Abstract
The human P2Y1 receptor was expressed in the yeast Saccharomyces cerevisiae strain MPY578q5, which is engineered to couple to mammalian G protein–coupled receptors (GPCRs) and requires agonist-induced activation for growth. A range of known P2Y1 receptor agonists were examined with the yeast growth assay system, and the results were validated by comparing with potencies in the transfected 1321N1 astrocytoma cell line, in which calcium mobilization was measured with a FLIPR (fluorescence-imaging plate reader). The data were also compared with those from phospholipase C activation and radioligand binding with the use of a newly available radioligand [3H]MRS2279 (2-chloro- N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate). In the yeast growth assay, the rank order of potency of 2-MeSADP (2-methylthioadenosine 5′-diphosphate), ADP (adenosine 5′-diphosphate), and ATP (adenosine 5′-triphosphate) is the same as those in other assay systems, i.e., 2-MeSADP>ADP>ATP. The P2Y1-selective antagonist MRS2179 (N6-methyl-2-deoxyadenosine-3′,5′-bisphosphate) was shown to act as an antagonist with similar potency in all systems. The results suggest that the yeast expression system is suitable for screening P2Y1 receptor ligands, both agonists and antagonists. The yeast system should be useful for random mutagenesis of GPCRs to identify mutants with certain properties, such as selective potency enhancement for small synthetic molecules and constitutive activity.
Keywords: Calcium mobilization assay, GPCR, nucleotides, nucleotide receptor, P2Y1 agonists, P2Y1 antagonist, P2Y1 receptor, signal transduction, yeast growth assay
Introduction
Extracellular purines are important signaling molecules responsible for biological effects mediated by cell surface receptors [1-3]. The family of purine receptors includes adenosine (P1 receptors) and P2 receptors. The P2 class, which primarily recognizes ATP (adenosine 5′-triphosphate) and ADP (adenosine 5′-diphosphate), has been subdivided into subfamilies of P2X ligand-gated ion channels and P2Y seven-transmembrane-spanning G protein-coupled receptors (GPCRs).
The P2Y1 receptor couples preferentially to Gq, which activates phospholipase Cβ (PLC), leading to formation of inositol trisphosphate (IP3) and mobilization of intracellular Ca2+. Various assays are available to measure the downstream signaling, with measurement of PLC being a common, classical technique [4]. Previous reports showed that assay results may vary significantly, depending on the signaling variable measured [5, 6]. In this investigation we set out to establish the yeast assay system and to compare measurement at this level of the signaling cascade, which is dependent on activation of the G protein, with other, subsequent levels, i.e., PLC and Ca2+. The compounds tested in this set of experiments included ADP, ATP, 2-MeSADP (2-methylthioadenosine 5′-diphosphate), ADP-β-S (adenosine 5′-O-(2-thio-diphosphate), HT-AMP (2-hexylthioadenosine 5′-monophosphate), PAPET-ATP (2-[4-amino(2-phenylethyl)thio]-ATP), and 3AM-ATP (3′-amino-3′-deoxy-ATP) [7, 8, 9]. The bisphosphate antagonist MRS2179 (N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate) [10], which is highly selective for P2Y1 receptors, was also tested.
The yeast Saccharomyces cerevisiae was used to measure functional effects at the G protein level. This yeast strain has a native signaling system to regulate mating [11]. Specifically, a GPCR (Ste2p) is activated by mating pheromones, which bind to Ste2p, triggering a cascade of events ultimately leading to up-regulation of mating-specific genes and down-regulation of other pathways. This particular system has been engineered to couple to mammalian GPCRs [12-16]. Several genetic modifications were required for this purpose, including modification of the G protein α subunit, deletion of certain genes, and insertion of reporter genes. Yeast assays for GPCRs have grown in popularity, primarily because of the well-known genetics of yeast, making manipulations straightforward, and the ability to directly analyze a particular receptor in the absence of other receptor subtypes. The speed of yeast growth allows broad screening and use of random mutagenesis of GPCRs to identify mutants with certain properties, such as selective potency enhancement for small synthetic molecules and constitutive activity [16, 17].
The results from the yeast system were compared to pharmacological parameters reflecting later steps along the signaling cascade, measured for the same compounds: activation of PLC and calcium mobilization. PLC results were obtained from the 1321N1 astrocytoma cell line expressing P2Y1 receptors as reported previously [18, 19], and calcium levels were monitored in the same cell line. We set out to explore the yeast system for P2Y receptors, not only for conventional compound screening at the native receptors, but also to develop genetically engineered receptors. Because it is possible to introduce random mutagenesis and to select for responsive clones [16, 17] by cell growth, yeast promises to be a unique screening tool. One possible use is identification of mutant receptors (e.g., neoceptors) with enhanced pharmacological properties [20].
Materials and Methods
Materials
Yeast media components were purchased from Qbiogene, Inc (Carlsbad, CA, USA). The calcium assay kit was from Molecular Devices (Sunnyvale, CA, USA). The compounds 3-amino-1,2,4-triazole (3-AT), probenecid, ADP, ATP, 3AM-ATP, 2-MeSADP, ADP-β-S, and MRS2179 were from Sigma (St. Louis, MO, USA); HT-AMP, PAPET-ATP, and [3H]MRS2279 were synthesized as previously reported [21-23].
Yeast strain
The yeast Saccharomyces cerevisiae strain MPY578q5 (MATa GPA1 far1::LYS2 fus1::FUS1-HIS3 sst2::SST2-G418R ste2::LEU2 fus2::FUS2-CAN1 ura3 lys2 ade2 his3 leu2 trp1 can1) was used for expression experiments as previously described [24, 17]. This strain contains a mutant version of the GPA1 gene that codes for a Gα subunit in which the last five amino acids are derived from the mammalian αq (EYNLV). In addition, the deletion of the FAR1 gene allows yeast to grow despite activation of the pheromone pathway that normally leads to cell-cycle arrest. The SST2 gene was disrupted to prevent attenuation of G protein signaling mediated by the GTPase-activating protein activity of Sst2p. The STE2 gene coding for the yeast α factor receptor was deleted to prevent competition for G proteins. The FUS1-HIS3 reporter makes the production of the His3 protein dependent on receptor-mediated activation of the yeast pheromone pathway.
Yeast expression plasmid
Expression plasmids used were based on p416GPD [24], which is a single-copy plasmid containing the CEN/ARS element, the URA3 gene as a selection marker in yeast, the Amp gene for selection in Escherichia coli, and the yeast GAPDH (glyceraldehyde-3-phosphate dehydrogenase) promoter. In particular, human P2Y1 was subcloned from pCD-PS [25] into p416GPD to create p416-P2Y1, which was confirmed by DNA sequencing.
Yeast transformation and growth
Yeast cells were grown at 30°C at 180 rpm in synthetic complete (SC) media [26] unless otherwise noted. Yeast transformations were performed with a lithium acetate technique [27]. Transformants were selected and maintained in SC media lacking uracil (SC-URA).
Yeast liquid growth assay
Cells were grown overnight in 2 ml SC-URA. Cells were then washed once with PBS and used at a working concentration of ∼50000 cells/ml (based on the conversion OD 0.1 ∼3 × 106 cells/ml) in SC media lacking uracil and histidine (SC-URA-HIS) with 20 mM 3-AT. 3-AT is an inhibitor of imidazole glycerol-phosphate dehydrase, which decreases basal cell growth in histidine-deficient media because of leaky expression of FUS1-HIS3. A quantity of 180 μl cells was added to each well of a flat-bottom 96-well plate. Then 20 μl of either compound at the appropriate dilution or water (negative control) or histidine stock (positive control) was added to each well. For antagonist studies, 20 μl of both agonist and antagonist was added to each well. Plates were incubated at room temperature for several days. Immediately before measurement, the contents of each well were pipette mixed, and the optical density at 630 nm was measured with a plate reader. Samples were run in triplicate and averaged.
Calcium mobilization assay
Human 1321N1 astrocytoma cells stably expressing human P2Y1 receptors were cultured in Dulbecco's modified Eagle's medium (JRH Biosciences, Inc., Lenexa, KS, USA) and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units penicillin/ml, 100 μg streptomycin/ml, 2 μmol glutamine/ml, and 500 μg geneticin/ml. For the assay, cells were grown overnight in 100 μl media in 96-well flat-bottom plates at 37°C at 5% CO2 or until they reached ∼60–80% confluency. The calcium assay kit (Molecular Devices) was used as directed with no washing of cells and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were loaded with 50 μl dye with probenecid in each well and incubated for 45 min at room temperature. The compound plate was prepared with dilutions of various compounds in Hanks Buffer. For antagonist studies, both agonist and antagonist were added to the sample plate. Samples were run in duplicate with a Molecular Devices Flexstation I at room temperature. Cell fluorescence (excitation = 485 nm; emission = 525 nm) was monitored following exposure to compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
Binding assay
P2Y1 receptor binding experiments were performed as previously described [23]. Briefly, membranes (40 μg protein) from astrocytoma cells stably expressing human P2Y1 receptors were incubated with [3H]MRS2279 (8 nM) for 30 min at 4°C in a total assay volume of 200 μl. The radiolabeled-ligand concentration used in the assay approximated the Kd value in binding to the receptor. Binding reactions were terminated by filtration through Whatman GF/B glass-fiber filters under reduced pressure with a MT-24 cell harvester (Brandel, Gaithersburg, MD), and radioactivity was determined with a liquid scintillation counter (Packard, Downers Grove, IL).
Statistical analysis
Binding and functional parameters were estimated with GraphPad Prism software (GraphPad, San Diego, CA, USA). Antagonist potency was evaluated by Schild analysis [28]. The concentration ratio is equal to the EC50 of the agonist in the presence of the antagonist divided by the EC50 of the agonist alone. KB is the Schild constant of the antagonist. Data are expressed as mean ± standard error.
Results
Agonist effects on P2Y1 receptors in the yeast system via growth assays
A yeast expression system was used for measurement of G protein activation via P2Y1 receptors. To determine if the human P2Y1 receptor could effectively couple to a G protein, the haploid yeast (S. cerevisiae) strain MPY578q5 was used [24, 17]. The basic features of this strain include numerous modifications to the native yeast pheromone response pathway, including expression of a chimeric Gq protein, such that it required productive receptor–G protein coupling for growth in histidine-deficient media. This yeast strain harbors a mutant version of the GPA1 gene coding for a hybrid yeast/mammalian G protein subunit in which the last five amino acids of Gpa1p were replaced with the corresponding residues present in mammalian αq[24]. This strain was then transformed with the human P2Y1 gene in a yeast expression plasmid. To determine conditions for effective coupling, liquid growth assays were performed. Care was taken to use 3-AT as shown previously to suppress basal growth [24, 16]. To validate the effectiveness of the system, we first tested two agonists, including the endogenous P2Y1 receptor agonist ADP and the classical agonist 2-MeSADP. Basal growth was low in the absence of any exogenously added nucleotides and in the presence of UDP (data not shown), which was shown to be relatively inactive at the P2Y1 receptor. The positive control (addition of histidine to the media to check cell viability) showed that the agonists examined were fully efficacious. These data showed that the chimeric G protein was effectively coupled to the human P2Y1 receptor, which was important to establish because many chimeras do not effectively couple in yeast [29].
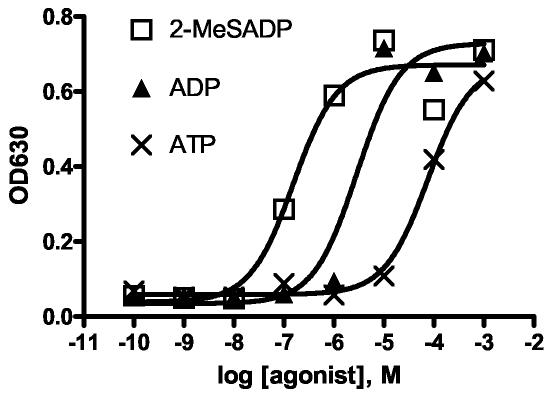
To compare present results with the available PLC data, 2-MeSADP, ADP, and ATP were studied. As shown in Figure 1, the rank order of these three compounds was similar to that of the published PLC data (Table 1).
Figure 1.
Yeast growth assays in the presence of 2-MeSADP, ADP, ATP. Representative data exhibiting the concentration-dependent growth (represented as OD630) of the engineered yeast strain expressing P2Y1 receptors in the presence of 2-MeSADP, ADP, and ATP ranging in concentration from 10−10 to 10−3 (M). The EC50 values averaged over at least three experiments are 2-MeSADP (130 nM) > ADP (1800 nM) > ATP (28000 nM). Experiments were performed at least three times with representative data shown. Within each experiment each data point was done in triplicate and averaged.
Table 1.
Potencies of adenine nucleotide derivatives measured in three functional assays of human P2Y1 receptors and in a receptor binding assay.a
| Functional Potency (EC50, nM) | Binding (Ki, nM) | |||
|---|---|---|---|---|
| Yeast growth | PLC (Astrocytoma) | Calcium (astrocytoma) | Astrocytoma | |
| ADP | 1800 ± 650 | 660 b | 58 ± 17 | 900 b |
| 2-MeSADP | 130 ± 43 | 36 b | 1.7 ± 0.2 | 57 b |
| ATP | 28,000 ± 18000 | 2600 b | 5400 ± 3600 | 1200 b |
| ADP-β-S | 21,000 ± 9800 | ND | 86 ± 42 | 710 |
| HT-AMP | 24,000 ± 3900 | 1400c | 16 ± 11 | 1700 |
| PAPET-ATP | 17,000 ± 6500 | ND | 28 ± 13 | 1100 |
| 3AM-ATP | 9200 (n=1) | 10,000d | 3000 ± 1500 | ND |
Unless noted, the following methods were used, as described in Materials and Methods (n = 3, unless noted): The parameter measured in the engineered yeast cells was growth, indicated by turbidity determinations. The activity designated PLC in 1321N1 astrocytoma cells stably expressing the human P2Y1 receptor represents the accumulation of tritiated inositol phosphates isolated with ion exchange columns [4, 25]. Calcium transients in the same cells were measured with a fluorescent chelating dye method on a FlexStation in 96-well format. Ki values refer to competition for binding of [3H]MRS2279 [23].
Data from Gao et al., 2004 (astrocytoma cells) [19].
Data from Hoffmann et al., 1999 (COS-7 cells) [18].
Data from Moro et al., 1999 (COS-7 cells) [34]. ND = not determined.
To further test the yeast system, ADP-β-S, HT-AMP, and two amine-derivatized agonists, PAPET-ATP and 3AM-ATP, were included. The amine-derivatized agonists were demonstrated to be moderately potent in a PLC assay or have moderate affinity in binding [18, 19]. Because of the charged amino group at the 3′ position, 3AM-ATP has possible future applications in helping to determine interactions within the binding site at the molecular level (see Discussion). The resulting EC50 values for all the compounds are shown in Table 1. In general, the compounds were equally potent in the yeast assay and in the PLC assay.
Antagonist effects on P2Y1 receptors in the yeast system via growth assays
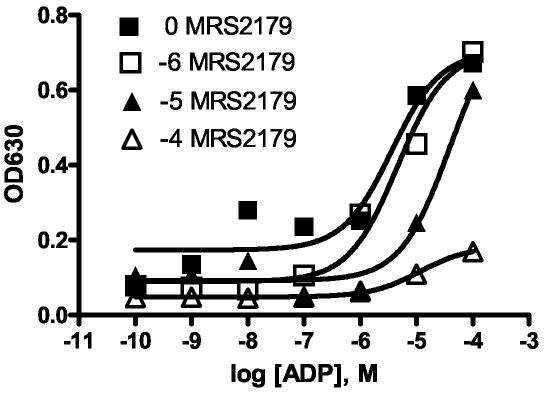
To show that the results obtained were specific to P2Y1 receptors in the yeast system, the P2Y1-selective antagonist MRS2179 was studied. The data obtained from the liquid growth assay are shown in Figure 2. In this experiment the agonist ADP was used as an activator of the P2Y1 receptor, and MRS2179 antagonized the effects of ADP by causing a right shift in the curve.
Figure 2.
Yeast growth assay in the presence of the antagonist MRS2179. MRS2179 (N6-methyl-2-deoxyadenosine-3′,5′-bisphosphate) was used to antagonize ADP (adenosine 5′-diphosphate) via a yeast growth assay (represented as OD630). The MRS2179 concentration was fixed at either 0, 10−6, 10−5, or 10−4 (M) over an ADP range of 10−10–10−4 M. Increasing amounts of antagonist (10−6, 10−5, 10−4 M) cause a right shift in the curves. Each experimental data point was done in triplicate and averaged.
Agonist effects on P2Y1 receptors in astrocytoma cells via calcium assays
We compared the agonist effects by using a different second messenger, the increase in intracellular calcium upon ligand exposure. These experiments were performed on astrocytoma cells expressing the human P2Y1 receptor. Upon addition of a nucleotide ligand, the calcium mobilization was determined by the increase in fluorescence in response to a calcium-sensitive dye. In control experiments, the absence of added nucleotides or the addition of UDP or UTP did not induce any response. In addition, astrocytoma cells (not expressing P2Y1 receptors) showed no response to either ATP or ADP (data not shown). The same set of compounds used in the yeast system was tested in the astrocytoma system (Table 1). As was seen with the yeast system and the PLC assays, the rank orders of 2-MeSADP, ADP, and ATP as agonists were consistent. The EC50 values for all the compounds are shown in Table 1. In the calcium system, the EC50 values were consistently lower than those in the PLC and binding assays and the yeast assay. The rank orders of several of the known compounds, 2-MeSADP, ADP, and ATP, were consistent across the systems. However, ADP-β-S, HT-AMP, and PAPET-ATP were more potent in the calcium measurement than in other assays.
Antagonist effects on P2Y1 receptors in the astrocytoma system
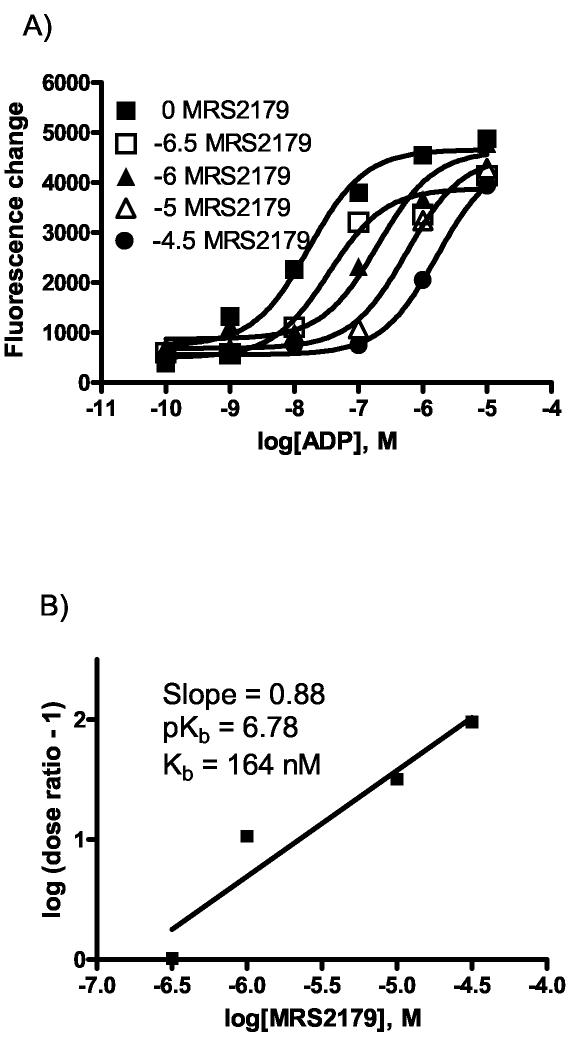
Intracellular calcium can be regulated by various stimuli; therefore, to show consistency and specificity for the P2Y1 receptor, the antagonist MRS2179 (with ADP as an agonist) was tested with the astrocytoma system (Figure 3a). As shown in Figure 3b, Schild analysis provided a KB value of 164 nM, which was comparable to the value of KB = 177 nM for MRS2179 previously reported [30]. Because this is a selective antagonist, it can be used as a control to show that the previous calcium results were specific and not artifacts of another receptor or pathway.
Figure 3.
Effects of MRS2179 on ADP-induced calcium mobilization in astrocytoma cells. a) MRS2179 was used to antagonize the effects of the agonist ADP in the calcium mobilization assay. The MRS2179 concentration was fixed at either 0, 5 × 10−6, 10−6, 10−5, or 5 × 10−4 (M) over an ADP range of 10−10–10−5 (M). Increasing amounts of antagonist cause a right shift in the curves. Each experimental data point was done in duplicate and averaged. b) A Schild analysis was performed to calculate a KB of 164 nM.
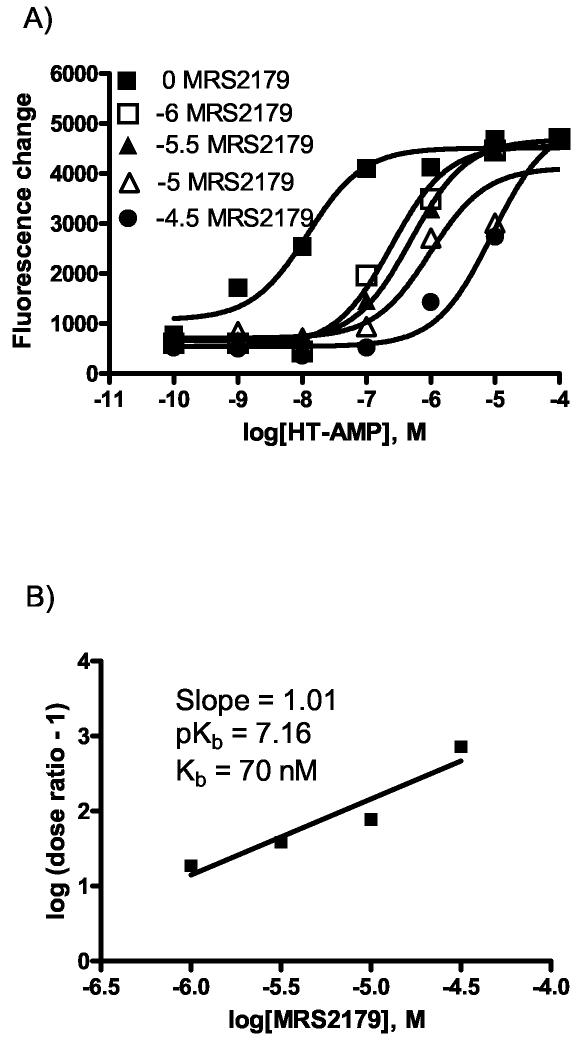
Because HT-AMP was much more potent in the calcium assay than in the yeast assay, it was important to demonstrate that HT-AMP acts as an agonist specifically on P2Y1 receptors to induce calcium mobilization. To confirm this, the antagonist effects of MRS2179 were tested with HT-AMP as the agonist (Figure 4a). Schild analysis gave a KB value of 70 nM, which was similar to that obtained with ADP as an agonist (Figure 4b).
Figure 4.
Effects of MRS2179 on calcium mobilization induced by HT-AMP in astrocytoma cells. a) MRS2179 was used to antagonize the effects of the agonist HT-AMP in the calcium mobilization assay. The MRS2179 concentration was fixed at either 0, 10−6, 5 × 10−5, 10−5, or 5 × 10−4 (M) over an HT-AMP range of 10−10–10−4 (M). Increasing amounts of antagonist cause a right shift in the curves. Each experimental data point was done in duplicate and averaged. b) A Schild analysis was performed to calculate a KB of 70 nM.
Competition of agonists for the [3H]MRS2279 binding to membranes from astrocytoma cells stably expressing human P2Y1 receptors
Because a clear discrepancy between the data for some agonists in the yeast assay and in the calcium assay was observed, we further examined the binding affinity of these compounds for the human P2Y1 receptor. HT-AMP, ADP-β-S, and PAPET-ATP were shown to be much less potent in the binding assay than in the calcium assay (Table 1). The antagonist radioligand was used in the binding assay to test the agonist potency, and the high-affinity and low-affinity binding sites of these agonists have been analyzed.
Discussion
It was shown that the yeast system showed good signal-to-noise ratios and low basal growth, suggesting that it can be used in screening ligands for P2Y1 receptors. The data from the yeast growth assays were generally consistent with those from the previous assays of inositol phosphates accumulation. The rank order for 2-MeSADP, ADP, and ATP is consistent for the different techniques. All of the compounds tested, including ATP, produced a maximal or nearly maximal effect in the yeast growth assay. However, we cannot conclude that ATP itself is a nearly full agonist in this system, because cleavage of ATP to form the full agonist ADP could not be ruled out. In all the systems, the antagonist MRS2179 was able to right-shift the concentration-response curve.
One application of the yeast growth assay involves the neoceptor concept [20] for the P2Y1 receptors. Because P2 receptors are widespread in most tissues, many side effects could potentially occur during therapeutic intervention. To overcome this problem, the use of receptor engineering, agonist design, and gene therapy has been proposed in what is known as the neoceptor concept [20]. One of the difficulties in this process is the identification of potential neoceptors, because site-directed mutagenesis can be laborious. Insight gained from modeling approaches [30] helps to predict potential mutagenesis sites, but this rational approach has met with only mild success. An alternative approach is to use the power of yeast genetics and screening to create libraries of potential neoceptors and then screen for possible candidates rapidly [31]. This technique was used in the identification of constitutively active mutant receptors and in other applications [16, 17]. In this study, 3AM-ATP was studied as a potential neoligand because of its charged nature caused by the amino group. Results showed that 3AM-ATP was only mildly potent in either the yeast or calcium measurements, thus leaving the possibility that the compound could potentially have enhanced affinity for a neoceptor via an electrostatic interaction.
A concern with the yeast assays is that the incubation period occurred over several days, during which the compound stability was questionable. For example, ATP could be broken down to ADP and further to adenosine. Previous work was performed with yeast systems and purinergic ligands [32, 33]. Our data in yeast (EC50: 2-MeSADP = 130 nM, ADP = 1800 nM) compare well with those of previous work [33] that used a yeast system (EC50: 2-MeSADP = 300 nM, ADP = 2300 nM), in which a different reporter system was used, allowing for shorter incubation times (24 h). Furthermore, the potencies of these compounds in the yeast system are similar to those from the assay of inositol phosphates (Table 1). The agreement among these data suggests that the longer incubation times in our studies did not significantly alter the results. The nature of the yeast assay is such that the cells multiply numerous times, requiring more agonist over time for continued growth. When measurements were recorded at an earlier time point, the same trends were observed, suggesting that agonist effect was persistent in the medium over time. This constancy in the effect may indicate that the agonist species is largely unchanged over time, although the stability of the nucleotides has not been directly determined.
In general, both the yeast and calcium data were obtained efficiently but should be analyzed with caution, as a clear discrepancy in the potency of some agonists was observed. The yeast system to measure G protein–level events may be a unique measure of these events, because the traditional GTPγS binding assay for G proteins has not been applied successfully to the P2Y1 receptor. As a complement to classical measurements (radioligand binding, PLC), these assays can provide another measure of functionality rapidly, and taken together the information could lead to the best ways to screen and characterize novel ligands.
In summary, the human P2Y1 receptor was functionally expressed in yeast, and for a variety of known agonists, the rank order of potencies was similar to that found in other assay systems. The results suggest that the yeast expression system is suitable for screening P2Y1 receptor ligands, both agonists and antagonists.
Acknowledgments
We thank Dr. Mark H. Pausch, Dr. Clarice Schmidt, and Dr. Aishe Chen for helpful discussions. We thank Dr. T. Kendall Harden (Univ. North Carolina) for the gift of 1321N1 human astrocytoma cells stably expressing human P2Y1 receptors. RTN was supported by NSF IGERT funding.
Abbreviations:
- ADP
adenosine 5′-diphosphate
- ADP-β-S
adenosine 5′-O-(2-thio-diphosphate)
- 3AM-ATP
3′-amino-3′-deoxy-ATP
- ATP
adenosine 5′-triphosphate
- 3-AT
3-amino-1,2,4-triazole
- FLIPR
fluorescence-imaging plate reader
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GPCR
G protein-coupled receptor
- HT-AMP
2-hexylthioadenosine 5′-monophosphate
- IP3
inositol trisphosphate
- 2-MeSADP
2-methylthioadenosine 5′-diphosphate
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2279
2-chloro- N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- PLC
phospholipase C
- PAPET-ATP
2-[4-amino(2-phenylethyl)thio]-ATP
- PBS
phosphate-buffered saline
- SC
synthetic complete
- SCURA
SC media lacking uracil
- SC-URA-HIS
SC media lacking uracil and histidine
- UDP
uridine 5′-diphosphate
- UTP
uridine 5′-triphosphate
References
- 1.Burnstock G, Fischer B, Hoyle C, Maillard M, Ziganshin AU, Brizzolara AL, von Isakovics A, Boyer JL, Harden TK, Jacobson KA. Structure activity relationships for derivatives of adenosine-5′-triphosphate as agonists at P2 purinoceptors—heterogeneity within P2X and P2Y subtypes. Drug Dev Res. 1994;31(3):206–19. doi: 10.1002/ddr.430310308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–92. [PubMed] [Google Scholar]
- 3.Jacobson KA, Jarvis MF, Williams M. Purine and pyrimidine (P2) receptors as drug targets. J Med Chem. 2002;45(19):4057–93. doi: 10.1021/jm020046y. [DOI] [PubMed] [Google Scholar]
- 4.Harden TK, Stephens L, Hawkins PT, Downes CP. Turkey erythrocyte membranes as a model for regulation of phospholipase C by guanine nucleotides. J Biol Chem. 1987;262(19):9057–61. [PubMed] [Google Scholar]
- 5.Niedernberg A, Tunaru S, Blaukat A, Harris B, Kostenis E. Comparative analysis of functional assays for characterization of agonist ligands at G protein-coupled receptors. J Biomol Screen. 2003;8(5):500–10. doi: 10.1177/1087057103257555. [DOI] [PubMed] [Google Scholar]
- 6.Bartfai T, Benovic JL, Bockaert J, Bond RA, Bouvier M, Christopoulos A, Civelli O, Devi LA, George SR, Inui A, Kobilka B, Leurs R, Neubig R, Pin JP, Quirion R, Roques BP, Sakmar TP, Seifert R, Stenkamp RE, Strange PG. The state of GPCR research in 2004. Nat Rev Drug Discov. 2004;3(7):577–626. doi: 10.1038/nrd1458. [DOI] [PubMed] [Google Scholar]
- 7.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischorfberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hechler B, Vigne P, Leon C, Breittmayer JP, Gachet C, Frelin C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol Pharmacol. 1998;53(4):727–33. [PubMed] [Google Scholar]
- 9.Palmer RK, Boyer JL, Schachter JB, Nicholas RA, Harden TK. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol Pharmacol. 1998;54(6):1118–23. [PubMed] [Google Scholar]
- 10.Camaioni E, Boyer JL, Mohanram A, Harden TK, Jacobson KA. Deoxyadenosine bisphosphate derivatives as potent antagonists at P2Y1 receptors. J Med Chem. 1998;41(2):183–90. doi: 10.1021/jm970433l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leberer E, Thomas DY, Whiteway M. Pheromone signalling and polarized morphogenesis in yeast. Curr Opin Genet Dev. 1997;7(1):59–66. doi: 10.1016/s0959-437x(97)80110-4. [DOI] [PubMed] [Google Scholar]
- 12.King K, Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Control of yeast mating signal transduction by a mammalian beta 2-adrenergic receptor and Gsalpha subunit. Science. 1990;250(4977):121–23. doi: 10.1126/science.2171146. [DOI] [PubMed] [Google Scholar]
- 13.Price LA, Kajkowski EM, Hadcock JR, Ozenberger BA, Pausch MH. Functional coupling of a mammalian somatostatin receptor to the yeast pheromone response pathway. Mol Cell Biol. 1995;15(11):6188–95. doi: 10.1128/mcb.15.11.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price LA, Strnad J, Pausch MH, Hadcock JR. Pharmacological characterization of the rat A2A adenosine receptor functionally coupled to the yeast pheromone response pathway. Mol Pharmacol. 1996;50(4):829–37. [PubMed] [Google Scholar]
- 15.Dowell SJ, Brown AJ. Yeast assays for G protein-coupled receptors. Recept Channels. 2002;8(56):343–52. [PubMed] [Google Scholar]
- 16.Beukers MW, van Oppenraaij J, van der Hoorn PP, Blad CC, den Dulk H, Brouwer J, IJzerman AP. Random mutagenesis of the human adenosine A2B receptor followed by growth selection in yeast. Identification of constitutively active and gain of function mutations. Mol Pharmacol. 2004;65(3):702–10. doi: 10.1124/mol.65.3.702. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt C, Li B, Bloodworth L, Erlenbach I, Zeng FY, Wess J. Random mutagenesis of the M3 muscarinic acetylcholine receptor expressed in yeast. Identification of point mutations that “silence” a constitutively active mutant M3 receptor and greatly impair receptor/G protein coupling. J Biol Chem. 2003;278(32):30248–60. doi: 10.1074/jbc.M304991200. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann C, Moro S, Nicholas RA, Harden TK, Jacobson KA. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J Biol Chem. 1999;274(21):14639–47. doi: 10.1074/jbc.274.21.14639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao ZG, Mamedova L, Tchilibon S, Gross AS, Jacobson KA. 2,2′-Pyridylisatogen tosylate antagonizes P2Y1 receptor signaling without affecting nucleotide binding. Biochem Pharmacol. 2004;68(2):231–37. doi: 10.1016/j.bcp.2004.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobson KA, Gao ZG, Chen A, Barak D, Kim SA, Lee K, Link A, Van Rompaey P, Van Calenbergh S, Liang BT. Neoceptor concept based on molecular complementarity in GPCRs: a mutant adenosine A3 receptor with selectively enhanced affinity for amine-modified nucleosides. J Med Chem. 2001;44(24):4125–36. doi: 10.1021/jm010232o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischer B, Boyer JL, Hoyle CV, Ziganshin AU, Brizzolara AL, Knight GE, Zimmet J, Burnstock G, Harden TK, Jacobson KA. Identification of potent, selective P2Y-purinoceptor agonists: structure activity relationships for 2-thioether derivatives of adenosine-5′-triphosphate. J Med Chem. 1993;36:3937–46. doi: 10.1021/jm00076a023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer JL, Siddiqi S, Fischer B, Romero-Avila T, Jacobson KA, Harden TK. Identification of potent P2Y-purinoceptor agonists that are derivatives of adenosine 5′-monophosphate. Br J Pharmacol. 1996;118(8):1959–64. doi: 10.1111/j.1476-5381.1996.tb15630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, Lacy J, Jacobson KA, Harden TK. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62(5):1249–57. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlenbach I, Kostenis E, Schmidt C, Hamdan FF, Pausch MH, Wess J. Functional expression of M1, M3 and M5 muscarinic acetylcholine receptors in yeast. J Neurochem. 2001;77(5):1327–37. doi: 10.1046/j.1471-4159.2001.00344.x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Q, Guo D, Lee BX, Van Rhee AM, Kim YC, Nicholas RA, Schachter JB, Harden TK, Jacobson KA. A mutational analysis of residues essential for ligand recognition at the human P2Y1 receptor. Mol Pharmacol. 1997;52(3):499–507. doi: 10.1124/mol.52.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 27.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 28.Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol. 1959;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AJ, Dyos SL, Whiteway MS, White JH, Watson MA, Marzioch M, Clare JJ, Cousens DJ, Paddon C, Plumpton C, Romanos MA, Dowell SJ. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein alpha-subunit chimeras. Yeast. 2000;16(1):11–22. doi: 10.1002/(SICI)1097-0061(20000115)16:1<11::AID-YEA502>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 30.Moro S, Guo D, Camaioni E, Boyer JL, Harden TK, Jacobson KA. Human P2Y1 receptor: molecular modeling and site-directed mutagenesis as tools to identify agonist and antagonist recognition sites. J Med Chem. 1998;41(9):1456–66. doi: 10.1021/jm970684u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mentesana PE, Dosil M, Konopka JB. Functional assays for mammalian G-protein-coupled receptors in yeast. Methods Enzymol. 2002;344:92–111. doi: 10.1016/s0076-6879(02)44708-8. [DOI] [PubMed] [Google Scholar]
- 32.Pausch MH, Lai M, Tseng E, Paulsen J, Bates B, Kwak S. Functional expression of human and mouse P2Y12 receptors in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 2004;324(1):171–77. doi: 10.1016/j.bbrc.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Gearing KL, Barnes A, Barnett J, Brown A, Cousens D, Dowell S, Green A, Patel K, Thomas P, Volpe F, Marshall F. Complex chimeras to map ligand binding sites of GPCRs. Protein Eng. 2003;16(5):365–72. doi: 10.1093/protein/gzg045. [DOI] [PubMed] [Google Scholar]
- 34.Moro S, Hoffmann C, Jacobson KA. Role of the extracellular loops of G protein-coupled receptors in ligand recognition: a molecular modeling study of the human P2Y1 receptor. Biochemistry. 1999;38(12):3498–507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]