Abstract
Background and Purpose
To determine if clinical and angioarchitectural features of brain AVMs differ between children and adults.
Materials and Methods
A prospectively collected institutional database of all patients diagnosed with brain AVMs since 2001 was queried. Demographic, clinical, and angioarchitecture information was summarized and analyzed with univariable and multivariable models.
Results
Results often differed when age was treated as a continuous variable as opposed to dividing subjects into children (≤18 years; n=203) versus adults (>18 years; n=630). Children were more likely to present with AVM hemorrhage than adults (59% vs. 41%, p<0.001). Although AVMs with a larger nidus presented at younger ages (mean of 26.8 years for >6 cm compared to 37.1 years for <3 cm), this was not significantly different between children and adults (p=0.069). Exclusively deep venous drainage was more common in younger subjects both when age was treated continuously (p=0.04), or dichotomized (p<0.001). Venous ectasia was more common with increasing age (mean, 39.4 years with ectasia compared to 31.1 years without ectasia) and when adults were compared to children (52% vs. 35%, p<0.001). Patients with feeding artery aneurysms presented at later average age (44.1 years) than those without such aneurysms (31.6 years); this observation persisted when comparing children to adults (13% vs. 29%, p<0.001).
Conclusion
Although children with brain AVMs were more likely to come to clinical attention due to hemorrhage than adults, venous ectasia and feeding artery aneurysms were underrepresented in children, suggesting that these particular high risk features take time to develop.
Introduction
An enormous diversity of brain vascular malformations occur in children. These include vein of Galen malformations, dural AV fistulas, non-Galenic pial AV fistulas, and nidal arteriovenous malformations (AVMs)[1]. AVMs are defined by a group of vessels with an abnormal low-resistance connection between arteries and veins occuring in a focal geographic area of the brain parenchyma – the nidus. Nidal AVMs in children have been described as being different when compared to those in adults. In fact, a diffuse nidus with intervening brain tissue is sometimes termed “juvenile” AVM angioarchitecture[1]. A smaller nidus size, presence of multiple large AV fistulas, preferential location deep within the brain, and more frequent deep venous drainage have also been described as occurring more commonly in children [2–4].
Children with brain AVMs are more likely to present with hemorrhage than adults, particularly including intraventricular hemorrhage [2]. Several studies have identified specific angioarchitectural features that confer higher risk for hemorrhage in adults, children, or both [3–7]. Using data obtained from a large, prospectively collected patient cohort, we sought to determine if the clinical and angioarchitectural features of brain AVMs differ by patient age. We conducted our analysis both using the age at presentation as a continuous variable, and dichotomized into children and adult groups. The former is more relevant with respect to expected gradual biological changes that occur over time and may affect AVM formation and symptom progression. The latter is a clinical convenience, as patients tend to be seen and treated by “pediatric” and “adult” groups, with varying degrees of overlap. Thus, we hope to provide information useful both to those interested in the underlying disease processes of brain vascular malformations as well as those who take care of patients based on somewhat arbitrary societal and administrative divisions of patient age.
Materials and Methods
Data Acquistion
Under an approved human research protocol, the Brain AVM Database prospectively collects demographic, clinical, and radiologic data for all patients with vascular malformations treated at UCSF. Only patients with nidal AVMs treated between 2001 and 2013 were included for analysis (n=833); those with primary diagnoses of vein of Galen malformation, dural AV fistula, or non-Galenic pial AV fistula [8] were excluded. Children were defined as ≤18 years of age at the time of the first angiogram on which the diagnosis of AVM was made. The earliest diagnostic angiogram available for each patient was evaluated by a neurointerventional radiologist and a structured list of angioarchitectural features was scored using methods recommended by the Joint Writing Group [9]. When available, the earliest MRI and CT examinations were also evaluated by a neurointerventional radiologist to confirm AVM nidus location and presence or absence of current or prior intracranial hemorrhage.
Statistical Methods
Demographic, clinical, and angioarchitectural information for 833 AVM patients was analyzed using Kaplan-Meier survival analysis and log-rank tests. Our primary analysis assumed the AVM was present from birth, starting survival time at date of birth and ending at date of AVM diagnosis with no censoring. We computed the median (p50) survival time to diagnosis (i.e., age at diagnosis) for each characteristic with associated 95% confidence intervals (95% CI) to see if characteristics were associated with younger or older patients. Secondary analysis compared angiographic characteristics of patients between children (≤18 years of age) and adults (>18 years) using Fisher’s exact test for categorical variables.
We performed univariable and multivariable Cox regression survival analyses, calculating hazard ratios (HR) and associated 95% CIs for the following predictors: AVM nidus size (cm), exclusively deep venous drainage, venous ectasia, central location, lobar location, posterior fossa location, and shunt-flow related aneurysms (i.e., aneurysms of arteries directly supplying the AVM or subjected to increased blood flow due to the AVM, such as the anterior communicating artery for frontal AVMs). These analyses were stratified by initial hemorrhagic presentation and ethnicity to allow baseline hazard ratio to vary and, thus, better adhere to proportional hazard assumption of the Cox model.
We considered p-values of <0.05 to be significant. All statistical analyses were performed using StataSE 12.0 [10].
Results
Baseline Demographics and Clinical Presentation
Demographic and clinical data are listed in Tables 1A and 1B, with the former considering age as a continuous variable (survival analysis) and the latter grouping patients into children versus adults. The median age at diagnosis for our sample was 33.8 years (95% CI: 32.7, 35.9). Survival distributions did not significantly differ between males and females (log-rank p=0.937); similarly, no gender difference was observed (p=0.687) between children (50% female) and adults (51% female). However, we observed significant differences in median age at diagnosis by race/ethnicity (log-rank p<0.001), with Asians and Hispanics having a younger median age at diagnosis (<30 years) than other race/ethnicities. Hispanics comprised 35% of the children in our cohort, but only 24% of the adults. An inverse trend was seen with non-Hispanic Caucasians (43% of children and 54% of adults). The difference in diagnosis age between those who presented with a hemorrhage and those who did not was particularly pronounced (log-rank p<0.001; Figure 1A). Those who presented with a hemorrhage had a median diagnosis age of 28.7 years (95% CI: 26.6, 32.2), which is almost nine years younger than those who did not (p50: 37.6; 95% CI: 35.3, 40.6). Children were also more likely to present with AVM hemorrhage than adults (59% versus 41%, p<0.001).
Table 1A.
Demographic Characteristics and Mode of Presentation (All Ages)
| Characteristic | No. (%) | Median Dx Age (yrs) | 95% CI | p-value |
|---|---|---|---|---|
| Overall | 833 | 33.8 | (32.7, 35.9) | n/a |
|
| ||||
| Gender | 0.937 | |||
| Female | 425 (51%) | 33.4 | (31.0, 35.7) | |
| Male | 408 (49%) | 34.3 | (31.5, 37.7) | |
|
| ||||
| Ethnicity | <0.001 | |||
| Asian / Pacific Islander | 113 (14%) | 29.9 | (25.9, 34.3) | |
| Black / African-American | 56 (5%) | 46.5 | (41.2, 50.3) | |
| Hispanic | 224 (27%) | 27.0 | (23.4, 29.8) | |
| Native American | 9 (1%) | 37.1 | (17.4, 47.1) | |
| Non-Hispanic Caucasian | 431 (52%) | 38.7 | (35.3, 42.6) | |
|
| ||||
| Hemorrhagic presentation | <0.001 | |||
| Yes | 375 (45%) | 28.7 | (26.6, 32.2) | |
| No | 458 (55%) | 37.6 | (35.3, 40.6) | |
|
| ||||
| HHT diagnosis | 0.695 | |||
| Yes | 12 (1%) | 38.3 | (1.8, 54.0) | |
| No | 821 (99%) | 33.7 | (31.6, 35.8) | |
P-values are from log-rank tests of survivor functions.
HHT = hereditary telangiectasia syndrome
Table 1B.
Demographic Characteristics and Mode of Presentation (Children vs. Adults)
| Characteristic | All (n=833) | Child (0–18 yrs) (n=203) | Adult (≥19 yrs) (n=630) | p-value |
|---|---|---|---|---|
| Age at diagnosis (years) | 35.1 ± 18.6 | 12.2 ± 4.7 | 42.6 ± 15.0 | n/a |
|
| ||||
| Female Gender | 425 (51%) | 101 (50%) | 321 (51%) | 0.687 |
|
| ||||
| Ethnicity | 0.014 | |||
| Asian / Pacific Islander | 113 (14%) | 31 (16%) | 82 (13%) | |
| Black / African-American | 11 (5%) | 11 (5%) | 42 (7%) | |
| Hispanic | 224 (27%) | 71 (35%) | 153 (24%) | |
| Native American | 9 (1%) | 1 (<1%) | 8 (1%) | |
| Non-Hispanic Caucasian | 431 (52%) | 88 (43%) | 343 (54%) | |
|
| ||||
| Hemorrhagic presentation | 375 (45%) | 119 (59%) | 256 (41%) | <0.001 |
|
| ||||
| HHT diagnosis | 12 (1%) | 4 (2%) | 8 (1%) | 0.500 |
Table entries are n (%) or mean ± sd. P-values are from Fisher’s exact test.
HHT = hereditary telangiectasia syndrome
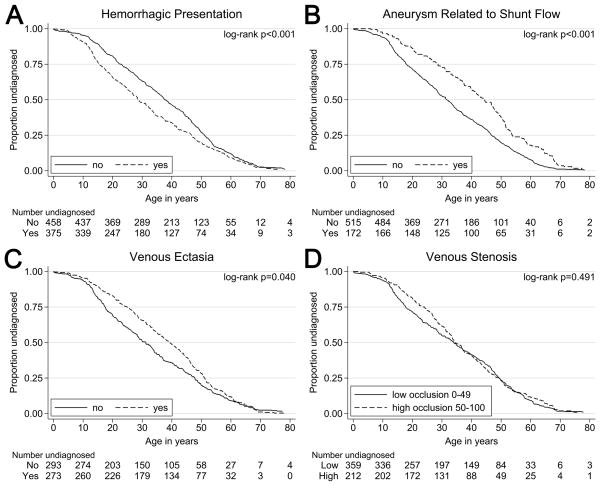
Figure 1. Age Related Differences in AVM Hemorrhagic Presentation, Aneurysms Related to Shunt Flow, Draining Venous Ectasia, and Draining Venous Stenosis.
Hemorrhagic presentation (A) was more prevalent at younger patient ages than non-hemorrhagic presentation. Conversely, presence of feeding artery aneurysms (B), and ectasia of draining veins (C) were more prevalent at older patient ages. There was not a significant difference the prevalence of of venous stenosis observed at presentation in older versus younger patients (D).
Nidus Morphology and Location
Angioarchitectural data are summarized in Table 2; with with age as a continuous variable (Table 2A) and data dichotomized into children versus adults (Table 2B). Larger AVMs (categorized as <3 cm, 3–6cm, and >6cm nidus size) were identified at younger ages than smaller AVMs (AVM >6cm p50: 26.8; 95% CI: 16.9, 33.9 versus AVM <3cm p50: 37.1; 95% CI: 34.1, 40.2, log-rank p=0.009). A comparison of AVM nidus size in children and adults was suggestive of an association, but not significant (p=0.069). Interestingly, large AVMs (nidus >6 cm) are twice as common in children (8%) as in adults (4%). The sharpness of the AVM border with adjacent brain on angiography, scored as “sharp” or “diffuse,” did not differ by continuous age (p=0.707) or between age groups (23% diffuse border in children versus 19% diffuse border in adults, p=0.218).
When data was analyzed using age at diagnosis as a continuous variable (Table 2A), AVMs found in lobar locations (as opposed to central locations) were marginally associated with older age (log-rank p=0.050) and AVMs in the posterior fossa were observed in older patients (log-rank p<0.001). No association with age based on either dural location (i.e., dural arterial supply to a parenchymal AVM, as opposed to a primary dural AV fistula which would have been excluded from this cohort) or central location could be determined (log-rank p=0.518 and log-rank p=0.617, respectively).
Draining Veins
Venous drainage patterns varied significantly by age of diagnosis (log-rank p=0.040). Patients with exclusively deep venous drainage had a median age of diagnosis of 26.8 (95% CI: 21.9, 32.2), while those with “superficial and deep” (p50: 31.5; 95% CI: 28.1, 35.1) and “superficial” (p50: 37.8; 95% CI: 34.9, 40.6) venous patterns were identified at older ages. Venous ectasia (Figure 1C) tended to be identified in older patients (log-rank p=0.040). A dichotomized venous stenosis measure (Figure 1D) did not have an association with age at diagnosis (log-rank p=0.491).
When age was dichotomized, the venous drainage of AVMs differed significantly between children and adults, but the location did not (Table 2B). Children were more likely to have exclusively deep venous drainage than adults (28% in children versus 14% in adults, p<0.001). Venous ectasia was also more prevalent in adults than in children (35% in children versus 52% in adults, p<0.001). There was a trend toward central, deep location of AVMs in children as compared to adults (p=0.075), but this did not reach statistical significance.
Aneurysms
There was a significant difference between the presence and absence of flow-related feeding artery aneurysms (log-rank p<0.001; Figure 1B), as these aneurysms tended to appear in older patients. We do not have sufficient data to support an association for intranidal aneurysms (log-rank p=0.143) and aneurysms not related to shunt-flow (log-rank p=0.069) with patient age. When age was dichotomized, feeding artery aneurysms related to flow were more prevalent in adults than in children (13% in children versus 29% in adults, p<0.001). Intranidal aneurysms were similar in frequency in both age groups (17% in children versus 15% in adults, p=0.537).
Regression Analysis
A multivariable Cox regression was performed on a subset of 550 patients (66%) in whom complete demographic, clinical and angiographic information was available (Table 3). As with the Kaplan-Meier analysis, larger AVMs (HR: 1.13; p<0.001) and centrally located AVMs (HR: 1.45; p=0.001) were more likely to be diagnosed earlier independent of other characteristics. In contrast, venous ectasia (HR: 0.75; p=0.003) and shunt-flow related aneurysms (HR: 0.53; p<0.001) were significantly associated with later AVM diagnosis. Posterior fossa location and exclusively deep venous drainage were not significant in multivariable analysis, although there is a suggestion that these characteristics may also be associated with later or earlier diagnosis, respectively (Table 3).
Table 2A.
Angioarchitecture (All Ages)
| Characteristic | No. (%) | Median Dx Age (yrs) | 95% CI | p-value |
|---|---|---|---|---|
| AVM nidus size | 0.009 | |||
| <3 cm | 417 (55%) | 37.1 | (34.1, 40.2) | |
| 3–6 cm | 305 (40%) | 30.7 | (27.9, 34.5) | |
| >6 cm | 38 (5%) | 26.8 | (16.9, 33.9) | |
|
| ||||
| AVM side | 0.087 | |||
| Right | 321 (44%) | 32.8 | (29.8, 35.9) | |
| Left | 363 (50%) | 34.1 | (32.1, 37.1) | |
| Middle | 39 (5%) | 37.2 | (27.6, 53.8) | |
|
| ||||
| Central location | 0.617 | |||
| Yes | 282 (38%) | 29.5 | (27.4, 33.6) | |
| No | 453 (62%) | 36.6 | (33.8, 38.4) | |
|
| ||||
| Dural location | 0.518 | |||
| Yes | 3 (<1%) | 25.9 | n/a | |
| No | 723 (>99%) | 34.1 | (32.2, 36.6) | |
|
| ||||
| Lobar location | 0.050 | |||
| Yes | 555 (76%) | 34.5 | (32.7. 37.0) | |
| No | 180 (24%) | 30.1 | (26.8, 39.8) | |
|
| ||||
| Posterior fossa location | <0.001 | |||
| Yes | 110 (15%) | 40.9 | (28.6, 47.8) | |
| No | 616 (85%) | 33.6 | (31.4, 35.7) | |
|
| ||||
| Eloquence | <0.001 | |||
| Yes | 452 (61%) | 31.5 | (29.0, 34.1) | |
| No | 292 (39%) | 37.4 | (34.3, 42.3) | |
|
| ||||
| AVM border | 0.707 | |||
| Compact | 573 (80%) | 35.1 | (33.2, 37.7) | |
| Diffuse | 140 (20%) | 31.2 | (27.4, 37.2) | |
|
| ||||
| Venous drainage | 0.040 | |||
| Superficial | 389 (51%) | 37.8 | (34.9, 40.6) | |
| Superficial and deep | 238 (31%) | 31.5 | (28.1, 35.1) | |
| Exclusively deep | 134 (18%) | 26.2 | (21.9, 32.2) | |
|
| ||||
| Number of draining veins | 0.572 | |||
| 1 vein | 208 (39%) | 33.2 | (28.4, 36.9) | |
| 2+ veins | 329 (61%) | 34.9 | (32.2, 37.5) | |
|
| ||||
| Number of veins reaching sinus | 0.678 | |||
| 0–1 veins | 171 (34%) | 32.3 | (28.3, 37.0) | |
| 2+ veins | 330 (66%) | 34.1 | (31.4, 37.0) | |
|
| ||||
| Venous stenosis (%) | 0.761 | |||
| 0–24 | 266 (47%) | 32.2 | (28.3, 36.1) | |
| 25–49 | 93 (16%) | 38.1 | (32.2, 42.4) | |
| 50–74 | 126 (22%) | 34.4 | (31.1, 40.0) | |
| 75–99 | 71 (12%) | 33.2 | (28.4, 39.0) | |
| 100 | 15 (3%) | 41.6 | (27.4, 50.3) | |
|
| ||||
| Venous thrombosis (%) | 0.551 | |||
| 0–24 | 504 (96%) | 34.0 | (32.1, 36.6) | |
| 25–49 | 4 (1%) | 21.1 | n/a | |
| 50–74 | 4 (1%) | 18.0 | n/a | |
| 75–99 | 1 (<1%) | n/a | n/a | |
| 100 | 14 (3%) | 34.1 | (12.2, 50.3) | |
|
| ||||
| Venous ectasia | 0.040 | |||
| Yes | 273 (48%) | 39.4 | (35.3, 42.8) | |
| No | 292 (52%) | 31.1 | (28.1, 33.9) | |
|
| ||||
| Venous reflux | 0.159 | |||
| Yes | 161 (29%) | 38.1 | (32.9, 43.4) | |
| No | 387 (71%) | 33.4 | (30.0, 35.8) | |
|
| ||||
| Moyamoya type changes | 0.659 | |||
| Yes | 8 (1%) | 27.4 | (21.4, 49.3) | |
| No | 563 (99%) | 33.9 | (31.5, 36.5) | |
|
| ||||
| Pial-to-pial collaterialization | 0.425 | |||
| Yes | 120 (21%) | 36.1 | (29.5, 39.8) | |
| No | 448 (79%) | 33.8 | (31.3, 36.6) | |
|
| ||||
| Aneurysm related to shunt-flow | <0.001 | |||
| Yes | 172 (25%) | 44.1 | (28.9, 33.9) | |
| No | 515 (75%) | 31.6 | (40.0, 47.8) | |
|
| ||||
| Aneurysm not related to shunt-flow | 0.069 | |||
| Yes | 16 (2%) | 47.1 | (32.1, 36.5) | |
| No | 670 (98%) | 33.9 | (34.5, 53.8) | |
|
| ||||
| Intranidal aneurysm | 0.143 | |||
| Yes | 107 (16%) | 31.9 | (26.6, 39.4) | |
| No | 575 (84%) | 34.6 | (32.8, 37.0) | |
Note that all variables are missing varying amounts of data.
P-values are from log-rank test of survivor functions.
Discussion
Using a large institutional cohort of patients with brain AVMs, we were able to describe the angioarchitectural features in detail, and also whether these features differ according to age at presentation, or the demographic group. As expected, the method of data analysis affected the results of our study. When age was examined as a continuous variable, patient ethnicity, presentation with hemorrhage, nidus size, lobar location, location in the posterior fossa, eloquent location, venous drainage, venous ectasia, and feeding artery aneurysms all differed by age (Tables 1A, 2A). When age was dichotomized into childhood and adult groups, only patient ethnicity, presentation with hemorrhage, venous drainage, number of draining veins, venous ectasia, and feeding artery aneurysms differed between children and adults (Tables 1B, 2B). When a multivariable Cox regression analysis was conducted on the 550 patients with complete data (Table 3), only AVM nidus size, central (deep) AVM location, venous ectasia, and feeding artery aneurysms differed by age of presentation.
In previously reported studies, factors that have been associated with hemorrhage at presentation in patients of all ages with AVMs include: supply by perforating arteries, nidal aneurysms, multiple aneurysms, supply by the posterior circulation, basal ganglia location, deep venous drainage, venous reflux, and venous stenosis [5, 11]. Specifically in children, a smaller AVM nidus, infratentorial nidus location, and exclusively deep venous drainage have previously been associated with increased risk of presentation with hemorrhage [2]. Although children with brain AVMs were more likely to present with an intracerebral hemorrhage [3], high risk features such as venous ectasia and feeding artery aneurysms were less frequent among the children in our cohort.
Brain AVMs are not static lesions; angioarchitectural features associated with hemorrhage can develop over time. It is reasonable to assume that venous stenosis, venous ectasia, and feeding artery aneurysms arise from chronic hemodynamic stresses, which may explain why they are underrepresented in children, who have not had sufficient time to develop these features. In our cohort, only 1 feeding artery aneurysm was found in a patient under 8 years of age, and AVM flow-related feeding artery aneurysms have been reported rarely in young children [12]. Lack of specific time-dependent high risk angioarchitectural features, similarly, may help explain why children with AVMs have been reported to have a lower risk of subsequent hemorrhage after initial presentation as compared to adults in longitudinal studies [3] despite the overrepresentation of AVMs in deep locations, which is typically a risk factor for increased incidence of subsequent hemorrhage [4, 13]. The presence of venous ectasia and feeding artery aneurysms may be an indirect method of estimating how long an AVM has been present in a given patient, and potentially provide insight into the congenital versus acquired nature of such lesions. Selection of surgical tissue samples from patients with particular angioarchitectural features may permit direct evaluation of the age of a given AVM through techniques such as radiocarbon dating[14].
AVMs and their draining veins were often located deep within the brain in children, raising the possibility that centrally-located AVMs may arise earlier in development or be more likely to come to clinical attention early in life than more peripherally located AVMs. Although angioarchitecturally distinct from nidal AVMs, vein of Galen malformations form early in embryonic development and are also centrally located in the brain. With the advent of fetal MRI and increasing use of MRI in children and adults, it may be possible to determine if there is a continuous progression from centrally-arising arteriovenous fistulas to peripherally located nidal AVMs in asymptomatic individuals. A limitation of our study is its cross-sectional nature. Ultimately, longitudinal studies such as the ARUBA Trial will provide more detailed natural history data for brain AVMs[15].
Conclusion
Although children with brain AVMs were more likely to come to clinical attention due to hemorrhage than adults, high-risk features such as venous stenosis and feeding artery aneurysms were underrepresented in children. AVMs and their draining veins tended to be in deep locations in children when compared to adults; raising the possibility that centrally-located AVMs may arise earlier in development or be more likely to come to clinical attention early in life.
Table 2B.
Angioarchitecture (Children vs. Adults)
| Characteristic | All (n=833) | Child (0–18 yrs) (n=203) | Adult (≥19 yrs) (n=630) | p-value |
|---|---|---|---|---|
| AVM nidus size | 0.069 | |||
| <3 cm | 417 (55%) | 95 (53%) | 322 (55%) | |
| 3–6 cm | 305 (40%) | 68 (38%) | 237 (41%) | |
| >6 cm | 38 (5%) | 15 (8%) | 23 (4%) | |
|
| ||||
| AVM side | 0.839 | |||
| Right | 321 (44%) | 79 (46%) | 242 (44%) | |
| Left | 363 (50%) | 84 (49%) | 279 (51%) | |
| Middle | 39 (5%) | 10 (6%) | 29 (5%) | |
|
| ||||
| Central location | 0.075 | |||
| Yes | 282 (38%) | 78 (44%) | 201 (36%) | |
| No | 453 (62%) | 98 (56%) | 355 (64%) | |
|
| ||||
| Dural location | 1.000 | |||
| Yes | 3 (<1%) | 0 (0%) | 3 (1%) | |
| No | 723 (>99%) | 174 (100%) | 549 (99%) | |
|
| ||||
| Lobar location | 0.131 | |||
| Yes | 555 (76%) | 125 (71%) | 430 (77%) | |
| No | 180 (24%) | 51 (29%) | 129 (23%) | |
|
| ||||
| Posterior fossa location | 0.809 | |||
| Yes | 110 (15%) | 25 (14%) | 85 (15%) | |
| No | 616 (85%) | 151 (86%) | 465 (85%) | |
|
| ||||
| Eloquence | 0.212 | |||
| Yes | 452 (61%) | 112 (65%) | 340 (59%) | |
| No | 292 (39%) | 60 (35%) | 232 (41%) | |
|
| ||||
| AVM border | 0.218 | |||
| Compact | 573 (80%) | 126 (77%) | 447 (81%) | |
| Diffuse | 140 (20%) | 38 (23%) | 102 (19%) | |
|
| ||||
| Venous drainage | <0.001 | |||
| Superficial | 389 (51%) | 67 (37%) | 322 (55%) | |
| Superficial and deep | 238 (31%) | 61 (34%) | 177 (30%) | |
| Exclusively deep | 134 (18%) | 51 (28%) | 83 (14%) | |
|
| ||||
| Number of draining veins | 0.046 | |||
| 1 vein | 208 (39%) | 58 (47%) | 150 (36%) | |
| 2+ veins | 329 (61%) | 66 (53%) | 263 (64%) | |
|
| ||||
| Number of veins reaching sinus | 0.117 | |||
| 0–1 veins | 171 (34%) | 46 (40%) | 125 (32%) | |
| 2+ veins | 330 (66%) | 68 (60%) | 262 (68%) | |
|
| ||||
| Venous stenosis | 0.144 | |||
| 0–24 | 266 (47%) | 75 (56%) | 191 (44%) | |
| 25–49 | 93 (16%) | 22 (16%) | 71 (16%) | |
| 50–74 | 126 (22%) | 24 (18%) | 102 (23%) | |
| 75–99 | 71 (12%) | 12 (9%) | 59 (14%) | |
| 100 | 15 (3%) | 2 (1%) | 13 (3%) | |
|
| ||||
| Venous thrombosis | 0.607 | |||
| 0–24 | 504 (96%) | 121 (96%) | 383 (96%) | |
| 25–49 | 4 (1%) | 0 (0%) | 4 (1%) | |
| 50–74 | 4 (1%) | 2 (2%) | 2 (1%) | |
| 75–99 | 1 (<1%) | 0 (0%) | 1(<1%) | |
| 100 | 14 (3%) | 3 (2%) | 11 (3%) | |
|
| ||||
| Venous ectasia | <0.001 | |||
| Yes | 273 (48%) | 45 (35%) | 228 (52%) | |
| No | 292 (52%) | 85 (65%) | 208 (48%) | |
|
| ||||
| Venous reflux | 0.075 | |||
| Yes | 161 (29%) | 29 (23%) | 132 (31%) | |
| No | 387 (71%) | 98 (77%) | 289 (69%) | |
|
| ||||
| Moyamoya type changes | 0.208 | |||
| Yes | 8 (1%) | 0 (0%) | 8 (2%) | |
| No | 563 (99%) | 135 (100%) | 428 (98%) | |
|
| ||||
| Pial-to-pial collaterialization | 0.090 | |||
| Yes | 120 (21%) | 21 (16%) | 99 (23%) | |
| No | 448 (79%) | 112 (84%) | 336 (77%) | |
|
| ||||
| Aneurysm related to shunt-flow | <0.001 | |||
| Yes | 172 (25%) | 21 (13%) | 151 (29%) | |
| No | 515 (75%) | 139 (87%) | 376 (71%) | |
|
| ||||
| Aneurysm not related to shunt-flow | 0.384 | |||
| Yes | 16 (2%) | 2 (1%) | 14 (3%) | |
| No | 670 (98%) | 158 (99%) | 512 (97%) | |
|
| ||||
| Intranidal aneurysm | 0.537 | |||
| Yes | 107 (16%) | 28 (17%) | 79 (15%) | |
| No | 575 (84%) | 134 (83%) | 441 (85%) | |
Table entries are n (%) or mean ± sd. Note that all variables are missing varying amounts of data.
P-values are from Fisher’s exact test.
Table 3.
Age at Diagnosis Cox Survival Analysis
| Univariable n = 550 |
Multivariable n = 550 |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | HR | 95% CI | p-value | HR | 95% CI | p-value |
| AVM nidus size (cm) | 1.05 | (1.00, 1.10) | 0.065 | 1.13 | (1.07, 1.20) | <0.001 |
| Exclusively deep venous drainage | 1.26 | (0.98, 1.63) | 0.077 | 1.27 | (0.95, 1.69) | 0.110 |
| Venous ectasia | 0.83 | (0.69, 0.99) | 0.034 | 0.75 | (0.62, 0.91) | 0.003 |
| Central location | 1.26 | (1.05, 1.52) | 0.014 | 1.45 | (1.16, 1.81) | 0.001 |
| Lobar location | 1.04 | (0.84, 1.30) | 0.716 | 1.10 | (0.75, 1.61) | 0.622 |
| Posterior fossa location | 0.76 | (0.59, 0.98) | 0.037 | 0.72 | (0.49, 1.06) | 0.099 |
| Aneurysm related to shunt-flow | 0.59 | (0.48, 0.71) | <0.001 | 0.53 | (0.43. 0.65) | <0.001 |
These are results for Cox regression analyses using age at diagnosis as the survival time and stratifying by ethnicity and hemorrhagic presentation. The Multivariable model includes all listed predictors.
Acknowledgments
We thank Nancy Quinnine, RN, Jeanne Scanlon, RN, Anne Federov, RN, Elizabeth Clain, and Diana Guo for their clinical support. We are deeply indebted to Bill Young, MD for his guidance and mentorship in this project. This project was supported by NIH grants: R01 NS034949 and UCSF-CTSI Grant Number UL1 TR000004. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Lasjaunias P, BA, Ter Brugge KG. Surgical Neuroangiography: Clinical and Interventional Aspects in Children. 2. Vol. 3. Berlin: Springer; 2001. [Google Scholar]
- 2.Ellis MJ, et al. Angioarchitectural features associated with hemorrhagic presentation in pediatric cerebral arteriovenous malformations. J Neurointerv Surg. 2013;5(3):191–5. doi: 10.1136/neurintsurg-2011-010198. [DOI] [PubMed] [Google Scholar]
- 3.Fullerton HJ, et al. Long-term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke. 2005;36(10):2099–104. doi: 10.1161/01.STR.0000181746.77149.2b. [DOI] [PubMed] [Google Scholar]
- 4.Stapf C, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006;66(9):1350–5. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 5.Turjman F, et al. Correlation of the angioarchitectural features of cerebral arteriovenous malformations with clinical presentation of hemorrhage. Neurosurgery. 1995;37(5):856–60. doi: 10.1227/00006123-199511000-00002. discussion 860–2. [DOI] [PubMed] [Google Scholar]
- 6.Stefani MA, et al. Angioarchitectural factors present in brain arteriovenous malformations associated with hemorrhagic presentation. Stroke. 2002;33(4):920–4. doi: 10.1161/01.str.0000014582.03429.f7. [DOI] [PubMed] [Google Scholar]
- 7.Kim H, et al. Racial/Ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38(9):2430–7. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 8.Hetts SW, et al. Pediatric intracranial nongalenic pial arteriovenous fistulas: clinical features, angioarchitecture, and outcomes. AJNR Am J Neuroradiol. 2012;33(9):1710–9. doi: 10.3174/ajnr.A3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atkinson RP, et al. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32(6):1430–42. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 10.StataCorp. Stata Statistical Software: Release 12. StataCorp LP; College Station, TX: 2011. [Google Scholar]
- 11.Nataf F, et al. Angioarchitecture associated with haemorrhage in cerebral arteriovenous malformations: a prognostic statistical model. Neuroradiology. 1997;39(1):52–8. doi: 10.1007/s002340050367. [DOI] [PubMed] [Google Scholar]
- 12.Ostergaard JR. Association of intracranial aneurysm and arteriovenous malformation in childhood. Neurosurgery. 1984;14(3):358–62. doi: 10.1227/00006123-198403000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Stefani MA, et al. Large and deep brain arteriovenous malformations are associated with risk of future hemorrhage. Stroke. 2002;33(5):1220–4. doi: 10.1161/01.str.0000013738.53113.33. [DOI] [PubMed] [Google Scholar]
- 14.Sarachine Falso MJBB. Bomb Pulse Biology. Nucl Instrum Methods Phys Res B. 2013;294:666–670. doi: 10.1016/j.nimb.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stapf C. The rationale behind “A Randomized Trial of Unruptured Brain AVMs” (ARUBA) Acta Neurochir Suppl. 2010;107:83–5. doi: 10.1007/978-3-211-99373-6_13. [DOI] [PMC free article] [PubMed] [Google Scholar]