Abstract
Angiopoietins were thought to be endothelial cell-specific via the tie2 receptor. We showed that angiopoietin-1 (ang1) also interacts with integrins on cardiac myocytes (CM) to increase survival. Since ang1 monomers bind/activate integrins (not tie2), we determined their function in vivo. We examined monomer/multimer expressions during physiologic and pathologic cardiac remodeling and overexpressed ang1 monomers in phenylephrine-induced cardiac hypertrophy. Cardiac ang1 levels (mRNA, protein) increased during post-natal development and decreased with phenylephrine-induced cardiac hypertrophy, while tie2 phosphorylations were unchanged. We found that most or all of the changes during cardiac remodeling were in monomers, offering an explanation for unchanged tie2 activity. Heart contains abundant ang1 monomers and little multimers (Western blotting). We generated plasmids which produce ang1 monomers (ang1-256), injected them into mice, and confirmed cardiac expression (immunohistochemistry, RT-PCR). Ang1 monomers localize to CM, smooth muscle cells and endothelial cells. In phenylephrine-induced cardiac hypertrophy, ang1-256 reduced LV/tibia ratios, fetal gene expressions (atrial/brain natriuretic peptides, skeletal actin, β-myosin heavy chain), and fibrosis (collagen III), and increased LV pro-survival signaling (akt, MAPKp42/44, AMPKT172), while tie2 phosphorylations were unchanged. Ang1-256 increased integrin-linked kinase, a key regulator of integrin signaling and cardiac health. Collectively these results suggest a role for ang1 monomers in cardiac remodeling.
Keywords: Cardiac Myocytes, Endothelial Cells, Integrin-linked kinase, AMP-activated protein kinase, Tie2
Introduction
Aging societies, increasingly prevalent risk factors such as obesity and diabetes, and improved survival from acute coronary syndromes have produced a growing population with reduced cardiac function. This triggers a process of cardiac remodeling whereby changes in heart composition and structure progress to counterbalance stress and salvage function. While initially stabilizing, the changes can not offset the impairment. The evolving cardiac phenotype becomes maladaptive and decompensates, manifesting as heart failure. Once cardiac reserve is exhausted, mortality rates exceed 50%. With heart failure the fastest growing form of cardiovascular disease in developed countries, the need for new advances is pressing. As a final common pathway, cardiac remodeling has emerged as a primary therapeutic target for heart failure of all causes.
Ang1 is vital to cardiovascular development with a burgeoning role in heart disease. It is widely reported as an endothelial cell (EC)-specific regulator of vessel maturation since its receptor, tie2, is almost exclusive to EC(1, 2) However, our studies(3) and others(4) are revising this view. We showed that ang1 acts on cardiac and skeletal myocytes as a potent survival factor via select integrins, resulting in enhanced adhesion and activation of pro-survival signaling. Thus, ang1 is well-positioned at the vessel/tissue interface to mediate cardiac remodeling and preserve function.
Establishing that ang1/cardiac myocyte (CM) interactions promote myocyte survival invites re-interpretation of in vivo studies showing ang1 is cardioprotective to include broader mechanisms-of-action. Ang1 knockout mice have embryonic lethal cardiac defects (impaired endocardial development, trabeculae formation, vessel maturation)(5). In myocardial infarction (MI) models, ang1 overexpression reduced infarct zones and preserved ejection fractions(6). These benefits were attributed solely to the role of ang1 in angiogenesis causing increased capillary density(6–8). However, our data suggests that direct actions of ang1 on CM and SMC contribute to the protective effects.
Ang1 protein structure is complex with quaternary multimeric forms unique among growth factors. From amino to carboxy terminus, the domains are: superclustering(9); coiled-coil (oligomerization)(10); linker (binds ang1 to matrix)(11); and fibrinogen-like (binds tie2)(9, 12, 13). Coiled-coil domains join monomers into disulfide-linked ang1 homo-oligomers that multimerize about superclustering domains(9, 10).
Ang1’s quaternary protein structure is a determinant of its receptor targeting. Ang1 tetramers and pentamers bind and activate tie2(9). Ang1 trimers bind soluble tie2-Fc without phosphorylating tie2 (10, 12). Ang1 dimers weakly bind soluble tie2-Fc (63 fold < tetramer)(9) and do not phosphorylate EC tie2 (12). Ang1 monomers show little (290 fold < tetramer)(9) to no(12) binding to soluble tie2-Fc and do not activate EC tie2, but bind integrin α5β1 (13).
The findings that ang1 monomers bind integrins(13) whereas tie2 activation requires at least tetramers(9, 12) suggested to us that ang1 forms may be differentially regulated in vivo during remodeling and among organs. Here, we show that ang1 monomers are in adult tissues, shift during cardiac remodeling, and localize to cardiac myocytes, SMC, and EC in vivo. Recombinant ang1 monomers reduced murine cardiac hypertrophy, activated pro-survival signaling and AMPKT172 (master regulator of cardiac energetics/ metabolism), and integrin-linked kinase (ILK) (central mediator of CM contractility/cell survival)(14). Thus, we propose that differential expression of ang1 quaternary forms is a unique regulatory mechanism for directing receptor/cell interactions in vivo and that ang1 monomer/integrin interactions have a cardioprotective role in remodeling.
Methods
Animals
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US NIH. C57BL/6J (C57) mice (Jackson Laboratory, Bar Harbor, ME, USA) were given free access to water and standard chow. Where indicated, seven-week-old male mice received phenylephrine hydrochloride (PE) (Sigma, St. Louis, MO, USA) in saline (75 mg/kg/d) or saline (control) via subcutaneous mini-osmotic pumps (Alzet, Cupertino, CA, USA). CM’s were isolated from Sprague-Dawley rats (Charles River Laboratory, Wilmington, MA, USA).
Recombinant Ang1 Constructs
Ang1 was cloned from C57 mouse heart as we described(15). Using pcDNA3.1/V5-His TOPO/TA (Invitrogen, Carlsbad, CA, USA) vector, we cloned in N-terminal hemagluttinin secretion signal (MKTIIALSYIFCLVFA) and FLAG tag (DYKDDDDK) fused to mouse ang1 from amino acid 256–498 (ang1-256/pcDNA). The vector contains C-terminal V5 and 6X polyhistidine tags. Plasmids were double-purified with Endotoxin-free plasmid DNA purification kits (Qiagen, Valencia, CA, USA) and sequenced. Mice were retroorbitally injected with ang1-256/pcDNA or pcDNA (40 µg) using InVivo Jet-polyethylenimine (PEI) transfection agent plus glucose (ISC Bioexpress, Kaysvilla, UT, USA).
Cell Culture/Isolations
C2C12 myoblast cell lines were maintained as we described(3). Myoblasts were differentiated into myocytes by confluency or by 3% horse serum / DMEM(16). Rat neonatal CM (RNCM) and rat neonatal cardiac fibroblasts (RNCF) were isolated from ventricles of 2d-old pups. RNCM were cultured as we described(3) except for pre-platings (2x, 1 h), cytosine-β-D-arabinofuranoside (cβDa) (10 µg/ml) added to media and 1% gelatin coating on plates. RNCF were isolated using published methods(17). Media was changed to low glucose (LG)-DMEM/10% fetal bovine serum (FBS)/1% penicillin-streptomycin- glutamine (PSG). Cells were grown to confluency and passaged to eliminate residual non-fibroblasts. Rat adult cardiac myocytes (RACM) were isolated from 8 week-old rat ventricles using published procedures(18). Hearts were sterilely removed, hung by the aorta (23 gauge blunt needle) and secured (4.0 gauge suture). Hearts were retrograde perfused with Ca2+-free Krebs-Henseleit buffer (KHB) / 11 mM glucose / 25 mM NaHCO3 at pH 7.4 until clear and perfusion digested (20 min., 37°C) with Enzyme I solution (0.33 mg/ml type II collagenase (Worthington, Lakewood, NJ, USA) / 0.3 mg/ml type II hyaluronidase / Ca2+-free KHB / 11 mM glucose / 25 mM NaHCO3). Ventricles were minced and further digested with Enzyme I / 0.02 mg/ml trypsin type IX / 0.02 mg/ml DNAse (Worthington) (20 min., 37°C with shaking). Tissues were filtered (80 µm nylon mesh) into 1:1 Ca2+-free KHB / LG-DMEM, centrifuged, supernatant removed and cell pellet resuspended in 1:1 Ca2+-free KHB / LG-DMEM. Cells were placed in Ca2+-free KHB / 6.5% bovine serum albumin (BSA) (Fisher Scientific, Morris Plains, NJ, USA) until myocytes settled. Supernatant was removed and myocytes plated on 10 µg/ml laminin-coated plates in Medium199 / 5 mM creatine / 2 mM carnitine / 5 mM taurine / 10 µM cβDa / 4% FBS / 1% PSG (37°C, 5% CO2, 1 d).
PCR
Real-time mouse [ang1, ang2, tie1, tie2, atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), β-myosin heavy chain (βMHC), skeletal actin, smooth muscle actin (SMA), collagen III, GAPDH](19) and RT-PCR mouse [ang1, ang2, tie2, PECAM, VEGF(20), VEGFR1, VEGFR2], rat [ang1 (forward mouse ang1 (15), reverse 5’ (21))], and GAPDH primers (Integrated DNA Technology, Coralville, IA, USA) were used for PCR as we described(15). Ang1-256 mRNA was amplified with forward 5’-TACAAAGACGATGACGACAAGGACACA-3’ and reverse 5’-AGGGTTAGGGATAGGCTTACCTTCGAA-3’ primers. GAPDH mRNA levels were stable among groups and used for normalization.
Western Blotting / Cell Signaling
Protein lysates were made as we described(3) except 50 mM NaF was added. Some lysates were PNGaseF-treated (Sigma) following manufacturer’s instructions to assess for N-linked glycosylation. SDS-PAGE analyses were conducted under nonreduced and reduced conditions. Tissue (100 µg) and recombinant human ang1 (R&D Systems, Minneapolis, MN, USA) (rhAng1/R&D) lysates received sample loading buffer (Biorad, Hercules, CA, USA). Some lysates received reducing agent [(Biorad) or 0.435 M βMe] and were heated (5 min, 95°C). Samples were loaded on 3–8% Tris-Acetate Criterion gels (Biorad), separated by SDS-PAGE and transferred to nitrocellulose (Perkin Elmer, Boston, MA, USA). Membranes were probed for ang1 with antibodies: [Rockland, Gilbertsville, PA (100-401-403), R&D Systems (MAB050 or AF923), Sigma (A0604), Santa Cruz, Santa Cruz, CA, USA (sc6319), Abcam, Cambridge, MA, USA (ab8451), Chemicon/Millipore, Tennecula, CA, USA (AB3120)]. Blots were blocked (30 min.), incubated in primary antibody in block (1 h), rinsed 3x in 10 mM Tris-base / 150 mM NaCl / 0.1% Tween20 (TBST), incubated in appropriate HRP-conjugated secondary antibody (anti-mouse, antirabbit IgG, 1:1500) in block (1 h), rinsed (3x, TBST), and exposed to chemiluminescence using ECL kits (Amersham, Piscataway, NJ, USA or Perkin Elmer). Blots were reprobed for GAPDH (Santa Cruz) as we described(3). Blots were probed for phosphorylation of aktS473, MAPKp42(T202)/44(T204), AMP-activated protein kinase (AMPK)T172, AMPKS485/S491, ILK, (Cell Signaling, Danvers, MA, USA), and FAKT397 (BD Pharmingen, Billerica, MA, USA), stripped with Restore Stripping Buffer (Pierce Biotechnology, Rockford, IL, USA), and reprobed for total akt, AMPK α1/α2 (Cell Signaling), MAPKp42/44 (Chemicon/Millipore), FAK (BD Pharmingen), or GAPDH (Santa Cruz) as we described(3).
Tie2 immunoprecipitation
Protein lysates (1000 µg) from mouse LV were incubated (overnight, 4°C) with 7 µg mouse anti-tie2 monoclonal clone AB33 (Upstate, Waltham, MA, USA), protein G PLUS agarose beads (Santa Cruz) added (4 h, 4°C), pellets rinsed (modified RIPA buffer, 3x) and sample loading buffer / Biorad reducing agent added. Membranes were probed with mouse anti-phosphotyrosine monoclonal recombinant 4G10 (1:200) (Upstate) and HRP-linked anti-mouse IgG (1:2000). Blots were stripped with Restore Stripping Buffer (45 min.), rinsed in PBS / 0.05% Tween20 and re-probed with rabbit anti-tie2 polyclonal (1:400) (sc324, Santa Cruz) and HRP-linked anti-rabbit IgG (1:1500).
Ang1 immunoprecipitation
C2C12 myocytes, RNCM, and RNCF were cultured in antibiotic-free media (3 d). Media (90 ml) was collected, centrifuged and protease and phosphatase inhibitors added as described(3). Media was pre-cleared with agarose beads and anti-ang1 antibody (2 µg) (R&D monoclonal) was added (2 h, 4°C) followed by protein A/G PLUS agarose beads (overnight, 4°C). Beads were centrifuged, washed in modified RIPA buffer (3 min., 3x) and sample loading buffer was added. Samples were divided in half, prepared as nonreduced and reduced (0.435 M βMe) and analyzed by SDS-PAGE. Membranes were probed with anti-ang1 antibody. Negative controls included substituting mouse IgG for primary antibody. When these control samples were probed with ang1 antibodies on a Western blot, there were no bands (unpublished observations).
Hanging Heart Perfusions
Mice were given intraperitoneal injections of heparin (4 units/g) / 2.5% Avertin. For some studies, anesthetized mice were retroorbitally injected (30 gauge needle) with 10% fluorescein-isothiocyanate (FITC)-tomato lectin (Vector, Burlingame, CA, USA)/ PBS and given 5 minutes to allow lectin to circulate. Hearts were removed, dipped in KHB / 25 mM NaHCO3 / 1.4 mM CaCl2, and hung by the aorta (23 gauge blunt needle). Aortas were tied (4.0 gauge suture), retrograde perfused (KHB/ 25 mM NaHCO3 / 1.4 mM CaCl2 / 40 mM KCl / 4 mM sodium nitroprusside dihydrate) until exiting perfusates cleared and perfusion-fixed (2% formaldehyde/ PBS, 10 min.). Where indicated, hearts were then perfused with tissue-marking dye (Triangle Biomedical, Durham, NC, USA). Hearts were trimmed to the ventricles. The LV/RVs were filled with then immersed into warmed (37°C) TissueTek O.C.T Compound (Fisher Scientific), and frozen (−80°C).
Immunohistochemistry
Endogenous LV Ang1
Ventricles embedded in O.C.T. compound were cryosectioned (5µg, −20°C) and mounted on Superfrost Plus slides. Sections were air dried (warming plate), post-fixed (ice-cold acetone, 2 min.), washed in TBS and blocked in appropriate normal serum buffer. Tissues were incubated (overnight, 4°C) in primary antibodies ang1 (Rockland, Santa Cruz) or goat polyclonal troponin I (Santa Cruz) (1:100) in antibody diluent (Dako, Carpinteria, CA, USA). Negative controls incubated in the appropriate IgG or sera (Vector) showed no signal (unpublished observations). Slides were washed in TBS, blocked (serum buffer), and incubated (2 h) in rabbit anti-goat Texas Red- (Jackson Immunoresearch, West Grove, PA, USA) or rabbit anti-goat AlexaFluor 647, or goat anti-rabbit AlexaFluor 568-conjugated secondary antibodies (Invitrogen) (1:200, serum block buffer). Slides were washed (TBS) and coverslips placed (Fluoromount G antifade mounting media, Southern Biotech, Birmingham, Alabama, USA).
Alternatively, 100 µm thick sections were cut using an oscillating tissue slicer, washed (buffer/ 0.1% TritonX100) and incubated in secondary antibody (4 h). Slides were scanned in fluorescence mode with a Leica PL APO 40x N.A.1.25 oil immersion objective lens using Leica TCS-NT laser scanning confocal microscope fitted with argon and krypton lasers at room temperature. Serial optical sections of ventricle were recorded beginning at the ventricular apex using confocal microscopy to capture images digitally using a photomultiplier tube detector and Leica confocal software. Where indicated, the resultant stacks were rendered in three-dimensions using VoxelView 2.5.1 software (Vital Images, Minnetonka, MN, USA).
Recombinant Ang1-256
Mouse heart tissues were fixed in 4% paraformaldehyde / PBS, dehydrated through a graded ethanol series, embedded in paraffin, and cut into 8 µm sections. Sections were rehydrated, epitopes retrieved with 10 mM sodium citrate buffer / 0.05% Tween-20 (pH 6.0) (20 min., 95°C), rinsed in PBS, blocked with normal goat serum in PBS (0.5 h), then primary antibody or relevant control (IgG or normal sera) were added in block. FLAG rabbit polyclonal antibody (Sigma) was added (1:50, overnight at 4°C). SMA mouse monoclonal antibody (Sigma) (1:100, 45 min), von Willebrand factor (vWF) rabbit polyclonal antibody (Dako) (1:150, 1 h), or phalloidin-conjugated to AlexaFluor488 (1:100, 0.5 h) (Invitrogen) were added. Slides were rinsed (PBS) and the appropriate secondary antibodies added [for FLAG, goat anti-rabbit AlexaFluor647 (1:200, 1 h, Invitrogen); for SMA, goat anti-mouse AlexaFluor568 (1:200, 45min.); for VWF biotinylated anti-rabbit IgG (1:100, 1 h, Vector) / strepavidin-conjugated AlexaFluor568 (1:200, 1 h, Invitrogen)]. Slides were rinsed and VECTASHIELD Mounting Medium with DAPI added (Vector). Optical sections were taken with a Leica TCS SP2-AOBS attached to a DMIRE-2 inverted microscope with a 63X objective.
Statistical Analysis
Results are reported as mean ± standard deviation (SD) and two-tailed Student’s t-Tests with two sample equal variance and p≤0.05 considered a significant difference between groups.
Results
Ang1 mRNA Levels Shift during Cardiac Remodeling
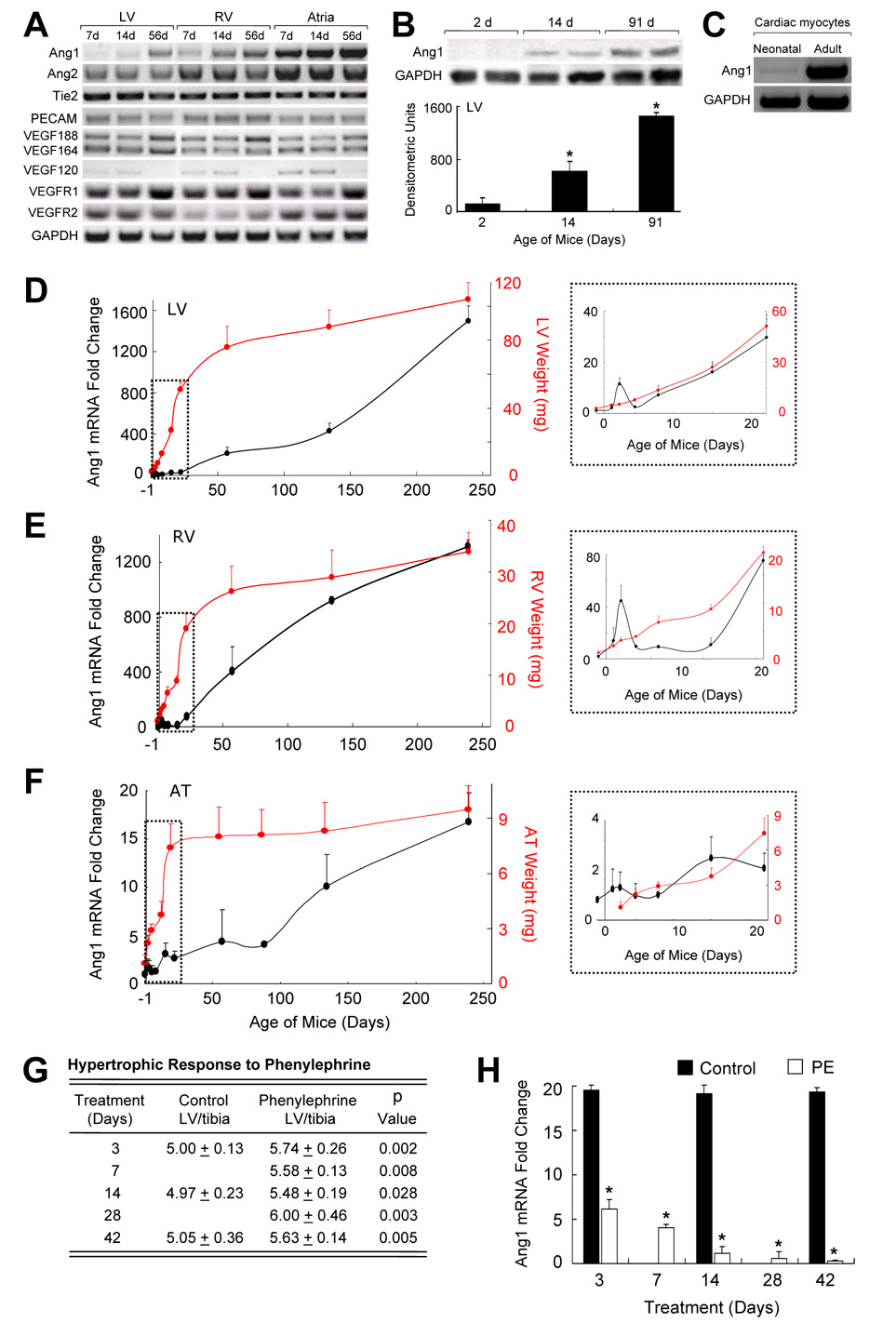
We examined angiopoietin ligands/receptors in different aged C57 mouse hearts during physiologic remodeling [RT-PCR, GAPDH (control), PECAM (EC marker)]. Ang1 mRNA levels increased with age in the left ventricle (LV), right ventricle (RV) and atria (AT), while ang2, tie2 and PECAM levels were stable (Fig 1A). Western blotting showed parallel increases in ang1 LV protein levels (Fig 1B) with stable tie2 protein levels (unpublished observations). Rat neonatal CM’s had trace ang1 mRNA, while adult CM’s expressed ang1 strongly (Fig 1C).
Fig. 1.
Ang1 mRNA Levels Change during Cardiac Remodeling. (A) mRNA transcripts were assessed (RT-PCR) in mouse LV, RV and AT. From neonate to adult, ang1 and VEGF188 mRNA expressions increased, while VEGF120 mRNA decreased. (B) Western blotting showed corresponding increases in ang1 protein levels in mouse LV with age (*p≤0.05 versus 2 d). (C) Ang1 mRNA levels were trace in neonatal and abundant in adult rat CM. (A–C, n=4/group; studies done in duplicate). Ang1 mRNA levels (real-time PCR) in mouse (D) LV, (E) RV, and (F) AT increased with age. Insets (dashed box) magnify days - 1 to 21. (n=5/group; studies done in triplicate). (G–H) Mice were given PE or saline (control). (G) PE increased LV/tibia ratios. (H) LV ang1 mRNA levels (real-time PCR) decreased with PE-induced hypertrophy.
To quantify ang1 mRNA increases, we conducted real-time PCR with LV, RV and AT from different aged mice [(−1 d = embryonic 20 d), 1, 2, 4, 7, 14, 21, 56, 133, 238 d]. Relative fold increases in ang1 were graphed alongside increasing chamber weights. Ang1 mRNA levels increased over a thousand fold in LV (Fig 1D) and RV (Fig 1E) with smaller atrial increases (Fig 1F). A small spike in LV and RV ang1 occurred between 1–4 d which returned to near baseline, then climbed. During the fetal to adult transition, there were similar ang1 increases (LV, RV, AT) in female and male mice with stable ang2 and tie2 mRNA levels (unpublished observations).
To assess ang1 in pathologic cardiac remodeling, mice were given PE to induce hypertrophy, or saline (controls). PE increased LV/tibia ratios (Fig 1G) and LV mRNA levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), β-myosin heavy chain (βMHC), collagen III, skeletal actin and smooth muscle actin (SMA) (Supp. Fig 1A–F), and decreased ang1 mRNA levels in LV (Fig 1H) and RV, while ang2 and tie2 were stable (real-time PCR) (unpublished observations).
Integrin-Binding Ang1 Monomers are Abundant in Heart
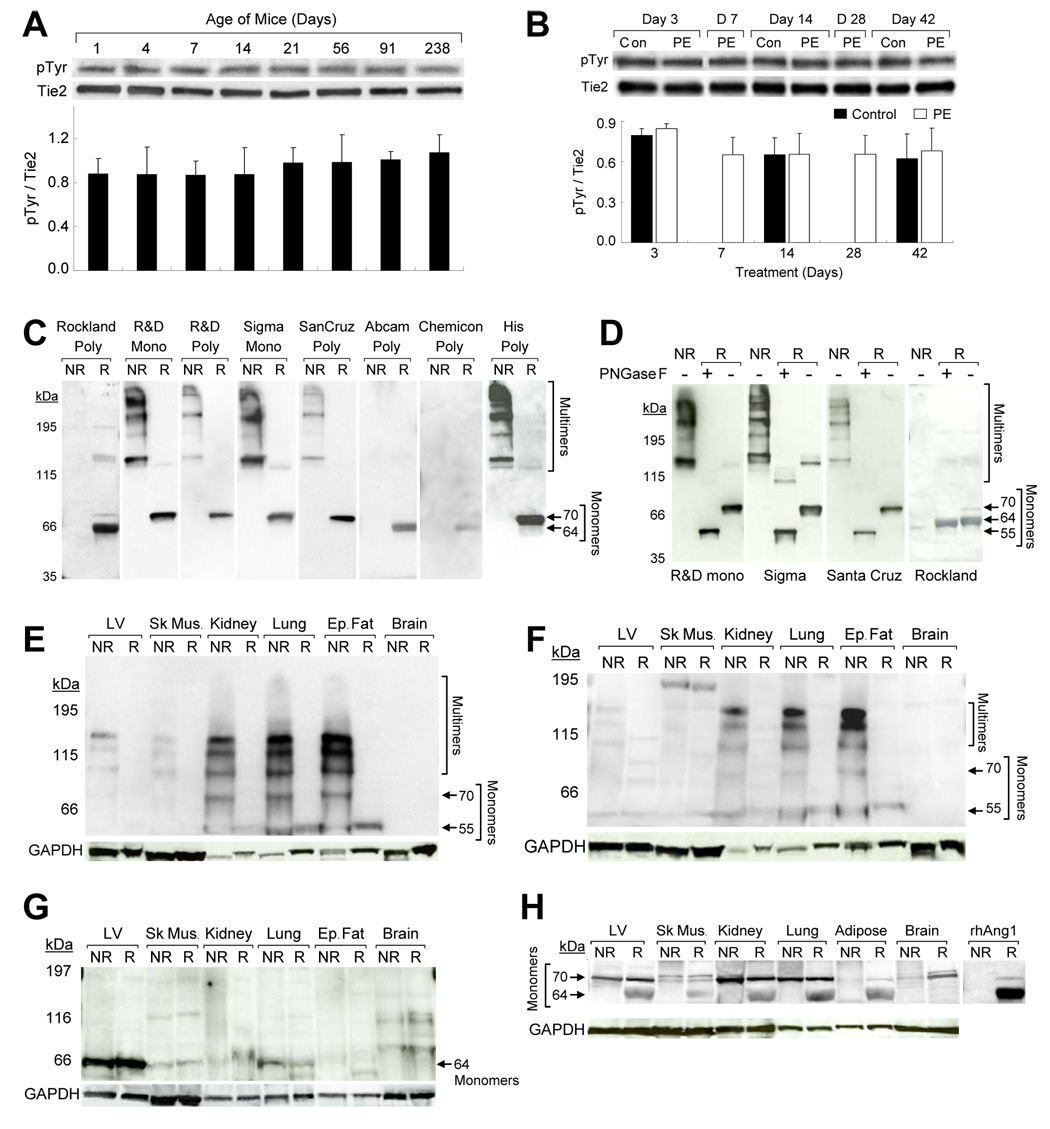
Using immunoprecipitation/Western blotting, we found that tie2 phosphorylation was stable in different aged (Fig 2A) and PE-treated (Fig 2B) mice, despite substantial shifts in ang1 (Fig 1). Thus, we proposed that integrins are the target for ang1 shifts.
Fig. 2.
Tie2 Activation is Stable during Cardiac Remodeling and Heart has Abundant Ang1 Monomers compared to Other Tissues. Tie2 phosphorylation [immunoprecipitation/Western blotting for phosphotyrosine (pTyr) and tie2] was unchanged during (A) physiologic and (B) pathologic (PE-induced) LV remodeling, remaining stable in (A) neonatal through adult and (B) control versus hypertrophic LVs (n=3/group; studies done in triplicate). (C–D) Western blotting was conducted with rhAng1/R&D (0.09 µg) in nonreduced (NR) and reduced (R) (βMe) conditions. Blots were probed with anti-ang1 monoclonal (mono) or polyclonal (poly) antibodies (studies done in duplicate). (C) The ang1 monomers and/or multimers detected varied and are indicated by arrows. (D) PNGase F-treatment of rhAng1/R&D revealed that some ang1 forms were glycosylated. Protein lysates from (E–G) mouse and (H) human LV, skeletal muscle (Sk. Mus), kidney, lung, epididymal fat tissue (Ep. Fat), adipose tissue, and brain were evaluated in nonreduced and reduced (βMe) conditions (Western blotting). Blots were probed with anti-ang1 [(E) R&D or (F) Sigma monoclonal or (G–H) Rockland polyclonal] and anti-GAPDH (studies done in duplicate). Ang1 monomers and/or multimers were in (E–G) mouse and (H) human adult tissues. Heart/skeletal muscle have (E–F) low multimer/ (G) high monomer versus other tissues.
We first established whether integrin-binding ang1 monomers and various multimers are in heart. We defined the ang1 forms recognize by available ang1 antibodies [monoclonal (R&D Systems, Sigma), polyclonal (Abcam, Chemicon/Millipore, R&D Systems, Rockland, Santa Cruz) using recombinant human ang1 (R&D Systems) (rhAng1/R&D). Using Western blotting under nonreducing conditions, monoclonal (R&D, Sigma) and polyclonal (R&D, Santa Cruz) ang1 antibodies detected several multimers (Fig 2C). Using a 6x polyhistidine antibody to the polyhistidine tag on rhAng1/R&D, we detected a similar pattern of multimers as observed with ang1 antibodies (Fig 2C). In reduced conditions, rhAng1/R&D monomer was found at 64kDa (Rockland, Abcam, Chemicon polyclonals) and 70kDa (R&D and Sigma monoclonals, Santa Cruz, R&D, Rockland polyclonals)] similar to a prior report(12).
Ang1 has potential glycosylation sites. We PNGase-F-treated rhAng1/R&D and conducted Western blotting with ang1 antibodies. In reduced conditions, PNGase-F-treated rhAng1/R&D monomer shifted from 70kDa to 55kDa (R&D and Sigma monoclonal, Santa Cruz polyclonal) (Fig 2D) as was shown(22). However, the Rockland anti-ang1 antibody detects ang1 monomer at 64 kDa. PNGase-F-treatment did not alter the mobility of the 64kDa monomer, indicating that this form is not glycosylated (Fig 2D).
To establish whether ang1 monomers and multimers exist in vivo, we conducted Western blotting with ang1 and GAPDH (control) antibodies on adult mouse (Fig 2E–G) and human [Fig 2H (USBiological, Swampscott, MA, USA), (Imgenex, San Diego, CA , USA, unpublished observations)] tissues. Also, rhAng1/R&D (nonreduced/ reduced) was run on these blots to confirm the location of ang1 bands (Fig 2H, unpublished observations). In nonreduced conditions, mouse tissues probed with R&D (Fig 2E) and Sigma (Fig 2F) monoclonal antibodies revealed ang1 monomers (70kDa, 55kDa), dimers and trimers but little larger forms. Human tissues had only trace ang1 multimers (R&D and Sigma monoclonals, unpublished observations), perhaps due to degradation/instability of higher complexes(9, 12, 13) or poor epitope recognition.
With βMe, all ang1 forms reduced to 55kDa with R&D (Fig 2E) and Sigma (Fig 2F) monoclonal anti-ang1 and PNGase-F-treatment shifted all ang1 forms to 55kDa (Supp. Fig 2A–B), similarly to rhAng1/R&D. Mouse LV and skeletal muscle had trace ang1 multimers (Fig 2E–F). Multimers were abundant in kidney, lung, and adipose tissue, but without appreciable expression in brain.
Using Rockland anti-ang1, mouse LV highly expressed 64kDa monomer versus other tissues (Fig 2G), and ang1 monomers were in all human tissues tested (Fig 2H). and PNGase-F did not alter mobility of the 64kDa form in tissue or with rhAng1/R&D (Supp Fig 2C). Thus, integrin-binding ang1 monomers are broadly produced in vivo with tissue-specific differential expressions of ang1 forms.
Nonendothelial cells produce ang1, so we assessed whether cardiac myocytes and fibroblasts and skeletal myocytes produce ang1 monomers and multimers. We collected rat neonatal cardiac myocyte and fibroblast and differentiated C2C12 skeletal myocyte media and immunoprecipitated ang1. In nonreduced conditions, Western blotting showed that neonatal CM and cardiac fibroblasts, and C2C12 myocytes produced ang1 monomer (70kDa), dimer, trimer, tetramer, and trace larger forms and are compared to rhAng1/R&D [(Sigma anti-ang1, Supp. Fig 2D), R&D anti-ang1 (unpublished observations)]. In reduced conditions, different ang1 forms converted to 55 kDa monomer.
Ang1 Monomers Shift During Cardiac Remodeling and Interact with Cardiac Myocytes and EC In Vivo
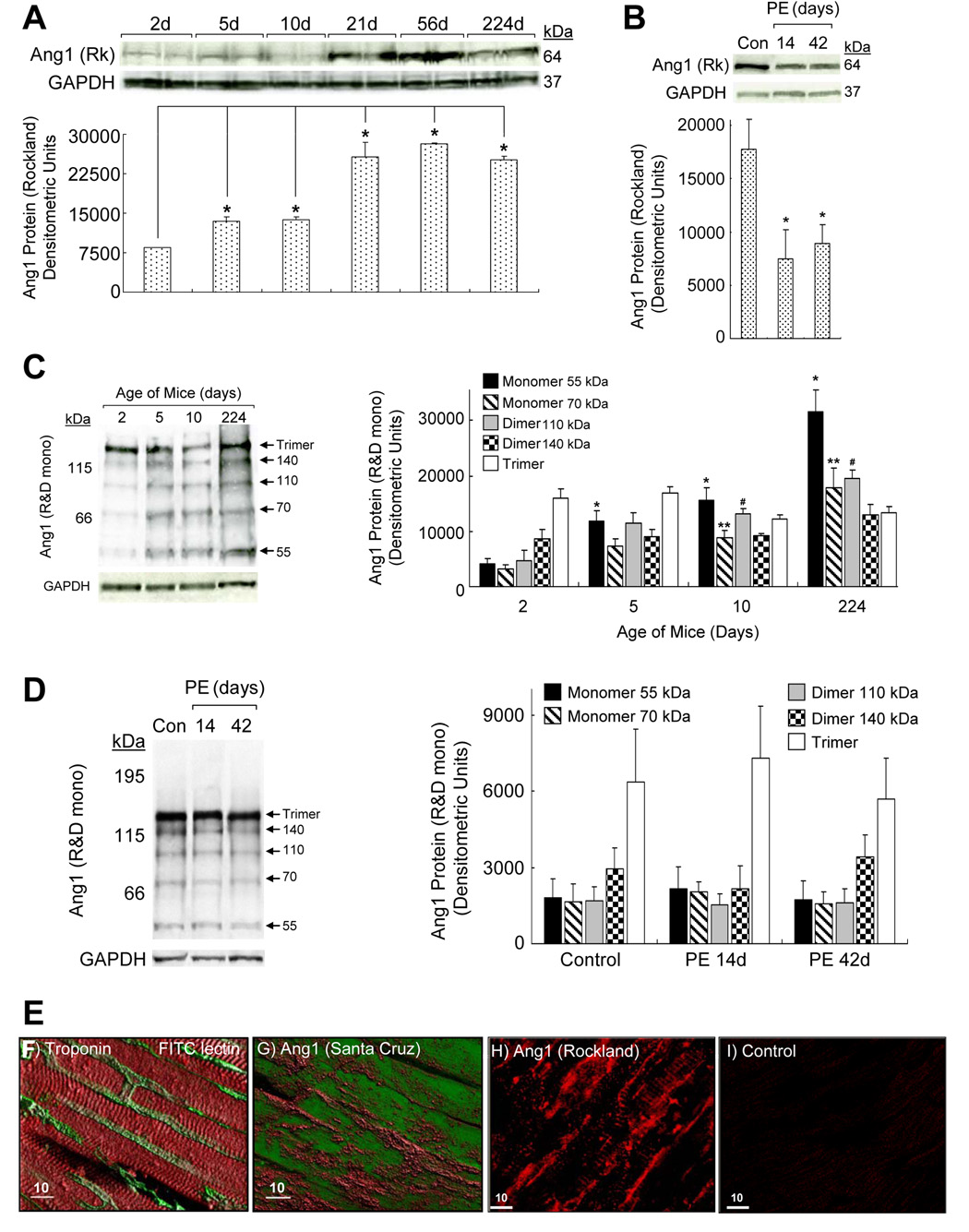
We conducted Western blotting with LV protein lysates from different aged mice or mice treated with PE or saline (control). Under nonreduced conditions, 64kDa (Rockland polyclonal, Fig 3A), 55kDa and 70kDa (R&D monoclonal, Fig 3C) ang1 monomers increased during the neonatal to adult transition. At 10d, 110kDa ang1 dimers increased, but ang1 dimers (140kDa) and trimers were stable (Fig 3C). With hypertrophy, in nonreduced conditions (Fig 3B), 64kDa (Rockland anti-ang1) monomer declined, but 55 and 70kDa monomers, dimer and trimer (R&D monoclonal anti-ang1) levels were stable (Fig 3D). Reduced conditions converted various ang1 forms to monomers (unpublished observations). Thus, shifts in ang1 during physiologic and pathologic cardiac remodeling are primarily in integrin-binding monomers.
Fig. 3.
Ang1 Monomers Shift during Cardiac Remodeling. Western blotting was conducted under nonreduced conditions with LV from (A, C) neonate to adult mice or those with (B, D) PE-induced hypertrophy compared to saline-treated controls (con). Blots were probed with anti-ang1 (A–B) Rockland (Rk) polyclonal or (C, D) R&D monoclonal antibodies (n=3/group; studies done in duplicate). (A) Ang1 monomers increased with age (left to right, *p=0.015, 0.036, 0.013, 0.00002, 0.0007). (B) Ang1 monomers declined during PE-induced remodeling (*p≤0.03). (C) Western blotting with anti-ang1 (R&D monoclonal) showed that monomers (55kDa, left to right *p=0.023, 0.017, 0.011) and (70kDa, left to right, **p=0.038, 0.029) and dimers (110kDa, left to right, #p=0.029, 0.014) increased with age, while other dimers/trimers were stable (ang1 forms are indicated by arrows). (D) In PE-induced remodeling, blots probed with anti-ang1 (R&D monoclonal) showed that monomer (55kDa, 70kDa), dimer, and trimer levels were unchanged. (E) Fluorescent immunohistochemistry/confocal imaging of ang1 forms in perfusion-fixed mouse LV (studies done in triplicate). (F) CM were labeled with troponin-I linked to Texas Red (red) and EC were FITC-lectin (green) tagged. (G) LV was labeled with anti-ang1 (Santa Cruz) conjugated to AlexaFluor647 (red) and CM autofluoresce (green). CM and ECs were labeled, indicating that ang1 multimers interact with both cell types. (H) Anti-ang1 (Rockland) conjugated to AlexaFluor568 (red), which recognizes only monomers, immunostained CM and EC in vivo. (I) Rabbit sera (negative control) were substituted for anti-ang1 (Rockland). Bar=10 microns.
To identify in vivo interactions of ang1 forms with cardiac cells, we conducted fluorescent immunohistochemistry on perfusion-fixed mouse LV (Fig 3E). Confocal images of CM labeled with troponin I conjugated to AlexaFluor647 (red) show a typical ladder pattern (Fig 3E–F). EC’s perfusion-stained with fluorescein isothiocyanate (FITC)-conjugated ulex lectin (green) border CM’s (Fig 3E–F). Anti-ang1 [Santa Cruz, detects multimers/monomers (70, 55kDa)] conjugated to AlexaFluor647 (red) appeared to label CM (autofluoresce green) and the vasculature (Fig 3E, G). Anti-ang1 [Rockland, detects monomers (70, 64kDa)] conjugated to AlexaFlour568 (red) also appeared to label CM (ladder and punctuate pattern) and EC (Fig 3E, H). Rabbit IgG substituted for primary antibody was negative control (Fig 3E, I). We propose that ang1 monomers interact with adult CM and cardiac EC in vivo likely via integrins.
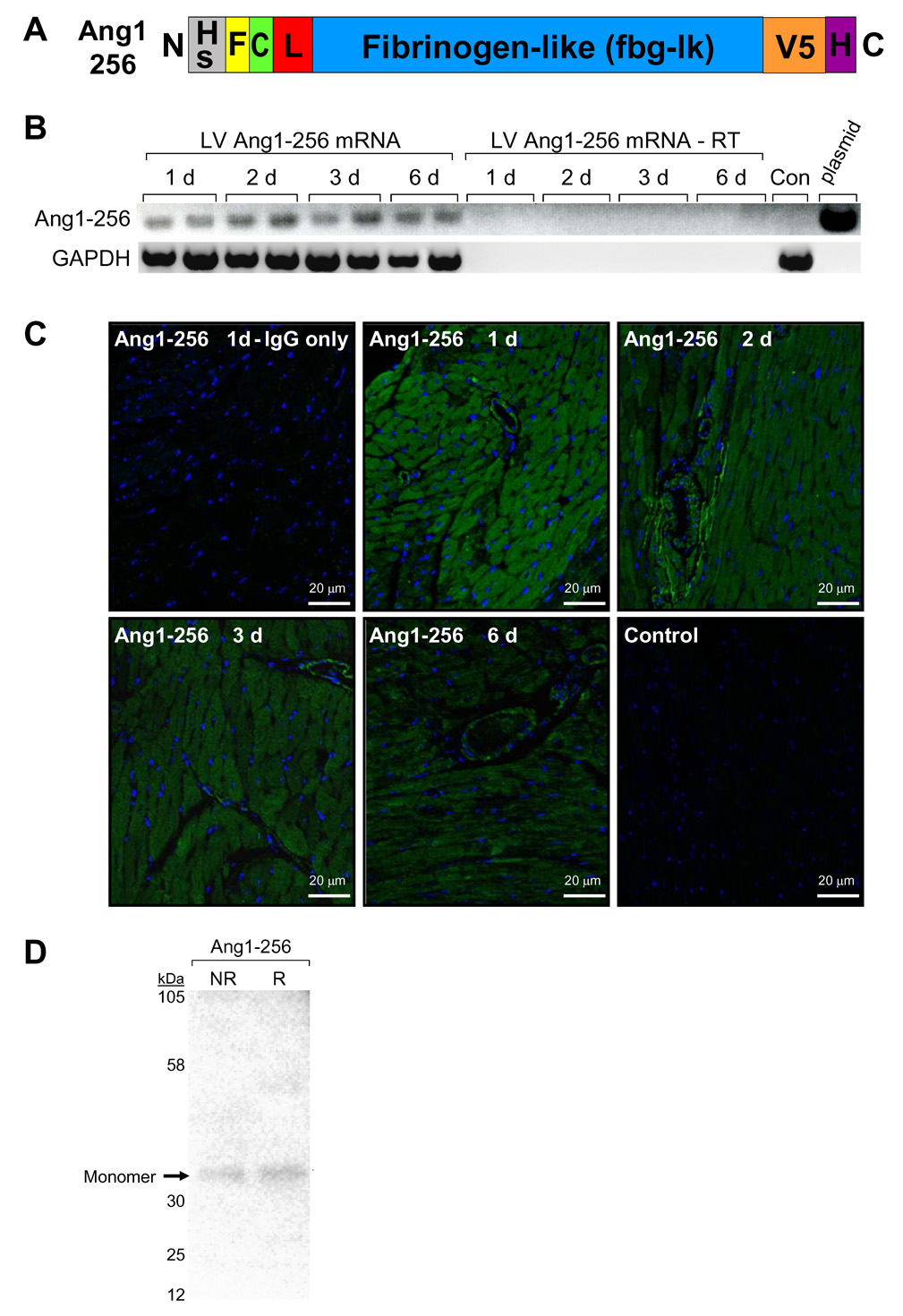
Ang1-256 Monomer Expression in Heart
To determine the function of integrin-binding ang1 monomers in cardiac remodeling, we generated a truncated recombinant ang1 form (ang1-256) known to generate only monomers(12). Ang1-256 consists of ang1’s amino acids 256–498, N-terminal hemagluttinin secretion signal and FLAG tag, and C-terminal V5 and polyhistidine tags (Fig 4A). Using pcDNA plasmid-based expression, we transfected HEK293 cells with ang1-256/pcDNA and purified ang1-256 protein. Western blotting with an anti-V5 antibody showed a single ang1 monomer band (34kDa) under nonreduced and reduced conditions (Fig 4D), verifying that ang1-256/pcDNA generates only ang1 monomers. We retroorbitally injected ang1-256/pcDNA or pcDNA vector alone using In Vivo-Jet-PEI into C57 mice and examined expression of ang1-256 mRNA (RT-PCR) and protein (fluorescent immunohistochemistry/confocal imaging). This method of delivering plasmids to heart is very effective, reliable, efficient, nontoxic, and used in clinical trials to deliver cancer gene therapy (ISC Bioexpress)(23–25). Ang1-256 mRNA was detected in mouse LV at all timepoints tested (Fig 4B). Control (Con) mice injected with pcDNA alone were negative for ang1-256 mRNA as were LV’s from mice injected with ang1-256 where cDNA synthesis reactions were conducted without reverse transcriptase (Fig 4B). Ang1-256 plasmid was used as a positive control. Ang1-256 protein expression was detected using Flag antibody linked to AlexaFluor647 (green) and nuclei were stained with DAPI (blue). Ang1-256 protein was observed at all timepoints with the highest expression on day 1 (Fig 4C). Ang1-256 appeared to be present in CM and cardiac EC. Negative controls include pcDNA alone-injected mice (control) and rabbit IgG substituted for primary antibody in LV sections from ang1-256-injected mice.
Fig. 4.
Ang1-256 Monomers are Expressed in Heart. (A) Recombinant truncated ang1-256 has a modified influenza hemagluttinin secretion signal (HS) and FLAG (F) tag fused to ang1 at amino acid 256. Ang1-256 has 6 amino acids of the coiled coil (C), linker (L) and fibrinogen-like domains of ang1 followed by C-terminal V5 and 6X polyhistidine (H) tags. (B–C) Ang1-256/pcDNA (ang1-256) or pcDNA control (Con) were retroorbitally injected into C57 mice (studies done in triplicate). (B) LV ang1-256 mRNA was detected at all timepoints. cDNA synthesis reactions were conducted ± reverse transcriptase (RT) with ang1-256/pcDNA plasmid (positive control). (C) In mouse heart, ang1-256 protein was detected at all timepoints using fluorescent immunohistochemistry/confocal imaging. Ang1-256 was labeled using FLAG antibody linked to AlexaFluor647 and nuclei labeled (DAPI). Negative controls included substituting rabbit IgG for primary antibody and injecting mice with pcDNA vector (control) (1 d). (D) HEK293 cells were transfected with ang1-256/pcDNA. Ang1-256 proteins were purified by anti-FLAG M1 affinity column chromatography (Sigma), dialyzed, and concentrated. Western blots probed with V5 antibody show ang1-256 monomer protein at the predicted MW (34 kDa) in nonreduced (NR) and reduced (R) conditions.
Ang1-256 Protein Localizes to Cardiac and Smooth Muscle Myocytes and EC
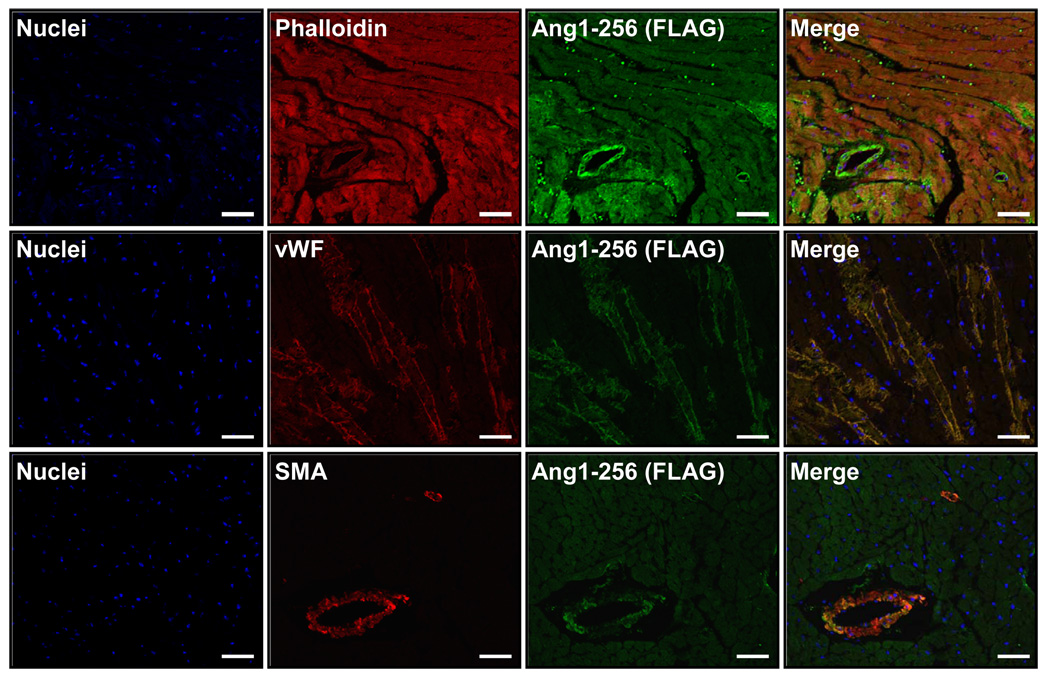
We conducted fluorescent immunohistochemistry/confocal imaging to discern the location of ang1-256 on day 1 in mouse hearts. Phalloidin-linked AlexaFluor488 (red) stained F-actin, von Willebrand factor (vWF) antibody linked to biotinylated anti-rabbit IgG and strepavidin-linked AlexaFluor488 labeled EC, αSMA antibody linked to anti-mouse AlexaFluor568 labeled smooth muscle cells (SMC), flag antibody linked to anti-rabbit AlexaFluor647 tagged ang1-256, and nuclei were labeled with DAPI. Negative controls included LV ang1-256 (day 1) where the appropriate sera and/or IgG’s were substituted for primary antibodies. These sections had no appreciable signal (unpublished observations). In merged images, we found that ang1-256 localizes with phalloidin on CM’s, vWF on EC’s, and SMA on SMC’s (Fig 5).
Fig. 5.
Ang1-256 Protein in Heart Localizes to Cardiac/Smooth Muscle Myocytes and EC. Ang1-256/pcDNA (ang1-256) was retroorbitally injected into C57 mice (1 d) and fluorescent immunohistochemistry/confocal imaging conducted on heart (studies done in triplicate). CM were labeled with AlexaFluor488-linked phalloidin (red), EC tagged with vWF antibody and biotinylated anti-rabbit IgG linked to strepavidin-linked AlexaFluor568 (red), SMC tagged with α-SMA antibody linked to AlexaFluor568 (red), ang1-256 immunostained with FLAG antibody conjugated to AlexaFluor647 (green), and DAPI stained nuclei (blue). Merged images show that ang1-256 localized to CM, EC, and SMC. Bar = 50 microns.
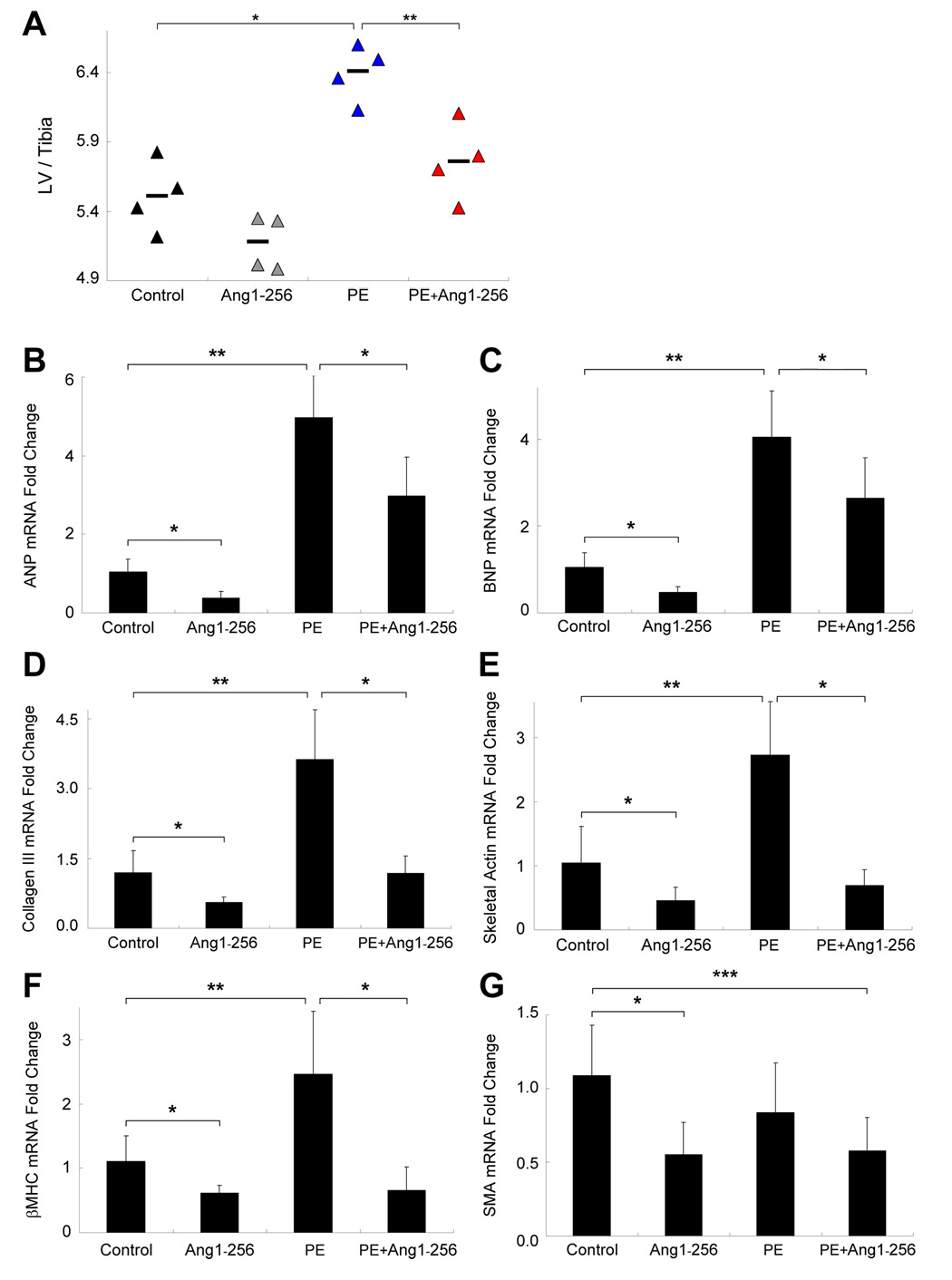
Ang1-256 Reduces Phenylephrine-Induced Cardiac Hypertrophy
Ang1 monomer levels decrease in PE-induced cardiac hypertrophy (Fig 3B), so we determined whether replacement of ang1 monomer attenuates cardiac hypertrophy. We retroorbitally injected C57 mice with ang1-256/pcDNA or pcDNA alone, and the next day, implanted osmotic pumps containing PE or saline. Mice in control and PE groups received pcDNA. Mice in control and ang1-256 groups received saline-filled pumps. On day 6, we found that PE increased LV/tibia ratios, while mice given PE+ang1-256 had significantly lower LV/tibia ratios (Fig 6A). We examined mRNA levels (real-time PCR) of fetal genes that increase during cardiac hypertrophy. PE increased mRNA levels of ANP (Fig 6B), BNP (Fig 6C), collagen III (Fig 6D), skeletal actin (Fig 6E) and βMHC (Fig 6F). In contrast, ang1-256 decreased mRNA levels for each of those fetal genes (Fig 6B–F) as well as for SMA (Fig 6G) mRNA levels ± PE treatment. Ang1-256 protein completely blocked the PE-induced increases in collagen III, skeletal actin and βMHC, maintaining normal transcript levels.
Fig. 6.
Ang1-256 Monomer Reduces Cardiac Hypertrophy In Vivo. Ang1-256/pcDNA (ang1-256) or pcDNA (control) were retroorbitally injected into C57 mice. On day 2, mice were given PE or saline via pumps (n=4/group, studies done in duplicate). On day 6, (A) PE increased LV/tibia ratios (*p=0.009) and ang1-256 monomer blunted PE-induced increases in LV/tibia ratios (**p=0.02). PE upregulated mRNA levels of (B) ANP, (C) BNP, (D) collagen III, (E) skeletal actin and (F) βMHC (**p≤0.02), but not (G) SMA (real-time PCR). Ang1-256 monomer reduced ANP, BNP, collagen III, skeletal actin, βMHC, and SMA mRNA levels in the absence (*p≤0.03; ****p=0.006) or presence of PE (***p≤0.03).
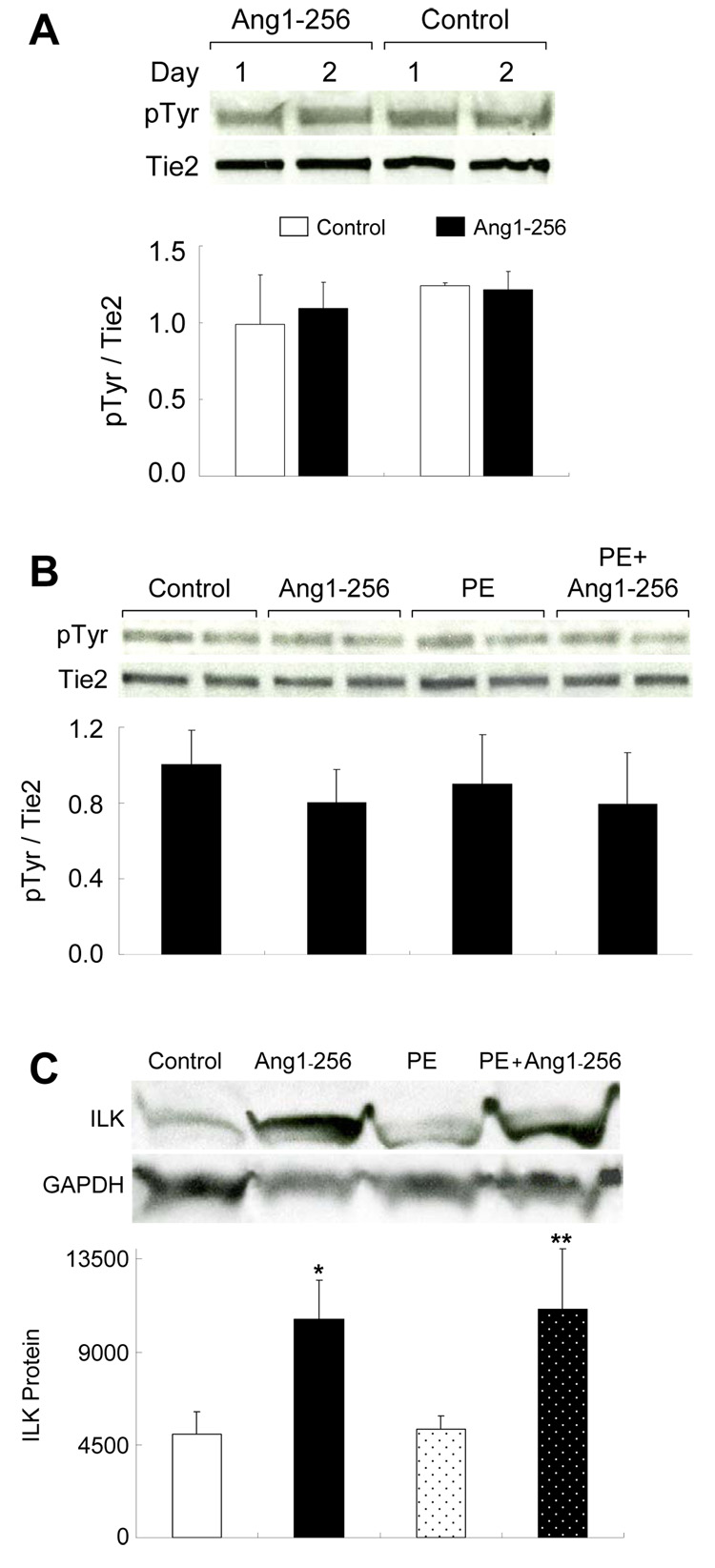
Ang1-256 Does Not Change Tie2 Phosphorylation, but Upregulates ILK
Ang1 monomers do not bind/activate tie2. However, ang1 binding to EC α5β1 integrin in vitro induces complexation and activation of tie2 (26). Thus, we determined whether ang1-256 plasmid-based expression in heart changes tie2 phosphorylation by conducting tie2 immunoprecipitation and Western blotting on hearts from C57 mice injected with ang1-256/pcDNA or pcDNA (control). At 1 and 2 days post-plasmid injection, levels of tie2 phosphorylation were similar in hearts from control and ang1-256/pcDNA-injected mice (Fig 7A). We also measured tie2 phosphorylation in hearts of mice with phenylephrine-induced cardiac hypertrophy. On day 6, levels of tie2 phosphorylation were similar among the groups (Fig 7B). We examined (Western blotting) the effect of ang1-256 on mouse LV ILK protein levels. Ang1-256 ± PE increased ILK (Fig 7C), a major regulator of integrin subunit β1 and β3 based signaling, survival and CM contractility(14) suggesting ang1-256 actions are integrin-mediated.
Fig. 7.
Ang1-256 Monomer Does Not Change LV Tie2 Phosphorylation, but Increased ILK. (A–C) Ang1-256/pcDNA (ang1-256) or pcDNA (control) were retroorbitally injected into C57 mice. Using tie2 immunoprecipitation and Western blotting for pTyr and tie2 (studies done in duplicate), we found that tie2 phosphorylations were unchanged in adult heart on (A) days 1 and 2 post plasmid injection and (B) day 6 in PE-induced cardiac hypertrophy. (C) Six days after plasmid injection, Western blotting of mouse LV shows that ILK protein levels increased by ang1-256 either alone or with PE-induced cardiac hypertrophy (n=4/group, studies done in duplicate, *p=0.01, **p=0.03). ILK was normalized to GAPDH.
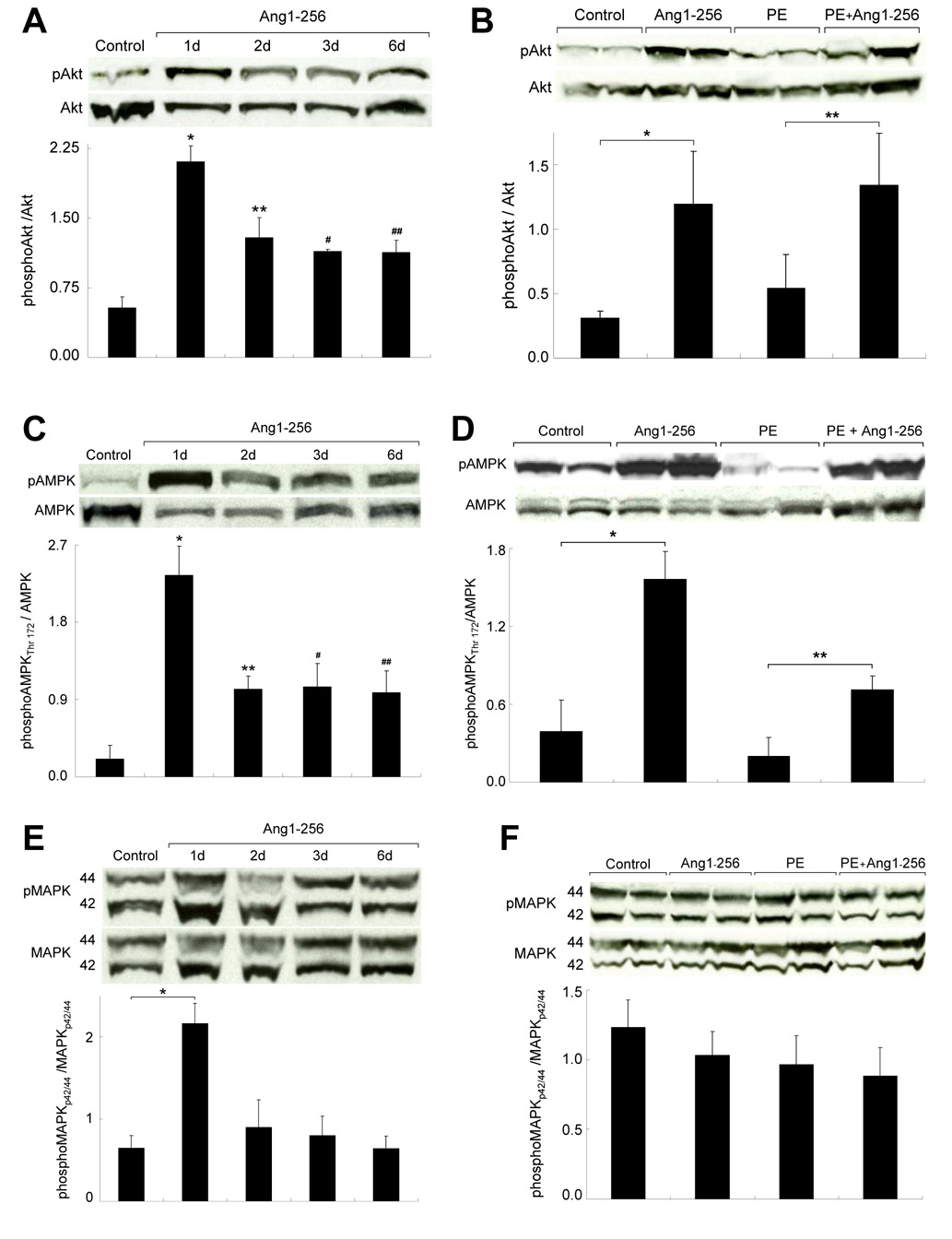
Ang1-256 Monomer Activates Akt, MAPKp42/44 and AMPK
To assess the effect of ang1-256 monomer on cardiac function, we examined major cardioprotective regulators of survival and/or energetics (akt, MAPKp42/44, AMPK, FAK). We tracked LV levels of phosphorylated versus total receptor over time (day 1–6) (Fig 8A, C, E) and ± PE-induced cardiac hypertrophy (Fig 8B, D, F). Ang1-256 monomer phosphorylated aktS473 and AMPKT172 with the largest increases on day 1, and lesser increases on days 2–6 (Fig 8A, C) and ± PE treatment (Fig 8B, D). PE-induced decreased AMPKT172 phosphorylation (Fig 8D) is consistent with increased protein synthesis during pathologic cardiac hypertrophy and reduced cardiac energetics. Ang1-256-mediated increases in AMPKT172 phosphorylation suggest reduced protein synthesis. Ang1-256 monomer increased phosphorylation of MAPKp42(T202)/44(T204) on day 1 only (Fig 8E–F). Ang1-256 monomer did not alter FAKY397 or AMPKS485/S491 phosphorylation on days 1–6 or ± PE-induced cardiac hypertrophy (unpublished observations).
Fig. 8.
Ang1-256 Monomer Activates Cardioprotective Signals Akt, AMPK, and MAPKp42/44. Ang1-256/pcDNA (ang1-256) or pcDNA (control) were retroorbitally injected into C57 mice. On day 2 (B, D, F), mice were given PE or saline (control) via pumps. Western blotting on mouse hearts measured phosphorylated versus total protein (n=4/group, studies done in duplicate). Ang1-256 activated (A) aktS473 and (C) AMPKT172 at 1 (*p≤0.01), 2 (**p≤0.03), 3 (#p≤0.05) and 6 (##p≤0.04) days, and (E) MAPKp42(T202)/44(T204) on day 1 (*p=0.05) only. On day 6 (B, D, F), ang1-256 monomers phosphorylated LV (B) akt S473 and (D) AMPKT172 without (*p≤0.02) or with PE treatment (**p≤0.01), but did not alter (F) MAPKp42/44 phosphorylation.
Discussion
Ang1 mRNA/protein (Fig 1) levels shift during physiologic and pathologic cardiac remodeling without changes in tie2 phosphorylation (Fig 2, 7). This disparity was also seen in an MI model(27). These findings support a novel mechanism-of-action for ang1 in heart, which our prior work suggested is integrin-mediated(3) and herein we show is associated with changes in ILK, a molecule central to integrin-based signaling.
We show that integrin-binding ang1 monomers are abundant in mouse/human hearts (Fig 2), shift during cardiac remodeling (Fig 3) and bind cardiac myocytes and EC (Fig 3). These findings suggest a major role for ang1 monomer/integrin interactions in cardiac health and disease. Ang1-256 increased ILK in mouse LV (Fig 7C). ILK binds the cytoplasmic tail of β1/β3 integrins, and we identified CM β1 and β3 subunits as those that interact with ang1 (3). ILK is abundant in human and mouse hearts, regulates physiologic cardiac hypertrophy, and ILK ablation in murine heart causes cardiomyopathy and heart failure(14, 28). In humans, loss of ILK causes a dilated cardiomyopathy. ILK promotes cell survival via aktS473. We showed that full-length ang1 (3) and ang1-256 monomers phosphorylate aktS473 (Fig 8A–B) in mouse heart and isolated CM. ILK is important to CM, which rely on ILK/integrin based interactions to support the force of contraction. Increased ILK blocks cardiac fibrosis, and we show that ang1-256 blocked phenylephrine-induced increases in collagen III (Fig 6D).
Here, we introduce a new regulatory mechanism for ang1 receptor targeting that is based on changes in different ang1 quaternary forms. EC tie2 activation requires ang1 tetramers or larger(9, 29). Cartilage oligomeric matrix protein fused to ang1 linker/fibrinogen-like domains (COMP-ang1) forms mainly tetramers/pentamers and increases EC tie2 phosphorylation more than native ang1(29, 30). COMP-ang1, but not native ang1, tracks with tie2 in adult heart and other tissues. Our data may explain this finding in that we show native ang1 in mouse and human heart and other tissues is predominately composed of monomers, dimers, and trimers (Fig 2), and these forms may act chiefly via integrins.
Ang1 monomer/multimer expressions were tissue-specific. Multimers were abundant in adipose tissue, lung and kidney (Fig 2E–F). Monomers were prevalent in heart (Fig 2G–H). In support of this finding, COMP-ang1 weakly stains heart, but prominently labels lung(30). Further, brain has little tie2 (31), and we did not detect ang1 multimers in brain, only monomers (Fig 2). Ang1 has an anti-permeability effect in brain, and integrin-binding recombinant ang1 monomers blocked transendothelial cell permeability(13). Thus, our data suggests a new role for ang1 monomer/integrin interactions in brain.
We found ang1 monomers/dimers in all tissues tested (Fig 2), however, other studies using soluble tie2-Fc identified ang1 trimers, tetramers, and pentamers(10, 12). Since monomers/dimers show weak/no tie2 binding, they may not be detected with tie2-Fc. Use of soluble tie2-Fc to block ang1’s actions may not disrupt monomer/dimer functions and may prevent ang1 from binding integrins and/or tie1. Also, our studies demonstrated the importance of ang1 antibody selection, since they differ in the ang1 forms they detect (Fig 2).
Akt and MAPKp42/44 (32) activities are decreased in failing human hearts(33). Akt phosphorylation prevents CM apoptosis and positively affects CM inotropism(34). Our studies show that integrin-binding ang1-256 monomer retains at least some of the functions of full-length ang1 (3), including akt and MAPK p42/44 activation (Fig 8A–B, E–F). Another recombinant truncated ang1 that produces monomers/dimers, binds integrins, but does not phosphorylate tie2, also has some capacities of full-length ang1 (promotes EC adhesion, activates MAPKp42/44,reduces EC permeability)(13). Besides CM, ang1-256 localized to cardiac EC and SMC indicating that these monomers act on vasculature likely via integrins. Ang1 binds HUVEC integrins(4), and ang1-driven angiogenesis in chorioallantoic membrane assays was inhibited completely by anti-α5β1 and partly by anti-αvβ3 function-blocking antibodies(26). Thus, ang1 monomer/integrin interactions may mediate some EC functions in vivo.
Here, we introduced new functions for ang1-256 monomers, including AMPK phosphorylation and decreased cardiac hypertrophy and fetal gene expressions. There are several implications for these new cardioprotective actions, all of which are consistent with improved cardiac function and energetics in injured myocardium.
AMPK phosphorylation is largely determined by α subunit phosphorylation at threonine 172 (35) and increases energy production and inhibits apoptosis, protecting the heart during stress(36). AMPK is a key regulator of energetics and is known as the “guardian of energy status”(37) in heart. AMPK responds to stress by activating ATP-generating pathways (glycolysis, glucose uptake, fatty acid oxidation, glycogenolysis) and downregulating ATP-consuming pathways (protein synthesis, hypertrophy).
MI, hypertension, cardiac hypertrophy and heart failure are associated with increases in collagen III. Increased collagen III causes fibrosis and myocardial stiffening(38). Ang1-256 monomer’s blunts PE-induced increase in collagen III which may decrease pathologic cardiac remodeling, preserving LV function.
In summary, we show that integrin-binding ang1 monomers have a regulatory role in cardiac remodeling. Ang1-256 monomers localize to cardiac myocytes, EC and SMC, and block PE-induced cardiac hypertrophy, activating cardioprotective signaling pathways. This data introduces a novel mechanism of ang1 regulation whereby quaternary forms are differentially expressed in vivo as a function of tissue type and remodeling state to direct receptor/cell type interactions. Such a mechanism may serve to coordinate ang1’s actions as a CM survival factor and vascular regulator during cardiac remodeling to preserve function and impede the development of heart failure.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by U.S. National Institutes of Health (NIH) grant K02-HL071840-01, American Heart Association Grant-in-Aid 0455824T, grant R21-CA107976-01, and a Thomas Smith Award to M.A.R; and grant K01-DK063970-01 and a David M. Bray Scholars in Medicine Award to S.M.D; and NIH grant P01-CA45548 and philanthropic funds provided to Dr. Judah Folkman (Children’s Hospital, Boston, MA, USA).
References
- 1.Dumont DJ, Yamaguchi TP, Conlon RA, Rossant J, Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- 2.Korhonen J, Partanen J, Armstrong E, Vaahtokari A, Elenius K, Jalkanen M, Alitalo K. Enhanced expression of the tie receptor tyrosine kinase in endothelial cells during neovascularization. Blood. 1992;80:2548–2555. [PubMed] [Google Scholar]
- 3.Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96:e8–e24. doi: 10.1161/01.RES.0000158285.57191.60. [DOI] [PubMed] [Google Scholar]
- 4.Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem. 2001;276:26516–26525. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- 5.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis [see comments] Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Ito Y, Morikawa M, Kobune M, Huang J, Tsukamoto M, Sasaki K, Nakamura K, Dehari H, Ikeda K, Uchida H, Hirai S, Abe T, Hamada H. Adenoviral-delivered angiopoietin-1 reduces the infarction and attenuates the progression of cardiac dysfunction in the rat model of acute myocardial infarction. Mol Ther. 2003;8:584–592. doi: 10.1016/s1525-0016(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui AJ, Blomberg P, Wardell E, Hellgren I, Eskandarpour M, Islam KB, Sylven C. Combination of angiopoietin-1 and vascular endothelial growth factor gene therapy enhances arteriogenesis in the ischemic myocardium. Biochem Biophys Res Commun. 2003;310:1002–1009. doi: 10.1016/j.bbrc.2003.09.111. [DOI] [PubMed] [Google Scholar]
- 8.Chen SL, Zhang BR, Mei J, Xu ZY, Zhu JL, Cai KH, Huang SD, Liu YL. Induction of angiogenesis in ischemic myocardium by adenovirus mediated angiopoietin-1 gene transfer, an experimental study. Zhonghua Yi Xue Za Zhi. 2003;83:637–640. [PubMed] [Google Scholar]
- 9.Davis S, Papadopoulos N, Aldrich TH, Maisonpierre PC, Huang T, Kovac L, Xu A, Leidich R, Radziejewska E, Rafique A, Goldberg J, Jain V, Bailey K, Karow M, Fandl J, Samuelsson SJ, Ioffe E, Rudge JS, Daly TJ, Radziejewski C, Yancopoulos GD. Angiopoietins have distinct modular domains essential for receptor binding, dimerization and superclustering. Nat Struct Biol. 2003;10:38–44. doi: 10.1038/nsb880. [DOI] [PubMed] [Google Scholar]
- 10.Procopio WN, Pelavin PI, Lee WM, Yeilding NM. Angiopoietin-1 and -2 coiled coil domains mediate distinct homo-oligomerization patterns, but fibrinogen-like domains mediate ligand activity. J Biol Chem. 1999;274:30196–30201. doi: 10.1074/jbc.274.42.30196. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biol Chem. 2001;276:34990–34998. doi: 10.1074/jbc.M103661200. [DOI] [PubMed] [Google Scholar]
- 12.Kim K-T, Choi H-H, Steinmetz MO, Maco B, Kammerer RA, Ahn SY, Kim H-Z, Lee GM, Koh GY. Oligomerization and Multimerization Are Critical for Angiopoietin-1 to Bind and Phosphorylate Tie2. J. Biol. Chem. 2005;280:20126–20131. doi: 10.1074/jbc.M500292200. [DOI] [PubMed] [Google Scholar]
- 13.Weber CC, Cai H, Ehrbar M, Kubota H, Martiny-Baron G, Weber W, Djonov V, Weber E, Mallik AS, Fussenegger M, Frei K, Hubbell JA, Zisch AH. Effects of Protein and Gene Transfer of the Angiopoietin-1 Fibrinogen-like Receptor-binding Domain on Endothelial and Vessel Organization. J. Biol. Chem. 2005;280:22445–22453. doi: 10.1074/jbc.M410367200. [DOI] [PubMed] [Google Scholar]
- 14.Hannigan GE, Coles JG, Dedhar S. Integrin-linked kinase at the heart of cardiac contractility, repair, and disease. Circ Res. 2007;100:1408–1414. doi: 10.1161/01.RES.0000265233.40455.62. [DOI] [PubMed] [Google Scholar]
- 15.Dallabrida SM, Zurakowski D, Shih SC, Smith LE, Folkman J, Moulton KS, Rupnick MA. Adipose tissue growth and regression are regulated by angiopoietin-1. Biochem Biophys Res Commun. 2003;311:563–571. doi: 10.1016/j.bbrc.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 16.McMahon DK, Anderson PA, Nassar R, Bunting JB, Saba Z, Oakeley AE, Malouf NN. C2C12 cells: biophysical, biochemical, and immunocytochemical properties. Am J Physiol. 1994;266:C1795–C1802. doi: 10.1152/ajpcell.1994.266.6.C1795. [DOI] [PubMed] [Google Scholar]
- 17.Kim NN, Villarreal FJ, Printz MP, Lee AA, Dillmann WH. Trophic effects of angiotensin II on neonatal rat cardiac myocytes are mediated by cardiac fibroblasts. Am J Physiol Endocrinol Metab. 1995;269:E426–E437. doi: 10.1152/ajpendo.1995.269.3.E426. [DOI] [PubMed] [Google Scholar]
- 18.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/s0008-6363(98)00128-x. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld JR, Vasser M, Jhurani P, Ng P, Hunter JJ, Ross J, Jr, Chien KR, Lowe DG. Distinct molecular phenotypes in murine cardiac muscle development, growth, and hypertrophy. J Mol Cell Cardiol. 1998;30:2269–2280. doi: 10.1006/jmcc.1998.0787. [DOI] [PubMed] [Google Scholar]
- 20.Kim I, Ryan AM, Rohan R, Amano S, Agular S, Miller JW, Adamis AP. Constitutive Expression of VEGF, VEGFR-1, and VEGFR-2 in Normal Eyes. Invest. Ophthalmol. Vis. Sci. 1999;40:2115–2121. [PubMed] [Google Scholar]
- 21.Goede V, Schmidt T, Kimmina S, Kozian D, Augustin HG. Analysis of blood vessel maturation processes during cyclic ovarian angiogenesis. Lab Invest. 1998;78:1385–1394. [PubMed] [Google Scholar]
- 22.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning [see comments] Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 23.Bragonzi A, Boletta A, Biffi A, Muggia A, Sersale G, Cheng SH, Bordignon C, Assael BM, Conese M. Comparison between cationic polymers and lipids in mediating systemic gene delivery to the lungs. Gene Ther. 1999;6:1995–2004. doi: 10.1038/sj.gt.3301039. [DOI] [PubMed] [Google Scholar]
- 24.Chemin I, Moradpour D, Wieland S, Offensperger WB, Walter E, Behr JP, Blum HE. Liver-directed gene transfer: a linear polyethlenimine derivative mediates highly efficient DNA delivery to primary hepatocytes in vitro and in vivo. J Viral Hepat. 1998;5:369–375. doi: 10.1046/j.1365-2893.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari S, Pettenazzo A, Garbati N, Zacchello F, Behr JP, Scarpa M. Polyethylenimine shows properties of interest for cystic fibrosis gene therapy. Biochim Biophys Acta. 1999;1447:219–225. doi: 10.1016/s0167-4781(99)00153-0. [DOI] [PubMed] [Google Scholar]
- 26.Cascone I, Napione L, Maniero F, Serini G, Bussolino F. Stable interaction between alpha5beta1 integrin and Tie2 tyrosine kinase receptor regulates endothelial cell response to Ang-1. J Cell Biol. 2005;170:993–1004. doi: 10.1083/jcb.200507082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, Kuliszewski MA, Kutryk MJ, Stewart DJ. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–124. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 28.White DE, Coutu P, Shi YF, Tardif JC, Nattel S, St Arnaud R, Dedhar S, Muller WJ. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. COMP-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci U S A. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho CH, Kammerer RA, Lee HJ, Yasunaga K, Kim KT, Choi HH, Kim W, Kim SH, Park SK, Lee GM, Koh GY. Designed angiopoietin-1 variant, COMP-Ang1, protects against radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101:5553–5558. doi: 10.1073/pnas.0307575101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong AL, Haroon ZA, Werner S, Dewhirst MW, Greenberg CS, Peters KG. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–574. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- 32.Ravingerova T, Barancik M, Strniskova M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247:127–138. doi: 10.1023/a:1024119224033. [DOI] [PubMed] [Google Scholar]
- 33.Baba HA, Stypmann J, Grabellus F, Kirchhof P, Sokoll A, Schafers M, Takeda A, Wilhelm MJ, Scheld HH, Takeda N, Breithardt G, Levkau B. Dynamic regulation of MEK/Erks and Akt/GSK-3beta in human end-stage heart failure after left ventricular mechanical support: myocardial mechanotransduction-sensitivity as a possible molecular mechanism. Cardiovasc Res. 2003;59:390–399. doi: 10.1016/s0008-6363(03)00393-6. [DOI] [PubMed] [Google Scholar]
- 34.Latronico MVG, Costinean S, Lavitrano ML, Peschle C, Condorelli G. Regulation of Cell Size and Contractile Function by AKT in Cardiomyocytes. Annals of the New York Academy of Sciences. 2004;1015:250–260. doi: 10.1196/annals.1302.021. [DOI] [PubMed] [Google Scholar]
- 35.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 36.Russell RR, 3rd, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, Giordano FJ, Mu J, Birnbaum MJ, Young LH. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardie DG. AMP-activated protein kinase: the guardian of cardiac energy status. J Clin Invest. 2004;114:465–468. doi: 10.1172/JCI22683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedak PW, Verma S, Weisel RD, Li RK. Cardiac remodeling and failure From molecules to man (Part II) Cardiovasc Pathol. 2005;14:49–60. doi: 10.1016/j.carpath.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.