Abstract
Purpose
To (a) evaluate the response of hepatocellular carcinoma (HCC) to chemoembolization after initial nonresponse, as determined with European Association for the Study of the Liver (EASL) criteria and modified Response Evaluation Criteria in Solid Tumors (mRECIST), and (b) compare posttreatment survival of initial nonresponders versus that of initial responders.
Materials and Methods
The institutional review board approved this retrospective study, which was compliant with HIPAA. A total of 116 consecutive patients (96 men, 20 women; mean age, 63 years) with unresectable HCC who underwent at least two chemoembolization procedures were included. The chemoembolization mixture consisted of 100 mg of cisplatin, 50 mg of doxorubicin, and 10 mg of mitomycin C mixed 1:1 with iodized oil. Tumor response at magnetic resonance imaging was evaluated after each chemoembolization procedure according to EASL criteria and mRECIST. The survival rate in each subgroup was calculated and correlated with response. The Wilcoxon test was used to test group comparability. Kaplan-Meier estimators were used to generate survival curves and compared by using the log-rank test.
Results
No response to initial chemoembolization was seen in 43% and 50% of patients according to EASL criteria and mRECIST, respectively. After a second chemoembolization procedure, 44% (EASL) and 47% (mRECIST) of initial nonresponders showed a significant response. With EASL criteria, the 1-, 2-, and 3-year survival rates (±standard error of the mean) after two chemoembolization procedures were 39% ± 10, 14% ± 7, and 0%, respectively, for non-responders and 68% ± 10, 50% ± 11, and 37% ± 11 for responders (P = .036, P = .006, and P < .005 at 1, 2, and 3 years). With mRECIST, the 1-, 2-, and 3-year survival rates after two chemoembolization procedures were 49% ± 9, 20% ± 8, and 7% ± 6 for nonresponders and 67% ± 9, 44% ± 10, and 36% ± 9 for responders (P = .174, P = .046, and P = .011 at 1, 2, and 3 years).
Conclusion
Patients who underwent chemoembolization for HCC showed a response (with both EASL criteria and mRECIST) and improved survival after the second chemoembolization treatment. At least two chemoembolization procedures should be performed in the same targeted lesions before further treatment is abandoned.
Since the studies by Llovett et al (1), Cammà et al (2), and Lo et al (3), chemoembolization has been established as the mainstay treatment for unresectable hepatocellular carcinoma (HCC). It is now recommended as part of the guidelines of the American Association for the Study of Liver Diseases for intermediate-stage HCC. Imaging assessment of tumor response to chemoembolization has, however, not been standardized. Various groups use World Health Organization criteria (bidimensional), Response Evaluation Criteria in Solid Tumors (RECIST, unidimensional), and European Association for the Study of the Liver (EASL) criteria (which measure lack of contrast material enhancement within the tumor as a surrogate of tumor cell death) interchangeably. More recently, modified RECIST (mRECIST, which measure residual contrast enhancement within the tumor, indicating persistent viability) has been suggested as a possible universal method for evaluating response (4,5).
Studies have shown little correlation between size (World Health Organization criteria and RECIST) and enhancement (EASL criteria) measures after chemoembolization (6–8). Furthermore, Keppke et al (8) showed that HCC response after yttrium 90 radioembolization was partially dependent on method of measurement. In the same cohort of patients, they recorded responses of 23%, 26%, and 57% with use of RECIST, World Health Organization criteria, and EASL criteria, respectively. Further confounding the issue of determining response is the fact that a large percentage of patients treated with chemoembolization do not respond at all, irrespective of response criteria used. Specifically, up to 70% and 35% of patients evaluated on the basis of RECIST and EASL criteria, respectively, do not respond to a single chemoembolization treatment (9). The clinical implications and thus the appropriate treatment plan for this large percentage of patients are unclear.
This issue has indeed been partly addressed for patients with neuroendocrine liver metastasis. Varker et al (10) concluded that initial nonresponders, when treated with a second chemoembolization procedure, experience benefits (imaging and symptomatic response) similar to those of patients who responded after the first procedure. The validity of Varker and colleagues’ conclusion for metastatic neuroendocrine tumors cannot be expanded to cover HCC, however, because of the important differences between the two diseases in terms of biology, symptoms, and natural history. The objectives of our study were to (a) evaluate response of HCC to chemoembolization after initial nonresponse, as determined with EASL criteria and mRECIST, and (b) compare posttreatment survival of initial nonresponders versus that of initial responders.
Materials and Methods
Patients
Our institutional review board approved this study, which was compliant with the Health Insurance Portability and Accountability Act, and data were collected prospectively. From January 2006 to December 2009, 164 patients with unresectable HCC were treated with two or more chemoembolization procedures. Patients were included in this study if they had not previously undergone surgical or local-regional treatment. Exclusion criteria included an Eastern Cooperative Group score greater than 2, a total bilirubin level of more than 4 mg/dL, an international normalized ratio (INR) of more than 1.8 or platelet count less than 50 000 per microliter, or encephalopathy. In addition, we excluded patients with lobar or main portal venous thrombosis (bland or neoplastic) or extrahepatic disease even though those conditions are not considered absolute contraindications to chemoembolization (11). We elected to exclude such patients to minimize confounding variables and increase the statistical power of the study. Forty-eight of the 164 patients were excluded. Thus, our study population consisted of 116 patients (96 men, 20 women; mean age, 63 years). At baseline, all patients underwent dual-phase magnetic resonance (MR) imaging of the liver, along with physical examination, and a clinical history and relevant laboratory values were obtained. Patients were treated by four interventional radiologists, including three authors (C.G., J.F.G., K.H.; mean experience in performing chemoembolizations, 6 years).
Treatment Protocol
The patients were given nothing by mouth for 8 hours. Patients received intravenous administration of 2 g of cefotetan (AstraZeneca, Wilmington, Del) or piperacillin and tazobactam (Zosyn; Wyeth Pharmaceuticals, Philadelphia, Pa) before chemoembolization. Patients who were allergic to penicillin received intravenous administration of 600 mg of clindamycin (Pharmacia & Upjohn, Kalamazoo, Mich). At the initial chemoembolization, arteriography of the superior mesenteric artery was performed during the portal venous phase to document possible portal venous thrombosis and show variant hepatic arterial supply. The celiac axis was selected with a 0.035-inch guidewire (Glidewire; Terumo Medical, Somerset, NJ) and Simmons-1 catheter (Angiodynamics, Latham, NY), and the desired hepatic arterial branch was subselected depending on tumor location. In many patients, a 3.0-F Renegade High-Flo catheter (Boston Scientific, Natick, Mass) was introduced coaxially over a 0.014-inch Transend or Fathom wire (Boston Scientific) because of the need to place the catheter in close proximity to the tumor. A 7–10-mL chemotherapy solution (100-mg cisplatin [Bristol Myers Squibb, Princeton, NJ], 50-mg doxorubicin (Adriamycin, Pharmacia & Upjohn), and 10-mg mitomycin C [Bedford Laboratories, Bedford, Ohio]) was infused in a 1:1 or 2:1 volume ratio with iodized oil (Ethiodol; Savage Laboratories, Melville, NY). With the latter approach, twice as much chemotherapy solution was given compared with iodized oil, depending on flow characteristics, to avoid complete stasis within the selected hepatic artery. Next, we infused 1–4 mL of 100–300-µm microsphere particles (Embosphere; Biosphere Medical, Boston, Mass).
The end point of the procedure was achieved when all the chemotherapy solution was delivered and the infused microspheres resulted in visibly slowed arterial flow (two to five heart the beats were necessary to clear the contrast material column in the selected artery) or when further infusion of chemotherapy would probably result in reflux. Nonbuffered lidocaine (10–20 mL, 1:100) was also given intraarterially after chemoembolization for pain control. After chemoembolization, patients were admitted to the hospital for an overnight stay; unenhanced computed tomography (CT) of the liver was performed before discharge to document iodized oil distribution. The procedure was considered a technical success if it was completed and follow-up CT showed distribution of iodized oil in the targeted lobe or segment.
Follow-up
All patients were seen in the clinic 4–6 weeks after each chemoembolization; at that point, dual-phase MR imaging of the liver was performed, along with a physical examination, and relevant laboratory values were obtained. MR imaging has been shown to be superior to CT in the follow-up of patients with HCC after treatment (12) and is therefore our follow-up imaging study of choice. The index lesion method was used to determine response; this method is validated for follow-up of patients with HCC (6) and is recommended by the American Association for the Study of Liver Diseases (4,5). The response of the index lesion to chemoembolization was documented according to RECIST, mRECIST, and EASL criteria. However, response was analyzed on the basis of EASL criteria and mRECIST alone for many reasons. First, the degree of response according to RECIST is significantly less than that for methods that use residual enhancement, thereby limiting the statistical power (5). According to the RECIST guidelines, only one patient in our cohort showed a response after the first chemoembolization. Second, the response documented with EASL manifests sooner than that documented with RECIST (1.6 vs 7.7 months, respectively) (6). Third, after local-regional treatments for HCC (7), response criteria that include percentage tumor necrosis (ie, EASL criteria) have been shown to be superior to those that do not include percentage of tumor necrosis. Posttreatment evaluation was performed by a nontreating radiologist.
EASL evaluation
With use of EASL criteria, patients were categorized into four groups according to the percentage enhancement of their index lesion: Group I had 0%–25% enhancement, group II had 25%–50% enhancement, group III had 50%–75% enhancement, and group IV had 75%–100% enhancement. A patient was classified as a responder if the category for the index lesion decreased by at least one group after chemoembolization. Repeat chemoembolization was planned if there was more than 25% tumor viability according to EASL criteria. Further treatment was abandoned if the patient developed any of the contraindications to chemoembolization or if residual enhancement after treatment was 0%–25%.
mRECIST evaluation
The mRECIST used herein are detailed by Lencioni and Llovet (5). The relevant variable for mRECIST is the longest diameter of the viable portion of the target lesion, as defined during the arterial phase of MR imaging (or CT). Complete response is defined as no residual contrast enhancement, partial response as a decrease in the diameter of the arterially enhancing portion of the target lesion of at least 30% (with the maximal diameter of the arterially enhancing lesion at baseline as reference), and progressive disease as an increase of at least 20% in the diameter of the arterially enhancing portion of the target lesion (smallest diameter of the arterially enhancing lesion since treatment initiation). Disease was considered stable if findings were between those for partial response and progressive disease.
Statistical Analysis
Definitions
Patients were categorized according to response as follows: N1, patients who did not respond to the first chemoembolization; R1, patients who responded to the first chemoembolization; N1N2, patients who did not respond to either the first or second chemoembolization; N1R2, patients who did not respond to the first chemoembolization but responded to the second chemoembolization; R1N2, patients who responded to the first chemoembolization but not to the second chemoembolization; and R1R2, patients who responded to both the first and second chemoembolizations.
Comparability of groups
Patients who responded to the initial chemoembolization (R1) were compared with those who did not (N1). For continuous characteristics, we compared the medians and 15% and 85% quantiles and compared the group distributions by using the Wilcoxon test. For categorical characteristics, we examined the distributions by using the Fisher exact test.
The same methods were used to compare the two subgroups among patients who did not respond to initial chemoembolization (ie, those who did [N1R2] and those who did not [N1N2] respond to the second chemoembolization). A matrix describing the responses classified with use of EASL criteria and mRECIST before and after the second chemoembolization procedure was generated. Exact confidence intervals (CIs) for binomial proportions were calculated for the N1R2 group. Although no patients deteriorated clinically during the follow-up period according to EASL staging and only three patients deteriorated on the basis of mRECIST, an exact CI was also calculated for the generalizable population from this subgroup.
Survival analysis
Survival curves were generated by using Kaplan-Meier estimators for the N1 and R1 groups as well as the N1N2 and N1R2 subgroups. The log-rank test was used to compare the survival curves between the independent groups during the entire follow-up period. For years 1, 2, and 3 (which were selected prospectively as standard reporting times for oncologic outcomes), the survival probabilities between independent groups were compared nonparametrically by using the difference of the corresponding Kaplan-Meier estimates divided by the square root of the sum of the squares of the standard error of the mean of the two Kaplan-Meier estimates.
Results
Group Comparability
EASL criteria
Of the 116 patients, 50 (43%) did not respond to the first chemoembolization (initial nonresponders, N1). Of those 50 patients, 22 (44%; 95% CI: 30%, 59%) responded to the second chemoembolization (N1R2) and the groups were tested for comparability. remaining 28 (56%) did not respond to the second chemoembolization (N1N2). None of the patients (0%; 95% CI: 0%, 7%) showed progression of the targeted lesion or lesions during the follow-up period from baseline MR imaging (median, 220 weeks). Table 1 shows the variables for which the groups were tested for comparability. In the N1 and R1 groups, all tested variables except INR were similar. However, the INR in both groups was within the normal range (1.1 and 1.0, respectively) and therefore no clinical effect would be expected. The two N1 subgroups (N1N2 vs N1R2) differed significantly with regard to the number of tumors. All groups were similar for all other variables.
Table 1.
Group Comparability according to EASL Criteria
| Variable | R1 (n = 66) | N1 (n = 50) | P Value | |||
|---|---|---|---|---|---|---|
| All (n = 50) | N1R2 (n = 22) | N1N2 (n = 28) | Groups R1 vs N1 | Subgroups N1R2 vs N1N2 | ||
| Age (y)* | 63 (51, 74) | 64 (51, 72) | 67 (55, 76) | 60 (49, 71) | .93 | .19 |
| M:F ratio | 54:12 | 41:9 | 17:5 | 25:3 | .81 | .28 |
| No. of tumors (%) | .57 | .03 | ||||
| One | 48 | 46 | 27 | 61 | ||
| Two | 11 | 14 | 23 | 7 | ||
| Three | 11 | 4 | 0 | 7 | ||
| More than three | 30 | 36 | 50 | 25 | ||
| Tumor morphology (%) | .99 | .17 | ||||
| Single | 39 | 38 | 27 | 47 | ||
| Multifocal | 44 | 44 | 59 | 32 | ||
| Infiltrative | 17 | 18 | 14 | 21 | ||
| Child-Pugh class (%) | .65 | .93 | ||||
| A | 73 | 67 | 68 | 67 | ||
| B | 25 | 33 | 32 | 33 | ||
| C | 2 | 0 | 0 | 0 | ||
| Total bilirubin level (mg/dL)* | 0.8 (0.4, 1.8) | 0.9 (0.5, 2.0) | 0.8 (0.4, 2.5) | 1.0 (0.5, 1.0) | .24 | .41 |
| Albumin level (g/dL)* | 3.8 (3.1, 4.3) | 3.8 (3.0, 4.2) | 3.8 (3.1, 4.2) | 3.7 (3.0, 4.2) | .92 | .72 |
| Creatinine level (mg/dL)* | 0.8 (0.6, 1.2) | 0.8 (0.7, 1.1) | 0.8 (0.6, 1.1) | 9 (0.7, 1.0) | .89 | .79 |
| INR* | 1.0 (0.9, 1.3) | 1.1 (1.1, 1.3) | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.3) | .04 | .99 |
| MELD*† | 7 (6, 12) | 8 (6, 13) | 8 (6, 14) | 8 (6, 13) | .26 | .75 |
| AFP level (ng/mL)* | ||||||
| At baseline | 64 (4, 9958) | 88 (4, 29 896) | 563 (14, 30 892) | 21 (3, 18 548) | .48 | .07 |
| After first chemoembolization | 32 (5, 2423) | 95 (11, 24 666) | 93 (12, 24 019) | 491 (10, 22 237) | .09 | .96 |
| Follow-up duration (mo)* | 43 (27, 91) | 62 (24, 94) | 54 (26, 90) | 66 (24, 97) | .53 | .51 |
| Patients with a third chemoembolization (%) | 23 | 50 | 36 | 61 | <.01 | .15 |
Note.—The two main groups (N1 and R1) were matched in all tested variables shown except number of chemoembolizations and INR. The number of total chemoembolizations had no influence on the statistical calculations because they were based on data after the second chemoembolization for all groups. Despite differences in the INR, those values were within normal limits and are not expected to have made a difference. The two initial nonresponder subgroups (N1R2 and N1N2) were matched in all variables except number of tumors, with the group that did not respond to a second chemoembolization having more patients with a single tumor. N1 = patients who did not respond to the first chemoembolization, N1N2 = patients who did not respond to either chemoembolization, N1R2 = patients who did not respond to the first chemoembolization but did respond to the second chemoembolization, R1 = patients who responded to the first chemoembolization.
Data are medians, with 15% and 85% quantiles in parentheses.
MELD = Model for End-Stage Liver Disease.
mRECIST
Of the 116 patients, 58 (50%) did not respond to the first chemoembolization (initial nonresponders, N1). Of those 58 patients, 27 (47%; 95% CI: 33%, 60%) responded to the second chemoembolization (N1R2) and the remaining 31 (53%) did not respond to the second chemoembolization (N1N2). Three patients showed disease progression of the targeted lesion after the second chemoembolization (5%; 95% CI: 1%, 14%). Table 2 shows the variables for which the groups were tested for comparability. The N1 and R1 groups were similar in all tested variables except INR, as was seen with the analysis using EASL criteria. In both groups, however, the INR was within the normal range (1.1 and 1.0, respectively). In addition, patients who responded to the second chemoembolization (N1R2) had a higher baseline α-fetoprotein (AFP) level than did those who did not (N1N2).
Table 2.
Group Compatibility according to mRECIST
| Variable | R1 (n = 58) | N1 (n = 58) | P Value | |||
|---|---|---|---|---|---|---|
| All (n = 58) | N1R2 (n = 27) | N1N2 (n = 31) | Groups R1 vs N1 | Subgroups N1R2 vs N1N2 | ||
| Age (y)* | 63 (51, 74) | 64 (51, 74) | 67 (54, 75) | 61 (50, 72) | .84 | .14 |
| M:F ratio | 46:12 | 50:8 | 22:5 | 28:3 | .46 | .48 |
| No. of tumors (%) | .38 | .24 | ||||
| One | 47 | 48 | 41 | 55 | ||
| Two | 12 | 12 | 18 | 7 | ||
| Three | 12 | 4 | 0 | 7 | ||
| More than three | 29 | 36 | 41 | 32 | ||
| Tumor morphology (%) | 1.00 | .65 | ||||
| Single | 40 | 38 | 33 | 42 | ||
| Multifocal | 43 | 45 | 52 | 39 | ||
| Infiltrative | 17 | 17 | 15 | 19 | ||
| Child-Pugh class (%) | .28 | .95 | ||||
| A | 75 | 66 | 67 | 65 | ||
| B | 23 | 34 | 33 | 35 | ||
| C | 2 | 0 | 0 | 0 | ||
| Total bilirubin level (mg/dL)* | 0.7 (0.4, 1.9) | 1.0 (0.5, 1.8) | 1.0 (0.6, 1.8) | 0.9 (0.5, 1.8) | .17 | .94 |
| Albumin level (g/dL)* | 3.8 (3.0, 4.3) | 3.7 (3.1, 4.2) | 3.8 (3.1, 4.2) | 3.7 (3.0, 4.2) | .71 | .81 |
| Creatinine level (mg/dL)* | 0.8 (0.6, 1.2) | 0.8 (0.7, 1.1) | 0.8 (0.7, 1.2) | 0.9 (0.7, 1.0) | .53 | .53 |
| INR* | 1.0 (0.9, 1.1) | 1.1 (1.1, 1.3) | 1.1 (1.0, 1.3) | 1.1 (0.9, 1.3) | .01 | .68 |
| MELD*† | 7 (6,11) | 8 (6, 13) | 8 (6, 14) | 8 (6, 12) | .16 | .93 |
| AFP level (ng/mL)* | ||||||
| At baseline | 31 (4, 7573) | 105 (4, 29 894) | 876 (18, 26 519) | 20 (3, 29 894) | .21 | .04 |
| After first chemoembolization | 25 (5, 2659) | 95 (10, 23 452) | 93 (12, 24 019) | 491 (4, 21 023) | .10 | .98 |
| Follow-up duration (mo)* | 43 (27, 91) | 60 (24, 94) | 50 (26, 91) | 66 (24, 96) | .54 | .33 |
| Patients with a third chemoembolization (%) | 19 | 50 | 37 | 61 | <.01 | .11 |
Note.—The two main groups (N1 and R1) were matched in all tested variables shown except number of chemoembolizations and INR. The number of total chemoembolizations had no influence on the statistical calculations because they were based on data after the second chemoembolization for all groups. Despite differences in the INR, those values were within normal limits and are not expected to have made a difference. The two initial nonresponder subgroups (N1R2 and N1N2) were matched in all variables except AFP level, with those who responded to a second chemoembolization having a significantly higher level than those who did not (P = .04). N1 = patients who did not respond to the first chemoembolization, N1N2 = patients who did not respond to either chemoembolization, N1R2 = patients who did not respond to the first chemoembolization but did respond to the second chemoembolization, R1 = patients who responded to the first chemoembolization.
Data are medians, with 15% and 85% quantiles in parentheses.
MELD = Model for End-Stage Liver Disease.
Response
Table 3 shows the response assessed with EASL criteria after the second chemoembolization for the 50 patients who did not respond to the first chemoembolization. Twenty-two patients (44%) responded to the second chemoembolization despite showing no response to initial treatment.
Table 3.
Response after Second Chemoembolization according to EASL for the 50 Patients Who Did Not Respond to First Chemoembolization
| Response after First Chemoembolization |
Response after Second Embolization | All Patients | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| II | 3* | 0 | 0 | IV | 3 |
| III | 1* | 2* | 5 | 0 | 8 |
| IV | 7* | 4* | 5* | 0 | 39 |
| Total | 11 | 6 | 10 | 23 | 50 |
Note.—Data are numbers of patients.
Patients who responded to second chemoembolization (22 patients, 44%). For example, the third row describes the response to the second chemoembolization for the 39 patients in the EASL IV group. Of those patients, 23 remained stable at EASL IV after the second chemoembolization, five showed EASL III response, four showed EASL II response, and seven showed EASL I response. No patients progressed during the follow-up period (median, 220 weeks).
Table 4 shows the response assessed with mRECIST after the second chemoembolization for the 58 patients who did not respond to the first chemoembolization. Twenty-seven patients (47%) responded to the second chemoembolization despite showing no response to initial treatment.
Table 4.
Response after First and Second Chemoembolizations according to mRECIST
| Response after First Chemoembolization |
Response after Second Chemoembolization | All Patients | |||
|---|---|---|---|---|---|
| Progressive Disease | Stable Disease | Partial Response | Complete Response | ||
| Progressive disease | 1 | 0 | 1* | 0 | 2 |
| Stable disease | 2 | 28 | 15* | 11* | 56 |
| Partial response | 0 | 6 | 18 | 18 | 42 |
| Complete response | 0 | 0 | 1 | 15 | 16 |
| Total | 3 | 34 | 35 | 44 | 116 |
Note.—Data are numbers of patients.
Patients who responded to second chemoembolization from the subgroup of initial nonresponders (27 of 58 patients, 47%). Only three patients from the initial nonresponder group (5%) showed disease progression during the follow-up period (median follow-up, 61 weeks) despite a second chemoembolization.
Survival
Assessment according to EASL criteria
Survival data and curves are shown in Table 5 and the Figure, respectively. Overall survival times for the N1 and R1 groups were similar (P = .279). The median survival times for the N1 and R1 groups (±standard error of the mean) were 370 days ± 65 and 551 days ± 88, respectively (P = .06). For the two subgroups who did not respond to initial chemoembolization (N1N2 and N1R2), the median survival durations were 328 days ± 53 and 625 days ± 268, respectively (P = .10). The survival curves were significantly different at certain time points (log rank = 9.5 in 1 df, P = .002).
Table 5.
Survival Data at 1, 2, and 3 Years according to EASL Criteria
| Group or Subgroup | 1-year Survival | 2-year Survival | 3-year Survival |
|---|---|---|---|
| R1 | 66 ± 6 | 41 ± 6 | 25 ± 6 |
| N1 | 53 ± 7 | 31 ± 7 | 19 ± 6 |
| P value for R1 vs N1 | .16 | .28 | .49 |
| N1R2 | 68 ± 10 | 50 ± 11 | 37 ± 11 |
| N1N2 | 39 ± 10 | 14 ± 7 | .0* |
| P value for N1R2 vs N1N2 | .036 | .006 | <.005† |
Note.—Unless otherwise noted, data are mean survival rates (in percentages) ± standard errors of the mean. Among patients who did not respond to initial chemoembolization according to EASL criteria, those who responded to the second chemoembolization had significantly better 1-, 2-, and 3-year survival rates than did those who did not. N1 = patients who did not respond to the first chemoembolization, R1 = patients who responded to the first chemoembolization, N1N2 = patients who did not respond to either the first or second chemoembolization, N1R2 = patients who did not respond to the first chemoembolization but did respond to the second chemoembolization, R1N2 = patients who responded to the first chemoembolization but not to the second chemoembolization, R1R2 = patients who responded to both the first and second chemoembolizations.
Standard error of the mean was unavailable because only one patient was in the last risk set.
P = .005 for the survival comparison of 3 years for N1R2 group versus 788 days for N1N2 group, so P = .005 for the comparison of 3 years for N1R2 group versus 3 years for N1N2 group.
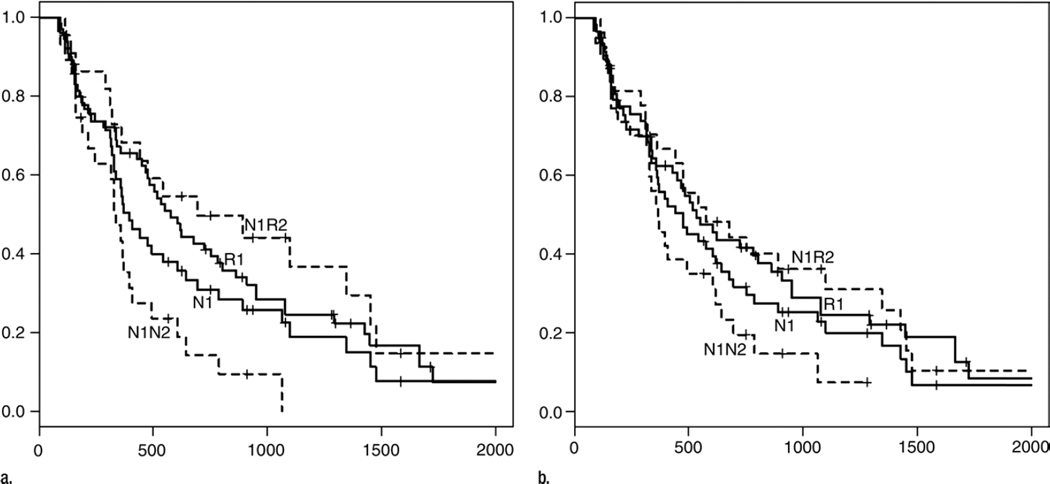
Figure.
Kaplan-Meier survival curves obtained with (a) EASL criteria and (b) mRECIST. Although patients who responded to first chemoembolization (R1) showed a tendency toward longer survival compared with those who did not (N1), the difference was not statistically significant (P = .16, P = .28, and P = .49 at 1, 2, and 3 years, respectively, with EASL criteria; P = .518, P = .276, and P = .849 with mRECIST). After second chemoembolization, N1 patients were divided into two groups: those who responded to second chemoembolization (N1R2) and those who did not (N1N2). N1R2 patients had significantly greater 1-, 2-, and 3-year survival times than did N1N2 patients. With EASL criteria, 1-, 2-, and 3-year survival rates were 39% ± 10, 14% ± 7, and 0%, respectively, for N1N2 patients and 68% ± 10, 50% ± 11, and 37% ± 11 for N1R2 patients (P = .036, P = .006, and P < .005 at 1, 2, and 3 years). With mRECIST, 1-, 2-, and 3-year survival rates were 49% ± 9, 20% ± 8, and 7% ± 6 for N1N2 patients and 67% ± 9, 44% ± 10, and 36% ± 9 for N1R2 patients (P = .174, P = .046, and P = .011 at 1, 2, and 3 years). Numbers on horizontal axis are days after first chemoembolization.
The 1-, 2-, and 3-year survival rates were 53% ± 7, 31% ± 7, and 19% ± 6, respectively, for the N1 group and 66% ± 6, 41% ± 6, and 25% ± 6 for the R1 group. The differences were not statistically significant (P = .16, P = .28, and P = .49 at 1, 2, and 3 years, respectively). There was, however, a statistically significant survival difference between the two subgroups of patients who did not respond to initial chemoembolization at the 1- and 2-year mark. The 1-, 2-, and 3-year survival rates were 39% ± 10, 14% ± 7, and 0%, respectively, for the N1N2 group and 68% ± 10, 50% ± 11, and 37% ± 11 for the N1R2 group (P = .036, P = .006, and P < .005 at 1, 2, and 3 years).
Assessment according to mRECIST criteria
Survival data and curves are shown in Table 6 and the Figure, respectively. Overall survival durations were similar for the N1 and R1 groups (P = .343). The median survival times for the N1 and R1 groups were 441 days ± 79 and 517 days ± 107, respectively (P = .44). For the two subgroups of patients who did not respond to initial chemoembolization (N1N2 and N1R2), median survival durations were 358 days ± 68 and 541 days ± 183 (P = .35), respectively. The survival curves were significantly different at certain time points (log rank = 4.1 in 1 df, P = .044).
Table 6.
Survival Data at 1, 2, and 3 Years according to mRECIST
| Group or Subgroup | 1-year Survival | 2-year Survival | 3-year Survival |
|---|---|---|---|
| R1 | 62 ± 7 | 42 ± 7 | 24 ± 6 |
| N1 | 58 ± 7 | 32 ± 6 | 23 ± 6 |
| P value for R1 vs N1 | .52 | .28 | .85 |
| N1R2 | 67 ± 9 | 44 ± 10 | 36 ± 9 |
| N1N2 | 49 ± 9 | 20 ± 8 | 7 ± 6 |
| P value for N1R2 vs N1N2 | .174 | .046 | .011 |
Note.—Unless otherwise noted, data are mean survival rates (in percentages) ± standard errors of the mean. Among patients who did not respond to the initial chemoembolization, those who responded to the second chemoembolization had significantly longer 2- and 3-year survival times than did those who did not. N1 = patients who did not respond to the first chemoembolization, R1 = patients who responded to the first chemoembolization, N1N2 = patients who did not respond to either the first or second chemoembolization, N1R2 = patients who did not respond to the first chemoembolization but did respond to the second chemoembolization, R1N2 = patients who responded to the first chemoembolization but not to the second chemoembolization, R1R2 = patients who responded to both the first and second chemoembolizations.
The 1-, 2-, and 3-year survival rates were 58% ± 7, 32% ± 6, and 23% ± 6, respectively, for the N1 group and 62% ± 7, 42% ± 7, and 24% ± 6 for the R1 group. The differences were not statistically significant (P = .52, P = .28, and P = .85 at 1, 2, and 3 years, respectively). Conversely, there was a significant survival difference between the two subgroups of patients who did not respond to initial chemoembolization at the 2- and 3-year marks. The 1-, 2-, and 3-year survival rates were 49% ± 9, 20% ± 8, and 7% ± 6, respectively, for the N1N2 group and 67% ± 9, 44% ± 10, and 36% ± 9 for the N1R2 group (P = .174, P = .046, and P = .011 at 1, 2, and 3 years, respectively).
Discussion
In our study, patients with HCC who did not respond to the first chemoem bolization did not necessarily fail to respond to the second chemoembolization. In keeping with results of published series (8,9), our study showed that a significant proportion of patients with HCC did not respond to the initial chemoembolization procedure (43% and 50% according to EASL criteria and mRECIST, respectively). Many of our patients who did not respond to the initial chemoembolization responded favorably after the second treatment (44% and 47% according to EASL criteria and mRECIST, respectively). Furthermore, the survival time of the subgroup of patients who responded to the second chemoembolization was significantly greater than that of the subgroup of patients who did not respond to the first or second chemoembolization. Considering that one cannot predict response (or lack thereof) to the second treatment after failure to respond to the initial chemoembolization, our findings argue against abandoning treatment for patients who show no response to initial chemoembolization.
Of note, the patients who did not respond to the first chemoembolization but did respond to the second chemoembolization had a tendency for a longer survival duration compared with those who responded to the initial treatment. This finding was not statistically significant and is probably spurious. Some of our data suggest that disease biology may indeed play an important role in outcomes after chemoembolization. Despite several advances in the treatment of HCC, staging remains controversial; staging systems show little correlation with treatment outcome or survival (13). Although Barcelona Clinic Liver Cancer staging has been shown to be better correlated with survival for patients with HCC compared with other staging systems, significant limitations in classifying patients with respect to response to chemoembolization and other liver-directed therapies remain. Most staging systems use tumor structure or liver disease stage while ignoring variables that measure disease biology. For example, in our study, the trend in AFP level followed the EASL and survival trends. The AFP level has been shown to be an independent predictor of survival, and a significant decrease in the AFP level is probably correlated with tumor necrosis and possibly longer survival. In addition, initial nonresponders tended toward shorter survival despite undergoing more chemoembolization procedures than those who did respond. Finally, patients who did not respond to the first and second chemoembolizations had a shorter survival than those who responded to the second chemoembolization despite receiving more treatments.
Our study had some limitations, including its retrospective nature. Longer follow-up may have revealed a correlation with the response assessed with RECIST. Patients with previous treatment, extrahepatic disease, and portal venous thrombosis were excluded from analysis, although in general these are not strict exclusion criteria. Such exclusion limits the applicability of our conclusions to those patient groups.
In conclusion, 43% and 50% of patients in our study showed no response to the first chemoembolization with EASL criteria and mRECIST, respectively. More importantly, approximately 50% of patients with HCC who did not respond to the initial chemoembolization showed both response and improved survival after a second chemoembolization. We therefore encourage interventional radiologists to perform at least two chemoembolization procedures in the same targeted lesion or lesions before abandoning treatment.
Advances in Knowledge.
-
■
Almost 50% of patients with hepatocellular carcinoma (HCC) who do not respond to initial chemoembolization show a significant response after a second chemoembolization procedure.
-
■
In addition, such patients demonstrate a significant survival benefit compared with patients who do not respond to the first or second chemoembolization.
Implications for Patient Care
-
■
Patients with HCC treated with chemoembolization should undergo at least two treatments of the same targeted lesion before being classified as nonresponders.
-
■
Premature abandonment of treatment will preclude many patients from receiving a survival benefit.
Abbreviations
- AFP
α-fetoprotein
- CI
confidence interval
- EASL
European Association for the Study of the Liver
- HCC
hepatocellular carcinoma
- INR
international normalized ratio
- mRECIST
modified RECIST
- RECIST
Response Evaluation Criteria in Solid Tumors
Footnotes
Author contributions:
Guarantor of integrity of entire study, C.G.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, C.G., N.H., A.H.P., E.L., K.H., I.K., C.F.; clinical studies, C.G., J.F.G., N.H., A.H.P., I.K.; statistical analysis, J.F.G., N.H., A.H.P., Z.W., C.F.; and manuscript editing, C.G., J.F.G., E.L., K.H., I.K., C.F.
Disclosures of Potential Conflicts of Interest: C.G. No potential conflicts of interest to disclose. J.F.G. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for Biocompatibles, Bayer Healthcare, Guerbet, Nordion, Merit, Abbott, and Jennerex; institution has grants or grants pending from Biocompatibles, Genentech, Bayer Healthcare, Philips Medical, Nordion, Context Vision, and CeloNova. Other relationships: none to disclose. N.H. No potential conflicts of interest to disclose. A.H. No potential conflicts of interest to disclose. E.L. No potential conflicts of interest to disclose. K.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a paid consultant for Boston Scientific, WL Gore, and SureFire Medical; receives payment for lectures including service on speakers bureaus from Boston Scientific; owns stock/stock options in SureFire Medical. Other relationships: none to disclose. Z.W. No potential conflicts of interest to disclose. I.K. No potential conflicts of interest to disclose. C.F. No potential conflicts of interest to disclose.
References
- 1.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 2.Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 3.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100(10):698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 5.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 6.Riaz A, Miller FH, Kulik LM, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. JAMA. 2010;303(11):1062–1069. doi: 10.1001/jama.2010.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616–623. doi: 10.1002/cncr.24050. [DOI] [PubMed] [Google Scholar]
- 8.Keppke AL, Salem R, Reddy D, et al. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am J Roentgenol. 2007;188(3):768–775. doi: 10.2214/AJR.06.0706. [DOI] [PubMed] [Google Scholar]
- 9.Lewandowski RJ, Mulcahy MF, Kulik LM, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010;255(3):955–965. doi: 10.1148/radiol.10091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varker KA, Martin EW, Klemanski D, Palmer B, Shah MH, Bloomston M. Repeat transarterial chemoembolization (TACE) for progressive hepatic carcinoid metastases provides results similar to first TACE. J Gastrointest Surg. 2007;11(12):1680–1685. doi: 10.1007/s11605-007-0235-7. [DOI] [PubMed] [Google Scholar]
- 11.Georgiades CS, Hong K, D’Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol. 2005;16(12):1653–1659. doi: 10.1097/01.RVI.0000182185.47500.7A. [DOI] [PubMed] [Google Scholar]
- 12.Kloeckner R, Otto G, Biesterfeld S, Oberholzer K, Dueber C, Pitton MB. MDCT versus MRI assessment of tumor response after transarterial chemoembolization for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33(3):532–540. doi: 10.1007/s00270-009-9728-y. [DOI] [PubMed] [Google Scholar]
- 13.Georgiades CS, Liapi E, Frangakis C, et al. Prognostic accuracy of 12 liver staging systems in patients with unresectable hepatocellular carcinoma treated with transarterial chemoembolization. J Vasc Interv Radiol. 2006;17(10):1619–1624. doi: 10.1097/01.RVI.0000236608.91960.34. [DOI] [PubMed] [Google Scholar]