Abstract
Up to a third of the world's population is infected with Toxoplasma gondii. Natural infection in humans can be life threatening during pregnancy and in immunocompromised individuals. TLR11 is the mouse innate sensor that recognizes T. gondii profilin; however in humans the TLR11 gene leads to transcription of no functional protein. Herein, by using a multiple sequence alignment phylogenetic analysis program between human and mouse species we found that human TLR5 seems to be evolutionarily closest member of the TLR gene family related to mouse tlr11. We, therefore asked whether human TLR5 could mediate IL-6, IL-8 and IL-12p70 production in response to the T. gondii profilin. We found that this was the case both in human cell lines as well as peripheral blood monocytes. Moreover, TLR5 neutralization and gene silencing mediated specific ablation of cytokine production after profilin exposure. Finally, peripheral blood monocytes carrying the TLR5 R392X mutation failed to produce cytokines in response to stimulation with profilin. Taken together, the results presented herein reveal a previously unappreciated cross-recognition of a relevant human pathogen-derived PAMP.
Introduction
Microbial recognition by the innate immune system is mediated by a multitude of cellular and endosomal membrane-bound, as well as intracellular receptors. Toxoplasma gondii-derived pattern activation molecular patterns (PAMP's), namely cyclophilin-18 and profilin have been shown to be recognized by receptors present in macrophages and dendritic cells, triggering cell activation and production of pro-inflammatory cytokines, including IL-1β, IL-6 and IL-12. While cyclophilin-18 is recognized by both mouse and human CCR5 [1,2], profilin has been shown to mediate powerful cytokine production from mouse dendritic cells via activation of TLR11 [3]. In fact, TLR11 that was previously found to mediate recognition of uropathogenic bacteria has been identified as a major component and is essential for development of protective immune response in infected mice through the induction of massive IL-12 production by dendritic cells. IL-12-mediated induction of type 1-immunity is crucial for containing parasite replication and mediating long-term immunity to infection. However, due to the presence of several stop codons, transcription of the human TLR11 gene does not produce a functional protein [4]. Yet, as we show here, human cells are responsive to T. gondii profilin. Therefore, we asked whether there could be a functional ortholog for mouse TLR11 that is responsible for recognition of T. gondii profilin in humans. To do so, we performed evolutionary genetic taxa comparisons. We found that TLR11 is, perhaps, the most ancient TLR family member and that the following members of this family of genes were derived from successive gene duplications. Both human and mouse TLR5 seemed to be evolutionarily the oldest relatives to mouse TLR11. This result led us to hypothesize that human TLR5 could have conserved (or rescued) mouse TLR11 biological function and mediate T. gondii profilin recognition. To test this hypothesis, we systematically examined whether human cell lines as well as peripheral blood monocytes expressed functional TLR5, followed by examining their cytokine response to T. gondii profilin in the absence of TLR5 through loss-of-function approaches (Ab-mediated neutralization and siRNA gene silencing). Our results conclusively show that T. gondii profilin induces a TLR5-dependent pro-inflammatory response by human monocytes.
Material and Methods
Reagents and cells
IgA anti-human TLR5, recombinant flagellin and recombinant T. gondii profilin were purchased from Invivogen and proteinase K from Roche. HEK293 cells were purchased from ATCC (CRL-1573.3) and grown in 10% FCS RPMI medium. Peripheral CD14+ blood monocytes were purified from healthy whole blood donors using Ficoll density gradient and highly specific monocyte isolation kit (CD14+ antibody magnetic labeled beads, Miltenyii). Proteinase K digestion of flagellin and profilin were performed as described previously [5,6]. Briefly, proteinase K-agarose was reconstituted in endotoxin-free water to 10 mg/mL, incubated at 4°C for 2 hr, and washed five times with endotoxin-free water. Digestion buffer was prepared by supplementing PBS with 2.7 mM KCl, 1.5 mM K2 PO4 , 137 mM NaCl, and 8.1 mM Na2 PO4 . 100 μg of Flagellin or profilin were incubated in digestion buffer with Proteinase K-agarose slurry on a shaking platform for 3 hr at 37°C, followed by centrifugation and harvesting supernatants. Both cell lines or human peripheral blood monocytes were cultured overnight with native or proteinase K pre-digested PAMP's, with or without anti-huTLR5 Ab. Culture supernatants were harvested and stored at −40°C until assayed for cytokine production.
Evolutionary relationships of taxa
The evolutionary history was inferred using the Neighbor-Joining method [7]. The evolutionary distances were computed using the Poisson correction method [8] and are in the units of the number of amino acid substitutions per site. The analysis involved 20 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 102 positions in the final dataset. Evolutionary analyses were conducted in MEGA5 [9,10] and with ClustalW2-Phylogeny [11].
Human cytokine measurements
Human IL-6, IL-8, IL-12p40 and IL-12p70 levels were evaluated in culture supernatants using ELISA Duo-Set kits from R&D.
TLR5 flow cytometry analysis
HEK293 cells and human peripheral blood monocytes were incubated with mouse R-PE-labeled anti-human TLR5 mAb (clone 85B152.5 – Enzo life sciences) or isotype mouse IgG2a-PE control Ab in FACS buffer (surface staining) or PermWash solution (surface and intracellular staining) (BD) for 30 minutes. Cells were then washed in FACS buffer, re-suspended and acquired for flow cytometry analysis. Data was analyzed using FlowJo software.
siRNA TLR5 gene silencing
Control (sc-37007) and TLR5-specific (sc-40253) siRNA oligos were obtained from Santa Cruz Biotechnology. Gene silencing was performed using transfection kit from Amaxa, following their specific instructions. Briefly, highly enriched peripheral blood CD14+ monocytes were transfected with control and TLR5-specific siRNAs using a nucleofector device and transfection reagent (Amaxa) in media, afterwards cells were placed in a 24 well plate with pre-warmed transfection media and incubated for 24 hrs. GFP-labeled empty vector control was used to determine the transfection efficiency by flow cytometry. To verify the TLR5 gene silencing, we analyzed TLR5 expression in transfected monocytes by flow cytometry using mouse RPE-labeled anti-human TLR5 (Enzo Life Sciences). In order to test the functional ablation of TLR5 expression, transfected monocytes that showed decreased TLR5 protein levels were stimulated with flagellin and/or profilin (1 μg/mL) for 24 hrs, supernatants were harvested and assayed for cytokine production by ELISA.
TLR5 (R392X) genotyping
Genomic DNA samples (25ng) from 35 peripheral blood monocytes were isolated and screened for TLR5 (R392X, rs5744168). Genotyping was carried out by allelic discrimination real-time PCR using the primers: WT TLR5 T5-10, forward, 5’-ATGGGAGACCACCTGGACCTTCTCC-3’; T5-30, reverse, 5’-GGAGATGGTTGCTACAGTTTGCAACGG-3’. PCR primers for TLR5 (R392X) were T5-10, forward and T5-31, reverse, 5’-GAGATCCAAGGTCTGTAATTTTTCCAGG-3’ [12]. End-point analysis was made by high-resolution melting curve analysis using LightCycler 480 software (Roche).
In vitro Hu-TLR5 ectodomain binding assay
Assay was performed as indicated by the manufacturer's instructions, as follows. Flagellin and profilin (Invivogen) (100 ng/mL in PBS) were incubated overnight in 96-well ELISA plates. Wells were washed three times with PBS and incubated with titration curves of Hu-TLR5-Fc (Invivogen) (100-3.125ng/mL) with PBS alone or with Flagellin or with Profilin (100 ng/mL). After a 2hr incubation, wells were washed 5 times with PBS and incubated with anti-Human IgG1-HRP conjugates for 1hr. Wells were developed with TMB substrate and OD measured at 405nm. Non-linear regression curves were plotted, normalized and analyzed using Prism software.
Statistical analysis
Student t test was performed to determine statistical significance of differences (p < 0.05) between control and treated groups, using the GraphPad software.
Results
Human TLR5 and mouse tlr11 and tlr12 are part of an ancient cluster within the TLR phylogenetic tree
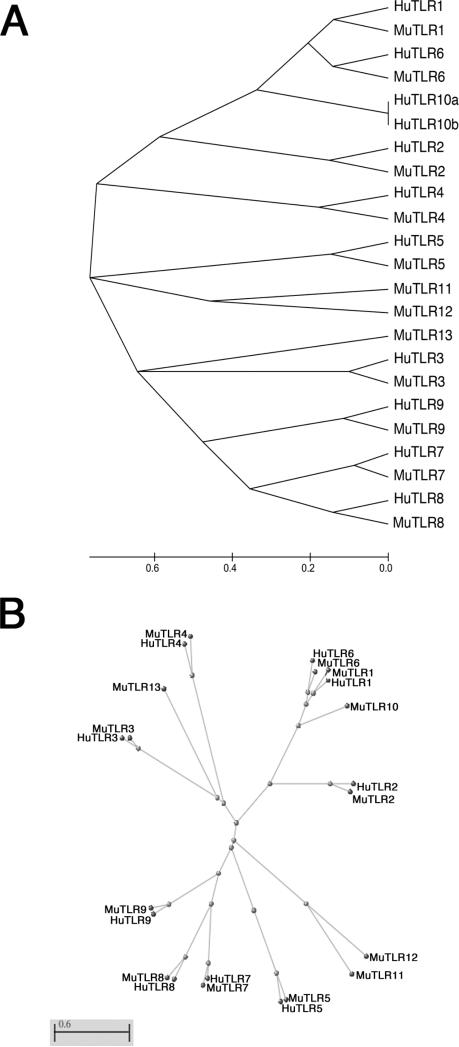
Human innate immune system cells can recognize the presence of T. gondii parasites and produce pro-inflammatory cytokines, including IL-12 [13-15]. T. gondii profilin was shown to be a major component of innate recognition by mouse innate cells via activation of TLR11 [3]. Human TLR11 gene is not translated due to the presence of a stop codon within its coding region [4] and to this date, there are no homologs to mouse tlr12. We hypothesized that T. gondii profilin activates human cells through interaction with another TLR present in human cells. The approach to select which candidates to test was to examine the comparative evolutionary background of the TLR gene family between humans and mice. Figure 1 shows a phylogenetic tree comparing the amino-acid sequences for TLR's 1 through 13 using Neighbors-Joining method. Interestingly, the data indicate that mouse TLR11 is the most ancient member of this family, with all subsequent clusters derived from gene duplications and amino acid substitutions. In this regard, the oldest event gave origin to a cluster with mouse TLR12 and with human and mouse TLR5. Later, clusters containing TLR's 1, 2, 3, 4, 6 and 10 and, more recently, another cluster containing TLR's 7, 8 and 9 were derived. Based on these observations, we hypothesized that human TLR5 could potentially perform the microbial recognition executed by mouse TLR11. Although this method is limited with regards to interpretations that indicate complete evolutionary estimation, for the question posed in this manuscript, we consider that it fulfilled its potential as a general sequence comparison analysis of gene family evolution between the two species based on amino acid sequences. We therefore raised the hypothesis that human TLR5 is involved in innate recognition and induction of cytokine production by T. gondii-derived profilin.
Figure 1. Evolutionary relationship comparison of Toll-like receptor gene family between human and mouse.
The evolutionary history was inferred using the Neighbor-Joining method using MEGA5 cladogram tree (A) or ClustalW2-Phylogeny radial tree (B). The optimal tree with the sum of branch length = 7.94970641 is shown. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site. The analysis involved 20 amino acid sequences. All positions containing gaps and missing data were eliminated. There were a total of 102 positions in the final dataset.
HEK293 cells are TLR5+ and respond to both flagellin and profilin in a TLR5-dependent manner
Next, we have focused on investigating the potential involvement of human TLR5 in the recognition of T. gondii profilin. We adopted a widely known approach using human embryonic kidney (HEK) 293 cell lines transfected with the respective TLR's. However, to our surprise, we noticed that in the presence of both T. gondii profilin and the prototypical TLR5 ligand, flagellin, there was significant IL-8 production from nontransfected cells, independent of the presence of TLR5-containing plasmid. At this point, we followed up on testing whether HEK293 cells expressed detectable amounts of human TLR5. As shown in figure 2A, we found significant levels of TLR5 in HEK293 cells. On the other hand, THP-1 cells did not express detectable levels of TLR5 above isotype control Ab staining. These results suggest that the profilin-triggered IL-8 response in HEK293 cells could be derived from activation of this receptor.
Figure 2. Endogenously-expressed human TLR5 in HEK293 cells mediate IL-8 responses to Flagellin and T. gondii-derived profilin.
(A) HEK293 or THP-1 cells were suspended in FACS buffer with PE-labeled anti-human TLR5 antibody. Cells were then washed and acquired for flow cytometry. Data shown are histogram overlays of isotype antibody control (IgG2a-PE – red histograms) and anti-TLR5 (blue histograms) stained samples. (B, C and D) Following, cells were stimulated with several concentrations of recombinant flagellin C (B), recombinant T. gondii profilin (C) or LPS (D) for 24hrs. HEK293 cells were plated and incubated in the presence of medium alone or with anti-TLR5 mAb, as indicated, followed by stimulation with flagellin (E), profilin (F) or LPS (G) for 24 hrs. Supernatants were harvested and assayed for the presence of IL-8 by ELISA. Regression curves, r2 and IC50 for anti-TLR5 mAb-mediated inhibition of IL-8 responses to flagelin, profilin or LPS are shown. Data shown is representative of, at least, three independent experiments.
In fact, figure 2B shows that both flagellin and profilin triggered a dose-dependent IL-8 production from HEK293 cells, but not in THP-1 cells (Figure 2B). Upon transfection with human, but not mouse, TLR5 HEK293 cells produced extremely high levels of IL-8 in response to flagellin (Figure 2C) and profilin (Figure 2D). Such potent, yet non-physiological response over-shadows the endogenous TLR5-triggered cytokine production. Moreover, mAb-mediated neutralization of human TLR5 inhibited IL-8 production by HEK293 cells in response to flagellin and profilin, but not to LPS stimulation (Figures 2E-G). Therefore, this data clearly indicate that TLR5 expressed in HEK293 cells triggers IL-8 production in response to both flagellin and T. gondii-derived profilin.
Human peripheral blood-derived CD14+ monocytes produce pro-inflammatory cytokines in response to flagellin and profilin in a TLR5-dependent manner
To establish a role for human TLR5 in the recognition of T. gondii profilin in a more physiological context, we next aimed to evaluate the production of pro-inflammatory cytokines by peripheral blood monocytes in response to flagellin, profilin and LPS. TLR5 surface expression profile was established by flow cytometry. Freshly isolated human peripheral blood monocytes (CD14+ cells) displayed membrane (solid line) as well as intracellular (dotted line) TLR5 above background staining (gray histogram) (Figure 3A). Upon exposure to flagellin, profilin and LPS, we observed significant induction of IL-6 and IL-12p70 by peripheral CD14+ monocyte cultures (upward triangles, Figures 3B-G), while cells incubated with medium alone showed almost undetectable production (squares, Figures 3B-G). Moreover, pre-incubation with a neutralizing anti-TLR5 mAb abolished cytokine induction by flagellin and profilin, but not by LPS, thus confirming the specific TLR5 activation by both flagellin and profilin (diamonds, Figure 3B-G). Notably, pre-digestion of both flagellin and profilin using proteinase K also prevented cytokine induction in these cultures, but not with LPS-treated ones (inverted triangles, Figure 3B-G), therefore ruling out the potential effect of non-peptide contaminants in the induction of cytokine production. Taken together, these results provide solid evidence that human peripheral blood monocytes are activated by T. gondii profilin in a TLR5-sensitive manner.
Figure 3. T. gondii-derived profilin triggers a TLR5-sensitive human peripheral blood-derived monocyte pro-inflammatory cytokine production.
(A) Peripheral blood monocytes were purified and stained for intact (solid line) and permeabilized cells (dotted line). CD14-MACS beads purified peripheral blood monocytes were suspended in FACS buffer with or without Perm/wash buffer followed by incubation with PE-conjugated isotype control IgG2a (gray histogram) or with anti-TLR5 mAb. (B – G) Cells were plated and incubated with medium alone (squares), flagellin (B-C), profilin (D-E) or LPS (F-G) in PBS (upward triangles), PAMP pre-digested with proteinase K (downward triangles) or with anti-human TLR5 mAb (1-0.1μg/mL) (diamonds). After 24-hr incubation, supernatants were harvested and assayed for IL-6 (B, D and F) or IL-12p70 (C, E and G) by ELISA. Data shown is representative of three independently performed experiments.
TLR5 gene silencing inhibits response of human monocytes to flagellin and profilin
To further establish the role of TLR5 in mediating cytokine induction by human monocytes, we inhibited TLR5 gene expression by transfection with siRNA-coding plasmids. Figure 4A shows the effect of TLR5 siRNA transfection (solid line) versus control siRNA transfection (dotted line) over the cell membrane TLR5 expression levels as determined by flow cytometry. Figures 4B-C show that while control siRNA-transfected cells presented production of IL-6 and IL-12p70 in response to all microbial stimulants, there was a significant reduction in cytokine production by cells transfected with TLR5 siRNA after stimulation with both flagellin (black bars) and profilin (gray bars). Taken together, these results indicate that TLR5 is a required component of human monocytes response to T. gondii-derived profilin.
Figure 4. siRNA-mediated silencing of human TLR5 inhibits profilin and flagellin-mediated pro-inflammatory cytokine production by human peripheral blood-derived monocytes.
Peripheral blood-derived monocytes were isolated and electroporated in the presence of medium alone, control siRNA or human TLR5 siRNA oligos. Cells were then stained for TLR5 as described in figure 3. (A) Transfected cells were gated and TLR5 expression analyzed. A histogram overlay of isotype control stained cells (gray histogram), control siRNA transfected cells (dotted line) or TLR5 siRNA transfected cells (solid line) is shown. Cells were plated and incubated in the presence of medium alone (white bars), flagellin (black bars), profilin (gray bars) or LPS (white bars) for 24 hrs. Culture supernatants were then harvested and assayed for IL-6 (B) and IL-12p70 (C) by ELISA. Data shown is representative of three independently performed experiments.
TLR5 (R392X) peripheral blood monocytes are unresponsive to T. gondii profilin stimulation and hyporresponsive to tachyzoite exposure in vitro
Human polymorphisms of the TLR5 gene had been described previously to be relevant in several infectious diseases and chronic inflammatory diseases, including Legionnaires’ disease [12], Crohn's disease [16], cystic fibrosis [17] and obesity [18]. In particular, the mutation R392X – which leads to the insertion of a stop codon at the position 392, leads to complete loss of TLR5 protein expression. R392X is a highly frequent (up to 10%) mutation among Caucasians of European background [12]. Notably, TLR5 (R392X) cells were shown be unresponsive to flagellin stimulation [12]. Here, we aimed to further establish a more physiological model to further dissect the function of TLR5 in mediating monocyte cytokine responses to T. gondii profilin. To do so, we determined TLR5 expression in purified CD14+ monocytes. Figure 5A shows a histogram overlay profile from monocytes that expressed low (light gray filled histogram– CTDC C42) and high (black filled histogram – CTDC C14) levels of TLR5, empty histograms represent isotype control staining (Gray line, isotype control Ab for CTDC C42 and black line, isotype control Ab for CTDC C14). Figure 5B shows the MFI of such samples and the low/high profiles of TLR5 expression within CD14+ cells. We then confirmed by real-time genotyping that the cells that showed low-levels of TLR5 staining also showed high detection using primers containing the R392X mutation (Supplementary figure 1). We then followed to examine their cytokine profile in response to LPS, flagellin and profilin. Figures 5C-D show IL-6 and IL-12p40 levels induced above background (unstimulated control) values. LPS stimulation triggered increased production of all cytokines tested in cell from both donors (white bars). On the other hand, flagellin and profilin triggered IL-6 and IL-12p40 production from TLR5high (CTDC C14), but not from TLR5 R392X (CTDC C42) cells (gray and black bars – Figures 5C-D), thus providing evidence that a fully functional TLR5 is required for monocyte response to T. gondii profilin. To further establish the biological relevance of TLR5-mediated recognition of T. gondii profilin, we exposed TLR5WT (CTDC C14) and TLR5R392X (CTDC 42) to live T. gondii Rh strain tachyzoites at several m.o.i.'s and assayed for IL-6 and IL-12p40 by ELISA. Figures 5E (IL-6) and 5F (IL-12p40) show that TLR5WT (filled circles) or TLR5R392X (empty squares) peripheral blood monocytes presented cytokine production in response to tachyzoite exposure in a m.o.i.-dependent manner, however TLR5R392X monocytes showed significant reduction in cytokine production at 1 m.o.i. (Figures 5E-F). Thus, suggesting a minor, but nonetheless relevant role for TLR5-mediated cytokine response to live parasite in monocytes. In light of these results, we exposed HEK293 cells to live T. gondii Rh strain tachyzoites (same m.o.i. range as in figure 5E and F) in the presence of isotype control Ab or with neutralizing anti-TLR5 mAb and assayed for IL-8 production, as described in figure 2. Figure 5G shows that HEK293 cells produced IL-8 in response to tachyzoite exposure in a m.o.i.-dependent manner while in the presence of isotype control Ab (filled circles). However, human TLR5 neutralization completely abolished HEK293 IL-8 response to live tachyzoites in vitro (empty squares). Thus, suggesting that epithelial cells (such as HEK293) which have a more limited range of PAMP recognition machinery use mostly TLR5/profilin interaction for inducing cytokine production. On the other hand, monocytes that express a wider spectrum of pattern recognition receptors are capable of responding to live tachyzoites through TLR5-independent pathways.
Figure 5. TLR5 (R392X) mutation abolishes monocyte cytokine production after to T. gondii profilin stimulation.
Peripheral blood CD14+ monocytes were purified and stained as described in figure 3. Panel A shows histogram overlays of isotype control versus TLR5 staining of CD14+ monocytes obtained from two donors (CTDC C42 – gray histograms and CTDC C14 – black histograms). Line empty histograms represent isotype control Ab staining (Black line for CTDC C14 and gray line for CTDC C42). Mean fluorescence intensity from the samples shown are plotted in panel B (CTDC C42 – black bar and CTDC C14 – white bar). Cell suspensions from the same donors were then cultured in the presence of medium alone, LPS (white bars), flagellin (gray bars) or profilin (black bars) (all at 1 μg/mL) for 24hrs. Supernatants were harvested and assayed for IL-6 (C) and IL-12p40 (D) by ELISA. Peripheral blood monocytes from CTDC C14 (filled circles) and CTDC C42 (empty squares) were incubated in the presence of live T. gondii Rh strain tachyzoites (m.o.i. ranging from 1000 to 0.0001), 24 hrs later supernatants were harvested and assayed for IL-6 (E) and IL-12p40 (F). HEK293 cells were plated and incubated in the presence of isotype control Ab (filled circles) or anti-HuTLR5 mAb (empty squares) and live tachyzoites as indicated in panels E-F. Culture supernatants were harvested and assayed for IL-8 by ELISA. Data shown is representative of triplicate samples from two independent experiments performed. Asterisks indicate p<0.05 between donors or between isotype control and anti-HuTLR5 mAb treatment as determined by t test.
Flagellin and profilin share common binding sites within the ectodomain of human TLR5
Our results consistently show a human TLR5-dependent cytokine response to T. gondii profilin within both myeloid and non-myeloid compartments. The relative contribution of this pathway remains to be established in human toxoplasmosis, however it suggests profilin as a novel ligand for human TLR5. To document such interaction, we took advantage of binding assays using human TLR5 ectodomain/human IgG Fc (huTLR5-Fc) fusion protein. Figure 6 show binding curves of huTLR5-Fc pre-incubated with BSA to both flagellin (solid red line) and profilin (solid blue line). In order to investigated whether flagellin could compete for profilin TLR5 binding sites (and vice versa), we pre-exposed huTLR5-Fc to the competitor prior to incubating to the plate bound ligand. Interestingly, we found minor cross-competition between flagellin and profilin (Figure 6, dotted lines). Thus, suggesting distinct binding sites among the two ligands with minor overlap within TLR5.
Figure 6. Flagellin and profilin bind to the ectodomain of human TLR5 in vitro.
Flagellin (red lines) or profilin (blue lines) (1μg/mL) were immobilized to ELISA plates. Wells were then incubated with increasing concentrations of HuTLR5-Fc fusion protein (ranging from 1.5 to 200μg/mL) in the presence of 1μg/mL of BSA (solid lines), profilin (red dotted line) or flagellin (blue dotted line) for 2hrs. Wells were washed 3× with PBSTween 0.5%, followed by incubation with anti-human IgG-HRP conjugates. HuTLR5-Fc binding was determined colorimetrically using TMB substrate in an ELISA plate reader. Data was then normalized to percentage of maximum values and non-linear regression curve fit using Prism. Data shown is mean of triplicate samples from one of two independent experiments performed.
Discussion
Some studies have shown an overlap of TLR5 and TLR11 in the mouse system with TLR5-dependent responses to previously assigned TLR11 ligands [19-21] and vice-versa [22]. This set of overlapping activity might be rooted on the selective pressure for recognition of PAMP's from pathogens well-adapted to their hosts. Our functional clustering of the TLR gene family from humans and mice suggests an older relationship between TLR11 (supposedly the oldest TLR in both species) and TLR5 – the first gene theoretically product of an ancient tlr11 gene duplication event. Despite the evolutionary distance, our results suggest that function and microbial ligand affinity is conserved between human TLR5 and mouse TLR11. Interestingly, there has been previously reported overlap with regard to mouse TLR5 and TLR11 ligand specificity; however a thorough comparative study of TLR5 and TLR11 ligands in mouse cells has not yet been conducted to date.
Previous literature vastly relied on HEK293 transfection system to test ligand specificity with a great degree of reproducibility. Nevertheless, our manuscript raises a central issue for the correct interpretation of these results. We have shown here strong evidence supporting the endogenous expression of TLR5 in non-transfected HEK293 cells. Past analysis of TLR/PAMP interaction in transfected HEK293 cells were certainly made under very high gene expression levels (more than 1,000 fold higher than baseline) by comparing untreated versus stimulated cells (usually represented as fold-increase over control). However, this method introduces a bias in the interpretation of the results due to the fact that TLR activation by PAMP in non-transfected or mock-transfected cells is proportionally increased. However, the magnitude of TLR activation signal is too high in transfected cells as for the signal levels observed in non-transfected cells to be appreciated after ligand exposure. Another potential complicating factor with this method of analysis is that the signals coming from subtle affinity changes between receptor and PAMP's are minimized by the extremely high activation threshold over baseline. In fact, the commercial source for the use of HEK293 cells in a TLR/NOD reporter assay alerts for the endogenous baseline levels of TLR3, TLR5 and NOD1 in these cells (Invivogen Cat# 293-LacZ). Moreover, several previous reports indicated increased endogenous TLR5 expression in HEK293 cells [23-25]. Our results, therefore, are consistent with several lines of published data.
Human cells show an obvious response to T. gondii profilin that is independent of any cognate signal (i.e., CD40L, IFN-γ) – an observation that highlights the innate character of this interaction. However, it is not clear that profilin is the only PAMP from this protozoan to trigger human innate cytokine response in vivo. The mouse model suggests a very complex scenario, where several receptor/ligand pairs play a relevant role early after infection in vivo. As such, TLR11 is required for profilin-triggered cytokine production [3], while TLR9 has been shown to mediate some response [26]. However both TLR11- and TLR9-deficient mice show resistance to acute infection, while MyD88-deficient mice quickly succumb to infection [27]. Moreover, we and others have shown the activation of CCR5-dependent cytokine dendritic cell responses by exposure to cyclophillin-18 from T. gondii [1,28]. CCR5-deficient mice also showed high mortality upon infection concomitant to lower type-1 cytokine production [1].
More recently, a series of studies have shown that TLR11-mediated response to T. gondii is compounded by co-activation of TLR12, as well as TLR7/TLR9 triggering by parasite RNA/DNA [29]. In the absence of all these pathways combined, mice show a susceptibility phenotype that resembles T. gondii-infected MyD88-deficient hosts [29]. Such complex response can be further supported by the observations using UNC93B1-deficient mice, in which the activation of TLR's 3/7/9 by RNA/DNA is abolished [30]. Taking all these observations together with the fact that humans have a truncated nonfunctional TLR11 gene and no homolog for mouse tlr12, we propose here that TLR5 “fills-in” for the absent human TLR11. Further interactions resulting from recognition of parasite RNA and DNA in the context of profilin-initiated responses remain to be further characterized. Our experiments were performed using recombinant profilin to focus on a specific ligand/receptor interaction, although crude parasite lyzates (STAg) can trigger monocyte cytokine production (J.A. personal observations). Furthermore, proteinase K digestion of recombinant profilin completely abolished cytokine induction by this molecule, thus suggesting that potential nucleotide, polysaccharides or other non-peptide contamination by is unlikely.
The relative contribution of TLR5 to the protection against toxoplasmosis in humans, especially within populations in which there is high frequency of the TLR5 R392X mutant remains to be fully investigated. Finally, the biological implications of the studies presented here open a new venue for PAMP-based vaccine adjuvants. Vaccine research using the mouse system has not accounted for the potential role of TLR5/profilin interaction seen in human cells, as we showed here. The use of profilins as vaccine adjuvants has been proposed previously [31]. Our results clearly identify the receptor/ligand interaction involved in profilin recognition in humans is, therefore highly relevant for the future development of PAMP-based vaccine adjuvants as well as other clinical applications.
Supplementary Material
Acknowledgments
Rosa Maria Salazar Gonzalez, Hesham Shehata, Yanfen Yang and Michael J. O'Connell designed and performed experiments. Maria E. Moreno-Fernandez and Claire A. Chougnet provided vital reagents. Julio Aliberti designed, performed experiments and wrote the manuscript. This work was supported by NIH grants AI078969 and AI075038.
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
References
- 1.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Sousa C, Fairlamb A, Ribeiro JM, Sher A. Molecular mimicry of a ccr5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 2.Yarovinsky F, Andersen JF, King LR, Caspar P, Aliberti J, Golding H, Sher A. Structural determinants of the anti-hiv activity of a ccr5 antagonist derived from toxoplasma gondii. J Biol Chem. 2004 doi: 10.1074/jbc.M410550200. [DOI] [PubMed] [Google Scholar]
- 3.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. Tlr11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 4.Ishii KJ, Uematsu S, Akira S. ‘Toll’ gates for future immunotherapy. Curr Pharm Des. 2006;12:4135–4142. doi: 10.2174/138161206778743484. [DOI] [PubMed] [Google Scholar]
- 5.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 6.Luong M, Zhang Y, Chamberlain T, Zhou T, Wright JF, Dower K, Hall JP. Stimulation of tlr4 by recombinant hsp70 requires structural integrity of the hsp70 protein itself. J Inflamm (Lond) 2012;9:11. doi: 10.1186/1476-9255-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 8.Zuckerkandl E, Pauling L. Molecules as documents of evolutionary history. J Theor Biol. 1965;8:357–366. doi: 10.1016/0022-5193(65)90083-4. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Nei M, Dudley J, Tamura K. Mega: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in bioinformatics. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura K, Dudley J, Nei M, Kumar S. Mega4: Molecular evolutionary genetics analysis (mega) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 11.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at embl-ebi. Nucleic Acids Res. 2010;38:W695–699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hawn TR, Verbon A, Lettinga KD, Zhao LP, Li SS, Laws RJ, Skerrett SJ, Beutler B, Schroeder L, Nachman A, Ozinsky A, Smith KD, Aderem A. A common dominant tlr5 stop codon polymorphism abolishes flagellin signaling and is associated with susceptibility to legionnaires' disease. J Exp Med. 2003;198:1563–1572. doi: 10.1084/jem.20031220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subauste CS, Wessendarp M. Human dendritic cells discriminate between viable and killed toxoplasma gondii tachyzoites: Dendritic cell activation after infection with viable parasites results in cd28 and cd40 ligand signaling that controls il-12-dependent and - independent t cell production of ifn-gamma. J Immunol. 2000;165:1498–1505. doi: 10.4049/jimmunol.165.3.1498. [DOI] [PubMed] [Google Scholar]
- 14.Seguin R, Kasper LH. Sensitized lymphocytes and cd40 ligation augment interleukin-12 production by human dendritic cells in response to toxoplasma gondii. J Infect Dis. 1999;179:467–474. doi: 10.1086/314601. [DOI] [PubMed] [Google Scholar]
- 15.Aldebert D, Durand F, Mercier C, Brenier-Pinchart MP, Cesbron-Delauw MF, Pelloux H. Toxoplasma gondii triggers secretion of interleukin-12 but low level of interleukin-10 from the thp-1 human monocytic cell line. Cytokine. 2007;37:206–211. doi: 10.1016/j.cyto.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Gewirtz AT, Vijay-Kumar M, Brant SR, Duerr RH, Nicolae DL, Cho JH. Dominant-negative tlr5 polymorphism reduces adaptive immune response to flagellin and negatively associates with crohn's disease. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G1157–1163. doi: 10.1152/ajpgi.00544.2005. [DOI] [PubMed] [Google Scholar]
- 17.Blohmke CJ, Park J, Hirschfeld AF, Victor RE, Schneiderman J, Stefanowicz D, Chilvers MA, Durie PR, Corey M, Zielenski J, Dorfman R, Sandford AJ, Daley D, Turvey SE. Tlr5 as an anti-inflammatory target and modifier gene in cystic fibrosis. J Immunol. 2010;185:7731–7738. doi: 10.4049/jimmunol.1001513. [DOI] [PubMed] [Google Scholar]
- 18.Al-Daghri NM, Clerici M, Al-Attas O, Forni D, Alokail MS, Alkharfy KM, Sabico S, Mohammed AK, Cagliani R, Sironi M. A nonsense polymorphism (r392x) in tlr5 protects from obesity but predisposes to diabetes. J Immunol. 2013;190:3716–3720. doi: 10.4049/jimmunol.1202936. [DOI] [PubMed] [Google Scholar]
- 19.Chassin C, Tourneur E, Bens M, Vandewalle A. A role for collecting duct epithelial cells in renal antibacterial defences. Cell Microbiol. 2011;13:1107–1113. doi: 10.1111/j.1462-5822.2011.01614.x. [DOI] [PubMed] [Google Scholar]
- 20.Arikawa K, Nishikawa Y. Interleukin-8 induction due to diffusely adherent escherichia coli possessing afa/dr genes depends on flagella and epithelial toll-like receptor 5. Microbiol Immunol. 2010;54:491–501. doi: 10.1111/j.1348-0421.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- 21.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: Tlr5−/− mice are more susceptible to escherichia coli urinary tract infection. J Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 22.Mathur R, Oh H, Zhang D, Park SG, Seo J, Koblansky A, Hayden MS, Ghosh S. A mouse model of salmonella typhi infection. Cell. 2012;151:590–602. doi: 10.1016/j.cell.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D, Chen Q, Su SB, Zhang P, Kurosaka K, Caspi RR, Michalek SM, Rosenberg HF, Zhang N, Oppenheim JJ. Eosinophil-derived neurotoxin acts as an alarmin to activate the tlr2-myd88 signal pathway in dendritic cells and enhances th2 immune responses. J Exp Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, Hartmann G. Quantitative expression of toll-like receptor 1-10 mrna in cellular subsets of human peripheral blood mononuclear cells and sensitivity to cpg oligodeoxynucleotides. J Immunol. 2002;168:4531–4537. doi: 10.4049/jimmunol.168.9.4531. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Kikkawa S, Kohase M, Miyake K, Seya T. Establishment of a monoclonal antibody against human toll-like receptor 3 that blocks double-stranded rna-mediated signaling. Biochem Biophys Res Commun. 2002;293:1364–1369. doi: 10.1016/S0006-291X(02)00380-7. [DOI] [PubMed] [Google Scholar]
- 26.Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, Buzoni-Gatel D, Kasper LH. Tlr9 is required for the gut-associated lymphoid tissue response following oral infection of toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- 27.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: Myd88 is required for resistance to toxoplasma gondii infection and regulates parasite-induced il-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 28.Aliberti J, Reis e Sousa C, Schito M, Hieny S, Wells T, Huffnagle GB, Sher A. Ccr5 provides a signal for microbial induced production of il-12 by cd8 alpha+ dendritic cells. Nat Immunol. 2000;1:83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 29.Andrade WA, Souza Mdo C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. Combined action of nucleic acid-sensing toll-like receptors and tlr11/tlr12 heterodimers imparts resistance to toxoplasma gondii in mice. Cell Host Microbe. 2013;13:42–53. doi: 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melo MB, Kasperkovitz P, Cerny A, Konen-Waisman S, Kurt-Jones EA, Lien E, Beutler B, Howard JC, Golenbock DT, Gazzinelli RT. Unc93b1 mediates host resistance to infection with toxoplasma gondii. PLoS Pathog. 2010;6:e1001071. doi: 10.1371/journal.ppat.1001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial cd4+ t cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.