Abstract
Exposure to nicotine during pregnancy via maternal cigarette smoking is associated with visual deficits in children. This is possibly due to activation of nicotinic acetylcholine receptors (nAChRs) in the occipital cortex which are important in the development of visual mapping. Using a baboon model we explored the effects of prenatal nicotine on parameters in the primary and associated visual cortices. Pregnant baboons were infused with nicotine (0.5 mg/hr, i.v.) or saline from 86 days gestation. At 161 days gestation fetal brains were collected (n=5/group) and the occipital lobe assessed for nAChRs and markers of the serotonergic and catecholaminergic systems using tissue autoradiography and/or high performance liquid chromatography. Neuronal nAChRs and serotonergic markers were expressed in a region and subunit dependent manner. Prenatal nicotine exposure was associated with increased binding for 3H-epibatidine sensitive nAChRs in the primary visual cortex (BA 17) and BA 18, but not BA 19, of the associative visual cortex (p<0.05). Markers of the serotonergic or catecholaminergic systems were not significantly altered. Thus, prenatal nicotine exposure is associated with alterations in the cholinergic system in the occipital lobe which may aid in the explanation of the appearance of visual deficits in children from mothers who smoke during pregnancy.
Keywords: Visual cortex, Smoking, Nicotinic receptors, Pregnancy, Serotonin, Calcarine sulcus
INTRODUCTION
Despite extensive health warnings, 20–25% of women in the general population smoke during pregnancy [8, 44]; with numbers exceeding 76% in high risk populations [14]. Maternal cigarette smoking has been linked to adverse effects on fetal brain development, resulting in sensory, cognitive, and behavioral impairments, even into adolescence and adulthood [26, 28, 33]. Nicotine is especially recognized to have selective effects on visual processing [60], and human prenatal nicotine exposure is associated with visual deficits in the offspring, including in visual acuity in adolescents [28]. The cognitive and sensory deficits associated with prenatal nicotine exposure are driven in part by binding of exogenous (excessive) nicotine to endogenous nicotinic acetylcholine receptors (nAChRs) in the developing brain, after nicotine’s crossing of the placental-fetal and fetal blood-brain barriers [25].
Neuronal nicotinic receptors are pentameric, ligand-gated ion channels comprised of heteromeric or homomeric combinations of subunits encoded by 9α (α2-10) and 3β (β2-4) genes [22], with α7 and α9 being the only subunit known to form homomeric receptors. They are present in the human brain as early as 4-6 weeks gestation [24], and play a key role in neuronal differentiation and synaptogenesis, as well as neurotransmission [30]. The activation of nAChRs by exogenous nicotine during fetal life has been shown in different experimental paradigms to profoundly affect the development of the neuroanatomical organization of the brain [36], with persistent adverse consequences postnatally [21, 56]. Because the different subtypes of nAChRs are known to have unique distribution patterns, developmental profiles [13], and pharmacological properties [22], their activation by exogenous nicotine is likely to result in complex effects in different regions of the brain and at different stages of development.
In the following study in fetal baboons, we compared the effects of prenatal exposure to nicotine on nAChR subunit binding in different Brodmann Areas (BAs) of the occipital lobe, i.e., BA 17, 18, and 19. While these areas are adjacent to one another and are all involved in visual processing, they subserve different aspects of this function -primary (BA 17) versus associative (BA 18 and BA 19) (see [43]). We hypothesized that exposure of the baboon fetus to exogenous nicotine would alter the binding to its receptors depending upon nAChR subunit composition and BA (primary versus associative) in the occipital cortex as measured by tissue receptor autoradiography. Because prenatal nicotine exposure has been shown in other experimental paradigms to alter the developmental trajectory of other neurotransmitter systems, particularly the serotonergic (5-HT) [15, 56] and catecholaminergic [38] systems, we sought to determine if the putative altered nAChR binding was associated with abnormalities in the spatial distribution and/or binding levels of the 5-HT1A and 5-HT2A receptors and the serotonin transporter (5-HTT), as measured by tissue receptor autoradiography or 5-HT, catecholamines and/or their metabolites, as measured by high performance liquid chromatography (HPLC), in the same fetal baboon cases.
MATERIALS AND METHODS
Animals
All animal care and experimental procedures were reviewed and approved by the IACUC of Columbia University and adhered to the guidelines of the American Association for Accreditation of Laboratory Animal Care and U.S. Department of Agriculture. Individually caged baboons (Papio papio) were maintained at the Institute of Comparative Medicine, Columbia University, New York City, NY. Animals were exposed to a 12 hour light/dark cycle and fed twice daily with high protein primate chow, supplemented with fruits and vegetables, with free access to water. During the receptive period of their cycle, females were placed with a male for a period of up to 5 days with conception defined as the midpoint of breeding. This date was then used to calculate all subsequent days of gestation (dg) where term is ~180 days. After ultrasound confirmation of pregnancy animals were assigned to receive either nicotine or saline. The data pertaining to findings in the brainstem from this cohort of animals have been reported previously [15]. In this study subsequent analyses of cortical findings are presented from these animals.
Surgical Procedures and Tethering
Long-term monitoring of pregnant baboons was accomplished with a light weight backpack and tethering system to which the animals were pre-conditioned. Description of this system, animal maintenance, breeding, surgery and post-operative care have been reported previously in detail [59]. In brief, at ~85 dg maternal catheters were implanted into the femoral artery and vein under general anesthesia (isoflurane 0.5-3% with nitrous oxide:oxygen 60:40%) for saline or drug administration (initiated between 2-5 days after surgery) and sampling of maternal blood. At ~132 dg surgery was again performed to catheterize the amniotic fluid, fetal jugular vein, fetal carotid artery, and fetal trachea and placement of electrocardiographic (ECG) leads. Catheters and leads were exteriorized, passed into a maternal backpack, and attached to a flexible tether cable for access to fetal blood samples and recording of cardiorespiratory signals. The entire system rotated around a swivel, permitting mothers to move freely with continuous monitoring of the fetus. Cefazolin (antibacterial prophylaxis) and morphine (analgesia) were administered for 2-4 days post-operatively. During this period fetal well-being was monitored via samples of arterial blood (0.5ml) for acid-base and blood gases (Radiometer, ABL-30). Analysis of fetal physiological parameters from these animals have been published previously [15].
Nicotine Exposure
Beginning at 86±3 dg (second trimester), five pregnancies were exposed to nicotine by maternal intravenous infusion up until one hour prior to delivery which continued for a mean of 71 days (range 62-80 days; variability based on some fetuses needing to be delivered early due to maternal signs of impending labour). This time point was chosen to reduce the risk of spontaneous abortion. Solutions were prepared by adding Nicotine tartrate (N5260, Sigma Aldrich, St. Louis, MO) to 1L saline and a continuous round the clock infusion at 0.5 mg/h for 24 hours per day of nicotine (approximately 12mg per day which is equivalent to 15 cigarettes) administered with pre-calibrated peristaltic infusion pumps (P720, Instech Laboratories, Inc., Plymouth Meeting, PA). The only exception was when infusions were stopped for approximately 4 hours while fetal catheter surgery was performed at 132 dg. During the infusion period blood was sampled from mother and fetus for nicotine concentrations. A comparable four control pregnancies were infused with saline alone. At ~161 days of gestation (range 156-165 days) fetuses were delivered via cesarean section, sacrificed and the brain extracted. This range is driven by the fact that some fetuses were delivered early due to concerns about mothers going into labour. The weight of the placenta and fetal body, brain, and liver were recorded and were not different between groups [15]. Fetal baboons were sacrificed at ~161 dg (~90% gestation), a time point developmentally equivalent to term gestation in humans (37-42 gestational weeks) [27]. Due to the difficulty in acquiring instrumented tissues an additional non-instrumented control fetus was delivered at the same gestation. All fetal measures were equivalent to instrumented controls, and data for this uninstrumented animal were included with those of the saline infused group (n=5 controls total).
Measurement of Nicotine and Cotinine Concentrations in Plasma
Blood samples (0.6ml) were collected weekly in heparinized microtainers (Becton Dickinson, Franklin Lakes, NJ) from indwelling catheters for plasma nicotine and cotinine levels. Following centrifugation plasma was stored at −20°C. Samples of maternal or fetal plasma were pooled to obtain 1 ml of plasma for analysis. Maternal samples were divided into pre- and post-fetal catheterization. Wherever possible, pooled fetal samples were matched with maternal plasma obtained during the same time interval. Samples were assayed for nicotine and cotinine levels using Gas Chromatography-Mass Spectrometry at the Analytical Psychoanalytical Laboratories, Nathan Kline Institute, NY as described in detail [15]. The curve and quality controls were processed similarly with each batch of samples. For nicotine the inter-assay precision was determined by testing plasma controls containing 4, 40 and 80ng/ml of nicotine on six separate days. The relative standard deviations were 8.6%, 7.4%, and 8.3%, respectively. For cotinine the inter-assay precision was detected by testing plasma controls containing 4, 40 and 8 ng/ml of cotinine. The relative standard deviations for cotinine concentrations were 6.4%, 5.9%, and 6.8%, respectively.
The average dose of nicotine delivered to the pregnant baboons was 0.76 ± 0.04mg/kg/day (range from 0.64–0.9mg/kg/day). This resulted in a mean nicotine concentration in the mother of 14.4 ± 1.9ng/ml which is similar to afternoon levels reported in pregnant women smoking an average of 15 cigarettes per day or wearing a 22mg/day nicotine patch [37]. Maternal nicotine levels were not different to mean levels in the fetus (13.9 ± 1.5ng/ml) nor was there a difference in the fetal-to-maternal ratio (0.99 ± 0.09) suggesting nicotine crosses the placenta. Similarly, there was no difference in mean cotinine levels between the mother (166 ± 12ng/ml) and fetus (154 ± 8ng/ml), nor the fetal-to-maternal ratio (0.94 ± 0.50). For individual animal data see our prior report [15].
Analysis of the Fetal Baboon Occipital Lobe
A block of fresh frozen brain tissue (~3mm thick) from the right occipital lobe beginning immediately caudal to the parieto-occipital sulcus was serially-sectioned at 20μm on a motorized Leitz cryostat, mounted on glass microscopic slides, and stored at −20°C until required. A minimum of 2 sections from each case were stained with hematoxylin-and-eosin for histological analysis. One saline fetus had a cortical infarct on the left side of the brain, the origin of which could not be determined, however, as autoradiographic data from this case was within the range for its group, data were combined with the other fetuses for analysis.
Tissue Section Autoradiography
Tissue from the occipital lobe was surveyed for “markers” of the cholinergic (nAChRs) and 5-HT system using tissue section autoradiography (Table 1). For nAChRs, the subunit specific ligands 3H-epibatidine (α2-4, β2, β4) [29] and 125I-bungarotoxin (α7) [29] were used. For markers of the 5-HT system, 3H-8-OH-DPAT (8-hydroxy-2-(di-n-propylamino)tertraline) (5-HT receptors), 125 1A I-DOI (2,5-dimethoxy-4-iodoamphetamine) (5-HT2A receptors) and 125I-RTI-55 (3 beta-(4-iodophenyl)tropan-2 beta-carboxylic acid methyl ester) (the 5-HT transporter) were used. For each radioligand the general methods of tissue autoradiography were the same and all ligands were purchased from PerkinElmer (Waltham, MA). In brief, alternating sections were preincubated in incubation buffer (30-60min), followed by incubation with the radioligand diluted in the same buffer (Table 1). Sections were washed in a series of buffers and distilled water, to remove unbound ligand, then dried under a stream of anhydrous air. Sections were then exposed to125I-sensitive film (Kodak BoiMax MR) or 3H-sensitive phosphoimage plates (BAS TR2025 system). In a subset of sections non-specific binding was determined via incubation with the radioligand and excess “cold” displacer (Table 1).
Table 1.
Radioligands used to assess nAChRs and 5-HT markers in the fetal baboon occipital lobe of the cerebral hemisphere
| Ligand | Concentration | Displacer | Imaging | Detects |
|---|---|---|---|---|
| 3H-Epibatidine | 0.5nM (56 Ci/mmol); 60min at RT |
300μM nicotine (Sigma-Aldrich, St. Louis, MO) |
BAS system – 3 wks |
heteromeric α2- 4, β2, β4 nAChRs |
| 125I-Bungarotoxin | 1.5nM (120 Ci/mmol); 2hrs at 4°C |
1mM nicotine (Sigma-Aldrich, St. Louis, MO) |
125I film - 7 days | homomeric α7 nAChRs |
| 3H-8-OH-DPAT | 4nM (120 Ci/mmol); 1hr at RT |
10μM serotonin (Sigma-Aldrich, St. Louis, MO) |
BAS system – 4 wks |
5-HT1A autoreceptor |
| 125I-DOI | 86.3pM (2200 Ci/mmol); 60min at RT |
10−6M ritanserin (Sigma-Aldrich, St. Louis, MO) |
125I film - 7 days | 5-HT2A receptor |
| 125I-RTI-55 | 0.15nM (2200 Ci/mmol); 90min at RT |
100nM citalopram (Sigma-Aldrich, St. Louis, MO) |
125I film – 9 hours | 5-HT transporter, 5- HTT |
Receptor binding density (expressed as the specific activity of tissue-bound ligand) was analyzed in the calcarine sulcus of the visual cortex (BA 17), and within the associative cortices BA 18 and BA 19 (Figure 1) in line with previous definitions in other primate species [20, 46]. For ligands where cortical patterns could be clearly discerned individual cortical laminae were measured and the cortical layers they represented determined relative to H&E stained sections from the same animal (see Table 2). The selected regions were analyzed from 5 randomly selected sections per ligand (4 specific and 1 nonspecific for each ligand). The MCID Elite 6 imaging system (Imaging Research Inc., Ontario, CA) was used to perform quantitative densitometry of autoradiographs. Receptor binding density was determined in each region by digitizing the boundaries of regions upon images of the autoradiogram displayed on the computer monitor and compared to hematoxylin-and-eosin counterstained sections. A set of 3H- or 125I-standards (Amersham, Piscataway, NJ) were included in each X-ray cassette to permit the conversion of the optical densities of silver grains in autoradiograms to specific activity of tissue-bound ligand to femtomole per milligram (fmol/mg) of tissue. Calculations to equate for nonspecific binding were made if needed. Specific activity data are presented as computer-generated mosaics with an increasing linear color step scale for each ligand.
Figure 1. Region of interest.
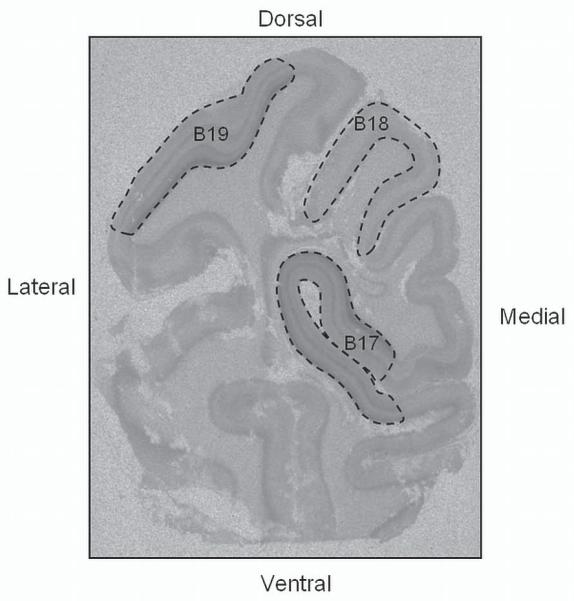
Coronal section of baboon occipital lobe with 3H-8-OH-DPAT binding to 5-HT1A receptors highlighting the regions of interest: primary visual cortex BA 17 and associate visual cortices BA 18 and BA 19.
Table 2.
nAChR and 5-HT markers in the occipital lobe of the cerebral hemisphere in the fetal baboon model of prenatal nicotine exposure.
| Measure | Control Mean of Means ± SEM (range) (n=5) |
Nicotine-exposed Mean of Means ± SEM (range) (n=5) |
FDR corrected p-value |
|---|---|---|---|
| α2-4, β2 or β4 nAChR Binding (fmol/mg) | |||
| Primary visual cortex (BA 17): total |
7.26 ± 1.22 (4.0, 10.8) |
13.81 ± 0.94 (11.3, 16.1) |
0.04* |
| Associate visual cortex (BA 18): total |
7.99 ± 1.25 (4.3, 12.1) |
15.01 ± 1.14 (11.2, 17.6) |
0.04* |
| Associate visual cortex (BA 19): total |
6.09 ± 1.83 (2.3, 10.7) |
11.72 ± 2.32 (4.5, 17.9) |
0.28 |
| α7 nAChR Binding (fmol/mg) | |||
| Primary visual cortex (BA 17): total |
1.85 ± 0.43 (0.4, 2.8) |
2.66 ± 0.84 (0, 4.5) |
0.50 |
| Associate visual cortex (BA 18): total |
1.99 ± 0.70 (0, 4.0) |
3.19 ± 0.90 (0.8, 5.6) |
0.43 |
| Associate visual cortex (BA 19): total |
2.23 ± 0.65 (0.3, 3.8) |
3.55 ± 0.78 (1.6, 5.9) |
0.40 |
| 5-HT1A Receptor Binding (fmol/mg) | |||
| Primary visual cortex (BA 17): total |
43.38 ± 8.60 (11.6, 59.6) |
39.94 ± 4.72 (28.5, 49.8) |
0.73 |
| B A 17 layers I-II | 30.07 ± 4.24 (16.1, 40.0) |
30.31 ± 9.43 (0.6, 55.0) |
0.99 |
| BA 17 layer III | 50.11 ± 5.16 (33.2, 61.7) |
46.93 ± 8.39 (15.3, 60.1) |
0.99W |
| BA 17 layer IV | 29.71 ± 3.02 (21.3, 36.4) |
34.73 ± 6.26 (10.5, 45.7) |
0.99 |
| BA 17 layer V | 48.08 ± 5.64 (33.5, 60.2) |
55.36 ± 8.89 (25.2, 73.4) |
0.99 |
| BA 17 layer VI | 82.06 ± 9.55 (55.8, 105.8) |
88.50 ± 12.99 (44.6, 112.2) |
0.99 |
| Associate visual cortex (BA 18): total |
13.23 ± 2.78 (3.8, 20.4) |
10.30 ± 3.19 (5.7, 22.7) |
0.60 |
| BA 18 layers I-II | 12.43 ± 2.15 (5.0, 17.5) |
11.92 ± 2.35 (3.6, 17.3) |
0.99 |
| BA 18 layers III-IV | 7.63 ± 1.41 (3.4, 11.7) |
8.79 ± 1.60 (3.0, 12.2) |
0.99 |
| BA 18 layers V-IV | 17.11 ± 2.23 (11.5, 21.8) |
18.22 ±2.95 (8.0, 25.2) |
0.99 |
| Associate visual cortex (BA 19): total |
37.15 ± 8.44 (13.1, 50.4) |
32.31 ± 4.32 (19.8, 41.9) |
0.65 |
| BA 19 layers I-II | 22.69 ± 3.18 (13.2, 26.9) |
22.04 ± 6.03 (5.0, 30.8) |
0.99 |
| BA 19 layer III | 36.69 ± 4.11 (25.3, 44.7) |
38.19 ± 8.86 (12.4, 51.4) |
0.99 |
| BA 19 layer IV | 22.60 ± 3.19 (14.0, 29.3) |
22.13 ± 5.56 (5.6, 28.8) |
0.99W |
| BA 19 layer V | 41.29 ± 4.89 (28.6, 49.4) |
46.19 ± 8.94 (20.3, 58.4) |
0.99 |
| BA 19 layer VI | 86.88 ± 6.04 (69.0, 94.5) |
82.38 ± 17.68 (34.6, 117.4) |
0.99 |
| 5-HT2A Receptor Binding (fmol/mg) | |||
| Primary visual cortex (BA 17): total |
0.92 ± 0.12 (0.6, 1.2) |
1.43 ± 0.20 (1.0, 2.0) |
0.24 |
| BA 17 layers I-III | 0.75 ± 0.14 (0.5, 1.2) |
1.42 ± 0.23 (1.0, 2.2) |
0.46 |
| BA 17 layers IV-VI | 0.90 ± 0.10 (0.7, 1.3) |
1.51 ± 0.20 (1.1, 2.2) |
0.46 |
| Associate visual cortex (BA 18): total |
0.70 ± 0.13 (0.4, 1.2) |
1.22 ± 0.22 (0.7, 1.9) |
0.25 |
| B18 layers I-III | 0.78 ± 0.18 (0.4, 1.4) |
1.28 ± 0.17 (0.9, 1.8) |
0.46 |
| B18 layers IV-VI | 0.71 ± 0.10 (0.6, 1.1) |
1.08 ± 0.15 (0.8, 1.5) |
0.46 |
| Associate visual cortex (BA 19): total |
0.48 ± 0.04 (0.4, 0.6) |
0.88 ± 0.22 (0.3, 1.4) |
0.42W |
| BA19 layers I-III | 0.48 ± 0.06 (0.4, 0.7) |
0.98 ± 0.26 (0.3, 1.5) |
0.99W |
| BA 19 layers IV-VI | 0.60 ± 0.07 (0.4, 0.8) |
0.97 ± 0.23 (0.3, 1.5) |
0.62 |
| 5-HTT (fmol/mg) | |||
| Primary visual cortex (BA 17): total |
3.36 ± 0.68 (1.4, 5.2) |
4.31 ± 0.33 (3.8, 5.5) |
0.40 |
| B17 layers I-IV | 3.02 ± 0.72 (0.9, 5.2) |
3.99 ± 0.32 (3.4, 5.2) |
0.72 |
| B17 layers V-IV | 4.16 ± 0.75 (2.4, 6.3) |
5.56 ± 0.40 (4.7, 6.8) |
0.54 |
| Associate visual cortex (BA 18): total |
2.23 ± 0.41 (1.1, 3.0) |
3.38 ± 0.18 (2.9, 3.8) |
0.21 |
| Associate visual cortex (BA 19): total |
2.13 ± 0.39 (1.1, 2.9) |
3.02 ± 0.28 (2.2, 3.6) |
0.28 |
| BA 19 layers I-IV | 2.43 ± 0.55 (1.2, 4.2) |
3.29 ± 0.66 (1.6, 4.5) |
0.87 |
| BA 19 layers V-IV | 3.30 ± 0.48 (1.7, 4.5) |
4.67 ± 0.54 (3.1, 5.3) |
0.46 |
Legend:p<0.05 is considered significant.
Wilcoxon Rank Sum used instead of t-test (see Materials and Methods). FDR: false discovery rate. Note: Laminar data presented where binding could be determined.
High Pressure Liquid Chromatography (HPLC)
A 3mm block of fresh frozen tissue from the left occipital lobe (corresponding to the same region as the right hemisphere) was dissected. Using a 2mm micropunch (Harris Uni-core, Electron Microscopy Sciences, Hatfield, PA; 4-5 punches per block along the horizontal plane), tissue from the primary visual cortex (BA 17, calcarine sulcus) was collected and stored at −80°C. Samples were homogenized on ice in 1ml Perchloric Acid solution (0.1M HC104, 1mM EDTA, 1mM Na2SO3) with a cell disruptor (Branson Sonifier 200, power 4) for 1min of continuous sonification. The homogenate was then centrifuged (Sorvall RC-5B refrigerated superspeed centrifuge, Du Pont Instruments) at 9500×G at 4°C for 15min. The supernatant was filtered through sterile 0.45μm and 0.2μm syringe filters and used immediately for analytical procedures (see below). The protein pellet was resuspended in 1M NaOH and the protein concentration determined by the Lowry method [34] read at a wave length of 660nm.
Analytical procedure
Analyses of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), norepinephrine (NE), dopamine (DA) and 3,4-dihydroxyphenylacetic acid (DOPAC) were performed. Analyses used a HPLC system with electrochemical detection (EC; ESA Coulochem II, Bedford, MA) equipped with a C-18 reverse-phase column (3μm, 4.6mm diameter, 100mm length; Microsorb MV Varian, Walnut Creek, CA) as described previously [16]. The mobile phase consisted of 90mM NaH2PO4, 50mM citric acid, 50μM EDTA, 1.7mM 1-octane sulfonic acid, and 3% acetonitrile at a pH of 3.0 which was pumped through the column at a rate of 1.0ml/min. Column temperature was maintained at a constant 36°C throughout. To equate for the sensitivity of the system, for 5-HT 25μl of sample was run at 5nA and for all other compounds 5μl of sample was run at 10nA. This allowed for the measurement of the eluents with a sensitivity of 0.5fmol/sample. 5-HT, 5-HIAA, NE, DA, and DOPAC eluted from the column with approximate retention times of 40, 8.6, 3.6, 4.6 and 11.7 min respectively.
The area under the curve was compared with fresh appropriate standards (dilutions of 5×10−7, 10−7, 5×10−8, and 10−8 M, Sigma Chemical, St Louis, MO, USA) injected onto the column at the beginning of each day. The use of Justice Innovations ChromPerfect (Palo Alto, CA) software allowed determination of regression for the standard (minimum correlation coefficient of 0.99). The concentrations of eluents were estimated by comparing peak areas from the samples with those of the external standards. Values were corrected for protein concentrations to give a final value in pmol/mg protein.
Statistical Analyses
Prior to testing group differences, Shapiro Wilks tests were used to examine normality of all variables due to the small sample size. The Wilcoxon Rank Sum test was used in measures that failed the normality test. T-tests were done to evaluate normally distributed measures. All tests were two-tailed with an alpha level of 0.05; the mean (plasma and HPLC) or mean of means (autoradiography) ± standard error of the mean are presented for all continuous measures. All p-values were adjusted to account for multiple testing using the false discovery rate method. The false discovery rate method adjusts for multiple testing by controlling for the proportion of significant results that are false positives. Essentially, a corrected p-value is calculated by adjusting the observed p-values based on the total number of tests conducted and on the ranking of the observed p-values [5]. The total number of tests was determined by the type of measure. For instance, each marker’s raw p-value is adjusted by the total number of 5-HT and nicotinic marker tests conducted.
RESULTS
Distribution of nAChR and 5-HT Markers in Fetal Baboon Occipital Lobe
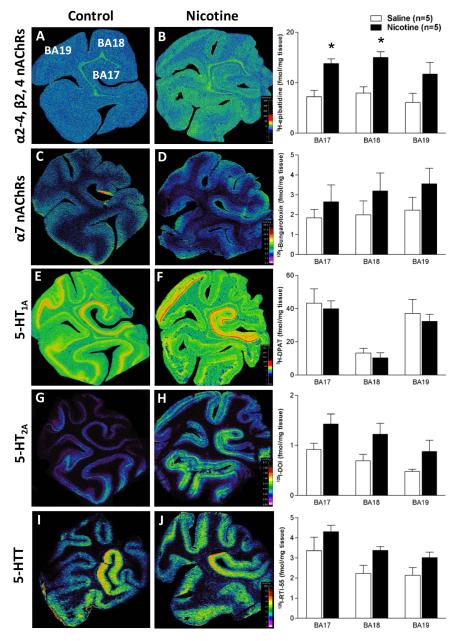
In control animals (n=5) the ligands used for different nAChR subunits, i.e., 3H-epibatidine preferential for α2-4, β2, and β4, and 125I-bungarotoxin for α7, demonstrated diffuse binding across the occipital lobe (Table 2) with no differential binding present in cortical layers (Figure 2). For 3H-epibatidine the highest binding was located in BA 18, followed by BA 17, with the lowest binding in BA 19 (Table 2). Binding for α7 nAChRs was highest in associate visual cortex BA 19, followed by BA 18 and lowest in BA 17 (Table 2).
Figure 2. nAChR and 5-HT markers in the fetal baboon occipital lobe.
Pseudocolored autoradiographic images of (A) 3H–epibatidine binding to α2-4, β2, β4 nAChRs, (B) 125I-bungrotoxin binding to α7 nAChRs, (C) 3H-8-OH-DPAT binding to 5-HT1A receptors, (D) 125I-DOI binding to 5-HT2A receptors, and (E) 125I-RTI-55 binding to the 5-HT transporter in the fetal baboon occipital lobe with specific activity scales for control (left) and nicotine-exposed (right) fetuses with corresponding graphs of binding levels for each group (fmol/mg tissue). Nicotine exposure resulted in a region specific increase in 3H-epibatidine binding (B). *p<0.05, mean of means ± SEM. Legend: BA 17: primary visual cortex, BA 18 and BA 19: associated visual cortices.
The highest binding for all markers of the 5-HT system was located in the primary visual cortex (BA 17). For 5-HT1A receptors the next highest binding was observed in BA 19 with the lowest binding in BA18 (Table 2, Figure 2), with discrete changes in binding at cytoarchitectual boarders of BA 17 and B19 (Figure 2E). Furthermore, at both regions there was a distinct laminar binding pattern in layers I and II, III, IV, V and VI, with layer VI displaying the highest binding in both regions (Table 2). This laminar distribution was different in associate visual cortex BA 18 where binding could only be distinguished in 3 laminar patterns (layers I and II, III to IV, V and VI) with layers V and VI combined displaying the highest binding (Table 2, Figure 2).
Following binding in the primary visual cortex (BA 17), binding for 5-HT2A receptors and 5-HTT was highest in associate visual cortex BA 18 and lowest in BA 19 (Table 2, Figure 2). In the cortex laminar distribution for 5-HT2A receptor binding was different to that for 5-HT1A receptors, with only 2 distinct laminar patterns being present in all cortical areas (layers I to III and IV to VI) (Table 2). Interestingly, binding in BA 17 and BA 19 was highest in layers IV to VI while binding was slightly higher in layers I to III in BA 18. Laminar distribution for 5-HTT was present in the primary visual cortex (BA 17) and associate visual cortex BA19 in 2 distinct laminar -layers I to IV and V to VI, the later displaying the highest binding at both sites. No distinct laminar distribution of binding could be determined in associate visual cortex BA 18 (Table 2, Figure 2).
Effects of Prenatal Nicotine-Exposure on nAChR and 5-HT Markers
The most robust and consistent difference in the occipital lobe in nicotine-exposed (n=5) compared to control fetuses was up to a 2 fold increase in 3H-epibatidine binding for nAChRs containing combinations of α2-4, β2, and/or β4 subunits. This increase was significant (p<0.05) in the primary visual cortex (BA 17) and associate visual cortex BA 18, but not in BA 19 (Table 2, Figure 2). In comparison there were no significant differences in 125I-bungarotoxin binding for nAChRs containing α7 subunits at any site despite mean binding values being higher in nicotine-exposed compared to control animals (Table 2, Figure 2). Furthermore, while nicotine-exposed animals displayed higher mean binding values for 5-HT2A receptors and 5-HTT, there were no significant differences between control and nicotine-exposed fetuses for any of the 5-HT markers used to identify 5-HT1A and 5-HT2A receptors and 5-HTT (Table 2), even when assessed based on laminar distribution (Table 2).
Serotonin, Catecholamine, and Metabolite Levels
Analysis of the primary visual cortex (BA 17) using HPLC indicated the highest levels of any measured parameter were present for the metabolite DOPAC, even though dopamine levels in this region were the lowest of any of the neurotransmitters (Table 3). While NE and DA levels were nearly 3-2 fold lower in BA 17 (respectively), and both the mean 5-HIAA:5-HT and DOPAC:DA ratios were increased in nicotine-exposed fetuses compared to controls, there were no significant differences after correcting for multiple testing in the levels of DA, 5-HT, NE, 5-HIAA or DOPAC between control and nicotine-exposed fetuses (Table 3). There was also no difference between groups in the turnover of neurotransmitters, as indicated by metabolite to neurotransmitter ratio (Table 3).
Table 3.
High-performance liquid chromatography measurements for markers of the serotonin and catecholamine systems in the occipital cortex in the fetal baboon model of prenatal nicotine exposure
| Measure | Control Mean ± SEM (range) (n=5) |
Nicotine-exposed Mean ± SEM (range) (n=5) |
FDR corrected p-value |
|---|---|---|---|
| 5-HT (pmol/mg protein) | 4.33 ± 0.59 (3.2, 6.1) |
3.65 ± 0.40 (2.9, 5.1) |
0.40 W |
| 5-HIAA (pmol/mg protein) | 11.06 ± 1.45 (8.2, 16.6) |
14.04 ± 1.23 (10.7, 17.0) |
0.35 |
| Norepinephrine (pmol/mg protein) |
5.53 ± 2.63 (0, 15.1) |
1.81 ± 0.50 (0.5, 3.4) |
0.42W |
| Dopamine (pmol/mg protein) | 3.25 ± 0.45 (1.9, 4.5) |
1.81 ± 0.14 (1.3, 2.1) |
0.21 |
| DOPAC (pmol/mg protein) | 36.88 ± 7.59 (18.6, 64.5) |
34.76 ± 7.67 (21.6, 64.1) |
0.71W |
| 5-HIAA:5HT ratio | 2.62 ± 0.24 (1.9, 3.2) |
4.11 ± 0.65 (2.1, 5.3) |
0.24 |
| DOPAC: dopamine ratio | 12.26 ± 2.70 (4.9, 19.2) |
21.17 ± 7.11 (11.2, 49.3) |
0.50W |
Legend: Wilcoxon Rank Sum used instead of t-test (see Materials and Methods). FDR: false discovery rate.
DISCUSSION
Exposure to nicotine during pregnancy via maternal cigarette smoke is a substantial health care consequences for the offspring including preterm birth, low birth weight, an increased 3 fold risk for SIDS [35] and long-term association with decreased IQ and cognitive deficits (see [26]). In addition, studies have reported that sensory cortices, including the visual cortex, may be more vulnerable to the effects of prenatal nicotine exposure [50, 60]. However, the underlying pathologies resulting from prenatal exposure to nicotine and how these relate to altered visual function in young adolescents [28] and adults [45] delivered after maternal smoking during pregnancy have not been extensively explored. As a step towards deciphering the underlying basis of the effects of exogenous nicotine on the developing visual cortices, we explored the effects of chronic prenatal nicotine exposure from mid to late gestation in the fetal baboon on the expression of cholinergic markers in the occipital lobe. In addition, we also explored the serotonergic and catecholaminergic systems to investigate whether prenatal nicotine may affect multiple neurotransmitter systems in this region. We have previously published results of changes to both cholinergic and serotonergic markers in the brainstem (specifically the medulla oblongata) from these animals [15]. Thus, we are able to compare the regional differences between the occipital lobe and the brainstem on the same population of baboons based on the findings from this study.
The effects of prenatal nicotine on the cholinergic system in the occipital lobe
As reported previously in the rodent [2], the regional and laminar distribution of nAChRs was relatively homogenous throughout the cortex irrespective of which subunit was targeted. In line with other studies in the brain from primates and rodents [15, 52, 60] prenatal exposure to nicotine resulted in a dramatic two fold increase in 3H-epibatidine receptor binding in the occipital lobe, similar increases were also observed across the entire medulla of these animals [15]. Epibatidine binds heteromeric nAChRs containing combinations of α2-4, β2, β4 subunits including α4β2, which are the most abundant nAChRs in the brain. Nicotine has high affinity for receptors containing both of these subunits [41], thus our findings were as expected. While our data are in line with studies reporting increased mRNA for α4 and β2 subunits in the brain following prenatal nicotine exposure [51], further work is needed to confirm whether increased binding in response to nicotine relates to altered receptor expression or efficacy of binding. Indeed, it is suggested that in response to nicotine these receptors become desensitized, despite elevated expression. Based on the fact that the majority of nAChRs are located presynaptically this could lead to diminished neurotransmitter release at nerve terminals, as has been shown for gamma-aminobutyric acid (GABA) and glycine (see [19, 62]). However receptors are also located postsynaptically and thus, acetylcholine mediated synaptic transmission could be affected [30]. Nicotinic receptors are present in the brain from as early as 4-6 weeks gestation in humans [24], and the downstream actions of acetylcholine are largely dependent on the location and activation of its receptors. Thus, the changes observed here would suggest altered cholinergic transmission in the occipital region which may have profound physiological effects [53]. These effects have been shown to persist into the postnatal period [21, 56].
In the visual cortex cholinergic activity influences neuronal firing [12, 63], receptor field properties [63], vision based learning [12] and visual information processing by controlling response gain [58]. Activity dependent development of retinal mapping in the visual cortex occurs via spontaneous wave activity generated by nAChRs which is present prior to the onset of visual experience [1, 49]. Subsequent stimulation of the cholinergic system in association with visual stimulation indicates a role of nAChRs in facilitating visual responsiveness [31] and contrast sensitivity [6]. Proper visual functioning in adulthood is dependent on the regional and developmental expression of nAChRs in the visual cortex, in spite of normal retinal processing [49]. Thus the changes to nAChR binding in this study following nicotine exposure may aid in the explanation of the appearance of discrete changes to visual function, such as poor stereovision [42] and a 5% decrease in visual performance accuracy at ~16 years of age [28], which are reported in human patients whose mothers smoked during pregnancy. As cholinergic processes project throughout the entire brain, changes in receptor functionality due to maternal cigarette smoking during pregnancy may impact other functions including motor control [33], audition [28] and cognition (see [26]).
In the occipital lobe 3H-epibatidine binding was significantly increased following prenatal nicotine exposure which was specific to the primary visual cortex (BA 17) and associated visual cortex (BA 18) but not BA 19, suggesting region specific effects of prenatal nicotine. The difference among the responses of these regions may be due to their different functional roles and thus neurochemical profiles. The primary visual cortex (BA 17) processes sensory information from the ipsilateral lateral geniculate nucleus integrating the spatiotemporal features of vision. Information is then relayed from this site to the associated visual cortices which are involved with processing aspects of visual information including object recognition (BA 18) and shape recognition and multisensory integration (B19) (though these processes do not appear isolated to one region) (see [43]). Thus the BA specific changes observed in our study may aid in the explanation of why discrete changes to visual acuity are seen in children whose mothers smoked [28]. It is also possible that the apparent differences across BA’s may be associated with different sensitivities, stoichiometry or expression patterns [9] of nAChR subunits expressed in these regions. We observed a subunit specific effect on heteromeric receptors containing combinations of α2-4, β2, β4 subunits as binding for α7 subunits was not significantly different between groups. A similar observation was apparent in the medulla from these animals [15]. The subunit specific effects of nicotine observed in this study may be attributed to several possibilities. For example, as previously mentioned, α4β2 containing nAChRs are the most prevalent nicotinic receptor expressed in the brain with high affinity for nicotine [41]. In comparison α7 containing nAChRs have low affinity for nicotine and thus usually require exposure at high concentrations to cause significant changes in expression [10, 61]. Indeed, in rodents changes to 125I-bungarotoxin following exposure to nicotine appear smaller in magnitude [10, 61] and less robust after chronic treatment to nicotine with an increase in binding occurring in fewer brain regions than 3H-nicotine binding [39]. Furthermore, nAChR subunits are developmentally expressed [13] and have different roles in the visual cortex; α4 is thought to contribute to refinement of pathways in this region independent of visual activity; whereas, α7 is involved in activity dependent synaptic plasticity following visual stimulation [3]. This is an important consideration should exposure to cigarette smoke continue after birth. A final possibility of the subunit specific vulnerability of nAChRs is demonstrated by the recent work of Dau and colleagues who showed that the assembly and functional expression of nAChR subunits at the cell surface is dependent on molecules such as chaperone resistant to inhibitors of acetylcholinesterase (RIC-3) which differentially regulates nAChR expression in a subunit dependent manner [11], which in turn is also regulated by exposure to nicotine [57].
The effects of prenatal nicotine on the serotonergic system in the occipital lobe
In this study we demonstrated that 5-HT markers in the occipital lobe in the fetal baboon are regionally and laminarly expressed with clear boundaries present between cytoarchitecturally different cortical regions. These boundaries were clearly evident for 5-HT1A receptor binding in BA17 and BA19. The highest binding for all markers (5-HT1A, 5-HT2A and 5-HTT) was present in the primary visual cortex (BA 17) though binding intensities were dependent on the marker across BA 18 and BA 19 (see Table 2). The clearest laminar distribution was observed for 5-HT1A receptors for which discrete cortical layers could be identified in all regions, the highest binding in layers VI or V/VI combined (region BA 18). Binding for both 5-HT2A and 5-HTT was lower than 5-HT1A and could be separated into 2 discrete laminar patterns, the highest binding in regions incorporating layers V and VI (the exception being 5-HT2A binding in BA 18). Laminar distribution of 5-HT markers suggests discrete neurochemical organization of this neurotransmitter system in the visual cortex. During the refinement and integration of interconnected cortical regions synaptic modification may occur in a laminar dependent fashion. For example, it has been demonstrated that 5-HT dependent long-term potentiation is increased in layer IV compared to layers II and III following high frequency stimulation in the cat visual cortex [32]. Other research has shown a dissociable contribution of 5-HT1A and 5-HT2A receptors to functional outcomes in other cortical regions [7]. While the functional consequences corresponding to differential receptor expression in the visual cortex are currently unknown, the laminar and regional distribution of 5-HT markers in the visual cortex in early brain development suggest a role for 5-HT in the organization of regional circuitry.
Laminar distribution for 3H-5-HT (binds to type 1 receptors) and/or 3H-ketanserin (binds to type 2 receptors) has previously been reported in the primary visual cortex of 2 month old rhesus monkeys [47] and adult marmosets [20]. There are some discrepancies between the intensity of binding across different cortical layers in this report compared with those in other non-human primates [20, 47] and cats [17]. Discrepancies across studies may be a function of the different receptor ligands used or due to the changes that occur in receptor distribution between fetal and postnatal development [4]. In humans 5-HT1A receptors display a 3-4 fold increase in density in the fetal cortex compared to the adult [4] and visual cortex specific changes have been shown in the expression of both 5-HT1A and 5-HT2 receptors in the cat during postnatal development [17]. Changes in receptor expression correspond loosely to 5-HT innervation of the cortex and suggest that each receptor subtype plays a distinct role during development.
While mean binding values for 5-HT2A receptors and 5-HTT were higher following prenatal exposure to nicotine, we did not observe a significant difference in binding or neurotransmitter levels for markers of the 5-HT system. This is in contrast to the studies of Slotkin et al., who have shown long-term significant effects on the 5-HT system, including receptor expression following prenatal exposure to nicotine [54, 55]. However these studies have investigated nicotine-induced 5-HT changes within homogenates of cortical tissues and postnatally, where it is possible that subsequent changes following the prenatal period have occurred that could not be examined in the current study.
Overlap between neurotransmitter systems in the occipital lobe
In support of other studies [18, 20], this study of the fetal primate found a clear overlap in the distribution of both cholinergic and 5-HT markers both across cortical regions and within laminae. Thus while we observed significant changes in nAChR binding following prenatal nicotine exposure, it is possible that this response has downstream consequences on other neurotransmitter systems. For example there was a 67% decrease in mean norepinephrine levels and a 44% decrease in mean dopamine levels on BA 17 in our study in fetuses exposed to nicotine. Furthermore the turnover of neurotransmitters, as indicated by the mean metabolite to neurotransmitter ratio was higher in nicotine-exposed fetuses. Thus while these parameters were not significant, due to the small sample size in this study they warrant further investigation.
Comparison between changes in the occipital lobe and brainstem in our model
Following prenatal nicotine exposure only the fetal occipital lobe had an increase in 3H-epibatidine binding which most likely represented increased expression of nicotine sensitive α4β2 nAChRs. This increase may aid in the explanation of visual deficits present after birth in people whose mothers smoked during pregnancy [28]. Our previous assessment of the medulla in these animals showed that the exposure paradigm resulted in increased nACh and 5-HT1A receptor binding specifically in the raphé obscurus of the brainstem. Despite the fact that 5-HT innervation of the cortex originates from neurons in the brainstem, we did not observe alterations to markers of the 5-HT system in the occipital lobe. Thus suggesting that nicotine has discrete affects in the brain and that 5-HT neurons, or the regions in which they are located, may be more vulnerable to toxic insults than cortical neurons or downstream projection sites [48]. In the raphé we demonstrated that 5-HT neurons express nAChRs and thus provided a possible site of interaction where prenatal nicotine could affect the 5-HT system. We reported that dysfunctional interactions between the 5-HT and cholinergic systems occurring at this site may be paramount in the pathogenesis of the parasympathetic abnormalities present in these animals [15]. Given the overlap between selective changes in the raphé obscurus in our baboon model and reported nAChR [14] and 5-HT receptor [16] binding abnormalities in similar regions of the brainstem in SIDS infants, and the critical role of the brainstem in the modulation of homeostatic function [23, 40], the findings in the brainstem were particularly relevant to determining the basis of maternal smoking during pregnancy associated with SIDS risk. Thus we hypothesize that exposure to prenatal nicotine may increase the risk of SIDS via its actions in the brainstem, however, in children that survive past the period of vulnerability, subtle abnormalities in regions such as the visual cortex, and possibly other cortical regions, may lead to subtle long-term visual and cognitive deficits that manifest later on in life. This includes long-term altered sensitivities of these receptors as human patients who were exposed to nicotine during pregnancy have reduced visual attention performance accuracy which is further reduced should they subsequently be exposed to nicotine during adolescence [28].
Potential limitations
This study was limited to the examination of the fetus and thereby precluded examination of postnatal changes that are likely to have an impact on long-term outcomes. Fetuses exposed to cigarette smoke have been exposed to a multitude of potential neurotoxins during and after pregnancy and not pure nicotine as in these studies of the fetal baboon. Nevertheless, the effects seen here suggest specific changes that have the potential for long-term adverse consequences. Another potential limitation of this study is the small sample size due to the difficulty in obtaining fully instrumented chronic preparations in this model. Because these data are difficult to accrue, multiple parameters were obtained in each pregnancy. After correcting for multiple testing of primary variables, we found robust changes that underscore their potential validity as supported by the discrete changes to nAChRs and markers of the 5-HT system in the medulla from these same animals [15]. Thus, as a primary goal of our study was to investigate whether prenatal nicotine exposure would alter the binding to its receptors depending upon nAChR subunit composition and BA region, the significant differences observed in this study clearly indicate that the robust effects of prenatal nicotine are not uniform across different nAChR subunit compositions as originally hypothesized, despite the small sample size. However, while we highlight our robust findings for nAChRs, additional areas of potential differences to both the 5-HT and catecholaminergic systems may exist and warrant confirmation.
Summary
This study provides evidence that exogenous exposure to nicotine during gestation may result in an imbalance of central cholinergic processing in the occipital lobe. While the long-term effects after birth are still unknown, this imbalance has the potential to affect network formation and pathway signaling. Specifically, the results showing an influence of fetal exposure to nicotine on cortical regions associated with visual function point to a potential understanding of the visual deficits later in life following nicotine exposure via maternal smoking in pregnancy. Furthermore, the coherent distribution of nAChRs and markers of the 5-HT system suggest the potential for an impact on signal integration for both the cholinergic and serotonergic systems across cortical layers. The region specific effects of prenatal nicotine on the cholinergic and serotonergic markers presented here for the occipital lobe and previously for the medulla [15] suggest selective regional/nuclear, laminar and subunit specific changes to both systems following chronic exposure to nicotine during pregnancy and aid in the explanation of some of the behavioral effects induced by maternal cigarette smoking. Together these results suggest prenatal nicotine exposure results in an imbalance in central cholinergic (cortically and brainstem) and serotonergic (brainstem) systems. Findings underscore the potential of this fetal primate model for deciphering the cellular and molecular mechanisms underlying brain disorders associated with exposure to neurotoxic agents.
ACKNOWLEDGEMENTS
The authors thank May Yang (MPH) for assistance with the statistical analysis and Richard A. Belliveau, Tung-wah Kiu, and Salha S. Daniel for assistance with experimental aspects of this work. Thus study was supported by the First Candle/SIDS Alliance, CJ Martin Fellowship (National Health and Medical Research Council 359250), the Australian Research Council of which JRD is a Future Fellow (100100235), a grant from the National Institute of Child Health and Development (P01 HD13063, RIS) and the Victorian Government’s Operational Infrastructure Support Scheme.
Footnotes
There are no conflicts of interest.
REFERENCES
- 1.Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aracri P, Consonni S, Morini R, Perrella M, Rodighiero S, Amadeo A, Becchetti A. Tonic modulation of GABA release by nicotinic acetylcholine receptors in layer V of the murine prefrontal cortex. Cereb Cortex. 2010;20:1539–1555. doi: 10.1093/cercor/bhp214. [DOI] [PubMed] [Google Scholar]
- 3.Aztiria E, Gotti C, Domenici L. Alpha7 but not alpha4 AChR subunit expression is regulated by light in developing primary visual cortex. The Journal of comparative neurology. 2004;480:378–391. doi: 10.1002/cne.20358. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Peled O, Gross-Isseroff R, Ben-Hur H, Hoskins I, Groner Y, Biegon A. Fetal human brain exhibits a prenatal peak in the density of serotonin 5-HT1A receptors. Neuroscience letters. 1991;127:173–176. doi: 10.1016/0304-3940(91)90787-t. [DOI] [PubMed] [Google Scholar]
- 5.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approachto multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 6.Bhattacharyya A, Biessmann F, Veit J, Kretz R, Rainer G. Functional and laminar dissociations between muscarinic and nicotinic cholinergic neuromodulation in the tree shrew primary visual cortex. The European journal of neuroscience. 2012;35:1270–1280. doi: 10.1111/j.1460-9568.2012.08052.x. [DOI] [PubMed] [Google Scholar]
- 7.Carli M, Baviera M, Invernizzi RW, Balducci C. Dissociable contribution of 5-HT1A and 5-HT2A receptors in the medial prefrontal cortex to different aspects of executive control such as impulsivity and compulsive perseveration in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:757–767. doi: 10.1038/sj.npp.1300893. [DOI] [PubMed] [Google Scholar]
- 8.Chiolero A, Bovet P, Paccaud F. Association between maternal smoking and low birth weight in Switzerland: the EDEN study. Swiss medical weekly. 2005;135:525–530. doi: 10.4414/smw.2005.11122. [DOI] [PubMed] [Google Scholar]
- 9.Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins AC, Luo Y, Selvaag S, Marks MJ. Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. The Journal of pharmacology and experimental therapeutics. 1994;271:125–133. [PubMed] [Google Scholar]
- 11.Dau A, Komal P, Truong M, Morris G, Evans G, Nashmi R. RIC-3 differentially modulates alpha4beta2 and alpha7 nicotinic receptor assembly, expression, and nicotine-induced receptor upregulation. BMC neuroscience. 2013;14:47. doi: 10.1186/1471-2202-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dotigny F, Ben Amor AY, Burke M, Vaucher E. Neuromodulatory role of acetylcholine in visually-induced cortical activation: behavioral and neuroanatomical correlates. Neuroscience. 2008;154:1607–1618. doi: 10.1016/j.neuroscience.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Duncan JR, Paterson DS, Kinney HC. The development of nicotinic receptors in the human medulla oblongata: inter-relationship with the serotonergic system. Autonomic neuroscience : basic & clinical. 2008;144:61–75. doi: 10.1016/j.autneu.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan JR, Randall LL, Belliveau RA, Trachtenberg FL, Randall B, Habbe D, Mandell F, Welty TK, Iyasu S, Kinney HC. The effect of maternal smoking and drinking during pregnancy upon (3)H-nicotine receptor brainstem binding in infants dying of the sudden infant death syndrome: initial observations in a high risk population. Brain Pathol. 2008;18:21–31. doi: 10.1111/j.1750-3639.2007.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, Stark RI. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for sudden infant death syndrome. J Appl Physiol. 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA : the journal of the American Medical Association. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyck RH, Cynader MS. Autoradiographic localization of serotonin receptor subtypes in cat visual cortex: transient regional, laminar, and columnar distributions during postnatal development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13:4316–4338. doi: 10.1523/JNEUROSCI.13-10-04316.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eickhoff SB, Rottschy C, Zilles K. Laminar distribution and co-distribution of neurotransmitter receptors in early human visual cortex. Brain structure & function. 2007;212:255–267. doi: 10.1007/s00429-007-0156-y. [DOI] [PubMed] [Google Scholar]
- 19.Fregosi RF, Pilarski JQ. Prenatal nicotine exposure and development of nicotinic and fast amino acid-mediated neurotransmission in the control of breathing. Respiratory physiology & neurobiology. 2008;164:80–86. doi: 10.1016/j.resp.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebhard R, Zilles K, Schleicher A, Everitt BJ, Robbins TW, Divac I. Distribution of seven major neurotransmitter receptors in the striate cortex of the New World monkey Callithrix jacchus. Neuroscience. 1993;56:877–885. doi: 10.1016/0306-4522(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 21.Good CH, Bay KD, Buchanan RA, McKeon KA, Skinner RD, Garcia-Rill E. Prenatal exposure to cigarette smoke affects the physiology of pedunculopontine nucleus (PPN) neurons in development. Neurotoxicology and teratology. 2006;28:210–219. doi: 10.1016/j.ntt.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends in pharmacological sciences. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Haselton JR, Winters RW, Liskowsky DR, Haselton CL, McCabe PM, Schneiderman N. Cardiovascular responses elicited by electrical and chemical stimulation of the rostral medullary raphe of the rabbit. Brain research. 1988;453:167–175. doi: 10.1016/0006-8993(88)90155-2. [DOI] [PubMed] [Google Scholar]
- 24.Hellstrom-Lindahl E, Court JA. Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behavioural brain research. 2000;113:159–168. doi: 10.1016/s0166-4328(00)00210-2. [DOI] [PubMed] [Google Scholar]
- 25.Hellstrom-Lindahl E, Nordberg A. Smoking during pregnancy: a way to transfer the addiction to the next generation? Respiration. international review of thoracic diseases. 2002;69:289–293. doi: 10.1159/000063261. [DOI] [PubMed] [Google Scholar]
- 26.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and biobehavioral reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Inder T, Neil J, Kroenke C, Dieni S, Yoder B, Rees S. Investigation of cerebral development and injury in the prematurely born primate by magnetic resonance imaging and histopathology. Developmental neuroscience. 2005;27:100–111. doi: 10.1159/000085981. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- 29.Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. Journal of medicinal chemistry. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 30.Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends in neurosciences. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- 31.Kang JI, Vaucher E. Cholinergic pairing with visual activation results in long-term enhancement of visual evoked potentials. PloS one. 2009;4:e5995. doi: 10.1371/journal.pone.0005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kojic L, Dyck RH, Gu Q, Douglas RM, Matsubara J, Cynader MS. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1841–1844. doi: 10.1073/pnas.97.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsson M, Montgomery SM. Maternal smoking during pregnancy and physical control and coordination among offspring. Journal of epidemiology and community health. 2011;65:1151–1158. doi: 10.1136/jech.2008.085241. [DOI] [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.McDonnell-Naughton M, McGarvey C, O’Regan M, Matthews T. Maternal smoking and alcohol consumption during pregnancy as risk factors for sudden infant death. Irish medical journal. 2012;105:105–108. [PubMed] [Google Scholar]
- 36.Muhammad A, Mychasiuk R, Nakahashi A, Hossain SR, Gibb R, Kolb B. Prenatal nicotine exposure alters neuroanatomical organization of the developing brain. Synapse. 2012;66:950–954. doi: 10.1002/syn.21589. [DOI] [PubMed] [Google Scholar]
- 37.Ogburn PL, Jr., Hurt RD, Croghan IT, Schroeder DR, Ramin KD, Offord KP, Moyer TP. Nicotine patch use in pregnant smokers: nicotine and cotinine levels and fetal effects. American journal of obstetrics and gynecology. 1999;181:736–743. doi: 10.1016/s0002-9378(99)70521-1. [DOI] [PubMed] [Google Scholar]
- 38.Oliff HS, Gallardo KA. The effect of nicotine on developing brain catecholamine systems. Frontiers in bioscience : a journal and virtual library. 1999;4:D883–897. doi: 10.2741/oliff. [DOI] [PubMed] [Google Scholar]
- 39.Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. The Journal of pharmacology and experimental therapeutics. 1991;258:1127–1136. [PubMed] [Google Scholar]
- 40.Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflugers Archiv : European journal of physiology. 2001;442:78–86. doi: 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- 41.Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacology & therapeutics. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 42.Ponsonby AL, Brown SA, Kearns LS, MacKinnon JR, Scotter LW, Cochrane JA, Mackey DA. The association between maternal smoking in pregnancy, other early life characteristics and childhood vision: the Twins Eye Study in Tasmania. Ophthalmic epidemiology. 2007;14:351–359. doi: 10.1080/01658100701486467. [DOI] [PubMed] [Google Scholar]
- 43.Qiu L, Tian L, Pan C, Zhu R, Liu Q, Yan J, Zhao Q, Yuan H, Han Y, Yue W, Yan H, Zhang D. Neuroanatomical circuitry associated with exploratory eye movement in schizophrenia: a voxel-based morphometric study. PloS one. 2011;6:e25805. doi: 10.1371/journal.pone.0025805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raatikainen K, Huurinainen P, Heinonen S. Smoking in early gestation or through pregnancy: a decision crucial to pregnancy outcome. Preventive medicine. 2007;44:59–63. doi: 10.1016/j.ypmed.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Rahi JS, Cumberland PM, Peckham CS. Visual function in working-age adults: early life influences and associations with health and social outcomes. Ophthalmology. 2009;116:1866–1871. doi: 10.1016/j.ophtha.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Rakic P, Goldman-Rakic PS, Gallager D. Quantitative autoradiography of major neurotransmitter receptors in the monkey striate and extrastriate cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:3670–3690. doi: 10.1523/JNEUROSCI.08-10-03670.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rakic P, Lidow MS. Distribution and density of monoamine receptors in the primate visual cortex devoid of retinal input from early embryonic stages. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:2561–2574. doi: 10.1523/JNEUROSCI.15-03-02561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren Y, Feng J. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. Journal of neurochemistry. 2007;103:303–311. doi: 10.1111/j.1471-4159.2007.04741.x. [DOI] [PubMed] [Google Scholar]
- 49.Rossi FM, Pizzorusso T, Porciatti V, Marubio LM, Maffei L, Changeux JP. Requirement of the nicotinic acetylcholine receptor beta 2 subunit for the anatomical and functional development of the visual system. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6453–6458. doi: 10.1073/pnas.101120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seppa T, Salminen O, Moed M, Ahtee L. Induction of Fos-immunostaining by nicotine and nicotinic receptor antagonists in rat brain. Neuropharmacology. 2001;41:486–495. doi: 10.1016/s0028-3908(01)00093-4. [DOI] [PubMed] [Google Scholar]
- 51.Shacka JJ, Robinson SE. Exposure to prenatal nicotine transiently increases neuronal nicotinic receptor subunit alpha7, alpha4 and beta2 messenger RNAs in the postnatal rat brain. Neuroscience. 1998;84:1151–1161. doi: 10.1016/s0306-4522(97)00564-2. [DOI] [PubMed] [Google Scholar]
- 52.Slotkin TA, Pinkerton KE, Auman JT, Qiao D, Seidler FJ. Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain research Developmental brain research. 2002;133:175–179. doi: 10.1016/s0165-3806(02)00281-x. [DOI] [PubMed] [Google Scholar]
- 53.Slotkin TA, Seidler FJ, Qiao D, Aldridge JE, Tate CA, Cousins MM, Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Spindel ER. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C: neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:129–144. doi: 10.1038/sj.npp.1300544. [DOI] [PubMed] [Google Scholar]
- 54.Slotkin TA, Pinkerton KE, Tate CA, Seidler FJ. Alterations of serotonin synaptic proteins in brain regions of neonatal Rhesus monkeys exposed to perinatal environmental tobacco smoke. Brain research. 2006;1111:30–35. doi: 10.1016/j.brainres.2006.06.094. [DOI] [PubMed] [Google Scholar]
- 55.Slotkin TA, MacKillop EA, Rudder CL, Ryde IT, Tate CA, Seidler FJ. Permanent, sex-selective effects of prenatal or adolescent nicotine exposure, separately or sequentially, in rat brain regions: indices of cholinergic and serotonergic synaptic function, cell signaling, and neural cell number and size at 6 months of age. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:1082–1097. doi: 10.1038/sj.npp.1301231. [DOI] [PubMed] [Google Scholar]
- 56.Slotkin TA, Ryde IT, Tate CA, Seidler FJ. Lasting effects of nicotine treatment and withdrawal on serotonergic systems and cell signaling in rat brain regions: separate or sequential exposure during fetal development and adulthood. Brain research bulletin. 2007;73:259–272. doi: 10.1016/j.brainresbull.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Smith MA, Jr., Zhang Y, Polli JR, Wu H, Zhang B, Xiao P, Farwell MA, Pan X. Impacts of chronic low-level nicotine exposure on Caenorhabditis elegans reproduction: identification of novel gene targets. Reprod Toxicol. 2013;40:69–75. doi: 10.1016/j.reprotox.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soma S, Shimegi S, Osaki H, Sato H. Cholinergic modulation of response gain in the primary visual cortex of the macaque. Journal of neurophysiology. 2012;107:283–291. doi: 10.1152/jn.00330.2011. [DOI] [PubMed] [Google Scholar]
- 59.Stark RI, Daniel SS, James LS, MacCarter G, Morishima HO, Niemann WH, Rey H, Tropper PJ, Yeh MN. Chronic instrumentation and longterm investigation in the fetal and maternal baboon: tether system, conditioning procedures and surgical techniques. Laboratory animal science. 1989;39:25–32. [PubMed] [Google Scholar]
- 60.Tizabi Y, Perry DC. Prenatal nicotine exposure is associated with an increase in [125I]epibatidine binding in discrete cortical regions in rats. Pharmacology, biochemistry, and behavior. 2000;67:319–323. doi: 10.1016/s0091-3057(00)00379-8. [DOI] [PubMed] [Google Scholar]
- 61.van de Kamp JL, Collins AC. Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacology, biochemistry, and behavior. 1994;47:889–900. doi: 10.1016/0091-3057(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 62.Wonnacott S. The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends in pharmacological sciences. 1990;11:216–219. doi: 10.1016/0165-6147(90)90242-z. [DOI] [PubMed] [Google Scholar]
- 63.Zinke W, Roberts MJ, Guo K, McDonald JS, Robertson R, Thiele A. Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized Marmoset monkeys. The European journal of neuroscience. 2006;24:314–328. doi: 10.1111/j.1460-9568.2006.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]