Abstract
BACKGROUND
Little is known about the factors that predict for gastrointestinal stromal tumor (GIST) recurrence in patients treated with adjuvant imatinib.
METHODS
Risk factors for GIST recurrence were identified, and 2 risk stratification scores were developed using the database of the Scandinavian Sarcoma Group (SSG) XVIII trial, where 358 patients with high-risk GIST with no overt metastases were randomly assigned to adjuvant imatinib 400 mg/day either for 12 or 36 months after surgery. The findings were validated in the imatinib arm of the American College of Surgeons Oncology Group Z9001 trial, where 359 patients with GIST were randomized to receive imatinib and 354 were to receive placebo for 12 months.
RESULTS
Five factors (high tumor mitotic count, nongastric location, large size, rupture, and adjuvant imatinib for 12 months) were independently associated with unfavorable recurrence-free survival (RFS) in a multivariable analysis in the SSGXVIII cohort. A risk score based on these 5 factors had a concordance index with GIST recurrence of 78.9%. When a simpler score consisting of the 2 strongest predictive factors (mitotic count and tumor site) was devised, the groups with the lowest, intermediate high, and the highest risk had 5-year RFS of 76.7%, 47.5%, and 8.4%, respectively. Both scores were strongly associated with RFS in the validation cohort (P<.001 for each comparison).
CONCLUSIONS
The scores generated were effective in stratifying the risk of GIST recurrence in patient populations treated with adjuvant imatinib. Patients with non-gastric GIST with a high mitotic count are at a particularly high risk for recurrence.
Keywords: gastrointestinal stromal tumor, imatinib, adjuvant therapy, predictive score
INTRODUCTION
Gastrointestinal stromal tumor (GIST) is the most common sarcoma of the gastrointestinal tract.1,2 Activating mutations in KIT or PDGFRA oncogenes are considered the key molecular drivers of GIST pathogenesis.1 KIT mutations, found in 70% to 80% of GISTs, occur frequently in gene exon 11 and sometimes in exon 9, 13, or 17.1 PDGFRA mutation is present in approximately one-third of the GISTs that lack KIT mutation.3,4 GISTs that lack KIT and PDGFRA mutation are referred to as wild-type GISTs, although these tumors may harbor mutations in SDH (succinate dehydrogenase), B-RAF, or K-RAS.1
Most patients with GIST are cured by surgery alone,2 but administration of adjuvant imatinib at least for 3 years is now recommended when the risk of recurrence is considered significant.5,6 This recommendation is based on 3 randomized studies, the American College of Surgeons Oncology Group (ACOSOG) trial Z9001 (ClinicalTrials.gov identifier NCT00041197),7 the European Organization for Research and Treatment of Cancer (EORTC) sponsored randomized trial 62024 (NCT00103168),8 and the Scandinavian Sarcoma Group (SSG) XVIII/Arbeitsgemeinschaft Internistische Onkologie (AIO) trial (NCT00116935).9 In these studies, imatinib improved recurrence-free survival (RFS) as compared with placebo7 or observation,8 and the SSGXVIII/AIO study found 3 years of adjuvant imatinib to improve RFS and overall survival as compared with 1 year of imatinib in a patient population with KIT-positive high-risk GIST.9
Many studies have addressed the risk of GIST recurrence after surgery only,10,11 but most high-risk patients are now treated with surgery and adjuvant imatinib, and little data are available about the risk factors for GIST recurrence in this setting. Yet, such data are valuable for planning of patient follow-up and for counseling. We investigated the risk factors for GIST recurrence within the context of 2 of the 3 large randomized adjuvant trials performed (SSGXVIII/AIO and Z9001), and devised scores for estimation of the risk of recurrence.
MATERIALS AND METHODS
The SSGXVIII/AIO Trial
Patients who had undergone macroscopically complete surgery for KIT-immunopositive GIST and who had a high estimated risk for recurrence according to the modified National Institutes of Health (NIH) consensus criteria were eligible to the SSGXVIII/AIO trial.9,12 The exclusion criteria included metastatic or recurrent GIST, and neoadjuvant treatment for GIST.9 The study was approved by the national or institutional review commit-tees, and the patients provided written informed consent.
The patients were randomly allocated to receive adjuvant imatinib 400 mg daily either for 12 or 36 months between February 2004 and September 2008. The primary endpoint was RFS, considered as the time period from the date of randomization to the date of first detection of recurrence or death, whichever occurred first. The staging examinations at study entry included contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and the pelvis, and chest CT or radiograph. CT (or MRI) of the abdomen and the pelvis were performed at 6-month intervals during adjuvant imatinib treatment and after its completion in each group. Blood cell counts and chemistries were monitored, and physical examination was performed 4 weeks after study entry, every 3 months until 3 years on study, and subsequently every 6 months.9 Histological diagnosis of GIST and patient risk stratification were done at the participating institutes. Tumor histology was reviewed centrally after study entry by 2 pathologists, who also independently repeated tumor mitotic counting. KIT and PDGFRA were centrally screened for presence of mutations after study entry.9
The ACOSOG Z9001 Trial
The results obtained were validated in the patient population that received imatinib in the ACOSOG Z9001 trial.7 In Z9001, 713 patients with KIT-positive GIST ≥ 3 cm in diameter removed macroscopically completely at surgery were randomly assigned to receive either imatinib 400 mg/day (359 patients) or placebo (354 patients) for 12 months from 230 institutions in the United States and Canada between July 2002 and April 2007. The patients were free of tumor at study entry by imaging that included chest radiograph (or CT) and CT or MRI of the abdomen and pelvis. CT scan or MRI of the abdomen and pelvis was performed at 3-month intervals for the first 2 years and every 6 months for the next 3 years. The primary end-point was RFS. The study was approved by the institutional review board of each institution, and all patients provided written informed consent.
Statistical Methods
The Intention-To-Treat population of the SSGXVIII/AIO study consisted of 397 patients who signed informed consent. The current study was carried out in the Efficacy Population, which was formed when 3 patients who did not provide informed consent were excluded, as well as 15 patients who did not have GIST at central pathology review, and 24 patients who had 1 or more metastases removed in addition to the primary tumor at surgery. Of the 358 patients in the Efficacy Population, 181 received adjuvant imatinib for 12 months and 177 received it for 36 months. During a median follow-up time of 54 months, 72 and 42 patients had GIST recurrence in the 12-month and the 36-month groups, respectively.9
The factors defining the subgroups examined were predefined in the Statistical Analysis Plan of the SSGXVIII/AIO study,9 except for the subgroups based on the body mass index and the time from the date of surgery to the date of randomization, which were included as exploratory variables of potential interest. Frequency tables were analyzed using the chi-square test. Continuous distributions between groups were compared using Wilcoxon’s signed-rank test or the Mann-Whitney test, and mitotic counts determined locally and centrally with the Spearman rank correlation coefficient (R).
Survival between groups was compared using the Kaplan-Meier life-table method and unstratified Cox proportional hazards model (hazard ratios [HR] and P values). Patients who were alive without recurrence were censored on the date of last follow-up. Independence of prognostic factors was assessed using a stepwise Cox proportional hazards model. The Cox model was used to test the interactions between the duration of treatment and potential predictive factors by including each factor, one at a time, to the model together with treatment duration and the interaction term.
The concordance index for the model discriminative accuracy was calculated according to Harrell et al.13 Sub-population treatment effect pattern plot (STEPP) analyses were applied using the sliding window approach to investigate the correlation between RFS and the risk score.14 Sigmoidal nonlinear regression models were fitted to the STEPP curves to further illustrate the link between the RFS and the risk score. P values are 2-sided and not adjusted for multiple testing. Statistical analyses were performed with SAS version 9.2 for Windows (SAS Institute Inc., Cary, NC).
RESULTS
The 12 factors examined were balanced between the allocation groups (Table 1). The median tumor size was 10.0 cm, and did not differ between gastric and nongastric GISTs (9.5 cm and 10.0 cm, respectively; P=.716). The number of mitoses per 50 high-power fields of the microscope (HPFs) as determined locally or centrally showed strong correlation (R = 0.42, P<.001), but the median number was higher at local tumor histopathological assessment as compared with central review (9; interquartile range [IQR] of 5-20; versus 6, IQR of 2-17; respectively, P<.001). There was no difference in the mitotic counts between gastric and nongastric GISTs assessed either locally or centrally (P>.500 for each comparison).
TABLE 1.
Characteristics of Patients and Tumors in the SSGXVIII/AIO Series
| Factor | Enrolled to 12 mo of Imatinib (n = 181) No. (%)a |
Enrolled to 36 mo of Imatinib (n = 177) No. (%) |
|---|---|---|
| Sex | ||
| Women | 85 (47) | 89 (50) |
| Men | 96 (53) | 88 (50) |
| Age, y | ||
| ≤61 (median) | 89 (49) | 98 (55) |
| >61 | 92 (51) | 79 (45) |
| Body mass index, kg/m2 | ||
| ≤24.6 (median) | 91 (52) | 83 (49) |
| >24.6 | 85 (48) | 87 (51) |
| Not available | 5 | 7 |
| ECOG performance status | ||
| 0 | 155 (86) | 151 (85) |
| 1 or 2 | 24 (14) | 25 (15) |
| Not available | 2 | 1 |
| Completeness of surgery | ||
| Complete resection (R0) | 153 (85) | 146 (83) |
| Microscopic residual suspected (R1) |
27 (15) | 30 (17) |
| Not available | 1 | 1 |
| Time from surgery to randomization, days | ||
| ≤56 (median) | 85 (47) | 97 (55) |
| >56 | 95 (53) | 79 (45) |
| Not available | 1 | 1 |
| Tumor diameter, cm | ||
| ≤5.0 | 24 (13) | 16 (9) |
| 5.1-10.0 | 84 (47) | 73 (41) |
| 10.1-15.0 | 44 (24) | 60 (34) |
| >15.0 | 28 (16) | 27 (15) |
| Not available | 1 | 1 |
| Tumor mitotic count per 50 HPFs, local assessment | ||
| ≤5 | 51 (30) | 48 (30) |
| 6-10 | 45 (27) | 50 (31) |
| 11-15 | 21 (12) | 14(9) |
| 16-20 | 8(5) | 13(8) |
| 21-50 | 23 (14) | 26 (16) |
| >50 | 21 (12) | 11 (7) |
| Not available | 12 | 15 |
| Tumor mitotic count per 50 HPFs, central assessment | ||
| ≤5 | 80 (46) | 85 (51) |
| 6-10 | 27 (15) | 25 (15) |
| 11-15 | 19 (11) | 15 (9) |
| 16-20 | 8 (5) | 11 (7) |
| 21-50 | 29 (17) | 26 (16) |
| >50 | 12 (7) | 4 (2) |
| Not available | 6 | 11 |
| Tumor site | ||
| Stomach | 91 (51) | 100 (57) |
| Small intestine | 68 (38) | 55 (31) |
| Colon or rectum | 11 (6) | 15 (9) |
| Other | 10 (6) | 6 (3) |
| Not available | 1 | 1 |
| Tumor rupture prior to or at surgery | ||
| No | 149 (82) | 136 (77) |
| Yes | 32 (18) | 41 (23) |
| Rupture prior to surgery | ||
| No | 160 (89) | 153 (87) |
| Yes | 20 (11) | 23 (13) |
| Not available | 1 | 1 |
| Rupture at surgery | ||
| No | 163 (91) | 152 (86) |
| Yes | 17 (9) | 24 (14) |
| Not available | 1 | 1 |
| Tumor mutation type, central assessment | ||
| KIT exon 11 | 122 (70) | 119 (72) |
| KIT exon 9 | 12 (7) | 14 (8) |
| PDGFRA exon 18b | 20 (11) | 18 (11) |
| Other mutation | 5 (3) | 4 (2) |
| Wild type for KIT | 15 (9) | 10 (6) |
| and PDGFRA | ||
| Not available | 7 | 12 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; HPF, high-power field of the microscope; PDGFRA, platelet-derived growth factor receptor alpha.
The percentages may not sum up to 100 due to rounding.
A total of 16 (80%) of the 20 PDGFRA exon 18 mutations in the 12-month group and 13 (72%) of the 18 PDGFRA exon 18 mutations in the 36-month group were D842V substitution mutations.
Univariable Survival Analyses
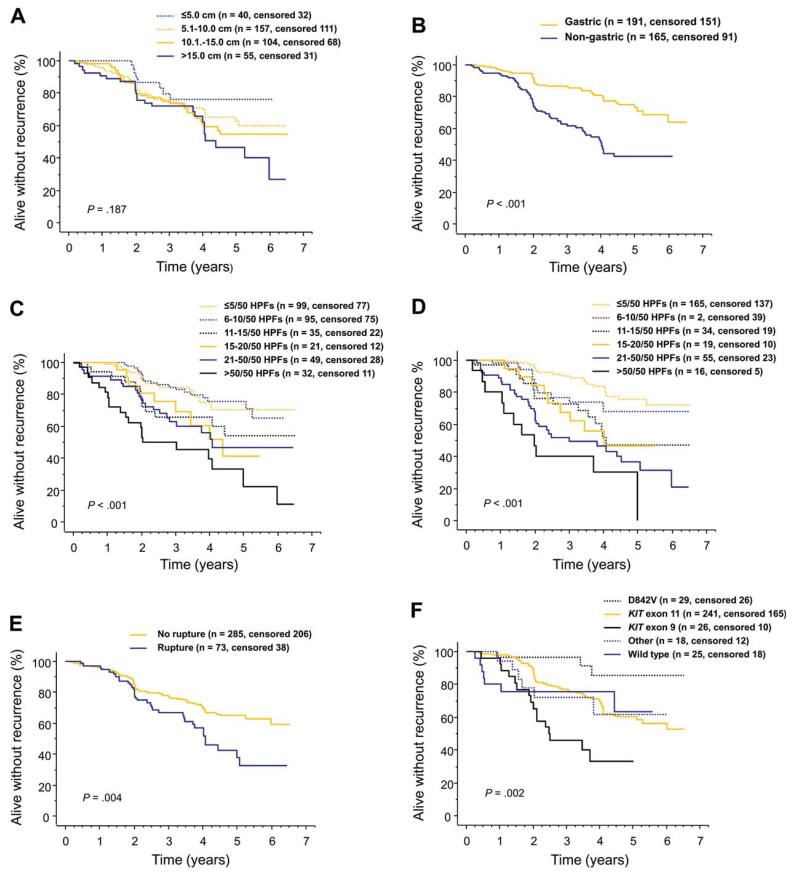
A low tumor mitotic count, gastric location, and patient allocation to the 3-year adjuvant imatinib arm were strongly associated with favorable RFS in univariable survival analyses (each P<.001; Fig. 1; Table 2). Tumor rupture, KIT exon 9 mutation, and a large body mass index were also associated with unfavorable RFS, whereas tumor PDGFRA mutation D842V was associated with favorable RFS (Table 2).
Figure 1.
Graphs show influence of gastrointestinal stromal tumor (GIST) (A) diameter, (B) site, (C) mitotic count assessed locally, (D) mitotic count assessed centrally, (E) rupture prior to or at surgery, and (F) mutation type on recurrence-free survival after surgery for GIST in patients treated with adjuvant imatinib in the SSGXVIII/AIO trial.
TABLE 2.
Univariable Survival Analyses in the SSGXVIII/AIO Series
| Factor | No.a | 5-y RFS | Hazard Ratio (95% CI) | P b |
|---|---|---|---|---|
| Sex | .562 | |||
| Women | 174 | 61.0 | 0.90 (0.62-1.30) | |
| Men | 184 | 57.5 | Referent | |
| Age, y | .090 | |||
| ≤61 (median) | 187 | 62.5 | 0.73 (0.50-1.05) | |
| >61 | 171 | 55.3 | Referent | |
| Body mass index, kg/m2 | .016 | |||
| ≤24.6 (median) | 174 | 67.5 | 0.62 (0.43-0.92) | |
| >24.6 | 172 | 51.2 | Referent | |
| ECOG performance status | .634 | |||
| 0 | 306 | 60.3 | 0.88 (0.52-1.49) | |
| 1 or 2 | 49 | 52.5 | Referent | |
| Completeness of surgery | .101 | |||
| R0 | 299 | 61.2 | 0.69 (0.45-1.07) | |
| R1 | 57 | 48.9 | Referent | |
| Time from surgery to randomization, days | .169 | |||
| ≤56 (median) | 182 | 61.8 | 0.77 (0.53-1.12) | |
| >56 | 174 | 56.2 | Referent | |
| Tumor diameter, cm | .187 | |||
| ≤5.0 | 40 | 76.0 | 0.46 (0.20-1.01) | .054 |
| 5.1-10.0 | 157 | 62.9 | 0.67 (0.41-1.10) | .114 |
| 10.1-15.0 | 104 | 54.9 | 0.78 (0.46-1.30) | .339 |
| >15.0 | 55 | 46.5 | Referent | |
| Mitotic count per 50 HPFs, local assessment | < .001 | |||
| ≤5 | 99 | 70.3 | 0.24 (0.13-0.43) | < .001 |
| 6-10 | 95 | 75.8 | 0.23 (0.12-0.42) | < .001 |
| 11-15 | 35 | 53.9 | 0.46 (0.23-0.92) | .028 |
| 16-20 | 21 | 41.6 | 0.53 (0.24-1.16) | .112 |
| 21-50 | 49 | 46.9 | 0.55 (0.30-1.00) | .051 |
| >50 | 32 | 22.1 | Referent | |
| Mitotic count per 50 HPFs, central review | < .001 | |||
| ≤5 | 165 | 75.7 | 0.13 (0.06-0.26) | < .001 |
| 6-10 | 52 | 68.2 | 0.23 (0.10-0.52) | < .001 |
| 11-15 | 34 | 46.9 | 0.39 (0.18-0.85) | .018 |
| 16-20 | 19 | 46.5 | 0.40 (0.17-0.97) | .043 |
| 21-50 | 55 | 36.6 | 0.60 (0.30-1.20) | .147 |
| >50 | 16 | 0.0 | Referent | |
| Tumor site | < .001 | |||
| Gastric | 191 | 73.1 | 0.36 (0.25-0.53) | |
| Nongastric | 165 | 42.3 | Referent | |
| Tumor rupture prior to or at surgery | .004 | |||
| No | 285 | 64.9 | 0.56 (0.37-0.83) | |
| Yes | 73 | 37.7 | Referent | |
| Rupture prior to surgery | .031 | |||
| No | 313 | 62.6 | 0.59 (0.37-0.95) | |
| Yes | 43 | 33.9 | Referent | |
| Rupture at surgery | .016 | |||
| No | 315 | 61.8 | 0.56 (0.35-0.89) | |
| Yes | 41 | 41.9 | Referent | |
| Tumor mutation type | .002 | |||
| KIT exon 11 | 241 | 59.6 | Referent | |
| KIT exon 9 | 26 | 33.4 | 2.54 (1.48-4.37) | < .001 |
| PDGFRA exon 18, D842V | 29 | 85.4 | 0.27 (0.09-0.87) | .028 |
| Other mutation | 18 | 61.9 | 1.10 (0.48-2.53) | .820 |
| Wild type for KIT and PDGFRA | 25 | 63.2 | 1.03 (0.47-2.23) | .944 |
| Treatment assignment | < .001 | |||
| 36 mo of adjuvant imatinib | 177 | 67.4 | 0.46 (0.31-0.68) | |
| 12 mo of adjuvant imatinib | 181 | 50.3 | Referent |
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HPF, high-power field of the microscope; PDGFRA, platelet-derived growth factor receptor alpha.
Data were not available for the body mass index, the ECOG performance status, completeness of surgery, the time from surgery to randomization, tumor diameter, local mitotic count, central mitotic count, tumor site, rupture prior to surgery, rupture at surgery, and mutation type in 12, 3, 2, 2, 2, 27, 17, 2, 2, 2, and 19 cases, respectively
P values and the hazard ratios were calculated using a Cox model. When a variable has >2 categories, both the overall P and the P value compared with a selected referent category are provided.
Multivariable Survival Analyses
In a stepwise Cox multivariable analysis a low tumor mitotic count (with central assessment), location in the stomach, and adjuvant imatinib for 36 months were the most important independent factors associated with favorable RFS (P<.001 for each factor). Small tumor size and absence of rupture (either prior to or at surgery) were also independently associated with favorable RFS (P=.004 and .010, respectively). Tumor mutation category, age at randomization, and time from surgery to randomization tended to be associated with RFS (P=.054, .061, and .087, respectively), whereas sex, Eastern Cooperative Oncology Group (ECOG) performance status, the body mass index, completeness of surgery (R0 versus R1), country, or the study site did not have independent prognostic value. No interaction was found between any of the studied factors and the duration of treatment.
Recurrence Risk Score Consisting of 5 Independent Factors
A risk score for GIST recurrence was next generated using the regression coefficients of the 5 independent factors identified in the Cox multivariable analysis as follows:
Recurrence risk score = 0.05316 × tumor mitotic count per 50 HPFs + 0.00000 (if gastric GIST) + 1.17607 (if nongastric GIST) + 0.00000 (adjuvant imatinib for 3 years) + 0.89619 (adjuvant imatinib for 1 year) + 0.00000 (if no tumor rupture) + 0.68533 (if tumor rupture) + 0.04460 × tumor size (cm).
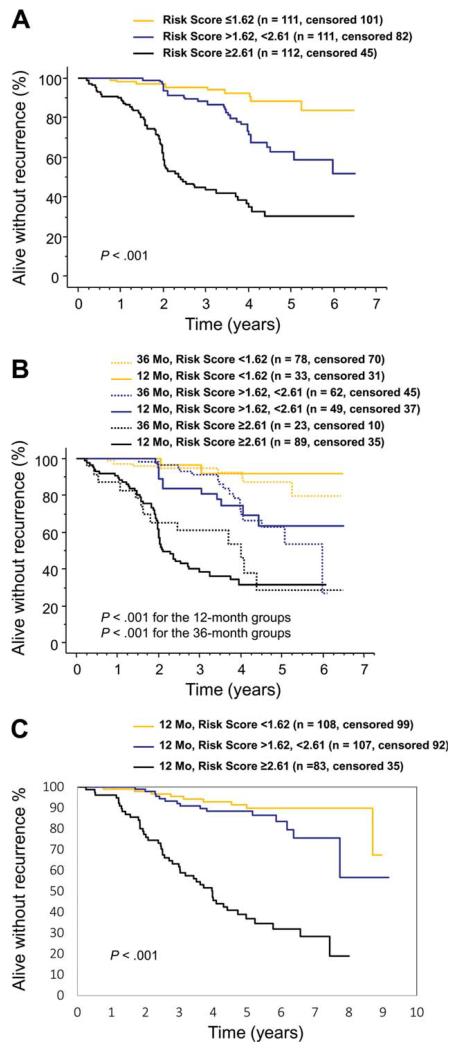
The few cases with a very high mitotic count (> 40 per 50 HPFs) were entered as 40 per 50 HPFs when designing the score. The score was strongly associated with the risk of GIST recurrence (shown for score tertiles in Fig. 2A). When the concordance index was computed, the discriminative accuracy of the score was 78.9%. The score predicted RFS well when stratified by the duration of adjuvant imatinib administered (Fig. 2B). Of note, a substantial proportion of the patients who had the score within the highest tertile (≥ 2.61) had GIST recurrence within the first 3 years from randomization despite adjuvant imatinib. The score was strongly associated with RFS in the Z9001 validation cohort (P< .001, Fig. 2C).
Figure 2.
A 5-factor risk score estimates the risk of gastrointestinal stromal tumor (GIST) recurrence during and after adjuvant imatinib. (A) Kaplan-Meier plots show the influence of the score on recurrence-free survival in the SSGXVIII/AIO trial. (B) Influence of the score on recurrence-free survival stratified by the duration of adjuvant imatinib administered in the SSGXVIII/AIO trial. (C) Kaplan-Meier plots show the influ-ence of the score on recurrence-free survival in the ACOSOG Z9001 trial.
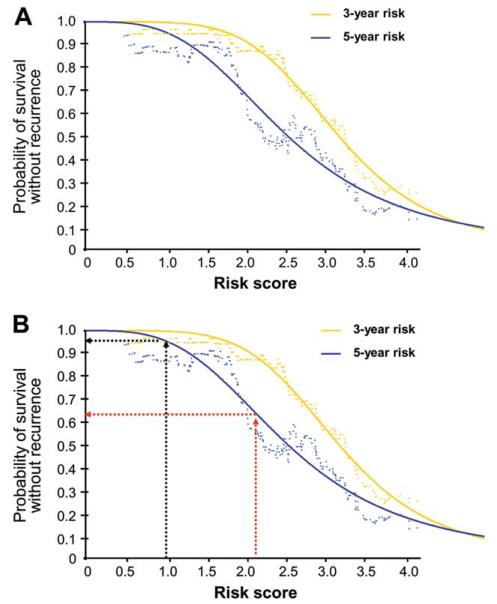
Plots estimating the risk of GIST recurrence at 3 or 5 years after initiation of adjuvant imatinib were generated with a STEPP curve showing the RFS as a function of the risk score and by fitting a nonlinear model to the STEPP curve (Fig. 3). For example, for a patient with nonruptured gastric GIST 10 cm in diameter and with 10 mitoses per 50 HPFs who received adjuvant imatinib for 3 years, the risk score is 0.98 (0.05316 × 10 [10 mitoses] + 0.00000 [gastric GIST] + 0.00000 [imatinib for 3 years] + 0.00000 [no rupture] + 0.044460 × 10 [size 10 cm]) corresponding to 96% probability of surviving for 5 years free from GIST recurrence, whereas for an otherwise similar tumor that is nongastric, the score is 2.15 and the 5-year RFS probability is 63%.
Figure 3.
Plots of the estimated the risk of gastrointestinal stromal tumor (GIST) recurrence after initiation of adjuvant imatinib as a function of the 5-factor risk score. (A) Probability of 3-year and 5-year recurrence-free survival. (B) Examples of calculating the 5-year probability of survival without GIST recurrence with the score value of 0.98 (black arrows) or 2.15 (red arrows).
Recurrence Score Consisting of Tumor Mitotic Count and Site
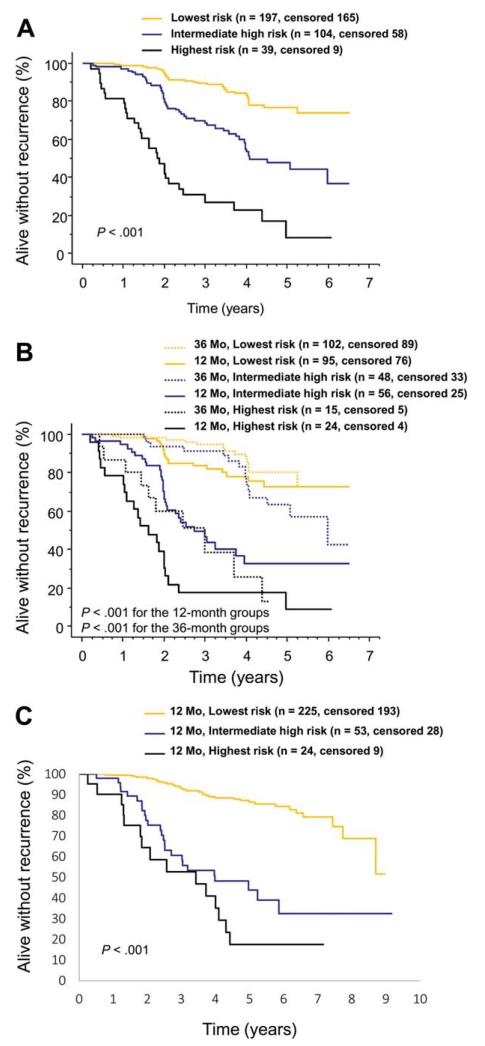
To generate a predictive score that does not require computations for risk estimation and does not include the planned duration of adjuvant imatinib as a parameter, we selected the 2 factors that were most strongly associated with RFS in the multivariable analysis for risk estimation (tumor mitotic count assessed centrally and tumor site). In this categorized 2-factor scheme, gastric GISTs with-≤10 mitoses and nongastric GISTs with ≤5 mitoses per 50 HPFs formed the lowest recurrence risk group, which consisted of 197 (57.9%) of the 340 tumors with data available on both factors. The group with intermediate high recurrence risk group consisted of gastric GISTs with 11 to 50 mitoses and of nongastric GISTs with 6 to 20 mitoses (n = 104 [30.6%]), and the highest risk group of gastric GISTs with > 50 mitoses and of nongastric GISTs with > 20 mitoses (n = 39 [11.5%]). These groups with the lowest, intermediate high, or the highest risk of recurrence were associated with 5-year RFS of 76.7% (95% confidence interval [CI] = 67.7%-83.5%), 47.5% (35.5%-58.5%), and 8.4% (0.8%-27.8%), respectively (P<.001; Fig. 4).
Figure 4.
A 2-factor score for estimating the risk of gastrointestinal stromal tumor (GIST) recurrence during and after adjuvant imatinib based on tumor mitotic count and site. (A) Kaplan-Meier plots show the influence of the score on recurrence-free survival (lowest risk, gastric GIST with ≤10 mitoses per 50 high-power fields of the microscope or nongastric GIST with ≤5 mitoses; intermediate high risk, gastric GIST with 11 to 50 mitoses or nongastric GIST with 6 to 20 mitoses; highest risk, gastric GIST with > 50 mitoses or nongastric GIST with > 20 mitoses per 50 high-power fields). (B) Influence of the predictive groups on recurrence-free survival stratified by the duration of adjuvant imatinib administered. (C) Kaplan-Meier plots show the influence of the score on recurrence-free survival in the ACOSOG Z9001 cohort.
When the 3 risk groups were stratified by the duration of adjuvant imatinib treatment given, patients who were allocated to 3 years of adjuvant imatinib had fewer RFS events in each group, but this reached statistical significance only in the intermediate high-risk group (hazard ratio [HR] = 0.30, 95% CI = 0.16-0.56, P<.001; lowest risk group HR = 0.57, 95% CI = 0.28-1.15, P=.114; highest risk group HR = 0.58, 95% CI = 0.27-1.25, P=.163). As many as 30 (76.9%) of the 39 patients with the highest risk had GIST recurrence during the follow-up, and most of such patients assigned to 3 years of adjuvant imatinib had recurrence while on imatinib (Fig. 4). The results remained largely similar when the locally assessed mitotic counts were used in place of the central counts, and when those patients who discontinued imatinib for adverse effects (n = 40) or patient wish (n = 10) were excluded from the analysis. This 2-factor risk score was strongly associated with RFS in the validation series (P<.001).
DISCUSSION
We generated 2 risk estimation scores for patients with GIST who were treated with adjuvant imatinib, one based on the 5 independent factors identified in a multivariable model and another simpler scheme based on tumor mitotic count and site only. The latter scheme is independent of the planned duration of adjuvant imatinib to be administered. To our knowledge, these are the first predictive schemes developed for this patient population, and may help in patient counseling, planning of follow-up, and selection of treatments.
GIST patients with the highest risk scores had a very high risk of GIST recurrence despite adjuvant imatinib, and recurrences were frequent both when the patients were on adjuvant imatinib and after its completion. This observation highlights a need for careful monitoring of patients with GIST with a high mitotic count and nongastric site of origin, and perhaps also those with KIT exon 9 mutation. Frequent CT or MRI examinations of the abdomen during adjuvant therapy and the follow-up there-after are likely beneficial for this subset of patients, and, obviously, more effective adjuvant therapy needs to be developed.
The most important single factors for recurrence were high tumor mitotic count, nongastric site of origin, and short duration of adjuvant imatinib treatment, but large tumor size and tumor rupture were also independently associated with RFS. Of note, the GIST mutation status and tumor rupture were not as strongly linked with unfavorable outcome as the mitotic count and site. Little data are available from other series about the factors associated with GIST recurrence during and after adjuvant imatinib, but in 1 nonrandomized series consisting of 106 patients who were treated with adjuvant imatinib at 400 mg daily for 12 months, GIST size, small bowel site, KIT exon 9 mutation, high mitotic rate, and older age were associated with poor RFS in a multivariable analysis.15
The reliability of mitotic counting is controversial. Potential limitations include different criteria for mitosis identification between pathologists, variance in the size of the microscope field-of-view at counting, and the influence of tissue fixation on the count.16,17 Several other methods have been evaluated for assessing GIST cell proliferation rate, such as immunostaining for the Ki-67 antigen18 or the mitotic checkpoint proteins,19 but none of these has replaced mitotic counting. Although the median mitotic count differed between the local and central pathologists, the local and central counts correlated strongly, and both were associated with RFS. Despite its limited reproducibility, the mitotic count may be the most important single prognostic factor in GIST.2
The study has some limitations. We were unable to study overall survival as the endpoint due to the small number of deaths encountered, and some of the sub-groups were relatively small in size. Because high-risk GIST was a study entry criterion in the SSGXVIII/AIO trial, the risk factors related to intermediate-risk GIST cannot be adequately addressed in this series, but the prognostic scores generated were strongly predictive also in the Z9001 trial series that also includes patients with lower risk GIST.7
We conclude that the scores generated were effective in stratifying of the risk of GIST recurrence in patient populations treated with adjuvant imatinib. GISTs with high mitotic count arising at nongastric sites recur frequently despite adjuvant imatinib, and some of such tumors recur when the patient is on imatinib suggesting that more efficient treatments need to be pursued. Such adjuvant strategies might include a higher than the standard 400 mg dose of imatinib, novel agents, or agents administered in combinations or in a sequence. Many GISTs recur soon after discontinuation of adjuvant imatinib, suggesting that treatment durations exceeding 3 years warrant evaluation.
Acknowledgments
FUNDING SOURCES
Supported by the Academy of Finland (grants 218068 and 131449), Helsinki University Research Funds (TYH7206), Cancer Society of Finland, and Sigrid Juselius Foundation.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The clinical research institute of Dr. Joensuu has received research funding from Novartis. Drs. Hohenberger, Al-Batran, and Bauer have received research support from Novartis; Drs. Ramadori, Hohenberger, Bono, and Reichardt have received honoraria from Novartis; Drs. Bauer and Reichardt have received honoraria from Pfizer and Bayer; Dr. Ramadori has received travel grants from Novartis; Drs. Hohenberger, Schlemmer, Bono, and Reichardt have served in advisory boards of Novartis; Dr. DeMatteo has been as a consultant to Novartis and Dr. Bauer for Pfizer; Dr. Reichardt has been on advisory boards of Pfizer and Bayer. All other authors declare no conflict of interest.
REFERENCES
- 1.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–878. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 2.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382:973–983. doi: 10.1016/S0140-6736(13)60106-3. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, Ohashi A, Nishida T, et al. Gain-of-function mutations of platelet-derived growth factor receptor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 5.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii49–vii55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Center [Accessed November 9, 2013];Clinical practice guidelines in oncology: Soft tissue sarcoma. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 7.DeMatteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–1104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casali P. Imatinib failure-free survival in patients with localized gasrointestinal stromal tumors (GIST) treated with adjuvant imatinib: The EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol. 2013;31(suppl):632s. [Google Scholar]
- 9.Joensuu H, Eriksson M, Sundby Hall K, et al. One versus three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 10.DeMatteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST) Cancer. 2008;112:608–615. doi: 10.1002/cncr.23199. [DOI] [PubMed] [Google Scholar]
- 11.Joensuu H, Vehtari A, Riihimäki J, et al. Risk of gastrointestinal stromal tumour recurrence after surgery: an analysis based on pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 14.Bonetti M, Gelber RD. Patterns of treatment effects in subsets of patients in clinical trials. Biostatistics. 2004;5:465–481. doi: 10.1093/biostatistics/5.3.465. [DOI] [PubMed] [Google Scholar]
- 15.DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) Intergroup phase 2 trial. Ann Surg. 2013;258:422–429. doi: 10.1097/SLA.0b013e3182a15eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gal R, Rath-Wolfson L, Rosenblatt Y, et al. An improved technique for mitosis counting. Int J Surg Pathol. 2005;13:161–165. doi: 10.1177/106689690501300206. [DOI] [PubMed] [Google Scholar]
- 17.Cross SS, Start RD, Smith JH. Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol. 1990;43:597–599. doi: 10.1136/jcp.43.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang HY, Huang WW, Lin CN, et al. Immunohistochemical expression of p16INK4A, Ki-67, and MDm2 proteins in gastrointestinal stromal tumors: prognostic implications and correlations with risk stratification of NIH consensus criteria. Ann Surg Oncol. 2006;13:1633–1644. doi: 10.1245/s10434-006-9188-4. [DOI] [PubMed] [Google Scholar]
- 19.Fujita A, Yamamoto H, Imamura M, et al. Expression level of the mitotic checkpoint protein and G2-M cell cycle regulators and prognosis in gastrointestinal stromal tumors in the stomach. Virchows Arch. 2012;460:163–169. doi: 10.1007/s00428-011-1181-z. [DOI] [PubMed] [Google Scholar]