Abstract
A reduction of heat loss to the environment through increased cutaneous vasoconstrictor (CVC) sympathetic outflow contributes to elevated body temperature during fever. We determined the role of neurons in the dorsomedial hypothalamus (DMH) in the increases in CVC sympathetic tone evoked by prostaglandin E2 (PGE2) into the preoptic area (POA) in chloralose/urethane-anesthetized rats. The frequency of axonal action potentials of CVC sympathetic ganglion cells recorded from the surface of the tail artery was increased by 1.8Hz following nanoinjections of bicuculline (50 pmol) into the DMH. PGE2 nanoinjection into the POA elicited a similar excitation of tail CVC neurons (+2.1Hz). Subsequent to PGE2 into the POA, muscimol (400 pmol/side) into the DMH did not alter the activity of tail CVC neurons. Inhibition of neurons in the rostral raphe pallidus (rRPa) eliminated the spontaneous discharge of tail CVC neurons, but only reduced the PGE2-evoked activity. Residual activity was abolished by subsequent muscimol into the rostral ventrolateral medulla. Transections through the neuraxis caudal to the POA increased the activity of tail CVC neurons which was sustained through transections caudal to DMH. We conclude that while activation of neurons in the DMH is sufficient to activate tail CVC neurons, it is not necessary for their PGE2-evoked activity. These results support a CVC component of the increased core temperature elicited by PGE2 in POA that arises from relief of a tonic inhibition, from neurons in POA, of CVC sympathetic premotor neurons in rRPa and is dependent on the excitation of CVC premotor neurons from a site caudal to DMH.
Introduction
In both humans (20, 43) (19) and rats (53), cutaneous vasoconstrictor (CVC) tone regulates heat loss to the environment and contributes importantly to the maintenance of normal body temperature and to the development of fever. The essential role for the generation of prostaglandin E2 (PGE2) in the preoptic area (POA) in the production of fever has led to the use of nanoinjections of PGE2 into the POA to understand the neural pathways controlling thermoregulatory effectors during the febrile response. Similarly, observations of thermal effector responses elicited by changes in skin or core temperature have provided important information on the central neural networks responsible for body temperature homeostasis. Such studies have been pursued most extensively with regard to the sympathetic neural regulation of non-shivering thermogenesis in rodent brown adipose tissue (BAT), a highly metabolic tissue in which heat production contributes significantly to the defense of body temperature in cold environments and to the elevation of body temperature during the febrile response. The neural circuit hypothesized to underlie the cooling-evoked and the PGE2-evoked stimulation of BAT thermogenesis involves relief, either through activation of the cold afferent pathway or through PGE2 binding to EP3 receptors, of a tonic GABAergic inhibition from the POA to neurons in the dorsomedial hypothalamus (DMH) and to neurons in the rostral raphe pallidus (rRPa) (23, 29, 35-38, 71). The synergistic effect of a reduced inhibition of BAT sympathetic premotor neurons in the rRPa and the increased excitatory input they receive from disinhibited DMH neurons is hypothesized to result in activation of BAT sympathetic premotor neurons in the rRPa (22, 30, 35, 36, 38) and thus to increases in BAT sympathetic nerve activity (SNA) and BAT thermogenesis.
Although control of cutaneous vasoconstriction and the accompanying changes in heat loss to the environment also have significant roles in the homeostatic regulation of body temperature and in the defended elevation in body temperature that defines fever, the functional organization of the central pathways regulating the CVC sympathetic outflow remains poorly understood. There are several similar qualities in the organization of the neural circuits controlling cutaneous vasoconstriction and BAT thermogenesis and in the responses of the neural outflows controlling their effector functions. Both effectors are regulated by sympathetic neural activity and activation of their respective populations of sympathetic premotor neurons, located principally in the rostral medullary raphe, is sufficient to strongly drive either BAT or tail CVC sympathetic outflows (31, 41, 46). Both sympathetic outflows are strongly modulated by the central respiratory pattern generator (11, 12, 31, 40). PGE2 injected into the POA activates both sympathetic outflows (23, 63), a response that is significantly dependent on the activation of sympathetic premotor neurons in the rRPa (18, 22). Additionally, neurons in the midbrain rostral ventromedial periaqueductal grey (rvmPAG) can provide an inhibitory influence on the sympathetic outflow to BAT (47) and on that to arterioles in the rat tail (73).
However, several distinctions, likely reflecting differences in the functional organization of their central regulatory circuits, have been observed between the sympathetic outflows controlling BAT and CVC. For example, CVC sympathetic outflow exhibits barosensitivity (15, 42), while BAT SNA does not (31, 33)Neuronal activity in the RVLM accounts for a portion of the excitation of tail CVC neurons (41, 46, 64), whereas, neuronal excitation in the RVLM is neither necessary nor sufficient for BAT SNA activation and may exert an inhibitory effect on BAT SNA (31). BAT SNA is more sensitive to variations in skin temperature than to those in core temperature, while the reverse is true for tail CVC activity (40). These differential responses could reflect fundamental differences in the pathways controlling these thermoregulatory effectors that may also contribute to the absence of a correlation, other than a shared respiratory modulation, between simultaneously recorded, cold-activated rat tail CVC and BAT sympathetic outflows (40).
The parallels between the functional roles of the sympathetic innervation to BAT and to the cutaneous vasculature, coupled with the organizational similarities described above in their central control circuits, have led to the suggestion that the neural circuits which underlie thermoregulatory reflex control of both tissues are likely to be broadly similar. Specifically, it has recently been suggested that the DMH may play an important role in the cold- and febrile-evoked activation of tail sympathetic outflow (6, 7). In the present study, we tested this hypothesis by inhibiting DMH neurons during the activation of tail CVC neurons elicited by application of PGE2 into the POA. We show that although disinhibition of neurons in the DMH does increase the excitation of tail CVC neurons, the activity of DMH neurons is not necessary for tail CVC neuronal activity driven by cool thermal afferents or for the increase in their activity evoked by PGE2 in the POA.
Experimental Procedures
All procedures conform to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Oregon Health & Science University. Male Sprague-Dawley rats (Charles River, Indianapolis, IN, USA, n = 25 rats) weighing 350-600gm were initially anaesthetized with isoflurane (2.5% in 100% O2). A femoral artery and vein and the trachea were cannulated for measurement of arterial pressure, the delivery of drugs and artificial respiration, respectively. Rats were transitioned to intravenous (iv) urethane (500mg/kg) and chloralose (80mg/kg) and the depth of anesthesia was monitored by the absence of a hindlimb withdrawal to noxious pinch and by the absence of a corneal reflex. Rats were positioned in a stereotaxic frame with the incisor bar at -4mm. Colonic (core) temperature (TC) was measured with a thermocouple (Physitemp) and thermocouple reader (Sable Systems) and maintained at 37°C with a heat lamp and a water-perfused blanket. Skin (TSK) and interscapular BAT (TBAT) temperatures were monitored with thermocouples placed subcutaneously in the umbilical region of the abdomen and inserted into the left interscapular BAT pad, respectively. Rats were artificially ventilated with 100% oxygen. End-expiratory CO2 was monitored (CWE) and resting levels were maintained at 3.0-4.0% by adjusting ventilatory volume (150-300 ml/min). Craniotomies were performed dorsal to the rRPa, the DMH and the POA, the dura was cut and reflected and the sagittal sinus was ligated in the area dorsal to the DMH.
The activity of tail CVC neurons was determined from that of their axons recorded using an arterial patch recording technique (16). The tail was rotated and placed in an organ bath filled with saline. An incision along the ventrolateral surface of the tail exposed the left side of the ventral artery after reflecting the tail skin. Axonal action potentials of CVC neurons were recorded from the left side of the tail artery, which is innervated primarily by the left ventral collector nerve. Nerve activity was recorded through a saline-filled glass micropipette with the tip (diameter <200μm) positioned on the ventrolateral surface of the ventral artery and a reference electrode placed in the bath. The extracellular axonal action potentials of CVC neurons were filtered (250-2000Hz), amplified (50,000 X, Axon Instruments Molecular Devices), digitized (20kHz, Cambridge Electronic Design Limited) and, along with the arterial pulse pressure, temperature and CO2 signals, recorded to computer hard drive.
In the rats where interscapular BAT SNA was recorded simultaneously with the activity of tail CVC neurons, a lower lumbar vertebra was clamped and a postganglionic sympathetic nerve bundle innervating the right interscapular BAT pad was dissected from the ventral surface of the BAT pad after sectioning it along the midline and reflecting it laterally (31). The nerve was placed on a bipolar hook electrode and maintained in a pool of mineral oil. BAT SNA was filtered (1-300 Hz), amplified (2,000-50,000 X) and digitized (1 kHz).
Nanoinjections of drugs into the brain were made with a pneumatic injection apparatus (Toohey) through micropipettes (tip diameter: 30-50 μm) and the injectate volume was measured by the movement of the fluid meniscus in the pipette using a microscope eyepiece graticule. The stereotaxic coordinates used to target nanoinjections to the POA were 0.5 mm caudal to bregma, 0.7 mm lateral to midline, 8.3 mm ventral to dura; to the DMH were 3.3 mm caudal to bregma, 0.5 mm lateral to midline, 8.5 mm ventral to dura; to the rRPa were 3 mm caudal to lambda, midline, 9.7 mm ventral to dura; to the rostral ventrolateral medulla (RVLM) were 3.5 mm caudal to lambda, 1.9 mm lateral to the midline and 9.3 mm ventral to dura (23, 30, 31). Fluorescent-labeled polystyrene microspheres (0.01% solids; diameter: 0.1 μm; Molecular Probes) were included in the injectate to mark the nanoinjection sites.
In 6 experiments, bicuculline (BIC, 30 pmol in 60 nl, Sigma) was nanoinjected into the DMH. In 11 rats, including 4 that had received BIC into the DMH, febrile responses were mimicked by nanoinjection of PGE2 (100 ng in 100 nl, Sigma) into the POA. Approximately 20 minutes after PGE2 nanoinjection into the POA, muscimol (MUSC, 400 pmol in 200 nl per side, Sigma) was nanoinjected bilaterally into the DMH. Muscimol (200 pmol in 100 nl) was subsequently nanoinjected into the rRPa within 10-40 min of the bilateral MUSC nanoinjections into the DMH and, in 5 of these rats, MUSC (200 pmol in 100 nl) was nanoinjected unilaterally into the right RVLM within 10 min after nanoinjection of MUSC into the rRPa. In 5 rats, MUSC was injected bilaterally into the DMH prior to nanoinjection of PGE2 into the POA. In 4 additional rats, MUSC was nanoinjected into the rRPa prior to PGE2 nanoinjection in the POA. The responses of some tail CVC neurons was observed following electrical stimulation (twin pulse, 1 ms duration, 6 ms interval, >50 μA) in the rRPa. In 4 rats, serial transections of the neuraxis were performed at 2 mm caudal to bregma (i.e., between POA and DMH), 4 mm caudal to bregma (i.e., caudal to DMH) and 6 mm caudal to bregma (i.e., caudal to rvmPAG) and at 13 mm caudal to bregma (i.e., caudal to rRPa). In all experiments, the activity of tail CVC neurons remaining after MUSC nanoinjections or transections was confirmed to be that of the axons of sympathetic ganglion cells by its elimination following hexamethonium injection (10 mg in 0.3 ml, iv).
Data were analyzed with Spike 2 (CED) software. Discrimination of the action potentials of tail CVC axons was accomplished by constructing templates based on signal height and shape and was confirmed by visual analysis. Action potentials were counted in 10-s bins and CVC neuronal responses were calculated as the average counts/10 s in three consecutive bins (30 s) at four time points: one minute prior to a treatment and at 1, 5 and 10 min after a treatment. Data are expressed as means ± SE. One-way, within-animal ANOVA or paired Student’s t-test was used to test for significant differences between control and post-treatment values of CVC discharge frequencies and other parameters. Student’s t-test was also used in post hoc analysis. The significance level was p<0.05.
Results
Properties of tail CVC postganglionic neurons
The rat tail CVC neurons recorded in this study showed properties consistent with those previously reported for CVC fiber populations (11, 12, 42). Tail CVC neuronal activity was increased in response to skin cooling effected by perfusing the water blanket with cold water. Shortly after obtaining recordings from the axons of tail CVC neurons, when core temperature averaged 37.3 ± 0.3°C and at a mean skin temperature of 36.6 ± 0.7°C, the basal firing rate of CVC neurons was 1.1 ± 0.4 Hz (n = 12 CVC neurons). After five minutes of cooling the trunk skin, TSK fell by -1.2 ± 0.3°C (TC fell by -0.4 ± 0.1°C) and the tail CVC neurons increased their mean firing rate to 2.7 ± 0.6 Hz.
The activity of tail CVC postganglionic neurons was modulated by the baroreceptor reflex. The spontaneous and evoked activity of tail CVC neurons was inhibited during the transient increase in mean arterial pressure (MAP) following iv injection of phenylephrine (5 μg in 0.1 ml): 5 CVC neurons were completely silenced (1.3 ± 0.35 Hz to 0.04 ± 0.04 Hz) at the peak of a phenylephrine-induced rise in MAP of +59 mmHg (peak MAP: 139 ± 17 mmHg). The spontaneous discharge of tail CVC neurons was also modulated over the course of the cardiac cycle: coincident with the late diastolic phase of the arterial pressure wave, there was a mean decline of 53 ± 8% (n = 6 CVC neurons) from the peak discharge frequency. This level of cardiac cycle modulation has been described as reflecting a “moderate correlation” between unit discharge and arterial pressure (11).
Bicuculline nanoinjection into the DMH activates tail CVC neurons
As an initial step in determining the role of DMH neurons in controlling the activation of CVC neurons, we determined whether CVC neuronal activity could be increased by activation of neurons in the DMH. Responses to nanoinjection of BIC (50 pmol) into the DMH were measured from 10 tail CVC neurons recorded in 6 rats. Bicuculline-evoked disinhibition of local neurons in the DMH increased tail CVC neuronal activity: in the example in Fig. 1, CVC unit activity increased from 1.1 Hz during control to 4.3 Hz at 1 min after BIC in the DMH. Within one minute of the nanoinjection of BIC into the DMH, mean tail CVC neuronal discharge increased (P < 0.05) from 0.3 ± 0.1 Hz (TSK=37.0 ± 0.6°C, TC=37.2 ± 0.4°C) to a peak level of 2.1 ± 0.8 Hz (Fig. 1B). There was no significant difference between the discharge rate of tail CVC neurons at 1 min and at 10 min after BIC nanoinjection into the DMH. In each of 4 cases, BIC nanoinjection into the DMH also increased BAT SNA recorded simultaneously with tail CVC neurons (Figure 1A). These data indicate the region within the DMH at which BIC nanoinjection activates tail CVC neurons overlaps with that eliciting excitation of BAT SNA and BAT thermogenesis (4, 72).
Fig. 1.
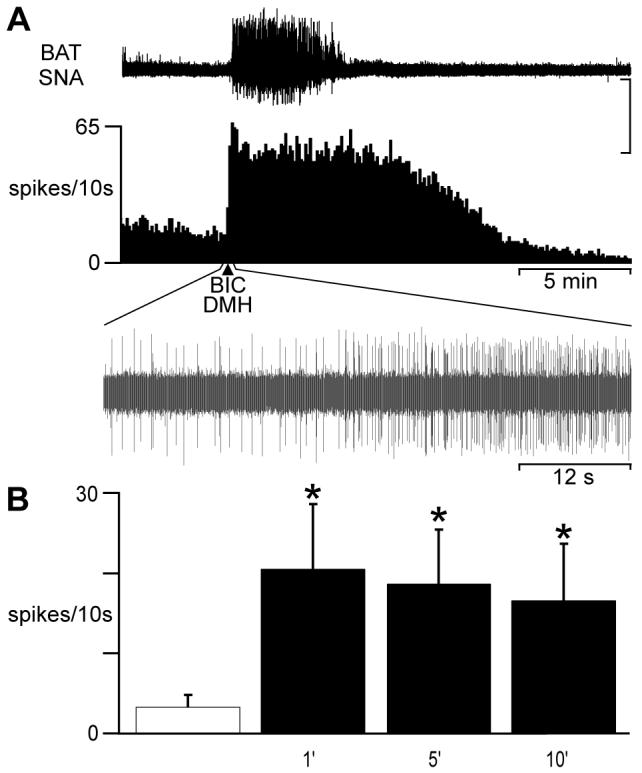
Bicuculline (BIC) into the dorsomedial hypothalamus (DMH) excites tail cutaneous vasoconstrictor (CVC) neurons. A: nanoinjection of BIC into the DMH (arrow) evoked a simultaneous increase in brown adipose tissue (BAT) sympathetic nerve activity (SNA) and in the discharge of a tail CVC neuron (see oscillographic trace of CVC neuron action potentials during the 1 min surrounding the BIC nanoinjection). Vertical scale: 250 μv for BAT SNA, 20 μv for neuronal action potentials. B: time course of the mean ± SE (n = 10 CVC neurons) CVC neuronal activity in response to BIC into the DMH. * indicates significantly (P < 0.05) greater discharge frequency than control (open cross-hatched bar).
Transections of the neuraxis
Following upon descriptions of the effects of transections of the neuraxis on BAT thermogenesis (5, 51, 57) and on the increases in BAT SNA produced by PGE2 in POA (32, 47), we used transections of the neuraxis to obtain a similar, indirect assessment of descending inhibitory and excitatory influences on CVC neuronal activity. Transection of the neuraxis immediately caudal to the POA activates BAT thermogenesis (5, 51) and a subsequent transection immediately caudal to the DMH reverses the increase in BAT SNA (32, 47). To test the hypothesis that the pathways descending from or through the POA and the DMH influencing tail CVC neuronal activity are organized similarly to those that influence BAT thermogenesis, we determined the changes in tail CVC neuronal activity following transections caudal to POA and to DMH. As shown in Fig. 2, transection caudal to the POA (bregma -2 mm) produced a strong and sustained excitation of tail CVC neurons: in this case, the discharge frequencies of two tail CVC neurons were increased from quiescence, induced by maintaining an elevated body temperature, to 4.0 Hz and 3.5 Hz. Maximal increases occurring within 5 min after transection and sustained at 10 min after transection (Figure 2A, 2B). At 5 min after transection, mean tail CVC neuronal activity (Fig. 2B) had increased (P < 0.05) from 1.0 ± 0.6 Hz to 6.1 ± 1.6 Hz (n = 5 CVC neurons).
Fig. 2.
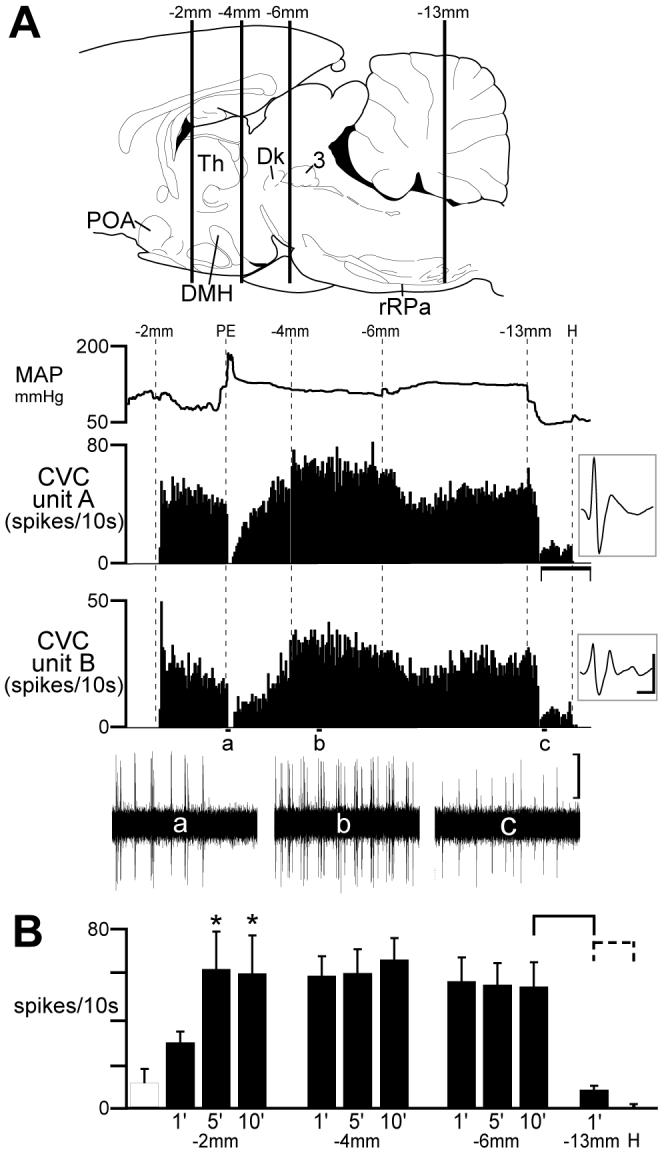
Effect of sequential transections of the neuraxis on the activity of tail cutaneous vasoconstrictor (CVC) postganglionic neurons. A: top image illustrates the approximate positions of serial (rostral to caudal) transections of the neuraxis by vertical lines (labeled as distance caudal to bregma) superimposed on a drawing of the rat brain (44). Traces indicate the time courses of the mean arterial pressure (MAP) and the action potential frequency of two discriminated tail CVC neurons (insets: average of 150 discriminated action potentials, vertical scale: 20 μv, horizontal scale: 2 ms). Phenylephrine (PE)-induced increase in MAP (+90 mmHg from 94 mmHg) elicited a baroreceptor-mediated inhibition of the discharge of the CVC neurons (trace a). Horizontal scale: 10 min. Oscillographic traces of unit action potentials (a-c) are 1 min duration, vertical scale: 20 μv. POA: preoptic area; DMH: dorsomedial hypothalamus; rRPa: rostral raphe pallidus; Th: thalamus; Dk: nucleus of Darkschewitsch; 3: occulomotor nucleus. B: Mean ± SE CVC neuronal discharge frequency following serial transections of the neuraxis. Open hatched bar indicates pre-transection firing frequency. * indicates a significant (P < 0.05) increase above pre-transection level resulting from cut at bregma -2 mm (caudal to the POA). After transections at bregma -4 or -6 mm, mean CVC neuronal activity was not different from that after transection at bregma -2 mm. Transection at bregma -13 mm (caudal to the rRPa) significantly reduced CVC neuronal activity (solid bracket: P < 0.01). Hexamethonium eliminated CVC neuronal activity (dashed bracket: P < 0.05).
Subsequent transections caudal to DMH at bregma -4 mm had no effect (P > 0.05) on the increased level of tail CVC neuronal activity (5.9 ± 1.6 Hz before vs 5.9 ± 1.1 Hz after, Fig. 2B). In the example in Fig. 2A (trace b), mean unit discharge rates were 6.2 Hz and 4.8 Hz after transection caudal to the DMH. There was also no effect (P >0.05) on the elevated level of tail CVC neuronal discharge from a subsequent transection at -6 mm caudal to bregma (6.5 ± 1.9 Hz before vs 5.5 ± 1.1 Hz after, Fig. 2B). In the example in Fig. 2A, mean unit discharge rates were 4.7 Hz and 4.5 Hz after transection caudal to the rostral ventromedial periaqueductal gray/ventral tegmental area (-6 mm). Transection of the neuraxis caudal to the rRPa markedly attenuated (P < 0.005) the evoked activity of each CVC neuron, with mean activity falling from 5.3 ± 1.1Hz to 0.7 ± 0.2 Hz (n = 5 CVC neurons) following this transection. In the example in Fig. 2A (trace c), mean unit discharge rates were reduced to 1.2 Hz and 0.9 Hz after transection caudal to the rRPa. Residual activity was eliminated by iv hexamethonium (Figs. 2A, 2B).
Muscimol in the DMH does not affect the PGE2-evoked increases in tail CVC neuronal activity
Finding that transection of the neuraxis caudal to POA increased CVC neuronal activity but that transection caudal to DMH did not reverse the evoked increase in CVC neuron activity strongly suggests that, contrary to the case for BAT SNA, a descending excitatory pathway from the DMH is not necessary for the activation of CVC neurons during the febrile response to PGE2 in POA. To test this directly, we inhibited neuronal activity bilaterally in the DMH during the increase in CVC neuronal activity produced by PGE2 in POA. Nanoinjection of PGE2 into the POA increased the discharge frequency of tail CVC neurons (Figs. 3, 4, 5). The activity of 14 individual tail CVC neurons recorded in 11 rats was increased (P < 0.005) from 1.0 ± 0.3 Hz to 1.6 ± 0.3 Hz within 1 min of the nanoinjection of PGE2 into the POA (Fig 3B), reaching a peak of 2.9 ± 0.5 Hz (P < 0.005) within 5 min (t-test(1′ vs 5′) P < 0.005) and sustained at 3.0 ± 0.5 Hz at 10 min (t-test(5′vs 10′) P > 0.05). The rate histogram in Figure 3A was derived from a multiunit recording of CVC neurons and illustrates the PGE2-evoked increase in discharge frequency from 13 spikes/10s in control (trace a) to 62 spikes/10s at 10 min (trace b) after PGE2. Approximately 20 min after the PGE2 nanoinjection into the POA, bilateral nanoinjection of MUSC (400 pmol, 200 nl) into the DMH had no significant effect (P > 0.05) on the PGE2-mediated elevation in the discharge of tail CVC neurons (Figs. 3 & 4). In the example in Fig. 3A, multiunit CVC neuronal discharge frequency was 73 spikes/10s prior to first nanoinjection of MUSC into DMH and 69 spikes/10s after bilateral nanoinjections. For the population of individual discriminated tail CVC neurons, mean discharge rate 10 min after the second nanoinjection of MUSC into the DMH was 2.7 ± 0.5 Hz (Fig. 3B), which was not significantly different (P > 0.8) from their mean activity (2.9 ± 0.6 Hz) at 1 min prior to the first nanoinjection of MUSC into the DMH. Muscimol also had no effect on the PGE2-evoked increases in tail CVC neuronal activity (data not shown) when nanoinjected into sites outside of the dorsal DMH area where BIC nanoinjections increased tail CVC neuronal activity (see Fig. 1).
Fig. 3.
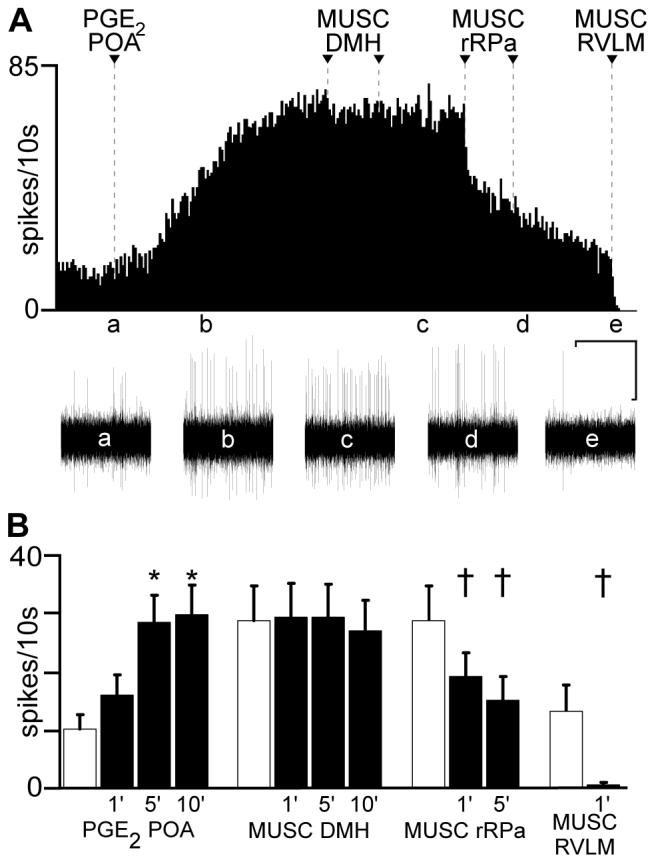
Increase in tail cutaneous vasoconstrictor (CVC) activity evoked by prostaglandin E2 (PGE2) into the preoptic area (POA) is not blocked by muscimol (MUSC) in the dorsomedial hypothalamus (DMH). A: rate histogram of multiunit tail CVC neuronal discharge following nanoinjection (arrowhead) of PGE2 into the POA. Traces a-e below the spike frequency histogram illustrate oscillographic records of neuronal activity at designated time points. Bilateral MUSC injections (arrowheads) were made into the DMH and two nanoinjections of MUSC (arrowheads) were made into the rostral raphe pallidus (rRPa). The residual activity remaining after MUSC into the rRPa was eliminated by nanoinjection of MUSC (arrowhead) into the right rostral ventrolateral medulla (RVLM). B: mean ± SE (n = 14 CVC neurons) discharge frequency of tail CVC neurons following PGE2 into the POA, MUSC into the DMH, MUSC into the rRPa and MUSC into the RVLM. Hatched bars represent mean discharge frequency during the 1 min control period prior to each treatment. The control discharge frequencies prior to MUSC into the DMH or into the rRPa were not different (P > 0.05) from that recorded 10 minutes after the PGE2-evoked activation. * and † indicate significant differences at P < 0.005 and P < 0.05, respectively, compared to respective control frequencies. Horizontal scale: 10 min for frequency histogram trace in A and 3.5s for oscillographic traces a-e; vertical scale bar: 20 μv for traces a-e.
Fig. 4.
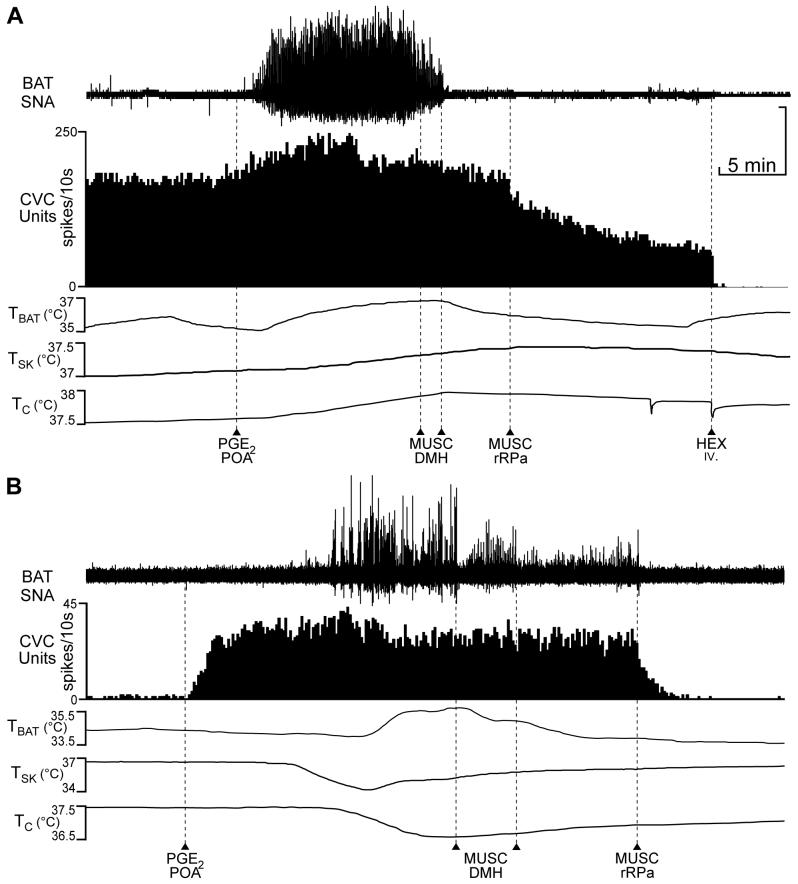
Bilateral muscimol (MUSC) into the dorsomedial hypothalamus (DMH) reverses the prostaglandin E2 (PGE2)- or PGE2/skin cooling-evoked increases in brown adipose tissue (BAT) sympathetic nerve activity (SNA) but has no effect on those in tail cutaneous vasoconstrictor (CVC) neuronal activity. A: PGE2 into the POA increased multiunit tail CVC neuronal activity, BAT SNA and BAT temperature (TBAT). Bilateral MUSC into the DMH (arrowheads) promptly reversed the PGE2-evoked increase in BAT SNA but had no effect on tail CVC neuronal activity. MUSC into the rRPa (arrowhead) reduced tail CVC neuronal activity. Intravenous (iv.) hexamethonium (HEX) eliminated the remaining tail CVC neuronal activity. TSK: skin temperature, TC: core temperature. B: PGE2 into the POA increased multiunit tail CVC neuronal activity, but had no effect on BAT SNA at skin and core temperatures necessary to reduce the basal CVC neuronal firing rate. Skin cooling elicited an increase in BAT SNA and TBAT. Bilateral MUSC into the DMH (arrowheads) reduced BAT SNA but had no effect on tail CVC neuronal activity. MUSC into the rRPa eliminated activity in both BAT SNA and tail CVC neurons. Vertical scale: 125 μv and 200 μv for BAT SNA in A and B, respectively.
Fig. 5.
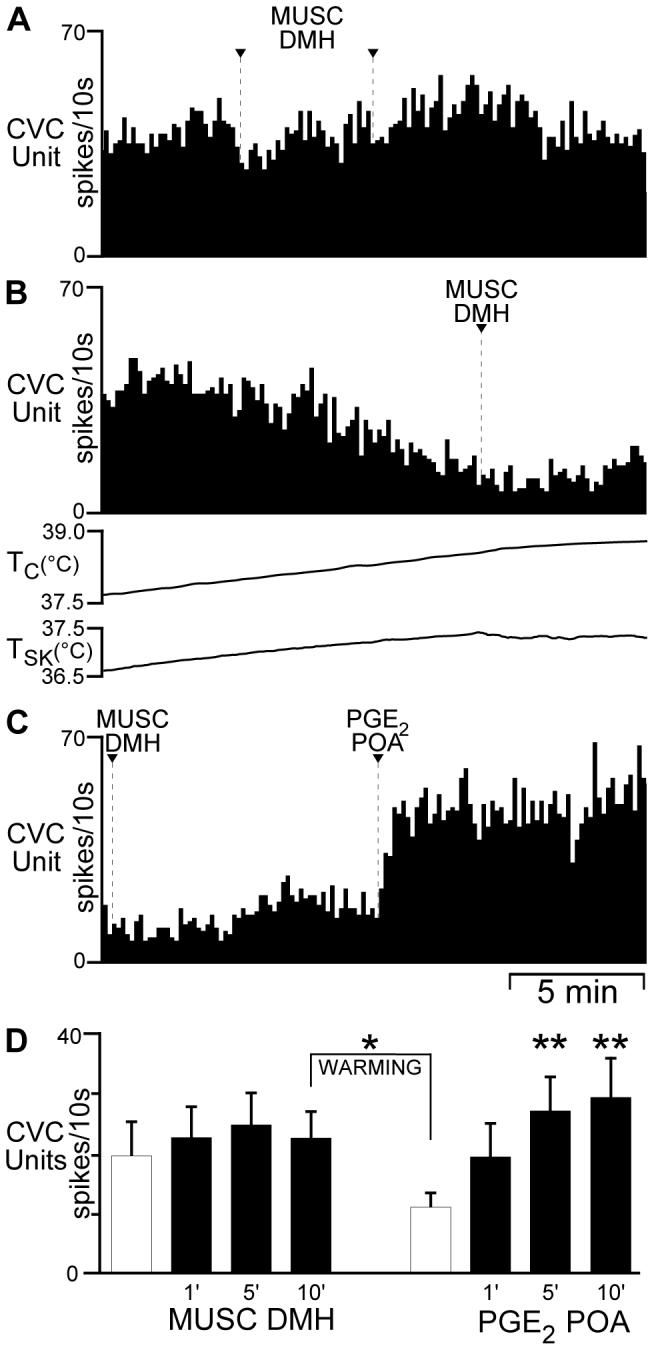
Bilateral muscimol (MUSC) into the dorsomedial hypothalamus (DMH) has no effect on the spontaneous or prostaglandin E2 (PGE2)-evoked activity of tail cutaneous vasoconstrictor (CVC) neurons. Panels A-C illustrate sequential segments of the activity recorded from a single tail CVC neuron. A: spontaneous discharge frequency (core temperature (TC): 37.4°C) was unaffected by bilateral MUSC (arrowheads) into the DMH. B: increasing TC reduced the tail CVC neuronal discharge frequency to 1.6 Hz. MUSC into the DMH (arrowhead) had no effect on tail CVC neuronal discharge. C: 10 min after the second MUSC into the DMH (repeated portion of B), PGE2 into the POA increased the tail CVC neuronal discharge frequency. D: mean ± SE (n = 6 CVC neurons) discharge frequency of tail CVC neurons following bilateral MUSC into the DMH. Hatched bars represent the mean control discharge frequency during the 1 min prior to MUSC into the DMH. Increasing TC (WARMING) reduced the tail CVC neuronal discharge frequency. * indicates P < 0.05 compared to 10 min after MUSC in the DMH. ** indicates a significant (P < 0.05) increase in tail CVC neuronal discharge frequency compared to before PGE2 into the POA.
To demonstrate that the DMH sites at which MUSC nanoinjections were ineffective in reducing the PGE2-mediated increases in tail CVC neuronal activity were, indeed, those that reversed the PGE2 in the POA- or skin cooling-evoked increases in BAT SNA (23, 36, 38, 71), simultaneous recordings of tail CVC neurons and BAT SNA were accomplished in 3 cases. These experiments were complicated by the differential core/skin temperature thresholds for these two thermoregulatory effectors (40, 42) and the interaction of core temperature and their sensitivities to mediators of the febrile response (50, 59). Thus, increasing the core temperature above 37.5°C to reduce the high discharge frequency of tail CVC neurons present at core temperatures below 37.5°C, and thereby allow observation of a marked increase in tail CVC neuronal discharge in response to PGE2 in the POA (Fig. 3A), frequently resulted in a nearly complete elimination of the BAT response to PGE2 in the POA (Fig. 4B). Illustrated in Fig. 4A is a case in which spontaneous tail CVC neuronal activity was high at a core temperature of 37.5°C, resulting in only a modest increase in discharge frequency (peak increase: +37%) following PGE2 in the POA, and in which BAT SNA exhibited a robust response (+4300%) to PGE2 in the POA at a core temperature of 37.5°C. Bilateral nanoinjections of MUSC into the DMH promptly terminated the PGE2-evoked increase in BAT SNA (-97% of the pre-MUSC level at 5 min after MUSC into the DMH) and BAT temperature, but had little effect on that in the tail CVC neuron (-9% of the pre-MUSC level at 5 min after MUSC in the DMH). In the case illustrated in Fig. 4B, spontaneous tail CVC neuronal activity, which was reduced by maintaining a core temperature of 37.5°C, markedly increased in discharge frequency (peak increase: +3.2 Hz) following PGE2 in the POA; however, the BAT SNA response to PGE2 in the POA was nearly completely inhibited at 37.5°C. A subsequent reduction in skin and core temperatures resulted in an increase in BAT SNA (peak: +870% of control), likely a combination of the effect of the PGE2 in the POA and of the thermoregulatory response to stimulation of cutaneous cool afferents. Bilateral nanoinjections of MUSC into the DMH nearly reversed the increase in BAT SNA (-85% of the pre-MUSC level at 5 min after bilateral MUSC into the DMH), but had no effect on the increase in tail CVC neuronal activity (-3% of the pre-MUSC level at 5 min after MUSC in the DMH). Although in this example, there was a slight increase in skin temperature during the BAT SNA response, this was insufficient to affect BAT SNA amplitude by comparison to the dramatic reductions in BAT SNA time-locked to the bilateral deliveries of MUSC into the DMH (Fig. 4B). In the 3 experiments in which BAT SNA could be simultaneously increased with tail CVC neuronal activity, bilateral MUSC nanoinjections into the DMH reduced (p < 0.05) the stimulated (PGE2 in POA and/or skin cooling) BAT SNA to 11 ± 8% of the pre-MUSC level at 5 min after bilateral MUSC into DMH, but did not alter the PGE2-evoked activation of tail CVC neuronal activity (94±12% of the pre-MUSC level at 5 min after bilateral MUSC into the DMH).
Muscimol into the DMH has no effect on spontaneous, temperature-sensitive tail CVC discharge
Having determined that DMH neuronal discharge was not required for the activation of tail CVC neurons by PGE2 in the POA, we sought to determine if DMH neurons play a role in the temperature-sensitive drive maintaining CVC neuronal discharge, also thought to be mediated through POA (17, 64). For 6 tail CVC neurons from 5 rats, bilateral nanoinjections of MUSC into the DMH were made prior to the nanoinjection of PGE2 into the POA (Fig. 5). At core and skin temperatures of 36.7 ± 0.6°C and 36.2 ± 0.8°C, respectively, the mean basal firing rate of the tail CVC neurons was 1.9 ± 0.5 Hz (Fig. 5, A and D). Bilateral nanoinjections of MUSC (400 pmol each in 200 nl) into the DMH had no significant effect (P >0.1) on the spontaneous tail CVC neuron discharge (2.4 ± 0.5 Hz after MUSC into the DMH; Fig. 5, A and D). In the example in Fig. 5A, the mean CVC neuronal discharge frequency was 4.1 Hz prior to and 4.7 Hz at 5 min following bilateral MUSC nanoinjections into DMH.
Tail CVC neuron activity remained sensitive to elevations in skin and core temperature following MUSC into the DMH. Ten min after bilateral MUSC into the DMH, the rats were warmed to increase core temperature to 38.3 ± 0.2°C (TSK = 38.1 ± 0.3°C), which resulted in a fall (P < 0.05) in mean tail CVC neuron activity (Fig. 5, B and D) to 1.1 ± 0.3 Hz from the level of spontaneous activity at 10 minutes after bilateral MUSC into the DMH (“WARMING” Fig. 5D). Subsequent nanoinjection of PGE2 into the POA increased (P < 0.05) tail CVC neuron activity (Fig. 5C) to 2.7 ± 0.6 Hz at 5 min after PGE2 nanoinjection (Fig. 5D). In the example in Fig. 5C, PGE2 into the POA increased the tail CVC neuron activity from 1.8 Hz to 4.6 Hz within one minute. The peak discharge rates of tail CVC neurons at 5 and 10 min following PGE2 into the POA of rats that had received prior bilateral nanoinjections of MUSC into the DMH (Fig. 5D) were not different (P > 0.8) from those at 5 and 10 min following PGE2 into the POA of naïve rats (Fig. 3B).
Muscimol in the rRPa attenuates the PGE2-evoked activity of tail CVC neurons
Having established that neurons in the same DMH locus as those required for BAT SNA responses were not necessary for tail CVC responses, we sought to determine if the activity of neurons in the rRPa, a locus of sympathetic premotor neurons for CVC drive (2, 39, 41, 58), is necessary for the activation of tail CVC neurons evoked by PGE2 into the POA by observing the effect on tail CVC neuronal discharge (n = 9 CVC neurons) of MUSC into the rRPa subsequent to PGE2 into the POA. These rats had received prior bilateral nanoinjections of MUSC into the DMH that were without effect on the neuronal activation elicited by PGE2 into the POA (Figs. 3, 4). One minute prior to nanoinjection of MUSC into the rRPa, tail CVC neuronal discharge (3.2 ± 0.7 Hz) was not different (P > 0.4) from that 10 min after the prior nanoinjections of PGE2 into the POA (3.4 ± 0.7 Hz). Nanoinjection of MUSC (200 pmol in 100 nl) into the rRPa reduced tail CVC neuronal discharge (Fig. 3A and Fig. 4, A and B) to 1.8 ± 0.4 Hz (P < 0.001, Fig. 3B) at 1 min and to 1.2 ± 0.4 Hz at 5 min after MUSC into the rRPa -- a decrease of more than 60%. In the example in Fig. 3A, CVC unit discharge frequency was reduced from 7.0 Hz prior to the first nanoinjection of MUSC into rRPa, to 3.2 Hz after the second nanoinjection. In Fig. 4A and 4B, CVC neuronal firing rate at 5 minutes after MUSC in rRPa was reduced by 38% and by 100%, respectively, from the pre-MUSC levels.
Muscimol into the RVLM eliminated tail CVC neuron activity remaining after MUSC into the rRPa
Since both rRPa and RVLM contain sympathetic premotor neurons controlling CVC neurons (41, 58), we sought to determine if the RVLM was the source of the residual drive supporting CVC neuronal discharge after inhibition of the rRPa. We assessed the role of neurons in the RVLM (41) as the potential source of the activity remaining in 5 tail CVC neurons after stimulation by PGE2 into the POA and the subsequent reduction following MUSC into the rRPa, by subsequently applying MUSC (200 pmol in 100 nl) into the RVLM. Unilateral nanoinjection of MUSC into the right RVLM eliminated the residual tail CVC neuronal discharge (Fig. 3A): the mean discharge frequency of 1.3 ± 0.4 Hz prior to MUSC into the RVLM was reduced (P < 0.001) to 0 ± 0.0 Hz at 1 min after MUSC into the RVLM (Fig. 3B, n = 5 CVC neurons in 5 rats). The discharge of tail CVC neurons in animals that did not receive MUSC into the RVLM was eliminated by ganglionic blockade with iv hexamethonium (Fig. 4A).
Muscimol into the rRPa prior to PGE2 into the POA eliminates spontaneous tail CVC activity and prevents its PGE2-evoked activation
In 4 different tail CVC neurons recorded at TC = 37.8 ± 0.3°C and TSK = 37.1 ± 0.5°C, we observed that MUSC into the rRPa completely inhibited (P < 0.001) their spontaneous discharge (Fig. 6, mean discharge frequency: 1.6 ± 0.1 Hz at 1 min prior to MUSC into rRPa, 0 ± 0.0 Hz at 5 min after MUSC into rRPa). Both subsequent nanoinjection of PGE2 into the POA and subsequent transections of the neuraxis caudal to the POA and to the DMH failed to evoke increases in tail CVC neuronal activity (Fig. 6).
Fig. 6.
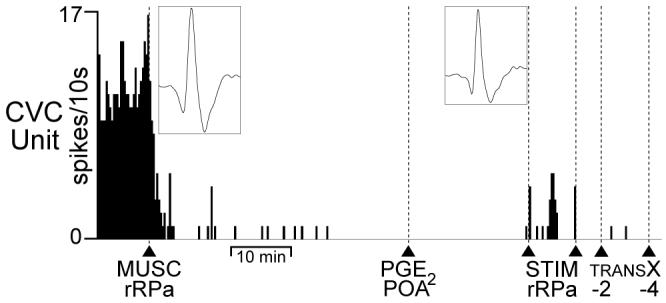
Inhibition of neurons in the rostral raphe pallidus (rRPa) eliminates spontaneous tail cutaneous vasoconstrictor (CVC) neuronal activity and prevents tail CVC neuron activation by prostaglandin E2 (PGE2) in the preoptic area (POA). Spike frequency record of a tail CVC neuron illustrates elimination of spontaneous CVC neuronal activity following muscimol (MUSC) into rRPa (arrowhead). Left inset is the average of 30 spontaneous CVC neuron action potentials prior to MUSC in rRPa (trace duration: 5.5 ms, peak amplitude: 30 μv). Subsequent PGE2 into the POA (arrowhead) failed to evoke tail CVC neuronal activity. Single electrical stimuli (STIM rRPa, between arrowheads) in the rRPa evoked CVC neuron action potentials (right inset: average of 30 stimulus-evoked CVC neuron action potentials, trace duration: 5.5 ms, peak amplitude: 23 μv), indicating that the CVC neuronal recording was maintained. Transections (TRANS X) at bregma -2 mm and -4 mm did not evoke tail CVC neuron activity after MUSC into the rRPa.
Localization of nanoinjection sites in the DMH, the rRPa and the RVLM
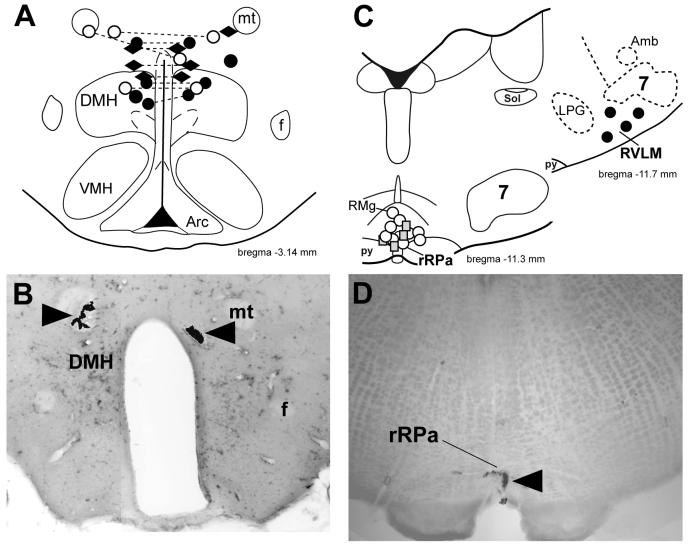
The anatomical locations of the MUSC nanoinjection sites targeting the DMH were confirmed histologically in 12 rats (Fig. 7, A and B). The nanoinjection sites at which MUSC was ineffective in altering the PGE2-elevated tail CVC discharge in experiments involving simultaneous recordings of a tail CVC neuron and BAT SNA were located among those that were similarly ineffective in experiments in which only tail CVC neurons were recorded. The sites of the bilateral MUSC nanoinjections in the DMH were located within the subregions of the DMH (a) at which bicuculline nanoinjection elicited a large increase in CVC neuronal activity (present study, Fig. 1), (b) which contain neurons necessary for the expression of PGE2- and skin cooling-evoked increases in BAT SNA (7, 23, 36, 38, 71) and (c) at which bicuculline nanoinjections elicit increases in BAT SNA as well as in renal SNA and heart rate (4, 13, 32, 52).
Fig. 7.
Sites of nanoinjections of muscimol into the dorsomedial hypothalamus (DMH), the rostral raphe pallidus (rRPa) and the rostral ventrolateral medulla (RVLM). A: locations of nanoinjections of fluorescent beads contained in the muscimol solution injected into the DMH before (diamonds) or after (circles) PGE2 into the POA. Connected symbols indicate identified bilateral injection sites. Open circles indicate the injection sites from the three animals where simultaneous recordings from BAT and tail cutaneous vasoconstrictor neurons were made. B: photomicrograph of a histological section (same rat as data in Figs 2 & 5) through the DMH containing bilateral nanoinjections (arrowheads) of fluorescent beads (shown in black) contained in the muscimol solution. C: locations of nanoinjection sites of muscimol into the rRPa after muscimol injection into the DMH (open circles) or prior to PGE2 injection into the POA (grey squares). The 4 identified sites of injections into the RVLM (shown as filled circles) were at a level that included a very caudal portion of the facial nucleus. D: photomicrograph of a histological section through the rRPa containing a nanoinjection (arrowhead) of fluorescent beads (shown in black) contained in the muscimol solution. Arc, arcuate nucleus; f, fornix; 7, facial nucleus; mt, mammillothalamic tract; py, pyramidal tract; RMg: nucleus raphe magnus; Sol: nucleus of the solitary tract; VMH, ventromedial hypothalamic nucleus. Drawings are modified from (44).
The anatomical locations of the MUSC nanoinjection sites targeting the rRPa were confirmed histologically in 13 rats (Fig. 7, C and D). The rRPa injection sites were located within the region of the rostral ventromedial medulla containing putative sympathetic premotor neurons necessary for activation of cutaneous vasoconstriction (39, 41, 58) and BAT thermogenesis (3, 29, 30, 34, 35)
The anatomical locations of the MUSC nanoinjection sites targeting the RVLM were confirmed histologically in 4 rats (Fig. 7C). The RVLM injection sites were located within the subregion of the rostral ventrolateral medulla containing putative CVC sympathetic premotor neurons (58)and implicated in the control of CVC neuronal activity (41, 46), as well as visceral vasoconstriction (31).
Discussion
Our results demonstrate that although activation of neurons in the DMH is a potential source of the excitatory drive to increase the activity of rat tail CVC neurons and reduce cutaneous heat loss, the activity of neurons in the DMH is not necessary for the increase in tail CVC neuron discharge evoked by application of PGE2 to the POA, a model of the febrile response, a component of the acute phase immune response in which heat retention mediated by cutaneous vasoconstriction contributes to an increase in core temperature (19, 43, 53, 67). Our finding that disinhibition of DMH neurons increased CVC neuronal activity parallels the recent demonstration that excitatory amino acid injections into DMH can activate CVC fibers in the plantar nerve (62). Similarly, the basal discharge of tail CVC neurons, which is sensitive to skin and core temperatures, is unaffected by inhibition of neuronal discharge in the DMH. In contrast, the activity of neurons in the rRPa, presumably sympathetic premotor neurons for cutaneous vasoconstriction (2, 18, 46, 58), contributes significantly to basal (i.e., thermoregulated) CVC neuronal discharge and to the increase in CVC neuronal discharge evoked by PGE2 into the POA. The latter is consistent with the demonstration that GABA nanoinjection into the rRPa attenuates spontaneous and icv PGE1-evoked activity of rat tail vasoconstrictor nerves (18). A component of the PGE2-mediated activity of most tail CVC neurons is also dependent on the discharge of neurons in the RVLM, consistent with the existence of a descending excitatory pathway from the RVLM influencing tail CVC neuronal discharge (41).
The discovery that the skin and core temperature-driven and the PGE2 in the POA-evoked discharge of CVC neurons are not dependent on the activity of neurons in the DMH, as well as our finding that transections of the neuraxis at several points between the POA and the rRPa yielded a sustained increase in tail CVC neuronal discharge are in marked contrast to the effects of similar treatments on the sympathetic outflow to BAT, the other principal, sympathetically-regulated, cold defense effector. In naïve animals, brain transections immediately caudal to the POA (5) or in the vicinity of the pontomedullary junction (51, 57) dramatically increase BAT thermogenesis, while transections made in the region caudal to the DMH but rostral to the pontomedullary junction have no effect on basal levels of BAT thermogenesis in normothermic animals (51) and reverse PGE2 in the POA-mediated increases in BAT SNA (personal observation). These results, in combination with studies involving nanoinjection of MUSC into the DMH which show the dependence of cold-defense and febrile excitation of BAT SNA on the activity of neurons in the DMH (23, 32, 36, 38, 71) and the demonstration that the POA sends descending GABAergic projections to the DMH (38) and to the rRPa (35) have suggested a model for the cold-defense and febrile activations of BAT sympathetic outflow. In this model, skin or core cooling or the interaction of PGE2 with EP3 receptors in the POA act to relieve a tonic, inhibition from the POA to intermediate sympathoexcitatory neurons in the DMH and to sympathetic premotor neurons in the rRPa. This disinhibition of sympathetic premotor neurons in rRPa and their augmented excitatory drive from disinhibited DMH neurons are postulated to account for the increases in BAT thermogenesis (29, 35, 36, 38). Indeed, both cold exposure and LPS administration increase fos expression in neurons in the DMH (3, 54, 69). Our data reveal a differential dependence of BAT SNA and tail CVC activity on the discharge of neurons in the DMH.
For the regulation of CVC neuronal activity, the finding that transections caudal to the POA increase CVC neuronal discharge and that this activation remains unabated during subsequent transections made at levels between the POA and the rRPa is consistent with a major inhibitory regulation of CVC sympathetic premotor neurons in the rRPa by a long descending inhibitory input from the POA and with CVC sympathetic premotor neurons in the rRPa receiving their principal excitatory drive from neurons in the medulla. These findings support the view that PGE2-mediated febrile and cold-defense increases in CVC activity arise principally from the relief of the descending, GABAergic inhibition from the POA to CVC sympathetic premotor neurons in the rRPa. In contrast to the pathway regulating BAT SNA, the absence of an effect of inhibition of the DMH neurons on either the cold-defense or febrile activations of tail CVC neurons suggests that neither on-going activity in DMH neurons nor an increase in their activity due to disinhibition is a required source of the excitatory drive to the CVC sympathetic premotor neurons mediating these responses. Rather, these data are consistent with a model in which the requisite source of excitation of the sympathetic premotor neurons regulating cutaneous vasoconstriction and heat loss to the environment resides in the medulla, or potentially in a complement of membrane channels supporting their spontaneous discharge.
Our data could also be consistent with a PGE2-evoked and cooling-driven increase in CVC neuronal discharge arising from inhibition of POA neurons that excite neurons which are located between the POA and the rRPa and which inhibit CVC sympathetic premotor neurons in the rRPa. Several observations suggest that a population of such neurons may exist in the rostral PAG. Neurons in the rostral PAG receive input from neurons in the POA that are activated (increased Fos expression) by environmental warming, but not cooling (68). Environmental warming also increases Fos expression in the rostral PAG (69) and chemical excitation of neurons within the rostral PAG increases both tail temperature and blood flow(73), presumably due to inhibition of CVC sympathetic outflow. Together, these data suggest that warm-sensitive neurons in the POA could send excitatory projections to CVC sympathoinhibitory neurons in the rostral PAG. Further studies would be required to determine if rostral PAG neurons exert an inhibitory influence on tail CVC sympathetic premotor neurons in the rRPa and whether they are required for thermal and febrile activation of cutaneous vasoconstriction.
The present data add to the body of evidence suggesting a differential regulation of thermoregulatory effectors mediated by effector tissue-specific pathways from the POA (17, 49, 55). The finding that inhibition of neurons in the DMH did not affect the responses of tail CVC activity to either increased core temperature or to application of PGE2 into the POA is in stark contrast to the elimination of the PGE2-evoked and skin cooling-evoked increases in BAT SNA and BAT thermogenesis following MUSC nanoinjection into the DMH (7, 23, 36, 38, 71). However, while the contrasting effects (sustained increase in tail CVC activity shown here, no increase in BAT SNA (32)) of brain transections placed immediately caudal to the DMH suggest a differential regulation of tail CVC activity and BAT SNA by a tonically active descending inhibitory pathway through this region, our data do not rule out the possibility that tail CVC and BAT sympathetic premotor neurons in the rRPa share a common inhibitory input from the POA (but only those for BAT require activity in DMH neurons). The absence of coherence between the skin cooling-evoked discharges in BAT SNA and in tail CVC SNA (40) is consistent with the existence of separate populations of sympathetic premotor neurons controlling these thermoregulatory motor outputs and with an absence of synchronizing communication between the circuits that determine the burst discharge of these two populations of sympathetic premotor neurons. The current data provide new information on the differences within central pathways, particularly the sympathoexcitatory neurons in the DMH, which could contribute to the lack of non-respiratory-related coherence between BAT SNA and tail CVC discharge.
It is doubtful that the failure of MUSC to inhibit evoked tail CVC neuronal activity can be attributed to failure to target the DMH appropriately or to a lack of efficacy of MUSC. That the DMH was appropriately targeted is demonstrated by the large activation of tail CVC neurons immediately following bicuculline into the DMH (Fig. 2). Additionally, a positive control for both DMH localization and MUSC efficacy is provided by our finding that when BAT SNA and tail CVC neuronal activity were both increased in response to PGE2 (or to the combination of PGE2 and reduced body temperature), tail CVC neuronal activity was unaffected by bilateral MUSC into the DMH while the discharge in BAT SNA was eliminated. In contrast to the MUSC nanoinjections into the DMH, those into the rRPa were effective at attenuating the PGE2-evoked increases in tail CVC neuronal activity and at completely inhibiting spontaneous tail CVC neuronal discharge. These results demonstrate the effectiveness of the MUSC and that the DMH injection sites were in a region containing neurons capable of exciting tail CVC sympathetic premotor neurons in the rRPa as well as those essential for the elaboration of PGE2-evoked increases in BAT thermogenesis.
Our experiments were conducted in anesthetized animals and chloralose, one component of our anesthetic regimen, may have lowered the threshold temperature and reduced the response dynamics of thermoregulation in our rats (10). Thus, we expect that the thermoregulatory balance point temperature (48) and the response gains might be lower in our experiments than they would be in unanesthetized rats, but such differences would not affect the validity of our conclusions regarding the thermoregulatory pathways which we have investigated. It is possible, however, that anesthesia could mask the contribution of other brain regions to the febrile response. In this regard, experiments in awake animals have suggested a role for neurons in the hypothalamic paraventricular nucleus in the febrile response (14, 21, 56, 74) as well as a role for norepinephrine in the POA (8) and for neurons in the locus coeruleus (1) in the elaboration of fever. Additionally, the present study employed nanoinjections of PGE2 into the POA to activate descending fever-promoting pathways and it remains to be determined if the pathways derived using this stimulus also mediate the febrile responses to intravenous lipopolysaccaride or polyinosinic-cytidylic acid.
The large increase in tail CVC activity elicited by bicuculline nanoinjection into the DMH suggests that disinhibition of CVC sympathoexcitatory neurons in the DMH is involved in eliciting an as yet unidentified, cutaneous vasoconstrictor response. One such response may be the increase in CVC discharge and reduction of skin blood flow evoked by certain types of stress. Psychological stress has been shown to stimulate cutaneous vasoconstriction in rats (66), rabbits (70) and humans (26). Neurons in the DMH have been postulated to play an essential role in the psychologically-evoked activation of sympathetic outflow (6, 27). The activity of neurons in the DMH is required for restraint stress-evoked tachycardia (28, 61), but not for that evoked by contextual fear (9). The role of DMH neurons in mediating the increases in tail CVC activity in response to various stress paradigms remains to be determined.
Muscimol nanoinjection into the rRPa eliminated spontaneous tail CVC discharge and completely prevented the increase in tail CVC neuronal activity when MUSC was delivered prior to PGE2 in the POA, but not when nanoinjected during the PGE2-evoked tail CVC activation. These results are consistent with the earlier demonstration that GABA into the ventromedial medulla, including the rRPa, abolished spontaneous SNA to the rat tail, but only attenuated the increased tail SNA evoked by icv administration of PGE1 (18). Spinal administration of serotonin can evoke increases in rat tail SNA after spinal transection (25) and blockade of spinal serotonin receptors attenuates evoked sympathetic outflow to the cutaneous vessels in the rabbit ear (39). Nanoinjection of serotonin into the spinal intermediolateral nucleus produces a long-lasting potentiation of NMDA-evoked increases in BAT SNA, even at doses of serotonin that are subthreshold for evoking increases in BAT SNA (24). Thus, serotonin may produce a long-lasting increase in the excitability of sympathetic preganglionic neurons (45), including those regulating CVC. We hypothesize that PGE2 into the POA elicits a release of spinal serotonin, potentially from neurons in the rRPa (3, 60, 65), that does not occur to a significant degree under conditions of spontaneous CVC discharge and that potentiates the excitability of CVC sympathetic preganglionic neurons to inputs such as those from the rRPa and from the RVLM (41). If this is the case, nanoinjection of MUSC into the rRPa prior to PGE2 in the POA could prevent the activation of neurons in the rRPa, including those that release spinal serotonin. However, application of MUSC into the rRPa after PGE2 administration in the POA could eliminate the descending glutamatergic excitation from rRPa, but serotonin would already have increased the excitability of CVC sympathetic preganglionic neurons to other premotor inputs, such as that from the RVLM (41). Serotonergic potentiation of a drive to CVC preganglionic neurons from RVLM-spinal neurons would thus be consistent with the elimination of the residual activity of tail CVC neurons by MUSC nanoinjection into the RVLM (Fig. 3).
Overall, these data reveal that although the DMH contains neurons whose connections enable them to elicit increases in tail CVC neuronal activity, activation of neurons in the DMH is not necessary for the stimulation of CVC neuronal discharge and the decreased skin blood flow that contributes to the increase in body temperature during fever. In contrast, febrile activation of CVC sympathetic outflow requires the activity of neurons in the rRPa, likely the sympathetic premotor neurons located there. Our results are consistent with the existence of a descending inhibitory pathway from the POA that regulates the discharge of CVC sympathetic premotor neurons in the rRPa. We also suggest that the potentiation of CVC preganglionic neuronal excitability by serotonin contributes to the increase in tail CVC sympathetic outflow evoked by PGE2 in the POA and that such a potentiation of CVC preganglionic neuronal responses to their spinal inputs may determine the effectiveness of descending excitation from the RVLM in influencing tail CVC neuronal activity. These findings support the concept that the coordinated responses of distinct central circuits regulating different thermoregulatory effectors underlie the maintenance of body temperature homeostasis and the PGE2-driven deviation from homeostasis during fever.
Perspective and Significance
Increasing evidence has, for many years, supported the potential for central neurons to selectively activate the autonomic outflows to different effector target tissues and thus for central autonomic networks to exert the differential control of effectors that comprises the patterned responses we observe to homeostatic challenges. The present study reveals an aspect of the central thermoregulatory circuit controlling cutaneous vasoconstriction that differs markedly from that controlling thermogenesis: a differential role for neurons in the dorsomedial hypothalamus in the elaboration of cold-defense and febrile activations of these two sympathetic outflows.
One of the principal differences among thermoregulatory effectors is their temperature thresholds for activation, which seem to be inversely related to their energetic costs: cutaneous vasoconstriction, requiring little energy expenditure, is activated at a relatively high body temperature in comparison to brown adipose thermogenesis with a greater metabolic cost and a lower temperature threshold for activation. Our finding that cutaneous vasoconstriction may be regulated by preoptic inhibitory neurons that project to the rostral ventromedial medulla, in contrast to those that regulate brown adipose tissue which are hypothesized to project to the dorsomedial hypothalamus, is consistent with differential temperature thresholds for activation of these effector systems since neurons in the preoptic area are expected to play a significant role in determining the temperature threshold characteristics of the responses of different thermal effectors.
Differences in the functional organization of the central circuits controlling these thermoregulatory effectors may also relate to their different roles in non-thermoregulatory responses. The cutaneous vascular bed has a large capacity and its resistance characteristics can play a role in cardiovascular homeostasis, in contrast to the sympathetic outflow to brown adipose tissue which is not regulated by the baroreceptor reflex. The considerable ability of brown adipose tissue to consume lipid during thermogenesis implicates its sympathetically-regulated energy expenditure in overall metabolic homeostasis and regulation of energy stores.
Acknowledgements
This research was supported by NIH grant NS40987. We thank Mr. Brad Sugden for preparation of histological material and Dr. Kazuhiro Nakamura for critical review of the manuscript.
REFERENCES
- 1.Almeida MC, Steiner AA, Coimbra NC, Branco LG. Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J Physiol. 2004;558:283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blessing WW, Nalivaiko E. Raphe magnus/pallidus neurons regulate tail but not mesenteric arterial blood flow in rats. Neuroscience. 2001;105:923–929. doi: 10.1016/s0306-4522(01)00251-2. [DOI] [PubMed] [Google Scholar]
- 3.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 4.Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512(Pt 3):883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiMicco JA, Sarkar S, Zaretskaia MV, Zaretsky DV. Stress-induced cardiac stimulation and fever: common hypothalamic origins and brainstem mechanisms. Auton Neurosci. 2006;126-127:106–119. doi: 10.1016/j.autneu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R47–63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 8.Feleder C, Perlik V, Blatteis CM. Preoptic norepinephrine mediates the febrile response of guinea pigs to lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1135–1143. doi: 10.1152/ajpregu.00067.2007. [DOI] [PubMed] [Google Scholar]
- 9.Furlong T, Carrive P. Neurotoxic lesions centered on the perifornical hypothalamus abolish the cardiovascular and behavioral responses of conditioned fear to context but not of restraint. Brain Res. 2007;1128:107–119. doi: 10.1016/j.brainres.2006.10.058. [DOI] [PubMed] [Google Scholar]
- 10.Grewe W, Janig W, Kummel H. Effects of hypothalamic thermal stimuli on sympathetic neurones innervating skin and skeletal muscle of the cat hindlimb. J Physiol (Lond) 1995;488:139–152. doi: 10.1113/jphysiol.1995.sp020952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Habler H, Bartsch T, Janig W. Rhythmicity in single fiber postganglionic activity supplying the rat tail. J Neurophysiol. 1999;81:2026–2036. doi: 10.1152/jn.1999.81.5.2026. [DOI] [PubMed] [Google Scholar]
- 12.Habler HJ, Bartsch T, Janig W. Respiratory rhythmicity in the activity of postganglionic neurones supplying the rat tail during hyperthermia. Auton Neurosci. 2000;83:75–80. doi: 10.1016/S0165-1838(00)00156-9. [DOI] [PubMed] [Google Scholar]
- 13.Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol. 2004 doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- 14.Horn T, Wilkinson MF, Landgraf R, Pittman QJ. Reduced febrile responses to pyrogens after lesions of the hypothalamic paraventricular nucleus. The American journal of physiology. 1994;267:R323–328. doi: 10.1152/ajpregu.1994.267.1.R323. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CD, Gilbey MP. Effects of aortic nerve stimulation on discharges of sympathetic neurons innervating rat tail artery and vein. The American journal of physiology. 1998;275:R942–949. doi: 10.1152/ajpregu.1998.275.4.R942. [DOI] [PubMed] [Google Scholar]
- 16.Johnson CD, Gilbey MP. Sympathetic activity recorded from the rat caudal ventral artery in vivo. J Physiol. 1994;476:437–442. doi: 10.1113/jphysiol.1994.sp020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanosue K, Hosono T, Zhang Y-H, Chen X-M. Neuronal networks controlling thermoregulatory effectors. In: Sharma HS, Westman J, editors. Progress in Brain Research. Elsevier Science B.V.; Amsterdam: 1998. pp. 49–62. [DOI] [PubMed] [Google Scholar]
- 18.Korsak A, Gilbey MP. Rostral ventromedial medulla and the control of cutaneous vasoconstrictor activity following i.c.v. prostaglandin E(1) Neuroscience. 2004;124:709–717. doi: 10.1016/j.neuroscience.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Lenhardt R, Kurz A, Sessler DI. Thermoregulation and hyperthermia. Acta Anaesthesiol Scand Suppl. 1996;109:34–38. [PubMed] [Google Scholar]
- 20.Lenhardt R, Negishi C, Sessler DI, Ozaki M, Ettinger K, Bastanmehr H, Lobo E. The effect of pyrogen administration on sweating and vasoconstriction thresholds during desflurane anesthesia. Anesthesiology. 1999;90:1587–1595. doi: 10.1097/00000542-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- 23.Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- 24.Madden CJ, Morrison SF. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marina N, Taheri M, Gilbey MP. Generation of a physiological sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol. 2006;571:441–450. doi: 10.1113/jphysiol.2005.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marriott I, Marshall JM, Johns EJ. Cutaneous vascular responses evoked in the hand by the cold pressor test and by mental arithmetic. Clin Sci (Lond) 1990;79:43–50. doi: 10.1042/cs0790043. [DOI] [PubMed] [Google Scholar]
- 27.McDougall SJ, Widdop RE, Lawrence AJ. Central autonomic integration of psychological stressors: focus on cardiovascular modulation. Auton Neurosci. 2005;123:1–11. doi: 10.1016/j.autneu.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 28.McDougall SJ, Widdop RE, Lawrence AJ. Medial prefrontal cortical integration of psychological stress in rats. Eur J Neurosci. 2004;20:2430–2440. doi: 10.1111/j.1460-9568.2004.03707.x. [DOI] [PubMed] [Google Scholar]
- 29.Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News in Physiological Sciences. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- 30.Morrison SF. Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience. 2003;121:17–24. doi: 10.1016/s0306-4522(03)00363-4. [DOI] [PubMed] [Google Scholar]
- 31.Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. The American journal of physiology. 1999;276:R962–973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- 32.Morrison SF, Cao WH, Madden CJ. Dorsomedial hypothalamic and brainstem pathways controlling thermogenesis in brown adipose tissue. Journal of Thermal Biology. 2004;29:333–337. [Google Scholar]
- 33.Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. The American journal of physiology. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Matsumura K, Hubschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogkoi Z, Konig M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ootsuka Y, Blessing WW. Activation of slowly conducting medullary raphe-spinal neurons, including serotonergic neurons, increases cutaneous sympathetic vasomotor discharge in rabbit. Am J Physiol Regul Integr Comp Physiol. 2005;288:R909–918. doi: 10.1152/ajpregu.00564.2004. [DOI] [PubMed] [Google Scholar]
- 40.Ootsuka Y, McAllen RM. Comparison between two rat sympathetic pathways activated in cold defense. Am J Physiol Regul Integr Comp Physiol. 2006;291:R589–595. doi: 10.1152/ajpregu.00850.2005. [DOI] [PubMed] [Google Scholar]
- 41.Ootsuka Y, McAllen RM. Interactive drives from two brain stem premotor nuclei are essential to support rat tail sympathetic activity. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1107–1115. doi: 10.1152/ajpregu.00005.2005. [DOI] [PubMed] [Google Scholar]
- 42.Owens NC, Ootsuka Y, Kanosue K, McAllen RM. Thermoregulatory control of sympathetic fibres supplying the rat’s tail. J Physiol. 2002;543:849–858. doi: 10.1113/jphysiol.2002.023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmes ED, Park CR. The regulation of body temperature during fever. Arch Environ Health. 1965;11:749–759. doi: 10.1080/00039896.1965.10664295. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; Sydney: 1986. [DOI] [PubMed] [Google Scholar]
- 45.Pickering AE, Spanswick D, Logan SD. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994;480(Pt 1):109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathner JA, McAllen RM. Differential control of sympathetic drive to the rat tail artery and kidney by medullary premotor cell groups. Brain Res. 1999;834:196–199. doi: 10.1016/s0006-8993(99)01568-1. [DOI] [PubMed] [Google Scholar]
- 47.Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: a source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 48.Romanovsky AA. Do fever and anapyrexia exist? Analysis of set point-based definitions. Am J Physiol Regul Integr Comp Physiol. 2004;287:R992–995. doi: 10.1152/ajpregu.00068.2004. [DOI] [PubMed] [Google Scholar]
- 49.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 50.Romanovsky AA, Ivanov AI, Shimansky YP. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- 51.Rothwell NJ, Stock MJ, Thexton AJ. Decerebration activates thermogenesis in the rat. J Physiol. 1983;342:15–22. doi: 10.1113/jphysiol.1983.sp014836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons in rats. Am J Physiol Regul Integr Comp Physiol. 2004 doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- 53.Saper CB, Breder CD. The neurologic basis of fever. N Engl J Med. 1994;330:1880–1886. doi: 10.1056/NEJM199406303302609. [DOI] [PubMed] [Google Scholar]
- 54.Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, DiMicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur J Neurosci. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 55.Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201:16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- 56.Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shibata M, Benzi RH, Seydoux J, Girardier L. Hyperthermia induced by pre-pontine knife-cut: evidence for a tonic inhibition of non-shivering thermogenesis in anaesthetized rat. Brain Res. 1987;436:273–282. doi: 10.1016/0006-8993(87)91671-4. [DOI] [PubMed] [Google Scholar]
- 58.Smith JE, Jansen AS, Gilbey MP, Loewy AD. CNS cell groups projecting to sympathetic outflow of tail artery: neural circuits involved in heat loss in the rat. Brain Res. 1998;786:153–164. doi: 10.1016/s0006-8993(97)01437-6. [DOI] [PubMed] [Google Scholar]
- 59.Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herkenham M, Romanovsky AA. Thermoregulatory responses of rats to conventional preparations of lipopolysaccharide are caused by lipopolysaccharide per se-- not by lipoprotein contaminants. Am J Physiol Regul Integr Comp Physiol. 2005;289:R348–R352. doi: 10.1152/ajpregu.00223.2005. [DOI] [PubMed] [Google Scholar]
- 60.Stornetta RL, Rosin DL, Simmons JR, McQuiston TJ, Vujovic N, Weston MC, Guyenet PG. Coexpression of vesicular glutamate transporter-3 and gamma-aminobutyric acidergic markers in rat rostral medullary raphe and intermediolateral cell column. J Comp Neurol. 2005;492:477–494. doi: 10.1002/cne.20742. [DOI] [PubMed] [Google Scholar]
- 61.Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka M, McAllen RM. Functional topography of the dorsomedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R477–486. doi: 10.1152/ajpregu.00633.2007. [DOI] [PubMed] [Google Scholar]
- 63.Tanaka M, McAllen RM. A subsidiary fever center in the medullary raphe? Am J Physiol Regul Integr Comp Physiol. 2005;289:R1592–1598. doi: 10.1152/ajpregu.00141.2005. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphe in thermoregulatory vasomotor control in rats. J Physiol. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toth IE, Toth DE, Boldogkoi Z, Hornyak A, Palkovits M, Blessing WW. Serotonin-synthesizing neurons in the rostral medullary raphe/parapyramidal region transneuronally labelled after injection of pseudorabies virus into the rat tail. Neurochem Res. 2006;31:277–286. doi: 10.1007/s11064-005-9018-2. [DOI] [PubMed] [Google Scholar]
- 66.Vianna DM, Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci. 2005;21:2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]
- 67.Weinberg JR, Innes JA, Thomas K, Tooke JE, Guz A. Studies on the circulation in normotensive febrile patients. Q J Exp Physiol. 1989;74:301–310. doi: 10.1113/expphysiol.1989.sp003273. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida K, Maruyama M, Hosono T, Nagashima K, Fukuda Y, Gerstberger R, Kanosue K. Fos expression induced by warming the preoptic area in rats. Brain Res. 2002;933:109–117. doi: 10.1016/s0006-8993(02)02287-4. [DOI] [PubMed] [Google Scholar]
- 70.Yu YH, Blessing WW. Cutaneous vasoconstriction in conscious rabbits during alerting responses detected by hippocampal theta-rhythm. The American journal of physiology. 1997;272:R208–216. doi: 10.1152/ajpregu.1997.272.1.R208. [DOI] [PubMed] [Google Scholar]
- 71.Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]
- 72.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 73.Zhang YH, Hosono T, Yanase-Fujiwara M, Chen XM, Kanosue K. Effect of midbrain stimulations on thermoregulatory vasomotor responses in rats. J Physiol. 1997;503(Pt 1):177–186. doi: 10.1111/j.1469-7793.1997.177bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang YH, Lu J, Elmquist JK, Saper CB. Lipopolysaccharide activates specific populations of hypothalamic and brainstem neurons that project to the spinal cord. J Neurosci. 2000;20:6578–6586. doi: 10.1523/JNEUROSCI.20-17-06578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]