Abstract
There is considerable debate and controversy surrounding the cause(s) of Alzheimer disease (AD). To date, several theories have gained notoriety, however there has yet to be any one that garners universal acceptance. In this review, we provide evidence for the oxidative stress-induced AD cascade that posits aged mitochondria as the critical origin of neurodegeneration in AD.
Keywords: Alzheimer disease, amyloid-beta, free radicals, mitochondria, mitochondrial dynamics, oxidative stress
Introduction
Alzheimer disease (AD) is a neurodegenerative condition responsible for the cognitive deterioration of 26.6 million people in the world and it was projected that the worldwide prevalence of AD will grow four fold to 106.8 million with 1 in 86 people living with AD by the year 2050 where 59% of cases will be in Asia1. Age is the primary risk factor for AD such that the incidence of the disease is 15% among individuals over the age of 65 and almost 50% among those over 852. While many specific aspects of AD have been documented, there has yet to be any clear understanding of its pathological initiation and progression. To be sure, molecular aberrations in the cell elicit neuronal failure through a variety of well-established mechanisms, including: i) oxidative stress3; ii) abnormal protein folding and aggregation4; iii) cell cycle dysregulation5; iv) mitochondrial dysfunction6; v) synaptic failure7, 8; vi) inflammation9; vii) loss of calcium regulation10; viii) defective cholesterol metabolism11, 12; ix) vascular alterations13, 14; and x) neurotrophin deprivation11. However, the causal relationships governing these factors remain unknown.
AD is described by the successive degeneration of neurons first in the entorhinal cortex of the mediotemporal lobe, followed by those of the CA1 region of the hippocampus, the CA 2/3 regions, the CA4 region, and the neocortex15. Despite this predictable vulnerability, the molecular mechanisms governing it are unclear.
Amyloid-β (Aβ) and the hyperphosphorylated form of the microtubule-associated protein tau are two hallmark pathologies of AD that are critically involved in neuronal death. While the function of Aβ is unclear, its accumulation into insoluble fibrils and plaques increases over the course of AD progression, causing severe cellular burden. The peptide is formed by the proteolytic cleavage of its protein precursor, amyloid-β protein precursor (AβPP), via sequential enzymatic actions of β-site AβPP cleaving enzyme 1 (BACE-1) and γ-secretase, a protein complex with presenilins 1 and 2 at its catalytic core11, 16. Aβ is associated with multiple cascades that are thought to result in neuronal damage17, 18 and as such, Aβ is generally considered the primary mediator of neurodegeneration in AD19. The Amyloid Cascade Hypothesis maintains this position, although its lack of therapeutic translation has become a major criticism20.
The microtubule associated protein tau is similarly associated with AD progression, and its accumulation in the form of neurofibrillary tangles (NFTs) is a strong correlate of dementia in AD11, 21. NFTs are used as a post mortem diagnostic criterion for AD22. Tau becomes phosphorylated on serine and threonine residues that flank the microtubule binding domain. Upon hyperphosphorylation, tau is unable to bind to and stabilize cytoskeletal microtubules and instead self-aggregates into NFTs in and around the cell23.
AD is typically a sporadic condition. 90% of all cases are sporadic in nature and are characterized as late-onset AD (LOAD), with an age of onset of around 65 years. Familial AD (FAD), on the other hand, is an early-onset, genetic condition caused by several known autosomal dominant mutations; its age of onset is typically around ages 40–6024. Mutations that yield FAD all involve or result in an alteration in the ratio of Aβ42/Aβ40; this is perhaps the most touted evidence both for and against25, 26 the aforementioned Amyloid Cascade Hypothesis27. Dominantly inherited mutations in presenilin 1 (PS1), an essential component in the γ-secretase complex, account for the majority of FAD cases, and more than 170 such mutations have been identified in PS1 on chromosome 1428. In addition to PS1, mutations in presenilin 2 (PS2) (on chromosome 129) and AβPP (on chromosome 2130) also elicit FAD, although they are less prevalent. These mutations also affect the ratio of Aβ42/40. Importantly, both LOAD and FAD present identical brain lesions and patterns of neurodegeneration. Notably and somewhat contradictory, LOAD leads to a reduced ratio of Aβ42/40, whereas FAD leads to an increased ratio31.
Neuronal Oxidative Stress: Sources and Vulnerabilities
Aerobic respiration, like any other biochemical or physical process, is not 100% efficient. The mechanism by which mitochondria process carbohydrates and establish a proton gradient for the synthesis of ATP (i.e., the tri-carboxylic acid (TCA) cycle and oxidative phosphorylation) involves the controlled transfer of electrons from strong reducing agents to strong oxidizing agents. Some of the free radicals thus generated escape; rather than appropriately transporting their electron to the next molecule in the cascade, they abandon the inner membrane of the mitochondria and impose alterations on other macromolecules within the cell. These alterations are often detrimental: DNA/RNA oxidation may yield fragmentation and deficiencies in repair machinery32, 33; oxidative modification of enzymes and metabolic signaling proteins may lead to metabolic impairments34; and protein cross-linkages and impaired proteolysis network resulting from oxidative modification may render proteins insoluble and prone to aggregate abnormally35–37.
The ability of the cell to adapt to oxidative damage is robust, yet finite. Endogenous antioxidants are intended to sequester the formation of free radicals. Superoxide dismutase, for instance, catalyzes the conversion of the potent free radical superoxide to hydrogen peroxide and water. Other important oxidative stress proteins include catalase, glutathione peroxidase, glutathione reductase, and heme oxygenase38. Under normal conditions, the majority of free radicals are sequestered within the mitochondria (i.e., their place of origin). Notably, however, three important factors (age, metabolic demand, and disease) exacerbate the vulnerability of cells to oxidative burden.
Statistically, the longer a cell is respiring, the more reactive oxidative species (ROS) go unsequestered within that cell. It is estimated that, on average, cells utilize 1013 molecules of oxygen per day, with 1% of the associated reactions producing unsequestered ROS. Therefore, on average, 1011 molecules of ROS are generated in a given cell every day. Even with sufficiently efficient antioxidant mechanisms, some ROS will unavoidably escape and cause damage to the surroundings which may accumulate with time, especially in long living cells. Age is thus a great risk factor for oxidative stress. Likewise, high metabolic activity, which requires more oxidative phosphorylation per unit time, and thus involves a greater number of toxic species generated per unit time, also renders a cell more vulnerable to oxidative insults. A neuron, which requires energy for axonal transport, vesicular release, ion pump operation, electrochemical gradient maintenance, and the like, requires much more oxygen per unit time than any other cell in the body. Metabolic demand is therefore another risk factor for oxidative stress. Disease conditions that produce mutations in cellular antioxidant defensive machinery provide a third risk factor for oxidative damage.
Altogether, the brain represents a tremendously vulnerable region to oxidative damage. Despite comprising only 2–3% of total body mass, 20% of basal oxygen supply is utilized by the brain. Moreover, neurons are among the longest-lived cells in the body and thus must continue to survive and function for decades with the same metabolic machinery. The presence of transition metals in the brain that catalyze oxidative reactions furthers the appearance of oxidative pathologies. Iron and copper, for instance, increase the likelihood of an oxidation/reduction reaction taking place39, and the regional concentration increases of these metals in the brain compound the risk factors described above. Furthermore, the brain suffers a relative paucity of antioxidant systems as well as an increase in polyunsaturated fatty acids (prime targets for ROS)40. Given the aforementioned, an aging brain is tremendously susceptible to oxidative deterioration.
Oxidative Stress: Prominent and Early Feature of Alzheimer Disease
Free radical-induced damage to macromolecules has been well documented in AD and deposition of redox-active ions, capable of generating the most damaging hydroxyl radicals through Fenton reaction, likely exacerbates both the spectrum of molecules and cellular areas affected. DNA and RNA oxidation, marked by increased levels of 8-hydroxy-2-deoxyguanosine and 8-hydroxyguanosine (8-OHG), suggest a higher frequency of DNA fragmentation and aberration in DNA repair41. Oxidative modification of metabolic proteins, such as creatine kinase BB, cytochrome c oxidase (COX), and ketoglutarate dehydrogenase complex (KGDH), have been demonstrated by elevated levels of protein carbonyl and nitration of tyrosine residues 42 and indicate impaired metabolic activity. Lipid peroxidation, marked by thiobarbituric acid reactive substances, malondialdehydes, 4-hydroxy-2-transnonenal (HNE), and isoprostane, as well as by altered phospholipid composition, suggests altered membrane integrity. Oxidative modification of sugars, marked by increased glycation and glycooxidation, indicates impaired cellular ability to adequately process critical carbohydrates43. Each of these aspects is elevated in AD as compared to control cases42.
Moreover, mitochondrial abnormalities, attributed to oxidative stress-related damages, are well-established in AD. As stated above, specific enzymes involved in electron transport and the TCA cycle, such as KGDH and COX, have been documented as modified via ROS in AD44 45. Mitochondrial DNA (mtDNA) modification46 and calcium dysregulation47–49 occur in AD to a great extent, and increased numbers of mitochondria with broken cristae, altered size and shape, and aberrant intracellular localization are elevated in AD neurons47, 50, 51. These latter phenomena are the result of impaired mitochondrial dynamics that can be caused by and also amplifies oxidative stress52. Mitochondrial fission and fusion, the ongoing processes that ensure organelle stability within the dynamic cellular environment, rely on critical membrane proteins to successfully occur. Fusion enables the exchange of lipid membrane in inter-mitochondrial components (i.e., mtDNA and fission/fusion proteins) such that the cell is populated by healthy, normal mitochondria53. Fission, on the other hand, coupled with fusion and autophagy, enables the sequestration and elimination of irreversibly damaged mitochondria and mitochondrial content54. These dynamics are the necessary means by which the cell dampens its inevitable accumulation of oxidative free radical-induced damage. Over the lifetime of a cell, however, oxidative damages and transcriptional errors (due to alteration of mtDNA) reach a critical threshold. Oxidative stress ultimately propagates throughout the cell as mitochondria become abnormally shaped and localized47, 55–58.
Importantly, increasing evidence demonstrates that the oxidative modifications occurring in vulnerable neurons in AD occur prior to hallmark pathologies of the disease 35. That is, the markers of oxidative damage are found in the cytoplasm of neurons prior to any indication of degeneration in AD brains59, 60. In fact, the hallmark pathologies such as amyloid plaques and neurofibrillary tangles may be an adaptation in response to elevated oxidative stress. More recent studies found that patients who suffered from mild cognitive impairment (MCI), considered as prodromal stage of AD, demonstrated depleted antioxidants along with increased lipid peroxidation in the plasma and lymphocytes61. Elevated protein/RNA oxidation and increased redox-active iron were also documented in the brain of MCI32, 62, 63. Transgenic mouse models of AD also demonstrate oxidative damage prior to Aβ deposition64. The mitochondrial abnormalities described above provide considerable support for this primacy. Specifically, vulnerable neurons in AD and MCI exhibit severe metabolic deficiencies well before any clinical manifestations of disease. Neuroimaging studies and neuropsychological tests have indicated impaired cerebral metabolism prior to any evidence for functional impairment or brain atrophy induced by AD pathologies51, 65. This metabolic impairment is likely the result of the alterations in mitochondrial dynamics and intracellular localization66, which, in turn, increases the production of oxidative damage to mtDNA and membrane proteins leading to a vicious cycle.
Alzheimer-Specific Factors and Oxidative Stress
Besides the fact that oxidative stress characterizes aging, the greatest risk factor for AD, genetic and pathological factors associated with AD are also implicated in the regulation or being regulated by oxidative stress. For example, presenilins are needed for the import of around 50% of cellular copper and zinc67 and increased presenilin 2 expression increases DNA fragmentation and produces apoptotic changes68, which are both important consequences of oxidative damage. The translation of amyloid precursor protein (APP) is regulated by iron influx via an iron-responsive element (IRE) RNA stem loop in its 5'-untranslated region69. APP is the dedicated ferroxidase in neurons responsible for the export of irons and the elevation of redox-active iron in neurons in AD is likely due to reduced ferroxidase activity of APP inhibited by zinc70. ApoE is a strong chelator of copper and iron, both of which are important redox-active transition metals68. Apo E has a beneficial effect against free radicals since it reduced neuronal death caused by hydrogen peroxide and β-amyloid through antioxidant activity in an isoform-dependent manner with E4 being the least effective71. Interestingly, apo E is sensitive to attacks by free radicals, with the responses being isoform dependent such that E4 is most vulnerable72. Indeed, cerebral cortex of autopsied brain samples from patients with APP or presenilin gene mutations or apoE4 allele demonstrated higher oxidative stress73, 74.
Aβ may itself elicit oxidative damage to surrounding cells through, for example, activation of microglia and astrocytes that activate multiple ROS pathways75, including an upregulation of L-tryptophan metabolism through the kynurenine pathway76. Several intermediates in this pathway, namely 3-hydroxykynurenine and quinolinic acid, are neurotoxic, and the Aβ-induced increase in their production subjects surrounding neurons to oxidative damage77, 78. Aβ-induced lipid peroxidation yields several reactive aldehydes (including HNE) that are capable of harmfully modifying membrane proteins. HNE production specifically disrupts iron homeostasis by impairing glucose transporters79–82. Iron and copper accumulation within neuritic plaques (chiefly composed of Aβ) further facilitate free-radical generation83. When Aβ binds catalytic amounts of copper, hydrogen peroxide is created, which contributes to oxidative stress as it produces hydroxyl radical via the Fenton reaction.
Aβ has been demonstrated to have antioxidant capacities intracellularly and may actually be secreted as a compensatory measure against oxidative stress35, 84. That is, in vivo and in vitro studies of neuronal responses to oxidative stress indicate an increase in Aβ production85, which is followed by a corresponding reduction in oxidative stress86, 87. Moreover, markers of oxidative stress, such as 8-OHG, are known to be reduced in neuronal populations characterized by Aβ deposition, and elevated in vulnerable regions lacking Aβ 86, 87. The data clearly suggests that the neurons, in the former scenario, have been salvaged from oxidative stress by Aβ.
Tau hyperphosphorylation has been demonstrated as a pathological result of oxidative stress59, 84, 87–89. It is intriguing to note that most neuronal loss during the course of neurodegeneration occurs where the levels of oxidative stress are the highest and subsequent deposition of NFTs decreases these levels59. Of note, and supporting this, neurons that accumulate NFTs are able to survive for decades and be functionally integrated in cortical circuits90, 91. Mechanistically, phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin92. Regardless, the abnormal accumulation of hyperphosphorylated tau in the form of NFTs occurs subsequently to oxidative stress-induced damages.
Oxidative Stress and Alzheimer disease: Ubiquity vs. Specificity
Disease is defined by the deficits in specific functional output underlied by anatomical and biochemical/structural changes elicited by insults and adaptations and/or failure of adaptations. Interestingly, oxidative stress is a prominent biochemical change not only found in AD, but also found in almost all major neurodegenerative diseases including Parkinson disease, amyotrophic lateral sclerosis and Huntington disease93. Since oxidative stress is considered a major contributor to aging, the greatest risk factor for all the age-related neurodegenerative diseases, it is perhaps not surprising that oxidative stress is believed to be a causative factor or at least an ancillary factor in the pathogenesis of these diseases. However, given the different neuronal populations involved and distinct pathology that developsd in these various neurodegenerative diseases, such a ubiquity of the presence of oxidative stress raises the obvious question of specificity of its role in the pathogenesis of each of these diseases. For example, how oxidative stress specifically leads to neuronal death in hippocampus and cortex in AD while in substantia nigra in PD? One possibility is that the selective neuronal death may be caused by increased oxidative stress in selective brain regions in these diseases. For example, in AD, large body of evidence support the elevated oxidative stress in disease-affected area such as hippocampus and cortex but not in disease-spared area such as cerebellum42. Similarly, heightened oxidative stress is consistently demonstrated in substantia nigra but not in other unaffected brain region in PD94. If this is the case, the question becomes how selective oxidative stress occurs in these diseases, especially in the familial cases where causative mutations in specific genes affect all cells? It is likely the specific characteristics of various neuronal populations involved in different diseases contribute to such selectivity. Neurons with long axons and multiple synapses have higher metabolic demands that may render them more prone to oxidative attacks at a steady state level and thus become more vulnerable to additional disease-related changes. For example, CA1 neurons of hippocampus demonstrate higher levels of superoxide anion than parietal cortical or CA3 neurons which is at least one of the reasons that they are more vulnerable to global ischemia-induced dell death95. Dopaminergic neurons are exposed higher steady state levels of oxidative stress produced by metabolism of dopamine which make them more vulnerable to insults that affect the dopamine metabolism96. It is also possible the selectivity is due to the specificity of initiating factor(s). Such initiating factor(s) may not be a single event, but more likely a complex interaction between the genetic/epigenetic background of each individual and the environment which may determine the specific neuronal population affected. Overall, different diseases may each display a distinct oxidative stress pattern that likely will lead to a different homeostatic balance that is finely tuned to minimize cell death and more studies are clearly necessary to understand the role of oxidative stress in each disease.
The Age-Related Mitochondrial Cascade of Alzheimer Disease
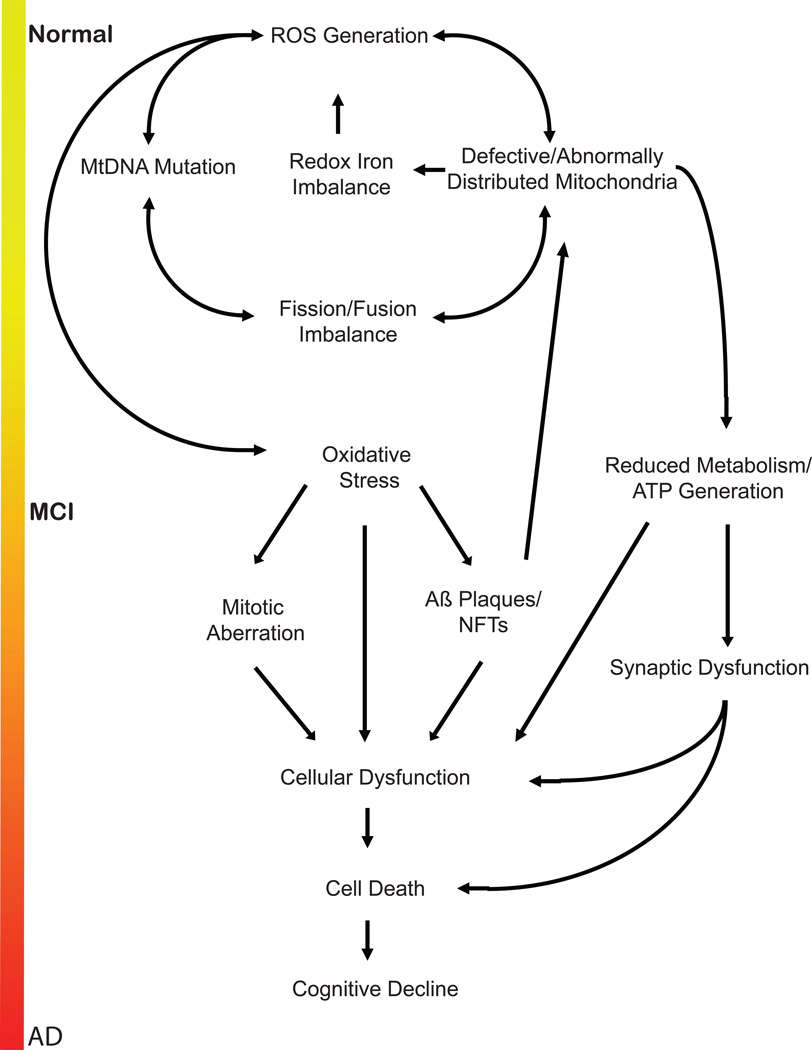
Combining the evidence listed above reveals the following: i) AD is primarily an age-related disorder; ii) the brain is the most metabolically demanding region of the body; iii) free radical release invariably results from the tremendous amount of oxidation/reduction reactions that take place within neurons; iv) resident antioxidant systems, though robust, are ultimately finite in capacity; v) mitochondria are the metabolic centers of the cell and experience the earliest detriments in AD; vi) Aβ peptides and NFTs accumulate after oxidative stress has already incurred severe damage, and seem to exert a compensatory response to cerebral oxidative stress. Fitting the pieces together, we assert that over the course of a lifetime, statistically guaranteed free radical damage accumulates within mitochondria, affecting mtDNA, membrane proteins, and calcium homeostasis. The antioxidant response of neurons is able to stagnate the effects of this stress for years; metabolic deficits resulting from mitochondrial dysfunctions and perturbed localizations do not appear until well into adult life, and are the very first aspects of dementia. However, the increased presence of transition metals and polyunsaturated fatty acids in the brain render neurons particularly susceptible to damage, and once mitochondrial mutations become widespread within the cell, compensatory responses, such as Aβ secretion, begin to appear to fight further damage. Eventually, secreted Aβ becomes subject to oxidative stress (i.e., dityrosine cross-linkages inhibit its solubility, promoting its aggregation62, 97). The cascade worsens as Aβ then induces neuronal detriments, producing further oxidative stress, inflammation, and synaptic dysfunction. The well-established pathophysiological inclusions of AD, including cell cycle aberration, inflammation, synaptic dysfunction, and loss of calcium regulation, become increasingly relevant, and affected neurons ultimately die. Thus, after decades of stress and increasing malfunction, cognitive decline spreads and yields clinical AD (Figure 1).
Figure 1.
Oxidative stress-induced cascade of Alzheimer disease. As stated in the text, the inevitable generation of free radicals within neurons eventually compromises the integrity of mitochondria, leading to a cascade of destructive events that elicits the hallmark features of Alzheimer disease. After years of accumulated burden, neuronal death and cognitive decline result.
Though its logic is quite appealing, this description of AD, like all previous hypotheses posited as explaining the disease, suffers some significant shortcomings. First, it does not adequately explain the predictable and consistent spread of neurodegeneration from the entorhinal cortex of the mediotemporal lobe, though the hippocampus, and into the neocortex. Perhaps this orderly passage reflects the metabolic demands of distinct regions within the brain. That is, the entorhinal cortex may require the highest metabolic activity due to its significant involvement in memory formation and retrieval (an ongoing process that occurs continuously throughout life). It would thus be the first victim of the oxidative stress accumulation that leads to degeneration by a matter of simple probability. The remaining neurons in the connective pathway would suffer by connective association, and the disease would spread. Notably, no evidence for this postulation exists, although its potential validity merits further investigation.
The mitochondrial cascade also seems to fail to explain the critical role of AD mutations in FAD and Down’s syndrome. A more careful look, however, ameliorates this misperception. In particular, the timeline of LOAD is such that Aβ accumulation and aggregation occurs late in adult life, incurring deficits on cognition typically after age 65. The mitochondrial cascade attributes this latency to the prolonged period of oxidative stress accumulation that takes years to elicit any real metabolic deficits and subsequent Aβ secretion. Once Aβ is secreted, however, and becomes oxidatively processed into insoluble fibrils, it exerts damage to surrounding cells via a variety of mechanisms. FAD represents a shortcut in this otherwise lengthy process. That is, mutations that increase the cleavage and processing of AβPP subject neurons to an early secretion and aggregation of Aβ. Affected patients thus skip the necessary accumulations of mitochondrial damage that typically take decades to elicit AD pathologies. Rather, Aβ is aberrantly cleaved regardless of the endogenous oxidative environment, and FAD patients experience clinical symptoms much earlier than do LOAD patients. Again, little experimental evidence has supported this postulation; however, it seems quite likely given the mountains of data.
Therapeutic Implications and Conclusions
We are currently faced with a strong incentive to produce a therapeutic agent that prevents AD onset. Based on the evidence here presented, the most likely method of adequate prevention should come from antioxidant administration. Preventing the accumulation of damaging species within vulnerable neurons would ideally protect them from the deleterious cascades that ROS accumulation yields. Unfortunately, there has been little success with antioxidant therapies thus far98. It must be emphasized that the failure of antioxidant clinical trials does not necessarily nullify the hypothesis that oxidative stress being a critical contributor to the disease because of the many limitations such as that in patient selection (it would be preferable to select patients with low antioxidants) and in monitor/analysis of the respondents (it would be preferable to monitor oxidative stress surrogate markers before and during the treatment to make certain the therapy compliance) involving such clinical studies. Better antioxidants, especially the ones that target the major ROS production sites, are needed. Several agents under investigation, such as the MitoQ, acetyl-L-carnitine, and α-lipoic acid, do show potential; however, an effective method of treatment is far from complete47, 99–110. Based on the many prospective studies and the complexity of the redox system in vivo, a balanced combination of several antioxidants may also be needed to exert a significant effect on the prevention of AD but it appears not much has been done on this aspect. Although agents that ameliorate secondary pathologies to AD are under increasing scrutiny22, these treatments would only stagnate the course of disease progression and could not achieve its full eradication. To do so, the community must acknowledge all aspects of disease pathogenesis and establish an unbiased understanding of its progression. Only then can research be adequately focused such that appropriate therapeutic intervention is possible. We here pose the oxidative stress looking glass as our best hope.
Acknowledgments
Funding Information
Work in the authors’ laboratories is supported by the National Institutes of Health (NS083385-01 to XZ) and the Alzheimer’s Association (IIRG-10-173358 to XZ and IIRG-10-173471 to GP).
References
- 1.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 2.Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. doi: 10.1016/s0074-7742(08)60607-8. [DOI] [PubMed] [Google Scholar]
- 3.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LC, Diamond MI, Duff KE, Hyman BT. Mechanisms of protein seeding in neurodegenerative diseases. JAMA Neurol. 2013;70:304–310. doi: 10.1001/jamaneurol.2013.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moh C, Kubiak JZ, Bajic VP, Zhu X, Smith MA, Lee HG. Cell cycle deregulation in the neurons of Alzheimer's disease. Results Probl Cell Differ. 2011;53:565–576. doi: 10.1007/978-3-642-19065-0_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu X, Perry G, Smith MA, Wang X. Abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2013;33(Suppl 1):S253–S262. doi: 10.3233/JAD-2012-129005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 8.Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer's disease. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krstic D, Knuesel I. Deciphering the mechanism underlying late-onset Alzheimer disease. Nat Rev Neurol. 2013;9:25–34. doi: 10.1038/nrneurol.2012.236. [DOI] [PubMed] [Google Scholar]
- 10.Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease. Biochim Biophys Acta. 2011;1813:965–973. doi: 10.1016/j.bbamcr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 12.Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ (Clinical research ed) 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Torre JC. Pathophysiology of neuronal energy crisis in Alzheimer's disease. Neurodegener Dis. 2008;5:126–132. doi: 10.1159/000113681. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X, Smith MA, Honda K, Aliev G, Moreira PI, Nunomura A, et al. Vascular oxidative stress in Alzheimer disease. J Neurol Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schonheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004;25:697–711. doi: 10.1016/j.neurobiolaging.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 17.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 18.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 20.Herrup K. Reimagining Alzheimer's disease--an age-based hypothesis. J Neurosci. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonda DJ, Lee HP, Lee HG, Friedlich AL, Perry G, Zhu X, et al. Novel therapeutics for Alzheimer's disease: an update. Curr Opin Drug Discov Devel. 2010;13:235–246. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis. 2013;33(Suppl 1):S123–S139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- 24.Goedert M, Strittmatter WJ, Roses AD. Alzheimer's disease. Risky apolipoprotein in brain. Nature. 1994;372:45–46. doi: 10.1038/372045a0. [DOI] [PubMed] [Google Scholar]
- 25.Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, Nakamura M, et al. Reexamining Alzheimer's disease: evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis. 2009;18:447–452. doi: 10.3233/JAD-2009-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau. Nat Neurosci. 1998;1:355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- 28.Schellenberg GD, Bird TD, Wijsman EM, Orr HT, Anderson L, Nemens E, et al. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992;258:668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- 29.Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KA, Weber JL, et al. A familial Alzheimer's disease locus on chromosome 1. Science. 1995;269:970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- 30.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 31.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 32.Nunomura A, Tamaoki T, Motohashi N, Nakamura M, McKeel DW, Jr, Tabaton M, et al. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley-Whitman MA, Timmons MD, Beckett TL, Murphy MP, Lynn BC, Lovell MA. Nucleic Acid Oxidation: An early feature of Alzheimer's disease. J Neurochem. 2013 doi: 10.1111/jnc.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardas SS, Sultana R, Clark AM, Beckett TL, Szweda LI, Murphy MP, et al. Oxidative modification of lipoic acid by HNE in Alzheimer disease brain. Redox Biol. 2013;1:80–85. doi: 10.1016/j.redox.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sayre LM, Smith MA, Perry G. Chemistry and biochemistry of oxidative stress in neurodegenerative disease. Curr Med Chem. 2001;8:721–738. doi: 10.2174/0929867013372922. [DOI] [PubMed] [Google Scholar]
- 36.Di Domenico F, Coccia R, Cocciolo A, Murphy MP, Cenini G, Head E, et al. Impairment of proteostasis network in Down syndrome prior to the development of Alzheimer's disease neuropathology: redox proteomics analysis of human brain. Biochim Biophys Acta. 2013;1832:1249–1259. doi: 10.1016/j.bbadis.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Castellani RJ, Moreira PI, Aliev G, Shenk JC, Siedlak SL, et al. Hydroxynonenal-generated crosslinking fluorophore accumulation in Alzheimer disease reveals a dichotomy of protein turnover. Free Radic Biol Med. 2012;52:699–704. doi: 10.1016/j.freeradbiomed.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aksenov MY, Tucker HM, Nair P, Aksenova MV, Butterfield DA, Estus S, et al. The expression of key oxidative stress-handling genes in different brain regions in Alzheimer's disease. J Mol Neurosci. 1998;11:151–164. doi: 10.1385/JMN:11:2:151. [DOI] [PubMed] [Google Scholar]
- 39.Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Curr Opin Chem Biol. 1999;3:220–225. doi: 10.1016/S1367-5931(99)80035-0. [DOI] [PubMed] [Google Scholar]
- 40.Pratico D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Nunomura A, Moreira PI, Castellani RJ, Lee HG, Zhu X, Smith MA, et al. Oxidative damage to RNA in aging and neurodegenerative disorders. Neurotox Res. 2012;22:231–248. doi: 10.1007/s12640-012-9331-x. [DOI] [PubMed] [Google Scholar]
- 42.Sultana R, Butterfield DA. Oxidative modification of brain proteins in Alzheimer's disease: perspective on future studies based on results of redox proteomics studies. J Alzheimers Dis. 2013;33(Suppl 1):S243–S251. doi: 10.3233/JAD-2012-129018. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibson GE, Shi Q. A mitocentric view of Alzheimer's disease suggests multi-faceted treatments. J Alzheimers Dis. 2010;20(Suppl 2):S591–S607. doi: 10.3233/JAD-2010-100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schon EA, Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng X, Kanki T, Fukuoh A, Ohgaki K, Takeya R, Aoki Y, et al. PDIP38 associates with proteins constituting the mitochondrial DNA nucleoid. J Biochem. 2005;138:673–678. doi: 10.1093/jb/mvi169. [DOI] [PubMed] [Google Scholar]
- 47.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, et al. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keller JN, Guo Q, Holtsberg FW, Bruce-Keller AJ, Mattson MP. Increased sensitivity to mitochondrial toxin-induced apoptosis in neural cells expressing mutant presenilin-1 is linked to perturbed calcium homeostasis and enhanced oxyradical production. J Neurosci. 1998;18:4439–4450. doi: 10.1523/JNEUROSCI.18-12-04439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coskun PE, Beal MF, Wallace DC. Alzheimer's brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726–10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonda DJ, Wang X, Perry G, Smith MA, Zhu X. Mitochondrial dynamics in Alzheimer's disease: opportunities for future treatment strategies. Drugs Aging. 2010;27:181–192. doi: 10.2165/11532140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Su B, Zheng L, Perry G, Smith MA, Zhu X. The role of abnormal mitochondrial dynamics in the pathogenesis of Alzheimer's disease. J Neurochem. 2009;109(Suppl 1):153–159. doi: 10.1111/j.1471-4159.2009.05867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer's disease patients. Am J Pathol. 2008;173:470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 54.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Su B, Lee HG, Li X, Perry G, Smith MA, et al. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Hum Mol Genet. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, et al. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer's disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, et al. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nunomura A, Tamaoki T, Motohashi N, Nakamura M, McKeel DW, Jr, Tabaton M, et al. The earliest stage of cognitive impairment in transition from normal aging to Alzheimer disease is marked by prominent RNA oxidation in vulnerable neurons. J Neuropathol Exp Neurol. 2012;71:233–241. doi: 10.1097/NEN.0b013e318248e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sultana R, Baglioni M, Cecchetti R, Cai J, Klein JB, Bastiani P, et al. Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radic Biol Med. 2013;65:595–606. doi: 10.1016/j.freeradbiomed.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW, Jr, Cohen ML, et al. Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimers Dis. 2010;19:363–372. doi: 10.3233/JAD-2010-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barone E, Di Domenico F, Sultana R, Coccia R, Mancuso C, Perluigi M, et al. Heme oxygenase-1 posttranslational modifications in the brain of subjects with Alzheimer disease and mild cognitive impairment. Free Radic Biol Med. 2012;52:2292–2301. doi: 10.1016/j.freeradbiomed.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, et al. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J Neurochem. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- 65.Blass JP. The mitochondrial spiral. An adequate cause of dementia in the Alzheimer's syndrome. Ann N Y Acad Sci. 2000;924:170–183. doi: 10.1111/j.1749-6632.2000.tb05576.x. [DOI] [PubMed] [Google Scholar]
- 66.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 67.Bush AI. The metal theory of Alzheimer's disease. J Alzheimers Dis. 2013;33(Suppl 1):S277–S281. doi: 10.3233/JAD-2012-129011. [DOI] [PubMed] [Google Scholar]
- 68.Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer's disease. Biochim Biophys Acta. 2000;1502:139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 69.Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, et al. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem. 2010;285:31217–31232. doi: 10.1074/jbc.M110.149161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giaccone G, Pedrotti B, Migheli A, Verga L, Perez J, Racagni G, et al. beta PP and Tau interaction. A possible link between amyloid and neurofibrillary tangles in Alzheimer's disease. Am J Pathol. 1996;148:79–87. [PMC free article] [PubMed] [Google Scholar]
- 72.Christen Y. Oxidative stress and Alzheimer disease. Am J Clin Nutr. 2000;71:621S–629S. doi: 10.1093/ajcn/71.2.621s. [DOI] [PubMed] [Google Scholar]
- 73.Nunomura A, Chiba S, Lippa CF, Cras P, Kalaria RN, Takeda A, et al. Neuronal RNA oxidation is a prominent feature of familial Alzheimer's disease. Neurobiol Dis. 2004;17:108–113. doi: 10.1016/j.nbd.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Bogdanovic N, Zilmer M, Zilmer K, Rehema A, Karelson E. The Swedish APP670/671 Alzheimer's disease mutation: the first evidence for strikingly increased oxidative injury in the temporal inferior cortex. Dement Geriatr Cogn Disord. 2001;12:364–370. doi: 10.1159/000051282. [DOI] [PubMed] [Google Scholar]
- 75.Combs CK, Karlo JC, Kao SC, Landreth GE. beta-Amyloid stimulation of microglia and monocytes results in TNFalpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonda DJ, Mailankot M, Stone JG, Garrett MR, Staniszewska M, Castellani RJ, et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer's disease. Redox Rep. 2010;15:161–168. doi: 10.1179/174329210X12650506623645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990;15:1101–1107. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- 78.Klivenyi P, Toldi J, Vecsei L. Kynurenines in neurodegenerative disorders: therapeutic consideration. Adv Exp Med Biol. 2004;541:169–183. doi: 10.1007/978-1-4419-8969-7_10. [DOI] [PubMed] [Google Scholar]
- 79.Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- 80.Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- 82.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 83.Butterfield DA, Drake J, Pocernich C, Castegna A. Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid beta-peptide. Trends Mol Med. 2001;7:548–554. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 84.Smith MA, Casadesus G, Joseph JA, Perry G. Amyloid-beta and tau serve antioxidant functions in the aging and Alzheimer brain. Free Radic Biol Med. 2002;33:1194–1199. doi: 10.1016/s0891-5849(02)01021-3. [DOI] [PubMed] [Google Scholar]
- 85.Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, et al. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- 86.Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, et al. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 2000;59:1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 87.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 88.Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 89.Kril JJ, Patel S, Harding AJ, Halliday GM. Neuron loss from the hippocampus of Alzheimer's disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol (Berl) 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- 90.Kuchibhotla KV, Wegmann S, Kopeikina KJ, Hawkes J, Rudinskiy N, Andermann ML, et al. Neurofibrillary tangle-bearing neurons are functionally integrated in cortical circuits in vivo. Proc Natl Acad Sci U S A. 2014;111:510–514. doi: 10.1073/pnas.1318807111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer's neurodegeneration. Proc Natl Acad Sci U S A. 2007;104:3591–3596. doi: 10.1073/pnas.0609303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 94.Sherer TB, Greenamyre JT. Oxidative damage in Parkinson's disease. Antioxid Redox Signal. 2005;7:627–629. doi: 10.1089/ars.2005.7.627. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lipski J, Nistico R, Berretta N, Guatteo E, Bernardi G, Mercuri NB. L-DOPA: a scapegoat for accelerated neurodegeneration in Parkinson's disease? Prog Neurobiol. 2011;94:389–407. doi: 10.1016/j.pneurobio.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 97.Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, et al. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30:447–450. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 98.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 99.Smith RA, Kelso GF, James AM, Murphy MP. Targeting coenzyme Q derivatives to mitochondria. Methods Enzymol. 2004;382:45–67. doi: 10.1016/S0076-6879(04)82003-2. [DOI] [PubMed] [Google Scholar]
- 100.Lu C, Zhang D, Whiteman M, Armstrong JS. Is antioxidant potential of the mitochondrial targeted ubiquinone derivative MitoQ conserved in cells lacking mtDNA? Antioxid Redox Signal. 2008;10:651–660. doi: 10.1089/ars.2007.1865. [DOI] [PubMed] [Google Scholar]
- 101.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 102.Tauskela JS. MitoQ--a mitochondria-targeted antioxidant. IDrugs. 2007;10:399–412. [PubMed] [Google Scholar]
- 103.Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer's disease. Free Radic Res. 2009;43:156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, et al. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Atamna H, Kuratsune H, Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci. 2002;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 106.Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, et al. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci U S A. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ames BN, Liu J. Delaying the mitochondrial decay of aging with acetylcarnitine. Ann N Y Acad Sci. 2004;1033:108–116. doi: 10.1196/annals.1320.010. [DOI] [PubMed] [Google Scholar]
- 110.Milgram NW, Araujo JA, Hagen TM, Treadwell BV, Ames BN. Acetyl-L-carnitine and alpha-lipoic acid supplementation of aged beagle dogs improves learning in two landmark discrimination tests. FASEB J. 2007;21:3756–3762. doi: 10.1096/fj.07-8531com. [DOI] [PubMed] [Google Scholar]