Introduction
Stroke is a leading cause of death and disability worldwide. Imaging plays a critical role in evaluating patients suspected of acute stroke and transient ischemic attack (TIA), especially prior to initiating treatment. Over the past few decades, major advances have occurred in stroke imaging and treatment, including Food and Drug Administration (FDA) approval of recanalization therapies for treatment of acute ischemic stroke. The primary goal of imaging patients with acute stroke symptoms is to distinguish between hemorrhagic and ischemic stroke. In ischemic stroke patients, secondary goals of imaging prior to initiating revascularization interventions with intravenous (IV) thrombolysis or endovascular therapies include identification of the location and extent of intravascular clot as well as the presence and extent of “ischemic core” (irreversibly damaged tissue) and “penumbra” (hypoperfused tissue at risk for infarction).1–3 In addition, early identification of the stroke etiology or mechanism (e.g., carotid atherosclerotic disease, vascular dissection or other treatable structural causes) is critical to treatment decisions and long-term management.
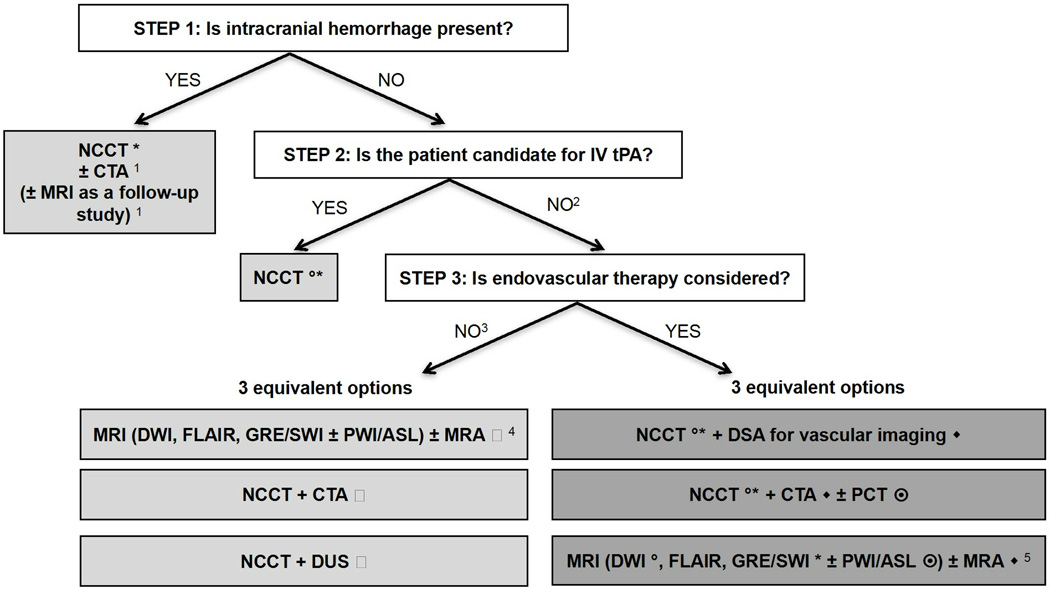
A wide variety of imaging techniques have become available to assess vascular lesions and brain tissue status in acute stroke patients. However, the practical challenge for physicians is to understand the multiple facets of these imaging techniques, including which imaging techniques to implement and how to optimally use them, given available resources at their local institution. Important considerations include constraints of time, cost, access to imaging modalities, preferences of treating physicians, availability of expertise, and availability of endovascular therapy. The choice of which imaging techniques to employ is impacted by both the time urgency for evaluation of patients and the complexity of the literature on acute stroke imaging. Ideally, imaging algorithms should incorporate techniques that provide optimal benefit for patient outcome without delaying treatment. Therefore, it is most practical and efficient to use a standardized imaging approach, with all relevant imaging studies conducted in as few sessions as possible (Figure 1).
Figure 1. Suggested imaging protocols for patients presenting with acute stroke symptoms based on the clinical scenario and the therapeutic options considered and available.
Each of the gray boxes represents one imaging strategy. In order not to delay treatment, a standardized imaging approach should be used: one imaging strategy (gray box) should be selected and all imaging studies belonging to this strategy should be performed upfront in as few sessions as possible.
1 To assess the etiology of the intracranial hemorrhage (CTA for vascular pathologies, such as aneurysms, arteriovenous malformations, vasculopathies; MRI for vascular malformations, neoplastic and other pathologies associated with hemorrhage)
2 Also if the patient is not a candidate for IV tPA (contraindication to tPA, outside time window for tPA) or if IV tPA failed or it is thought that it may fail.
3 For patients who are outside the time window for acute reperfusion therapies (> 4.5 hours at sites where only IV tPA is being considered; > 8 hours at sites where endovascular therapy is considered), and for patients with TIAs, emphasis is on secondary prevention, and their imaging work-up should be focused on vascular imaging (CTA, MRA of DUS) to assess carotid arteries as a possible cause of the ischemic stroke, with secondary prevention in mind. If MRA is obtained, it makes sense to concurrently obtain MRI with DWI, FLAIR and GRE/SWI. Echocardiography should also be obtained to assess for cardiac sources.
4 If available, MRI/MRA is the preferred imaging modality for TIA patients.
5 At institutions where MRI is available 24/7 and can be performed within a short time after admission.
* to assess for intracranial hemorrhage
◦ to assess the extent of ischemic core
◆ to assess the location and extent of the intravascular clot
to assess carotid atherosclerotic disease
to assess the extent of viable tissue
We performed a review of the evidence on the utility of various imaging techniques in acute stroke and TIA patients to establish best practices with standardization of imaging protocols. We indicated the quality of publications for diagnostic test and interventions by assigning levels of evidence (Table 1 and Table 2). These levels of evidence are based on the National Institute for Clinical Excellence (NICE), adapted from the Oxford Centre for Evidence-based Medicine Levels of Evidence (2001). The goal of this article is to present practical imaging recommendations for patients presenting with acute stroke and TIA across different practice settings, and to provide the rationale and evidence supporting their use. These recommendations are in agreement with the American College of Radiology (ACR) appropriateness criteria.4 We recognize that stroke imaging is a rapidly evolving field and that a number of the recommendations presented are the topic of continued investigation.
Table 1.
Levels of evidence for studies of the accuracy of diagnostic tests, adapted from The Oxford Centre for Evidence-based Medicine Levels of Evidence (2001) and the Centre for Reviews and Dissemination Report Number 4 (2001). © National Institute for Clinical Excellence February 2004
| Levels of evidence | Type of evidence |
|---|---|
| Ia | Systematic review (with homogeneity)* of level-1 studies† |
| Ib | Level-1 studies† |
| II | Level-2 studies‡ Systematic reviews of level-2 studies |
| III | Level-3 studies§ Systematic reviews of level-3 studies |
| IV | Consensus, expert committee reports or opinions and/or clinical experience without explicit critical appraisal; or based on physiology, bench research or ‘first principles’ |
Homogeneity means there are no or minor variations in the directions and degrees of results between individual studies that are included in the systematic review.
- that use a blind comparison of the test with a validated reference standard
- in a sample of patients that reflects the population to whom the test would apply.
- narrow population (the sample does not reflect the population to whom the test would apply)
- use a poor reference standard (defined as that where the ‘test’ is included in the ‘reference’, or where the ‘testing’ affects the ‘reference’)
- the comparison between the test and reference standard is not blind
- case–control studies.
Level-3 studies are studies that have at least two or three of the features listed above.
Table 2.
Levels of evidence for intervention studies. Reproduced from the Scottish Intercollegiate Guidelines Network. © National Institute for Clinical Excellence February 2004
| Levels of evidence | Type of evidence |
|---|---|
| 1++ | High-quality meta-analyses, systematic reviews of RCTs, or RCTs with a very low risk of bias |
| 1+ | Well-conducted meta-analyses, systematic reviews of RCTs, or RCTs with a low risk of bias |
| 1− | Meta-analyses, systematic reviews of RCTs, or RCTs with a high risk of bias* |
| 2++ | High-quality systematic reviews of case–control or cohort studies; High-quality case–control or cohort studies with a very low risk of confounding, bias or chance and a high probability that the relationship is causal |
| 2+ | Well-conducted case–control or cohort studies with a low risk of confounding, bias or chance and a moderate probability that the relationship is causal |
| 2− | Case–control or cohort studies with a high risk of confounding bias, or chance and a significant risk that the relationship is not causal* |
| 3 | Non-analytic studies (for example, case reports, case series) |
| 4 | Expert opinion, formal consensus |
Studies with a level of evidence ‘−‘ should not be used as a basis for making a recommendation
Rationale and Imaging Evidence for Patients Presenting with Acute Stroke Symptoms
The initial step in the evaluation of patients with symptoms of acute stroke is to differentiate between hemorrhagic and ischemic stroke (Figure 1). For patients with acute ischemic stroke who are candidates for IV tissue plasminogen activator (tPA), a non-contrast CT (NCCT) of the head should be obtained in order to determine eligibility for treatment. IV tPA can then usually be initiated without waiting for further imaging. In patients under consideration for endovascular therapy, three imaging options may be used: 1) NCCT followed immediately by digital subtraction angiography (DSA) for vascular assessment; 2) NCCT + CT angiography (CTA) ± perfusion CT (PCT); or 3) MRI+ magnetic resonance angiography (MRA) at institutions that can offer MRI 24/7 without delaying delay treatment. In patients who are not candidates for IV or endovascular therapy, and in patients with TIA, vascular imaging is recommended to guide management for secondary prevention of future stroke.
Imaging evidence for assessing intracranial hemorrhage
NCCT is the accepted standard-of-care imaging modality for exclusion of intracranial hemorrhage and has been incorporated in the inclusion criteria in randomized clinical trials evaluating the efficacy of IV thrombolysis.4, 5 NCCT is often referred to as the “reference standard” for detection of acute intracranial hemorrhage based on reports describing its accuracy with early CT scanners.6, 7 However, there are no recent studies that have used a true reference standard, such as surgical or pathological confirmation, to support Level I evidence. Therefore, the sensitivity and specificity of NCCT in detecting intracranial hemorrhage is unknown. The many advantages of NCCT in the emergent setting, as well as the proven benefit of IV thrombolysis in patients selected by NCCT4, 5, have led to its continued widespread use in acute stroke imaging (Table 3).
Table 3.
Advantages and limitations of CT and MR imaging
| Imaging characteristics | CT | MRI |
|---|---|---|
| Availability in the acute setting (0–6 hours) | ++ | − |
| Rapid image acquisition | ++ | + |
| Lack of vulnerability to motion artifact | + | − |
| Accessibility for patients with monitors and/or ventilators | ++ | − |
| Feasibility and safety for patients with metallic implants (pacemakers, implantable defibrillators) | ++ | − |
| Lower cost | + | − |
| Lack of ionizing radiation | − | ++ |
| Renal toxicity associated with contrast administration | + | + |
| Time for post-processing angiography and perfusion imaging | − | − |
| Sensitivity to lacunar and posterior fossa infarcts | − | ++ |
| Differentiation between acute and chronic ischemia | − | ++ |
| Ability to assess causes of ICH or SAH while in the scanner | + | + |
| Detection of chronic hemorrhage including microbleeds | − | + |
MRI T2*-weighted sequences have been studied for detection of acute and chronic hemorrhage in acute stroke patients. The accuracy of MRI techniques for detection of intracranial hemorrhage in the acute stroke setting (within 6 hours) has been reported as likely equivalent to NCCT (Level Ib).8, 9 Additionally, T2*-weighted sequences (including gradient-recalled echo [GRE] and susceptibility-weighted imaging [SWI] sequences) have superior accuracy in the detection of chronic microhemorrhages.9–11 In one large study as well as a meta-analysis, there was no statistically significant increased risk of symptomatic hemorrhage when patients with a small number of chronic microhemorrhages (<5) were treated with IV thrombolysis (Level Ia).12, 13 However, the risk of symptomatic hemorrhage in patients with numerous chronic microhemorrhages undergoing treatment with IV thrombolysis is unknown. It is important to recognize that the pivotal CT-based trials proving a benefit for IV tPA likely included patients with multiple microhemorrhages.
If intraparenchymal hemorrhage is present, as in 15% of all strokes, the imaging evaluation in the acute phase may include CTA of the intracranial arteries for evaluation of an underlying vascular malformation.14–16 CTA may demonstrate a “spot sign”, indicative of active bleeding, predictive of hematoma expansion, and strongly associated with poor outcomes.17–20 An MRI without and with contrast is sometimes obtained to assess for an underlying neoplastic or vascular mass, or associated microhemorrhages that may suggest amyloid angiopathy, multiple cavernous malformations, or septic emboli among other etiologies. In the acute phase, sensitivity of MRI may be limited by mass effect from the hematoma and the complex MRI signal of blood products that may obscure subtle enhancing lesions; its sensitivity is improved in the subacute phase once the hematoma has been resorbed.14–16 Please note that the imaging evaluation of patients with aneurysmal subarachnoid hemorrhage is beyond the scope of this article.
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients who are Candidates for IV Thrombolysis
Treatment options are considered for patients with acute ischemic stroke without intracranial hemorrhage present on imaging. FDA guidelines for administration of IV thrombolysis include imaging to exclude intracranial hemorrhage and its interpretation by a physician with appropriate expertise, while the completion of this initial imaging within 45 minutes of the patient admission to the emergency department is a CMS Hospital Outpatient Quality Reporting measure.21–23 There is strong evidence supporting the use of IV tPA as a recanalization therapy to improve clinical outcomes during the 0–3-hour time window (Level 1++)24–26 and during the 3–4.5-hour time window (Level 1+)27–29 This benefit is despite an increased risk of symptomatic intracranial hemorrhage after infusion. Overall, there is strong evidence (Level 1a) supporting the timely use of imaging of the brain to exclude hemorrhage in patients with the clinical diagnosis of stroke and before initiating IV thrombolytic therapy.4, 24 The primary goals of imaging during the 0–4.5-hour time window are to exclude the presence of intracranial hemorrhage and assess the presence and extent of ischemic changes. The presence of intracranial hemorrhage (excluding microbleeds) is an absolute contraindication to administering IV thrombolytic therapy. Early signs of ischemia involving more than one-third of the middle cerebral artery (MCA) territory in the 0–6-hour time window have been associated with large infarcted regions, increased risk of hemorrhagic transformation, and poor outcomes, and thus constitute a relative contraindication to IV thrombolysis.26, 30, 31
Imaging in patients who are potential candidates for IV thrombolysis should not delay administration of IV thrombolysis, as “time is brain”.22 Therefore, IV tPA decisions should be made immediately after the NCCT is completed. At institutions that offer endovascular treatment to IV tPA eligible patients with large artery occlusion (likely tPA failures), additional imaging can be performed while IV tPA is prepared/administered, in order to not delay treatment. From a logistics perspective, institutions should develop a standardized imaging algorithm based on their capabilities and interpretation of current evidence. This imaging protocol should be adhered to for all eligible patients in order to expedite the process and minimize delays in treatment. For instance, if NCCT, CTA and MRI constitute the imaging algorithm selected by an institution to evaluate for potential endovascular candidates, NCCT and CTA should be obtained in one imaging session to minimize imaging time. At institutions performing it regularly, the entire multimodal CT evaluation typically adds no more than 10–15 minutes to the time required to perform a NCCT. It does not delay IV thrombolysis, which can be performed directly in the CT scanner once the NCCT is completed and while the CTA and/or PCT are being obtained (Level 2+).32–35 Few institutions are able to perform MRI studies in the acute setting. Such MRI studies are usually performed after the NCCT has been completed (or used as a replacement for it) and are often obtained during or following IV tPA administration.
Imaging evidence for detection of ischemia
NCCT is also used to assess for early signs of infarction, including loss of gray-white differentiation, sulcal effacement, and hyperdense clot in the proximal vessels.30, 36 NCCT has been reported to have low sensitivity (39%) and high specificity (100%) for detection of ischemic changes (Level 1a).30, 37 However, the significance of these early signs detected on NCCT has been debated. In the European Cooperative Acute Stroke Studies (ECASS), large infarctions with early swelling had an increased incidence of hemorrhage and poor outcome following thrombolytic therapy.30, 37 Conversely, the National Institute of Neurological Disorders and Stroke rt-PA Stroke Study reported that extensive early signs of infarction on NCCT were associated with stroke severity but not with adverse outcome after thrombolysis.36 However, more recent studies have disagreed and recommended criteria for withholding IV thrombolytic therapy in the 0–3-hour time window for definite signs of ischemia involving more than one-third of the MCA territory.38
Detection of early signs of ischemia on NCCT varies amongst experienced observers39–41 depending on the size of the infarction, the time between symptom onset and imaging, and the CT window and level settings used. A more objective approach to define the extent of early ischemic changes has been described in the Alberta Stroke Program Early CT Score (ASPECTS), which is a 10-point scoring system of the MCA territory.42–44 Although ASPECTS showed superior inter-observer agreement, it only modestly improved accuracy for predicting functional outcome and performed the same as the one-third MCA rule for predicting symptomatic hemorrhage.42 Specifically, an ASPECTS score ≤7 has been shown to predict poor functional outcome with 78% sensitivity and 96% specificity, and symptomatic hemorrhage with 90% sensitivity and 62% specificity.23
Of note, the source images from CTA (CTA-SI) have been shown to have increased sensitivity relative to NCCT for detecting ischemic changes, except for infarcts that are small or in the posterior fossa (Level II)45, 46, although, with current technology (rapid CT acquisition), they tend to overestimate the size of the infarct.47 Importantly, CTA-SI maps are strongly dependent on the precise timing of the imaging, which may differ between centers and between individual patients.
MR diffusion-weighted imaging (DWI) is more sensitive for detecting ischemic changes compared with NCCT (Level Ia).48–53 Its sensitivity in detecting ischemia is reported as 99% with a high specificity of 92%.48, 50, 53–59 In anterior circulation strokes, the DWI lesion volume correlates well with baseline clinical stroke severity, final infarct volume, and clinical outcome (Level II).60–62 Although strong evidence suggests that MRI is superior to NCCT for confirming stroke within the first 24 hours (Level 1a)53, logistical issues related to performing MRI in the emergent setting, as well as the proven benefit of CT-based selection in randomized controlled trials, limit the use of MRI in the emergent setting (Table 3). Therefore, MRI may be reserved for select patients in whom the clinical diagnosis is uncertain or for centers that have MRI readily available 24/7 with streamlined protocols in order to limit imaging time within the standard-of-care guidelines for thrombolytic therapy.
SUMMARY: In acute stroke patients who are candidates for IV thrombolysis (0–4.5-hour time window), NCCT or MRI is recommended to exclude intracranial hemorrhage and determine the extent of ischemic changes.4, 63, 64
The presence of a large acute hypodensity on NCCT increases the risk of hemorrhagic transformation after thrombolytic therapy. This is considered a relative, not absolute, contraindication for IV tPA. MR DWI may be obtained for a more definitive estimate of the extent of ischemia, only if this does not delay IV thrombolysis.
The presence of a small number of MRI-detected chronic microhemorrhages (<5), in the absence of hemorrhage on NCCT, is not a contraindication to IV thrombolysis. However, the risk of hemorrhage in patients with multiple chronic microhemorrhages (>5) is unknown.
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients who are Candidates for Endovascular Revascularization
There is limited evidence supporting the use of IA thrombolytic agents up to 6 hours. Also, the evidence supporting improved clinical outcomes with first generation mechanical embolectomy devices up to 8 hours following symptom onset, compared to standard medical care, has recently been challenged by the results of the MR RESCUE65, IMS III66 and SYNTHESIS EXP trials67. Mechanical thrombectomy devices received FDA approval for use in patients presenting up to 8 hours from symptom onset, because of early recanalization being associated with a 4–5 fold improvement in clinical outcome.68 Further randomized, controlled trials are needed to test the clinical efficacy of new generation stent-retriever (“stentriever”) thrombectomy devices.
Initiation of endovascular revascularization therapy provides targeted treatment at the site of the clot. Due to the associated risks of the procedure, if this is considered, more information for appropriate patient selection is needed in order to achieve an acceptable risk-benefit ratio.69, 70 Poor response and poor outcomes with IV thrombolysis have been found with carotid terminus and large, proximal artery occlusions.71, 72 Additionally, the outcome after endovascular therapy is also influenced by the composition and location of the thrombus, with improved recanalization rates for more proximal rather than distal thrombus.73–77 Thus, there is some justification (Level II), for vascular imaging of acute stroke patients at the time of the initial brain imaging study in order to triage patients to best therapy and determine prognosis. This may also be the most practical and efficient time to obtain vascular imaging in stroke patients.
There are three major imaging strategies (and numerous combinations of these three strategies) used in acute ischemic stroke patients who are considered for endovascular revascularization therapy, with different underlying rationales (Figure 1). There is currently no definitive evidence supporting one strategy over the other. Some believe that more imaging provides additional, clinically relevant information, while others are concerned about the additional time resulting from the additional imaging and the potential delay to recanalization it might cause. The choice of imaging implemented may depend on physician preference and logistical factors (such as whether advanced imaging, especially MRI, can be performed quickly and on a 24/7 basis). In considering the underlying rationale for†endovascular therapy, additional imaging may be more justified in patients within the 4.5–8 hour time window. In patients with a contraindication to IV tPA within the 0–4.5 hour time window and in patients considered for endovascular therapy after IV tPA failure, imaging the volume of the infarct may be sufficient.
The first strategy consists of going to the angiography suite immediately after the initial NCCT. The rationale for this approach is to minimize the door-to-recanalization time. In this setting, the vascular patency status is assessed on the DSA that precedes the therapeutic portion of the procedure, prior to lysis or removal of the clot. Collateral patterns can also be demonstrated, although infarct volume can only be indirectly assessed by attention to flow, parenchymal blush, and arterial to venous transit times. The second strategy consists of obtaining a CTA to assess vascular patency, with or without perfusion imaging, in order to better characterize the site of occlusion and the ischemic tissue before making an endovascular treatment decision. The third strategy consists of using MRI/MRA, possibly with diffusion- and perfusion-weighted imaging at institutions where it can be performed quickly and on a 24/7 basis. The rationale of these latter approaches is that the extra time needed to perform this additional imaging may be justified by the information gathered, and the implications for decision-making.78, 79 Some studies have demonstrated that the extra time for imaging until treatment does not adversely affect outcomes.80–82
Imaging evidence for detection of intravascular clot
Vascular imaging of the acute stroke patient prior to endovascular therapy is necessary to determine whether an embolus/thrombus is present that is accessible and amenable to IA thrombolysis and/or mechanical thrombectomy. Imaging of the intracranial and extracranial vessels can be performed quickly and noninvasively using CTA and MRA. However, DSA is considered the “reference standard” for detection of vascular stenoses and occlusions. CTA has been reported to have high sensitivity (97–100%) and specificity (98–100%) for detecting intracranial stenoses and occlusions compared with DSA (Level Ib).83–90 MRA can also be used to characterize vascular patency (Level Ib).4, 64, 91 CTA has been shown to be slightly superior to MRA for this purpose, typically for distal vascular lesions.83, 84 Complete or partial signal void in regions of high and/or turbulent flow may occur on time-of-flight MRA, leading to an overestimation of stenosis. Window settings and presence of calcifications or adjacent bone can limit CTA evaluation.
CTA provides additional tissue information on the CTA-SI, initially thought to represent blood volume weighted data. However, with current, faster CTA protocols, a steady state is not always reached; the CTA-SI may be more blood flow-weighted and can frequently overestimate ischemic core relative to the DWI lesion volume.47 Hypodense regions on CTA-SI indicate early ischemic changes that may be seen to better advantage compared to NCCT. In one study, the combined information from the CTA and CTA-SI demonstrated marked improvement in localization of both the ischemic core and the occluded vessel compared with NCCT and clinical information.92 Another advantage of CTA is that it can be obtained immediately following NCCT, after initiation of IV thrombolytic therapy in the CT scanner, in order to avoid delaying treatment.
Imaging evidence for detection of viable tissue
Determination of tissue viability based on imaging has the potential to individualize thrombolytic therapy and extend the therapeutic time window for some acute stroke patients. Although perfusion imaging has been incorporated into acute stroke imaging algorithms at some institutions, its clinical utility has not been proven. The potential value of perfusion imaging has been assessed in the Desmoteplase in Acute Ischemic Stroke–phase II (DIAS-II) trial using MR diffusion/perfusion mismatch and a perfusion-CT mismatch as entry criteria to receive IV desmoteplase in patients presenting up to 9 hours from symptom onset.93 However, this trial failed to demonstrate superiority of treatment over placebo using penumbral imaging as a selection criterion. Other trials such as DEFUSE, DEFUSE-2 and EPITHET have shown promising results using a combination of diffusion and perfusion imaging to identify good candidates for revascularization therapy beyond 3 hours.78, 94, 95 The MR RESCUE trial failed to demonstrate any difference in outcome in stroke patients selected using penumbra imaging compared to no selection at all.65 Therefore, there is insufficient evidence at this point supporting the use of penumbra imaging to select patients for acute reperfusion therapy. Further randomized, controlled trials are needed to test the full spectrum of penumbra imaging selection for acute stroke therapies.
MR perfusion is employed at some institutions to assess the diffusion/perfusion mismatch. The presence of a perfusion abnormality larger than the DWI lesion (i.e., a mismatch) is a qualitative marker for potential infarct expansion.96,97, 98 However, the extent of mismatched tissue varies greatly, depending on the perfusion parameter selected and the threshold selected to represent the PWI abnormality (Level 2+).99,93, 100, 101 Individual studies have reported varying perfusion parameters as most predictive of tissue viability and clinical outcome, without clear consensus. Some studies have suggested that the Tmax parameter (time to peak of the residue function) using a threshold > 6 seconds is a good predictor of infarct growth in the absence of early recanalization.102–104
PCT is another method used to assess the ischemic core and penumbra. Similar to MR PWI, there is no clear consensus on the optimal perfusion parameter that is most predictive of tissue viability and outcome. A prospective multi-center study reported that an absolute cerebral blood volume (CBV) threshold reflected the ischemic core and that a relative mean transit time (MTT) threshold most accurately reflected the penumbra.105 However, in more recent and larger studies, relative cerebral blood flow (rCBF) was found to be more predictive of the ischemic core (nonviable tissue) than absolute CBV.106–108,109–114 As for PWI, there is a need for standardization of the PCT methods used to define the ischemic core and the penumbra.
It is important to note that perfusion imaging has many applications beyond characterization of the penumbra and triage of patients to acute revascularization therapy. The negative results of the MR RESCUE trial do not negate these potential benefits.65 These applications include, but are not limited to: (1) improving the sensitivity and accuracy of stroke diagnosis (in some cases, a lesion on PCT leads to more careful scrutiny and identification of a vascular occlusion that was not evident prospectively, particularly in the M2 and more distal MCA branches)46, 115–117, (2) excluding stroke mimics118, (3) better assessment of the ischemic core116 and collateral flow,119 and (4) prediction of hemorrhagic transformation and malignant edema.120, 121
Imaging evidence for the characterization of collateral vessels
The concept of collaterals as a vascular network that can potentially bypass devastating effects of a blocked cerebral artery has recently gained momentum. Collaterals have been shown to enhance recanalization and reperfusion, reduce the size of the core and ischemic lesion growth, decrease the risk of hemorrhagic transformation, and improve outcomes with IV and endovascular revascularization (Level III).119, 122 More specifically, a poor collateral pattern has a high specificity for poor tissue and clinical outcome (Level III).122
Several imaging approaches have been proposed to evaluate collaterals, including CTA, PCT, perfusion-weighted imaging (PWI), DSA, arterial spin labeling (ASL), and positron emission tomography (PET). Currently, none of these techniques is absolute nor is any established as a reference standard to assess and quantify collateral flow. Imaging techniques that include a serial, temporal assessment have a definitive advantage because of the dynamic nature of collateral perfusion. Optimized imaging analyses of collateral perfusion patterns may have to consider the underlying mechanism of arterial occlusion, as patterns may vary from intracranial atherosclerosis to cardioembolism.123 Thresholded volumes of hypoperfusion on perfusion maps may not be as informative as voxel-based measures that depict the heterogeneity of the penumbra.124
SUMMARY: In acute stroke patients who are candidates for endovascular therapy, vascular imaging (CTA, MRA, DSA) is strongly recommended during the initial imaging evaluation.4, 57, 63, 64 Perfusion imaging may be considered to assess the target tissue “at risk” for reperfusion therapy.4, 64 However, the accuracy and usefulness of perfusion imaging to identify and differentiate viable tissue have not been well established.
Acute large-vessel intracranial thrombus is accurately detected by CTA, MRA, and DSA.
Patients with large infarctions tend to have poor outcomes. The ischemic core is determined most accurately with DWI. Appropriately thresholded PCT-CBV and PCT-CBF can also be used to identify the ischemic core despite immediate reperfusion.
A poor collateral pattern has a high specificity for poor tissue and clinical outcome (Level III).
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients who are NOT Candidates for IV or Endovascular Therapy and Patients with Transient Ischemic Attacks (TIAs)
When acute revascularization therapy is not being considered, the role of imaging is primarily focused on diagnosis, prevention of immediate complications, and the identification of potentially treatable causes of future stroke. In patients with TIAs, multimodal MRI is preferred, and NCCT should be obtained only if MRI is not available, as NCCT has limited utility in patients whose symptoms have resolved.125 DWI can demonstrate lesions in approximately 40% of TIA patients,56, 126, 127 and DWI positivity in TIA patients is associated with a higher risk of recurrent ischemic events.128 The distribution of the DWI lesions can help with the determination of the stroke etiology (scattered emboli in multiple territories indicative of proximal embolic source (e.g. cardiac), watershed distribution of lesions suggestive of carotid disease, etc).129–131
MR-based perfusion imaging, either with dynamic susceptibility contrast (DSC) or ASL, may additionally identify a vascular etiology in TIA patients.132, 133
CTA or MRA of the intracranial and cervical arteries, and duplex ultrasound (DUS) for the cervical arteries, are used to identify stenosis and/or occlusion (Level 1b)125, and determine appropriate secondary prevention, such as extracranial carotid revascularization, for these patients. An appropriate evaluation for cardiac sources of TIA/stroke (e.g., echocardiography) should also be performed.
SUMMARY: When revascularization therapy is not indicated or available, multimodal neuroimaging of the brain and cerebrovasculature with MRI should be performed to confirm the diagnosis of stroke identify the underlying etiology, and assess immediate complications and risk of future stroke.125
Multimodal CT, including NCCT and CTA and possibly PCT, should be reserved for patients who have contraindications to MRI, or if MRI is not available.125
Rationale and Imaging Evidence for Acute Ischemic Stroke Patients with Wake-up Stroke or More Generally with Unknown Time-of-Onset
Acute stroke patients presenting without a definite time of symptom onset, such as wake-up stroke, may or may not proceed to thrombolytic treatment. If no acute reperfusion therapy is considered, NCCT is recommended to assess for intracranial hemorrhage. Further imaging evaluation is consistent with recommendations discussed in the previous sections.
However, if acute reperfusion therapy is considered, typically as part of a clinical trial, multimodal MRI (using the DWI-PWI mismatch or the DWI-FLAIR mismatch) or multimodal CT (NCCT, CTA and PCT) is required to assess the “tissue clock”, as the time clock concept does not apply.134–136
SUMMARY: In acute stroke patients without a definite time of symptom onset, imaging recommendations depend on whether acute reperfusion therapy may be performed.
If no acute reperfusion therapy will be performed, imaging recommendations are consistent with those in the previous sections.
If acute reperfusion therapy is considered, multimodal MRI or CT with perfusion imaging is recommended to evaluate viable tissue, as the time clock is not applicable. However, there is there is no firm evidence supporting imaging selection for treatment in this patient population.
Rationale and Imaging Evidence for Patients Suspected of Posterior Fossa Stroke
Acute stroke imaging in patients presenting with posterior fossa infarctions is quite similar to hemispheric ischemic stroke. A few aspects specific to the posterior fossa include the following:
NCCT is relatively insensitive in detecting acute and small cortical or subcortical infarctions, especially in the posterior fossa. PCT has very limited indications for the posterior fossa, as beam-hardening artifacts from the temporal bones limit the image quality. Additionally, the spatial resolution of PCT is challenged by the small size of ischemic lesions in the posterior fossa. Of further consideration, PCT imaging of the posterior fossa may involve inclusion of the ocular lenses in the cine imaging acquisition, which is associated with a non-negligible deterministic risk of cataract formation.
MRI with DWI is the optimal imaging technique to assess for ischemic lesions in the posterior fossa (Level Ia).132 It can assess the degree of brainstem infarction prior to IA treatment. However, due to the dismal prognosis of basilar occlusion, a higher risk is often tolerated to achieve recanalization at any time point.
CTA, MRA and DSA are the preferred imaging techniques to assess for basilar artery thrombosis (Level Ia).
SUMMARY: In acute stroke patients presenting with posterior fossa infarction, imaging recommendations are similar to hemispheric acute ischemic stroke.
MRI with DWI is the optimal imaging technique to assess the presence and extent of ischemia in the posterior fossa.
CTA and DSA are the preferred imaging techniques to assess for basilar artery thrombosis. MRA is an acceptable alternative for patients already undergoing an MRI examination.
Rationale and Evidence Supporting Imaging of the Cervical Arteries in Acute Stroke and TIA Patients
Imaging of the cervical arteries (and not only the intracranial arteries) should be performed routinely as part of the imaging evaluation of patients with acute ischemic stroke, but should not delay IV tPA administration in the first 4.5 hours.4, 64 Similarly, noninvasive imaging of the cervical arteries should be a routine component of the imaging work-up of patients with TIAs.4, 64 The primary goal of imaging the cervical arteries is to help identify the mechanism of the stroke, and thus potentially to prevent a recurrence.4, 64, 137 Several imaging techniques are available to assess the cervical arteries including DUS, CTA, MRA, and DSA.138–140 Each technique has its own advantages and limitations in specific clinical situations, but overall, these non-invasive techniques show general agreement with DSA in approximately 90% of cases (Level Ib).141–143 DSA is considered the “reference standard” imaging modality to assess the degree of stenosis and determine patient eligibility for carotid endarterectomy/angioplasty/stenting. The concordant results of two non-invasive techniques (DUS, CTA, and/or MRA) can be used to determine treatment eligibility, avoiding catheterization risks.144, 145 A 99% stenosis (the so called ”string sign”) is most accurately detected by DSA, followed closely by CTA and contrast-enhanced MRA.146
SUMMARY: In acute stroke patients, vascular imaging should be performed to evaluate the mechanism of stroke and assess risk of future stroke.1
Overall, vascular imaging with DUS, CTA, MRA or DSA has good agreement.
Concordant results from at least two noninvasive imaging techniques can be used to determine treatment eligibility for revascularization procedures.
Acknowledgement
We would like to thank Judy Burleson, MHSA, Director, Metrics, American College of Radiology and Christine Waldrip, RN, MHA, Program Manager, American College of Radiology Appropriateness Criteria, for the support they provided in the preparation of this manuscript.
References
- 1.Rowley HA. Extending the time window for thrombolysis: Evidence from acute stroke trials. Neuroimaging clinics of North America. 2005;15:575–587. doi: 10.1016/j.nic.2005.08.002. x. [DOI] [PubMed] [Google Scholar]
- 2.Sims J, Schwamm LH. The evolving role of acute stroke imaging in intravenous thrombolytic therapy: Patient selection and outcomes assessment. Neuroimaging clinics of North America. 2005;15:421–440. doi: 10.1016/j.nic.2005.06.001. xii. [DOI] [PubMed] [Google Scholar]
- 3.Schellinger PD. The evolving role of advanced mr imaging as a management tool for adult ischemic stroke: A western-european perspective. Neuroimaging clinics of North America. 2005;15:245–258. doi: 10.1016/j.nic.2005.06.003. ix. [DOI] [PubMed] [Google Scholar]
- 4.DeLaPaz RL, Wippold FJ, 2nd, Cornelius RS, Amin-Hanjani S, Angtuaco EJ, Broderick DF, et al. Acr appropriateness criteria(r) on cerebrovascular disease. Journal of the American College of Radiology : JACR. 2011;8:532–538. doi: 10.1016/j.jacr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 5.von Kummer R, Bourquain H, Bastianello S, Bozzao L, Manelfe C, Meier D, et al. Early prediction of irreversible brain damage after ischemic stroke at ct. Radiology. 2001;219:95–100. doi: 10.1148/radiology.219.1.r01ap0695. [DOI] [PubMed] [Google Scholar]
- 6.Paxton R, Ambrose J. The emi scanner. A brief review of the first 650 patients. The British journal of radiology. 1974;47:530–565. doi: 10.1259/0007-1285-47-561-530. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs L, Kinkel WR, Heffner RR., Jr Autopsy correlations of computerized tomography: Experience with 6,000 ct scans. Neurology. 1976;26:1111–1118. doi: 10.1212/wnl.26.12.1111. [DOI] [PubMed] [Google Scholar]
- 8.Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: A multicenter study on the validity of stroke imaging. Stroke; a journal of cerebral circulation. 2004;35:502–506. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of mri and ct for detection of acute intracerebral hemorrhage. JAMA : the journal of the American Medical Association. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 10.Kakuda W, Thijs VN, Lansberg MG, Bammer R, Wechsler L, Kemp S, et al. Clinical importance of microbleeds in patients receiving iv thrombolysis. Neurology. 2005;65:1175–1178. doi: 10.1212/01.wnl.0000180519.27680.0f. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Kang BS, Kim N, Roh JK. Does microbleed predict haemorrhagic transformation after acute atherothrombotic or cardioembolic stroke? Journal of neurology, neurosurgery, and psychiatry. 2008;79:913–916. doi: 10.1136/jnnp.2007.133876. [DOI] [PubMed] [Google Scholar]
- 12.Boulanger JM, Coutts SB, Eliasziw M, Gagnon AJ, Simon JE, Subramaniam S, et al. Cerebral microhemorrhages predict new disabling or fatal strokes in patients with acute ischemic stroke or transient ischemic attack. Stroke; a journal of cerebral circulation. 2006;37:911–914. doi: 10.1161/01.STR.0000204237.66466.5f. [DOI] [PubMed] [Google Scholar]
- 13.Fiehler J, Albers GW, Boulanger JM, Derex L, Gass A, Hjort N, et al. Bleeding risk analysis in stroke imaging before thrombolysis (brasil): Pooled analysis of t2*-weighted magnetic resonance imaging data from 570 patients. Stroke; a journal of cerebral circulation. 2007;38:2738–2744. doi: 10.1161/STROKEAHA.106.480848. [DOI] [PubMed] [Google Scholar]
- 14.Kidwell CS, Wintermark M. Imaging of intracranial haemorrhage. Lancet neurology. 2008;7:256–267. doi: 10.1016/S1474-4422(08)70041-3. [DOI] [PubMed] [Google Scholar]
- 15.Huisman TA. Intracranial hemorrhage: Ultrasound, ct and mri findings. European radiology. 2005;15:434–440. doi: 10.1007/s00330-004-2615-7. [DOI] [PubMed] [Google Scholar]
- 16.Hoggard N, Wilkinson ID, Paley MN, Griffiths PD. Imaging of haemorrhagic stroke. Clinical radiology. 2002;57:957–968. doi: 10.1053/crad.2002.0954. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Smith A, Hemphill JC, 3rd, Smith WS, Lu Y, Dillon WP, et al. Contrast extravasation on ct predicts mortality in primary intracerebral hemorrhage. AJNR. American journal of neuroradiology. 2008;29:520–525. doi: 10.3174/ajnr.A0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the ct-angiography spot sign (predict): A prospective observational study. Lancet neurology. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 19.Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, Schwab K, et al. Cta spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocritical care. 2012;17:421–428. doi: 10.1007/s12028-012-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koculym A, Huynh TJ, Jakubovic R, Zhang L, Aviv RI. Ct perfusion spot sign improves sensitivity for prediction of outcome compared with cta and postcontrast ct. AJNR. American journal of neuroradiology. 2012 doi: 10.3174/ajnr.A3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL. Time is brain--quantified. Stroke; a journal of cerebral circulation. 2006;37:263–266. doi: 10.1161/01.STR.0000196957.55928.ab. [DOI] [PubMed] [Google Scholar]
- 23.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Davalos A, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe implementation of thrombolysis in stroke-monitoring study (sits-most) Stroke; a journal of cerebral circulation. 2008;39:3316–3322. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 24.Tissue plasminogen activator for acute ischemic stroke. The national institute of neurological disorders and stroke rt-pa stroke study group. The New England journal of medicine. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 25.Adams HP, Jr, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, et al. Guidelines for thrombolytic therapy for acute stroke: A supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Circulation. 1996;94:1167–1174. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- 26.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ecass) JAMA : the journal of the American Medical Association. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 27.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. The New England journal of medicine. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 28.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0–4.5 h after onset of acute ischaemic stroke (ecass iii): Additional outcomes and subgroup analysis of a randomised controlled trial. Lancet neurology. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 29.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 30.von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, et al. Sensitivity and prognostic value of early ct in occlusion of the middle cerebral artery trunk. AJNR. American journal of neuroradiology. 1994;15:9–15. discussion 16–18. [PMC free article] [PubMed] [Google Scholar]
- 31.Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: A secondary analysis of the european-australasian acute stroke study (ecass ii) Stroke; a journal of cerebral circulation. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 32.Lee KH, Lee SJ, Cho SJ, Na DG, Byun HS, Kim YB, et al. Usefulness of triphasic perfusion computed tomography for intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Archives of neurology. 2000;57:1000–1008. doi: 10.1001/archneur.57.7.1000. [DOI] [PubMed] [Google Scholar]
- 33.Lev MH, Segal AZ, Farkas J, Hossain ST, Putman C, Hunter GJ, et al. Utility of perfusion-weighted ct imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: Prediction of final infarct volume and clinical outcome. Stroke; a journal of cerebral circulation. 2001;32:2021–2028. doi: 10.1161/hs0901.095680. [DOI] [PubMed] [Google Scholar]
- 34.Wintermark M, Reichhart M, Thiran JP, Maeder P, Chalaron M, Schnyder P, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Annals of neurology. 2002;51:417–432. doi: 10.1002/ana.10136. [DOI] [PubMed] [Google Scholar]
- 35.Koroshetz WJ, Lev MH. Contrast computed tomography scan in acute stroke:"You can't always get what you want but…You get what you need". Annals of neurology. 2002;51:415–416. doi: 10.1002/ana.10167. [DOI] [PubMed] [Google Scholar]
- 36.Patel SC, Levine SR, Tilley BC, Grotta JC, Lu M, Frankel M, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA : the journal of the American Medical Association. 2001;286:2830–2838. doi: 10.1001/jama.286.22.2830. [DOI] [PubMed] [Google Scholar]
- 37.von Kummer R, Nolte PN, Schnittger H, Thron A, Ringelstein EB. Detectability of cerebral hemisphere ischaemic infarcts by ct within 6 h of stroke. Neuroradiology. 1996;38:31–33. doi: 10.1007/BF00593212. [DOI] [PubMed] [Google Scholar]
- 38.Schellinger PD, Fiebach JB, Hacke W. Imaging-based decision making in thrombolytic therapy for ischemic stroke: Present status. Stroke; a journal of cerebral circulation. 2003;34:575–583. [PubMed] [Google Scholar]
- 39.Schriger DL, Kalafut M, Starkman S, Krueger M, Saver JL. Cranial computed tomography interpretation in acute stroke: Physician accuracy in determining eligibility for thrombolytic therapy. JAMA : the journal of the American Medical Association. 1998;279:1293–1297. doi: 10.1001/jama.279.16.1293. [DOI] [PubMed] [Google Scholar]
- 40.Grotta JC, Chiu D, Lu M, Patel S, Levine SR, Tilley BC, et al. Agreement and variability in the interpretation of early ct changes in stroke patients qualifying for intravenous rtpa therapy. Stroke; a journal of cerebral circulation. 1999;30:1528–1533. doi: 10.1161/01.str.30.8.1528. [DOI] [PubMed] [Google Scholar]
- 41.Kalafut MA, Schriger DL, Saver JL, Starkman S. Detection of early ct signs of >1/3 middle cerebral artery infarctions : Interrater reliability and sensitivity of ct interpretation by physicians involved in acute stroke care. Stroke; a journal of cerebral circulation. 2000;31:1667–1671. doi: 10.1161/01.str.31.7.1667. [DOI] [PubMed] [Google Scholar]
- 42.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Aspects study group. Alberta stroke programme early ct score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 43.Pexman JH, Barber PA, Hill MD, Sevick RJ, Demchuk AM, Hudon ME, et al. Use of the alberta stroke program early ct score (aspects) for assessing ct scans in patients with acute stroke. AJNR. American journal of neuroradiology. 2001;22:1534–1542. [PMC free article] [PubMed] [Google Scholar]
- 44.Demchuk AM, Coutts SB. Alberta stroke program early ct score in acute stroke triage. Neuroimaging clinics of North America. 2005;15:409–419. doi: 10.1016/j.nic.2005.06.008. xii. [DOI] [PubMed] [Google Scholar]
- 45.Schramm P, Schellinger PD, Fiebach JB, Heiland S, Jansen O, Knauth M, et al. Comparison of ct and ct angiography source images with diffusion-weighted imaging in patients with acute stroke within 6 hours after onset. Stroke; a journal of cerebral circulation. 2002;33:2426–2432. doi: 10.1161/01.str.0000032244.03134.37. [DOI] [PubMed] [Google Scholar]
- 46.Schramm P, Schellinger PD, Klotz E, Kallenberg K, Fiebach JB, Kulkens S, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke; a journal of cerebral circulation. 2004;35:1652–1658. doi: 10.1161/01.STR.0000131271.54098.22. [DOI] [PubMed] [Google Scholar]
- 47.Sharma M, Fox AJ, Symons S, Jairath A, Aviv RI. Ct angiographic source images: Flow- or volume-weighted? AJNR. American journal of neuroradiology. 2011;32:359–364. doi: 10.3174/ajnr.A2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet. 2007;369:293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. Ct and diffusion-weighted mr imaging in randomized order: Diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke; a journal of cerebral circulation. 2002;33:2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez RG, Schaefer PW, Buonanno FS, Schwamm LH, Budzik RF, Rordorf G, et al. Diffusion-weighted mr imaging: Diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology. 1999;210:155–162. doi: 10.1148/radiology.210.1.r99ja02155. [DOI] [PubMed] [Google Scholar]
- 51.Kucharczyk J, Mintorovitch J, Asgari HS, Moseley M. Diffusion/perfusion mr imaging of acute cerebral ischemia. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1991;19:311–315. doi: 10.1002/mrm.1910190220. [DOI] [PubMed] [Google Scholar]
- 52.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Annals of neurology. 1995;37:231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 53.Brazzelli M, Sandercock PA, Chappell FM, Celani MG, Righetti E, Arestis N, et al. Magnetic resonance imaging versus computed tomography for detection of acute vascular lesions in patients presenting with stroke symptoms. Cochrane database of systematic reviews. 2009:CD007424. doi: 10.1002/14651858.CD007424.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Lovblad KO, Laubach HJ, Baird AE, Curtin F, Schlaug G, Edelman RR, et al. Clinical experience with diffusion-weighted mr in patients with acute stroke. AJNR. American journal of neuroradiology. 1998;19:1061–1066. [PMC free article] [PubMed] [Google Scholar]
- 55.Marks MP, de Crespigny A, Lentz D, Enzmann DR, Albers GW, Moseley ME. Acute and chronic stroke: Navigated spin-echo diffusion-weighted mr imaging. Radiology. 1996;199:403–408. doi: 10.1148/radiology.199.2.8668785. [DOI] [PubMed] [Google Scholar]
- 56.Kidwell CS, Alger JR, Di Salle F, Starkman S, Villablanca P, Bentson J, et al. Diffusion mri in patients with transient ischemic attacks. Stroke; a journal of cerebral circulation. 1999;30:1174–1180. doi: 10.1161/01.str.30.6.1174. [DOI] [PubMed] [Google Scholar]
- 57.Ay H, Buonanno FS, Rordorf G, Schaefer PW, Schwamm LH, Wu O, et al. Normal diffusion-weighted mri during stroke-like deficits. Neurology. 1999;52:1784–1792. doi: 10.1212/wnl.52.9.1784. [DOI] [PubMed] [Google Scholar]
- 58.Barber PA, Darby DG, Desmond PM, Gerraty RP, Yang Q, Li T, et al. Identification of major ischemic change. Diffusion-weighted imaging versus computed tomography. Stroke; a journal of cerebral circulation. 1999;30:2059–2065. doi: 10.1161/01.str.30.10.2059. [DOI] [PubMed] [Google Scholar]
- 59.Lee LJ, Kidwell CS, Alger J, Starkman S, Saver JL. Impact on stroke subtype diagnosis of early diffusion-weighted magnetic resonance imaging and magnetic resonance angiography. Stroke; a journal of cerebral circulation. 2000;31:1081–1089. doi: 10.1161/01.str.31.5.1081. [DOI] [PubMed] [Google Scholar]
- 60.Warach S, Pettigrew LC, Dashe JF, Pullicino P, Lefkowitz DM, Sabounjian L, et al. Effect of citicoline on ischemic lesions as measured by diffusion-weighted magnetic resonance imaging. Citicoline 010 investigators. Annals of neurology. 2000;48:713–722. [PubMed] [Google Scholar]
- 61.Tong DC, Yenari MA, Albers GW, O'Brien M, Marks MP, Moseley ME. Correlation of perfusion- and diffusion-weighted mri with nihss score in acute (<6.5 hour) ischemic stroke. Neurology. 1998;50:864–870. doi: 10.1212/wnl.50.4.864. [DOI] [PubMed] [Google Scholar]
- 62.Lovblad KO, Baird AE, Schlaug G, Benfield A, Siewert B, Voetsch B, et al. Ischemic lesion volumes in acute stroke by diffusion-weighted magnetic resonance imaging correlate with clinical outcome. Annals of neurology. 1997;42:164–170. doi: 10.1002/ana.410420206. [DOI] [PubMed] [Google Scholar]
- 63.Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: A scientific statement from the american heart association. Stroke; a journal of cerebral circulation. 2009;40:3646–3678. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 64.Jauch E, Saver J, Adams HP, Connors JJ, Demaerschalk B, Bruno A, et al. Guidelines for the early management of patients with acute ischemic stroke. A guideline for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. doi: 10.1161/STR.0b013e318284056a. in press. [DOI] [PubMed] [Google Scholar]
- 65.Chelsea KJR, Gornbein J, Alger J, Nenov V, Ajani Z, Meyer B, Olson S, Schwamm L, Yoo A, Marshall R, Meyers P, Yagaval D, Wintermark M, Guzy J, Starkman S, Saver J. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013 doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. The New England journal of medicine. 2013 doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. The New England journal of medicine. 2013 doi: 10.1056/NEJMc1304759. [DOI] [PubMed] [Google Scholar]
- 68.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke; a journal of cerebral circulation. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 69.Caplan LR. Treatment of acute stroke: Still struggling. JAMA : the journal of the American Medical Association. 2004;292:1883–1885. doi: 10.1001/jama.292.15.1883. [DOI] [PubMed] [Google Scholar]
- 70.Caplan LR. Stroke thrombolysis: Slow progress. Circulation. 2006;114:187–190. doi: 10.1161/CIRCULATIONAHA.106.638973. [DOI] [PubMed] [Google Scholar]
- 71.Saqqur M, Uchino K, Demchuk AM, Molina CA, Garami Z, Calleja S, et al. Site of arterial occlusion identified by transcranial doppler predicts the response to intravenous thrombolysis for stroke. Stroke; a journal of cerebral circulation. 2007;38:948–954. doi: 10.1161/01.STR.0000257304.21967.ba. [DOI] [PubMed] [Google Scholar]
- 72.Sims JR, Rordorf G, Smith EE, Koroshetz WJ, Lev MH, Buonanno F, et al. Arterial occlusion revealed by ct angiography predicts nih stroke score and acute outcomes after iv tpa treatment. AJNR. American journal of neuroradiology. 2005;26:246–251. [PMC free article] [PubMed] [Google Scholar]
- 73.Ogawa A, Mori E, Minematsu K, Taki W, Takahashi A, Nemoto S, et al. Randomized trial of intraarterial infusion of urokinase within 6 hours of middle cerebral artery stroke: The middle cerebral artery embolism local fibrinolytic intervention trial (melt) japan. Stroke; a journal of cerebral circulation. 2007;38:2633–2639. doi: 10.1161/STROKEAHA.107.488551. [DOI] [PubMed] [Google Scholar]
- 74.Saver JL. Intra-arterial fibrinolysis for acute ischemic stroke: The message of melt. Stroke; a journal of cerebral circulation. 2007;38:2627–2628. doi: 10.1161/STROKEAHA.107.490417. [DOI] [PubMed] [Google Scholar]
- 75.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke; a journal of cerebral circulation. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 76.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. Ct and mri early vessel signs reflect clot composition in acute stroke. Stroke; a journal of cerebral circulation. 2011;42:1237–1243. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moftakhar P, English JD, Cooke DL, Kim WT, Stout C, Smith WS, et al. Density of thrombus on admission ct predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke; a journal of cerebral circulation. 2013;44:243–245. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 78.Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: The diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Annals of neurology. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 79.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet neurology. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schellinger PD, Bryan RN, Caplan LR, Detre JA, Edelman RR, Jaigobin C, et al. Evidence-based guideline: The role of diffusion and perfusion mri for the diagnosis of acute ischemic stroke: Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2010;75:177–185. doi: 10.1212/WNL.0b013e3181e7c9dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Breuer L, Schellinger PD, Huttner HB, Halwachs R, Engelhorn T, Doerfler A, et al. Feasibility and safety of magnetic resonance imaging-based thrombolysis in patients with stroke on awakening: Initial single-centre experience. International journal of stroke : official journal of the International Stroke Society. 2010;5:68–73. doi: 10.1111/j.1747-4949.2010.00410.x. [DOI] [PubMed] [Google Scholar]
- 82.Kohrmann M, Schellinger PD. Stroke-mri: Extending the time-window: Recent trials and clinical practice. International journal of stroke : official journal of the International Stroke Society. 2007;2:53–54. doi: 10.1111/j.1747-4949.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 83.Hirai T, Korogi Y, Ono K, Nagano M, Maruoka K, Uemura S, et al. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: Combined mr angiography and ct angiography compared with digital subtraction angiography. AJNR. American journal of neuroradiology. 2002;23:93–101. [PMC free article] [PubMed] [Google Scholar]
- 84.Katz DA, Marks MP, Napel SA, Bracci PM, Roberts SL. Circle of willis: Evaluation with spiral ct angiography, mr angiography, and conventional angiography. Radiology. 1995;195:445–449. doi: 10.1148/radiology.195.2.7724764. [DOI] [PubMed] [Google Scholar]
- 85.Knauth M, von Kummer R, Jansen O, Hahnel S, Dorfler A, Sartor K. Potential of ct angiography in acute ischemic stroke. AJNR. American journal of neuroradiology. 1997;18:1001–1010. [PMC free article] [PubMed] [Google Scholar]
- 86.Shrier DA, Tanaka H, Numaguchi Y, Konno S, Patel U, Shibata D. Ct angiography in the evaluation of acute stroke. AJNR. American journal of neuroradiology. 1997;18:1011–1020. [PMC free article] [PubMed] [Google Scholar]
- 87.Wildermuth S, Knauth M, Brandt T, Winter R, Sartor K, Hacke W. Role of ct angiography in patient selection for thrombolytic therapy in acute hemispheric stroke. Stroke; a journal of cerebral circulation. 1998;29:935–938. doi: 10.1161/01.str.29.5.935. [DOI] [PubMed] [Google Scholar]
- 88.Verro P, Tanenbaum LN, Borden NM, Sen S, Eshkar N. Ct angiography in acute ischemic stroke: Preliminary results. Stroke; a journal of cerebral circulation. 2002;33:276–278. doi: 10.1161/hs0102.101223. [DOI] [PubMed] [Google Scholar]
- 89.Graf J, Skutta B, Kuhn FP, Ferbert A. Computed tomographic angiography findings in 103 patients following vascular events in the posterior circulation: Potential and clinical relevance. Journal of neurology. 2000;247:760–766. doi: 10.1007/s004150070089. [DOI] [PubMed] [Google Scholar]
- 90.Nguyen-Huynh MN, Wintermark M, English J, Lam J, Vittinghoff E, Smith WS, et al. How accurate is ct angiography in evaluating intracranial atherosclerotic disease? Stroke; a journal of cerebral circulation. 2008;39:1184–1188. doi: 10.1161/STROKEAHA.107.502906. [DOI] [PubMed] [Google Scholar]
- 91.Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: Evaluation with ct angiography, mr angiography, and digital subtraction angiography. AJNR. American journal of neuroradiology. 2005;26:1012–1021. [PMC free article] [PubMed] [Google Scholar]
- 92.Pulli B, Schaefer PW, Hakimelahi R, Chaudhry ZA, Lev MH, Hirsch JA, et al. Acute ischemic stroke: Infarct core estimation on ct angiography source images depends on ct angiography protocol. Radiology. 2012;262:593–604. doi: 10.1148/radiol.11110896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hacke W, Furlan AJ, Al-Rawi Y, Davalos A, Fiebach JB, Gruber F, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by mri perfusion-diffusion weighted imaging or perfusion ct (dias-2): A prospective, randomised, double-blind, placebo-controlled study. Lancet neurology. 2009;8:141–150. doi: 10.1016/S1474-4422(08)70267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the echoplanar imaging thrombolytic evaluation trial (epithet): A placebo-controlled randomised trial. Lancet neurology. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 95.Lansberg MG, Straka M, Kemp S, Mlynash M, Wechsler LR, Jovin TG, et al. Mri profile and response to endovascular reperfusion after stroke (defuse 2): A prospective cohort study. Lancet neurology. 2012;11:860–867. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Butcher KS, Parsons M, MacGregor L, Barber PA, Chalk J, Bladin C, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke; a journal of cerebral circulation. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 97.Parsons MW, Barber PA, Chalk J, Darby DG, Rose S, Desmond PM, et al. Diffusion- and perfusion-weighted mri response to thrombolysis in stroke. Annals of neurology. 2002;51:28–37. doi: 10.1002/ana.10067. [DOI] [PubMed] [Google Scholar]
- 98.Sorensen AG, Copen WA, Ostergaard L, Buonanno FS, Gonzalez RG, Rordorf G, et al. Hyperacute stroke: Simultaneous measurement of relative cerebral blood volume, relative cerebral blood flow, and mean tissue transit time. Radiology. 1999;210:519–527. doi: 10.1148/radiology.210.2.r99fe06519. [DOI] [PubMed] [Google Scholar]
- 99.Perkins CJ, Kahya E, Roque CT, Roche PE, Newman GC. Fluid-attenuated inversion recovery and diffusion- and perfusion-weighted mri abnormalities in 117 consecutive patients with stroke symptoms. Stroke; a journal of cerebral circulation. 2001;32:2774–2781. doi: 10.1161/hs1201.099634. [DOI] [PubMed] [Google Scholar]
- 100.Furlan AJ, Eyding D, Albers GW, Al-Rawi Y, Lees KR, Rowley HA, et al. Dose escalation of desmoteplase for acute ischemic stroke (dedas): Evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke; a journal of cerebral circulation. 2006;37:1227–1231. doi: 10.1161/01.STR.0000217403.66996.6d. [DOI] [PubMed] [Google Scholar]
- 101.Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, et al. The desmoteplase in acute ischemic stroke trial (dias): A phase ii mri-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke; a journal of cerebral circulation. 2005;36:66–73. doi: 10.1161/01.STR.0000149938.08731.2c. [DOI] [PubMed] [Google Scholar]
- 102.Lansberg MG, Lee J, Christensen S, Straka M, De Silva DA, Mlynash M, et al. Rapid automated patient selection for reperfusion therapy: A pooled analysis of the echoplanar imaging thrombolytic evaluation trial (epithet) and the diffusion and perfusion imaging evaluation for understanding stroke evolution (defuse) study. Stroke; a journal of cerebral circulation. 2011;42:1608–1614. doi: 10.1161/STROKEAHA.110.609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mlynash M, Lansberg MG, De Silva DA, Lee J, Christensen S, Straka M, et al. Refining the definition of the malignant profile: Insights from the defuse-epithet pooled data set. Stroke; a journal of cerebral circulation. 2011;42:1270–1275. doi: 10.1161/STROKEAHA.110.601609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Olivot JM, Mlynash M, Thijs VN, Kemp S, Lansberg MG, Wechsler L, et al. Optimal tmax threshold for predicting penumbral tissue in acute stroke. Stroke; a journal of cerebral circulation. 2009;40:469–475. doi: 10.1161/STROKEAHA.108.526954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, et al. Perfusion-ct assessment of infarct core and penumbra: Receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke; a journal of cerebral circulation. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 106.Mayer TE, Hamann GF, Baranczyk J, Rosengarten B, Klotz E, Wiesmann M, et al. Dynamic ct perfusion imaging of acute stroke. AJNR. American journal of neuroradiology. 2000;21:1441–1449. [PMC free article] [PubMed] [Google Scholar]
- 107.Bivard A, McElduff P, Spratt N, Levi C, Parsons M. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovasc Dis. 2011;31:238–245. doi: 10.1159/000321897. [DOI] [PubMed] [Google Scholar]
- 108.Arakawa S, Wright PM, Koga M, Phan TG, Reutens DC, Lim I, et al. Ischemic thresholds for gray and white matter: A diffusion and perfusion magnetic resonance study. Stroke; a journal of cerebral circulation. 2006;37:1211–1216. doi: 10.1161/01.STR.0000217258.63925.6b. [DOI] [PubMed] [Google Scholar]
- 109.Sobesky J, Zaro Weber O, Lehnhardt FG, Hesselmann V, Neveling M, Jacobs A, et al. Does the mismatch match the penumbra? Magnetic resonance imaging and positron emission tomography in early ischemic stroke. Stroke; a journal of cerebral circulation. 2005;36:980–985. doi: 10.1161/01.STR.0000160751.79241.a3. [DOI] [PubMed] [Google Scholar]
- 110.Bivard A, Levi C, Spratt N, Parsons M. Perfusion ct in acute stroke: A comprehensive analysis of infarct and penumbra. Radiology. 2012 doi: 10.1148/radiol.12120971. [DOI] [PubMed] [Google Scholar]
- 111.Bivard A, Spratt N, Levi C, Parsons M. Perfusion computer tomography: Imaging and clinical validation in acute ischaemic stroke. Brain : a journal of neurology. 2011;134:3408–3416. doi: 10.1093/brain/awr257. [DOI] [PubMed] [Google Scholar]
- 112.Bivard A, McElduff P, Spratt N, Levi C, Parsons M. Defining the extent of irreversible brain ischemia using perfusion computed tomography. Cerebrovascular Diseases. 2011;31:238–245. doi: 10.1159/000321897. [DOI] [PubMed] [Google Scholar]
- 113.Kamalian S, Kamalian S, Maas MB, Goldmacher GV, Payabvash S, Akbar A, et al. Ct cerebral blood flow maps optimally correlate with admission diffusion-weighted imaging in acute stroke but thresholds vary by postprocessing platform. Stroke; a journal of cerebral circulation. 2011;42:1923–1928. doi: 10.1161/STROKEAHA.110.610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Payabvash S, Souza LC, Wang Y, Schaefer PW, Furie KL, Halpern EF, et al. Regional ischemic vulnerability of the brain to hypoperfusion: The need for location specific computed tomography perfusion thresholds in acute stroke patients. Stroke; a journal of cerebral circulation. 2011;42:1255–1260. doi: 10.1161/STROKEAHA.110.600940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kloska SP, Nabavi DG, Gaus C, Nam EM, Klotz E, Ringelstein EB, et al. Acute stroke assessment with ct: Do we need multimodal evaluation? Radiology. 2004;233:79–86. doi: 10.1148/radiol.2331030028. [DOI] [PubMed] [Google Scholar]
- 116.Wintermark M, Fischbein NJ, Smith WS, Ko NU, Quist M, Dillon WP. Accuracy of dynamic perfusion ct with deconvolution in detecting acute hemispheric stroke. AJNR. American journal of neuroradiology. 2005;26:104–112. [PMC free article] [PubMed] [Google Scholar]
- 117.Scharf J, Brockmann MA, Daffertshofer M, Diepers M, Neumaier-Probst E, Weiss C, et al. Improvement of sensitivity and interrater reliability to detect acute stroke by dynamic perfusion computed tomography and computed tomography angiography. Journal of computer assisted tomography. 2006;30:105–110. doi: 10.1097/01.rct.0000187417.15321.ca. [DOI] [PubMed] [Google Scholar]
- 118.Keedy A, Soares B, Wintermark M. A pictorial essay of brain perfusion-ct: Not every abnormality is a stroke! Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2012;22:e20–e33. doi: 10.1111/j.1552-6569.2012.00716.x. [DOI] [PubMed] [Google Scholar]
- 119.Tan JC, Dillon WP, Liu S, Adler F, Smith WS, Wintermark M. Systematic comparison of perfusion-ct and ct-angiography in acute stroke patients. Annals of neurology. 2007;61:533–543. doi: 10.1002/ana.21130. [DOI] [PubMed] [Google Scholar]
- 120.Ryoo JW, Na DG, Kim SS, Lee KH, Lee SJ, Chung CS, et al. Malignant middle cerebral artery infarction in hyperacute ischemic stroke: Evaluation with multiphasic perfusion computed tomography maps. Journal of computer assisted tomography. 2004;28:55–62. doi: 10.1097/00004728-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Hom J, Dankbaar JW, Soares BP, Schneider T, Cheng SC, Bredno J, et al. Blood-brain barrier permeability assessed by perfusion ct predicts symptomatic hemorrhagic transformation and malignant edema in acute ischemic stroke. AJNR. American journal of neuroradiology. 2011;32:41–48. doi: 10.3174/ajnr.A2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke; a journal of cerebral circulation. 2011;42:693–699. doi: 10.1161/STROKEAHA.110.595256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim SJ, Seok JM, Bang OY, Kim GM, Kim KH, Jeon P, et al. Mr mismatch profiles in patients with intracranial atherosclerotic stroke: A comprehensive approach comparing stroke subtypes. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:1138–1145. doi: 10.1038/jcbfm.2009.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the american heart association/american stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardiovascular radiology and intervention; council on cardiovascular nursing; the interdisciplinary council on peripheral vascular disease. The american academy of neurology affirms the value of this statement as an educational tool for neurologists. Stroke; a journal of cerebral circulation. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 126.Coutts SB, Hill MD, Simon JE, Sohn CH, Scott JN, Demchuk AM. Silent ischemia in minor stroke and tia patients identified on mr imaging. Neurology. 2005;65:513–517. doi: 10.1212/01.wnl.0000169031.39264.ff. [DOI] [PubMed] [Google Scholar]
- 127.Restrepo L, Jacobs MA, Barker PB, Wityk RJ. Assessment of transient ischemic attack with diffusion- and perfusion-weighted imaging. AJNR. American journal of neuroradiology. 2004;25:1645–1652. [PMC free article] [PubMed] [Google Scholar]
- 128.Cucchiara BL, Messe SR, Taylor RA, Pacelli J, Maus D, Shah Q, et al. Is the abcd score useful for risk stratification of patients with acute transient ischemic attack? Stroke; a journal of cerebral circulation. 2006;37:1710–1714. doi: 10.1161/01.STR.0000227195.46336.93. [DOI] [PubMed] [Google Scholar]
- 129.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with toast stroke subtypes. Archives of neurology. 2003;60:1730–1734. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- 130.Wessels T, Wessels C, Ellsiepen A, Reuter I, Trittmacher S, Stolz E, et al. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR. American journal of neuroradiology. 2006;27:35–39. [PMC free article] [PubMed] [Google Scholar]
- 131.Rovira A, Grive E, Rovira A, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. European radiology. 2005;15:416–426. doi: 10.1007/s00330-004-2633-5. [DOI] [PubMed] [Google Scholar]
- 132.Mlynash M, Olivot JM, Tong DC, Lansberg MG, Eyngorn I, Kemp S, et al. Yield of combined perfusion and diffusion mr imaging in hemispheric tia. Neurology. 2009;72:1127–1133. doi: 10.1212/01.wnl.0000340983.00152.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zaharchuk G, Olivot JM, Fischbein NJ, Bammer R, Straka M, Kleinman JT, et al. Arterial spin labeling imaging findings in transient ischemic attack patients: Comparison with diffusion- and bolus perfusion-weighted imaging. Cerebrovasc Dis. 2012;34:221–228. doi: 10.1159/000339682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Thomalla G, Ebinger M, Fiehler J, Fiebach JB, Endres M, Gerloff C. [eu-funded treatment study: Wake-up : A randomized, placebo-controlled mri-based trial of thrombolysis in wake-up stroke] Der Nervenarzt. 2012;83:1241–1251. doi: 10.1007/s00115-012-3532-7. [DOI] [PubMed] [Google Scholar]
- 135.Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. Dwi-flair mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (pre-flair): A multicentre observational study. Lancet neurology. 2011;10:978–986. doi: 10.1016/S1474-4422(11)70192-2. [DOI] [PubMed] [Google Scholar]
- 136.Kang DW, Kwon JY, Kwon SU, Kim JS. Wake-up or unclear-onset strokes: Are they waking up to the world of thrombolysis therapy? International journal of stroke : official journal of the International Stroke Society. 2012;7:311–320. doi: 10.1111/j.1747-4949.2012.00779.x. [DOI] [PubMed] [Google Scholar]
- 137.Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, et al. Update to the aha/asa recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke; a journal of cerebral circulation. 2008;39:1647–1652. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buskens E, Nederkoorn PJ, Buijs-Van Der Woude T, Mali WP, Kappelle LJ, Eikelboom BC, et al. Imaging of carotid arteries in symptomatic patients: Cost-effectiveness of diagnostic strategies. Radiology. 2004;233:101–112. doi: 10.1148/radiol.2331030863. [DOI] [PubMed] [Google Scholar]
- 139.Lovett JK, Dennis MS, Sandercock PA, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke; a journal of cerebral circulation. 2003;34:e138–e140. doi: 10.1161/01.STR.0000080935.01264.91. [DOI] [PubMed] [Google Scholar]
- 140.Rothwell PM, Giles MF, Flossmann E, Lovelock CE, Redgrave JN, Warlow CP, et al. A simple score (abcd) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet. 2005;366:29–36. doi: 10.1016/S0140-6736(05)66702-5. [DOI] [PubMed] [Google Scholar]
- 141.Blakeley DD, Oddone EZ, Hasselblad V, Simel DL, Matchar DB. Noninvasive carotid artery testing. A meta-analytic review. Annals of internal medicine. 1995;122:360–367. doi: 10.7326/0003-4819-122-5-199503010-00007. [DOI] [PubMed] [Google Scholar]
- 142.Long A, Lepoutre A, Corbillon E, Branchereau A. Critical review of non- or minimally invasive methods (duplex ultrasonography, mr- and ct-angiography) for evaluating stenosis of the proximal internal carotid artery. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2002;24:43–52. doi: 10.1053/ejvs.2002.1666. [DOI] [PubMed] [Google Scholar]
- 143.Randoux B, Marro B, Koskas F, Duyme M, Sahel M, Zouaoui A, et al. Carotid artery stenosis: Prospective comparison of ct, three-dimensional gadolinium-enhanced mr, conventional angiography. Radiology. 2001;220:179–185. doi: 10.1148/radiology.220.1.r01jl35179. [DOI] [PubMed] [Google Scholar]
- 144.Johnston DC, Goldstein LB. Clinical carotid endarterectomy decision making: Noninvasive vascular imaging versus angiography. Neurology. 2001;56:1009–1015. doi: 10.1212/wnl.56.8.1009. [DOI] [PubMed] [Google Scholar]
- 145.Nederkoorn PJ, Mali WP, Eikelboom BC, Elgersma OE, Buskens E, Hunink MG, et al. Preoperative diagnosis of carotid artery stenosis: Accuracy of noninvasive testing. Stroke; a journal of cerebral circulation. 2002;33:2003–2008. doi: 10.1161/01.str.0000021900.58396.44. [DOI] [PubMed] [Google Scholar]
- 146.Lev MH, Romero JM, Goodman DN, Bagga R, Kim HY, Clerk NA, et al. Total occlusion versus hairline residual lumen of the internal carotid arteries: Accuracy of single section helical ct angiography. AJNR. American journal of neuroradiology. 2003;24:1123–1129. [PMC free article] [PubMed] [Google Scholar]