparities arose from altered phagocytic ability, we examined two lines from the Felix et al. study (numbers 387 and 437) that behaved differently in the clearance assay. Using a method described previously (Elrod-Erickson et al. 2000), we injected adult, virgin female flies with fluorescent Escherichia coli, and assayed fluorescence through the cuticle. Although flies from line 387 contained more fluorescence than those from line 437 at one week of age (1762 ±133 SEM (one standard error of the mean) versus 1415 ± 52 SEM in pixel intensity measurements, F1,35 = 23.0, p<0.0001), we saw no significant difference between young and old flies in either of the lines (F1,35 = 0.19, p=0.7; Table 1). However, this procedure measures global phagocytosis, and does not provide estimates of phagocytic events in individual hemocytes. Differences in blood cell numbers, body size, or pigmentation with age could confound the results. Thus, we sought an alternative way to measure phagocytic efficiency.
Table 1.
Fluorescent intensity measured through the cuticle of females infected with fluorescent E. coli shows differences by genotype but not age.
| Line | Number of flies | Age (weeks) | Whole abdomen fluorescent intensity | Intensity in region around dorsal vessel | |
|---|---|---|---|---|---|
| 387 | n=7 | 1 | mean | 1762.0 | 1991.9 |
| sem | 133.0 | 154.0 | |||
| 387 | n=10 | 5 | mean | 1736.4 | 2062.5 |
| sem | 90.0 | 138.0 | |||
| 437 | n=10 | 1 | mean | 1415.5 | 1487.3 |
| sem | 52.0 | 52.0 | |||
| 437 | n=12 | 5 | mean | 1376.7 | 1408.4 |
| sem | 27.0 | 32.0 |
Heart-associated hemocyte numbers decline with age but remain localized
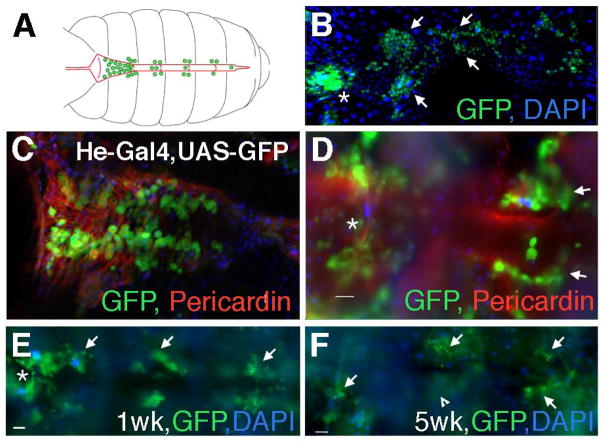
To observe the blood cells in greater detail, we dissected out the dorsal vessel (the fly heart) and adjacent body wall, where many hemocytes localize (Elrod-Erickson et al. 2000). Both circulating hemocytes, which flow with the pumped hemolymph through the heart, and sessile ones, which adhere to tissues, are fluorescently labeled in Hemese-Gal4 (He-Gal4), UAS-GFP flies (Kurucz et al. 2003; Zettervall et al. 2004). We dissected these flies to reveal the adherent hemocytes, and then stained the tissues. To identify the dorsal vessel, we added fluorescently-tagged phalloidin to mark Actin, or stained with an antibody recognizing the heart-enriched protein Pericardin (Figure 1A–B) (Chartier et al. 2002; Demerec 2008). We observed many hemocytes scattered individually along the dorsal abdominal wall. Some were trapped within the dorsal vessel itself, particularly in the first aeortic chamber (Figure 1A–C), and were presumed to be circulatory prior to fixation. Other hemocytes appeared in large clusters segmentally along the dorsal vessel (Figure 1A, B, D). These clusters that adhere to tissues neighboring the hemocoel are called sessile hemocytes.
Figure 1. Heart-associated hemocytes decline with age but not infection.
A) A schematic of the abdominal dorsal vessel (red) and associated blood cells (hemocytes, green). Hemocytes are enlarged for clarity. Anterior is to the left in this and subsequent panels. Many circulating hemocytes cluster in the first aeortic chamber of the dorsal vessel (left), while some can be seen in the other chambers. Adherent hemocytes localize just outside the dorsal vessel and along the body wall. B) Projection of multiple optical sections of a dissected dorsal vessel and associated blood cells from a one-week old female, marked by GFP (green) driven by Hemese-Gal4 (He-Gal4). Many circulating hemocytes are seen inside the first chamber (asterisk), and clusters of sessile hemocytes are found associated laterally along the dorsal vessel. Arrows indicate clusters of hemocytes along the second and third chambers. DAPI labels all nuclei in blue. C) An optical section of a dissected dorsal vessel from a one-week old female shows GFP-positive hemocytes (green, arrow) inside of the first chamber of the dorsal vessel, which is marked by staining with an antibody directed against Pericardin (red). D) In a different optical section, heart-associated hemocytes are observed adhering outside of the first chamber (asterisk), and laterally next to the second chamber of the dorsal vessel (arrows). Two blood cells are seen in the heart. E) A dorsal vessel from a one-week old He-Gal4, UAS-GFP female injected with E. coli displays hemocytes inside the first chamber (asterisk), and clusters of hemocytes laterally along the dorsal vessel (arrows). F) A dorsal vessel from a five-week old He-Gal4, UAS-GFP female injected with E. coli displays similar localization of hemocytes inside the first chamber (asterisk), and laterally along the dorsal vessel (arrows), although there are fewer blood cells. Arrowhead indicates an out-of-focus cluster of cells. All scale bars = 20μm.
A prior study demonstrated that the number of circulating hemocytes declines with age in mated females (Mackenzie et al. 2011), but did not analyze the sessile cells. We examined this population in young and old He-Gal4, UAS-GFP virgin female flies. GFP-positive cells clustered at the abdominal dorsal vessel in both one-week and five-week old flies but older flies possessed significantly fewer GFP-positive hemocytes than young ones (mean=145 ± 16 SEM cells in young, n=6 flies; 48 ± 7 SEM in old flies, n=8; F1,23 = 30.6, p<0.0001). In the same experiment, we examined the effect of infection on heart-associated hemocytes. Recent study in mosquitoes showed that infection status influences blood cell association with the dorsal vessel (King & Hillyer, 2012). Thus, we injected young and old flies with heat-killed E. coli, and at 90-minutes post infection, compared the results with age-matched, uninfected flies. We found no significant effect of infection on the numbers of GFP-positive hemocytes localized to the dorsal vessel at one week of age (F1,9 = 0.09, p = 0.78 infected mean= 140.5 ± 20 SEM, n=6) or at five weeks (F1,12 = 1.13, p = 0.30, infected mean= 54 ± 8 SEM, n=8).
A quantitative phagocytosis assay in adult flies
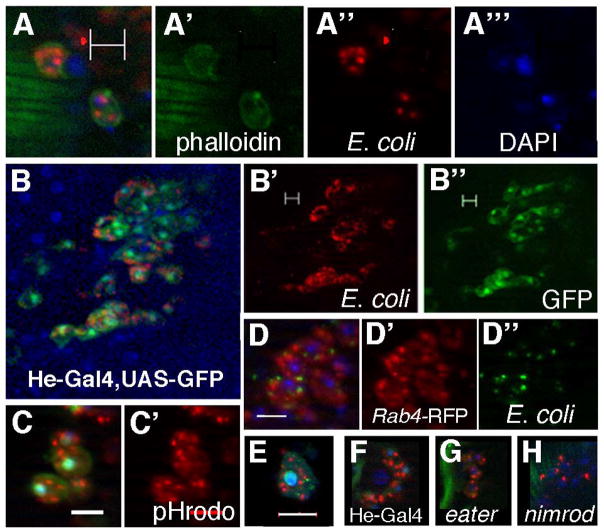
Next, we developed an assay to measure phagocytosis quantitatively. This assay combines the qualitative method described above and in (Elrod-Erickson et al. 2000) with labeling strategies previously used in cultured, larval, or circulating cells (Ramet et al. 2002; Kocks et al. 2005; Garver et al. 2006; Kurucz et al. 2007; Fauvarque & Williams 2011, Mackenzie et al. 2011). We injected Canton-S virgin female flies in the abdomen with heat-killed fluorescently-labeled E. coli, then dissected, fixed, and stained the tissues at 90 minutes post-injection. Unengulfed bacteria are washed away prior to fixation, so no fluorescent quenching step is required. Many dorsal vessel-associated cells contained phagocytic events, as observed through optical sectioning (Figure 2A–B) and consistent with previous assays. Phagocytic cells, outlined by phalloidin-stained cortical actin (Figure 2A) consistently measured ~10μm in diameter, the known size for hemocytes (Brehelin 1982; Zettervall et al. 2004), and were particularly concentrated in and around the first aeortic chamber of the heart.
Figure 2. A quantitative in vivo phagocytosis assay.
A) Hemocytes at the dorsal vessel from an adult Canton S female contain fluorescent E. coli 90 minutes after an infection. Scale bar in all panels = 10μm. In an optical section, Oregon-Green-Phalloidin staining (green, A′) indicates cortical Actin; Rhodamine reveals bacteria (red, A″); and DAPI staining shows the nuclei (blue A‴). B) Engulfed E. coli (red, B′) in the dissected dorsal vessel associate with blood cells, as marked by GFP (green, B″) via He-Gal4. C) E. coli associated with GFP-marked blood cells (green) have been engulfed, indicated by pHrodo-labeled E.coli, which fluoresce red (C′) when phagosomes fuse with acidic lysosomes. D) Some engulfed E. coli (green) colocalize with He-Gal4 driven UAS-Rab4-RFP (red), which marks endocytic vesicles. E) A circulating hemocyte, bled from an infected He-Gal4, UAS-GFP (green) female, contains E.coli (red) and shows similar phagocytic character as heart-associated hemocytes. F) Five hemocytes (distinguishable by DAPI) in the dorsal vessel show numerous phagocytic events (red) from a He-Gal4, UAS-GFP female. G) Four hemocytes each have fewer phagocytic events than controls when eater is knocked down by RNAi. H) Five hemocytes each have fewer phagocytic events than controls when nimrodC1 is knocked down by RNAi.
To verify that hemocytes accounted for observed phagocytic events, we infected He-Gal4, UAS-GFP flies (Kurucz et al. 2003; Zettervall et al. 2004). We observed fluorescent bacteria overwhelmingly in association with 10μm-diameter GFP-positive cells, many of which associated with the dorsal vessel (Figure 2B). Optical sectioning verified that the fluorescent bacteria were within the hemocytes and not merely on the cell surface. We confirmed that the bacteria were engulfed by utilizing pH-sensitive pHrodo-E.coli, which only fluoresces under acidic conditions, to indicate fusion between the phagosome and lysosome. The extent of phagocytic events per hemocyte was very similar using either label (compare Figure 2A and 2C). Next, we injected flies expressing the early endosome marker Rab4-GFP (Yang et al. 2011). In many cases, fluorescent bacteria co-localized with the Rab4-GFP protein (Figure 2D), indicating that the phagosomal cup transitioned into a vesicle. Finally, we collected circulating He-Gal4, GFP blood cells (Mackenzie et al. 2011) and found their size and phagocytic character to be qualitatively similar to those cells associated with the dorsal vessel (Figure 2E, compare to 2A, B). These data support the idea that our phagocytosis assay specifically reflects hemocyte engulfment activity.
To determine the cellular efficiency of phagocytosis in adult flies, we examined this process over time by counting the number of engulfed fluorescent bacteria per active hemocyte. Time course studies showed maximal and distinct phagocytic events at 90 minutes for E. coli, and the majority of GFP-positive hemocytes in He-Gal4, UAS-GFP adult heart tissue contained bacteria. Later time points, after 2.5 hours, revealed both punctate events and diffuse fluorescence, presumably due to degradation of the bacteria (see below). We found that GFP-positive hemocytes contained approximately 4.5 (± 0.18 SEM) events per cell at 90 minutes post-injection (Figure 3B). In separate experiments, we altered the concentrations of injected bacteria. Hemocytes contained similar numbers of phagocytic events when injected with either the original or one-fifth of the titers of bacteria (see below), but had too many events to count when the bacteria was five times more concentrated. This demonstrates that the initial titer was neither limiting nor elicited the maximal possible response. Overall, our assay gives high resolution, quantitative data in the form of bacteria engulfed per hemocyte.
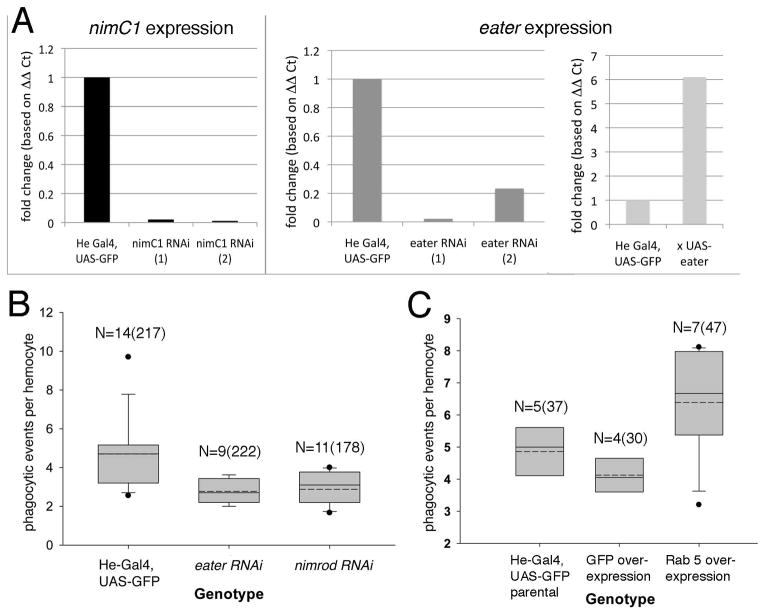
Figure 3. Genes required for efficient bacterial engulfment in adult Drosophila.
A) Quantitative RT-PCR confirms reduced expression of nimC1 and eater transcripts in young female flies of the genotypes He-Gal4, UAS-GFP; UAS-RNAi nimC1 (left) or eater (middle). Two biological replicates are shown. He-Gal4; UAS-GFP, UAS-Eater flies showed increased gene expression and served as a positive control (right). Fold changes were calculated using ΔΔ Ct values relative to the reference gene RpL32 (rp49) and the control genotype He-Gal4, UAS-GFP, using multiple technical replicates for each biological replicate. B–C) Box plots of phagocytic ability, measured as the number of fluorescent E. coli per active hemocyte at 90 minutes post injection. Boxes represent the middle two quartiles, separated by a line representing the median, and the whiskers show the 10th and 90th percentiles. Dots indicate outliers. A dashed line marks the mean value of events per cell. N= number of flies and (total cells) assayed. B) Reduced function of eater or nimC1 reduces bacterial uptake by adult hemocytes. The following genotypes were compared: He-Gal4, UAS-GFP, UAS-RNAi eater and He-Gal4, UAS-GFP, UAS-RNAi nimC1. The isogenic control strain (He-Gal4) showed significantly greater phagocytic ability compared to either knockdown line (p<0.05). C) Increased Rab5 increases the number of phagocytic events per hemocyte. There is a significant difference between He-Gal4 outcrossed to UAS-GFP control versus crossed to UAS-Rab5 wt (p<0.0001). The parental control strain (He-Gal4, UAS-GFP) is also shown.
Genes required for efficient phagocytosis by adult hemocytes
We tested whether genes that function in phagocytosis in larval and cell culture assays are also required for the process in adult flies. nimrod C1 (nimC1) is required for efficient bacterial uptake in hemocytes in larvae, and eater is necessary both in larval and adult blood cells (Avet-Rochex et al. 2005; Kocks et al. 2005; Kurucz et al. 2007). We disrupted these genes in hemocytes by RNA interference, verified gene knockdown by qRT-PCR (at least 4-fold reduction, Figure 3A), and measured phagocytic ability by counting the number of engulfed bacteria per hemocyte. While hemocytes from the control strain (He-Gal4, UAS-GFP alone) contained an average of 4.5 engulfment events each, cells in which eater or nimC1 were knocked down had fewer (averages of 2.9 and 3.2, respectively, Figure 2F–H, Figure 3B). A post-hoc Tukey test (Zar 2010) verified that the control strain differed significantly from the mutant strains (p< 0.05). Thus, reducing eater or nimC1 function in adult hemocytes results in poorer phagocytic ability per cell (Avet-Rochex et al. 2005; Kocks et al. 2005; Kurucz et al. 2007).
Next, we tested whether we could disrupt phagocytosis after the initial engulfment. We examined the effect of increasing Rab5, which is known to be required for the maturation of the early endosome/phagosome prior to fusion with the lysosome (Scott et al. 2003; Wucherpfennig et al. 2003; Zhang et al. 2007; Morrison et al. 2008) and can modulate engulfment of bacteria by fly cells in culture (Agaisse et al. 2005; Cheng et al. 2005; Philips et al. 2005; Peltan et al. 2012). Overexpressed Rab5 localizes to its normal subcellular structures (Zhang et al. 2007), can raise internalization rates, and can increase early endosomal structures (Nielsen et al. 1999; Wucherpfennig et al. 2003). When we overexpressed Rab5 in hemocytes, phagocytic events increased significantly, with the average events per cell increasing from 4.1 in the control to 6.4 events per cell in the Rab5 overexpressing strain (post hoc contrast analysis results: t = 2.52, df=12, p<0.05, Figure 3C). This is consistent with the idea that hemocytes engulf bacteria more rapidly with excess Rab5, but cannot complete phagocytic turnover as quickly, leading to accumulation. These genetic experiments further support the validity of the assay.
Phagocytic ability changes with age
We next used the adult phagocytosis assay to explore whether hemocytic function changes with age. A prior study suggested that both the number of circulating hemocytes and the proportion that were phagocytic declines with age in adult female, mated flies (Mackenzie et al. 2011). We determined whether the fraction of phagocytic cells differed between young and old, virgin, female He-Gal4, UAS-GFP flies, focusing on the cells localized to the heart. For these experiments, we used either our regular titer of bacteria or one-fiftieth of this concentration. We found no significant difference in the proportion of phagocytic cells across age at either titer (Table 2). 90 minutes after infection, 61% or 72% of GFP-positive cells at the dorsal vessel in 1 week old flies contained engulfed bacteria at low and regular titers, respectively. For 5 week old flies, 64% or 85% of the cells were phagocytically active at low and regular titers, respectively (Table 2). Interestingly, the mean percentage of phagocytically active cells was higher at both ages when more bacteria was injected. Thus, the proportion of hemocytes associated with the dorsal vessel that are phagocytic does not decline with age.
Table 2. No significant difference in proportion of phagocytically active hemocytes at one and five weeks of age.
He-Gal4, UAS-GFP flies injected with fluorescent E. coli assayed ninety minutes after infection. If GFP-positive cells had at least one phagocytic event, they were counted as active; GFP-positive cells with no events were classified as inactive. Only GFP-positive cells associated with the dorsal vessel were scored. We analyzed the data using a maximum likelihood analysis with nested logistic regression and a binomial distribution, and detected no significant difference in active proportion with age: Low titer (1:50th): χ2= 0.001, p=0.97; Regular titer: χ2=1.13, p=0.28. Ages given are either one week post eclosion (4–7 days) or 5 weeks post eclosion (35–39 days).
| Titer | Age | Number of flies and (number of GFP+ cells) analyzed | % Active | % Inactive |
|---|---|---|---|---|
| Low | 1 Week | n=5 (153 cells) | 61 | 39 |
| Low | 5 Weeks | n=4 (103 cells) | 64 | 36 |
| Regular | 1 Week | n=16 (253 cells) | 72 | 28 |
| Regular | 5 Weeks | n=11 (245 cells) | 85 | 15 |
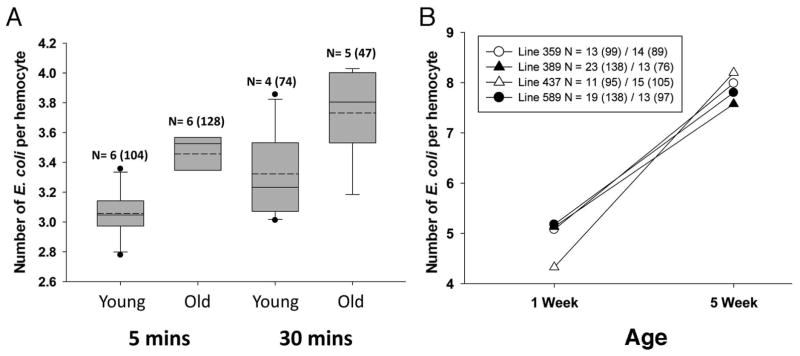
To look at phagocytosis on a cellular level, we assayed engulfment events per cell in young and old flies over time in our low titer experiment. At this concentration, not all cells with the potential to be phagocytic are active (Table 2). However, among active cells, we found a significant effect of age on initial uptake of bacteria and later numbers of engulfment events. We observed an average of 2.09 and 2.62 engulfed bacteria per cell at 5 minutes post-injection in 1-week and 5-week old flies, respectively (Figure 4A, F1,163=6.16, p=0.01). 30 minutes after a low-titer infection, hemocytes from older flies contained 3.01 events compared to 2.21 events per cell in younger flies (Figure 4A, F1,226 =4.90, p= 0.03). Thus, we saw on average fewer events per cell at low titer than regular titer (compare to Fig 3B–C). However, there were more events per active cell in older individuals at either time point.
Figure 4. Engulfed bacteria per hemocyte increases with age.
A) Box plots of phagocytic ability, measured as the number of fluorescent E. coli per active hemocyte in young (5–7 days) and old (35–39 days) He-Gal4, UAS-GFP flies, injected with low concentrations of E.coli. Dashed lines indicate the means. (See text for statistical analysis.) B) Results of the phagocytosis assay for four natural variant lines at one and five weeks of age. While all four lines show a significantly higher number of bacteria per active phagocytic cell with age (see text), genotypes differ slightly in the way that age affects this trait. Values shown are averages for each genotype at 1 and 5 weeks, respectively; N=number of flies and (number of cells) assayed at 1 and 5 weeks.
Prior work in twenty inbred fly lines derived from a natural population found extensive genetic variation in the ability to clear an E. coli infection at different ages (Felix et al. 2012). Thus, we wanted to determine if phagocytic ability specifically varied among lines and how it was influenced by age. It was possible that the increase in engulfment events per active cell was due to fewer hemocytes in older flies. Thus, we returned to our normal injection concentration (1 × 106 E.coli per μl), which provides an excess of E. coli per hemocyte, and assayed natural variant strains. We chose four lines to assay: two of these exhibited immunosenescence in the prior clearance assay, but the other two had improved clearance ability at four weeks of age (Felix et al. 2012).
All four natural variant lines revealed a strong, consistent effect of age on phagocytosis. Hemocytes in virgin female flies from the four lines had strikingly similar numbers of phagocytic events and displayed a significant difference in the number of engulfed bacteria between young and older ages (F1,113 = 43.7, p< 0.0001, Figure 4B). Surprisingly, in all lines, hemocytes of the 5 week old flies contained more engulfed bacteria (an average of 7.9 phagocytic events/cell) than the hemocytes of the 1 week old flies (5.0 events/cell; Figure 4B).
Several ideas, none mutually exclusive, could explain why older cells had more phagocytic events in these assays. One is that fewer hemocytes in old flies result in an effectively higher concentration of bacteria to engulf per cell. While this likely accounts for some differences, especially at low titers, the data when E. coli is in excess suggests this is not the only explanation. A second possibility is that older cells have improved phagocytic ability compared to younger flies, and engulfed more bacteria. A third alternative is that hemocytes from younger flies were able to clear the bacteria by completing phagocytosis, whereas completion of the process was delayed in older flies, leading to an accumulation of bacteria in the blood cells of older flies.
Completion of phagocytosis slows in older flies
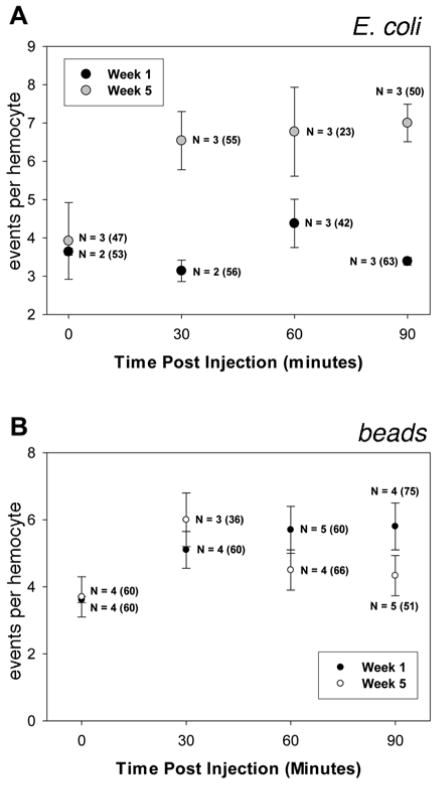
If phagocytic processing differed in young and old flies, it was possible that a more detailed time course would provide insight on these differences. Thus, we measured phagocytic ability at 1 and 5 weeks of age for one of the natural variant strains at different times post-injection. Notably, at an early time (5 minutes), the number of engulfment events in active cells was not significantly different (F1,96 = 3.01, p=0.09) with age (Figure 5A). This further supports that initial phagocytic uptake is not adversely affected by age, and demonstrates that immune-challenged hemocytes function rapidly and similarly in young and old flies. However, data from the remaining time points (with one exception at 60 minutes where we had fewer observations) showed significantly more engulfed bacteria in the hemocytes of 5 week old flies (Figure 5A, 30 mins: F1,107 = 3.01, 45.12, p<0.0001; 60 mins: F1,61 = 0.34, p=0.56; 90 mins: F1,109 = 39.37, p<0.0001). The apparently constant number of phagocytic events in younger cells over the time course could be due to the combination of degradation of older phagosomes and new events. Thus, the increase in phagocytic events over time in older cells suggested that there is an accumulation of phagosomes in older individuals due to a lack of degradation/turnover.
Figure 5. Phagocytic events accumulate in older flies.
A) One and five week old flies from the natural variant line 387 were injected with fluorescent E. coli and dissected at indicated times. Phagocytic events were counted for active cells only. Young flies exhibit a near-constant number of engulfed bacteria while older flies accumulate bacteria with time. N= number of flies and (total cells) assayed. B) Time course of bead engulfment. One and five week old flies from the natural variant line 387 were injected with fluorescently labeled plastic beads, which are not broken down by phagocytosis. Flies were dissected and fixed at indicated times. Hemocytes of young and old flies appeared to engulf and accumulate beads at a very similar rate, with no statistically significant difference between the ages. (See text for statistical analyses.)
Phagocytic turnover declines with age
To test the hypothesis that the higher number of phagocytic events in old blood cells was due to their inability to destroy bacteria efficiently, we employed a bead engulfment assay (Elrod-Erickson et al. 2000; Stroschein-Stevenson et al. 2006). The efficiency with which hemocytes engulf the beads depends on bead size, but is similar to microbial uptake (Mackenzie et al. 2011; Elrod-Erickson et al. 2000). Unlike bacteria, the fluorescent beads are not destroyed by the cell, and instead accumulate. We injected 1μm fluorescent beads into flies of the natural variant strain at 1 and 5 weeks of age, and blood cells associated with the dorsal vessel were examined at 5, 30, 60, and 90 minutes. At 5 minutes, the number of engulfed beads was similar to the number of events when E. coli was used (compare Figures 5A and 5B). We found that the number of engulfed beads was not significantly different between cells from young and old flies at any time point (Figure 5B, 5 mins: F1,116 = 1.28, p=0.26; 30 mins: F1,92 = 0.01, p=0.92; 60 mins: F1,125 = 2.24, p=0.14; 90 mins: F1,122 = 0.68, p=0.41), suggesting uptake occurs at a similar rate. Moreover, this suggests the change in cell number with age does not affect initial uptake. This suggests that old and young hemocytes were able to process engulfment events similarly, implying that the difference observed with E. coli was due to defective turnover in older cells.
Discussion
Immunosenescence is a common phenomenon, but the precise contributions of immune cells and molecular pathways to this decline are not yet resolved. Drosophila provide a powerful, genetic, model system for studying this issue, however definitive assays to measure age-related decline in immune response had not been worked out. Most prior studies were limited by their dependence on assaying indicators of immune function (e.g., (Zerofsky et al. 2005)) or summative aspects of immunity (Lesser et al. 2006; Ramsden et al. 2008; Felix et al. 2012) (but also see (Mackenzie et al. 2011)). We have developed a quantitative phagocytosis assay useful for understanding the functional effects of age on immune functions and for studying the genetic basis of age-related changes in the efficacy of phagocytosis. We show here that our method specifically measures phagocytosis by blood cells, and that adult fly blood cells require several of the same genes as hemocytes in larvae and other cell types for efficient phagocytic processing.
As was previously described (Mackenzie et al. 2011), we observed a decline in hemocyte number and function with age. However, our results do not entirely match those of Mackenzie et al., and several possibilities could explain these differences. One discrepancy was that we did not detect a significant decline in the proportion of cells that were phagocytically active with age. This could be due to variation in the assays, including the use of different genotypes, differences in mating status (our study used virgin instead of mated flies), varied infection titers and measurement time points, or more subtle differences in growth conditions. Moreover, our work focused on heart-associated cells, which may behave differently than the circulating cells characterized before. Indeed, in mosquitoes, phagocytic cells preferentially associate with the heart upon infection (King & Hillyer, 2012). In any case, fewer phagocytic cells at old age can contribute to immunosenesence by increasing the bacterial load on each hemocyte. Additionally, our data show that the completion of phagocytosis begins to fail on a cellular level.
While one could imagine that all cellular processes would deteriorate with age, we found this not to be the case. In particular, the proportion of heart-associated hemocytes engaged in phagocytosis and the rate of initial bacterial uptake were similar at either age. Instead, there appeared to be a decline in processing phagocytic vesicles. One possibility is that this phenotype results from a decrease in vesicle/membrane availability. Such a defect may arise due to changes in autophagy, which is responsible for normally destroying defective organelles and molecular debris, and has been strongly associated with aging in many organisms (Melendez & Neufeld 2008; Partridge 2008; Young & Narita 2010). Additional work is necessary to test this hypothesis.
Natural variant lines exhibit different abilities to clear infections with age (Felix et al. 2012). We tested four lines in our assay – two with improved bacterial clearance at older age, and two having worse clearance with age (Felix et al. 2012). We postulated these lines would have different phagocytic abilities, but they did not. Instead, the general effect of age on phagocytosis was the same among all genotypes tested: all lines showed a significant increase in the number of phagocytic events per hemocyte in older flies. Mackenzie et al. (2011) estimated that the number of hemocytes in old individuals was 90–66% of that in young flies, which contributes to an increase in bacteria per blood cell. However, given that our injected E. coli titers were not limiting at either age, and that more cells are active at higher infection levels, we do not believe that fewer hemocytes sufficiently explains why there are more events per cell. Instead we propose the difference with age is largely due to a decrease in the rate of destruction of engulfed bacteria. An accumulation of phagocytic events in older flies may explain why the change with age was not detected when we used the assay by Elrod-Erickson et al. (2000) - fewer hemocytes with more events in old flies looked similar to more blood cells with fewer events each in young animals. Fewer cells combined with poorer phagocytic processing likely gives rise to an overall poorer immune response with age. Mechanistically, the differences in bacterial clearance observed in the natural variant lines reported by Felix et al. (2012) must be due to a combination of both arms of the immune response, and future studies should address these components simultaneously.
A detailed characterization of the ability of hemocytes to engulf and degrade bacteria is important in understanding the cellular aspects of the innate immune response. We have shown a potential mechanism that contributes to immunosenescence, above and beyond the loss of blood cells with age. Much remains to be done to parse the relative influence of phagocytosis and AMP production on immune function with age. The combination of the quantitative phagocytosis assay and extensive genetic resources in Drosophila will enable us to determine key components contributing to immunosenescence, and to implicate molecules that may have conserved functions in humans.
Experimental Procedures
in vivo phagocytosis assay
Flies were injected into the abdomen with heat-killed fluorescent E. coli (Bioparticles, tetrarhodamine, Alexa Fluor 488-, or Alexa Fluor 594-conjugated, or pHrodo (E-2862, E-13231, E-23370, P35361). Invitrogen/Life Technologies, Carlsbad, CA) using an Eppendorf Fempto-jet micro-injector set for the following conditions: The FemptoJet parameters were pi [PSI] = 0.46, ti[s] = 0.2, pc [PSI] = 0.20. The stock solution contained 6 × 109 E. coli particles per mL, which was diluted 1:6 for injections, unless otherwise indicated. For bead assays, we used Alexa Fluor 568-labelled 1μm beads (Invitrogen, F-13083) at the same concentration. For natural variant comparisons, injections were always carried out in the morning to avoid changes in immune function due to circadian rhythms (e.g., as observed in (Lazzaro et al. 2004)). Post injection, flies recovered at room temperature for 90 minutes or the times indicated prior to dissection and fixation.
The dorsal vessel and attached abdominal cuticle of each fly was dissected in Schneider’s Media (Invitrogen) supplemented with 15% Fetal Bovine Serum and 0.6x Pen/strep, fixed in 4% formaldehyde (MeOH-free, Electron Microscopy Sciences, Hatfield, PA) for 15 minutes, and washed in PBS + 0.1% Tween. For cortical actin staining, we used Oregon Green 488 Phalloidin (1:8, Invitrogen O-7466) in PBS. Abdomens were mounted on slides dorsal side down in 70% glycerol. In trials with the natural variant line 397, we did not use Phalloidin staining. In these assays we counted only bacteria within a 10μm diameter circle centered on the cell nucleus.
Images were acquired using the Zeiss AxioImager.Z1 fluorescent microscope. The ApoTome (Zeiss, Germany) structural interference system was used generate optical sections; images were acquired and analyzed with AxioVision software. Images were oriented and formatted in Adobe Photoshop and Adobe Acrobat.
Genetic strains
As Hemese (He) expression is confined to blood cells, we used He-Gal4 driving GFP (w*; P{w[+mC]=He-Gal4.Z}85 or w*; P{w[+mC]=He-Gal4.Z}85, P{w[+mC]=UAS-GFP.nls}8 (Zettervall et al. 2004). For the Rab4-RFP assay, we crossed He-Gal4 virgin females to w[*]; P{w[+mC]=UAS-Rab4-mRFP}2 (Yang et al. 2011) (flybase.org). For knockdown of eater and nimrodC1, He-Gal4, UAS-GFP females were crossed to y1, v1; P{y[+t7.7] v[+t1.8]=TRiP.JF01884}attP2 or y1, v1; P{y[+t7.7] v[+t1.8]=TRiP.JF01793}attP2, (Ni et al. 2008) respectively, or to UAS-eater (Kocks et al. 2005) for a qRT-PCR control. For Rab5 experiments, He-Gal4, UAS-GFP virgin females were crossed to y[1] w[*]; P{w[+mC]=UASp-YFP.Rab5}Pde8[08b] (Zhang et al. 2007). Stocks listed above were provided by the Bloomington Stock Center, Bloomington, IN. The four inbred lines derived from the natural population of Drosophila in Raleigh, NC, (359, 387, 437, and 589), were kindly provided by the laboratory of Trudy Mackay at North Carolina State University (Mackay et al. 2012).
Quantitative RT-PCR
Total RNA was isolated from He-Gal4, UAS GFP, and He-Gal4, UAS GFP; TRiP UAS-RNAi nimC1, and He-Gal4, UAS GFP; TRiP UAS-RNAi eater, and He-Gal4, UAS GFP; UAS-eater females using the RNeasy Isolation Kit (Qiagen). cDNA synthesis from total RNA was performed using the iScript cDNA Synthesis kit (BioRad), and potential genomic DNA contamination was removed using Turbo DNA-free (Ambion). Quantitative RT-PCR was performed with iTaq Universal SYBR-Green Supermix (BioRad) with a BioRad iCycler iQ system. The following primer pairs were used: eater: 5′-GGAAGTGGCTTCTGCACGAAAC-3′ and 5′-CGACTACATCCCTTGCAGTAGGG-3′ nimC1 (RA) 5′ GTTTGTAACCGATCGCAGGTGG and 5′-TCGTAGCCCTCACAGCAACTG-3′ RpL32 (rp49): 5′-GTGAAGAAGCGCACCAAGCAC-3′ and 5′-ACGCACTCTGTTGTCGATACCC-3′. Serial dilutions confirmed the amplification rates and delta Ct analysis was carried out relative to RpL32 gene expression. At least two sets of five flies from each genotype were assayed, with multiple technical replicates.
Statistical Analysis
To account for possible small variation in injection volumes between experiments, each experiment was completed on a single day and repeated. We calculated phagocytic event data using multiple individuals per genotype at each age as indicated, and at least 10 blood cells from each individual. Box and whiskers plots illustrate the ranges of numbers of phagocytic events. Differences in phagocytic events were analyzed with either fixed effects ANOVA or mixed model nested ANOVA (Proc Mixed in SAS V9.2, (Zar 2010)). Mixed models were used to test the main fixed effects of age and genotype and the random effect of individuals nested within genotype and age (whenever multiple blood cell counts were used per individual in the phagocytosis assays). We used independent contrasts (Judd & McClelland 1989) in post hoc tests following mixed model analyses to test for significant differences among targeted genotypes. Blood cell counts were transformed to √x + √x +1 to satisfy assumptions of ANOVA (Snedecor & Cochran 1989). We used maximum likelihood to estimate the effects of age on the proportion of blood cells that had engulfed bacteria using a nested logistic regression model with individuals nested within age (implemented by Proc Genmod in SAS V9.2, (Allison 1999)).
Antibodies
Antibody staining and DAPI staining (1:1000, Invitrogen/Life Technologies, D1306) were executed using standard procedures. The following antibodies were used: rabbit anti-GFP (1:1000) (Invitrogen, A6455), Alexa Fluor 488 anti-rabbit (1:400) (Invitrogen, A11008), mouse monoclonal antibody directed against Pericardin, EC11 (1:5) (Chartier et al. 2002)(Developmental Studies Hybridoma Bank), Alexa Fluor 568 anti-mouse (1:400) (Invitrogen, A11031).
Acknowledgments
We acknowledge Kathryn Bus for her technical assistance and contributions to the work. We thank Louisa Wu and her laboratory for training on the larval phagocytosis assay, and members of the fly community for helpful input. We acknowledge Departmental colleagues for assistance with qRT-PCR. This project was supported in part by an Undergraduate Research Award from UMBC (to LH), a March of Dimes Basil O’Connor Starter Scholar Award (to MSG), an NSF CAREER Award (1054422 to MSG) and NIH grant 5 R01 DK084219-02 (to JL). We thank Bloomington Stock Center for fly stocks, and FlyBase for genomic annotation. We acknowledge reagents from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA. We thank the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947) for providing transgenic RNAi fly stocks. We also thank Nicholas Gaiano for helpful comments on the manuscript.
Contributor Information
Lucas Horn, Email: lhorn1@umbc.edu.
Jeff Leips, Email: leips@umbc.edu.
Michelle Starz-Gaiano, Email: starz@umbc.edu.
References
- Allison P. Logistic Regression Using the SAS System: Theory and Application. Cary, NC: SAS Institute Inc; 1999. [Google Scholar]
- Agaisse H, Burrack LS, Philips JA, Rubin EJ, Perrimon N, Higgins DE. Genome-wide RNAi screen for host factors required for intracellular bacterial infection. Science. 2005;309:1248–1251. doi: 10.1126/science.1116008. [DOI] [PubMed] [Google Scholar]
- Akbar MA, Tracy C, Kahr WH, Kramer H. The full-of-bacteria gene is required for phagosome maturation during immune defense in Drosophila. J Cell Biol. 2011;192:383–390. doi: 10.1083/jcb.201008119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avet-Rochex A, Bergeret E, Attree I, Meister M, Fauvarque MO. Suppression of Drosophila cellular immunity by directed expression of the ExoS toxin GAP domain of Pseudomonas aeruginosa. Cell Microbiol. 2005;7:799–810. doi: 10.1111/j.1462-5822.2005.00512.x. [DOI] [PubMed] [Google Scholar]
- Brehelin M. Comparative study of structure and function of blood cells from two Drosophila species. Cell Tissue Res. 1982;221:607–615. doi: 10.1007/BF00215704. [DOI] [PubMed] [Google Scholar]
- Brennan CA, Delaney JR, Schneider DS, Anderson KV. Psidin is required in Drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr Biol. 2007;17:67–72. doi: 10.1016/j.cub.2006.11.026. [DOI] [PubMed] [Google Scholar]
- Charroux B, Royet J. Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc Natl Acad Sci U S A. 2009;106:9797–9802. doi: 10.1073/pnas.0903971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier A, Zaffran S, Astier M, Semeriva M, Gratecos D. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development. 2002;129:3241–3253. doi: 10.1242/dev.129.13.3241. [DOI] [PubMed] [Google Scholar]
- Cheng LW, Viala JP, Stuurman N, Wiedemann U, Vale RD, Portnoy DA. Use of RNA interference in Drosophila S2 cells to identify host pathways controlling compartmentalization of an intracellular pathogen. Proc Natl Acad Sci U S A. 2005;102:13646–13651. doi: 10.1073/pnas.0506461102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin SJ, Nehme NT, Limmer S, Liegeois S, Pospisilik JA, Schramek D, Leibbrandt A, de Simoes RM, Gruber S, Puc U, Ebersberger I, Zoranovic T, Neely GG, von Haeseler A, Ferrandon D, Penninger JM. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M. The Biology of Drosophila. New York: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- Elrod-Erickson M, Mishra S, Schneider D. Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol. 2000;10:781–784. doi: 10.1016/s0960-9822(00)00569-8. [DOI] [PubMed] [Google Scholar]
- Fauvarque MO, Williams MJ. Drosophila cellular immunity: a story of migration and adhesion. J Cell Sci. 2011;124:1373–1382. doi: 10.1242/jcs.064592. [DOI] [PubMed] [Google Scholar]
- Felix TM, Hughes KA, Stone EA, Drnevich JM, Leips J. Age-specific variation in immune response in Drosophila melanogaster has a genetic basis. Genetics. 2012;191:989–1002. doi: 10.1534/genetics.112.140640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Wu J, Wu LP. The peptidoglycan recognition protein PGRP-SC1a is essential for Toll signaling and phagocytosis of Staphylococcus aureus in Drosophila. Proc Natl Acad Sci U S A. 2006;103:660–665. doi: 10.1073/pnas.0506182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haine ER, Moret Y, Siva-Jothy MT, Rolff J. Antimicrobial defense and persistent infection in insects. Science. 2008;322:1257–1259. doi: 10.1126/science.1165265. [DOI] [PubMed] [Google Scholar]
- High KP. Infection as a cause of age-related morbidity and mortality. Ageing Res Rev. 2004;3:1–14. doi: 10.1016/j.arr.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Judd CM, McClelland GH. Data Analysis: A Model Comparison Approach. Harcourt Brace Jovanovich, Inc; N.Y: 1989. [Google Scholar]
- Kadandale P, Stender JD, Glass CK, Kiger AA. Conserved role for autophagy in Rho1-mediated cortical remodeling and blood cell recruitment. Proc Natl Acad Sci U S A. 2010;107:10502–10507. doi: 10.1073/pnas.0914168107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JG, Hillyer JF. Infection-induced interaction between the mosquito circulatory and immune systems. PLoS Patho. 2012;8:1–15. doi: 10.1371/journal.ppat.1003058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocks C, Cho JH, Nehme N, Ulvila J, Pearson AM, Meister M, Strom C, Conto SL, Hetru C, Stuart LM, Stehle T, Hoffmann JA, Reichhart JM, Ferrandon D, Ramet M, Ezekowitz RA. Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell. 2005;123:335–346. doi: 10.1016/j.cell.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ, Palmer JL, Fortin CF, Fulop T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, Zettervall CJ, Hultmark D, Ando I. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17:649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Zettervall CJ, Sinka R, Vilmos P, Pivarcsi A, Ekengren S, Hegedus Z, Ando I, Hultmark D. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc Natl Acad Sci U S A. 2003;100:2622–2627. doi: 10.1073/pnas.0436940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro BP, Sceurman BK, Clark AG. Genetic basis of natural variation in D. melanogaster antibacterial immunity. Science. 2004;303:1873–1876. doi: 10.1126/science.1092447. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Lesser KJ, Paiusi IC, Leips J. Naturally occurring genetic variation in the age-specific immune response of Drosophila melanogaster. Aging Cell. 2006;5:293–295. doi: 10.1111/j.1474-9726.2006.00219.x. [DOI] [PubMed] [Google Scholar]
- Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RR, Barron M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu LL, Qu C, Ramia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu YQ, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, Mittelman D, Gibbs RA. The Drosophila melanogaster Genetic Reference Panel. Nature. 2012;482:173–178. doi: 10.1038/nature10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie DK, Bussiere LF, Tinsley MC. Senescence of the cellular immune response in Drosophila melanogaster. Exp Gerontol. 2011;46:853–859. doi: 10.1016/j.exger.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Melendez A, Neufeld TP. The cell biology of autophagy in metazoans: a developing story. Development. 2008;135:2347–2360. doi: 10.1242/dev.016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison HA, Dionne H, Rusten TE, Brech A, Fisher WW, Pfeiffer BD, Celniker SE, Stenmark H, Bilder D. Regulation of early endosomal entry by the Drosophila tumor suppressors Rabenosyn and Vps45. Mol Biol Cell. 2008;19:4167–4176. doi: 10.1091/mbc.E08-07-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme NT, Liegeois S, Kele B, Giammarinaro P, Pradel E, Hoffmann JA, Ewbank JJ, Ferrandon D. A model of bacterial intestinal infections in Drosophila melanogaster. PLoS Pathog. 2007;3:e173. doi: 10.1371/journal.ppat.0030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehme NT, Quintin J, Cho JH, Lee J, Lafarge MC, Kocks C, Ferrandon D. Relative roles of the cellular and humoral responses in the Drosophila host defense against three gram-positive bacterial infections. PLoS One. 2011;6:e14743. doi: 10.1371/journal.pone.0014743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni JQ, Markstein M, Binari R, Pfeiffer B, Liu LP, Villalta C, Booker M, Perkins L, Perrimon N. Vector and parameters for targeted transgenic RNA interference in Drosophila melanogaster. Nat Methods. 2008;5:49–51. doi: 10.1038/nmeth1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. Rab5 regulates motility of early endosomes on microtubules. Nat Cell Biol. 1999;1:376–382. doi: 10.1038/14075. [DOI] [PubMed] [Google Scholar]
- Nordenfelt P, Tapper H. Phagosome dynamics during phagocytosis by neutrophils. J Leukoc Biol. 2011;90:271–284. doi: 10.1189/jlb.0810457. [DOI] [PubMed] [Google Scholar]
- Ongradi J, Kovesdi V. Factors that may impact on immunosenescence: an appraisal. Immun Ageing. 2010;7:7. doi: 10.1186/1742-4933-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Some highlights of research on aging with invertebrates, 2008. Aging Cell. 2008;7:605–608. doi: 10.1111/j.1474-9726.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- Peltan A, Briggs L, Matthews G, Sweeney ST, Smith DF. Identification of Drosophila gene products required for phagocytosis of Leishmania donovani. PLoS One. 2012;7:e51831. doi: 10.1371/journal.pone.0051831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips JA, Rubin EJ, Perrimon N. Drosophila RNAi screen reveals CD36 family member required for mycobacterial infection. Science. 2005;309:1251–1253. doi: 10.1126/science.1116006. [DOI] [PubMed] [Google Scholar]
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Ramet M, Manfruelli P, Pearson A, Mathey-Prevot B, Ezekowitz RA. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature. 2002;416:644–648. doi: 10.1038/nature735. [DOI] [PubMed] [Google Scholar]
- Ramsden S, Cheung YY, Seroude L. Functional analysis of the Drosophila immune response during aging. Aging Cell. 2008;7:225–236. doi: 10.1111/j.1474-9726.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- Scott CC, Botelho RJ, Grinstein S. Phagosome maturation: a few bugs in the system. J Membr Biol. 2003;193:137–152. doi: 10.1007/s00232-002-2008-2. [DOI] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State University Press; 1989. [Google Scholar]
- Stroschein-Stevenson SL, Foley E, O’Farrell PH, Johnson AD. Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol. 2006;4:e4. doi: 10.1371/journal.pbio.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: learning on the fly. Nat Rev Immunol. 2008;8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- Ulvila J, Vanha-Aho LM, Ramet M. Drosophila phagocytosis - still many unknowns under the surface. APMIS. 2011;119:651–662. doi: 10.1111/j.1600-0463.2011.02792.x. [DOI] [PubMed] [Google Scholar]
- Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–416. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WK, Peng YH, Li H, Lin HC, Lin YC, Lai TT, Suo H, Wang CH, Lin WH, Ou CY, Zhou X, Pi H, Chang HC, Chien CT. Nak regulates localization of clathrin sites in higher-order dendrites to promote local dendrite growth. Neuron. 2011;72:285–299. doi: 10.1016/j.neuron.2011.08.028. [DOI] [PubMed] [Google Scholar]
- Young AR, Narita M. Connecting autophagy to senescence in pathophysiology. Curr Opin Cell Biol. 2010;22:234–240. doi: 10.1016/j.ceb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Zanet J, Payre F, Plaza S. Fascin for cell migration in Drosophila. Fly (Austin) 2009;3:281–282. doi: 10.4161/fly.10315. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 5. Pearson; 2010. p. 960. [Google Scholar]
- Zerofsky M, Harel E, Silverman N, Tatar M. Aging of the innate immune response in Drosophila melanogaster. Aging Cell. 2005;4:103–108. doi: 10.1111/j.1474-9728.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- Zettervall CJ, Anderl I, Williams MJ, Palmer R, Kurucz E, Ando I, Hultmark D. A directed screen for genes involved in Drosophila blood cell activation. Proc Natl Acad Sci U S A. 2004;101:14192–14197. doi: 10.1073/pnas.0403789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Schulze KL, Hiesinger PR, Suyama K, Wang S, Fish M, Acar M, Hoskins RA, Bellen HJ, Scott MP. Thirty-one flavors of Drosophila rab proteins. Genetics. 2007;176:1307–1322. doi: 10.1534/genetics.106.066761. [DOI] [PMC free article] [PubMed] [Google Scholar]