Abstract
We report a case of sudden death in a clinically stable adult with l-transposition of the great arteries (l-TGA). Sudden death has been reported to be the leading cause of death in l-TGA and is often attributed to arrhythmias in the absence of another identifiable cause. However, the contribution of nonarrhythmic causes to the burden of sudden death in this population is unknown. Comprehensive post-mortem investigation, including autopsy and pacemaker interrogation, demonstrated that the cause of death was massive pulmonary hemorrhage due to stenosis of the patient’s mechanical tricuspid (systemic AV) valve. This report highlights the important contribution of nonarrhythmic causes of sudden death in this population and the value of autopsy and device interrogation in determining true cause of death.
Keywords: autopsy, congenial heart disease, cardiac arrest/sudden death
The population of adults with congenital heart disease is increasing rapidly - with better surgical and medical treatments for children with congenital heart disease over the last few decades, the majority of these patients are living to adulthood. Arrhythmias are a substantial cause of morbidity and mortality in this population.1 Sudden cardiac death (SCD) is an increasing problem and most of these deaths are assumed to be due to ventricular arrhythmias. However, it is not known how often apparent SCDs are due to non-cardiac causes. L-transposition of the great arteries (l-TGA) has been reported to be associated with a particularly high incidence of SCD.1,2 This case report illustrates the important contribution of nonarrhythmic causes to the burden of sudden death in this population.
Methods
As a sudden death in the ongoing citywide Comprehensive UCSF SCD Study, the subject received systematic autopsy with detailed cardiac protocol, toxicology, histology, and device interrogation to determine true underlying cause of sudden death. Comprehensive standardized autopsy for diagnostic criteria for the underlying conditions causing sudden death was performed. A standardized, extensive cardiac examination was performed. Orthogonal dimensions of the atria and ventricles were recorded. Valves were examined for evidence of stenosis, acute leaflet insufficiency, or endocarditis. The major epicardial arteries and their main branches were cut transversely at 2-mm intervals and decalcified before sectioning. Significant coronary artery disease was defined as ≥75% cross-sectional area reduction in ≥1 coronary artery, or an active coronary lesion. Arrhythmic sudden death required documented VT/VF and/or absence of fatal non-cardiac (e.g., pulmonary embolism, lethal toxicology) or non-arrhythmic (e.g., tamponade) autopsy findings.
In addition to samples from areas of gross pathology, standardized samples for histology were taken according to a detailed protocol. Following tissue processing and sectioning, hematoxylin/eosin stains were obtained on lung and cardiac tissues. Trichrome stains were obtained on sections of heart.
Case Report
A 26-year-old male with l-TGA was found suddenly deceased at his home. He had been without complaint recently and was found at his laptop computer. Past medical history was notable for pulmonary atresia and a ventricular septal defect (VSD). At age 2, he had undergone a failed Blalock-Taussing shunt followed by a modified H-type Blalock-Taussig shunt. At age 11, the Blalock-Taussig shunt was taken down with complete repair of the VSD and placement of a homograft between the morphologic left ventricle and pulmonary artery. This was followed by replacement of the morphologic tricuspid valve (systemic AV valve) with a 23 mm St. Jude prosthesis due to progressive insufficiency. 11 months prior to his death, due to progressive pulmonary insufficiency, he underwent transcatheter Melody valve placement in the conduit. At that time, he also underwent stent repair of his proximal left pulmonary artery for severe proximal left pulmonary artery stenosis. At this time, there was moderate stenosis of the mechanical tricuspid valve and surgery to replace the prosthetic valve was deferred to assess his response to the pulmonary valve replacement. The patient had also received an epicardial pacemaker in 1998 for complete AV block. Due to failure of one of the leads, this was converted to a transvenous dual chamber pacemaker in 2004.
Transthoracic echocardiogram 5 months prior to his death showed moderate stenosis of the mechanical systemic AV valve with mean gradient 12 mm Hg. The systolic function of the systemic ventricle (morphologic right ventricle) was mildly reduced. At cardiac catheterization 11 months prior to his death, right ventricular end diastolic pressure was 23 mmHg with a 7 mmHg mean gradient across the tricuspid valve. Pulmonary artery systolic pressure was 54 mmHg and pulmonary vascular resistance was 1.6 Wood units. Medications included carvedilol, digoxin, furosemide, lisinopril, spironolactone, and warfarin. At his last clinic visit 6 weeks before his death, he was feeling well and device interrogation showed normal pacemaker function without recorded arrhythmias. His INR at that visit was 3.9, so his warfarin dose was decreased and he was scheduled for a repeat INR check.
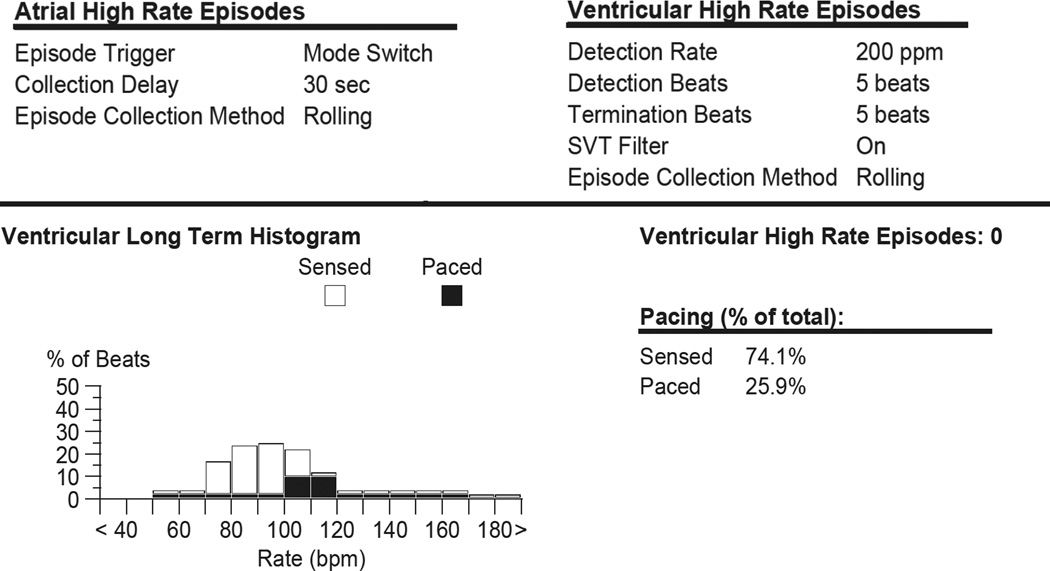
Autopsy revealed massive right pulmonary hemorrhage (Figure 1). In addition to hemorrhage, microscopy showed numerous hemosiderin-laden macrophages and dilated bronchial veins (Figure 2). Coronary arteries showed no significant stenosis, and there was no evidence of focal vascular rupture. The tricuspid valve orifice at autopsy (16 mm diameter) was very narrow for the size of the chambers. There was no pannus or thrombus and the leaflets were mobile. Suggestive of tricuspid stenosis, the left atrium was markedly dilated and hypertrophied. Postmortem pacemaker interrogation showed normal battery voltage, stable capture and sensing thresholds, and stable ventricular lead impedance prior to his death. There were no ventricular high rate episodes > 200 bpm (Figure 3), therefore arrhythmic causes of death were excluded. The cause of death was massive pulmonary hemorrhage due to pulmonary congestion in association with stenosis of the mechanical tricuspid (systemic AV) valve.
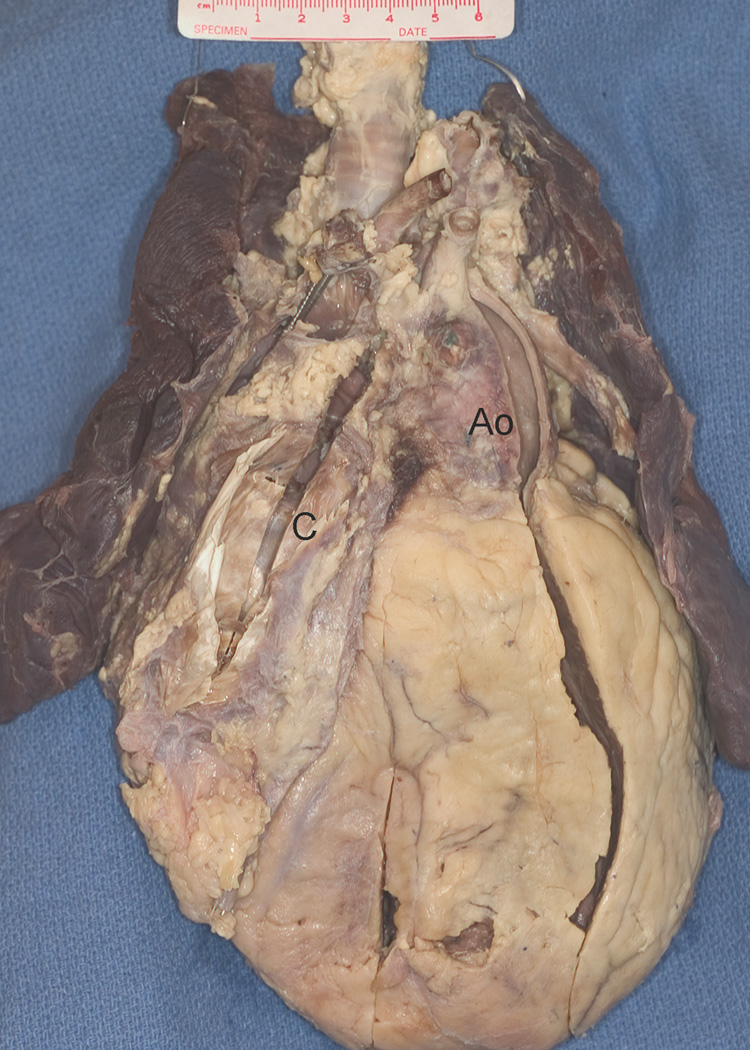
Figure 1.
From the anterosuperior surface the enlarged heart shows the leftwards and anterior aorta (Ao) characteristic of L-transposition, as well as the conduit (C). There is a pacing wire visible in the superior vena cava. The right lung is hemorrhagic.
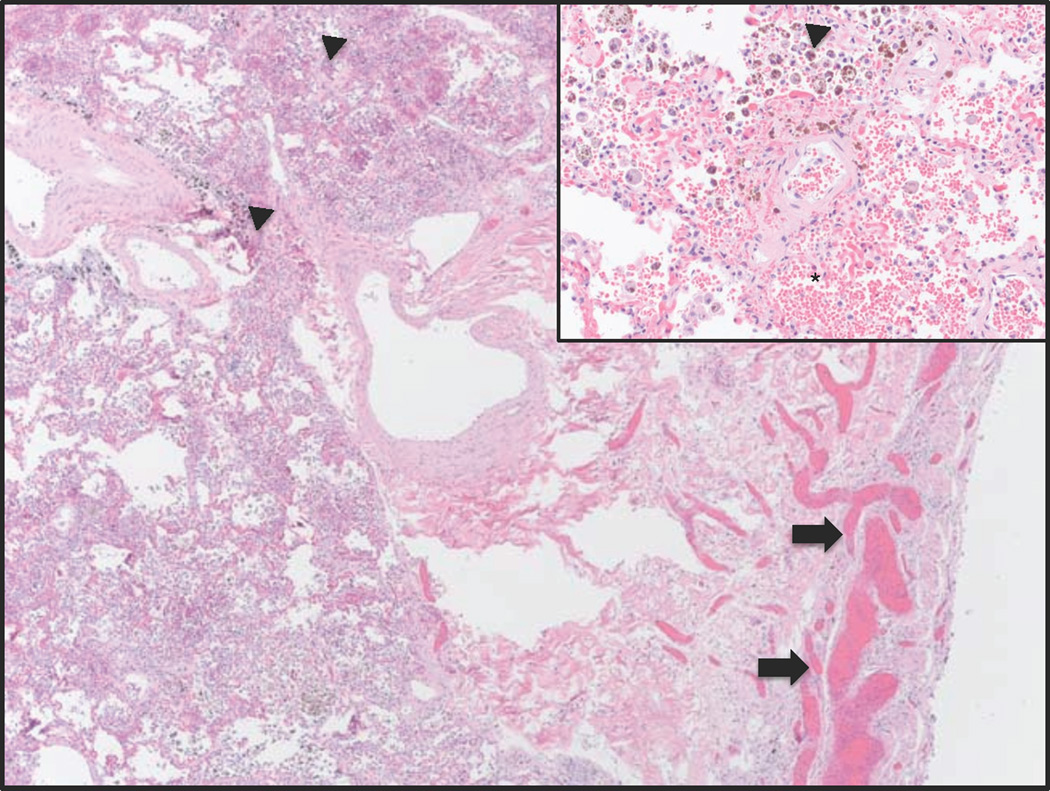
Figure 2.
Histology of lung (hematoxylin and eosin, 12.5X). There are very dilated bronchial veins (arrows) and hemosiderin-laden macrophages (arrowheads). Inset: High power (400×) view of hemosiderin-laden macrophages and intra-alveolar hemorrhage (*).
Figure 3.
Post-mortem pacemaker interrogation.
Discussion
Sudden death in this clinically stable adult with l-TGA was due to nonarrhythmic cause. In this case of out-of-hospital sudden death, comprehensive autopsy results, including pacemaker interrogation, were uniquely available because of the ongoing citywide Comprehensive UCSF SCD Study. In the absence of this evidence establishing a nonarrhythmic cause, this sudden death would have been assumed to be arrhythmic. Cause of sudden death in this case proved to be pulmonary hemorrhage due to pulmonary congestion. This in turn was a sequela of mechanical tricuspid stenosis due to patient-prosthesis mismatch.
In patients without congenital heart disease undergoing mitral valve replacement, patient-prosthesis mismatch has been associated with reduced survival in some but not all studies.3,4 Although hemoptysis is relatively common in patients with mitral stenosis, massive hemoptysis is uncommon5 and we found no reports in the literature of massive hemoptysis due to mitral stenosis resulting from patient-prosthesis mismatch. In this case of l-TGA with tricuspid (systemic AV) valve stenosis, elevated right ventricular end diastolic pressure further contributed to pulmonary vascular congestion and therapeutic anticoagulation likely predisposed this patient to massive pulmonary hemorrhage.
In patients with l-TGA, dual AV nodes are common and AV conduction most often occurs through the anteriorly situated AV node.6 It is thought that its length and position at the mitral-pulmonary continuity render it susceptible to fibrosis and the incidence of heart block is reported to be approximately 2% per year.7 This patient had undergone pacemaker implantation for complete AV block and pacemaker function and capture thresholds were normal at the time of his death. The incidence of ventricular arrhythmias is not precisely known, but has been reported to be 6–7% at approximately 10 years of follow-up.8,9
With advancing age, tricuspid regurgitation and systemic RV dysfunction are an increasing problem and approximately half of patients with l-TGA develop clinical heart failure by age 45.10,11 By recent published estimates, arrhythmias are assumed to be a common cause of death in adults with congenital heart disease,1,2 especially l-TGA. In l-TGA, sudden death has been associated with heart failure, systolic dysfunction of the systemic ventricle, and atrial arrhythmias.12 In one report of adults with congenital heart disease, l-TGA was the defect associated with the highest mortality rate. Sudden death has been reported to be the leading cause of death in l-TGA (40% of deaths) followed by perioperative death, congestive heart failure, and other cardiovascular death, however comprehensive evaluation of deaths including autopsies were not always performed.1
Sudden deaths in adults with congenital heart disease are often attributed to arrhythmias in the absence of another identifiable cause. In published studies,1,2,12,13 sudden deaths have been typically defined as death within 1 hour of symptom onset or unwitnessed death during sleep with the underlying cause assumed to be arrhythmic and/or cardiac despite few cases undergoing autopsy to exclude other causes. In studies of the congenital heart disease population, autopsies were rarely performed and rhythm documentation at the time of the event was available in the minority of cases.1,12,13 For incident out-of-hospital sudden deaths in the general population, typical prevailing autopsy rates have been in the range of 10–15% in the recent studies of community SCDs in Oregon14 and sudden deaths in VALIANT.15 These findings highlight the importance of autopsy even when the cause of death appears obvious. Had comprehensive autopsy not been performed in this case, the cause of death would have been presumed to be arrhythmic, promoting the overriding assumption that most common cause of death in adults with congenital heart disease is arrhythmic. Thus, the risks and benefits of ICDs in the ACHD population may evolve as underlying causes of sudden death are more accurately defined by our study and others.
Acknowledgments
The authors would like to thank the invaluable assistance and input of Valerie Bosco, NP and Phillip Moore, MD.
Disclosures: This work was supported in part by NIH/NHLBI 5R01 HL102090 (Dr. Tseng, Dr. Ursell).
References
- 1.Oechslin EN, Harrison DA, Connelly MS, et al. Mode of death in adults with congenital heart disease. Am J Cardiol. 2000;86:1111–1116. doi: 10.1016/s0002-9149(00)01169-3. [DOI] [PubMed] [Google Scholar]
- 2.Gallego P, Gonzalez AE, Sanchez-Recalde A, et al. Incidence and predictors of sudden cardiac arrest in adults with congenital heart defects repaired before adult life. Am J Cardiol. 2012;110:109–117. doi: 10.1016/j.amjcard.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 3.Jamieson WR, Germann E, Ye J, et al. Effect of prosthesis-patient mismatch on long-term survival with mitral valve replacement: assessment to 15 years. Ann Thorac Surg. 2009;87:1135–1141. doi: 10.1016/j.athoracsur.2009.01.056. discussion 42. [DOI] [PubMed] [Google Scholar]
- 4.Magne J, Mathieu P, Dumesnil JG, et al. Impact of prosthesis-patient mismatch on survival after mitral valve replacement. Circulation. 2007;115:1417–1425. doi: 10.1161/CIRCULATIONAHA.106.631549. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheimer BS, Schwartz SP. Paroxysmal pulmonary hemorrhages: the syndrome in young adults with mitral stenosis. American Heart Journal. 1933;9:12–25. [Google Scholar]
- 6.Anderson RH, Becker AE, Arnold R, et al. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911–923. doi: 10.1161/01.cir.50.5.911. [DOI] [PubMed] [Google Scholar]
- 7.Warnes CA. Transposition of the great arteries. Circulation. 2006;114:2699–2709. doi: 10.1161/CIRCULATIONAHA.105.592352. [DOI] [PubMed] [Google Scholar]
- 8.Rutledge JM, Nihill MR, Fraser CD, et al. Outcome of 121 patients with congenitally corrected transposition of the great arteries. Pediatr Cardiol. 2002;23:137–145. doi: 10.1007/s00246-001-0037-8. [DOI] [PubMed] [Google Scholar]
- 9.Presbitero P, Somerville J, Rabajoli F, et al. Corrected transposition of the great arteries without associated defects in adult patients: clinical profile and follow up. Br Heart J. 1995;74:57–59. doi: 10.1136/hrt.74.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham TP, Jr, Bernard YD, Mellen BG, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multi-institutional study. J Am Coll Cardiol. 2000;36:255–261. doi: 10.1016/s0735-1097(00)00682-3. [DOI] [PubMed] [Google Scholar]
- 11.Voskuil M, Hazekamp MG, Kroft LJ, et al. Postsurgical course of patients with congenitally corrected transposition of the great arteries. Am J Cardiol. 1999;83:558–562. doi: 10.1016/s0002-9149(98)00913-8. [DOI] [PubMed] [Google Scholar]
- 12.Koyak Z, Harris L, de Groot JR, et al. Sudden cardiac death in adult congenital heart disease. Circulation. 2012;126:1944–1954. doi: 10.1161/CIRCULATIONAHA.112.104786. [DOI] [PubMed] [Google Scholar]
- 13.Silka MJ, Hardy BG, Menashe VD, et al. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32:245–251. doi: 10.1016/s0735-1097(98)00187-9. [DOI] [PubMed] [Google Scholar]
- 14.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Pouleur AC, Barkoudah E, Uno H, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122:597–602. doi: 10.1161/CIRCULATIONAHA.110.940619. [DOI] [PubMed] [Google Scholar]